Abstract
Background
Protein bound solutes are poorly cleared by conventional hemodialysis because protein binding limits the ‘free’ solute concentration driving diffusion. This study tested the modeled prediction that clearances of bound solutes could be increased by raising the dialyzer mass transfer area coefficient (KoA) and dialysate flow (Qd) above the levels used in conventional practice.
Study Design
Pilot, cross-over trial.
Setting and Participants
Six stable long-term hemodialysis patients
Intervention
Study participants underwent an experimental dialysis treatment in which KoA and Qd were increased by using two dialyzers in series and supplying each dialyzer with a Qd of 800 ml/min by using two dialysis machines compared to those during a conventional treatment with a single dialyzer and Qd of 800 ml/min supplied by one machine.
Outcomes
Measured clearances of uremic solutes
Measurements
Clearances were measured for urea nitrogen (UN) and the bound solutes p-cresol-sulfate (PCS), indoxyl sulfate (IS), kynurenic acid (KYNA) and hippurate.
Results
Clearances for the bound solutes during conventional treatment were lower than for UN (clearance values: UN 255±16 ml/min; PCS 23±4 ml/min; IS 30±7 ml/min; KYNA 43±4 ml/min; hippurate 115±11 ml/min). Experimental treatment increased the clearances of all the solutes (clearance values: UN 318±19 ml/min; PCS 37±6 ml/min; IS 46±8 ml/min; KYNA 73±7 ml/min; hippurate 165±17 ml/min). The magnitude of the increases in clearance was greater for the bound solutes than for UN (increase in clearance: UN 25±6 %; PCS 66±19 %; IS 57±27 %; KYNA 69±5 %; hippurate 44±15 %).
Limitations
A longer term study would be required to determine if increased dialytic clearance of bound solutes leads to reduction in plasma solute levels.
Conclusions
The dialytic clearance of protein-bound solutes can be increased by raising KoA and Qd above conventional levels.
Keywords: Clearance, hemodialysis, protein-bound solutes
Conventional hemodialysis provides limited clearance of solutes which bind to plasma proteins (1–5). This is because only the unbound or “free” solute concentration contributes to the gradient driving diffusion across the dialysis membrane. Mathematical modeling predicts that the clearance of bound solutes can be increased by raising the dialyzer mass transfer area coefficient KoA and the dialysate flow rate Qd to exceed routinely prescribed values (6, 7). The goal of this study was to determine if the predicted increase in clearance can be achieved in practice. Dialytic clearances of urea nitrogen (UN) and the bound solutes p-cresol sulfate (PCS), indoxyl sulfate (IS), kynurenic acid (KYNA) and hippurate were measured in six stable hemodialysis patients. Measurements were made during a conventional treatment and during an experimental treatment in which KoA was increased by using two dialyzers in series and total Qd was increased by providing each of these dialyzers with a dialysate flow of 800 ml/min by using two dialysis machines. Results showed that the increase in KoA and Qd had the predicted effect of increasing the clearance of solutes which bind to plasma proteins.
Methods
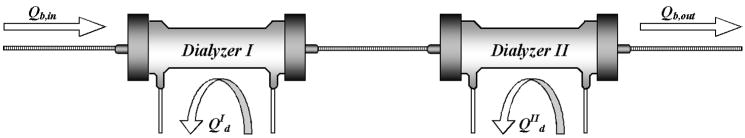
Studies were carried out in patients stably maintained on thrice weekly hemodialysis. The study was approved by the IRB and consent was obtained from all participants. Six patients were enrolled and all completed the study. Measurements were made during one conventional treatment and one experimental treatment with alternate participants receiving conventional/experimental and experimental/conventional treatment. Five participants were studied during midweek treatment sessions one week apart and the sixth was studied during the second and third session of the same week. Conventional treatment consisted of hemodialysis for the participant’s usual treatment time using a Fresenius F180NR dialyzer and Fresenius 2008H machine (FMC USA, Walnut Creek, CA) with the dialysate flow set at 800 ml/min. For the experimental treatment the participants received dialysis for the same time but using two Fresenius F200NR kidneys in series and using two dialysis machines so that each kidney could be supplied with a dialysate flow of 800 ml/min as depicted in Figure 1. Blood was pumped by the machine supplying dialysate to the second kidney using a standard line set (Medisystems Corporation. Seattle, WA) with pressure transducers attached in routine fashion. A “slave” machine supplied dialysate to the upstream kidney from which blood passed to the downstream kidney through a short connector (Recirculation Loop with Sampling Port MPC-660S. Molded Products, Harlan, IA). The arterial pressure transducer on the slave machine was connected to the arterial pressure transducer line on the pumping machine using a T connector. A fixed pressure set at approximately 100 mmHg above the venous pressure recorded in the line set was applied to the venous pressure transducer on the slave machine. This corrected for the pressure drop across the downstream kidney and prevented a transmembrane pressure alarm state which otherwise stopped provision of dialysate to the upstream kidney. The ultrafiltration goal was calculated from the patient’s dry weight and divided evenly between the two machines for the experimental treatment. Participants received their usual bolus heparin doses before both treatments and an additional continuous infusion of heparin at 1000 units per hour during the experimental treatment.
