Abstract
Background
MicroRNA (miRNA) plays a critical function in the progression of acute coronary syndrome (ACS) and is associated with major adverse cardiovascular events (MACEs) after undergoing percutaneous coronary intervention (PCI). This research was designed to probe the diagnostic accuracy of miR-483-5p in patients with ACS and its predictive value of MACEs.
Methods
118 patients with ACS (40 with unstable angina pectoris [UAP] and 78 with acute myocardial infarction [AMI]) and 75 healthy controls were enrolled. Serum miR-483-5p was detected in the subjects by reverse transcription-quantitative real-time PCR (RT-qPCR). ROC curve and logistic regression models were employed to estimate the diagnosis. Patients were monitored for 6 months after PCI to document the occurrence of MACEs. Kaplan-Meier survival was conducted to explore the predictive significance of miR-483-5p for the MACEs.
Results
Serum miR-483-5p levels were higher in ACS patients and associated with SYNTAX score and Gensini score. miR-483-5p was effective in identifying ACS patients from healthy individuals (AUC = 0.919) and AMI patients from ACS patients (AUC = 0.867), demonstrating a high diagnostic value, proven by logistic regression (OR = 9.664, 95%CI = 4.462–20.928, P < 0.001). The prevalence of MACEs during follow-up were 24.58%, and a higher prevalence of MACEs were observed in patients with elevated miR-483-5p (P = 0.01). miR-483-5p was also an effective predictor of MACE occurrence (HR = 5.955, 95%CI = 1.928–18.389, P = 0.002).
Conclusion
Expression of serum miR-483-5p can be utilized as a non-invasive marker for diagnosing ACS and predicting the onset of MACE after PCI.
Keywords: Acute coronary syndrome, miR-483-5p, Major adverse cardiovascular events, Diagnostic, Predicts
Background
Acute coronary syndrome (ACS) accounts for more than 1 million deaths worldwide each year [1]. The incidence of ACS in China is increasing annually and is predicted to reach 22.6 million patients by 2030 [2]. ACS commonly arises from the rupture or erosion of atherosclerotic plaques in the coronary arteries that supply blood to the heart, resulting in arterial thrombosis and subsequent myocardial ischemia. This is primarily manifested as acute myocardial infarction (AMI) and unstable angina pectoris (UAP) [3]. As a significant burden on global healthcare, ACS has an urgent attack, rapid illness, and heavy mortality rate, and earlier diagnosis and detection can help provide timely management measures for ACS patients [4]. Percutaneous coronary intervention (PCI) is currently the main method for ACS, significantly regaining correct coronary and reducing infarct size. However, 23% of patients exhibited a propensity for major adverse cardiovascular events (MACEs), such as recurrent angina and revascularization [5]. Hence, it is particularly important to identify dependable and consistent biomarkers for the diagnosis and evaluation of MACE.
MicroRNA, as an endogenous one-stranded non-coding RNA molecule, is involved in various biological processes by regulating target genes. Furthermore, miRNA has been proposed as a clinical biomarker for cardiovascular disease owing to its stable presence in various biological fluids (such as blood, serum, urine, and saliva) and its extracellular secretion that can be easily quantified by reverse transcription-quantitative real-time PCR (RT-qPCR). miR-142-3p [6], miR-941 [7], miR-3646 [8], and miR-497-5p [9] and miR-361-5p [10] were identified as promising biomarkers for ACS. miR-483-5p is a mature miRNA consisting of a 22 nucleotide (AAGACGGGAGGAAAGAAGGGAG) located on chromosome 11p15.5. Coronary plaque rupture is most commonly responsible for ACS [11]. Li et al. identified several miRNAs, including miR-483-5p, that exhibited differential expression before and after coronary plaque rupture [11]. Tian et al. demonstrated significant upregulation of miR-483-5p in both plaque arteries and normal coronary arteries via microarray assay [12]. Arrhythmias are a common complication of ACS, and miR-483-5p was reported to be markedly elevated in atrial fibrillation, the most common form of arrhythmia [13].
Herein, the present study aimed to assess the levels of serum miR-483-5p in patients with ACS and investigate its potential as a novel diagnostic biomarker for ACS. Additionally, it explored the predictive value of miR-483-5p about MACE following PCI. A first demonstration is that miR-483-5p expression can be used as a diagnostic marker for ACS and can non-invasively predict the occurrence of MACE after PCI.
Methods
Ethical statement
Subjects signed an informed consent form before enrollment. With the approval of the No.980 Hospital of PLA Joint Logistics Support Force Medical Ethics Committee, this research protocol strictly adhered to the Helsinki Declaration principles.
Study population
Patients aged 40–80 years old who visited the No.980 Hospital of PLA Joint Logistics Support Force for ACS with chest pain diagnosed as UAP, non-ST-elevation myocardial infarction (NSTEMI) and ST-elevation myocardial infarction (STEMI) from January 2018 to June 2019 were included. Inclusion criteria: (1) patients with ACS who met the European Society of Cardiology (ESC) [14] and American College of Cardiology (ACC)[15, 16] diagnostic criteria and had chest pain episodes of less than 24 h; (2) all patients with ACS were first-time episodes; (3) confirmed by coronary angiography with at least 1 coronary artery stenosis with > 75% stenosis (diameter method), requiring PCI treatment; (4) patients with complete clinical data. Exclusion criteria: (1) patients with recent use of immunosuppressants or immune enhancers; (2) combined with other cardiac insufficiencies, hematologic diseases, malignancies, or autoimmune diseases; (3) prior revascularization therapy [either PCI or coronary artery bypass grafting (CABG)]. The final 118 patients with ACS were included in this study, including 40 with UAP and 78 with AMI. The definition of UAP is clinical symptoms, Braunwald’s classification of class IIB and IIIB typical anterior chest pain, and no significant increase in serum creatine kinase concentration. The definition of AMI is characterized by clinical symptoms, coronary angiographic findings, electrocardiogram (ECG) suggestive of new onset, NSTEMI or STEMI, and serum creatine kinase (CK) concentrations were more than two-fold above the upper limit of the normative range. And cardiac troponin I (cTnI) values of more than 0.06 ng/ml were also confirmed for AMI. Furthermore, 75 healthy individuals matching the age and gender of the ACS patients and who were physically examined at the hospital served as controls. diastolic blood pressure (DBP) and systolic blood pressure (SBP) were measured by an Ormon Hem-7136 monitor. cTnI, as well as triglyceride (TG), total cholesterol (TC), low-density-lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C), were assessed by a Roche automated biochemical analyzer. Patient demographics and biochemical data were recorded in Table 1.
Table 1.
General information of the enroll participants
| Parameters | Controls (n = 75) |
ACS (n = 118) |
P values |
|---|---|---|---|
| Age, years | 60.12 ± 8.26 | 58.81 ± 7.96 | 0.275 |
| BMI, kg/m2 | 25.80 ± 3.77 | 26.75 ± 4.07 | 0.107 |
| Gender, male, n (%) | 45 (60.00) | 68 (57.63) | 0.766 |
| Smoking, n (%) | 46 (61.33) | 64 (54.24) | 0.372 |
| SBP, mmHg | 127.71 (124.74, 130.68) | 129.69 (123.75, 136.62) | 0.070 |
| DBP, mmHg | 78.90 (72.91, 82.89) | 82.17 (77.22, 88.11) | 0.002 |
| LDL-C, mmol/L | 2.59 ± 0.54 | 2.81 ± 0.80 | 0.040 |
| HDL-C, mmol/L | 1.36 (1.02, 1.64) | 1.35 (0.89, 1.63) | 0.278 |
| TC, mmol/L | 3.99 ± 0.58 | 4.12 ± 0.44 | 0.068 |
| TG, mmol/L | 1.48 ± 0.23 | 1.53 ± 0.56 | 0.437 |
| Creatine, µmol/L | 67.76 (60.35, 77.85) | 73.77 (68.48, 78.39) | 0.029 |
| WBC, ×109/L | 6.68 ± 0.50 | 7.41 ± 0.71 | 0.000 |
| UREA, mmol/L | 4.92 ± 0.37 | 4.88 ± 0.51 | 0.586 |
| NT-proBNP, pg/mL | 36.60 ± 6.04 | 110.59 ± 36.51 | 0.000 |
| hs-CRP, mg/L | - | 5.97 ± 1.39 | - |
| cTnI, ng/mL | - | 1.13 (0.05, 2.17) | - |
| SYNTAX score | - | 28.90 ± 10.95 | - |
| Gensini score | - | 49.13 ± 26.49 | - |
| Culprit lesion, n (%) | |||
| Left main | 1 (0.85) | ||
| Left anterior descending | 42 (35.59) | ||
| Left circumflex | 29 (24.58) | ||
| Right | 46 (38.98) | ||
| Baseline stenosis (%) | 94.08 ± 3.87 | ||
| MVD, n (%) | 73 (61.86) | ||
| Stent diameter (mm) | 3.06 ± 0.48 | ||
| Stent length (mm) | 26.50 ± 4.47 | ||
| Aspirin/clopidogrel, n (%) | 18 (15.25) | ||
| ACE inhibitor/ARB, n (%) | 31 (26.27) | ||
| β-blocker, n (%) | 25 (21.19) | ||
| Calcium Channel blocker, n (%) | 12 (10.17) | ||
| Stain, n (%) | 18 (15.25) |
Note: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; MVD, multivessel disease; TC, total cholesterol; TG, triglyceride; WBC, white blood cells; hs-CRP, high-sensitivity C-reactive protein; cTnI, cardiac troponin I; Date was presented as mean ± SD, or median (first quartile, third quartile), or N (%)
Clinical samples collection
5 mL of venous blood was obtained immediately on admission (within 24 h before PCI), and the upper serum was collected by centrifugation at 3000 g for 15 min at 4℃ into RNase-free EP tubes and stored in a -80℃ refrigerator for backup.
Coronary lesion evaluation and PCI procedure
Quantitative analysis of coronary lesions in ACS patients based on the Syntax score calculator (http://www.syntaxscore.com) and the left-right dominant classification of coronary arteries, lesion location, degree of stenosis, and pathological features based on coronary angiography by two experienced specialists [17]. As the score is higher, the more severe the coronary artery lesion becomes and the worse the outcome. Gensini score was employed to assess the degree of coronary stenosis and severity of atherosclerosis based on prior investigations [18].
PCI was performed in a standard manner, with adequate intraoperative balloon pre-dilation of the target lesion, balloon post-dilation after stent placement, and stents were selected from the new generation of drug-eluting stents, with images showing good stent deployment and less than 5% residual stenosis. Treatment with aspirin and clopidogrel during follow-up.
Follow up program
After PCI treatment discharge, the researchers followed the patients for 6 months using outpatient, telephone, and readmission visits. Follow-up endpoints were defined as the occurrence of MACEs such as sudden cardiac death, reinfarction, angina, clinically driven target vessel revascularization (including PCI and CABG), and new onset heart failure, and were judged by the investigators based on ECG, ischemic symptoms, and cardiac enzyme levels. Angina was defined as the recurrence of typical angina pectoris after PCI, manifested as retrosternal or precardiac pain at activity or rest, usually lasting no more than 20 min, and the onset was caused by changes in electrocardiogram ST-T and no increase in myocardial injury indexes. Reinfarction was defined as a significant increase in markers of myocardial injury during follow-up, ECG evidence of short ST elevation of more than 1 mm in two or more adjacent, or new left bundle branch blocks, and new pathological Q wave on ECG. Coronary angiography was utilized to determine the location of obstructive lesions in all patients with Reinfarction. New-onset heart failure was defined as the presence of dyspnea from PCI procedures, including exertional dyspnea, terminal and nocturnal paroxysmal dyspnea, signs of pulmonary edema or peripheral edema, ventricular enlargement, and echocardiographic systolic insufficiency.
Reverse transcription-quantitative real-time PCR (RT-qPCR)
600 µL of Trizol LS was mixed with the serum and left to stand at room temperature. The RNA was precipitated by adding chloroform as well as isopropanol, washed with 75% ethanol after centrifugation, and the RNA precipitate was dissolved in 30 µL of RNA-free water. The purity and concentration of isolated and extracted RNA were assessed using Nanodrop spectrophotometry. The miRNA cDNA synthesis kit (CW2141, Cwbiotech, Beijing, China) was performed at 37℃ for 15 min for miRNA addition (A) tail, followed by synthesis of miRNA cDNA at 42℃ for 50 min and 85℃ for 5 min. After mixing cDNA, primers, and miRNA qPCR Assay kit, amplification reactions were performed in a LightCycler 480 machine (Roche Applied Science). U6 served as an internal control and the relative level of miR-483-5p was obtained after three replicates with the 2−ΔΔCt method.
Statistical analysis
Kolmogorov-Smirnov was applied to examine the normal distribution of data. Normally distributed variables were illustrated as mean ± SD and analyzed by Student’s t-test, while non-normally distributed continuous data were indicated using median and quartiles [M(Q1-Q3)], and the Mann-Whitney U test was performed. One-way ANOVA and post hoc Tukey’s test were performed to detect differences between multiple groups. Categorical factors were characterized by [n (%)] and analyzed by the χ2 test. ROC was performed to examine the diagnostic value, whereas the Youden index was conducted to define the threshold value of ROC. Data were evaluated using SPSS 22.0 and GraphPad Prism 6.0 for analysis. Bilateral P < 0.05 was illustrated as statistically meaningful.
Results
Demographic and clinicopathological data of the subjects
Table 1 presents the demographic and clinicopathological data of the subjects. No statistical differences in age, body mass index (BMI), gender, smoking, SBP, HDL-C, TG, TC, and UREA between ACS patients and controls (P > 0.05). However, the levels of DBP, LDL-C, creatine, white blood cell (WBC), and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were typically higher in the ACS groups (P < 0.05).
Upregulated serum miR-483-5p was positively associated with SYNTAX score and Gensini score
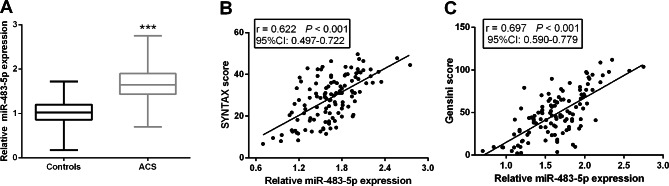
Serum miR-483-5p in ACS patients before PCI was significantly higher than that in the controls (P < 0.01, Fig. 1A). Additionally, the SYNTAX score based on the anatomical structure was found to be associated with the severity of coronary lesions [19]. Pearson correlation coefficient analysis confirmed that miR-483-5p was positively associated with SYNTAX score (r = 0.622, P < 0.001, 95% CI: 0.497–0.722, Fig. 1B). Gensini score was established as a quantitative method for assessing coronary artery stenosis [9], and serum miR-483-5p was found to be positively associated with the Gensini score (r = 0.697, P < 0.001, 95% CI: 0.590–0.779, Fig. 1C).
Fig. 1.
Serum miR-483-5p levels in ACS patients and correlation with different scores. A. Serum miR-483-5p levels of miR-483-5p in controls and ACS patients were explored by RT-qPCR. Pearson correlation coefficient was employed to evaluate the correlation of miR-483-5p with SYNTAX score (B) and Gensini score (C). *** P < 0.001 vs. Controls
Serum mir-483-5p has high diagnostic efficacy for ACS
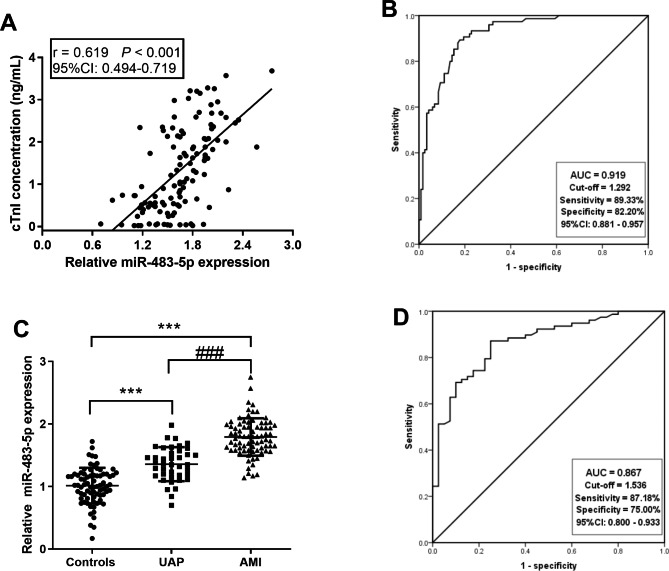
Serum miR-483-5p levels were positively correlated with cTnI levels (r = 0.619, P < 0.001, 95%CI = 0.494 − 0.719), a common biomarker of ACS (Fig. 2A). The diagnostic performance of biomarkers is usually assessed by ROC. Figure 2B confirmed that the AUC of miR-483-5p was 0.919, and the sensitivity and specificity of differentiating ACS patients from controls were 89.33% and 82.20% at a cut-off value of 1.292, demonstrating a feasibility diagnostic value. RT-qPCR furthermore verified that miR-483-5p levels were elevated in both UAP and AMI compared to controls, and the AMI group was markedly elevated compared to the UAP group (P < 0.01, Fig. 2C). ROC also confirmed that the AUC for miR-483-5p to identify AMI patients from ACS patients was 0.867, and the sensitivity and specificity were 87.18% and 75.00%, respectively, when the cut-off value was 1.536 (Fig. 2D).
Fig. 2.
Diagnostic accuracy of serum miR-483-5p in patients with ACS. A. The correlation of miR-483-5p levels with cTnI concentrations in ACS patients. B. ROC based on miR-483-5p levels in controls and ACS patients. C. RT-qPCR detection of miR-483-5p levels in ACS patients with UAP and AMI. D. ROC based on miR-483-5p levels in UAP and AMI patients in the ACS group. *** P < 0.001 vs. Controls, ### P < 0.001 vs. UAP
What’s more, the ACS and controls were treated as independent dichotomous variables in logistic analysis that included serum miR-483-5p levels and related clinical indicators. As presented in Table 2, miR-483-5p (OR = 9.664, 95%CI: 4.462–20.928, P < 0.001) and DBP (OR = 2.203, 95%CI: 1.053–4.609, P = 0.036) both independently contributed to the development of ACS.
Table 2.
Relation of different parameters to the occurrence of ACS
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| Age | 0.703 | 0.323–1.527 | 0.373 |
| Gender | 0.491 | 0.233–1.035 | 0.062 |
| BMI | 0.698 | 0.324 − 1.503 | 0.358 |
| Smoking | 0.850 | 0.404–1.789 | 0.669 |
| SBP | 0.523 | 0.245–1.116 | 0.094 |
| DBP | 2.203 | 1.053–4.609 | 0.036 |
| LDL-C | 0.553 | 0.263– 1.161 | 0.117 |
| HDL-C | 0.919 | 0.419–2.013 | 0.832 |
| TC | 0.555 | 0.261– 1.181 | 0.127 |
| TG | 0.998 | 0.473– 2.107 | 0.995 |
| Creatine | 2.024 | 0.944–4.339 | 0.070 |
| WBC | 2.017 | 0.955–4.258 | 0.066 |
| UREA | 0.684 | 0.327–1.428 | 0.312 |
| MiR-483-5p | 9.664 | 4.462– 20.928 | 0.000 |
Note: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride; WBC, white blood cells
Significance of serum mir-483-5p in predicting MACE post-PCI in ACS patients
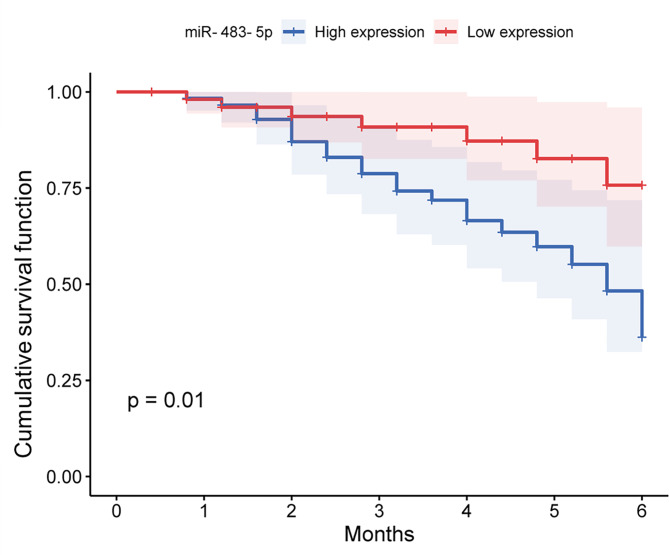
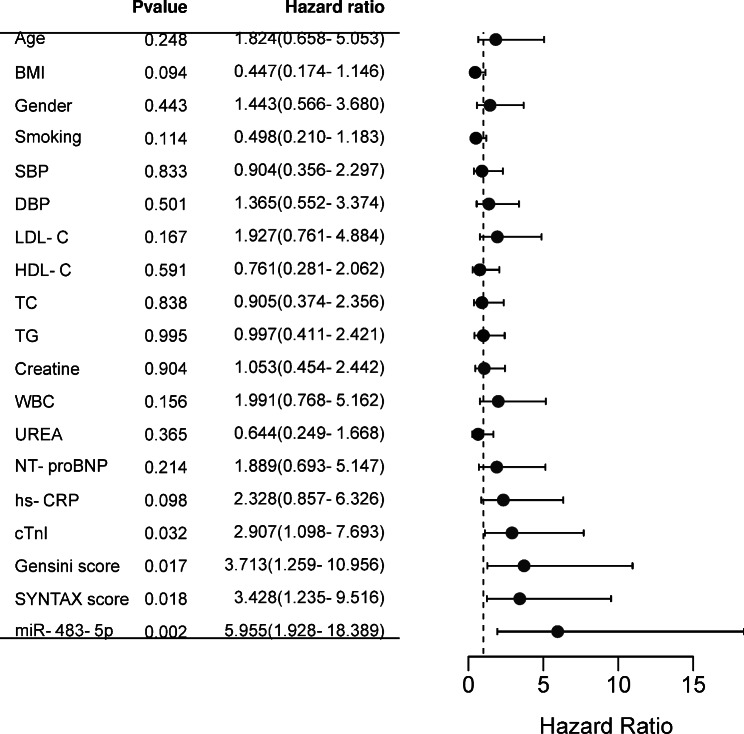
During the 6-month follow-up period after PCI, the incidence of MACE was 24.58%, including 3 cardiac death, 6 revascularizations, 9 recurrent angina, 4 reinfarctions, and 7 heart failure. Divide ACS patients into high miR-483-5p group (n = 62) and low miR-483-5p group (n = 56) based on the mean miR-483-5p levels (1.64 ± 0.36). As shown in Table 3, high levels of miR-483-5p were significantly associated with LDL-C, HDL-C, NT-proBNP, cTnI, SYNTAS score, Gensini score, Baseline stenosis, and stent Diameter (P < 0.05). Furthermore, the high miR-483-5p level was found to be associated with a higher incidence of MACEs after PCI (P = 0.018, Table 4). Kaplan-Meier analysis confirmed the same results (P = 0.01, Fig. 3). COX regression analysis was performed as shown in Fig. 4, and similar to cTnI, Gensini score, and SYNTAX score, miR-483-5p (HR = 5.955, 95%CI: 1.928–18.389, P = 0.002) could be used as an independent predictor of MACE occurrence.
Table 3.
Correlation of miR-483-5p levels with clinical information of ACS patients
| Parameters | low miR-483-5p group (n = 56) |
high miR-483-5p group (n = 62) |
P values |
|---|---|---|---|
| Age, years | 59.68 ± 8.24 | 58.02 ± 7.64 | 0.259 |
| BMI, kg/m2 | 26.25 ± 4.09 | 27.225 ± 3.95 | 0.191 |
| Gender, male, n (%) | 30 (53.57) | 38 (61.29) | 0.489 |
| Smoking, n (%) | 33 (58.93) | 31 (50.00) | 0.318 |
| SBP, mmHg | 130.68 (123.75, 135.63) | 128.70 (123.50, 136.62) | 0.612 |
| DBP, mmHg | 82.20 (77.45, 87.10) | 82.20 (74.98, 88.10) | 0.983 |
| LDL-C, mmol/L | 2.70 (2.00, 3.05) | 2.95 (2.38, 3.53) | 0.025 |
| HDL-C, mmol/L | 1.38 (1.02, 1.68) | 1.24 (0.83, 1.57) | 0.019 |
| TC, mmol/L | 4.14 ± 0.49 | 4.08 ± 0.40 | 0.429 |
| TG, mmol/L | 1.23 ± 0.56 | 1.50 ± 0.57 | 0.437 |
| Creatine, µmol/L | 74.39 (68.62, 78.50) | 73.25 (69.08, 78.21) | 0.602 |
| WBC, ×109/L | 7.42 ± 0.67 | 7.40 ± 0.75 | 0.859 |
| UREA, mmol/L | 4.92 ± 0.52 | 4.85 ± 0.49 | 0.479 |
| NT-proBNP, pg/mL | 102.74 ± 36.64 | 118.36 ± 34.63 | 0.019 |
| hs-CRP, mg/L | 5.82 ± 1.52 | 6.11 ± 1.26 | 0.255 |
| cTnI, ng/mL | 0.55 (0.28, 1.63) | 0.71 (1.00, 2.38) | 0.000 |
| SYNTAX score | 26.03 ± 10.60 | 30.83 ± 10.65 | 0.016 |
| Gensini score | 40.80 ± 22.13 | 55.96 ± 28.53 | 0.002 |
| Culprit lesion, n (%) | |||
| Left main | 0 (0.00) | 1 (1.61) | 0.340 |
| Left anterior descending | 17 (30.36) | 25 (40.32) | 0.259 |
| Left circumflex | 10 (17.86) | 19 (30.65) | 0.107 |
| Right | 28 (50.00) | 18 (29.03) | 0.412 |
| Baseline stenosis (%) | 92.09 ± 3.70 | 95.81 ± 3.11 | 0.000 |
| MVD, n (%) | 34 (60.72) | 39 (62.90) | 0.807 |
| Stent diameter (mm) | 2.90 ± 0.48 | 3.21 ± 0.44 | 0.003 |
| Stent length (mm) | 25.86 ± 4.54 | 27.08 ± 4.36 | 0.139 |
| Aspirin/clopidogrel, n (%) | 7 (12.50) | 11 (26.81) | 0.429 |
| ACE inhibitor/ARB, n (%) | 15 (26.79) | 16 (25.81) | 0.904 |
| β-blocker, n (%) | 11 (19.64) | 14 (22.58) | 0.679 |
| Calcium Channel blocker, n (%) | 5 (8.93) | 7 (11.29) | 0.672 |
| Stain, n (%) | 5 (8.93) | 13 (20.97) | 0.069 |
| Subgroups of ACS patients | |||
| UAP/AMI | 35/21 | 5/57 | 0.000 |
Note: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; MVD, multivessel disease; TC, total cholesterol; TG, triglyceride; WBC, white blood cells; hs-CRP, high-sensitivity C-reactive protein; cTnI, cardiac troponin I; Date was presented as mean ± SD, or median (first quartile, third quartile), or N (%)
Table 4.
Major adverse cardiac events according to miR-483-5p expression levels
| Variables | Cases No. (n = 118) |
miR-483-5p expression | P | |
|---|---|---|---|---|
| High (n = 62) | Low (n = 56) | |||
| Total MACEs | 29 (24.58) | 21 (33.87) | 8 (14.29) | 0.018 |
| Death | 3 (2.54) | 2 (3.23) | 1 (1.79) | 0.538 |
| Revascularization | 6 (5.08) | 5 (8.06) | 1 (1.79) | 0.129 |
| Angina | 9 (7.63) | 7 (11.29) | 2 (3.57) | 0.108 |
| Reinfarction | 4 (3.39) | 3 (4.84) | 1 (1.79) | 0.349 |
| Heart failure | 7 (5.93) | 4 (6.45) | 3 (5.36) | 0.557 |
Note: Data are represented as n (%); MACE, Major adverse cardiac event
Fig. 3.
Kaplan Meier monitored the influence of miR-483-5p levels on ACS patients undergoing MACE during 6 months after PCI
Fig. 4.
Cox regression analysis of potential influences factors affecting the occurrence of MACE after PCI in patients with ACS was performed and visualized with forest plots
Discussion
Current standard diagnostic methods for ACS included typical symptoms, ECG findings, and myocardial troponin levels[20]. However, in the emergency, ECG patterns diagnostic of ACS (insignificant ECG changes in the presence of persistent acute myocardial ischemia) are not present in some patients [21]. Moreover, myocardial troponin is not consistently elevated in AMI and lacks sensitivity in the first few hours due to its delayed release into the bloodstream, resulting in a “troponin blind period” [22]. As reported, the diagnostic sensitivity of cardiac troponin for myocardial injury within the first 3 h after admission to the emergency department is only 19–43% [23], and it can only be detected 6–12 h after coronary artery occlusion [24]. Additionally, myocardial troponin changes are common in chronic renal failure, acute pulmonary embolism, acute inflammatory myocarditis, and arrhythmias [25]. These pose significant obstacles to the diagnosis of ACS. To overcome this shortcoming, we have focused on miRNAs that are relevant to the pathogenesis of plaque rupture and can be easily detected and quantified.
As an endogenous RNA molecule that participated in the regulatory control of a range of developmental and physiological processes, miRNA dysregulation has been recognized as a useful marker for a wide range of diseases. This plays to the potential advantages of stable expression, ease of detection, and relevance to the clinicopathology of miRNAs. For example, miR-142-3p [6], miR-3464 [8], and miR-4286 [26] were identified as potential diagnostic or predictive biomarkers for ACS.
miR-483-5p, one of many miRNAs, has been suggested by several studies to have potential relevance to ACS. Firstly, coronary plaque rupture is the highest incidence of ACS, and Li et al. identified miRNAs significantly associated with plaque rupture, including miR-483-5p [11]. Atherosclerosis serves as the pathological foundation of ACS, and microarray analysis was conducted on both normal coronary arteries and arteries with plaque, where differential expression miRNAs included miR-483-5p [12]. Arrhythmia is a common complication of ACS, and miR-483-5p is involved in regulating atrial fibrillation, which is common in postoperative arrhythmias [13]. miR-483-3p, originating from the same precursor as miR-483-5p but located on the opposite arm of the pre-miRNA, exhibits consistent upregulation in heart failure patients with implanted left ventricular assist devices[27]. The results of previous studies indicate that miR-483-5p may be correlated with ACS, and in this preliminary study, we explore its diagnostic value for ACS. First, we evaluated miR-483-5p expression in 75 controls and 118 ACS patients. It was evidenced that miR-483-5p was typically elevated in ACS patients, which concurred with the results reported above. ROC is widely used to determine the accuracy of diagnostic biomarkers. Our results found that miR-483-5p has high sensitivity and specificity to identify ACS patients from controls. ACS was defined as AMI and UAP, and we also found higher levels of miR-483-5p in AMI patients than in UAP. And ROC established that serum miR-483-5p markedly distinguished AMI patients from UAP patients in ACS patients, demonstrating a high diagnostic potential.
PCI has become a common strategy for the treatment of ACS [28, 29], which can significantly restore coronary perfusion, reduce infarct size, and decrease cardiovascular mortality and disability [30], but some patients still develop MACE after performing PCI. SYNTAX score has been developed as an anatomy-based tool that can be used to define the complexity and progression of coronary artery disease and guide decision-making for PCI, as well as risk prediction for MACE [31]. miR-483-5p was confirmed to be positively correlated with the SYNTAX score. Additionally, the Gensini score, another widely used score for quantitative analysis of coronary lesions, is simpler and more scientific than the SYNTAX score and is more applicable to ACS patients treated with emergency PCI, enabling rapid assessment of coronary lesions and identification of high-risk patients, and timely treatment [18]. Our study also revealed a significant positive correlation between the Gensini score and miR-483-5p. Given the important role of miR-483-5p, we sought to explore the effect of miR-483-5p on MACE after PCI. To reflect the discrete profile of patients, we divided the patients into high miR-483-5p group and low miR-483-5p group based on their mean serum miR-483-5p values and found that the number of patients in the high miR-483-5p group was higher. In this preliminary study of ACS patients undergoing PCI with a 6-month follow-up, 29 patients experienced MACE, and most of them were patients with high miR-483-5p expression. Cox regression analysis confirmed that, together with cTnI, SYNTAX score, and Gensini score, miR-483-5p was an independent predictor of the experiencing MACE in patients after PCI. Finally, there are some limitations in this study. Because serum samples were collected from ACS patients at only time point, it was not possible to determine the time-dependent pattern of miR-483-5p expression in patients, which will be addressed in the next studies. New onset heart failure was determined by clinical signs and symptoms in a physical examination and on cardiac ultrasound and chest radiography. What’s more, multiple MACE events in a single patient were not identified in the follow-up MACE events due to the short follow-up time and small sample size, but we will expand the sample size and keep an eye on the occurrence of MACE events. Additionally, a significant positive correlation was observed between miR-483-5p and cTnI, a widely used biomarker for ACS. However, due to the lack of cTnI data from healthy individuals, this pilot study could not compare the diagnostic performance of cTnI and miR-483-5p in ACS patients. Collectively, our study determined the clinical diagnostic potential of miR-483-5p in patients with ACS, as well as its predictive accuracy for MACE after performing PCI.
Acknowledgements
N/A.
Authors’ contributions
All authors designed this study. XX S, YZ M and X L conducted the experiment. YY Z and YH P analyzed the data. YY Z wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Medical Science Research Project of Hebei Provincial Health Commission (20200230, 20200237).
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
There is no conflict of interest in this study.
Ethics approval and consent to participate
Subjects signed an informed consent form before enrollment. With the approval of the No.980 Hospital of PLA Joint Logistics Support Force Medical Ethics Committee, this research protocol strictly adhered to the Helsinki Declaration principles.
Consent for publication
N/A.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shin M, Mun S, Park SH, Lee J, Kang HG. Serum biomarker discovery related to pathogenesis in acute coronary syndrome by proteomic approach. Biosci Rep 2021, 41. [DOI] [PMC free article] [PubMed]
- 2.Shi Z, Zhao C, Hu J, Dai Q, Guan M, Zhong C, Tian G, Shang H. The application of traditional chinese medicine injection on patients with acute coronary syndrome during the perioperative period of percutaneous coronary intervention: A systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med 2020, 2020:3834128. [DOI] [PMC free article] [PubMed]
- 3.Liu L, Ding X, Han Y, Lv J. Effects and safety of sacubitril/valsartan for patients with myocardial infarction: a systematic review and meta-analysis. J Healthc Eng. 2022;2022:7840852. doi: 10.1155/2022/7840852. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Wang L, Jin Y. Noncoding rnas as biomarkers for acute coronary syndrome. Biomed Res Int 2020, 2020:3298696. [DOI] [PMC free article] [PubMed]
- 5.Mol JQ, Belkacemi A, Volleberg RH, Meuwissen M, Protopopov AV, Laanmets P, Krestyaninov OV, Dennert R, Oemrawsingh RM, van Kuijk JP, et al. Identification of anatomic risk factors for acute coronary events by optical coherence tomography in patients with myocardial infarction and residual nonflow limiting lesions: Rationale and design of the pectus-obs study. BMJ Open. 2021;11:e048994. doi: 10.1136/bmjopen-2021-048994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szelenberger R, Karbownik MS, Kacprzak M, Maciak K, Bijak M, Zielinska M, Czarny P, Sliwinski T, Saluk-Bijak J. Screening analysis of platelet mirna profile revealed mir-142-3p as a potential biomarker in modeling the risk of acute coronary syndrome. Cells 2021, 10. [DOI] [PMC free article] [PubMed]
- 7.Bai R, Yang Q, Xi R, Li L, Shi D, Chen K. Mir-941 as a promising biomarker for acute coronary syndrome. BMC Cardiovasc Disord. 2017;17:227. doi: 10.1186/s12872-017-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J, Li Y, Leng D, Cao C, Yu Y, Wang Y. Microrna-3646 serves as a diagnostic marker and mediates the inflammatory response induced by acute coronary syndrome. Bioengineered. 2021;12:5632–40. doi: 10.1080/21655979.2021.1967066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen T, Zhang X, Qian W, Zhou R, Su M, Ma Y. Serum mir-497-5p serves as a diagnostic biomarker for acute coronary syndrome and predicts the occurrence of major adverse cardiovascular events after percutaneous coronary intervention. Bioengineered. 2022;13:8266–76. doi: 10.1080/21655979.2022.2051885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, Chang G, Cao L, Ding G. Dysregulation of serum mir-361-5p serves as a biomarker to predict disease onset and short-term prognosis in acute coronary syndrome patients. BMC Cardiovasc Disord. 2021;21:74. doi: 10.1186/s12872-021-01891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S, Lee C, Song J, Lu C, Liu J, Cui Y, Liang H, Cao C, Zhang F, Chen H. Circulating micrornas as potential biomarkers for coronary plaque rupture. Oncotarget. 2017;8:48145–56. doi: 10.18632/oncotarget.18308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian S, Cao Y, Wang J, Bi Y, Zhong J, Meng X, Sun W, Yang R, Gan L, Wang X, et al. The mir-378c-samd1 circuit promotes phenotypic modulation of vascular smooth muscle cells and foam cells formation in atherosclerosis lesions. Sci Rep. 2021;11:10548. doi: 10.1038/s41598-021-89981-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harling L, Lambert J, Ashrafian H, Darzi A, Gooderham NJ, Athanasiou T. Elevated serum microrna 483-5p levels may predict patients at risk of post-operative atrial fibrillation. Eur J Cardiothorac Surg. 2017;51:73–8. doi: 10.1093/ejcts/ezw245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Task Force M, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, et al. 2013 esc guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the european society of cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 15.Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE, Jr, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H, Lincoff AM, et al. 2011 accf/aha focused update of the guidelines for the management of patients with unstable angina/ non-st-elevation myocardial infarction (updating the 2007 guideline): a report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2011;123:2022–60. doi: 10.1161/CIR.0b013e31820f2f3e. [DOI] [PubMed] [Google Scholar]
- 16.Thygesen K. Ten commandments’ for the fourth universal definition of myocardial infarction 2018. Eur Heart J. 2019;40:226. doi: 10.1093/eurheartj/ehy856. [DOI] [PubMed] [Google Scholar]
- 17.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–72. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 18.Wang KY, Zheng YY, Wu TT, Ma YT, Xie X. Predictive value of gensini score in the long-term outcomes of patients with coronary artery disease who underwent pci. Front Cardiovasc Med. 2021;8:778615. doi: 10.3389/fcvm.2021.778615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujiwara R, Yahiro R, Horio T, Miyauchi M, Yoshimura R, Matsuoka Y, Yokouchi G, Sakamoto Y, Matsumoto N, Fukuda K, et al. Achilles tendon thickness is associated with coronary lesion severity in acute coronary syndrome patients without familial hypercholesterolemia. J Cardiol. 2022;79:311–7. doi: 10.1016/j.jjcc.2021.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Bergmark BA, Mathenge N, Merlini PA, Lawrence-Wright MB, Giugliano RP. Acute coronary syndromes. Lancet. 2022;399:1347–58. doi: 10.1016/S0140-6736(21)02391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garg P, Morris P, Fazlanie AL, Vijayan S, Dancso B, Dastidar AG, Plein S, Mueller C, Haaf P. Cardiac biomarkers of acute coronary syndrome: from history to high-sensitivity cardiac troponin. Intern Emerg Med. 2017;12:147–55. doi: 10.1007/s11739-017-1612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abd El Baky Mahmoud M, Shaaban MAA, Ali Ramzy A. Clinical role of serum copeptin in acute coronary syndrome. Egypt Heart J. 2018;70:155–9. doi: 10.1016/j.ehj.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raskovalova T, Twerenbold R, Collinson PO, Keller T, Bouvaist H, Folli C, Giavarina D, Lotze U, Eggers KM, Dupuy AM, et al. Diagnostic accuracy of combined cardiac troponin and copeptin assessment for early rule-out of myocardial infarction: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care. 2014;3:18–27. doi: 10.1177/2048872613514015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Yang Y, Wang L, Qiao S, Lu X, Wu Y, Xu B, Li H, Gu D. Plasma mir-122 and mir-3149 potentially novel biomarkers for acute coronary syndrome. PLoS ONE. 2015;10:e0125430. doi: 10.1371/journal.pone.0125430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong KL, Mahmood Zuhdi AS, Wan Ahmad WA, Vanhoutte PM, de Magalhaes JP, Mustafa MR, Wong PF. Circulating micrornas in young patients with acute coronary syndrome. Int J Mol Sci 2018, 19. [DOI] [PMC free article] [PubMed]
- 26.Shen M, Xu X, Liu X, Wang Q, Li W, You X, Peng R, Yuan Y, Long P, Niu R, et al. Prospective study on plasma microrna-4286 and incident acute coronary syndrome. J Am Heart Assoc. 2021;10:e018999. doi: 10.1161/JAHA.120.018999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morley-Smith AC, Mills A, Jacobs S, Meyns B, Rega F, Simon AR, Pepper JR, Lyon AR, Thum T. Circulating micrornas for predicting and monitoring response to mechanical circulatory support from a left ventricular assist device. Eur J Heart Fail. 2014;16:871–9. doi: 10.1002/ejhf.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan W, Zhang Y, Gao X, Liu Y, Shi F, Liu J, Sun L. The prognostic value of a derived neutrophil-lymphocyte ratio in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Clin Appl Thromb Hemost. 2021;27:10760296211034579. doi: 10.1177/10760296211034579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo JT, Zeng CM, Zhao YM, Zeng ZY. The relationship between homocysteine and cardiopulmonary exercise testing in patients with acute coronary syndrome after percutaneous coronary intervention. BMC Cardiovasc Disord. 2023;23:3. doi: 10.1186/s12872-022-02976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong Z, Hou J, Zhang Q, Zhong W, Li B, Li C, Liu Z, Yang M, Zhao P. Assessment of the ldl-c/hdl-c ratio as a predictor of one year clinical outcomes in patients with acute coronary syndromes after percutaneous coronary intervention and drug-eluting stent implantation. Lipids Health Dis. 2019;18:40. doi: 10.1186/s12944-019-0979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vroegindewey MM, Schuurman AS, Oemrawsingh RM, van Geuns RJ, Kardys I, Ligthart J, Daemen J, Boersma E, Serruys PW, Akkerhuis KM. Syntax score ii predicts long-term mortality in patients with one- or two-vessel disease. PLoS ONE. 2018;13:e0200076. doi: 10.1371/journal.pone.0200076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.