Abstract
Fine needle aspiration biopsy does not permit to distinguish between benign and malignant follicular thyroid lesions (category IV in the Bethesda Cytopathology System). Some reports have suggested an association between increased serum TSH levels and thyroid cancer, so the aim of this study was to investigate the association between TSH levels and malignancy in patients with follicular thyroid nodules. Therefore, we conducted a retrospective study of all subjects who underwent surgical treatment for Bethesda IV thyroid nodules in a single center (years 2012–2017). A total of 127 patients were analyzed, and malignancy was present in 38.6% of the patients. Using ROC analysis, the best TSH cut-off point to differentiate benign from malignant disease was 2.1 mU/l and the age cut-off with better sensitivity and specificity was 47 years. The proportion of subjects with TSH ≥ 2.1 mU/l was greater among subjects with cancer than in those with benign diseases (65.3 vs 44.9%, P = 0.029). The concurrence of both cut-off points (TSH ≥ 2.1 mU/l and age ≥ 47 years) showed a higher diagnostic accuracy than either of the two variables separately. Therefore, the present study supports an association between serum concentrations of TSH and risk of malignancy among subjects with Bethesda IV thyroid nodules. TSH levels could modify the diagnostic and therapeutic approach of patients with Bethesda IV nodules.
Keywords: TSH levels, Bethesda IV, Malignancy, Nodular thyroid disease
Introduction
Nodular thyroid disease (NTD) is a common clinical problem. Its prevalence varies depending on the mode of discovery, ranging 2–6% by cervical palpation, 19–35% by ultrasonography, and 8–65% according to autopsy data [1]. Although it is a benign condition in a great majority of cases, the clinical importance of NTD lies in the need to rule out malignant thyroid neoplasms [2]. Differentiating benign from malignant disease in patients with NTD can be challenging. Several factors, including age, sex, nodule size, and a previous history of radiation have been traditionally considered for their potential in predicting thyroid malignancy [3]. Nowadays, ultrasound-guided fine needle aspiration biopsy (FNAB) is the gold standard of diagnosis [4] and the Bethesda System for Reporting Thyroid Cytopathology, launched in 2009 [5], is the most commonly used method for reporting and interpreting aspiration cytology results. However, despite being a safe, accurate, and cost-effective method [6], a significant proportion of FNABs are inconclusive [7]. Most of them correspond to “follicular neoplasms” or “suspicious for follicular neoplasms” (category IV in the Bethesda Cytopathology System), a cytologic pattern observed in both malignant (mainly follicular carcinoma and follicular variant of papillary carcinoma) and benign lesions (follicular adenoma and adenomatous goiter), which represents 2–25% of all FNAB results [8]. In these cases, the risk of malignancy ranges between 10 and 40% [9] and it is usually necessary to perform a surgical biopsy for definitive diagnosis [10]. Different strategies have been proposed to diagnose malignancy in patients with indeterminate nodules, including the assessment of clinical, ultrasonographic, and scintigraphic features [11–14], as well as the examination of new immunohistochemical and molecular markers in FNAB specimens [15]. Among the latter, the specific point mutations C250T and C228T in the telomerase reverse transcriptase (TERT) promoter region seem to be particularly promising because they can exhibit spatial heterogeneity in follicular thyroid carcinoma, providing insights to molecular underpinnings as well as clinical management of the disease [16, 17]. However, these markers have not been found to be accurate enough to be incorporated into routine clinical practice [18].
In the last decade, a growing number of studies have shown that there is a significant association between increased TSH levels and the risk of thyroid cancer in patients with NTD [19–23]. However, most of them have not considered whether the prognostic value of TSH changes among groups with different types of cytology results, and specifically, among patients with indeterminate follicular lesions. The aim of this study was to evaluate whether TSH levels can help to differentiate benign from malignant disease in patients with Bethesda IV category nodules.
Methods
Subjects
All patients who underwent thyroid surgery following a Bethesda type IV cytology diagnosis on ultrasound-guided-FNBA in our institution between January 1, 2012, and December 31, 2017, were retrospectively reviewed. Patients in whom cytology results were not reported in the standard Bethesda format and those without an available preoperative measure of TSH were excluded.
Demographic and clinical data, dimensions and ultrasonographic characteristics suggesting thyroid cancer (namely hypoechogenicity compared with the surrounding thyroid, irregular borders, central vascularization, shape taller than wide, and microcalcifications), presence of additional thyroid nodules, final pathological diagnosis, and serum TSH concentration in the last determination before thyroid surgery were recorded. The volume of the nodule was calculated using the formula to calculate the volume of an ellipsoid. Nodules were further classified in five ultrasound patterns with different risk of malignancy, following the recommendations of the American Thyroid Association Guidelines: high suspicion, intermediate suspicion, low suspicion, very low suspicion, and benign [2, 4].
