Abstract
Background:
The disturbance of colonized trees and soil, such as through forestry activities, has been proposed to disperse soil- and tree-inhabiting fungal pathogens. Cryptococcus gattii sensu lato is one such pathogen that was detected on Vancouver Island, British Columbia, Canada, beginning in 1999 and caused human and animal illness.
Objectives:
Our aim was to determine if C. gattii s.l. human case incidence on Vancouver Island was correlated with the intensity of landscape-level tree harvesting occurring near human settlement areas.
Methods:
We created buffers around human settlement areas with radii increments of , from 2.5 to , and summed the area of annual tree harvests occurring within each buffer zone. We then performed Spearman rank–order correlation to measure the association between case incidence and annual tree harvest intensity at each radius from 1998 through 2014.
Results:
The incidence of C. gattii was positively correlated with tree harvesting intensity only at distances of (, ) and (, ) from human settlement areas. As annual tree harvesting area increased between 1999 and 2003, so did annual C. gattii incidence in humans, before both plateaued around 2002 and decreased after 2007.
Discussion:
Our findings suggest that tree harvesting plays a role in the spread of C. gattii on Vancouver Island. This may be due to tree cutting or soil disturbance facilitating the aerosolization of spores to increase infection risk. This research also illustrates the contribution that geographic information systems can make to public health research on environmental disturbance and disease outbreaks. https://doi.org/10.1289/EHP12396
Introduction
The disruption of colonized soil and trees has been proposed as a key factor in the dispersal of soil- and tree-inhabiting fungal pathogens. Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum, which cause infection through inhalation of aerosolized spores,1–3 have shown increased dispersal following forest and soil disruption. Blastomyces spores are released following mechanical disruption of soil and other organic matter.4 Coccidioidomycosis (commonly known as Valley fever) outbreaks occurred following archaeological activities,5,6 an earthquake,7 and a windstorm8 in the southwestern United States. Land activities that disturb forests and soil, such as cultivation and urbanization, are predicted to have increased the geographic ranges of Histoplasma.9 The spread of fungal species is aggravated by forestry activities, including tree harvesting, where trees are cut and sold as lumber while the land does not undergo a land-use change.10
Another soil- and tree-dwelling fungal pathogen, Cryptococcus gattii sensu lato, was detected on Vancouver Island, British Columbia, in 1999 and caused a multispecies outbreak of cryptococcosis.11,12 Cryptococcosis, a potentially fatal infection of the lungs and central nervous system in mammals, including humans, is caused by inhalation of Cryptococcus fungal spores or desiccated yeast cells.13 The incubation period of C. gattii s.l., hereafter referred to as C. gattii, ranges from 2 to 11 months, with a median of 6–7 months,14 although it has been reported to last up to 3 y.15 On Vancouver Island, soil and trees are the principal reservoirs of C. gattii colonization.16 Following the Vancouver Island outbreak, the effects of logging on C. gattii dispersal were explored, and tree harvesting was found to increase spore dispersal at the neighborhood level. The felling and chipping of individual trees colonized with C. gattii on Vancouver Island led to an increase in airborne concentrations of this pathogen.17 Dogs and cats residing within of a logging site or other area of commercial soil disturbance on Vancouver Island had an increased risk of developing cryptococcosis.18 Studies of the spatiotemporal dynamics of forestry activities and C. gattii at the landscape scale, however, have not yet been attempted owing to the lack of large-scale forestry data. Moreover, few studies have addressed the maximum potential distance of C. gattii spore dispersal.
In the province of British Columbia, Canada (population 19), soil and tree disturbance are largely due to tree harvesting. British Columbia has the second largest provincial forestry industry in Canada.20 The province has a total of hectares of forest area,21 nearly equivalent to the area of France, and as of 2014, cuts an average of hectares per year.22 More than 90% of trees harvested in coastal British Columbia, including Vancouver Island, are coniferous trees, including cedar, fir, hemlock, pine, and spruce.23,24 On Vancouver Island, trees from which C. gattii has been isolated include the western red cedar (Thuja plicata), Douglas fir (Pseudotsuga menziesii), western hemlock (Tsuga heterophylla), and grand fir (Abies grandis).25,26 During first-attempt sampling on the island, even greater proportions of positive C. gattii isolations were made from soil samples close to trees rather than direct tree samples (e.g., swabs of tree hollows, stumps, cut logs).16 It is hypothesized that forestry activities on Vancouver Island may have affected the emergence of C. gattii as a human and animal pathogen by disturbing colonized trees and soil. Tree harvesting on Vancouver Island occurring before and during the C. gattii outbreak was conducted largely for the British Columbia forestry industry but also included some construction projects, including the construction of the Inland Island Highway, a highway built between Campbell River and Parksville along the eastern side of the island that was completed in 1999.27 Although tree disturbance has been directly assessed at the level of individual trees, broader-scale studies using remotely sensed forestry data have never been compared with C. gattii infection risk. Here, we analyze the correlation between human C. gattii incidence and the size of tree harvesting areas at various distances and time frames to explore the role of tree harvesting on the emergence of C. gattii as a human pathogen on Vancouver Island.