Figure 1.
The arrangement of dialyzers and dialysate flows for the experimental treatments using two kidneys. The dialyzers were hooked in series and each provided with a separate dialysate stream flowing countercurrent to the blood at 800 ml/min.
Plasma samples were obtained pre- and post-dialysis. Post-dialysis blood samples were collected from the arterial port after blood flow had been slowed to 50 ml/min for 15 seconds and then stopped. Samples of plasma ultrafiltrate were prepared with Nanosep 30K Omega separators (Pall, Ann Arbor, MI) for measurement of free levels of the bound solutes. The combined dialysate and ultrafiltrate from each dialyzer was collected in a 200 liter barrel and volume was determined by weight. The protein bound solutes were assayed by HPLC. For PCS and IS plasma samples were deproteinized using 3:1 methanol:plasma. PCS was measured using a 4.6×150 mm C18 column and fluorescence detection with excitation 214 nm and emission 306 nm. Buffer flow was 1ml/min using methanol (A) and ammonium formate (50mM, pH 4) (B) with a gradient from 15%A/85%B to 75%A/25%B over 15 min. IS was measured using the same column with excitation 250 nm and emission 410 nm. Buffer flow was 1ml/min using methanol (A) and ammonium formate (50mM, pH 4) (B) with a gradient from 0%A/100%B to 80%A/20%B over 25min. KYNA was measured using a method derived from Mackay et al. (19). Plasma was prepared by adding 240 μM of 3-Nitro-L-Tyrosine, 2 mM ascorbic acid and 4M PCA and spun at 1000G for 3 min. The pellet was washed twice with water and 4M PCA and KYNA was measured in the combined supernatants using a 4.6×250 mm C18 column with excitation 344 nm and emission 404 nm. Buffer flow was 1 ml/min using 100 nM zinc acetate with 3% acetonitrile and 50mM acetic acid. For hippurate plasma was deproteinized using 2:1 water:plasma and heating to 90°C for 30 minutes. Hippurate was measured using a 4.6 ×150 mm C18 column and UV detection at 254 nm. Buffer flow was 1 ml/min using methanol (A) and ammonium formate (50mM, pH 4) (B) with a gradient from 0%A/100%B to 80%A/20%B over 25 min. UN was measured using a commercial kit (1770-500; Thermo Electron Corp., Melbourne, Australia). Protein in the dialysate was measured by the Coomassie blue method. Free T4, vitamin B12, and amino acid levels were measured in clinical laboratories using standard techniques.
Qd values were calculated by subtracting the ultrafiltrate volume from the total fluid recovered and then dividing by the treatment time. Free fractions of each solute were calculated as the ultrafiltrate concentration divided by the total plasma concentration and pre- and post-dialysis values were averaged to obtain a value for each study session. Clearances were calculated as
| (1) |
where R is the amount of solute removed, t is the length of the dialysis session, and Clm is the log mean solute concentration given by
| (2) |
where Cpre and Cpost are the pre- and post-dialysis plasma solute concentrations, respectively. Modeled clearance values were obtained as previously described from the nominal blood flow Qb as determined by the blood pump speed, Qd, the ultrafiltration rate Qf, hematocrit, protein binding, and the KoA for the solute of interest (6). Clearance values for the two kidneys in series were calculated as:
| (3) |
where ktotal is the calculated total clearance and kind is the modeled clearance of each of the individual dialyzers, and where Q is blood water flow for UN and plasma flow for the bound solutes. At Qb 400 ml/min, blood transit time was approximately 15 seconds through the F180NR dialyzer (blood side volume 98 ml) and 35 seconds through the two F200NR kidneys (blood side volume 112 ml each). KoA values for each solute for the F180NR and F200NR dialyzers were determined by in vitro experiments (n=3 each dialyzer) in which solutes in buffered saline were dialyzed out of a 40L reservoir with reservoir fluid flow 400 ml/min and dialysate flow 800 ml/min. Arterial-venous solute concentration differences were multiplied by the fluid flow to obtain clearance values and KoA values were obtained using the relation described by Michaels (20). Statistical comparisons between experimental and conventional treatment were made using the Wilcoxon signed-rank sum and comparison between individual solutes were made using the Student-Newman-Keuls test (nonparametric).
