Abstract
Background
Probiotics are often used to prevent antibiotic-induced low-diversity dysbiosis, however their effect is not yet sufficiently summarized in this regard. We aimed to investigate the effects of concurrent probiotic supplementation on gut microbiome composition during antibiotic therapy.
Methods
We performed a systematic review and meta-analysis of randomized controlled trials reporting the differences in gut microbiome diversity between patients on antibiotic therapy with and without concomitant probiotic supplementation. The systematic search was performed in three databases (MEDLINE (via PubMed), Embase, and Cochrane Central Register of Controlled Trials (CENTRAL)) without filters on 15 October 2021. A random-effects model was used to estimate pooled mean differences (MD) with 95% confidence intervals (CI). This review was registered on PROSPERO (CRD42021282983).
Results
Of 11,769 identified articles, 15 were eligible in the systematic review and 5 in the meta-analyses. Quantitative data synthesis for Shannon (MD = 0.23, 95% CI: [(−)0.06–0.51]), Chao1 (MD = 11.59 [(−)18.42–41.60]) and observed OTUs (operational taxonomic unit) (MD = 17.15 [(−)9.43–43.73]) diversity indices revealed no significant difference between probiotic supplemented and control groups. Lacking data prevented meta-analyzing other diversity indices; however, most of the included studies reported no difference in the other reported α- and ß-diversity indices between the groups. Changes in the taxonomic composition varied across the eligible studies but tended to be similar in both groups. However, they showed a potential tendency to restore baseline levels in both groups after 3–8 weeks.
This is the first meta-analysis and the most comprehensive review of the topic to date using high quality methods. The limited number of studies and low sample sizes are the main limitations of our study. Moreover, there was high variability across the studies regarding the indication of antibiotic therapy and the type, dose, and duration of antimicrobials and probiotics.
Conclusions
Our results showed that probiotic supplementation during antibiotic therapy was not found to be influential on gut microbiome diversity indices. Defining appropriate microbiome diversity indices, their standard ranges, and their clinical relevance would be crucial.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-023-02961-0.
Keywords: Antibiotics, Probiotics, Gastrointestinal microbiome, Meta-analysis, Diversity
Background
Antibiotic treatment affects the gut bacterial microbiota quantitatively and qualitatively, causing a decrease or even extinction of certain species, leading to a low-diversity microbiome, and allowing some potentially harmful bacteria to become dominant, e.g., Clostridium perfringens, Staphylococcus aureus, or Clostridioides difficile [1, 2]. This microbial imbalance is called dysbiosis. The deviation from the normal microbiome has been linked to obesity, malnutrition, inflammatory bowel disease, neurological dysfunctions, and cancer [3]. The gut microbiota can spontaneously recover, but it is influenced by various host and external factors like age, health status, the geographical area of origin of patients, dose, duration, and the spectrum of antibiotic treatment [4–6]. Young, healthy adults have stable microbial community functions [7], but repeated perturbation of the ecosystem is particularly detrimental if there is insufficient time for recovery after the initial impairment. Previous research has shown that the gut microbiota recovers within about 2 weeks after a single antibiotic exposure in adults, but repeated exposures can significantly prolong the recovery time [4, 8–10].
Probiotics are preparations containing live micro-organisms, typically composed of microbes that are also found in the natural gut flora. Probiotics may contain bacteria, yeasts, or a mixture of them [11]. These products are used to prevent dysbiosis; however, the effects of concurrent probiotic supplementation on fecal microbiota diversity and taxonomical composition during antibiotic therapy are not fully understood. The effects of these products on clinical outcomes during antibiotic therapy have been intensely researched; however, most research did not focus on investigating the composition of the gut microbiome. This aspect is also missing from the current guidelines on the use of probiotics of the American Gastroenterological Association (AGA) and World Gastroenterology Organization (WGO) [11, 12].
So far, reported results have been highly variable both in terms of the reported outcomes and the conclusion about the efficacy of probiotics on microbiota restoration after antibiotic therapy. The biggest challenges in analyzing it are the lack of a consensus definition for ‘normal’ microbiota, generally accepted diversity indicators, and standard measurement methods [13–16]. Moreover, the significant inter-individual variation in microbial species makes it difficult to define the normal microbiota. Several types of diversity indices have been described so far [17]. Diversity indices commonly used in ecology are used to characterize microbiome diversity. α-diversity indices reflect the diversity of a single sample, measuring species richness (number of species) and/or distribution (evenness of species). Each alpha diversity index is calculated differently, depending on factors like how the presence or absence of certain rare species is assessed and interpreted. In contrast, β-diversity indices can be used to compare different samples and communities. It can consider both the overall abundance per sample and the abundance of each taxon [17]. In simple terms, α-diversity represents a within-sample diversity, whereas β-diversity describes similarity or dissimilarity between samples [3, 14, 15].
We aimed to systematically review and meta-analyze the effect of probiotics on antibiotic-induced dysbiosis in randomized controlled trials.
Methods
We designed the study according to the Cochrane recommendations [18]. We previously submitted our study protocol to the International prospective register of systematic reviews (PROSPERO) (CRD42021282983) and applied it consistently (Additional File 1: Supplementary Methods S1). When reporting our results, we followed the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Statement [19] (Additional File 2: Table S1).
Systematic search and selection
The PICO-S format (population, intervention, comparison, outcome, and study design) was used to formulate our clinical question and establish the eligibility criteria. We included all the studies that met the following eligibility criteria: population (P) — people treated with antibiotics regardless of indication; intervention (I) — probiotic supplementation along with antibiotic treatment; comparison group (C) — no probiotic supplementation. The assessed outcomes (O) were gut microbial diversity and composition (any diversity indices reported) at the end of the intervention and after a follow-up period, as reported in each study. No restrictions were applied regarding sex, age, ethnicity, or associated comorbidities. Only randomized controlled trials (RCTs) were included.
The systematic search was conducted without filters or restrictions in three medical databases — MEDLINE (via PubMed), Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) using the search key detailed in Additional File 1: Supplementary Methods S2 until 15 October 2021. We manually screened the reference lists of the studies included in the review for additional eligible articles. If our search did not retrieve the published protocols for the identified eligible studies, we tried to find them at https://www.clinicaltrialsregister.eu/ and https://clinicaltrials.gov/.
The selection was performed with the reference management program EndNote X9 (Clarivate Analytics, Philadelphia, PA, USA). After automatic and manual duplicate removal, two independent investigators manually selected the articles stepwise, first by title and abstract and subsequently by full-text contents adhering to the predefined eligibility criteria. Cohen’s kappa coefficient was calculated at each selection step to quantify the agreement between assessors. Disagreements were solved by consensus.
Data collection
Two independent authors (AJÉ and VB) extracted the data in each article manually and crosschecked each other’s data pool. Disagreements were solved by consensus. The information was summarized in a standardized data collection form (Microsoft Excel, Microsoft Office 365, Redmond, WA, USA). The following data were extracted: study characteristics (first author, year of publication, country, number of centers, and setting), population description (sample size, sex distribution, age, and indication for antibiotic therapy), therapy details for both probiotics and antibiotics (drug/probiotic type, dose, and duration), and outcomes as reported in each article. Outcomes are detailed in Additional File 2: Table S2 [20–36]. When data were available in graphic format only, we performed the extraction with GetData Graph Digitizer software (v. 2.26.0.20.) [37].
Synthesis methods
The statistical analysis was performed by a biostatistician using the R software [38] with meta [39] and dmetar [40] packages. A meta-analysis was performed if the evaluated outcome was reported in at least three articles. For the effect size measure, we calculated mean differences (MD, probiotic and antibiotic minus only antibiotic treatment) with 95% confidence intervals (CIs). The mean and the corresponding standard deviations (SD) were extracted from each study if available. In other cases, to estimate the mean and standard deviation based on 0,1,2,3,4 quartiles (extracted from box plots), Luo [41] and Shi [42] methods were used as implemented in the meta package. On the basis of the article of Oh et al. [43], where the raw data of Shannon, Chao1, and observed OTUs (operational taxonomic units) diversity indices were given, we could assume that the distribution of these indices did not differ from a normal distribution in relevant amount, and therefore the estimation of mean and SD from the quantiles could be acceptable. As the main result, we pooled the values of Shannon, Chao1, and observed OTUs diversity indices after treatment and used the inverse variance weighting method to each of them separately. We included only RCTs; therefore, we could assume that the characteristics before the treatment were not different in the intervention and control groups. As an additional sensitivity analysis, we performed a separate analysis for data before the treatment and a meta-analysis for the “before-after” change values. For the change calculations, we used the correlation coefficient determined from the data of Oh et al. [43]. As we anticipated considerable between-study heterogeneity, a random-effects model was used to pool the effect sizes. We did not apply the Hartung-Knapp adjustment [44, 45]. The maximum-likelihood estimator was applied with the Q profile method for confidence interval to estimate the heterogeneity variance measure τ 2 [46]. Additionally, between-study heterogeneity was described using the Cochran’s Q test and Higgins&Thompson’s I 2 statistics [47]. As the study number was low (< 10), we could not assess the publication bias or additional influence analysis (e.g., leave-one-out analyses).
