Abstract
Case series
Patients: —
Final Diagnosis: Donor livers had replace right hepatic artery
Symptoms: Liver had replaced right hepatic artery
Clinical Procedure: —
Specialty: Anatomy • Surgery
Objective:
Unusual clinical course
Background:
The presence of anatomical variations of the hepatic artery poses a challenge for normothermic machine per-fusion (NMP). Here, we describe our experience of creating a single arterial cannulation for NMP in 3 donor livers with replaced right hepatic artery.
Case Report:
Three donor livers with replaced right hepatic artery were perfused using NMP (OrganOx® metra®) for liver transplantation. To maintain hepatic artery integrity and establish an intact arterial vascular inflow for NMP, a single vasculature was created to allow single arterial cannulation for NMP. A piece of intravenous-line tubing was used as a bridge from the splenic artery to the superior mesenteric artery during the back-table preparation. After 1 h of NMP, the lactate of 2 livers decreased from >10.0 to about 1.0 mmol/L, and the lactate of 1 liver decreased from >4.0 to <0.4 mmol/L. Three livers made >100 mL of bile after 4 h of NMP and were successfully implanted after >10 h of NMP. The recipients spent 2, 3, and 4 days in the Intensive Care Unit and were discharged home at 6, 7, and 9 days, respectively. None of the patients experienced early allograft dysfunction or any early technical complication or non-anastomotic biliary stricture.
Conclusions:
Creating an intravenous-line tubing bridge from the splenic artery to the superior mesenteric artery prior to NMP of liver grafts associated with replaced right hepatic artery could reduce the cold ischemia time associated with vessel reconstruction and reduce bleeding risk during NMP. This is feasible, safe, and effective.
Keywords: Biliary Atresia, Cold Ischemia, Hepatic Artery, Liver Transplantation, Perfusion
Background
Non-anastomotic biliary stricture (NABS) significantly reduces graft and patient survival after liver transplantation [1,2]. One of the most important risk factors for the development of NABS after liver transplantation is the longer ischemia time, which includes warm ischemia time (WIT) during donation after circulatory death (DCD) recovery and cold ischemia time (CIT) after cross-clamping [3–5]. Reduced WIT could decrease the risk of NABS [5]. The American Society of Transplant Surgeons recommended in 2009 that the DCD donor WIT should be <30 to 45 min and functional donor WIT (MAP <60 mm Hg) be a maximum of 20 to 30 min to achieve better outcomes [6]. CIT of liver grafts can be successfully reduced by using normothermic machine perfusion (NMP) [7]. However, the presence of anatomical variations of the hepatic artery pose challenges during back-table preparation for NMP because the graft requires ex vivo arterial reconstruction [8]. Arterial reconstruction increases non-oxygenic CIT as a single arterial cannulation for NMP on the back-table is created. In addition, it also increases the risk of bleeding from anastomosis of arteries, as heparin is continuously administered during NMP. Here, we describe our experience of creating a single arterial cannulation to keep an intact hepatic arterial vascular inflow for NMP in 3 donor livers with replaced right hepatic artery.
Case Report
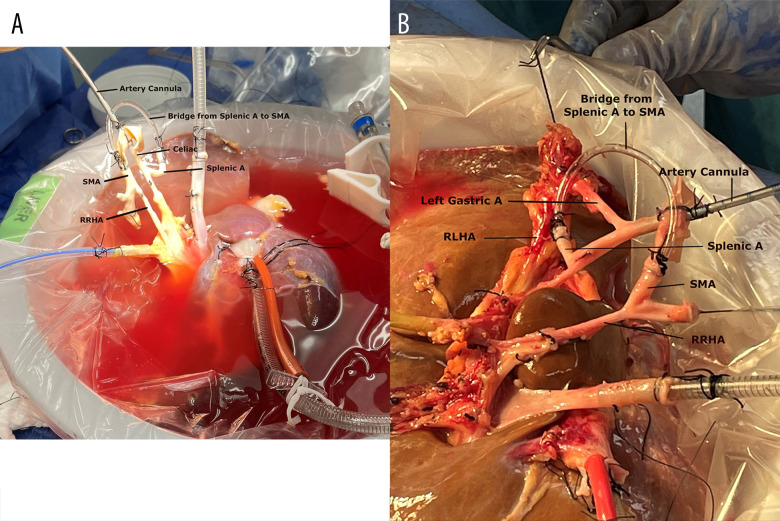
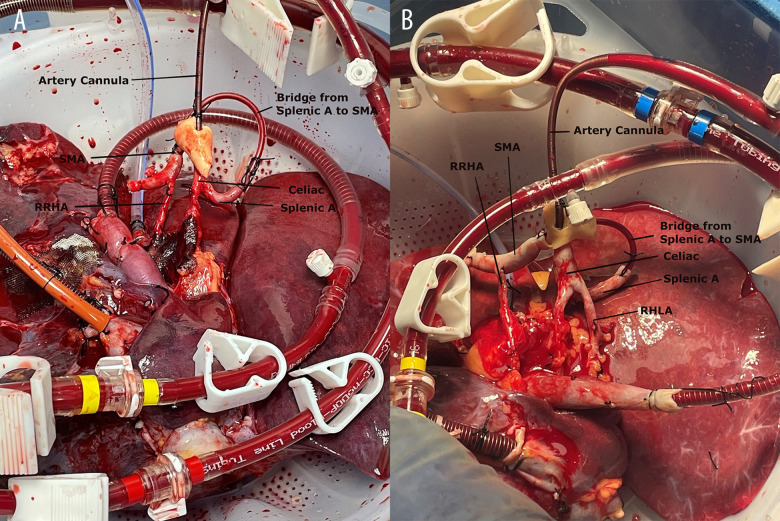
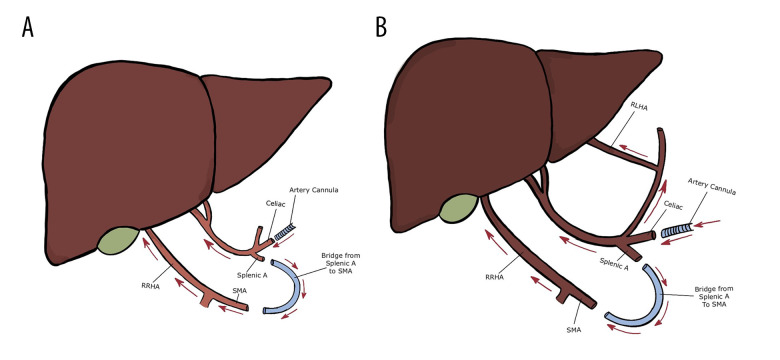
In our center, a series of 11 fatty donor livers (8 DCD and 3 donation after brain death [DBD] for liver transplantation) were perfused using NMP (OrganOx® metra®) for liver transplantation. Three livers (2 DCD livers with 29.8 kg/m2 and 26.7 kg/m2 body mass indexes had 24 min and 26 min of WIT, respectively; 1 DBD with 45.6 kg/m2 body mass index) had anatomical variations of the hepatic artery. One liver had right replaced hepatic artery (RRHA), and 2 livers had RRHA and replaced left hepatic artery (RLHA). To maintain hepatic artery integrity and establish an intact arterial vascular inflow for NMP, a single vasculature was created to allow single arterial cannulation for NMP. Figure 1 shows a piece of intravenous-line tubing that was used as a bridge from the splenic artery to the superior mesenteric artery (SMA) during the back-table preparation. Figure 2 showcases the liver after creating the bridge from the splenic artery to the SMA during NMP. Figure 3 shows the intravenous-line tubing “bridge” from the splenic artery to the SMA and the arrow points are the direction of arterial flow. The arterial and portal flows for 3 livers were 0.4 to 0.6 L/min and 1.0 to 1.5 L/min during NMP. After 1 h of NMP, the lactate of the 2 DCD livers decreased from >10.0 mmol/L to <1.0 mmol/L in 1 liver and from >10.0 mmol/L to 1.3 mmol/L in another liver; the lactate of the DBD liver decreased from >4.0 mmol/L to <0.4 mmol/L. Three livers made >100 mL of bile after 4 h of NMP. These 3 livers were successfully implanted after >10 h of NMP. The recipients spent 2, 3, and 4 days in the Intensive Care Unit (ICU) and were discharged home in 6, 7, and 9 days, respectively. None of the patients experienced early allograft dysfunction or any early technical complication or NABS.
Figure 1.
Donor liver at the back-table preparation. (A) Liver with replaced right hepatic artery (RRHA); (B) Liver with RRHA and replaced left hepatic artery (RLHA). A piece of intravenous-tube as a bridge from splenic artery (splenic A) to superior mesenteric artery (SMA) creates a single arterial cannulation for normothermic machine perfusion.
Figure 2.
Donor livers on the normothermic machine perfusion. (A) liver with replaced right hepatic artery (RRHA); (B) liver with RRHA and replaced left hepatic artery (RLHA). The bridge from the splenic artery (splenic A) to the superior mesenteric artery (SMA) creates a single arterial cannulation and maintains an intact hepatic arterial vascular inflow for the liver grafts.
Figure 3.
Schematics of donor livers. (A) liver with replaced right hepatic artery (RRHA); (B) liver with RRHA and replaced left hepatic artery (RLHA). A piece of intravenous tube as a bridge from splenic artery (splenic A) to superior mesenteric artery (SMA) creates a single arterial cannulation for normothermic machine perfusion. Arrow points are the direction of arterial blood flow.
Discussion
Following The American Society of Transplant Surgeons recommendation in 2009 for DCD donors [6], 30 min of functional donor WIT is the cut-off time for acceptance for liver transplantation. Accordingly, 24 min and 26 min of WIT in our 2 DCD livers should not have increased the risk of NABS.
NMP could successfully decrease the length of CIT, resulting in reduced complication of NABS, especially for medically complex donor livers (eg, DCD and steatosis)[7]. However, anatomical variations of the hepatic artery of donor livers pose a challenge for NMP. Ex vivo vascular reconstruction, required prior to NMP, results in a longer CIT. Anatomical variations of the hepatic artery can be seen in up to 30% of liver grafts, and the most common variation was the replaced right hepatic artery (about 11–21%), which is the chief source of blood supply to the bile duct [9,10]. To keep an intact hepatic arterial vascular inflow, creating a single vascular conduit is necessary for NMP [11]. Several techniques have been reported by different centers for this mandatory reconstruction of all vessels of abnormal hepatic arterial anatomy [3,8,11]. However, these techniques add non-oxygenated CIT to perform and require more specialized instruments during the back-table reconstruction in the donor operation room. Our technique can be performed in about 30 min, resulting in a shorter non-oxygenated CIT during the back-table preparation. In addition, there is no bleeding risk from the bridge. More than 30% of arterial flow of total hepatic blood flow can be observed during NMP. More than 100 mL of bile can be produced after 4 h of NMP. This means the biliary system has adequate flow using this described technique. The patients in this small case series had short ICU and hospital stays, and less complications were observed. These observations suggest this technique did not negatively impact short-term outcomes.
This technique allows the implanting surgeons and their team to perform anastomosis between the splenic artery and SMA (or RRHA) to maintain intact arterial vascular inflow after the transplant liver is re-perfused by portal flow during recipient surgery. Because this anastomosis is not performed under a non-oxygenated situation, it should not increase the risk of NABS. Most transplant centers that this procurement team works with prefer that the arterial anastomosis be performed by the recipient center’s team. This technique allows local recovery and perfusion of the liver, while maintaining the opportunity for anastomosis to be done by the liver accepting center.
This technique can also easily be adapted for other variations of hepatic arterial anatomy because there are 3 relatively large sizes of branches (ie, left gastric artery, splenic artery, and gastro-duodenary artery) from the orifice of the celiac trunk to the proper hepatic artery. A bridge from one of these branches to an aberrant hepatic artery will allow for a single vasculature creating the required single arterial cannulation site required for NMP.
Conclusions
NMP reduces the CIT of liver grafts following recovery. Using intravenous-line tubing to make a bridge between the splenic artery and SMA during the back-table preparation is simple and adequate to prepare for arterial cannulation of the liver grafts with RRHA prior to NMP. The technique could reduce the CIT and bleeding risk during NMP, and this case series suggests it is feasible, safe, and effective.
Acknowledgments
We appreciate all the excellent technical assistance provided by Robert Hanes, Chris Cook, and Meagan Lenhart. We thank Mike Latka for providing excellent anatomical schematics drawings.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration About Informed Consent
The study was approved by the LifeShare Transplant Donor Services of Oklahoma, Inc. All the patients consented preoperatively to participate in this study.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Foley DP, Fernandez LA, Leverson G, et al. Biliary complications after liver transplantation from donation after cardiac death donors: An analysis of risk factors and long-term outcomes from a single center. Ann Surg. 2011;253(4):817–25. doi: 10.1097/SLA.0b013e3182104784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ito T, Botros M, Aziz A, et al. Nonanastomotic biliary strictures after liver transplantation. Am Surg. 2020;86(10):1363–67. doi: 10.1177/0003134820964461. [DOI] [PubMed] [Google Scholar]
- 3.Lantinga VA, Buis CI, Porte RJ, de Meijer VE, van Leeuwen OB. Reducing cold ischemia time by donor liver “back-table” preparation under continuous oxygenated machine perfusion of the portal vein. Clin Transplant. 2022;36(8):e14762. doi: 10.1111/ctr.14762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paterno F, Guarrera JV, Wima K, et al. Clinical implications of donor warm and cold ischemia time in donor after circulatory death liver transplantation. Liver Transpl. 2019;25(9):1342–52. doi: 10.1002/lt.25453. [DOI] [PubMed] [Google Scholar]
- 5.Kalisvaart M, Croome KP, Hernandez-Alejandro R, et al. Donor warm ischemia time in DCD Liver Transplantation-Working Group report from the ILTS DCD, Liver Preservation, and Machine Perfusion Consensus Conference. Transplantation. 2021;105(6):1156–64. doi: 10.1097/TP.0000000000003819. [DOI] [PubMed] [Google Scholar]
- 6.Reich DJ, Mulligan DC, Abt PL, et al. ASTS Standards on Organ Transplantation Committee. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant. 2009;9(9):2004–11. doi: 10.1111/j.1600-6143.2009.02739.x. [DOI] [PubMed] [Google Scholar]
- 7.van Beekum CJ, Vilz TO, Glowka TR, et al. Normothermic machine perfusion (NMP) of the liver – current status and future perspectives. Ann Transplant. 2021;26:e931664. doi: 10.12659/AOT.931664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasralla D, Lembach H, Mergental H, et al. Ex situ arterial reconstruction during normothermic perfusion of the liver. Transplant Direct. 2020;6(9):e596. doi: 10.1097/TXD.0000000000001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noussios G, Dimitriou I, Chatzis I, Katsourakis A. The main anatomic variations of the hepatic artery and their importance in surgical practice: Review of the literature. J Clin Med Res. 2017;9(4):248–52. doi: 10.14740/jocmr2902w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu X, Kang J, Liu Y, et al. A rare hepatic artery variant reporting and a new classification. Front Surg. 2022;9:1003350. doi: 10.3389/fsurg.2022.1003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker F, Kneifel F, Riegel A, et al. Ex situ arterial reconstruction prior normothermic machine perfusion of liver grafts. Langenbecks Arch Surg. 2022;407(8):3833–41. doi: 10.1007/s00423-022-02611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]