In all cases, serum TSH was determined using a two-site inmunoenzymatic (“sandwich”) assay (Beckman Coulter, France) (normal range, 0.38–5.33 mIU/l).
Statistical Analysis
Continuous variables were presented as mean ± SD or median (ranges), according to data distribution. The Kolmogorov-Smirnov test was used to determine whether data were normally distributed. Discrete variables were represented as percentages. Comparisons between groups with malignant and benign disease were performed using the Student t test, the Mann-Whitney test, or the chi-square test, as appropriate. ROC curves were analyzed to evaluate cut-off points with predictive power for the preoperative diagnosis of malignancy. A logistic regression model was performed to assess the independent effect of elevated TSH values on the risk of malignancy. Results were presented as odds ratio (OR) with 95% confidence intervals (CI) and P values. Diagnostic tests (sensitivity, specificity, predictive values, likelihood ratio, and accuracy) were derived from conventional 2 × 2 contingency tables.
Results
A total of 127 subjects (111 women) were identified. Eighteen of them (14.2%) had a diagnosis of primary hypothyroidism and were taking levothyroxine treatment before thyroid surgery. Forty-nine patients (38.6%) showed malignancy upon definitive histopathological examination (21 cases of follicular variant of papillary carcinoma, 19 of follicular thyroid carcinoma, 8 with other types of papillary carcinoma, and 1 with medullary carcinoma). The main demographic and clinical characteristics of the population and the ultrasound features of the category Bethesda IV dominant nodule that led to thyroid surgery are shown in Table 1, grouped according to the final histopathological diagnosis. Subjects with thyroid cancer were older than those with benign conditions. Prevalence of hypothyroidism, nodule dimensions, uni- or multinodular disease, and ultrasonographic characteristics usually considered to be associated with a higher risk of malignancy were very similar in groups with final diagnosis of benign or malignant disease.
Table 1.
Demographic, clinical characteristics, and ultrasound features of Bethesda IV dominant nodule, grouped according to the final histopathological diagnosis
| Total (N = 127) | Benign (n = 78) | Malignant (n = 49) | P | |
|---|---|---|---|---|
| Age (years) | 52.7 ± 14.0 | 50.1 ± 13.5 | 57.0 ± 13.7 | 0.007 |
| Male sex (%) | 12.6 | 14.1 | 10.2 | 0.59 |
| Postmenopausal status (women) (%) | 54.6 | 51 | 60 | 0.51 |
| Primary hypothyroidism (%) | 14.2 | 11.5 | 18.4 | 0.30 |
| TSH (mU/l) | 2.1 (0.09–37.00) | 2.3 (0.34–37.00) | 2.0 (0.09–10.94) | 0.30 |
| Ultrasound nodule features | ||||
| Maximum diameter (mm) | 26.6 | 26.9 ± 11.5 | 26.1 ± 12.3 | 0.71 |
| Volume (cc) | 3.2 (0.2–6.9) | 3.8 (0.3–46.9) | 2.9 (0.2–37.7) | 0.68 |
| Solitary nodule (%) | 37.0 | 34.6 | 40.8 | 0.57 |
| Hypoechogenicity (%) | 32.3 | 32.1 | 33.3 | 1 |
| Irregular margins (%) | 4.7 | 3.8 | 6.3 | 0.67 |
| Taller than wide shape (%) | 0.8 | 0 | 2.1 | 0.38 |
| Central vascularization (%) | 15.0 | 16.7 | 12.5 | 0.61 |
| Microcalcifications (%) | 4.7 | 5.1 | 4.2 | 1 |
| Ultrasound risk (ATA guidelines) | 0.91 | |||
| Benign (%) | 0.8 | 1.3 | 0 | |
| Very low suspicion (%) | 16.7 | 15.4 | 18.8 | |
| Low suspicion (%) | 50.0 | 51.3 | 47.9 | |
| Intermediate suspicion (%) | 27.0 | 26.9 | 27.1 | |
| High suspicion (%) | 5.6 | 5.1 | 6.3 | |
TSH level, assessed as a continuous variable, was not significantly higher in patients with thyroid cancer. Using ROC analysis, the best TSH cut-off point to differentiate benign from malignant disease was 2.1 mU/l (sensitivity 65.3%, specificity 55.1%, positive predictive value 47.8%, negative predictive value 71.7%, and area under the curve 0.55) (Fig. 1). The proportion of subjects with TSH ≥ 2.1 mU/l was greater among subjects with cancer than in those with benign diseases (65.3 vs 44.9%, P = 0.029). A logistic regression model including age and TSH ≥ 2.1 mU/l as independent variables showed that both of them retained independent association with malignancy. The OR for elevated TSH was 2.43 (95% CI, 1.131–5.21) (P = 0.023) and the OR for age was 1.049 (1.01–1.08) (P = 0.07). Given that the associations of malignancy with age and with increased TSH values were independent, a combination of both variables was also assessed to predict the risk of cancer. Using ROC analysis, the age cut-off with better sensitivity and specificity was 47 years. Among the 18 subjects younger than 47 years and with TSH values < 2.1 mU/l, only 2 of them had malignant nodules (11.1%). At the other end of the spectrum, malignant disease was diagnosed in 22 of 40 subjects with both age ≥ 47 years and TSH ≥ 2.1 mU/l (55%). The concurrence of both cut-off points (TSH ≥ 2.1 mU/l and age ≥ 47 years) as criterion to predict malignancy yielded a sensitivity of 44.9% and a specificity of 76.9%, providing a higher diagnostic accuracy than either of the two variables separately. The diagnostic performance of TSH and age is summarized in Table 2.