Results
Treatment parameters for the conventional and experimental hemodialysis sessions are summarized in Table 1. Conventional dialysis was performed with a single F180 dialyzer and an average dialysate flow of 793±5 ml/min, while experimental dialysis was performed with two F200 kidneys connected in series and each supplied with half of an average total dialysate flow of 1582±9 ml/min. Other parameters were the same for the two treatments.
Table 1.
Hemodialysis Treatment Parameters
| Conventional | Experimental | |
|---|---|---|
|
| ||
| Dialyzer | F180 | two F200’s in series |
| Total Qd (ml/min) | 793 ± 5 | 1582 ± 9 |
| Pre-dialysis weight (kg) | 84 ± 11 | 84 ± 10 |
| Net ultrafiltration (liters) | 2.9 ± 0.8 | 2.3 ± 0.4 |
| Mean Qb (ml/min) | 395 ± 13 | 404 ± 17 |
| Treatment time (min) | 195 ± 17 | 196 ± 15 |
| Pre-dialysis hematocrit (%) | 37 ± 5 | 36 ± 5 |
Values are mean ± sd. Abbreviations: Qb, whole blood flow; Qd, dialysate flow;
The effects of dialysis on plasma solute levels and the amount of each solute removed during dialysis are summarized in Table 2. Conventional dialysis reduced the plasma UN level by an average of 72±4 percent, consistent with current treatment standards. Experimental treatment in which blood passed serially through two large dialyzers with independent dialysate streams increased the fractional removal of UN only slightly. Results for the protein-bound solutes were quite different. Conventional dialysis reduced the plasma level of PCS, which was 94±2 percent bound to protein, by only 30±14 percent. The experimental treatment increased the reduction ratio to 48±7 percent and removed significantly more PCS. Similar results were obtained for IS and KYNA which exhibited nearly the same degree of protein binding as PCS. Hippurate was less tightly bound and its reduction ratio was 67±8 percent during conventional dialysis. Experimental treatment increased the reduction ratio to 79±4 percent.
Table 2.
Solutes in Plasma and Dialysate
| Conventional | Experimental | ||
|---|---|---|---|
|
| |||
| UN | plasma pre (mg/dl) | 58 ± 22 | 62 ± 20 |
| plasma post (mg/dl) | 16 ± 6 | 13 ± 6 | |
| reduction ratio (%) | 72 ± 4 | 80 ± 4 a | |
| removed in dialysate (mg) | 16 ± 6 × 103 | 19 ± 6 × 103a | |
|
| |||
| PCS | plasma pre (mg/dl) | 5.6 ± 0.9 | 5.0 ± 0.7 |
| plasma post (mg/dl) | 3.9 ± 0.9 | 2.6 ± 0.5 a | |
| % bound | 94 ± 2 | 94 ± 1 | |
| reduction ratio (%) | 30 ± 14 b | 48 ± 7 a,b | |
| removed in dialysate (mg) | 208 ± 65 | 261 ± 37 a | |
|
| |||
| IS | plasma pre (mg/dl) | 3.8 ± 1.0 | 3.9 ± 1.2 |
| plasma post (mg/dl) | 2.5 ± 0.7 | 1.8 ± 0.5 a | |
| % bound | 92 ± 2 | 93 ± 1 | |
| reduction ratio (%) | 36 ± 10 | 53 ± 7 a,b | |
| removed in dialysate (mg) | 177 ± 43 | 240 ± 54 a | |
|
| |||
| KYNA | plasma pre (μg/dl) | 17 ± 9 | 18 ± 5 |
| plasma post (μg/dl) | 11 ± 4 | 7 ± 2 | |
| % bound | 90 ± 2 | 91 ± 2 | |
| reduction ratio (%) | 34 ± 10 b | 58 ± 7 a | |
| removed in dialysate (mg) | 1.2 ± 0.7 | 1.7 ± 0.6 a | |
|
| |||
| Hippurate | plasma pre (mg/dl) | 5.5 ± 3.5 | 7.4 ± 3.3 |
| plasma post (mg/dl) | 1.8 ± 1.3 | 1.5 ± 0.6 | |
| % bound | 55 ± 4 | 56 ± 4 | |
| reduction ratio (%) | 67 ± 8 c | 79 ± 4 a,c,d | |
| removed in dialysate (mg) | 740 ± 521 | 1153 ± 398 | |
Values are mean ± sd.