Methods
Study Area
Our study area comprised Vancouver Island, along with the Gulf Islands, occupying (herein referred to as Vancouver Island). As of 2019, the islands had a human population of .28 They also have one of the highest annual incidences of C. gattii infections worldwide.29
Forest Disturbance Data Sets
We used Landsat-derived annual tree harvest layers from 1985 through 2014 with a spatial resolution of .10,30–32 These data were derived from a time series of Landsat-derived best-available-pixel image composites of surface reflectance acquired from 1 August d each year. These images were combined into a temporal stack from which a break point analysis was conducted across the years for each grid cell.31 An object-based image analysis approach10 was used to assess characteristics of each spectral change (i.e., changes in surface reflectance values). These characteristics included the magnitude of change (i.e., the difference in surface reflectance values before and after the change), the duration of the change (i.e., for how many years did the change last), and the change rate (i.e., the ratio of magnitude and duration). Objects (i.e., tree harvest, fire, road construction, or non–stand-replacing change, such as trees with diseases or water stress) would be created using a spatial aggregation of grid cells that experienced similar change characteristics. For example, grid cells characterized as tree harvests would exhibit a relatively large magnitude of change in surface reflectance values over a short period of time, such as only from 1 y to the next, compared with grid cells characterized as trees suffering from disease, where relatively smaller magnitudes of change in surface reflectance values would occur over a longer period, such as several years in a row.10
For our analysis, we used tree harvest data, although specific tree species that were harvested were not specified. For each grid cell that represented tree harvesting, we included the year of harvest and the level of confidence that tree harvesting occurred. The harvest year was the year the cell experienced the greatest spectral change between 1985 and 2015. Changes were assigned a high or low-confidence level based on the amount of data missing before, during, and after the change, with confidence decreasing as the amount of missing data increased.32 Confidence that an object (i.e., aggregation of grid cells) was correctly attributed to tree harvesting was calculated using a random forest classifier.10 This classifier determined how many votes from individual trees were received for each change type (e.g., tree harvest, fire) and calculated the proportion of votes of the second-most voted class (v2) and the proportion of votes of the assigned class (v1). If v2/v1, which ranged from 0 to 1, was , the change attribution was considered low confidence. Combined high- and low-confidence harvest area data were used for the present analysis (Figure S1 shows locations and years of harvests). However, high-confidence harvest data alone were tested as part of the sensitivity analysis.
Previous studies have found that C. gattii is unlikely to persist in areas at elevations of that have daily average winter temperatures below freezing.26 Specifically, the mountain hemlock and coastal mountain–heather alpine biogeoclimatic zones encapsulate these conditions (see Mak et al.26) (Figure 1). We therefore removed tree harvests that occurred within these biogeoclimatic zones.
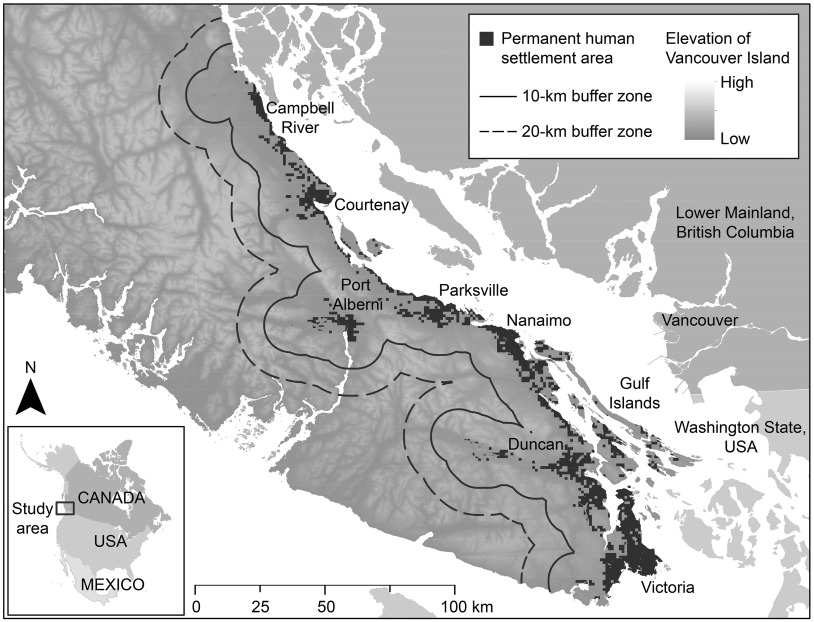
Figure 1.
Permanent human settlement areas on eastern Vancouver Island along with the border lines of the 10- and buffer zones. Permanent human settlement areas were created based on the 2015 raster data set from the European Commission Global Human Settlement–Settlement Model (SMOD) at a resolution (https://ghsl.jrc.ec.europa.eu/datasets.php). This figure was created using ArcGIS (version 10.7.1; ESRI).
Disease Surveillance Data
C. gattii-related cryptococcosis is a reportable disease in British Columbia. We used human case data reported to the British Columbia Centre for Disease Control between 1998 and 2014 following the University of British Columbia Research Ethics Board approval (H16-00118/2016). Public health officials interviewed each case using a standard questionnaire to record demographic information, travel history, and other factors.33 A confirmed case was defined as a culture-confirmed C. gattii infection using differential media and genotyping.33,34 A probable case was defined as laboratory evidence of infection in an human immunodeficiency virus (HIV)–negative person using antigen detection, histopathology, or microscopy. The number of new cases was recorded per year.
Case data from 1998 to 2009 included travel history from the 12 months before the onset of symptoms, whereas case data from 2010 to 2014 included travel history for the past 13 months.35 Travel histories were lacking for 229 of 378 () of cases from British Columbia. We therefore performed our analyses using all human cases of C. gattii infection with residences on Vancouver Island and the Gulf Islands regardless of travel history (). We assumed that individuals spent the majority of their time at home or within the eastern human settlement area of the island. However, as part of our sensitivity analysis, we excluded cases who reported travel outside of British Columbia in the 12–13 months prior to onset () (Tables S1 and S2) to account for possible exposure to C. gattii during travel. For these analyses, cases with no travel history were still included.
Permanent Human Settlement Areas and Buffer Zones
Permanent human settlement areas were mapped using the 2015 raster data set from the European Commission Global Human Settlement–Settlement Model (SMOD) at a resolution.36 Given that C. gattii has never been isolated in western Vancouver Island except in a porpoise, the human settlement areas were clipped to eastern Vancouver Island and the Gulf Islands (Figure 1).
The potential distance that aerosolized C. gattii spores can travel is unknown. We therefore created buffers around each human settlement area on Vancouver Island and the Gulf Islands with radii increments of , from 0 (i.e., within the human settlement areas) to . We calculated the total area of tree harvesting in square meters occurring within human settlement areas and cumulatively within each buffer zone annually from 1998 and 2014 (i.e., areas of tree harvesting were summed from , then , and so on). Within each buffer zone, we then examined whether annual C. gattii incidence was associated with the corresponding size of tree harvesting area for that year. We used as the maximum buffer radius owing to the presence of the Vancouver Island Ranges, which commence from the eastern shoreline, and given that no human cases of C. gattii have been reported west of this mountain range. In addition, we calculated the percentage of total annual tree harvest areas per buffer zone that was associated with the construction of the Inland Island Highway from 1991 to 1999. We calculated tree harvests directly associated with the highway by determining high-confidence as well as high- and low-confidence tree harvests occurring within of the highway.
Statistical Analysis
Our analysis data set consisted of the annual time series of total new cases, plus the corresponding harvest areas for each of the nine increasingly larger buffer zones. The correlation between annual C. gattii incidence and annual harvest area was assessed for each buffer zone using Spearman rank–order correlation.37 We separately examined confirmed as well as human cases of C. gattii infection with residences on Vancouver Island or Gulf Islands (Table S1). We were uncertain about the exact timing of tree harvests. Although candidate pixels for creating tree harvest data were acquired within July and August, harvests may have occurred within those months or up to a year prior. Owing to this uncertainty, and the potentially lengthy incubation period of C. gattii,14,15 we repeated the analyses comparing annual C. gattii incidence to tree harvests that occurred 1 y before case reporting. We therefore analyzed three sets of factors: a) travel history (all cases regardless of travel history vs. those who had no evidence of travel outside of British Columbia during their incubation period), b) confidence of the accuracy of the harvest data (high- and low-confidence data vs. high-confidence data), and c) the year of harvest relative to the time the case was reported (harvest occurred the same year the case was reported vs. harvest occurred the year before the case was reported). In the main analysis, we compared all human cases regardless of travel history with high- and low-confidence harvest data occurring in the same year as case reporting. The sensitivity analyses consisted of all other combinations of the three factors.
Given that each analysis consisted of multiple tests across buffer zones, we evaluated significant associations using Bonferroni adjusted -values. We used ArcGIS (version 10.7.1; ESRI) to map the georeferenced human records and the tree harvest data sets. Statistical analyses were conducted using R (version 3.0.2; R Development Core Team).
Results
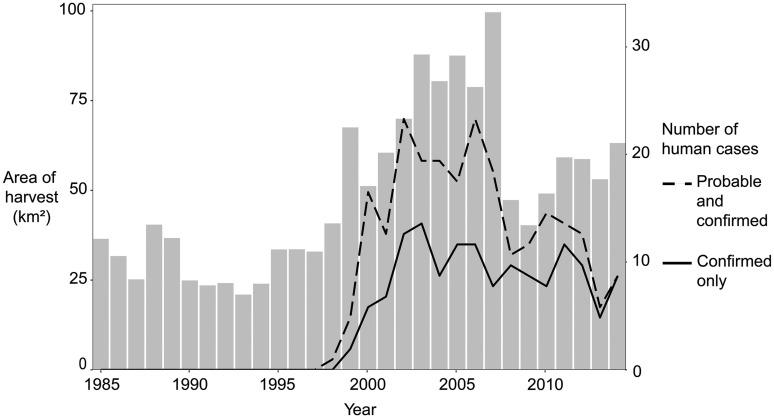
The annual incidence of C. gattii infection in humans on Vancouver Island (Table 1) increased in step with the annual increase in area of tree harvests within from human settlement areas from 1998 to 2002–2003 (Figure 2). The number of cases and the area of harvesting reached a plateau at around 2002 (for cases) and 2003 (for harvesting) that lasted until 2007 and both decreased thereafter. In 1999, when C. gattii was first confirmed in humans on Vancouver Island, the total annual tree harvesting area on the island was at least 47% higher than in any other prior year since 1985 across all buffer zones outside the human settlement area (Tables S3 and S4).
Table 1.
Summary of confirmed and probable human cases of C. gattii infection per year from 1998 through 2014 with residences on Vancouver Island, regardless of any travel history.
| Year | Confirmed | Probable | |
|---|---|---|---|
| 1998 | 0 | 1 | 1 |
| 1999 | 2 | 3 | 5 |
| 2000 | 6 | 11 | 17 |
| 2001 | 7 | 6 | 13 |
| 2002 | 13 | 11 | 24 |
| 2003 | 14 | 6 | 20 |
| 2004 | 9 | 11 | 20 |
| 2005 | 12 | 6 | 18 |
| 2006 | 12 | 12 | 24 |
| 2007 | 8 | 11 | 19 |
| 2008 | 10 | 1 | 11 |
| 2009 | 9 | 3 | 12 |
| 2010 | 8 | 7 | 15 |
| 2011 | 12 | 2 | 14 |
| 2012 | 10 | 3 | 13 |
| 2013 | 5 | 1 | 6 |
| 2014 | 9 | 0 | 9 |
| Total | 146 | 95 | 241 |
Note: A confirmed case was defined as a culture-confirmed C. gattii infection using differential media and genotyping. A probable case was defined as laboratory evidence of infection in an human immunodeficiency virus (HIV)–negative person using antigen detection, histopathology, or microscopy. Case data were provided through the British Columbia Centre for Disease Control Reportable Diseases Data Dashboard (http://www.bccdc.ca/health-professionals/data-reports/reportable-diseases-data-dashboard). Data from this table were used for the human case data in Figure 2.
Figure 2.
The total area of high-and low-confidence tree harvest events occurring within of the eastern human settlement area of Vancouver Island (in gray bars) compared with the number of confirmed (solid black line, ) and combined probable and confirmed human cases (dashed black line, ) with residences on Vancouver Island. Human cases regardless of travel history are shown. Tree harvests were assigned high or low confidence through a random forest classifier, based on how many votes from individual trees were received for each class type (e.g., tree harvest, fire). The proportion of votes of the second-most voted class (v2) was divided by the proportion of votes of the assigned class (v1). Tree harvests were considered high confidence if v2/v1 was and low confidence if v2/v1 was . Tree harvest data were provided by Hermosilla et al.10 (see https://opendata.nfis.org/mapserver/nfis-change_eng.html). Human settlement area data were mapped using the 2015 raster data set from the European Commission Global Human Settlement–Settlement Model (SMOD) at a resolution (https://ghsl.jrc.ec.europa.eu/datasets.php). Data for annual tree harvest areas is in Table S4 in the “” column. Data for annual human cases is in Table 1.
The tree harvesting area was positively correlated with C. gattii incidence among Vancouver Island residents within (, ) and (, ) (Table 2) from human settlement areas on Vancouver Island. When only assessing cases with no evidence of travel outside of British Columbia (Table S2), tree harvesting area was also positively correlated with C. gattii incidence among Vancouver Island residents, or approached significance, within (, ) and (, ) (Table S5). When assessing high-confidence tree harvesting area alone,10 tree harvesting area was positively correlated with C. gattii incidence among Vancouver Island residents, or approached significance, within both 7.5 and . This was true for cases regardless of travel history (, and , , respectively) and for cases with no evidence of travel outside of British Columbia (, and , , respectively) (Tables S6 and S7). Across all tests comparing harvest events occurring the same year as case reporting, confirmed cases alone showed no strong or significant correlations with tree harvesting area (Table 1; Table S5–S7). Tree harvest events occurring 1 y prior to case reporting showed no strong or significant correlations in any test (Tables S8–S11). However, correlations remained highest within distances of 7.5 and from human settlement areas in all sensitivity tests, consistent with analyses of tree harvest events occurring the same year as case reporting. Tree harvesting within of the highway construction zone from 1991 to 1999 was minimal compared with overall tree harvesting, consisting of of total high- or high- and low-confidence tree harvesting occurring within either 7.5 or of the human settlement areas (Figure S2 and Tables S12 and S13).
Table 2.
Spearman rank–order correlation results of annual high- and low-confidence harvest events occurring from 0 to of the human settlement area of eastern Vancouver Island and annual C. gattii cases on Vancouver Island occurring from 1998 through 2014 where cases were included regardless of travel history ( and 241 for confirmed and combined confirmed and probable cases, respectively).
| Buffer radius (km) | Confirmed cases | Probable and confirmed cases | ||
|---|---|---|---|---|
| Correlation | -Value | Correlation | -Value | |
| 0 | 0.19 | 0.47 | 0.50 | 0.041 |
| 2.5 | 0.15 | 0.56 | 0.46 | 0.061 |
| 5 | 0.23 | 0.38 | 0.55 | 0.022 |
| 7.5 | 0.42 | 0.091 | 0.66 | 0.004* |
| 10 | 0.43 | 0.085 | 0.64 | 0.005* |
| 12.5 | 0.44 | 0.080 | 0.61 | 0.009 |
| 15 | 0.46 | 0.063 | 0.59 | 0.012 |
| 17.5 | 0.43 | 0.088 | 0.57 | 0.018 |
| 20 | 0.41 | 0.098 | 0.54 | 0.027 |
Note: Annual human case numbers for confirmed and probable C. gattii infections are provided in Table 1. Here, harvest events occurring the same year as case reporting were assessed. A confirmed case was defined as a culture-confirmed C. gattii infection using differential media and genotyping. A probable case was defined as laboratory evidence of infection in an human immunodeficiency virus (HIV)–negative person using antigen detection, histopathology, or microscopy. Case data were provided through the British Columbia Centre for Disease Control Reportable Diseases Data Dashboard (http://www.bccdc.ca/health-professionals/data-reports/reportable-diseases-data-dashboard). Tree harvests were assigned high or low confidence through a random forest classifier, based on how many votes from individual trees were received for each class type (e.g., tree harvest, fire). The proportion of votes of the second-most voted class (v2) was divided by the proportion of votes of the assigned class (v1). Tree harvests were considered high confidence if v2/v1 was and low confidence if v2/v1 was . Tree harvest data were provided by Hermosilla et al.10 (see https://opendata.nfis.org/mapserver/nfis-change_eng.html). Annual human case numbers for confirmed and probable C. gattii infections are provided in Table 1. The -values were obtained from the Spearman correlation coefficient test.
Statistically significant after Bonferroni adjustment (, where was adjusted as 0.05/9).
Discussion
Summary of Results and Interpretation
Human C. gattii incidence on Vancouver Island was correlated with the tree harvesting area from 1998 through 2014. As the annual tree harvesting area increased between 1999 and 2003, so did the annual incidence of disease in the population. The correlation was strongest when tree harvesting occurred in the same year within 7.5 and from human settlement areas on the island. Our results support previous findings that tree disturbance may increase aerosolization of C. gattii spores. For instance, the felling and chipping of individual colonized trees has been shown to increase the concentration of C. gattii spores,17 whereas residing within of a logging or commercial soil disturbance site on Vancouver Island was a significant risk factor for the development of cryptococcosis in dogs and cats.18 Our results show the highest correlation between tree harvesting area and human C. gattii incidence occurred within 7.5 and from human settlement areas (and declined thereafter up to ), suggesting, as Duncan et al.18 did, that C. gattii spores may travel a few kilometers to cause infection in mammals.
In our study, the relatively low correlation between tree harvesting area and human C. gattii incidence that was found between 0 and could also suggest that microhabitats for C. gattii are most abundant from the coast. These findings may also suggest that, relative to broader-scale logging and commercial soil excavation, soil and tree disturbance activities that occur in (sub)urban areas are not intense enough to cause sufficient aerosolization of spores to increase infection risk. For instance, although living within of a commercial soil disturbance area was the most significant risk factor for the development of cryptococcosis in domestic dogs and cats, vegetation and soil disruption at the residence of these animals, such as chopping wood, gardening, or home construction, were not identified as significant risk factors.18,38 It is also possible that tree harvesting occurring within less than a radius is not sufficient in intensity to increase disease risk.
Possible Mechanisms of C. gattii Emergence and Spread
In 1999, when the first confirmed case of C. gattii was recorded on Vancouver Island, tree harvesting areas outside human settlements were at least 47% higher than in any prior year since 1985 across all buffer zones (Table S4). The increase in harvesting beginning in 1999 may have been related to the construction of the Inland Island Highway39 (Figure S1). Tree harvesting directly caused by the highway construction was minimal compared with general tree harvesting occurring in the area (Figure S2 and Tables S12 and S13). However, the highway construction may have indirectly contributed to C. gattii dispersal by facilitating the development of side roads for further construction and tree harvesting projects or by providing vehicle traffic that may have increased mechanical dispersal of spores.17
The consistent correlation found between tree harvesting area and human C. gattii incidence in our study suggests that soil and tree disturbance may have played a role in the emergence of C. gattii human and animal infections on Vancouver Island in 1999. The relative increase in tree harvesting beginning in 1999 may have aerosolized and spread fungal colonies that had previously experienced little to no disturbance. Harvested colonized trees may have also been chipped and transported to other areas of the island for landscaping, further introducing the fungus to new locations.17 However, determining the exact cause of the emergence of C. gattii on Vancouver Island is challenging given the uncertainty regarding when the fungus first appeared on the island, what source(s) introduced it, and its full geographic distribution.
Limitations and Future Work
Although some georeferenced environmental isolates of C. gattii on Vancouver Island and mainland British Columbia exist,16,26 C. gattii has not been systematically sampled across the island. We therefore do not know the full geographic extent of C. gattii colonization, nor do we know all the areas that may be permanently, intermittently, or only transiently colonized by C. gattii.17 This limited our ability to compare C. gattii cases with targeted forest areas of C. gattii colonization. We therefore assumed that C. gattii was present anywhere in the natural environment around and within human settlement areas along eastern Vancouver Island,17 and that physical disturbance of these environments through tree harvesting would increase the likelihood of dispersing these spores. For the purposes of this study, we examined the effects of overall tree harvesting area on C. gattii incidence. We summed all tree harvesting areas across each buffer zone and could therefore not discern the effects of harvest areas occurring within each zone. However, future research may analyze individual tree harvests along with their area and proximity to cases to account for the spatial complexity of harvests across a landscape. In addition, harvest events, although measured around 1 August of each year,10,30,32 could have occurred at any point between September of the previous year and August of the current year. It is possible that tree harvests recorded in a given year were compared with cases that were reported prior to the harvesting. Tree harvesting could also occur at any time of year in British Columbia and were not necessarily occurring in the same season each year, with summer months offering warmer conditions for workers and winter months providing harder grounds for the easier passage of machinery.40
The C. gattii case data used in this study do not necessarily represent the location where humans contracted the infection. Given the high mobility of humans, the lengthy incubation period of C. gattii, and the lack of complete travel histories, we were unable to assign exact locations of exposure. Instead, we accounted for travel where possible and calculated distances from eastern human settlement areas, as opposed to creating buffer zones around each specific location of residence. We assumed that most people infected with C. gattii on the island spent most of their time living and working within the eastern human settlement area, where most of the island population resides. We also did not have access to data specifying whether individuals developed C. gattii infections following immunocompromised status (e.g., cancer patients, organ transplant recipients). If we had removed these individuals from our analyses, we likely would have found stronger correlations between tree harvesting and C. gattii incidence. It is also important to note that we did not assess potential interactions between soil and tree disturbance and climatic factors, such as temperature. Future research should focus on how climatic changes, including climatic oscillations and global climate change, may interact with soil and tree disturbance to affect incidence rates of C. gattii and other fungal infections. Attention should also be paid to human settlement areas farther from the eastern coastline of the island to determine whether is indeed an optimal dispersal distance for spores or whether it indicates a distance from the shoreline where microhabitats for the fungus are most abundant. Tree harvest data sets with a finer temporal resolution would also be beneficial to better estimate timings between soil and tree disturbance and subsequent infection.
Conclusions
To the best of our knowledge, our study is the first to demonstrate a correlation between broad-scale tree harvesting and human C. gattii incidence. We found that annual tree harvesting within of human settlement areas on Vancouver Island were correlated with C. gattii incidence in the same year. Our findings suggest that tree harvesting may have played a role in the emergence of C. gattii on Vancouver Island by facilitating the aerosolization of spores. These findings further suggest that tree and soil disturbance activities may play a role in infection risk caused by similar fungal species that rely on spore aerosolization for dispersal. This research also illustrates the contribution that geographic information systems and remote sensing data can make to public health research on environmental disturbance and disease outbreaks.
Supplementary Material
Acknowledgments
We acknowledge and thank K. Bartlett, T. Hermosilla, and the two anonymous reviewers for their feedback. Research from this project was supported by a Vanier Canada Graduate Scholarship for the Natural Sciences and Engineering Research Council of Canada to E.S.A.
References
- 1.Bradsher RW, Chapman SW, Pappas PG. 2003. Blastomycosis. Infect Dis Clin North Am 17(1):21–40, PMID: , 10.1016/s0891-5520(02)00038-7. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen C, Barker BM, Hoover S, Nix DE, Ampel NM, Frelinger JA, et al. 2013. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin Microbiol Rev 26(3):505–525, PMID: , 10.1128/CMR.00005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, et al. 2007. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 45(7):807–825, PMID: , 10.1086/521259. [DOI] [PubMed] [Google Scholar]
- 4.Baumgardner DJ, Paretsky DP. 1999. The in vitro isolation of Blastomyces dermatitidis from a woodpile in north central Wisconsin, USA. Med Mycol 37(3):163–168, PMID: , 10.1080/j.1365-280X.1999.00214.x. [DOI] [PubMed] [Google Scholar]
- 5.Petersen LR, Marshall SL, Barton-Dickson C, Hajjeh RA, Lindsley MD, Warnock DW, et al. 2004. Coccidioidomycosis among workers at an archeological site, northeastern Utah. Emerg Infect Dis 10(4):637–642, PMID: , 10.3201/eid1004.030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werner SB, Pappagianis D. 1973. Coccidioidomycosis in Northern California. An outbreak among archeology students near Red Bluff. Calif Med 119(3):16–20, PMID: . [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider E, Hajjeh RA, Spiegel RA, Jibson RW, Harp EL, Marshall GA, et al. 1997. A coccidioidomycosis outbreak following the Northridge, Calif, earthquake. JAMA 277(11):904–908, PMID: , 10.1001/jama.277.11.904. [DOI] [PubMed] [Google Scholar]
- 8.Pappagianis D, Einstein H. 1978. Tempest from Tehachapi takes toll on Coccidioides conveyed aloft and afar. West J Med 129(6):527–530, PMID: . [PMC free article] [PubMed] [Google Scholar]
- 9.Maiga AW, Deppen S, Scaffidi BK, Baddley J, Aldrich MC, Dittus RS, et al. 2018. Mapping Histoplasma capsulatum exposure, United States. Emerg Infect Dis 24(10):1835–1839, PMID: , 10.3201/eid2410.180032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermosilla T, Wulder MA, White JC, Coops NC, Hobart GW. 2015. Regional detection, characterization, and attribution of annual forest change from 1984 to 2012 using Landsat-derived time-series metrics. Remote Sens Environ 170:121–132, 10.1016/j.rse.2015.09.004. [DOI] [Google Scholar]
- 11.Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, Fyfe M, et al. 2004. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc Natl Acad Sci USA 101(49):17258–17263, PMID: , 10.1073/pnas.0402981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephen C, Lester S, Black W, Fyfe M, Raverty S. 2002. Multispecies outbreak of cryptococcosis on southern Vancouver Island, British Columbia. Can Vet J 43(10):792–794, PMID: . [PMC free article] [PubMed] [Google Scholar]
- 13.Carter D, Campbell LA, Saul N, Krockenberger M. 2011. Sexual reproduction of Cryptococcus gattii: A population genetics perspective. In: Cryptococcus: From Human Pathogen to Model Yeast. Heitman J, Kozel TR, Kwon-Chung KJ, Perfect JR, Casadevall A, eds. Washington, DC: ASM Press, 299–311. [Google Scholar]
- 14.MacDougall L, Fyfe M. 2006. Emergence of Cryptococcus gattii in a novel environment provides clues to its incubation period. J Clin Microbiol 44(5):1851–1852, PMID: , 10.1128/JCM.44.5.1851-1852.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johannson KA, Huston SM, Mody CH, Davidson W. 2012. Cryptococcus gattii pneumonia. CMAJ 184(12):1387–1390, PMID: , 10.1503/cmaj.111346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kidd SE, Chow Y, Mak S, Bach PJ, Chen HM, Hingston AO, et al. 2007. Characterization of environmental sources of the human and animal pathogen Cryptococcus gattii in British Columbia, Canada, and the Pacific Northwest of the United States. Appl Environ Microbiol 73(5):1433–1443, PMID: , 10.1128/AEM.01330-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kidd SE, Bach PJ, Hingston AO, Mak S, Chow Y, MacDougall L, et al. 2007. Cryptococcus gattii dispersal mechanisms, British Columbia, Canada. Emerg Infect Dis 13(1):51–57, PMID: , 10.3201/eid1301.060823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan CG, Stephen C, Campbell J. 2006. Evaluation of risk factors for Cryptococcus gattii infection in dogs and cats. J Am Vet Med Assoc 228(3):377–382, PMID: , 10.2460/javma.228.3.377. [DOI] [PubMed] [Google Scholar]
- 19.BC Stats. 2021. 2021 Sub-Provincial Population Estimates Highlights. https://www2.gov.bc.ca/assets/gov/data/statistics/people-population-community/population/pop_subprovincial_population_highlights.pdf [accessed 4 April 2022].
- 20.Conference Board of Canada. 2020. Use of Forest Resources. https://www.conferenceboard.ca/hcp/provincial/environment/forest-resources.aspx [accessed 3 November 2021].
- 21.Gilani HR, Innes JL. 2020. The state of British Columbia’s forests: a global comparison. Forests 11(3):316, 10.3390/f11030316. [DOI] [Google Scholar]
- 22.BC Ministry of Environment. 2014. Forests: Trends in Silviculture in B.C. (1970–2012). https://www2.gov.bc.ca/assets/gov/environment/research-monitoring-and-reporting/reporting/envreportbc/archived-indicators/land/envreportbc_silviculture_july2014.pdf [accessed 5 January 2022].
- 23.Gregory C, McBeath A, Filipescu C. 2018. An Economic Assessment of the Western Redcedar Industry in British Columbia. Information report FI-X-017. https://cfs.nrcan.gc.ca/pubwarehouse/pdfs/39066.pdf [accessed 9 March 2023].
- 24.Rezaei H, Lim J, Sokhansanj S. 2020. Comparison of drying rates of ground western red cedar with hemlock, birch, aspen, and spruce/pine/Douglas fir. Appl Eng Agric 36(2):159–165, 10.13031/aea.13684. [DOI] [Google Scholar]
- 25.Acheson ES, Galanis E, Bartlett K, Mak S, Klinkenberg B. 2018. Searching for clues for eighteen years: deciphering the ecological determinants of Cryptococcus gattii on Vancouver Island, British Columbia. Med Mycol 56(2):129–144, PMID: , 10.1093/mmy/myx037. [DOI] [PubMed] [Google Scholar]
- 26.Mak S, Klinkenberg B, Bartlett KH, Fyfe M. 2010. Ecological niche modeling of Cryptococcus gattii in British Columbia, Canada. Environ Health Perspect 118(5):653–658, PMID: , 10.1289/ehp.0901448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morfitt GL. 1997. Vancouver Island Highway Project: Planning And Design. https://www.bcauditor.com/sites/default/files/publications/1996/report3/report/vancouver-island-highway-project-planning-and-design.pdf [accessed 4 April 2022].
- 28.Government of British Columbia. 2021. Municipal and sub-provincial areas population, 2011 to 2021. https://www2.gov.bc.ca/gov/content/data/statistics/people-population-community/population/population-estimates [accessed 3 November 2021].
- 29.Phillips P, Galanis E, MacDougall L, Chong MY, Balshaw R, Cook VJ, et al. 2015. Longitudinal clinical findings and outcome among patients with Cryptococcus gattii infection in British Columbia. Clin Infect Dis 60(9):1368–1376, PMID: , 10.1093/cid/civ041. [DOI] [PubMed] [Google Scholar]
- 30.Hermosilla T, Wulder MA, White JC, Coops NC, Hobart GW. 2018. Disturbance-informed annual land cover classification maps of Canada’s forested ecosystems for a 29-year Landsat time series. Can J Remote Sens 44(1):67–87, 10.1080/07038992.2018.1437719. [DOI] [Google Scholar]
- 31.Hermosilla T, Wulder MA, White JC, Coops NC, Hobart GW. 2015. An integrated Landsat time series protocol for change detection and generation of annual gap-free surface reflectance composites. Remote Sens Environ 158:220–234, 10.1016/j.rse.2014.11.005. [DOI] [Google Scholar]
- 32.Hermosilla T, Wulder MA, White JC, Coops NC, Hobart GW, Campbell LB. 2016. Mass data processing of time series Landsat imagery: pixels to data products for forest monitoring. Int J Digit Earth 9(11):1035–1054, 10.1080/17538947.2016.1187673. [DOI] [Google Scholar]
- 33.MacDougall L, Kidd SE, Galanis E, Mak S, Leslie MJ, Cieslak PR, et al. 2007. Spread of Cryptococcus gattii in British Columbia, Canada, and detection in the Pacific Northwest, USA. Emerg Infect Dis 13(1):42–50, PMID: , 10.3201/eid1301.060827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galanis E, Macdougall L, Kidd S, Morshed M, British Columbia Cryptococcus gattii Working Group. 2010. Epidemiology of Cryptococcus gattii, British Columbia, Canada, 1999–2007. Emerg Infect Dis 16(2):251–257, PMID: , 10.3201/eid1602.090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.BC Centre for Disease Control. 2021. Reportable Diseases Data Dashboard. http://www.bccdc.ca/health-professionals/data-reports/reportable-diseases-data-dashboard [accessed 4 November 2021].
- 36.Pesaresi M, Florczyk A, Schiavina MMM, Maffenini L. 2019. GHS settlement grid, updated and refined REGIO model 2014 in application to GHS-BUILT R2018A and GHS-POP R2019A, multitemporal (1975-1990-2000-2015), R2019A. European Commission, Joint Research Centre (JRC). https://ghsl.jrc.ec.europa.eu/datasets.php [accessed 3 November 2021].
- 37.Puth MT, Neuhäuser M, Ruxton GD. 2015. Effective use of Spearman’s and Kendall’s correlation coefficients for association between two measured traits. Anim Behav 102:77–84, 10.1016/j.anbehav.2015.01.010. [DOI] [Google Scholar]
- 38.Duncan CG. 2005. The Emergence of Cryptococcus gattii in British Columbia: Veterinary Aspects [master’s thesis]. Saskatoon, Saskatchewan, Canada: University of Saskatchewan. [Google Scholar]
- 39.Fyfe M, MacDougall L, Romney M, Starr M, Pearce M, Mak S, et al. 2008. Cryptococcus gattii infections on Vancouver Island, British Columbia, Canada: emergence of a tropical fungus in a temperate environment. Can Commun Dis Rep 34:1–12, PMID: . [PubMed] [Google Scholar]
- 40.Patterson PB. 2008. Attributions of danger and responses to risk among logging contractors in British Columbia’s southern interior: implications for accident prevention in the forest industry. In: The Economics of Health and Wellness: Anthropological Perspectives, Vol. 26. Wood DC, ed. Oxford, UK: Elsevier, 103–125. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.