Fig. 1.
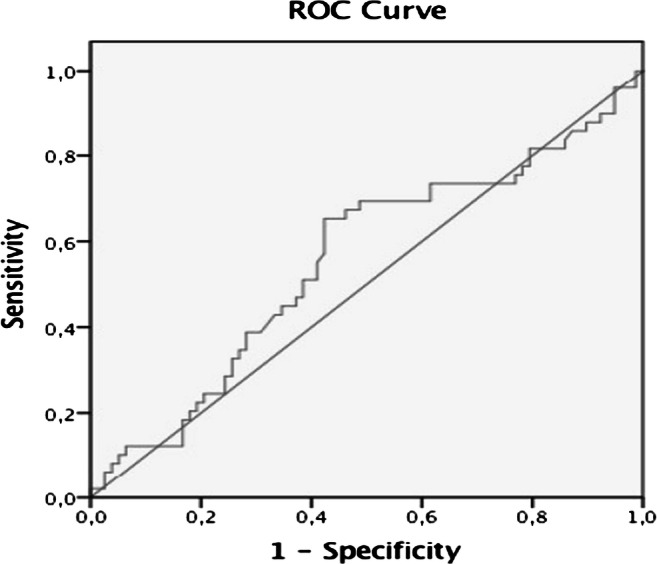
ROC curve for the TSH levels and the risk of malignancy in nodular thyroid disease
Table 2.
Diagnostic performance of TSH and age
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Odds ratio | Likelihood ratio | Accuracy (%) | |
|---|---|---|---|---|---|---|---|
| TSH ≥ 2.1 mU/l | 65.3 | 55.1 | 47.8 | 71.7 | 2.31 | 1.45 | 59.1 |
| Age ≥ 47 years | 75.5 | 42.3 | 45.1 | 73.3 | 2.26 | 1.31 | 55.1 |
| TSH ≥ 2.1 mU/l and age ≥ 47 years | 44.9 | 76.9 | 55.0 | 69.0 | 2.72 | 1.94 | 64.6 |
| TSH ≥ 2.1 mU/l and/or age ≥ 47 years | 95.9 | 20.5 | 43.1 | 88.9 | 6.06 | 1.21 | 49.6 |
PPV, positive predictive value; NPV, negative predictive value
Discussion
The present study was specifically focused to assess the potential of TSH measure to differentiate between benignity and malignancy in patients with Bethesda IV thyroid nodules. Although many studies have suggested that higher concentrations of TSH, even within the normal range, are associated with an increased risk of thyroid cancer in patients with NTD, in most cases the relationship between TSH and cancer was studied in all types of nodules. In fact, to our knowledge, only four previous studies have analyzed the association of TSH concentrations with risk of malignant disease in indeterminate follicular nodules. Within a large series of 845 patients with thyroid nodules undergoing surgical treatment, Haymart et al. [22] evaluated a small subset of 18 patients with FNAB cytology suspicious for malignancy. They found a trend for higher TSH levels among subjects with malignant final pathological diagnosis, although the difference was not significant because of the few number of studied patients. Castro et al. [23] reported that serum TSH levels were not a predictor of malignancy among 327 thyroid nodules with FNAB suspicious for follicular or Hürthle cell neoplasms. Muniz Fighera et al. [24] analyzed a sample of 622 patients of which 40 patients had follicular lesion. They found that follicular lesion had higher TSH levels compared with patients with benign cytological diagnosis. Finally, Husniye Baser et al. [16] investigated the association between concentrations of TSH and malignancy in euthyroid patients harboring thyroid nodules with different Bethesda categories. Patients with benign cytology had TSH levels significantly lower than those with other cytology results. Moreover, TSH levels increased as the Bethesda category did, rising from Bethesda categories II to VI. However, in contrast to other groups, among subjects within Bethesda IV category, TSH levels were similar between those with benign or malignant nodules. Nevertheless, it should be noted that the number of patients with Bethesda IV nodules was small compared with other groups.
As observed in previous studies, we also could not find significant differences between TSH levels in subjects with benign and malignant nodules, when TSH was assessed as a continuous variable. However, the risk of malignancy was higher in patients with TSH levels just above the median value (TSH levels ≥ 2.1 mU/l). The prognostic value of TSH was greater when combined to age, the only additional variable associated with thyroid cancer in our study. The concurrence of cut-off points with better diagnostic performance to predict malignancy for both TSH and age (TSH ≥ 2.1 mU/l and age ≥ 47 years) provided a higher diagnostic accuracy than either of the two variables separately.
The utilization of the TSH measurement in the estimation of the risk of malignancy could, logically, be of particular relevance in subjects with follicular nodules, since in this case the cytology does not allow to differentiate between benign and malignant nodules. Moreover, while thyroid ultrasonography can provide valuable markers of suspicion for papillary thyroid malignant neoplasms, it does not allow to differentiate so clearly between follicular adenomas or carcinomas, which represent the great majority of nodules with Bethesda IV cytology. In our study, neither ultrasonographic features nor other variables previously associated with malignant follicular nodules, such as uninodular goiter [25] and hypothyroidism requiring thyroid hormone substitution [23], were associated with malignant disease.
In the recent years, other new diagnostic approaches based on histopathological, imaging, and genetic biomarkers have been researched for diagnostic use in thyroid nodules with indeterminate cytology. Although these emerging tools have been validated in different studies, and some of them have been already introduced in clinical settings [26], they are expensive and only accessible to few centers [27–30]. On the other hand, there are few data on the integration of the information provided by these methods with that obtained from conventional clinical and biochemical variables. Further studies should be performed to assess if TSH levels, whose measure is very cheap and universal, can improve the diagnostic performance and the rational utilization of these novel tests.
Our study has some limitations. First, the sample size was small and this could have influenced on the results. On the other hand, it is a retrospective study and several possible confounding covariables, some of which have also been related to an increased risk of malignancy, such as antithyroperoxidase autoantibodies and serum T4 levels, [31, 32] were only available in a small proportion of patients and were not evaluated.
In conclusion, TSH levels above the median value were significantly associated with a higher risk of cancer in subjects with NTD and indeterminate Bethesda IV cytology results. Further studies are needed to investigate the potential of this easy and cheap technique to increase the accuracy of other emerging diagnostic tests for management of these lesions.
Funding Information
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Compliance with Ethical Standards
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee of the Hospital Universitario De Gran Canaria Dr. Negrin (CEI/CEIM HUGCDN: 2018-270-1.) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2008;22(6):901–911. doi: 10.1016/j.beem.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin J, Machekano R, McHenry CR. The utility of preoperative serum thyroid-stimulating hormone level for predicting malignant nodular thyroid disease. Am J Surg. 2010;199(3):294–298. doi: 10.1016/j.amjsurg.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 5.Cibas ES, Ali SZ. The bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009;132(5):658–665. doi: 10.1309/AJCPPHLWMI3JV4LA. [DOI] [PubMed] [Google Scholar]
- 6.Danese D, Sciacchitano S, Farsetti A, Andreoli M, Pontecorvi A. Diagnostic accuracy of conventional versus sonography guided fine needle aspiration biopsy of thyroid nodules. Thyroid. 1998;8(1):15–21. doi: 10.1089/thy.1998.8.15. [DOI] [PubMed] [Google Scholar]
- 7.Yassa L, Cibas ES, Benson CB, Frates MC, Doubilet PM, Gawande AA, Moore FD, Jr, Kim BW, Nosé V, Marqusee E, Larsen PR, Alexander EK. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer. 2007;111(6):508–516. doi: 10.1002/cncr.23116. [DOI] [PubMed] [Google Scholar]
- 8.Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The Bethesda system for reporting thyroid cytopathology: a meta-analysis. Acta Cytol. 2012;56(4):333–339. doi: 10.1159/000339959. [DOI] [PubMed] [Google Scholar]
- 9.Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. J Am Soc Cytopathol. 2017;6(6):217–222. doi: 10.1016/j.jasc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Baloch ZW, Fleisher S, LiVolsi VA, Gupta PK. Diagnosis of “follicular neoplasm”: a gray zone in thyroid fine-needle aspiration cytology. Diagn Cytopathol. 2002;26(1):41–44. doi: 10.1002/dc.10043. [DOI] [PubMed] [Google Scholar]
- 11.De Roy van Zuidewijn DB, Songun I, Hamming J, Kievit J, van de Velde CJ, Veselic M. Preoperative diagnostic tests for operable thyroid disease. World J Surg. 1994;18(4):506–510. doi: 10.1007/BF00353749. [DOI] [PubMed] [Google Scholar]
- 12.Maia FF, Zantut-Wittmann DE. Thyroid nodule management: clinical, ultrasound and cytopathological parameters for predicting malignancy. Clinics (Sao Paulo) 2012;67(8):945–954. doi: 10.6061/clinics/2012(08)15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brito JP, Gionfriddo MR, Al Nofal A, Boehmer KR, Leppin AL, Reading C, Callstrom M, Elraiyah TA, Prokop LJ, Stan MN, Murad MH, Morris JC, Montori VM. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99(4):1253–1263. doi: 10.1210/jc.2013-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ianni F, Campanella P, Rota CA, Prete A, Castellino L, Pontecorvi A, Corsello SM. A meta-analysis-derived proposal for a clinical, ultrasonographic, and cytological scoring system to evaluate thyroid nodules: the “CUT” score. Endocrine. 2016;52(2):313–321. doi: 10.1007/s12020-015-0785-5. [DOI] [PubMed] [Google Scholar]
- 15.Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. 2013;381(9871):1058–1069. doi: 10.1016/S0140-6736(13)60109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stenman A, Hysek M, Jatta K, Bränström R, Darai-Ramqvist E, Paulsson JO, Wang N, Larsson C, Zedenius J, Juhlin CC. TERT promoter mutation spatial heterogeneity in a metastatic follicular thyroid carcinoma: implications for clinical work-up. Endocr Pathol. 2019;30(3):246–248. doi: 10.1007/s12022-019-09580-7. [DOI] [PubMed] [Google Scholar]
- 17.Hysek M, Paulsson JO, Jatta K, Shabo I, Stenman A, Höög A, Larsson C, Zedenius J, Juhlin CC. Clinical routine TERT promoter mutational screening of follicular thyroid tumors of uncertain malignant potential (FT-UMPs): a useful predictor of metastatic disease. Cancers (Basel) 2019;11(10):1443. doi: 10.3390/cancers11101443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baser H, Topaloglu O, Tam AA, Evranos B, Alkan A, Sungu N, Dumlu EG, Ersoy R, Cakir B. Higher TSH can be used as an additional risk factor in prediction of malignancy in euthyroid thyroid nodules evaluated by cytology based on Bethesda system. Endocrine. 2016;53(2):520–529. doi: 10.1007/s12020-016-0919-4. [DOI] [PubMed] [Google Scholar]
- 19.Boelaert K, Horacek J, Holder RL, Watkinson JC, Sheppard MC, Franklyn JA. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab. 2006;91(11):4295–4301. doi: 10.1210/jc.2006-0527. [DOI] [PubMed] [Google Scholar]
- 20.Fiore E, Rago T, Provenzale MA, Scutari M, Ugolini C, Basolo F, Di Coscio G, Miccoli P, Grasso L, Pinchera A, Vitti P. L-thyroxine-treated patients with nodular goiter have lower serum TSH and lower frequency of papillary thyroid cancer: results of a cross-sectional study on 27,914 patients. Endocr Relat Cancer. 2010;17(1):231–239. doi: 10.1677/ERC-09-0251. [DOI] [PubMed] [Google Scholar]
- 21.Gul K, Ozdemir D, Dirikoc A, Oguz A, Tuzun D, Baser H, Ersoy R, Cakir B. Are endogenously lower serum thyroid hormones new predictors for thyroid malignancy in addition to higher serum thyrotropin? Endocrine. 2010;37(2):253–260. doi: 10.1007/s12020-010-9316-6. [DOI] [PubMed] [Google Scholar]
- 22.Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, Chen H. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab. 2008;93(3):809–814. doi: 10.1210/jc.2007-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castro MR, Espiritu RP, Bahn RS, Henry MR, Gharib H, Caraballo PJ, Morris JC. Predictors of malignancy in patients with cytologically suspicious thyroid nodules. Thyroid. 2011;21(11):1191–1198. doi: 10.1089/thy.2011.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fighera TM, Perez CL, Faris N, Scarabotto PC, da Silva TT, Cavalcanti TC, