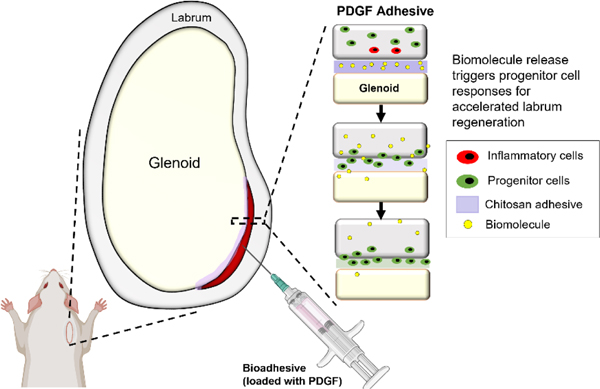
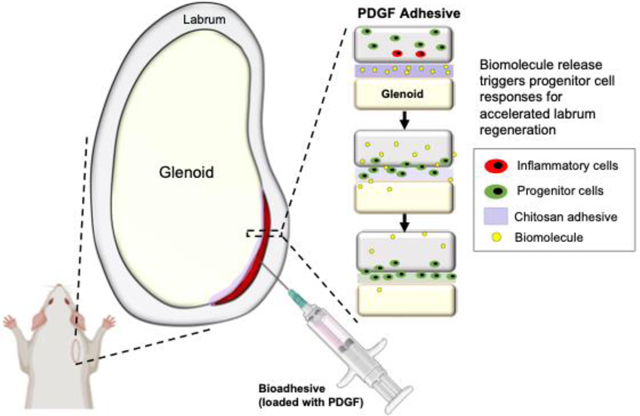
Abstract
Glenoid labral tears occur with repetitive dislocation events and are common injuries observed in shoulder arthroscopic procedures. Although surgery can restore shoulder anatomy, repair is associated with poor clinical outcomes, which may be attributed to the poor regenerative capability of glenoid labral fibrocartilage. Thus, this study was designed to assess whether in situ tissue regeneration via biomolecule-stimulated recruitment of progenitor cells is a viable approach for the regeneration of labral tears. We developed a click chemistry-based bioadhesive to improve labral repair and reduce local inflammatory responses due to trauma. Additionally, we previously identified the presence of progenitor cells in the human labrum, which can be recruited by platelet-derived growth factor (PDGF). Thus, we hypothesized that PDGF-releasing adhesives could induce the regenerative responses of progenitor cells at the injury site to improve labral healing. In a rat glenoid labral tear model, we evaluated the effect of PDGF-releasing adhesives on promoting progenitor cells to participate in labral tear healing. After 3 and 6 weeks, the labrum was histologically analyzed for inflammatory responses, progenitor cell recruitment, proliferation, and extracellular matrix (ECM) production (collagen and glycosaminoglycan). Our results showed that adhesives alone considerably reduced local inflammatory responses and labral tissue dissolution. PDGF-releasing adhesives significantly increased progenitor cell recruitment, proliferation, and ECM production. These results demonstrate that by accelerating autologous progenitor cell responses, PDGF-releasing adhesives represent a novel clinically relevant strategy to improve the healing of glenoid labral tears.
We report the creation of a platelet-derived growth factor (PDGF)-releasing bioadhesive for repairing the torn labrum, promoting labral tissue regeneration by triggering progenitor cell recruitment and extracellular matrix (ECM) production. Our proof-of-concept in vivo studies indicate that the application of a chitosan bioadhesive reduced labral tissue inflammation following injury, thereby mitigating the tissue degeneration process. Subsequently, the local delivery of PDGF enhanced tissue repair by recruiting nearby progenitor cells while also promoting cell proliferation and differentiation at the injury site.
Keywords: glenoid labrum, labral tear, progenitor cells, bioadhesive, PDGF
Graphical Abstract
1. Introduction
The glenoid labrum is a fibrocartilaginous structure that is peripherally attached to the glenoid rim. The labrum participates in glenohumeral joint stability by deepening the glenoid cavity.1 Glenoid labral tears or lesions can occur in several distinct locations—anterior, superior, posterior, or multidirectional—with variable morphologies—detachment from the bone or intrasubstance—and have been associated with chronic shoulder pain and/or shoulder instability.2 Clinically, both nonsurgical and surgical interventions are available for the treatment of glenoid labral tears. Nonsurgical treatments include the use of non-steroidal anti-inflammatory medications and physical therapy. Unfortunately, these methods are often unsuccessful (>50%) owing to the limited healing capacity of the labral fibrocartilage.3 Thus, arthroscopic repair serves as the standard for patients who wish to return to their usual level of activity. Glenoid labral tears are repaired with suture anchors, by which the labrum is repaired within its substance or reattached to the glenoid rim.4 Such a surgical procedure is associated with several risks such as nerve injury, anchor migration, anchor loosening or failure, and an inflammatory reaction potentially leading to osteolysis/chondrolysis.5 Furthermore, the surgical repair of glenoid labral tears aims to restabilize the shoulder while restoring complex shoulder mechanics but still does not address the poor regenerative property of the labrum and underlying degenerative processes following injury. Specifically, the fibrocartilage microenvironment at the tear site is disrupted by inflammation and extracellular matrix (ECM) degeneration, particularly in superior labrum from anterior to posterior (SLAP) tears.6 Consequently, revision surgery for persistent shoulder instability is a clinical challenge and is indicated in approximately 20% of these patients.7,8 Therefore, there is a need to develop new therapeutic strategies to repair and promote tissue regeneration in the torn glenoid labrum.
An increasing amount of effort has been made to develop stem cell-based therapies for tissue regeneration.9 Transplantation of endogenous and exogenous stem cells, which has been widely explored, is not available clinically because of limited cell lifespan, immune rejection, tumorigenic potential, and pathogen transfer.10 To overcome these limitations, our laboratory was the first to develop scaffolds capable of triggering autologous stem cell responses.11–13 These approaches allow us to harness host progenitor cell responses without the need for cell isolation, culture expansion, or transplantation. Many combinations of bioactive cues, scaffold designs, and growth factors have been explored for the healing of other tissues, such as the knee meniscus,14,15 bone tissue,12 cartilage,13 and other musculoskeletal tissues.16 Despite these exciting advancements, similar strategies for labral tear regeneration have not yet been investigated. By analyzing the cells inside and isolated from human acetabular labrum tissue, we identified the presence of progenitor cells (CD90+/CD105+).17 Additionally, these cells can be recruited by platelet-derived growth factor (PDGF) and then differentiated into fibrochondrocytes in vitro, using a high-density cell pellet model.17 Based on these recent findings, we hypothesized that progenitor cells could be recruited to regenerate glenoid labral tears.