Forest plots were used to summarize the results graphically. Individual study confidence intervals were presented on the plot using t-distribution estimation. We report the results as (MD, [95% CI lower limit – 95% CI upper limit]).
Risk of bias assessment
Risk-of-bias assessment was performed by two independent authors using the revised Cochrane risk-of-bias tool (RoB2) [48]. Disagreements were solved by consensus. Domains assessed biases resulting from the randomization process, deviations from the intended intervention, missing data, the measurement of the outcome, and the selection of the reported results. The investigators rated each domain, and the risk level was automatically calculated by the algorithm, which could be characterized as low, some concerns, or high.
Certainty assessment
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) tool was applied by two independent investigators to assess the quality of the best available evidence [49]. Disagreements were resolved by consensus.
Results
Study selection
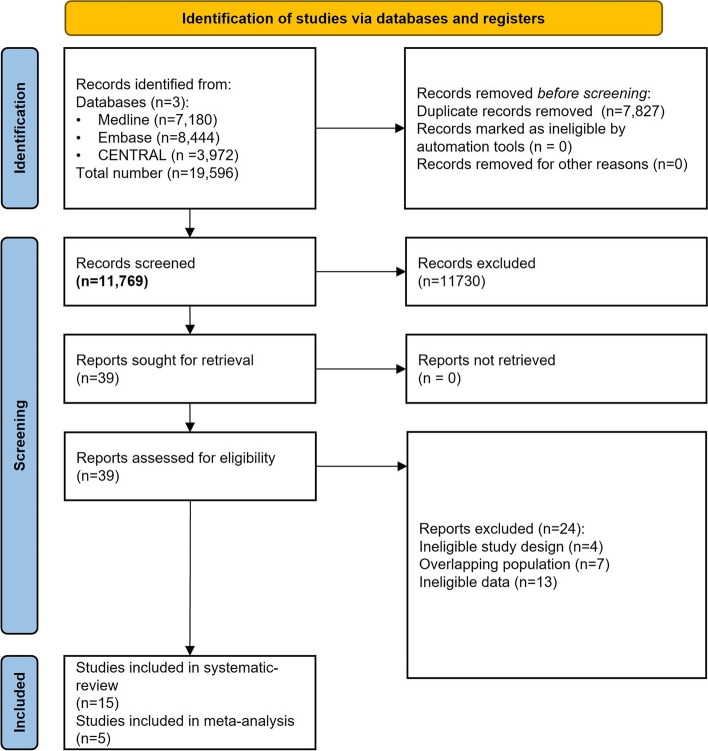
The results of the search and selection processes are summarized in Fig. 1. Our search key identified 19,596 records. Cohen’s kappa index for the title and abstract selection was 0.86, whereas it was 0.95 for the full-text selection. Of the 15 articles eligible for the qualitative synthesis (877 patients), five were suitable for the quantitative synthesis of the Shannon diversity index (335 patients) [43, 50–53] and three for the quantitative synthesis of Chao1 and observed OTUs indices (236 patients) [43, 50, 52]. No additional articles were found by screening the reference lists of the included papers. We included only non-overlapping populations in our review. Most of the studies investigated adult populations. One article investigated neonates [54], and one study included an adolescent population aged 15 years [50]. In eight of the studies, the indication of antibiotic therapy was Heliobacter pylori eradication [43, 50, 52, 55–59]. One study focused on Clostridioides difficile infection [53], and two investigated patients with various infections outside the gastrointestinal tract [54, 60]. Four studies investigated healthy populations without any medical indication for antibiotic therapy [51, 61–63]. For the investigation of the microbial composition, nine studies used the 16S rRNA sequencing technique [43, 50–56, 61], three used standard microbiological culturing techniques [57–59], one study combined DNA-based terminal restriction fragment length polymorphism (TRFLP) analysis and standard culturing methods [62], and two studies used other polymerase chain reaction (PCR)-based techniques [60, 63]. All included articles were available in full text and were published in peer-reviewed journals, except the study by Amarri et al. [60], which was available as a report on the EU Clinical Trials Register website.
Fig. 1.
PRISMA flowchart of the selection process
Study characteristics (Table 1)
Table 1.
The main characteristics of the included studies
| Study | Country | Study design a | Population | Antibiotic (and additional) treatment | Probiotic supplementation | Microbiome analysis method | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of randomized patients (female %) | Age (years - mean ± SD) in the intervention (and control) groups | Indication | Type and dose | Duration (days) | Type and dose | Duration (days) | ||||
| Cárdenas et al. (2020) [55] | Ecuador | single-blinded RCT | 38 (60.5) |
37.9 ± 7.2 (39.5 ± 10.7) |
Helicobacter pylori infection |
Amoxicillin 1 g tid, tinidazole 1 g qid, omeprazole 40 mg bid | 14 |
Saccharomyces boulardii CNCM I-745 22.5 × 109 CFU/day |
14 | 16S rRNA sequencing |
| Chen et al. (2018) [56] | China | open-label RCT | 70 (78.5) |
43.89 ± 12.50 (43.20 ± 12.45) |
Helicobacter pylori infection |
Pantoprazole 40 mg, amoxicillin 1000 mg, furazolidone 100 mg, colloidal bismuth pectin 0.4 g, bid | 14 |
Clostridium butyricum 3 × 40 mg/day |
14 | 16S rRNA sequencing |
| De Wolfe et al. (2018) [53] | USA | double-blinded, placebo controlled RCT | 31 (N.D.) |
N.D. (N.D.) |
Clostridioides difficile infection | Standard of care antibiotics (vancomycin, metronidazole, or fidaxomicin) | 28 |
Lactobacillus acidophilus NCFM® (ATCC 700396), Lactobacillus paracasei Lpc-37 (ATCC SD5275), Bifidobacterium lactis Bi-07 (ATCC SC5220), and Bifidobacterium lactis Bl-04 (ATCC SD5219). 1.7 × 1010 CFU/day |
28 | 16S rRNA sequencing |
| Kabbani et al. (2017) [61] | USA | open-label RCT | 24 (59) |
N.D. (N.D.) |
Healthy volunteers no indication |
Amoxicillin-clavulanate 875/125 mg, bid | 7 |
Saccharomyces boulardii (SB) CNCM I-745 (syn. CBS 5926) 2 × 500 mg/day |
14 | 16S rRNA gene pyrosequencing (bTEFAP). |
| Kakiuchi et al. (2020) [50] | Japan | open-label RCT | 65 (44.6) |
15.31 ± 0.32 (15.08 ± 0.28) |
Helicobacter pylori infection |
Vonoprazan 20 mg, amoxicillin 750 mg, clarithromycin 400 mg bid | 7 |
Enterococcus faecium 129 BIO 3B-R. 3 tablets/day |
7 | 16S rDNA sequencing |
| MacPherson et al. (2018) [51] | Canada | double-blinded, placebo-controlled RCT | 70 (N.D.) |
N.D. (N.D.) |
Healthy volunteers no indication |
Amoxicillin trihydrate 875 mg, potassium clavulanate 125 mg | 7 |
Lactobacillus rhamnosus R0011 and Lactobacillus helveticus R0052 3.8 × 109 CFU and 0.2 × 109 CFU/day |
14 | 16S rRNA gene amplicon, shotgun metagenomics sequencing |
| Oh et al. (2016) [43] | Korea | RCT | 20 (30) |
51.7 ± 0.79 (49.3 ± 3.56) |
Helicobacter pylori infection |
Clarithromycin 500 mg, amoxicillin 1000 mg, lansoprazole 30 mg bid | 14 |
Streptococcus faecium and Bacillus subtilis 2 × (9 × 108 and 1 × 108)/day |
14 | 16S rRNA gene-pyrosequencing |
| Tang et al. (2021) [52] | China | placebo-controlled, multi-center RCT | 151 (34.4) |
43.29 ± 11.30 (45.32 ± 10.98) |
Helicobacter pylori infection |
Esomeprazole 20 mg, amoxicillin 1000 mg furazolidone 100 mg, bismuth potassium citrate 220 mg bid | 14 |
Enterococcus faecium and Bacillus subtilis 3 × (4.5 × 108 and 5.0 × 107) CFU/day |
28 | 16S rRNA high-throughput sequencing |
| Zhong et al. (2021) [54] | China | open-label parallel RCT | 42 (52.4) |
all neonates (all neonates) |
15 neonates with neonatal pneumonia 5 neonates with urinary tract infection 35 neonates with non-specific infection |
Piperacillin–tazobactam 100 mg/kg bid | 7 |
Bifidobacterium longum, Lactobacillus acidophilus, and Enterococcus faecalis 3 × 1.0 × 107 CFU/day |
7 | High-throughput sequencing of 16S rRNA amplicons |
| Engelbrektson et al. (2009) [62] | USA | placebo-controlledRCT | 40 (77.5) |
36.5 ± N.D. (39.5 ± N.D.) |
Healthy volunteers – no indication | Augmentin (amoxicillin and clavulanic acid) 875 mg bid | 7 |
Bifidobacterium lactis Bl-04 (5 × 109 CFU), Bifidobacterium lactis Bi-07 (5 × 109 CFU), Lactobacillus acidophilus NCFM (5 × 109 CFU) Lactobacillus paracasei Lpc-37 (5 × 109 CFU) and Bifidobacterium bifidum Bb-02 (5 × 108 CFU) 2 × 2.05 × 1010 CFU/day |