, p < 0.05 experimental vs conventional treatment. Significance of differences between different solutes is indicated for the reduction ratios only;
, p < 0.05 vs UN;
, p < 0.05 vs PCS;
, p < 0.05 vs IS. The reduction ratio is calculated as (plasma pre – plasma post)/plasma pre. Note: Conversion factors for units: Plasma UN in mg/dl to mmol/L, x0.357; plasma PCS in mg/dl to mmol/L, ×0.053; plasma IS in mg/dl to mmol/L, ×0.047; plasma KYNA in μg/dl to μmol/L, ×0.053; plasma Hippurate in mg/dl to mmol/L, ×0.056.
Abbreviations: UN, urea nitrogen; PCS, p-cresol-sulfate; IS, indoxyl sulfate; KYNA, kynurenic acid.
Clearance values calculated from plasma solute levels and the amounts of solute removed are summarized in Table 3. The UN clearance during the conventional treatments averaged 255±16 ml/min, and the use of two kidneys with separate dialysate streams increased this value by only 25±6 percent. As expected, clearance values for the tightly bound solutes PCS, IS, and KYNA were much lower than the UN clearance during conventional dialysis, and ranged from 23±4 to 43±4 ml/min. Increases in the clearances of these solutes achieved with the experimental treatment averaged from 57 to 69 percent, and were greater than the increase in UN clearance. The clearance of the less tightly bound solute hippurate during conventional dialysis fell between the clearance of UN and the clearances of the more tightly bound solutes. Experimental treatment raised the hippurate clearance by 44±15 percent to 165±17 ml/min. For all solutes, the clearance during the experimental treatment was greater than the clearance during the conventional treatment in each of the study participants as shown in Fig S1 (provided as online supplementary material available with this article at www.ajkd.org).
Table 3.
Clearance Values
| Conventional Clearance ml/min | Experimental Clearance ml/min | Experimental Increase % | ||
|---|---|---|---|---|
|
| ||||
| Observed | UN | 255 ± 16 | 318 ± 19 a | 25 ± 6 |
| PCS | 23 ± 4 b | 37 ± 6 a,b | 66 ± 19 b | |
| IS | 30 ± 7 b | 46 ± 8 a,b | 57 ± 27 | |
| KYNA | 43 ± 4 | 73 ± 7 a | 69 ± 5 b | |
| Hippurate | 115 ± 11 c | 165 ± 17 a,c | 44 ± 15 | |
|
| ||||
| Modeled | UN | 351 ± 9 | 399 ± 16 a | 14 ± 6 |
| PCS | 33 ± 8 b | 57 ± 11 a,b | 78 ± 28 b | |
| IS | 41 ± 11 b | 66 ± 12 a,b | 65 ± 25 b | |
| KYNA | 47 ± 6 | 79 ± 15 | 71 ± 28 b | |
| Hippurate | 155 ± 8 c | 218 ± 15 a,c | 41 ± 10 | |
Values are mean ± sd and clearances are expressed as ml/minute of plasma. Modeled values for treatment with a single kidney were obtained as described in reference 6 and modeled values for the experimental treatment were obtained from the modeled values for treatment with a single dialyzer using equation 3.
, p < 0.05 experimental vs. conventional treatment;
, p < 0.05 vs. UN;
, p < 0.05 vs. PCS. Abbreviations: UN, urea nitrogen; PCS, p-cresol-sulfate; IS, indoxyl sulfate; KYNA, kynurenic acid.
Clearance values obtained using a previously described mathematical model for the dialysis of bound solutes are also summarized in Table 3. The modeled values slightly exceeded the observed values but followed the same pattern. The increases in the clearance of the bound solutes predicted by the model ranged from 41 to 78 percent, as compared to the observed increases of 44 to 69 percent. Estimates of KoA for the F180NR dialyzers used for the conventional treatment and the F200NR dialyzers used for the experimental treatment were required to model the solute clearances. These KoA values were determined by in vitro dialysis experiments as summarized in Table 4. As would be expected on the basis of their slightly larger molecular size, the bound solutes had lower in vitro KoA values than urea.
Table 4.
In Vitro KoA Values
| MW Daltons | KoA F180NR ml/min | KoA F200NR ml/min | |
|---|---|---|---|
|
| |||
| Urea | 60 | 1196 ± 46 | 1275 ± 52 |
| PCS | 188 | 819 ± 9 | 865 ± 23 |
| IS | 213 | 791 ± 18 | 848 ± 33 |
| KYNA | 189 | 692 ± 18 | 748 ± 24 |
| Hippurate | 179 | 674 ± 17 b | 729 ± 27 b |
Values are mean ± sd. MW; molecular weight.
, p < 0.05 F200NR vs. F180NR;
, p < 0.05 vs. urea. Abbreviations: UN, urea nitrogen; PCS, p-cresol-sulfate; IS, indoxyl sulfate; KYNA, kynurenic acid.
Additional measurements were made to assess the effects of the experimental treatment on valuable substances, as summarized in Table 5. Total amino acid removal averaged 9.9±3.1 g during the experimental treatment and 7.7±1.5 g during conventional treatment. This difference did not attain conventional significance (p=0.12) in our study of six participants. But we should note that study of ten participants would be required to provide 80% power to detect a 25 percent change in amino acid removal at five percent level of significance. There was no notable difference between the effect of experimental and conventional dialysis on the levels of the 20 individual amino acids which were assessed (Table S1). Levels of the protein bound substances T4 and vitamin B12 were not significantly reduced by either conventional or experimental treatment. Total protein removed was slightly increased by the experimental treatment, averaging 242±83 mg per treatment during conventional dialysis and 343±128 mg per treatment during experimental dialysis. The decline in plasma potassium was the same (1.4±0.7 mEq/l conventional; 1.4±0.4 mEq/l experimental) in the five of six participants in whom the dialysate potassium was the same for both treatments.
Table 5.
Levels of Valuable Substances
| Conventional | Experimental | ||
|---|---|---|---|
|
| |||
| Total Amino Acids | plasma pre (mg/dl) | 34 ± 4 | 35 ± 6 |
| plasma post (mg/dl) | 21 ± 4 b | 20 ± 5 b | |
| removed in dialysate (g) | 7.7 ± 1.5 | 9.9 ± 3.1 | |
|
| |||
| Free T4 | plasma pre (ng/dl) | 0.8 ± 0.2 | 0.7 ± 0.1 |
| plasma post (ng/dl) | 0.8 ± 0.2 | 0.8 ± 0.2 | |
|
| |||
| Vitamin B12 | plasma pre (pg/ml) | 494 ± 202 | 500 ± 184 |
| plasma post (pg/ml) | 529 ± 216 | 544 ± 193 b | |
|
| |||
| Protein | removed in dialysate (mg) | 242 ± 83 | 343 ± 128 a |
Values are mean ± sd.
, p < 0.05 experimental vs. conventional treatment.
, p < 0.05 post vs. pre-dialysis value. Note: Conversion factors for unit: T4 in ng/dl to pM, ×12.9; vitamin B12 in pg/ml to pM, ×0.738.
Discussion
The adequacy of hemodialysis is currently assessed by measuring urea removal, and treatment guidelines call for the removal of about two thirds of the body urea content at each of three weekly sessions. But many solutes are removed much less effectively than urea. Among these are those that bind to plasma proteins. Their dialytic clearance is limited because only the free solute concentration contributes to the gradient driving diffusion across the dialysis membrane. Their plasma levels therefore fall less than the urea level during conventional hemodialysis treatment even though they often have smaller volumes of distribution (4, 8, 9).
Mathematical modeling has suggested that the clearance of bound solutes can be increased by raising KoA and Qd above the levels used for conventional hemodialysis (6, 7). In theory, increasing Qd reduces the solute concentration in the dialysate and allows the gradient favoring diffusion to approach the maximum imposed by the free plasma solute concentration while increasing KoA provides more solute diffusion while the gradient is thus limited. The current study was performed to determine whether the predicted effect of increasing KoA and Qd on bound solute clearances could be achieved in practice.
To increase KoA and Qd we had to overcome the limitations of standard dialysis equipment, which is designed to provide urea clearances that are approximately 80 percent of an access blood flow of 400 ml/min. Dialysate flows and urea KoA’s of approximately 800 ml/min are sufficient to accomplish this, and increasing Qd and KoA above this level cannot raise the clearance of urea much higher. Current machines therefore do not provide dialysate flows greater than 800 ml/min. Many dialyzers provide somewhat larger urea KoA’s but no available dialyzer provides the increase in KoA or is engineered to handle the total dialysate flow which we wished to test. To increase effective KoA in the current study, we therefore substituted two F200NR kidneys (surface area 2.0 m2 each) for the single F180NR kidney (surface area 1.8 m2) used for standard treatments in our unit. The two dialyzers were hooked in series and each supplied with a separate stream of dialysate running countercurrent to the blood at 800 ml/min, as depicted in Figure 1. Applying a previously published model of the dialysis of bound solutes, we would predict that for a solute that is 94 percent bound to plasma protein and has a dialyzer KoA of 800 ml/min, this should provide about 95 percent of the clearance that would be obtained if the two kidneys could be combined into a single unit served by a dialysate flow of 1600 ml/min. This prediction is based on the further assumption that the fractional clearance provided by each of two kidneys in series is equal so that the total clearance achieved with the pair can be calculated using equation (3).
In accord with its predicted effect, increasing KoA and Qd to values which were effectively near double those used for conventional dialysis substantially increased the clearance of bound solutes. The average increases in the clearance of PCS, IS, and KYNA, which were each slightly more than 90 percent bound to protein, ranged from 57 to 69 percent. In contrast, the increase in UN clearance averaged only 25 percent and the increase in the clearance of hippurate, which was approximately 50 percent bound to protein, averaged 44 percent. The observed clearance values for the individual solutes were less than modeled clearances and the observed increases in the bound solute clearances were of slightly smaller magnitude than the modeled increases. Observed UN clearances may fall below modeled values in part because real Qb values are less than those calculated from the pump speed and because effective urea KoA values during treatment are less than those measured in vitro (10, 11). Recent studies further suggest that equilibration of UN across the erythrocyte membrane, which is assumed in clearance models, may not in fact be complete with modern dialysis prescriptions (12). Our “observed” UN clearance values may also underestimate true clearance values because compartmentation of urea in the body results in an average plasma urea concentration during dialysis which is less than the logarithmic mean of the pre- and post-dialysis plasma concentrations which was used to calculated clearances in this study. These various sources of error should have less effect on the calculated clearances of bound solutes than of UN. But the clearances of these solutes, like UN, would be reduced below modeled values by failure of KoA values to equal those measured in vitro. In addition, our model assumes rapid dissociation of bound solutes from protein and equilibration of the free solute concentrations across the lumen of the dialyzer fibers. Any departure from these assumptions would also tend to reduce observed clearances below the modeled values.
It should be emphasized that the clearance of unbound solutes as well as bound solutes will be increased by performing dialysis twice in series on the same blood line as was done in the current study. But for small unbound solutes the clearance with standard dialysis is a large fraction of the plasma flow, and thus cannot be increased much further by replicate dialysis in series. Based on the KoA values in Table 4 we would estimate, for instance, that an unbound solute of the same size (mw 213 Daltons) as IS would have a clearance of more than 80 percent of a plasma flow of 240 ml/min, and so serial treatment would afford little added clearance. The clearance of unbound solutes which have a low fractional clearance with standard dialysis due to larger size could be increased proportionally more. But the clearance of such solutes, unlike the clearance of bound solutes, could be increased almost as much by using two dialyzers to increase membrane surface area and KoA without increasing the total dialysate flow.
Three previous studies have assessed the effect of manipulating the dialysis prescription on the clearance of bound solutes. Lesaffer et al. (9) initially demonstrated that the clearance of bound solutes was not increased by use of a high-flux as compared to a low-flux membrane. This finding was not unexpected, as the well known and commonly studied bound solutes are of low molecular weight. The increased permeability of high-flux membranes to large solutes thus does not increase the flux of the unbound solute fraction, and the membranes do not provide significant flux of protein-solute complexes. Bammens et al. (2) subsequently assessed the extent to which the clearance of bound solutes could be increased by adding convection to dialysis. They found that a convective flow of 260 ml/min with substitution in the pre-dilution mode increased the clearance of p-cresol by about 50 percent over the value obtained with standard dialysis. Their finding was consistent with the prediction that only the unbound solute fraction is carried across the membrane by convection. Most recently, De Smet et al. (3) reported that the clearance of the bound solutes IS and indolacetic acid was increased by approximately 20 percent when a super-flux membrane was substituted for a low-flux membrane having the same nominal surface area. Increased clearance of the bound solutes could not be attributed to passage of protein-solute complexes through the membrane, and its mechanism remains to be established.
Together with those of previous studies, our results indicate that the removal of protein-bound solutes can be increased without increasing the number or duration of treatment sessions. But at present, to obtain a large increase in clearance requires either hemodiafiltration with a high convective flow as described by Bammens et al. (2) or large increases in KoA and Qd as described in the current study. Devices which pass albumin over sorbents have been developed but not yet successfully employed in patients with renal failure (13). Increasing KoA and Qd to the levels we used would require modification of existing dialysis equipment. It should be noted, however, that many centers routinely use dialysate flow rates less than the maximum 800 ml/min which can be obtained with most machines. This is particularly the case when average treatment duration is in the neighborhood of four hours, and target UN Kt/V values can be obtained with a Qd of 500 ml/min. In this setting, we would predict that an increase in bound solute clearances can be obtained with existing equipment. Increasing bound solute clearances during dialysis will increase removal of valuable protein-bound substances as well as uremic solutes. For substances like T4 and B12 which bind tightly to specific carrier proteins, the predicted losses are a negligible portion of the average daily production. There could be more significant losses of some drugs and of valuable endogenous substances which are not known to us. Adverse effects of blood-membrane contact could also be increased if membrane area is enlarged to improve the clearance of bound solutes. But the major unanswered question is whether increasing the removal of protein-bound uremic solutes would be beneficial. Various bound solutes have been shown to have toxic effects in vitro and recent studies have revealed an association between levels of p-cresol and poor outcomes in dialysis patients (1, 14–18). Our pilot study shows one means by which the clearance of bound solutes can be increased. Longer term studies will be required to determine the extent to which an increase in dialytic clearance lowers the plasma levels of bound solutes and whether reducing solute levels improves clinical outcomes.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the help of the staff of the dialysis unit at the VA Palo Alto HCS.
Support: Frank Luo was supported by a National Institutes of Health (NIH) Training Grant (T32 DK7357) and subsequently by a National Kidney Foundation Fellowship. Kajal Patel was supported by the Nephrology Division of the Stanford School of Medicine. Ilian Marquez was supported by a fellowship grant from Amgen. Thomas Hostetter was supported by a NIH Grant (R21 DK077326). Timothy Meyer and Natalie Plummer were supported by an NIH Grant (R33 DK071251).
Footnotes
Financial Disclosure: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vanholder R, De Smet R, Lameire N. Protein-bound uremic solutes: the forgotten toxins. Kidney Int. 2001;(suppl 78):S266–70. doi: 10.1046/j.1523-1755.2001.59780266.x. [DOI] [PubMed] [Google Scholar]
- 2.Bammens B, Evenepoel P, Verbeke K, Vanrenterghem Y. Removal of the protein-bound solute p-cresol by convective transport: a randomized crossover study. Am J Kidney Dis. 2004;44:278–85. doi: 10.1053/j.ajkd.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 3.De Smet R, Dhondt A, Eloot S, Galli F, Waterloos MA, Vanholder R. Effect of the super-flux cellulose triacetate dialyser membrane on the removal of non-protein-bound and protein-bound uraemic solutes. Nephrol Dial Transplant. 2007;22:2006–12. doi: 10.1093/ndt/gfm065. [DOI] [PubMed] [Google Scholar]
- 4.Martinez AW, Recht NS, Hostetter TH, Meyer TW. Removal of P-cresol sulfate by hemodialysis. J Am Soc Nephrol. 2005;16:3430–6. doi: 10.1681/ASN.2005030310. [DOI] [PubMed] [Google Scholar]
- 5.Brunet P, Dou L, Cerini C, Berland Y. Protein-bound uremic retention solutes. Adv Ren Replace Ther. 2003;10:310–20. doi: 10.1053/j.arrt.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Meyer TW, Leeper EC, Bartlett DW, et al. Increasing dialysate flow and dialyzer mass transfer area coefficient to increase the clearance of protein-bound solutes. J Am Soc Nephrol. 2004;15:1927–35. doi: 10.1097/01.asn.0000131521.62256.f0. [DOI] [PubMed] [Google Scholar]
- 7.Meyer TW, Walther JL, Pagtalunan ME, et al. The clearance of protein-bound solutes by hemofiltration and hemodiafiltration. Kidney Int. 2005;68:867–77. doi: 10.1111/j.1523-1755.2005.00469.x. [DOI] [PubMed] [Google Scholar]
- 8.Fagugli RM, De Smet R, Buoncristiani U, Lameire N, Vanholder R. Behavior of non-protein-bound and protein-bound uremic solutes during daily hemodialysis. Am J Kidney Dis. 2002;40:339–47. doi: 10.1053/ajkd.2002.34518. [DOI] [PubMed] [Google Scholar]
- 9.Lesaffer G, De Smet R, Lameire N, Dhondt A, Duym P, Vanholder R. Intradialytic removal of protein-bound uraemic toxins: role of solute characteristics and of dialyser membrane. Nephrol Dial Transplant. 2000;15:50–7. doi: 10.1093/ndt/15.1.50. [DOI] [PubMed] [Google Scholar]
- 10.Langsdorf LJ, Krankel LG, Zydney AL. Effect of blood-membrane interactions on solute clearance during hemodialysis. Asaio J. 1993;39:M767–72. [PubMed] [Google Scholar]
- 11.Depner TA, Greene T, Daugirdas JT, Cheung AK, Gotch FA, Leypoldt JK. Dialyzer performance in the HEMO Study: in vivo K0A and true blood flow determined from a model of cross-dialyzer urea extraction. Asaio J. 2004;50:85–93. doi: 10.1097/01.mat.0000104824.55517.6c. [DOI] [PubMed] [Google Scholar]
- 12.Eloot S, Torremans A, De Smet R, et al. Complex compartmental behavior of small water-soluble uremic retention solutes: evaluation by direct measurements in plasma and erythrocytes. Am J Kidney Dis. 2007;50:279–88. doi: 10.1053/j.ajkd.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Meijers BK, Weber V, Bammens B, et al. Removal of the uremic retention solute p-cresol using fractionated plasma separation and adsorption. Artif Organs. 2008;32:214–9. doi: 10.1111/j.1525-1594.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 14.De Smet R, Van Kaer J, Van Vlem B, et al. Toxicity of free p-cresol: a prospective and cross-sectional analysis. Clin Chem. 2003;49:470–8. doi: 10.1373/49.3.470. [DOI] [PubMed] [Google Scholar]
- 15.Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006;69:1081–7. doi: 10.1038/sj.ki.5000115. [DOI] [PubMed] [Google Scholar]
- 16.Schepers E, Meert N, Glorieux G, Goeman J, Van der Eycken J, Vanholder R. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol Dial Transplant. 2007;22:592–6. doi: 10.1093/ndt/gfl584. [DOI] [PubMed] [Google Scholar]
- 17.Dou L, Jourde-Chiche N, Faure V, et al. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost. 2007;5:1302–8. doi: 10.1111/j.1538-7836.2007.02540.x. [DOI] [PubMed] [Google Scholar]
- 18.Taki K, Tsuruta Y, Niwa T. Indoxyl sulfate and atherosclerotic risk factors in hemodialysis patients. Am J Nephrol. 2007;27:30–5. doi: 10.1159/000098542. [DOI] [PubMed] [Google Scholar]
- 19.Mackay GM, Forrest CM, Stoy N, et al. Tryptophan metabolism and oxidative stress in patients with chronic brain injury. Eur J Neurol. 2006;13:30–42. doi: 10.1111/j.1468-1331.2006.01220.x. [DOI] [PubMed] [Google Scholar]
- 20.Michaels AS. Operating parameters and performance criteria for hemodialyzers and other membrane-separation devices. Trans Am Soc Artif Intern Organs. 1966;12:387–92. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.