To test the feasibility of in situ labral tissue regeneration via progenitor cell recruitment, a tissue adhesive was used as the growth factor carrier. Tissue adhesives offer a non-invasive approach to joining injured tissues, rather than suturing, and have been applied in a wide range of biomedical applications such as wound healing, internal tissue closure, and drug delivery.18–20 Moreover, given the versatility of their design and ease of use, bioadhesives have gained widespread attention in the fields of tissue engineering and regeneration because they can be loaded with various biomolecules. Bioadhesives approved for clinical use are generally categorized as naturally derived adhesives (i.e., fibrin glue, gelatin, or protein-based adhesives) or synthetic polymer-based adhesives (i.e., cyanoacrylates, albumin, as well as glutaraldehyde-based and polyethylene glycol [PEG]-based hydrogels).18,21 Unfortunately, in some cases, these bioadhesives are cytotoxic, induce inflammation affecting tissue fragility, or are unable to achieve sufficient adhesive strength.22 Although considerable research efforts have been made to develop safe and effective bioadhesives to overcome these limitations, no adhesives have been developed to actively promote glenoid labral repair and regeneration.
Recently, a novel injectable click chemistry-based chitosan (CS) bioadhesive, developed with a fast gelation time and minimal cellular toxicity, has been shown to accelerate wound healing in a mouse skin incision model—faster than fibrin glue.23 Additionally, previous studies have shown that CS adhesives are able to re-attach labral tissue with better mechanical force than commercially available fibrin glue.17 In addition, PDGF-releasing CS adhesives were found to promote the recruitment of endogenous progenitor cells and increase the production of type II collagen (COL2) and glycosaminoglycan (GAG) in human acetabular labrum tissue.17 Although these results are promising, these studies were carried out ex vivo and for a short term. The function of PDGF-releasing CS adhesives in living organisms has not yet been investigated, which was the main objective of this study. Therefore, this study was designed to assess whether in situ tissue regeneration via biomolecule-stimulated recruitment of endogenous labral progenitor cells is a viable approach for the repair of labral tears.
A series of studies were carried out to test the hypothesis that PDGF-releasing adhesives can be engineered to accelerate glenoid labral healing in vivo through host progenitor cell responses. Using a glenoid labral tear rat model, we investigated the influence of various treatments on labral tissue regeneration at 3- and 6-weeks post injury. Specifically, injured glenoid labral tissues were isolated at specific time points and then used to analyze the extent of tissue erosion, inflammatory cell infiltration, progenitor cell recruitment, and ECM production.
2. Materials and Methods
2.1. Fabrication of click chemistry-based CS bioadhesives
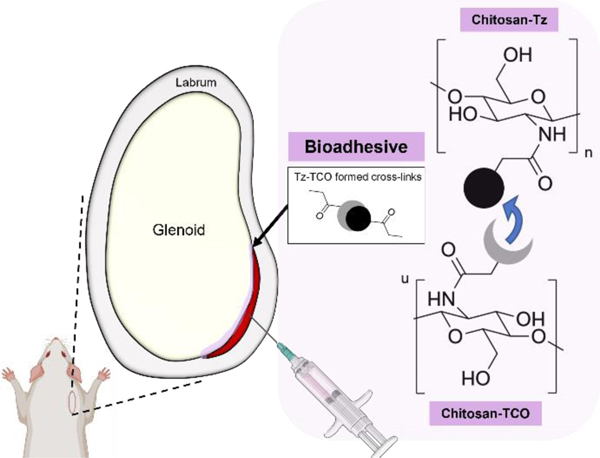
The CS bioadhesives were synthesized using previously published methods.17,23 Two precursors, CS-Tz and CS-TCO, were produced through carbodiimide crosslinking between primary amine groups on the CS polymer backbone (low MW, Sigma-Aldrich, St Louis, MO) and N-hydroxysuccinimide (NHS) groups in tetrazine-NHS (Tz; Click Chemistry Tools, Scottdale, AZ) and trans-cyclooctene-NHS ester (TCO; Click Chemistry Tools). The Tz/TCO-NHS ester was dissolved in 2 mL dimethyl sulfoxide (62 mM) and mixed with 2 mL CS solution (10 mg/mL in 50 mM MES buffer at pH 6.0). The obtained solution was continuously stirred overnight. The conjugated CS with Tz and TCO solution was purified first via precipitation in excess acetone, washed in dimethylformamide, and then exhaustively dialyzed (molecular cutoff: 2 kDa) against 1% acetic acid and deionized water. After dialysis, CS-Tz/TCO solutions were lyophilized and stored at 4°C until further use. Freeze-dried CS-Tz/TCO was reconstituted in 50 mM MES buffer at 10 mg/mL. To form the bioadhesive, CS-Tz, CS-TCO, and 90 mg/mL co-crosslinker 4-arm PEG-propionaldehyde (10 kDa MW, Laysan Bio, Arab, AL) were mixed in a 5:5:1 ratio.
2.2. Characterization of adhesives
The degradation and release properties of CS adhesives were measured in vitro as described earlier.17,23 For degradation, adhesives were lyophilized, weighed (W0), and incubated in 1 mL phosphate buffered saline (PBS, 100 mM, pH 7.4) with or without lysozyme from chicken egg white (0.8 mg/mL, Sigma Aldrich) at 37 ºC with shaking. At each time point, the adhesives were lyophilized and measured for the relative weight (Wt). The degradation curve was generated by calculating the % initial weight remaining at each time point using the following equation: (Wt/W0) x 100%. Next, the PDGF releasing property of the CS adhesives was characterized as documented earlier.17 Briefly, adhesives were loaded with PDGF-AB (Shenandoah Biotechnology, Warminster, PA) (2 μL, 0.2 mg/mL) prior to incubation in 1mL PBS. Supernatant was collected every other day for 2 weeks and stored at −80 ºC. The releasing medium was replenished with fresh PBS. The amount of released PDGF and remaining PDGF in the adhesives were measured using Human PDGF-AB ELISA kit (Catalog # EHPDGFAB, Thermo Fisher Scientific, Waltham, MA). The cumulative release percentage (%) was calculated as the total amount of PDGF released into the medium at a specific time relative to the initial loading amount.
2.3. Surgical procedure
The regenerative activity of the adhesives was evaluated using a rat labral tear model adapted from previous studies.24,25 Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 200–300 g were used in this experiment and divided (male and female assigned to groups randomly) into three groups: injured with no treatment control (labeled as “Injured”), injured with adhesive only (labeled as “Adhesive”), and injured with PDGF-releasing adhesive (labeled as “PDGF Adhesive”); n = 3 animals for each group and each time point (18 total). Hydrogel precursors were injected and allowed to mix in situ to form adhesives through click chemistry reaction (Figure 1).
Figure 1.
Schematic illustration of injection of chitosan-based bioadhesives crosslinked via tetrazine/trans-cyclooctene (Tz/TCO) click chemistry reaction. Created with BioRender.com.
All animals were treated according to the standard guidelines approved by the Institutional Animal Care and Use Committee at the University of Texas at Arlington in accordance with the Animal Welfare Act and the Guide for Care and Use of Laboratory Animals. Briefly, the animals were anesthetized using ~2–3% isoflurane. An intramuscular dose of cefazolin (Covetrus, Portland, ME) and subcutaneous sustained-release (SR) buprenorphine (ZooPharm, Laramie, WY) was administered. After preparation of the surgical site, a 2–3-cm incision was made along the posterior aspect of the shoulder. The muscle tissue surrounding the glenohumeral joint was separated to expose the rotator cuff and joint capsule. Arthrotomy was performed, and the shoulder was dislocated to expose the glenoid cavity and labrum (Figure 2A). A no. 12 scalpel was used to create a small laceration in the anterior aspect of the labrum (at the 2–4 o’clock position, with the 12 o’clock position designating the insertion of the biceps tendon in the supraglenoid tubercle) at the level of the glenoid rim (Figure 2B). For the bioadhesive groups, the respective bioadhesive (~10 μL) was prepared using a 28-gauge syringe needle and immediately injected into the injury site (Figure 2C).
Figure 2.
Surgical procedure to generate an anterior labral detachment at 2 to 4 o’clock and application of adhesives: (A) laceration at the glenoid rim, (B) complete detachment of labrum (black arrow), and (C) injection of bioadhesive into the space between lacerated labrum (black arrow show the excessive adhesive which was later removed). Labels: G: glenoid cavity, L: labrum, HH: humeral head.
In the growth factor group, 100 ng/mL PDGF-AB (Shenandoah Biotechnology) was loaded into the adhesives via physical incorporation. After tissue joining, the shoulder was reduced, and the capsulotomy, rotator cuff interval, and subcutaneous tissue were repaired using 4–0 absorbable sutures (Vicryl, Ethicon, Johnson & Johnson, Raritan, NJ). In the control group, the shoulder joint was reduced after labral injury and the tissues were closed in a similar fashion. The skin was closed using wound clips and a subcutaneous dose of meloxicam (Covetrus) was administered. Buprenorphine SR and meloxicam were administered every 72 h for 6 days. The animals were monitored daily, allowed free caged activities, and free access to food and water. On postoperative day 7, the wound clips were removed.
2.4. Histology and immunohistochemistry
At each time point (3 and 6 weeks), the animals were euthanized, and the scapula of each animal was harvested and assessed for gross morphology. Soft tissue surrounding the glenohumeral joint from all animals was removed without compromising the joint capsule, and the joint capsule was dissected from its humeral insertions. Shoulders without surgery and without treatment were used as healthy tissue controls. To ensure the treatment assignments will be blinded to the investigators who participate in the material implantation, the treatment assignments were blinded to the person(s) who performed the tissue analysis. Tissue samples from the healthy and injured shoulders were fixed in 4% w/v buffered paraformaldehyde, decalcified with 10% w/v ethylenediaminetetraacetic acid for ~2 weeks, embedded in paraffin wax, and then sectioned using a manual rotary microtome. Hematoxylin and eosin Y (H&E) staining was carried out for morphological assessment, and immunohistochemical staining was carried out to evaluate the inflammatory response using antibodies against CD11b (sc-6614, Santa Cruz Biotechnology, Dallas, TX) and proinflammatory cytokines - matrix-metallopeptidase 13 (MMP-13; 18165–1-AP, Thermo Fisher Scientific) and interleukin 1-beta (IL-1β; BS-20449R, Thermo Fisher Scientific).25 Toluidine blue and Masson’s Trichrome (HT15, Sigma-Aldrich) staining were used to evaluate GAG formation and collagen production, respectively. Lastly, the neo-fibrocartilage tissues were assessed using a modified Pauli’s score histological grading system based on sections stained with Safranin-O (02782–25, PolySciences) and H&E.26–28 Diaminobenzidine, a chromogen, was used to develop the color for CD11b+, MMP-13+, and IL-1β+ staining. To determine the presence of progenitor cells near the injury site, the sections were immunohistochemically stained with CD44 (sc-7946, Santa Cruz Biotechnology) and CD73 (sc-25603, Santa Cruz Biotechnology), since progenitor cells in the rat meniscus have been shown to possess CD44 and CD73 marker.29 Ki-67 (ab16667, Abcam, Cambridge, United Kingdom) was used to assess the proliferation of progenitor cells. PDGF responsive cell type was determined by the expression of PDGF α-receptors (PDGFRα) and β-receptors (PDGFRβ) on the progenitor cells (ab32570, Abcam). Fluorescein isothiocyanate and rhodamine fluorescent dye-conjugated secondary antibodies were used to visualize the positive staining. 4′,6-Diamidino-2-phenylindole (DAPI; Fisher Scientific, Hampton, NH) was used as a counterstain for cell nuclei. Images were acquired and analyzed using the ImageJ software (NIH). The tissue directly adjacent to the articular cartilage and joint capsule was identified as labral tissue and was the focus of histological analyses. No animals from each experimental group were excluded from the study and analysis (n=3 animals in each experimental group analyzed).
2.5. Statistical analyses
All statistical analyses were performed using GraphPad Prism version 9.0.0 (GraphPad Software, San Diego, CA). The sample size was determined using a power of 0.80 and α value of 0.05 using previous results (based on progenitor cell responses) from our pilot study (calculated using G*Power). Specifically, the Kruskal-Wallis one way analysis of variance was used to describe results for Modified Pauli’s Score (Figure 3C). For the remaining quantifications, the normal distribution was confirmed using the Shapiro-Wilk normality test. Comparisons were made using analysis of variance and Tukey’s post hoc tests and were considered statistically significant when p ≤ 0.05. The results are presented as the mean ± standard deviation (SD). Study data are available upon request.
Figure 3.
Characterization of CS bioadhesives in vitro. (A) Degradation of CS bioadhesives over 2 weeks in PBS solution with or without lysozyme (0.8 mg/mL). (B) Controlled release curve of PDGF from CS adhesives over 2 weeks in PBS solution quantified using ELISA. Data presented as mean ± SD (n=3).
3. Results
3.1. In vitro degradation and PDGF release of CS adhesives
The degradation study showed that 48±8 % of the CS adhesives remained after 14 days in PBS, while ~4.7±0.1% remained after 14 days in lysozyme degradation media (Figure 3A). For PDGF release, our results showed a burst release during the first 48 hours due to physical encapsulation of the biomolecule in CS adhesives. Following, sustained release of PDGF lasted for up to 14 days in PBS (Figure 3B).
3.2. Gross assessment of the labra with various treatments
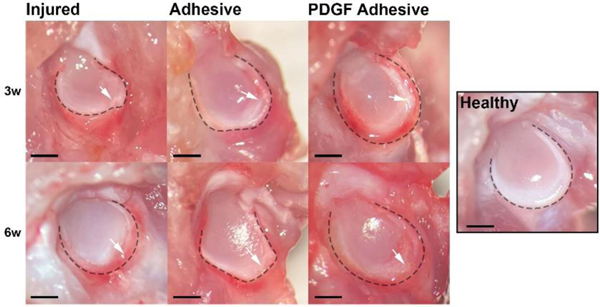
We first grossly examined the harvested scapula with portions of the joint capsule intact from the different groups of animals. By comparing with healthy tissue, we found that there was tissue degeneration at the site of the injured labra compared with the uninjured labra at both time points, with significant inflammation and tissue erosion noted in the injured group (Figure 4).
Figure 4.
Gross evaluation of injured labrum with or without adhesive treatment compared with healthy labrum. Representative images of injured labra at 3- and 6- weeks demonstrate highly inflamed tissue and/or degenerated labrum with no treatment. Irregular peripheral edge shows tissue damage. Outer edge of labrum identified with black dashed line. White arrows indicate tear site. Scale bar = 1 mm.
By contrast, less inflammation and tissue degeneration were found in the adhesive and PDGF adhesive groups compared to that in the injured group. At 6 weeks, the PDGF adhesive tissue was similar to healthy tissue.
3.3. CS bioadhesive treatment on tissue degeneration in the injured labra
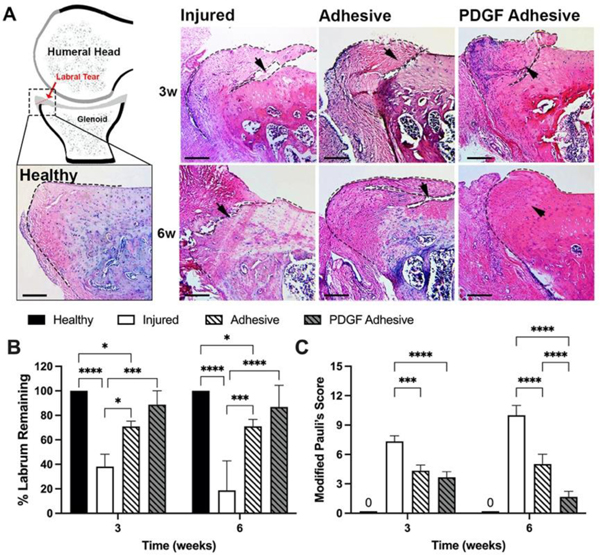
The histological results were quantified to assess the extent of tissue degeneration and regeneration. As expected, the labral tissue of the injured group showed significant erosion at both time points compared to the healthy labra (Figure 5A). Compared with healthy tissue, the remaining percentage of labral tissues were quantified using H&E images to determine the local extent of labral degeneration in response to different treatments. For each tissue section image, the boundaries of the remaining labra were traced, the surface area measured, and taken as a percentage of the surface area of normal healthy labrum. As expected, the extent of tissue degeneration in the adhesive group was substantially less than that in the injured control at the same time points (70.92±4.37 % vs. 38.19±10.02% and 71.10±5.57 % vs. 18.75±24.05 % labrum remaining at week 3 and 6, respectively) (Figure 5B). In addition, the PDGF adhesive group showed minimal tissue degeneration (88.71±11.34 % labrum remaining at week-3 and 86.78±17.63 % at week-6), which was similar to that in the adhesive group. Using a modified Pauli’s histological scoring method, we examined the overall effectiveness of the various treatments for reducing labral tissue degeneration (Figure 5C). At 6 weeks post-surgery, the PDGF adhesive group achieved lower scores than the adhesive only and injured control groups. This indicates that localized PDGF release significantly reduced labral tissue degeneration.
Figure 5.
Histological evaluation of healthy and injured rat labrum with or without adhesive treatment. (A) Schematic demonstrates histological area that was analyzed and representative H&E images of labral healing at 3- and 6-weeks. Tear site indicated by black arrows. Black dashed line represents traced boundary of healthy or injured labrum. Scale bar = 200 μm. (B) Percent labrum remaining after 3- and 6-weeks when compared to healthy labrum. (C) PDGF adhesive group had significantly lower Modified Pauli’s Scores compared to adhesive alone and no treatment groups. Healthy labrum would have a score of 0. Data presented as mean ± SD (n=3), *, **, ***, and **** indicate p<0.05, p<0.01, p<0.001, and p<0.0001, respectively.
3.4. CS bioadhesive treatment on inflammatory responses in the injured labra
Since inflammatory responses and their products—including the presence of CD11b cells and the production of MMP-13 and IL-1β—are responsible for tissue degeneration,25 we analyzed the influence of CS adhesive treatment on the presence of these inflammatory indicators in the injured labra (Figure S-1–S-4). First, we found little to no presence of CD11b+ cells, and low levels of MMP-13+ and IL-1b+, in the healthy labrum (or no tear control). The injured labra in all groups displayed hypercellularity at week 3, but not week 6, compared to the healthy labra. At week 3, the PDGF adhesive group exhibited approximately 2X more cell density compared to that in the adhesive alone group and significantly higher cell density compared to injured group (3X more, p=0.0036) (Figure 6A). Next, to compare the inflammatory activities in various groups, we found that the injured labral tissue exhibited a significantly higher number of CD11b+ inflammatory cells compared to healthy labrum (~20X more, p=0.0009) at week 3 (Figure 6B).
Figure 6.
Immunohistochemical evaluation of host inflammatory response with or without adhesive treatment. (A) Cell density of remaining labrum at 3- and 6-weeks. (B) Number of CD11b+ inflammatory cells at 3- and 6-weeks. (C) Labral matrix metalloproteinase 13 (MMP-13) expression at 3- and 6-weeks. (D) Number of Interleukin-1 beta (IL-1β+) cells at 3- and 6-weeks. Data presented as mean ± SD (n=3), *, **, and *** indicate p<0.05, p<0.01, and p<0.001, respectively.
Similarly, injured labrum possessed a significant increase in production of MMP-13 (~4X more, p=0.0030) (Figure 6C) and IL-1β+ cells (10X more, p=0.0019) (Figure 6D) compared to healthy labrum. At week 6, the injured labral tissue had significantly more MMP-13 production (>3X more, p=0.0195), but not CD11b+ and IL-1β+ cells, compared to healthy labrum. Interestingly, with adhesive alone, the presence of CD11b+ inflammatory cells was considerably reduced (~2X lower) at 3 weeks compared to the injured group, demonstrating that the hypercellularity observed in response to adhesive treatment was not associated with the inflammatory response (Figure 6B). Additionally, adhesive group had a ~2X lower production of both MMP-13 and IL-1β in compared to that in the injured labra at 3 weeks (Figure 6C&D). At week 6, CD11b+ cell count, MMP13 expression, and IL-1β+ cell count of adhesive treated tissue was not statistically significant compared to injured labra. Lastly, among all treatment groups, the PDGF adhesive-treated group had the lowest CD11b+ cell count, MMP-13 production, and IL-1β+ cell count at both time points. At week 3, the CD11b+ cell count, MMP-13 production, and IL-1β+ cell count in PDGF adhesive group were significantly reduced compared to those in injured labra with >6X (p=0.0009), 2X (p=0.0155), and >5X (p=0.0018) less, respectively. At week 6, the PDGF adhesive treated labral tissue had a significantly less MMP-13 production (>2X less, p=0.0201), but not CD11b+ and IL-1β+ cell counts, compared to injured labrum. These results support the use of CS adhesives and PDGF-releasing adhesives to reduce the initial inflammatory responses in the injured glenoid labra, which may lead to reduced labral degeneration.
3.5. Effect of bioadhesive treatments on progenitor cell responses
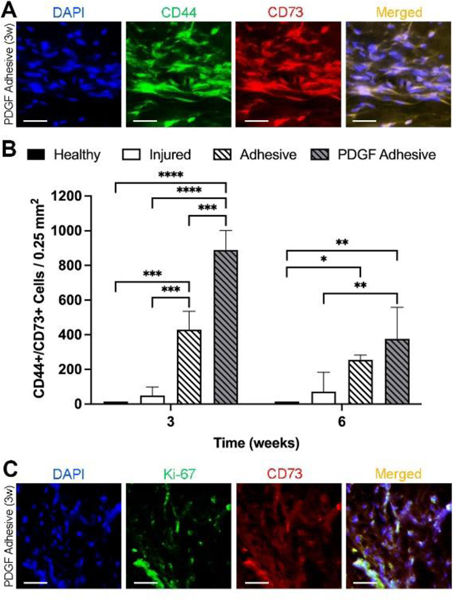
Since the PDGF-releasing adhesive was designed to elicit autologous stem cell responses, further tissue analyses were performed to assess the influence of the various treatments on progenitor cell (CD44+/CD73+) recruitment. As anticipated, the injured labrum and adhesive-treated labrum had sparse progenitor cells at week 3 (Figure S-5), whereas a high density of progenitor cells was observed with the PDGF adhesive (Figure 7A).
Figure 7.
Assessment of progenitor cell recruitment in rat labrum following injury and different treatments. (A) Representative fluorescent images for DAPI, CD44, and CD73 in PDGF adhesive treated labrum at 3 weeks after surgery. Scale bar = 50 μm. (B) Number of CD44+/CD73+ cells in healthy labrum and labrum with different treatments after 3- and 6-weeks post-surgery. Data presented as mean ± SD (n=3), *, **, ***, and **** indicate p<0.05, p<0.01, p<0.001, and p<0.0001, respectively. (C) Representative fluorescent images for DAPI, Ki-67, and CD73 in PDGF adhesive treated labrum at 3 weeks after surgery. Scale bar = 50 μm.
There were significantly more CD44+/CD73+ cells in the PDGF adhesive group at 3 weeks compared to that in the adhesive alone (2X, p=0.0001) and injured (8X, p<0.0001) groups (889±112 vs. 429±107 and 146±144 cells/0.25mm2, respectively) (Figure 7B). These results indicate that the hypercellularity observed in PDGF adhesive group in Figure 5A was associated with the active recruitment of progenitor cells. Furthermore, the PDGF adhesive group had 1.3X and 5X more CD44+/CD73+ cells at 6 weeks compared to the adhesive alone and injured labrum groups, respectively (376±17 vs. 254±29 and 138±182 cells/0.25mm2, respectively).
Additionally, Ki-67 staining showed that the localized release of PDGF promoted progenitor cell proliferation at 3 weeks (Figure 7C). To confirm that progenitor cell responses were elicited by PDGF release, tissues were stained with PDGF α- and β-receptors (PDGFRα + PDGFRβ). We found that more than 80% of PDGFR+ cells were progenitor cells (Figure S-6). Collectively, these results support that localized PDGF release induces progenitor cell migration and proliferation.
3.6. Histological evaluation of labral regeneration
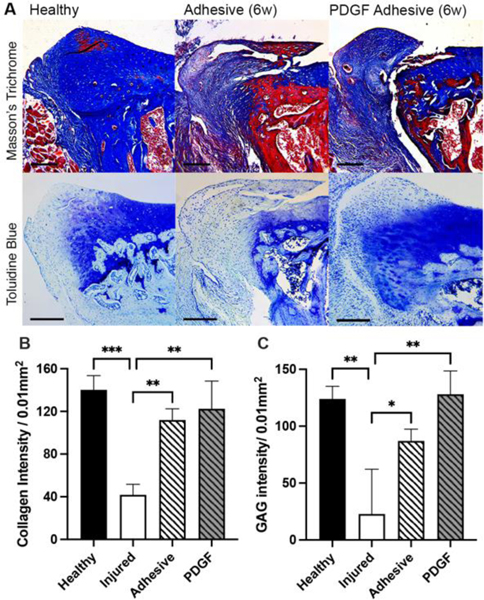
Subsequent analyses were performed to assess whether bioadhesive treatments would increase labral regeneration by increasing ECM production in the injured labrum.13,30 The labral ECM composition was assessed using Masson’s Trichrome (collagen) and toluidine blue (glycosaminoglycan (GAG)) and quantified using ImageJ. Color Deconvolution plugin was used to separate colors. As expected, there was major collagen and GAG loss observed in the injured group compared to healthy by week 6. At week 6, the adhesive only group showed more (~2.6X higher intensity) collagen production compared to that in the injured control group. Finally, we found that the PDGF adhesive group had ~3X higher intensity of collagen than the injured control groups (Figure 8B). Further analysis found that collagen intensity of PDGF adhesive and healthy tissues were not statistically significant (p = 0.5724). Similarly, the PDGF adhesive group had the highest overall amount of GAG production, with a ~1.5X and ~6X higher intensity of GAG staining than that in the adhesive alone and injured groups (Figure 8C). The results support the overall objective that PDGF adhesive treatment promotes labral regeneration and ECM production.
Figure 8.
ECM analysis of healthy rat labrum and injured rat labral regeneration with adhesive or PDGF adhesive treatment at 6 weeks post-surgery. (A) Representative images for Masson’s Trichrome (collagen) and toluidine blue (GAG) staining. Scale bar = 200 μm. (B) Labral collagen quantification using ImageJ. (C) GAG intensity quantification using ImageJ. Data presented as mean ± SD (n=3), *, **, and *** indicate p<0.05, p<0.01, and p<0.001, respectively.
3.7. Source of progenitor cells
While the PDGF-releasing adhesive was found to increase progenitor cell recruitment and enhance labral tissue regeneration, it was not clear where the progenitor cells came from and how they triggered tissue regeneration. Interestingly, the distribution of progenitor cells in the PDGF adhesive group was densely localized to the capsule edge of the labrum at 3 weeks, suggesting the origin of the cells to be a nearby tissue adjacent to the capsular edge of the labrum (Figure S-5). To determine the origin of these progenitor cells, we examined healthy shoulder joint tissue and identified an abundance of endogenous CD44+/CD73+ progenitor cells in the glenohumeral joint 3 weeks after the procedures. Specifically, CD44+/CD73+ cells were found primarily in the joint capsule and not the glenoid labrum itself (Figure S-7), which suggests the substantial increase in progenitor cells present in PDGF adhesive-treated labra occurred via cell migration from nearby capsule. To demonstrate PDGF-mediated progenitor cell migration from the joint capsule to the injured labrum, the differential distribution of CD44+/CD73+ cells was quantified and compared between the injured and healthy labra. At 3 weeks, the interfacial cellularity (at 0 μm) was substantially higher for PDGF adhesive group than for the injured, adhesive alone, and healthy groups (Figure 9A). Furthermore, the progenitor cell density of the PDGF adhesive group gradually increased from the distal edge of the capsule to the labral tear site (red dashed line); thereafter, the cell density decreased at distances beyond the tear. By contrast, the injured control group showed a significant reduction in progenitor cells in the joint capsule and few to no cells in the labrum. Progenitor cell density of adhesive group remained constant from the joint capsule to labrum which indicate mild recruitment possibly by injury mediated inflammatory signals. This change in progenitor cell distribution across the capsule and injured labrum suggests that progenitor cells were actively recruited from the capsule to the injury site with the localized release of PDGF. Finally, to determine whether the progenitor cells in the labrum actively contributed to labral tissue repair, we stained the tissue for CD44, CD73, and COL2 and found that the progenitor cells and COL2 colocalized near the injury site (Figure 9B). These results show that PDGF-releasing adhesives can enhance the recruitment of progenitor cells that trigger ECM production and contribute to labral regeneration.
Figure 9.
Migratory origin of progenitor cells. (A) Differential distribution of CD44+/CD73+ progenitor cells in healthy, injured, adhesive, and PDGF adhesive treated labra, ranging from the outer capsular edge to the labral tear. Red dashed line represents labral tear site. Data are presented as the mean ± SD (n = 3). (B) Representative colocalization of CD44+ cells and collagen type II (COL2) demonstrates active contribution of progenitor cells to labral regeneration. Scale bar = 10 μm.
4. Discussion
Here, we report the creation of a PDGF-releasing bioadhesive for repairing the torn labrum, promoting labral tissue regeneration by triggering progenitor cell recruitment and ECM production. Our proof-of-concept in vivo studies indicate that the application of a CS bioadhesive can reduce labral tissue inflammation following injury, thereby mitigating the tissue degeneration process. Subsequently, the local delivery of PDGF can enhance tissue repair by recruiting nearby progenitor cells while also promoting cell proliferation and differentiation at the injury site (Figure 10).
Figure 10.
Summary of progenitor cell-mediated labral regeneration. Incorporation of PDGF in the adhesives were able to elicit host progenitor cell responses to the injury for enhanced labral regeneration. Of note, inflammatory cells (red) and progenitor cells (green) are not present in healthy uninjured labra and likely originated from surrounding joint capsule during injury.
One major finding of this study is the discovery of progenitor cells within the shoulder joint capsule, which may be recruited to improve and accelerate labral healing. Furthermore, application of adhesive reduced labral inflammation with minimal CD11b+ inflammatory cells at the tear site, suggesting that the adhesive may block inflammatory cell infiltration and thus reduce labral tissue degeneration following tear.
In situ tissue regeneration harnesses the regenerative potential of the body and facilitates tissue repair. For this to be possible, tissue scaffolding, and drug delivery systems must minimize the deleterious effects of injury while supporting the body’s reparative process. As a proof-of-concept, a PDGF-releasing CS adhesive was developed for glenoid labral regeneration. All the components used in the adhesive are biocompatible and possess unique functions and activities. The CS polysaccharide is a well-established biomaterial owing to its biocompatibility, low toxicity, and bioadhesion to wet tissue surfaces.31 CS hydrogels have been shown to accelerate the healing of various tissues.32 The low immunogenicity, adhesive properties, and injectability of CS make it an attractive material for labral repair. In the present study, we observed that the application of the CS adhesive to labral tears reduced the level of tissue degeneration compared to that in the injured control. This may be attributed to the adhesive narrowing of the tissue repair gap while creating a sealed barrier at the injury site to prevent the invasion of synovial fluid, which is populated with inflammatory cells following injury.33,34
PDGF serves as a bioactive agent that enhances labral regeneration by eliciting host cell responses; PDGF and its receptors are abundantly expressed in many organs.35,36 It is known to have strong chemotactic activity toward progenitor cells and has been used in the healing of different tissues.14,37,38 Furthermore, previous in vitro studies conducted in our laboratory demonstrated that PDGF had a substantial effect on the proliferation, migration, and differentiation of human labral cells.17 Other chemokines and growth factors, such as stromal cell-derived factor 1 alpha protein (SDF-1α), transforming growth factor beta-3 (TGFβ−3), fibroblast growth factor 2 (FGF-2), and bone morphogenetic protein 7 (BMP-7), which have been explored in cartilage and meniscus regeneration,39 may also elicit similar progenitor cell responses. Of note, PDGF has been implicated in the development of inflammation and fibrosis in many organs (i.e., lung, kidney, heart, liver, skin) through recruitment of various cell types.40 However, it should be noted that such effects are often associated with overactivity of PDGF signaling or systemic injection of large doses of PDGF.41,42 To overcome such unwanted responses, localized delivery of PDGF has been established as an effective means to avoid toxicity to other organs, produce favorable angiogenic responses, and promote tissue regeneration as documented in many previous publications.14,38,43–45 In agreement, several lines of evidence support that the PDGF-releasing adhesive approach may induce labral regenerative responses without adverse systemic complications. First, PDGF has been shown to trigger labral progenitor cell migration and proliferation in vitro, and PDGF-mediated cell migration to the injured labrum was observed using human labral explants cultured ex vivo.17 Secondly, while hypercellularity present+ in PDGF treated tissues (Figure 6A), the number of recruited CD11b+ inflammatory cells significantly reduced in the PDGF adhesive group compared to injured controls (85% reduction, p=0.0009) by 3 weeks (Figure 6B). Similarly, PDGF adhesive treatment group was found to produce significantly lesser inflammatory products, such as MMP-13 (~55% less) and IL-1β (~80% less), than injured controls at 3 weeks. (Figure 6C&D). On the other hand, the number of recruited cells that possess progenitor cells markers (CD44+/CD73+) in PDGF adhesive treated group is significantly more than that of injured control (889±112 vs. 146±144 cells/0.25mm2, p<0.0001) (Figure 7B). In addition, IHC staining supports that PDGF release may be responsible for the recruitment of progenitor cells, since the majority (>80%) of PDGF receptor positive cells (PDGFR+) possess progenitor cell markers (CD44+) (Figure S-6). Lastly, PDGF adhesive treatment was found to increase the production of collagen and glycosaminoglycans (Figure 8). These results support that localized release of PDGF in labrum tissue is likely to contribute to labrum tissue regeneration, but not inflammatory and fibrotic reactions. Nonetheless, further investigations into the dose-dependent effects of PDGF would advance the efficacy of our targeted therapeutic approach to glenoid labral repair.
The use of progenitor cells for in situ tissue regeneration has been explored in different tissues such as cartilage, bone, and meniscus;13,14,30,46 however, there is limited knowledge of their roles in labral injuries and healing. The discovery of cells with regenerative potential in or near the labrum provides a potential therapeutic avenue for the repair and regeneration of a torn labrum. While we observed the presence of CD44+/CD73+ cells in the injured labrum, with a significantly higher number in the PDGF-treated labrum, we did not observe these cells in the healthy labrum. Instead, we discovered that the joint capsule provided a rich reservoir of progenitor cells. Since many reports have observed the glenoid labrum of various animal species and humans to be continuous with the joint capsule in various regions around the glenoid rim (primarily anteriorly and posteriorly),47 cells located in these attachment areas may migrate to the labrum injury site. Nonetheless, further studies are needed to determine the exact source of progenitor cells.
This model has several limitations. To create a labral tear, there is substantial surgical trauma to the joint capsule and surrounding muscle, which may not simulate labral tears in humans. While the effect of external injuries on labral tear repair warrants further investigation, we believe that this approach is feasible in humans where the injury is localized to the tear site. Additionally, the current model may not simulate the clinical environment of labral tears because labral tear repair using adhesives occurs immediately following injury. While it is rare to do so in humans, the severity of the surgical procedure would not permit allocating time between injury and repair in this model. Rats are an established animal model for glenoid labral tears, because of the similarities in shoulder anatomy and pathology with humans;24,25,48 however, we did not perform a biomechanical analysis to assess shoulder function owing to the small size and irregular shape of the glenohumeral joint. For the same reason, our technique for the injured controls did not include the suture anchor procedure performed in humans because of the lack of suitable suture anchors in small animals. Thus, the potential interaction between the bioadhesive and anchoring devices has not been evaluated.
It should be noted that hydrogel-based adhesives generally have low mechanical strength compared to suture materials; therefore, we envision that biomolecule-releasing bioadhesives could be used in conjunction with the current suture anchoring techniques, as the bioadhesive developed here serves to elicit regenerative activities in the injured labrum. Additionally, the degradation and protein release properties of the adhesives were characterized in solution in vitro. Since the adhesives were applied and pressed between lacerated labra tissue in vivo, this limits the adhesive’s exposure to potential degradative environment (synovial fluid) – a potential degradative environment. Therefore, the “real” degradation rate of CS bioadhesive in this model is likely to be significantly reduced. We estimate that 100% degradation of the bioadhesive would take ~4 weeks. The degradation kinetics is appropriate since the adhesive is designed to be infiltrated and then replaced by recruited progenitor cells during weeks 0–4. This infiltration is then expected to be followed by increasing cell mass and ECM production by week 6. Similarly, it is likely that the release of PDGF would have lasted longer than the previously reported 14 days since drug release from hydrogel matrices depends on several variables including volume of drug reservoir, rate of diffusion of water, and area of mass transfer.49–51 Indeed, our results support that the application of PDGF releasing CS adhesives was able to accelerate labral healing with a significant increase in labral progenitor cells and ECM production at week 3 and 6, respectively. Further development and optimization of CS adhesive is needed for maximizing labrum tissue regenerative capability. Furthermore, while we speculate that the recruited cells originated from the joint capsule because of the dense localization of cells on the capsular side of the labrum at 3 weeks (early process), we cannot exclude the activity of synovium-derived progenitor cells, which are CD44+/CD73+ and have been implicated in the progression of bone and joint diseases.52,53
Although progenitor cells in the joint capsule and their subsequent migration to the injury site have been observed in rats, it is not clear whether this phenomenon would occur in humans as well. Further investigations are needed to determine whether progenitor cells in the human joint capsule can serve as a source of regenerative cells as required for this approach. Nonetheless, this work may improve our understanding of glenoid labral tears and provide insight into the restorative mechanisms of labral healing, which could be used for the development of novel adjuncts to current surgical procedures for the repair of labral injuries. The presence of previously unknown progenitor cells within the joint capsule strongly supports the feasibility of this new technology for glenoid labral repair.
Supplementary Material
5. Acknowledgments
We thank Vicki Ea for assistance in conducting part of the in vitro degradation work. One the authors has affiliated with a commercial entity not related to this particular study and orthopaedic surgery but related to another branch of medicine. The authors acknowledge the support from the Research and Scholarship Excellent fund from the University of Texas at Arlington and National Heart, Lung, and Blood Institute through award number NIH T32 HL134613 (C.C.). The content is the sole responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank Editage (www.editage.com) for English language editing.
References
- 1.Cooper DE et al. Anatomy, histology, and vascularity of the glenoid labrum. An anatomical study. (1992) J Bone Joint Surg Am 74, 46–52. [PubMed] [Google Scholar]
- 2.Alexeev M, Kercher JS, Levina Y, Duralde XA Variability of glenoid labral tear patterns: a study of 280 sequential surgical cases. (2021) J Shoulder Elbow Surg 30, 2762–2766. [DOI] [PubMed] [Google Scholar]
- 3.Edwards SL et al. Nonoperative Treatment of Superior Labrum Anterior Posterior Tears: Improvements in Pain, Function, and Quality of Life. (2010) Am J Sports Med 38, 1458–1461. [DOI] [PubMed] [Google Scholar]
- 4.Petrera M, Patella V, Patella S, Theodoropoulos J. A meta-analysis of open versus arthroscopic Bankart repair using suture anchors. (2010) Knee Surg. Sports Traumatol. Arthrosc 18, 1742–1747. [DOI] [PubMed] [Google Scholar]
- 5.Matsuki K, Sugaya H. Complications after arthroscopic labral repair for shoulder instability. (2015) Curr Rev Musculoskelet Med 8, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clavert P. Glenoid labrum pathology. (2015) ORTHOP TRAUMATOL-SUR 101, S19–S24. [DOI] [PubMed] [Google Scholar]
- 7.Probyn LJ, White LM, Salonen DC, Tomlinson G, Boynton EL Recurrent symptoms after shoulder instability repair: Direct MR arthrographic assessment - Correlation with second-look surgical evaluation. (2007) Radiology 245, 814–823. [DOI] [PubMed] [Google Scholar]
- 8.Barron OA, Bigliani LU Revision instability surgery. (1995) Clin Sports Med 14, 955–972. [PubMed] [Google Scholar]
- 9.Mihalceanu S, Aitzetmüller MM, Machens H-G, Duscher D. Stem Cell Therapies for Tissue Regeneration and Wound Healing: Strategies to Enhance Therapeutic Effectiveness. (Springer, Cham, 2019). in Regenerative Medicine and Plastic Surgery 187–199 doi: 10.1007/978-3-030-19958-6_18. [DOI] [Google Scholar]
- 10.Kim HJ, Park J-S Usage of Human Mesenchymal Stem Cells in Cell-based Therapy: Advantages and Disadvantages. (2017) Dev Reprod 21, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thevenot PT et al. The effect of incorporation of SDF-1α into PLGA scaffolds on stem cell recruitment and the inflammatory response. (2010) Biomaterials 31, 3997–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair AM et al. The effect of erythropoietin on autologous stem cell-mediated bone regeneration. (2013) Biomaterials 34, 7364–7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hakamivala A. et al. Recruitment of Endogenous Progenitor Cells by Erythropoietin Loaded Particles for In Situ Cartilage Regeneration. (2020) Bioact. Mater 5, 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee K. il Olmer M, Baek J, D’Lima DD, Lotz MK Platelet-derived growth factor-coated decellularized meniscus scaffold for integrative healing of meniscus tears. (2018) Acta Biomater. 76, 126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarafder S. et al. Engineered Healing of Avascular Meniscus Tears by Stem Cell Recruitment. (2018) Sci. Rep 8, 8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nie H. et al. Musculoskeletal tissue engineering by endogenous stem/progenitor cells. (2012) Cell Tissue Res. 347, 665–676. [DOI] [PubMed] [Google Scholar]
- 17.Li S. et al. Biomolecules-releasing click chemistry-based bioadhesives for repairing acetabular labrum tears. (2022) J. Orthop. Res 1–10 doi: 10.1002/jor.25290. [DOI] [PubMed] [Google Scholar]
- 18.Pei X, Wang J, Cong Y, Fu J. Recent progress in polymer hydrogel bioadhesives. (2021) J Polym Sci 59, 1312–1337. [Google Scholar]
- 19.Zhu W, Chuah YJ, Wang DA Bioadhesives for internal medical applications: A review. (2018) Acta Biomater. 74, 1–16. [DOI] [PubMed] [Google Scholar]
- 20.Mehdizadeh M, Yang J. Design Strategies and Applications of Tissue Bioadhesives. (2013) Macromol Biosci 13, 271–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhagat V, Becker ML Degradable Adhesives for Surgery and Tissue Engineering. (2017) Biomacromolecules 18, 3009–3039. [DOI] [PubMed] [Google Scholar]
- 22.Leggat PA, Smith DR, Kedjarune U. Surgical applications of cyanoacrylate adhesives: A review of toxicity. (2007) ANZ J. Surg 77, 209–213. [DOI] [PubMed] [Google Scholar]
- 23.Li S. et al. Injectable Click Chemistry-based Bioadhesives for Accelerated Wound Closure. (2020) Acta Biomater. 110, 95–104. [DOI] [PubMed] [Google Scholar]
- 24.Packer JD et al. Ibuprofen impairs capsulolabral healing in a rat model of anterior glenohumeral instability. (2018) J Shoulder Elbow Surg 27, 315–324. [DOI] [PubMed] [Google Scholar]
- 25.Mulcahey MK et al. Factors Expressed in an Animal Model of Anteroinferior Glenohumeral Instability. (2015) Orthop. J. Sports Med. 3, 2325967115599733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pauli C. et al. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. (2011) Osteoarthr. Cartil 19, 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishida Y. et al. Intra-Articular Injection of Stromal Cell-Derived Factor 1α Promotes Meniscal Healing via Macrophage and Mesenchymal Stem Cell Accumulation in a Rat Meniscal Defect Model. (2020) Int. J. Mol. Sci 21, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Co CM et al. Click chemistry-based pre-targeting cell delivery for cartilage regeneration. (2021) Regen Biomater 8, rbab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He S. et al. Characterization and Comparison of Postnatal Rat Meniscus Stem Cells at Different Developmental Stages. (2019) Stem Cells Transl Med 8, 1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lienemann PS et al. Smart Hydrogels for the Augmentation of Bone Regeneration by Endogenous Mesenchymal Progenitor Cell Recruitment. (2020) Adv. Sci 7, 1903395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mati-Baouche N. et al. Chitosan as an adhesive. (2014) Eur. Polym. J 60, 198–212. [Google Scholar]
- 32.Hamedi H, Moradi S, Hudson SM, Tonelli AE Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. (2018) Carbohydr. Polym 199, 445–460. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura K. et al. Dynamic analysis of inflammatory cells in synovial fluid after index anterior cruciate ligament reconstruction surgery. (2016) Osteoarthr. Cartil 24, S340. [Google Scholar]
- 34.Garcia J. et al. The synovial fluid from patients with focal cartilage defects contains mesenchymal stem/stromal cells and macrophages with pro- and anti-inflammatory phenotypes. (2020) Osteoarthr. Cartil. Open 2, 100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. (2008) Genes Dev. 22, 1276–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Betsholtz C, Karlsson L, Lindahl P. Developmental roles of platelet-derived growth factors. (2001) BioEssays 23, 494–507. [DOI] [PubMed] [Google Scholar]
- 37.Mishima Y, Lotz M. Chemotaxis of Human Articular Chondrocytes and Mesenchymal Stem Cells. (2008) J. Orthop. Res 26, 1407–1412. [DOI] [PubMed] [Google Scholar]
- 38.Kang S. et al. Long-term local PDGF delivery using porous microspheres modified with heparin for tendon healing of rotator cuff tendinitis in a rabbit model. (2019) Carbohydr. Polym 209, 372–381. [DOI] [PubMed] [Google Scholar]
- 39.Forriol F. Growth factors in cartilage and meniscus repair. (2009) Injury 40, S12–S16. [DOI] [PubMed] [Google Scholar]
- 40.Klinkhammer BM, Floege J, Boor P. PDGF in organ fibrosis. (2018) Mol Aspects Med 62, 44–62. [DOI] [PubMed] [Google Scholar]
- 41.Bonner JC Regulation of PDGF and its receptors in fibrotic diseases. (2004) Cytokine Growth Factor Rev 15, 255–273. [DOI] [PubMed] [Google Scholar]
- 42.Heldin CH Targeting the PDGF signaling pathway in the treatment of non-malignant diseases. (2014) Journal of Neuroimmune Pharmacology 9, 69–79. [DOI] [PubMed] [Google Scholar]
- 43.Hsieh PCH, MacGillivray C, Gannon J, Cruz FU, Lee RT Local Controlled Intramyocardial Delivery of Platelet-Derived Growth Factor Improves Postinfarction Ventricular Function Without Pulmonary Toxicity. (2006) Circulation 114, 637–644. [DOI] [PubMed] [Google Scholar]
- 44.de la Riva B. et al. Local controlled release of VEGF and PDGF from a combined brushite–chitosan system enhances bone regeneration. (2010) Journal of Controlled Release 143, 45–52. [DOI] [PubMed] [Google Scholar]
- 45.Baek J, Lee E, Lotz MK, D’Lima DD Bioactive proteins delivery through core-shell nanofibers for meniscal tissue regeneration. (2020) Nanomedicine 23, 102090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo W. et al. Cell-Free Strategies for Repair and Regeneration of Meniscus Injuries through the Recruitment of Endogenous Stem/Progenitor Cells. (2018) Stem Cells Int 2018, 5310471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Como CJ, Rothrauff BB, Alexander PG, Lin A, Musahl V. Common animal models lack a distinct glenoid labrum: a comparative anatomy study. (2021) J. Exp. Orthop 8, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gereli A. Animal Models for Research on Shoulder Pathologies. (Springer, Cham, 2022). in Fundamentals of the Shoulder 15–21 doi: 10.1007/978-3-030-94702-6_3. [DOI] [Google Scholar]
- 49.Lee PI Kinetics of drug release from hydrogel matrices. (1985) Journal of Controlled Release 2, 277–288. [Google Scholar]
- 50.Lin CC, Metters AT Hydrogels in controlled release formulations: Network design and mathematical modeling. (2006) Adv Drug Deliv Rev 58, 1379–1408. [DOI] [PubMed] [Google Scholar]
- 51.Siepmann J, Göpferich A. Mathematical modeling of bioerodible, polymeric drug delivery systems. (2001) Adv Drug Deliv Rev 48, 229–247. [DOI] [PubMed] [Google Scholar]
- 52.Li N. et al. Synovial membrane mesenchymal stem cells: past life, current situation, and application in bone and joint diseases. (2020) Stem Cell Res. Ther 11, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGonagle D, Baboolal TG, Jones E. Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. (2017) Nat Rev Rheumatol 13, 719–730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.