21 | DNA-based TRFLP analysis and culture-based microbiological techniques |
| Forssten et al. (2014) [63] | Finland | double-blinded, parallel RCT | 80 (50) |
33.7 ± 9.4 (30.9 ± 10.3) |
Healthy volunteers – no indication | Amoxicillin 875 mg, clavulanate 125 mg | 7 |
Lactobacillus acidophilus (L. acidophilus) ATCC 700396 and Bifidobacterium animalis (B. animalis) ssp. Lactis ATCC SD5220 (Danisco) 12.5 × 109 and 12.5 × 109 CFU/day |
14 | qPCR and flow cytometry |
| Madden et al. (2005) [57] | UK | pilot-scale, double-blinded RCT | 13 (53.8) |
60 ± N.D. (49 ± N.D.) |
Helicobacter pylori infection | Amoxycillin 500 mg qid, metronidazole 400 mg tid, lansoprazole 30 mg bid | 8 |
Lactobacillus acidophilus (CLT60 and CUL21) and two strains of Bifidobacterium bifidum (CUL17 and B. bifidum Rhodia 2.5 × 1010 CFU/day |
14 | Culture-based microbiological techniques |
| Plummer et al. (2005) [58] | UK | double-blinded RCT | 155 (N.D.) |
N.D. N.D. |
Helicobacter pylori infection | Amoxicillin 1 g bid, clarithromycin 500 mg bid, lansoprazole 30 mg bid; in case of penicillin allergy, metronidazole 400 mg tid was substituted | 7 |
Lactobacillus acidophilus (CUL60 and CUL21) and two strains of Bifidobacterium spp 2.5 × 1010 CFU/day |
21 | Culture-based microbiological techniques |
| Wang et al. (2017) [59] | China | double-blinded RCT | 20 (45) |
37.1 ± 12.3 (42.8 ± 13.8) |
Helicobacter pylori infection | Esomeprazole 20 mg bid, amoxicillin 1000 mg bid, clarithromycin 500 mg bid, tinidazole 500 mg bid | 14 |
Saccharomyces boulardii CNCM I-745® 2 × 500 mg |
14 | Culture-based microbiological techniques |
| Amarri et al. (2008) [60] | Italy | open-label, national, parallel RCT | 58 (50) | 40 ± 18.9 months (42.1 ± 18.9 months) | Bacterial upper respiratory tract infections | Amoxicillin 50 mg/kg/day divided in 3 daily doses | 5-10 |
Antibiotic-resistant Bacillus clausii 2 × 2 × 109 CFU/day |
12-17 | PCR-DGGE |
Abbreviations: RCT Randomized controlled trial, USA United States of America, UK United Kingdom, N.D. No data, bid Twice a day; tid, three times a day, qid Four times a day, bTEFAP Bacterial tag–encoded FLX amplicon pyrosequencing, DNA Deoxyribonucleic acid, TRFLP Terminal restriction fragment length polymorphism, qPCR Quantitative real-time polymerase chain reaction PCR-DGGE Polymerase chain reaction denaturing gradient gel electrophoresis, rRNA Ribosomal ribonucleic acid, CFU Colony forming unit
aIf not otherwise mentioned, the studies were single centers
The impact of probiotic supplementation during antibiotic therapy on the Shannon diversity index
We identified eight eligible articles reporting the results of the Shannon diversity index [43, 50–56], but only six provided the data (in numerical or boxplot form) for meta-analysis [43, 50–54]. The article that reported on the neonate population exclusively [54] was not included in the meta-analysis due to the impact on the indirectness of our results [18].
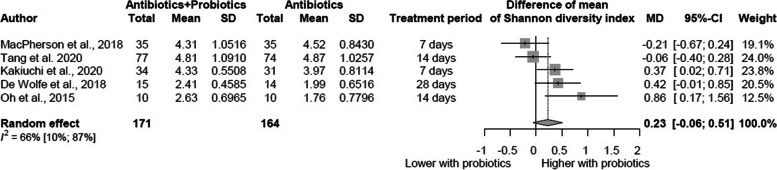
The results of the meta-analysis including five articles with 335 patients are summarized in Fig. 2. On the basis of our results, the gut microbiome diversity was not significantly different between the probiotic-supplemented and antibiotic-only treated groups when measured immediately at the end of antibiotic treatment. The mean difference in Shannon diversity index between the intervention and control groups was 0.23 [(−)0.06 – 0.51].
Fig. 2.
After antibiotic treatment, the Shannon diversity index is not significantly higher in patients receiving concurrent probiotic supplementation than in those treated with antibiotics alone, as measured immediately after antibiotic treatment. CI, confidence interval; MD, mean difference
Although all the included studies were RCTs, the baseline values in the article by De Wolfe et al. [53] showed a marked difference between the probiotic and control groups (MD = 0.64 [(0.05–1.22]). As a sensitivity analysis, we performed a separate calculation for data before the treatment and for the “before-after” values change in each study (Additional File 3: Fig. S1-2). We did not find any significant difference in the change values between the experimental and control groups regarding the Shannon diversity index (MD = 0.07 [(−)0.19–0.32]).
The impact of probiotic supplementation during antibiotic therapy on the Chao1 index
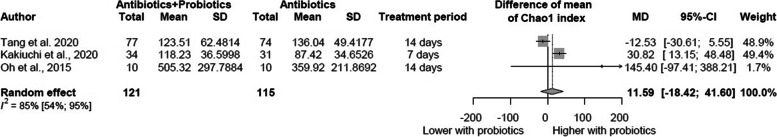
We identified three eligible articles with 236 patients in total for the meta-analysis of Chao1 index, [43, 50, 52]. The results are presented in Fig. 3. The results of Kabbani et al. were previously excluded due to the time point of measurement, which was not reported precisely [61]. According to our results, the mean difference of Chao1 index between the intervention and control groups was 11.59 [(−)18.42–41.60], meaning that the diversity of the intestinal flora of the two groups did not significantly differ from each other.
Fig. 3.
The Chao1 index is not significantly higher in the group receiving concurrent probiotic supplementation than in the group treated with antibiotics alone as measured immediately after antibiotic treatment. CI, confidence interval; MD, mean difference
As there was a large difference in the baseline values between the intervention and control groups in the article of Kakiuchi et al. [50] (MD = 21.57 [3.47–39.68]), here, we also performed an additional sensitivity analysis for the baseline values and the changes (Additional File 3: Fig. S3-4) with no significant difference in the latter between the groups (MD = 3.77 [(−)10.17–17.71]).
The impact of probiotic supplementation during antibiotic therapy on observed OTUs
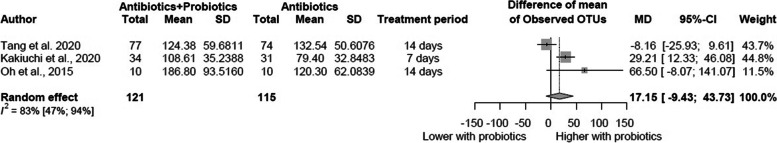
Three of the six articles reporting on Observed OTUs were eligible for quantitative analysis [43, 50, 52]. Others were excluded due to qualitative data reporting [55], not precisely defined time point of measurement [61], or due to the age of the population (neonates) [54]. Results are presented in Fig. 4. According to our results, probiotic supplementation did not result in a significantly different microbiome diversity compared to the antibiotic-only treated group. The mean difference of observed OTUs between the intervention and control groups was 17.15 [(−)9.43–43.73].
Fig. 4.
The number of Observed OTUs is not significantly higher in the group receiving concurrent probiotic supplementation than in the group treated with antibiotics alone, as measured immediately after antibiotic treatment. OTU, operational taxonomic unit; CI, confidence interval; MD, mean difference
The additional sensitivity analysis for the baseline and the change values revealed no significant difference between groups either (Additional File 3: Fig. S5-6) (change: MD = 8.09 [(−)3.87–20.05]).
Qualitative synthesis
The impact of simultaneous probiotic supplementation during antibiotic treatment on α-diversity indices
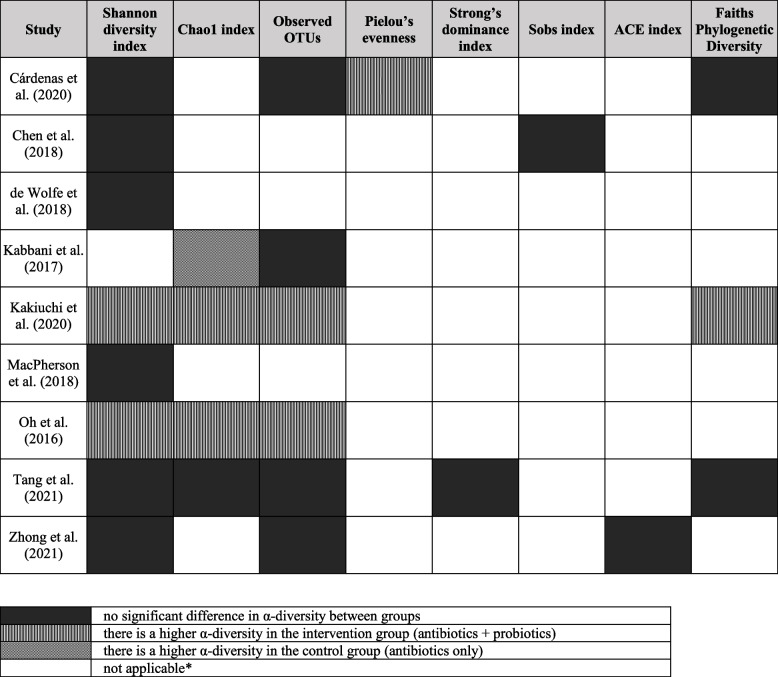
The results of α-diversity indices of the studies, adding those that were not included in the meta-analysis, are summarized in Table 2. The α-diversity indices were lower after the antibiotic administration in both the intervention and the control groups. The three articles — that were not included in the meta-analysis — reporting on the Shannon diversity index revealed no significant difference between the groups [54, 55, 64]. As for the observed OTUs, the three articles not included in the meta-analysis reported no significant difference between the two groups [54, 55, 61]. Regarding the Chao1 index, Kabbani et al. reported significantly higher values in the control group [61]. For most of the α-diversity indices that were not suitable for meta-analysis, the studies did not reveal a significant difference (5% significance level) between the probiotic and control groups. Overall, from the nine studies reporting on α-diversity indices, three were able to show a significant effect of probiotics on at least one index [43, 50, 55]. However, we did not find any common but distinguishable aspects that could explain the similar results.
Table 2.
Changes in the microbiome α-diversity indices as measured after the antibiotic treatment
The impact of simultaneous probiotic supplementation during antibiotic treatment on β-diversity indices
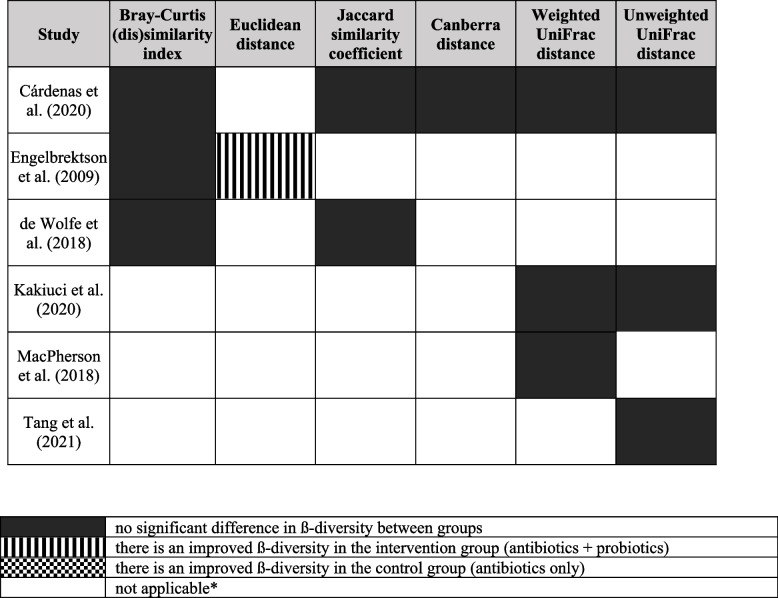
The summarized results of ß-diversity indices are presented in Table 3. The most used ß-diversity indices were Bray-Curtis dissimilarity index and both weighted and unweighted UniFrac (unique fraction metric) distances. Most studies found no significant difference (5% significance level) between the groups. Only Engelbrektson et al. reported a significantly improved ß-diversity by the Euclidean distance [62] in the intervention group. After antibiotic therapy, almost no change occurred in the probiotic group, while there was a large shift toward diminished ß-diversity in the control group. None of the studies reported significant differences between the two groups regarding other indices [50–53, 55].
Table 3.
The systematic review of the microbiome ß-diversity indices as measured immediately after the completion of antibiotic treatment
Taxonomic analysis of microbiome composition
At phylum level, a decreasing trend in the proportion of Firmicutes and Bacteroidetes with a higher relative abundance of Proteobacteria was observed after antibiotic therapy in both groups, regardless of probiotic supplementation. There was also a reduction in the Bacteroidetes:Firmicutes (B:F) ratio at the cessation of treatments. This was confirmed by several studies; however, in the study of Oh et al., the reduction was significantly greater in the control group [43, 52, 56]. Importantly, these changes in phyla abundances disappeared at day 56 in the studies of Chen et al. and Tang et al. [52, 64].
Changes in the level of Enterobacteriaceae family were inconsistent across the studies. Several articles reported an increasing trend of Enterobacteriaceae in the probiotic supplemented group only [55, 62]; however, according to other studies [57, 58], this increase was observed only in the control group. Meanwhile, Forssten et al. and MacPherson et al. reported a higher relative abundance of Enterobacteriaceae in both groups after antibiotic treatment, which normalized after 2 weeks of follow-up [51, 63]. Changes in the other bacterial families were heterogeneously reported (see Tables S3 and S4).
At genus level, Bacteroides showed a decreasing trend in the probiotic supplemented group [53, 55, 57]. However, some studies reported a reduction of Bacteroides in both groups after antibiotic treatment, which showed a re-growing tendency during 3–8 weeks of follow-up [52, 58]. Patients with probiotic supplementation had a higher proportion of Escherichia spp. according to Cárdenas et al. [55], while two other studies reported that the addition of probiotics reduced the overgrowth of Escherichia compared to the control group [43, 61]. According to the study of Tang et al., where probiotic supplementation was continued for two more weeks after antibiotics cessation, the abundance of genus Enterococcus increased at weeks 2 and 4 of follow-up in the intervention group only [52]. Meanwhile, Wang et al. reported this increasing tendency in both groups at week 2. In their study, probiotics were suspended after the antibiotic cessation. However, by weeks 6, 8, and 9 of follow-up, the enrichment of Enterococcus had disappeared in both intervention and control groups as reported in both of the studies [52, 59]. Probiotic supplementation seems to help maintain the level of Bifidobacterium genus [50, 54, 62]. According to Plummer et al., Bifidobacterium decreased in both groups during antibiotic therapy but tended to increase after therapy cessation to day 35 of follow-up [58]. In the study of Kabbani et al., Roseburia prevalence was decreased by antibiotic treatment only; however, Tang et al. reported a significant reduction in both groups [52, 61]. Probiotic supplementation resulted either in an increase of Blautia in the intervention group or decrease in the control group only according to two studies [50, 54]. However, Tang et al. described a lower abundance of Blautia in both groups after antibiotic treatment, with a re-growing tendency with time regardless of probiotic supplementation [52].
The summarized results of the taxonomic analysis of microbiome composition, as measured immediately at the end of simultaneous antibiotic and probiotic treatment, are presented in Additional File 2: Table S3. The results of the follow-up measurements (after cessation of antibiotic and probiotic treatments) are summarized in Additional File 2: Table S4.
Risk of bias assessment
The results of the risk of bias assessment are detailed in Additional File 2: Tables S5-6 and Additional File 3: Fig. S7-12. The overall risk of bias was low to high for the indices included in the meta-analyses. The high risk of bias was caused mainly by the baseline differences between interventional and control groups regarding some of the diversity indices [50, 53].
On the basis of the GRADE assessment, the quality of evidence for the meta-analyses was low (Additional File 2: Table S7).
Discussion
This study is the first systematic review and meta-analysis that summarizes the results of the currently available randomized controlled trials investigating the effect of probiotic supplementation during antibiotic treatment on the gut microbiome. Probiotic supplementation to prevent antibiotic-induced dysbiosis is not supported by our results.
The imbalance of the bacterial composition in the gut microbiome is called dysbiosis. One form of this can be low-diversity dysbiosis, which is often caused by broad-spectrum antibiotic therapy [1]. Decreased gut microbiome diversity has been associated with obesity, inflammatory bowel disease, liver disease, and recurrent Clostridioides difficile infection, among other pathologies. Maintaining the gut microbial diversity during periods of potential impairment seems important [3, 65]. Probiotics are widely used to prevent this dysbiotic state during antibiotic therapy; however, their role and effect on the gut microbiome are still in question.
The results of our systematic review and meta-analysis do not support probiotic supplementation during antibiotic therapy in order to prevent low-diversity dysbiosis. The meta-analysis of Shannon, Chao1, and observed OTUs diversity indices did not show a significant effect of probiotics on maintaining diversity. According to the current evidence, a single index is insufficient to describe bacterial communities [13–15]. Our quantitative results of three alpha diversity indices indicate a lack of significant effect of probiotic supplementation on gut microbiome diversity during antibiotic therapy. We could not include many of the identified reported data in the quantitative analysis as several studies provided only narrative results. However, these data confirm the findings of the meta-analyses as most studies concluded that there were no significant differences in diversity between the probiotic supplemented and the antibiotics alone groups after antibiotic therapy. As for other indices describing α- and ß-diversity, most studies found no significant difference between the two groups, especially when comparing ß-diversities. In conclusion, according to currently available data, there is no evidence that probiotic supplementation has a relevant effect on gut bacterial diversity during antibiotic therapy.
Antibiotic-induced changes in the gut microbial communities, such as decreased Bacteroidetes:Firmicutes (B:F) ratio has been associated with obesity and the metabolic syndrome [66, 67]. This tendency of reduction in the B:F ratio was observed regardless of probiotic supplementation during antibiotic therapy according to several included studies, but it also normalizes in both groups during follow-up [52, 64]. Increased proportion of Proteobacteria was reported by studies in both groups, which is a possible microbial signature of several diseases, such as metabolic disorders and inflammatory bowel disease [68]. These changes, however, showed a restoration tendency after 8 weeks of follow-up [43, 52, 56].
The enrichment of the Enterobacteriaceae family is commonly associated with specific antibiotic resistance genes for aminoglycosides, beta-lactams, and carbapenems, thus being a potentially dangerous source of antibiotic resistance gene transfer [51, 69]. Although the members of this family are considered normal intestinal residents, some may become opportunistic pathogens. They have a higher abundance in inflammatory bowel disease patients, but their underlying pathological mechanisms are still under investigation [70]. Changes in the level of Enterobacteriaceae family were inconsistently reported in the included articles; therefore, we cannot draw strong conclusions about the consequences of probiotic supplementation. The tendency of abundance normalization after the cessation of the antibiotic treatment suggests that the changes in Enterobacteriaceae induced by treatment are transient [51, 63].
Reduced abundance of several species in Bacteroides might be associated with the risk of Clostridioides difficile infection [71]. The reduction of this genus was prevalent in the probiotic supplemented group in several cases, the background of which is unclear [53, 55, 57]. The re-growth of these bacteria during follow-up suggests that the changes are not permanent [52, 58].
Escherichia coli and Enterococcus family species are commensal inhabitants of the gastrointestinal tract that may become pathogens in a dysbiotic environment for several diseases, such as antibiotic-associated diarrhea, vomiting, or permanent intestinal inflammation. Moreover, they are characterized by antibiotic resistance [72, 73]. Probiotic supplementation seems to reduce Escherichia overgrowth during antibiotic therapy according to Kabbani et al. and Oh et al. [43, 61]. Nevertheless, the level of both Escherichia and Enterococcus tends to normalize after antibiotics cessation regardless of probiotics supplementation. This brings the efficacy of probiotics in preventing this type of antibiotic induced dysbiosis into question [52, 59, 61].
Probiotic supplementation seems to maintain the level of Bifidobacteria during antibiotic therapy [50, 54, 62]. Several species and/or strains of this genus may be useful for health, including modulating gut microbial homeostasis, inhibiting pathogens, and modulating immune responses. They can suppress the oncogenic activity within the microbiome, and they are able to produce vitamins and transform food compounds into bioactive molecules [74]. Bifidobacteria also play a crucial role during early life. They are among the first colonizers of the human gut. According to previous studies, children with allergic diseases have a reduced gut microbial diversity with lower abundance of Bifidobacterium, Lactobacillus, and Bacteroides compared to healthy controls [75]. Therefore, the results of Zhong et al. are especially important as they showed that probiotic supplementation was able to maintain the level of Bifidobacteria in newborns during antibiotic therapy [54].
Some of the included articles suggested that probiotic supplementation during antibiotic therapy has a protective effect on Blautia and Roseburia spp. levels [50, 54, 61]. Recently, Blautia has been associated with the alleviation of inflammatory and metabolic diseases by regulating host health, and it has also been characterized by antibacterial activity [76]. Gut Roseburia spp. produce short-chain fatty acids, modulate colonic motility, support immunity, and have anti-inflammatory effects [77]. These findings suggest that probiotic supplementation may have some benefits but the tendency for Blautia levels to normalize spontaneously after antibiotic discontinuation casts doubt on them [52].
Implication for practice and research
The summary of the available literature facilitates the utilization of scientific results in daily practice, which is crucially important [78, 79]. According to our findings, probiotics have only a minimal and temporary effect on the composition and diversity of gut microbiome during antibiotic therapy and are not suitable for preventing antibiotic-induced low-diversity dysbiosis. In this regard, strain-specific probiotic supplementation with antibiotics may be considered especially for vulnerable groups to prevent Clostridioides difficile infection or antibiotic-associated diarrhea, as advised by the current guideline of the AGA and WGO on the use of probiotics [11, 12]. These findings were however not connected to gut microbial compositions. Given our results, which describe a low moderating effect on gut flora, the question arises as to what exactly is the mechanism by which probiotics help prevent these conditions. A recent meta-analysis also points out that some strains may be more effective in the prevention of diarrhea and that the effect depends on the initial risk level. According to this, patients with a low baseline diarrhea risk do not benefit from probiotic supplementation during antibiotic treatment [80]. Our findings do not suggest any further benefit regarding microbiome composition. Further evaluation of the relation between clinical manifestations, microbial diversity indices, and taxonomic composition will bring a more comprehensive understanding of the role of gut microbes in human health and how different factors affect it. The standardization of methods for microbiome diversity measurement and the definition of its optimal value are key factors in generating more homogenous data with increased clinical relevance. A measurement after a standard follow-up period should be considered for all future similar studies to determine the long-term effects.
The relatively small number of publications and the wide range of methods and diversity indices used across the eligible articles indicate that microbiome diversity is an under-researched area and that professional consensus is still lacking. Although recommendations for the conduct and reporting of microbiome research have been published, there are no standards for the choice of diversity indices [81, 82]. Moreover, every year, new approaches to characterize microbiome composition are emerging, making standardization increasingly difficult [83]. Similarly, the relationship of microbiome composition and its changes with physiological functions and clinical symptoms is not well understood yet: no evident clinical characteristics or symptoms can be attributed to the different diversity index values, especially their numerical variation. Our results suggest that the routine use of probiotics is not justified for maintaining gut microbial balance and diversity. This is particularly important for outpatients who are at low risk and usually start taking probiotics for this purpose. This finding is in accordance with the current AGA recommendation, which also highlights that patients with low risk would reasonably select no probiotics, thus avoiding potential harm and additional costs [12]. In order to determine the exact role of probiotics in the clinic, these questions need to be answered through further professional discussion and intensive research.
Strengths and limitations
The main strength of the study is the high level of evidence for our quantitative results as we included only randomized controlled trials in our review and meta-analysis. Moreover, to our knowledge, this is the first meta-analysis and the most comprehensive review of the topic to date. We followed the strict guidelines of Cochrane recommendations [18] and PRISMA Statement [19] when performing our systematic review and meta-analysis, which is strengthening our results.
Although we have identified all relevant studies published on the topic without setting restrictions on microbial variables, we acknowledge the limited availability of eligible articles. Our strict inclusion criterion of using only results from randomized controlled trials ensured the highest level of evidence, despite potentially reduced the number of included articles and cases. This emphasizes the necessity for further high-quality studies. Due to the small sample sizes and the limited number of studies, the results of the meta-analysis of the investigated diversity indices should be handled with criticism. We could not perform a quantitative synthesis of much of the data either due to insufficient reporting and high variability regarding the methods and indices used to measure and describe the gut microbial composition and diversity. ASVs (amplicon sequence variant) are generally considered more accurate method to represent groups of DNA sequences than OTUs as they do not rely on arbitrary similarity thresholds and can identify individual variants within a taxon. However, due to the lack of available studies meeting inclusion criteria using ASV analysis, our meta-analysis utilized OTU data, which did not however hinder the interpretation of consistent results obtained from before-and-after comparisons. Factors such as the use of different bacterial strains as probiotics, varied type and dose of antibiotics, inclusion of subjects with different health conditions (including both diseased and healthy individuals without infections), as well as variations in age across the included studies, could have contributed to the lack of conclusive evidence regarding efficacy. These factors should be taken into consideration when interpreting the results and highlight the need for further research to better understand the impact of these variables on the outcomes.
Conclusions
The summarized results of the currently available randomized controlled trials cannot support probiotic supplementation during antibiotic therapy to prevent low-diversity dysbiosis. The meta-analyses of Shannon, Chao1, and observed OTUs diversity indices showed no significant effect of probiotics on maintaining diversity. Although we could not analyze all the identified results quantitatively, a tendency of no modulating effect of probiotics was observed for other reported α- and β-diversity indices as well. Changes in the taxonomic composition tend to be similar in the intervention and control groups; however, it varies between the different studies The tendency of microbiome restoration after a 3–8-week follow-up period, regardless of probiotic supplementation and remission of the differences between the intervention and control groups, challenges the questions on the benefits of routine probiotic supplementation during antibiotic treatment. There is a strong need to standardize methods and indicators, to build professional consensus, and to continue intensive research on clinical relevance.
Supplementary Information
Additional file 1: Supplementary Methods S1. Details of the study protocol; Supplementary Methods S2. Details of the systematic search.
Additional file 2: Table S1. PRISMA checklist 2020; Table S2. Definitions of gut microbiome diversity outcomes reported in the included studies; Table S3. The summarized results of taxonomic analysis of microbiome composition as measured immediately at the end of simultaneous antibiotic and probiotic treatment; Table S4. Outcomes of follow-up as reported in each study; Table S5. Risk of bias assessment for all outcomes - Assignment to intervention; Table S6. Risk of bias assessment for all outcomes - Adhering to intervention; Table S7. GRADE assessment for the meta-analyses of Shannon, Chao1 and Observed OTUs diversity indices.
Additional file 3: Fig. S1. Additional sensitivity analysis for the baseline values of Shannon diversity index; Fig. S2. Additional sensitivity analysis for the change between the “before-after” values of Shannon diversity index; Fig. S3. Additional sensitivity analysis for the baseline values of Chao1 index; Fig. S4. Additional sensitivity analysis for the change between the “before-after” values of Chao1 index; Fig. S5. Additional sensitivity analysis for the baseline values of Observed OTUs; Fig. S6. Additional sensitivity analysis for the change between the “before-after” values of Observed OTUs; Fig. S7. Risk of bias assessment for the main meta-analysis of Shannon diversity index - Assignment to intervention; Fig. S8. Risk of bias assessment for the main meta-analysis of Shannon diversity index - Adhering to intervention ; Fig. S9. Risk of bias assessment for the meta-analysis of Chao1 index - Assignment to intervention; Fig. S10. Risk of bias assessment for the meta-analysis of Chao1 index - Adhering to interventionFig. S11. Risk of bias assessment for the meta-analysis of Observed OTUs - Assignment to intervention; Fig. S12. Risk of bias assessment for the meta-analysis of Observed OTUs - Adhering to intervention.
Acknowledgments
Not applicable.
Abbreviations
- 16S rRNA
16S ribosomal ribonucleic acid
- ACE
Abundance-based coverage estimator
- AGA
American Gastroenterological Association
- ASV
Amplicon sequence variant
- B:F ratio
Bacteroidetes:Firmicutes ratio
- CFU
Colony forming unit
- bid
Twice a day
- CI
Confidence interval
- DNA
Deoxyribonucleic acid
- GRADE
The Grading of Recommendations, Assessment, Development and Evaluation
- MD
Mean difference
- OTU
Operational taxonomic unit
- PCR
Polymerase chain reaction
- PCR-DGGE
Polymerase chain reaction denaturing gradient gel electrophoresis
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
International prospective register of systematic reviews
- RCT
Randomized controlled trial
- RoB
Risk of bias
- tid
Three times a day
- TRFLP
Terminal restriction fragment length polymorphism
- UK
United Kingdom
- UniFrac
Unique fraction metric
- USA
United States of America
- qid
Four times a day
- qPCR
Quantitative real-time polymerase chain reaction
- WGO
World Gastroenterology Organization
Authors’ contributions
AJÉ: study design, search strategy design, literature screening, data extraction and interpretation, preparation of figures, draft of the manuscript, review of the manuscript; VB: search strategy design, literature screening, data extraction, review of the manuscript; CP, DD: literature screening, review of the manuscript; DSV: statistical analysis, preparation of figures, review of the manuscript; SB, BE, PH: study design, search strategy design, review of the manuscript; LFN, KL: study idea, study design, search strategy design, data interpretation, review of the manuscript. All authors certify that they have participated sufficiently in this work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by Semmelweis University. This project was supported by an ITM NRDIF grant (TKP2021-EGA-23). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. The original datasets used in this study can be found in the full-text publications included in the systematic review and meta-analysis.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anna Júlia Éliás, Email: elias.anna.julia@gmail.com.
Viktória Barna, Email: simon.viktoria2@semmelweis.hu.
Cristina Patoni, Email: patonicristina@gmail.com.
Dóra Demeter, Email: dora.de.dora@gmail.com.
Dániel Sándor Veres, Email: daniel.s.veres@gmail.com.
Stefania Bunduc, Email: stfnbndc@gmail.com.
Bálint Erőss, Email: dr.eross.balint@gmail.com.
Péter Hegyi, Email: hegyi2009@gmail.com.
László Földvári-Nagy, Email: foldvari-nagy.laszlo@semmelweis.hu.
Katalin Lenti, Email: lenti.katalin@semmelweis.hu.
References
- 1.Larcombe S, Hutton ML, Lyras D. Involvement of bacteria other than Clostridium difficile in antibiotic-associated diarrhoea. Trends Microbiol. 2016;24:463–76. doi: 10.1016/j.tim.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro CFA, Silveira GGDOS, Cândido EDS, Cardoso MH, Espínola Carvalho CM, Franco OL. Effects of antibiotic treatment on gut microbiota and how to overcome its negative impacts on human health. ACS Infect Dis. 2020;6:2544–59. doi: 10.1021/acsinfecdis.0c00036. [DOI] [PubMed] [Google Scholar]
- 3.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–30. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Relman DA. The human microbiome: ecosystem resilience and health. Nutr Rev. 2012;70(SUPPL):1. doi: 10.1111/j.1753-4887.2012.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koo H, Hakim JA, Crossman DK, Kumar R, Lefkowitz EJ, Morrow CD. Individualized recovery of gut microbial strains post antibiotics. Biofilms Microbiomes. 2019;5:1–6. doi: 10.1038/s41522-019-0103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horii T, Suzuki S, Takano C, Shibuya H, Ichijima R, Kusano C, et al. Lower impact of vonoprazan–amoxicillin dual therapy on gut microbiota for Helicobacter pylori eradication. J Gastroenterol Hepatol. 2021;36:3314–21. doi: 10.1111/jgh.15572. [DOI] [PubMed] [Google Scholar]
- 7.Becattini S, Taur Y, Pamer EG. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol Med. 2016;22:458–78. doi: 10.1016/j.molmed.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan H, Yu L, Tian F, Zhai Q, Fan L, Chen W. Antibiotic-induced gut dysbiosis and barrier disruption and the potential protective strategies. Crit Rev Food Sci Nutr. 2022;62:1427–52. doi: 10.1080/10408398.2020.1843396. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79:471–89. doi: 10.1016/j.jinf.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108(SUPPL. 1):4554–61. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guarner F, Sanders ME, Szajewska H, Cohen H, Eliakim R, Herrera C, et al. WGO Practice Guideline. Probiotics and Prebiotics. World Gastroenterol Organ Glob Guidel. 2023:1–53.
- 12.Su GL, Ko CW, Bercik P, Falck-ytter Y, Sultan S, Weizman AV, et al. CLINICAL PRACTICE GUIDELINES AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology. 2020;159:697–705. doi: 10.1053/j.gastro.2020.05.059. [DOI] [PubMed] [Google Scholar]
- 13.Kim BR, Shin J, Guevarra RB, Lee JH, Kim DW, Seol KH, et al. Deciphering diversity indices for a better understanding of microbial communities. J Microbiol Biotechnol. 2017;27:2089–93. doi: 10.4014/jmb.1709.09027. [DOI] [PubMed] [Google Scholar]
- 14.Roswell M, Dushoff J, Winfree R. A conceptual guide to measuring species diversity. Oikos. 2021;130:321–38. doi: 10.1111/oik.07202. [DOI] [Google Scholar]
- 15.Xu S, Böttcher L, Chou T. Diversity in biology: definitions, quantification and models. Phys Biol. 2020;17:031001. [DOI] [PMC free article] [PubMed]
- 16.Gilbert JA, Lynch SV. Community ecology as a framework for human microbiome research. Nat Med. 2019;25:884–9. doi: 10.1038/s41591-019-0464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andermann T, Antonelli A, Barrett RL, Silvestro D. Estimating alpha, beta, and gamma diversity through deep learning. Front Plant Sci. 2022;13:839407. [DOI] [PMC free article] [PubMed]
- 18.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2022. www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
- 19.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021:372. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taur Y, Jenq RR, Perales MA, Littmann ER, Morjaria S, Ling L, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124:1174–82. doi: 10.1182/blood-2014-02-554725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rain R, Czernia D, Bowater J. Shannon diversity index calculator. Omni Calculator. 2022. https://www.omnicalculator.com/ecology/shannon-index.
- 22.Lankelma JM, van Vught LA, Belzer C, Schultz MJ, van der Poll T, de Vos WM, et al. Critically ill patients demonstrate large interpersonal variation in intestinal microbiota dysregulation: a pilot study. Intensive Care Med. 2017;43:59–68. doi: 10.1007/s00134-016-4613-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heip C. A new index measuring evenness. J Mar Biol Assoc United Kingdom. 1974;54:555–7. doi: 10.1017/S0025315400022736. [DOI] [Google Scholar]
- 24.Blaxter M, Mann J, Chapman T, Thomas F, Whitton C, Floyd R, et al. Defining operational taxonomic units using DNA barcode data. Philos Trans R Soc B Biol Sci. 2005;360:1935–43. doi: 10.1098/rstb.2005.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao A. Nonparametric estimation of the number of classes in a population. Scand J Stat. 1984;11:265–70. [Google Scholar]
- 26.Faith DP. Conservation evaluation and phylogenetic diversity. Biol Conserv. 1992;61:1–10. doi: 10.1016/0006-3207(92)91201-3. [DOI] [Google Scholar]
- 27.Strong WL. Assessing species abundance unevenness within and between plant communities. Community Ecol. 2002;3:237–46. doi: 10.1556/ComEc.3.2002.2.9. [DOI] [Google Scholar]
- 28.Pielou EC. The measurement of diversity in different types of biological collections. J Theor Biol. 1966;13 C:131–44. doi: 10.1016/0022-5193(66)90013-0. [DOI] [Google Scholar]
- 29.Gotelli NJ, Colwell RK. Estimating species richness. In: Diversity Biological., editor. Frontiers in Measurement and Assessment. Oxford University Press: United Kingdom; 2011. pp. 39–54. [Google Scholar]
- 30.Chao A, Lee SM. Estimating the number of classes via sample coverage. J Am Stat Assoc. 1992;87:210–7. doi: 10.1080/01621459.1992.10475194. [DOI] [Google Scholar]
- 31.Chao A, Yang MCK. Stopping rules and estimation for recapture debugging with unequal failure rates. Biometrika. 1993;80:193–201. doi: 10.1093/biomet/80.1.193. [DOI] [Google Scholar]
- 32.Bray JR, Curtis JT, Roger J. This content downloaded from 147.8.31.43 on Mon. Source Ecol Monogr. 1957;27:325–49. doi: 10.2307/1942268. [DOI] [Google Scholar]
- 33.Jaccard P. The distribution of the flora in the alpine zone. New Phytol. 1912;11:37–50. doi: 10.1111/j.1469-8137.1912.tb05611.x. [DOI] [Google Scholar]
- 34.Zeleny D. Ecological resemblance notes. 1966;:1–10. en:similarity https://www.davidzeleny.net/anadat-r/doku.php/en:similarity.
- 35.Lance GN, Williams WT. Computer programs for hierarchical polythetic classification (“similarity analyses”) Comput J. 1966;9:60–4. doi: 10.1093/comjnl/9.1.60. [DOI] [Google Scholar]
- 36.Lozupone CA, Hamady M, Kelley ST, Knight R. Quantitative and qualitative β diversity measures lead to different insights into factors that structure microbial communities. Appl Environ Microbiol. 2007;73:1576–85. doi: 10.1128/AEM.01996-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fedorov S. GetData Graph Digitizer. Accesed 18 Dec 2021.
- 38.R Core Team . R: a language and environment for statistical computing. 2021. [Google Scholar]
- 39.Schwarzer Guido. Meta: general package for meta-analysis. 2022. [Google Scholar]
- 40.Cuijpers P, Furukawa T, Ebert DD DD. Dmetar: companion r package for the guide doing meta-analysis in r. 2020. [Google Scholar]
- 41.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 42.Shi J, Luo D, Weng H, Zeng XT, Lin L, Chu H, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. 2020;11:641–54. doi: 10.1002/jrsm.1429. [DOI] [PubMed] [Google Scholar]
- 43.Oh B, Kim BS, Kim JW, Kim JS, Koh SJ, Kim BG, et al. The effect of probiotics on gut microbiota during the Helicobacter pylori eradication: Randomized Controlled Trial. Helicobacter. 2016;21:165–74. doi: 10.1111/hel.12270. [DOI] [PubMed] [Google Scholar]
- 44.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 45.Inthout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:1–12. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7:55–79. doi: 10.1002/jrsm.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 48.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:1–8. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 49.GRADEpro GDT: GRADEpro Guideline Development Tool. 2021. https://gradepro.org.
- 50.Kakiuchi T, Mizoe A, Yamamoto K, Imamura I, Hashiguchi K, Kawakubo H, et al. Effect of probiotics during vonoprazan-containing triple therapy on gut microbiota in Helicobacter pylori infection: a randomized controlled trial. Helicobacter. 2020;25:1–8. doi: 10.1111/hel.12690. [DOI] [PubMed] [Google Scholar]
- 51.MacPherson CW, Mathieu O, Tremblay J, Champagne J, Nantel A, Girard SA, et al. Gut bacterial microbiota and its resistome rapidly recover to basal state levels after short-term amoxicillin-clavulanic acid treatment in healthy adults. Sci Rep. 2018;8:1–14. doi: 10.1038/s41598-018-29229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang B, Tang L, Huang C, Tian C, Chen L, He Z, et al. The effect of probiotics supplementation on gut microbiota after Helicobacter pylori eradication: a multicenter randomized controlled trial. Infect Dis Ther. 2021;10:317–33. doi: 10.1007/s40121-020-00372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Wolfe TJ, Eggers S, Barker AK, Kates AE, Dill-McFarland KA, Suen G, et al. Oral probiotic combination of lactobacillus and bifidobacterium alters the gastrointestinal microbiota during antibiotic treatment for clostridium difficile infection. PLoS One. 2018;13:1–13. doi: 10.1371/journal.pone.0204253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong H, Wang XG, Wang J, Chen YJ, Qin HL, Yang R. Impact of probiotics supplement on the gut microbiota in neonates with antibiotic exposure: an open-label single-center randomized parallel controlled study. World J Pediatr. 2021;17:385–93. doi: 10.1007/s12519-021-00443-y. [DOI] [PubMed] [Google Scholar]
- 55.Cárdenas PA, Garcés D, Prado-Vivar B, Flores N, Fornasini M, Cohen H, et al. Effect of Saccharomyces boulardii CNCM I-745 as complementary treatment of Helicobacter pylori infection on gut microbiome. Eur J Clin Microbiol Infect Dis. 2020;39:1365–72. doi: 10.1007/s10096-020-03854-3. [DOI] [PubMed] [Google Scholar]
- 56.Chen L, Xu W, He J, Lee A, Si J, Chen S. The impact of Helicobacter pylori infection, eradication therapy, and probiotic supplementation on gut microenvironment homeostasis: an open-label, prospective clinical trial. J Gastroenterol Hepatol. 2018;33:54–221. doi: 10.1016/j.ebiom.2018.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Madden JAJ, Plummer SF, Tang J, Garaiova I, Plummer NT, Herbison M, et al. Effect of probiotics on preventing disruption of the intestinal microflora following antibiotic therapy: a double-blind, placebo-controlled pilot study. Int Immunopharmacol. 2005;5:1091–7. doi: 10.1016/j.intimp.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 58.Plummer SF, Garaiova I, Sarvotham T, Cottrell SL, Le Scouiller S, Weaver MA, et al. Effects of probiotics on the composition of the intestinal microbiota following antibiotic therapy. Int J Antimicrob Agents. 2005;26:69–74. doi: 10.1016/j.ijantimicag.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Wang ZJ, Chen XF, Zhang ZX, Li YC, Deng J, Tu J, et al. Effects of anti-Helicobacter pylori concomitant therapy and probiotic supplementation on the throat and gut microbiota in humans. Microb Pathog. 2017;109:156–61. doi: 10.1016/j.micpath.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 60.Amarri S, Morelli L. Evaluation of the effects of Enterogermina, 2 billion Bacillus clausii spores, on the intestinal flora of children antibiotic treated for bacterial upper respiratory tract infections: open, pilot study. 2008. [Google Scholar]
- 61.Kabbani TA, Pallav K, Dowd SE, Villafuerte-Galvez J, Vanga RR, Castillo NE, et al. Prospective randomized controlled study on the effects of Saccharomyces boulardii CNCM I-745 and amoxicillin-clavulanate or the combination on the gut microbiota of healthy volunteers. Gut Microbes. 2017;8:17–32. doi: 10.1080/19490976.2016.1267890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Engelbrektson A, Korzenik JR, Pittler A, Sanders ME, Klaenhammer TR, Leyer G, et al. Probiotics to minimize the disruption of faecal microbiota in healthy subjects undergoing antibiotic therapy. J Med Microbiol. 2009;58:663–70. doi: 10.1099/jmm.0.47615-0. [DOI] [PubMed] [Google Scholar]
- 63.Forssten S, Evans M, Wilson D, Ouwehand AC. Influence of a probiotic mixture on antibiotic induced microbiota disturbances. World J Gastroenterol. 2014;20:11878–85. doi: 10.3748/wjg.v20.i33.11878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen L, Xu W, Lee A, He J, Huang B, Zheng W, et al. The impact of Helicobacter pylori infection, eradication therapy and probiotic supplementation on gut microenvironment homeostasis: an open-label, randomized clinical trial. EBioMedicine. 2018;35:87–96. doi: 10.1016/j.ebiom.2018.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kriss M, Hazleton KZ, Nusbacher NM, Martin CG, Lozupone CA. Low diversity gut microbiota dysbiosis: drivers, functional implications and recovery. Curr Opin Microbiol. 2018;44:34–40. doi: 10.1016/j.mib.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Indiani CMDSP, Rizzardi KF, Castelo PM, Ferraz LFC, Darrieux M, Parisotto TM. childhood obesity and firmicutes/bacteroidetes ratio in the gut microbiota: a systematic review. Child Obes. 2018;14:501–9. doi: 10.1089/chi.2018.0040. [DOI] [PubMed] [Google Scholar]
- 67.Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, et al. The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020;12:1474. [DOI] [PMC free article] [PubMed]
- 68.Rizzatti G, Lopetuso LR, Gibiino G, Binda C, Gasbarrini A. Proteobacteria: a common factor in human diseases. Biomed Res Int. 2017;2017:9351507. [DOI] [PMC free article] [PubMed]
- 69.Rozwandowicz M, Brouwer MSM, Fischer J, Wagenaar JA, Gonzalez-Zorn B, Guerra B, et al. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J Antimicrob Chemother. 2018;73:1121–37. doi: 10.1093/jac/dkx488. [DOI] [PubMed] [Google Scholar]
- 70.Janda JM, Abbott SL. The changing face of the family Enterobacteriaceae (order: “Enterobacterales”): new members, taxonomic issues, geographic expansion, and new diseases and disease syndromes. Clin Microbiol Rev. 2021:1–45. [DOI] [PMC free article] [PubMed]
- 71.Schubert AM, Rogers MAM, Ring C, Mogle J, Petrosino JP, Young VB, et al. Microbiome data distinguish patients with clostridium difficile infection and non-c Difficile-associated diarrhea from healthy controls. MBio. 2014;5:1–9. doi: 10.1128/mBio.01021-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Allocati N, Masulli M, Alexeyev MF, Di Ilio C. Escherichia coli in Europe: an overview. Int J Environ Res Public Health. 2013;10:6235–54. doi: 10.3390/ijerph10126235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hanchi H, Mottawea W, Sebei K, Hammami R. The genus Enterococcus: between probiotic potential and safety concerns-an update. Front Microbiol. 2018;9:1–16. doi: 10.3389/fmicb.2018.01791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tojo R, Suárez A, Clemente MG, De Los Reyes-Gavilán CG, Margolles A, Gueimonde M, et al. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol. 2014;20:15163–76. doi: 10.3748/wjg.v20.i41.15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cukrowska B, Bierła JB, Zakrzewska M, Klukowski M, Maciorkowska E. The relationship between the infant gut microbiota and allergy. The role of Bifidobacterium breve and prebiotic oligosaccharides in the activation of anti-allergic mechanisms in early life. Nutrients. 2020;12:946. [DOI] [PMC free article] [PubMed]
- 76.Liu X, Mao B, Gu J, Wu J, Cui S, Wang G, et al. Blautia—a new functional genus with potential probiotic properties? Gut Microbes. 2021;13:1–21. doi: 10.1080/19490976.2021.1875796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zohre T-S, Imen S, Latifa B, Olivier L, Vincent M, Shao Bing F, et al. Roseburia spp.: a marker of health? Future Microbiol. 2017;12:157–170. doi: 10.2217/fmb-2016-0130. [DOI] [PubMed] [Google Scholar]
- 78.Hegyi P, Erőss B, Izbéki F, Párniczky A, Szentesi A. Accelerating the translational medicine cycle: the Academia Europaea pilot. Nat Med. 2021;27:1317–9. doi: 10.1038/s41591-021-01458-8. [DOI] [PubMed] [Google Scholar]
- 79.Hegyi P, Petersen OH, Holgate S, Erőss B, Garami A, Szakács Z, et al. Academia europaea position paper on translational medicine: the cycle model for translating scientific results into community benefits. J Clin Med. 2020;9(5):1532. [DOI] [PMC free article] [PubMed]
- 80.Goodman C, Keating G, Georgousopoulou E, Hespe C, Levett K. Probiotics for the prevention of antibiotic-associated diarrhoea: a systematic review and meta-analysis. BMJ Open. 2021;11:1–14. doi: 10.1136/bmjopen-2020-043054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mirzayi C, Renson A, Furlanello C, Sansone SA, Zohra F, Elsafoury S, et al. Reporting guidelines for human microbiome research: the STORMS checklist. Nat Med. 2021;27:1885–92. doi: 10.1038/s41591-021-01552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qian XB, Chen T, Xu YP, Chen L, Sun FX, Lu MP, et al. A guide to human microbiome research: Study design, sample collection, and bioinformatics analysis. Chin Med J (Engl) 2020;133:1844–55. doi: 10.1097/CM9.0000000000000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gupta VK, Kim M, Bakshi U, Cunningham KY, Davis JM, Lazaridis KN, et al. A predictive index for health status using species-level gut microbiome profiling. Nat Commun. 2020;11:4635. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Methods S1. Details of the study protocol; Supplementary Methods S2. Details of the systematic search.
Additional file 2: Table S1. PRISMA checklist 2020; Table S2. Definitions of gut microbiome diversity outcomes reported in the included studies; Table S3. The summarized results of taxonomic analysis of microbiome composition as measured immediately at the end of simultaneous antibiotic and probiotic treatment; Table S4. Outcomes of follow-up as reported in each study; Table S5. Risk of bias assessment for all outcomes - Assignment to intervention; Table S6. Risk of bias assessment for all outcomes - Adhering to intervention; Table S7. GRADE assessment for the meta-analyses of Shannon, Chao1 and Observed OTUs diversity indices.
Additional file 3: Fig. S1. Additional sensitivity analysis for the baseline values of Shannon diversity index; Fig. S2. Additional sensitivity analysis for the change between the “before-after” values of Shannon diversity index; Fig. S3. Additional sensitivity analysis for the baseline values of Chao1 index; Fig. S4. Additional sensitivity analysis for the change between the “before-after” values of Chao1 index; Fig. S5. Additional sensitivity analysis for the baseline values of Observed OTUs; Fig. S6. Additional sensitivity analysis for the change between the “before-after” values of Observed OTUs; Fig. S7. Risk of bias assessment for the main meta-analysis of Shannon diversity index - Assignment to intervention; Fig. S8. Risk of bias assessment for the main meta-analysis of Shannon diversity index - Adhering to intervention ; Fig. S9. Risk of bias assessment for the meta-analysis of Chao1 index - Assignment to intervention; Fig. S10. Risk of bias assessment for the meta-analysis of Chao1 index - Adhering to interventionFig. S11. Risk of bias assessment for the meta-analysis of Observed OTUs - Assignment to intervention; Fig. S12. Risk of bias assessment for the meta-analysis of Observed OTUs - Adhering to intervention.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. The original datasets used in this study can be found in the full-text publications included in the systematic review and meta-analysis.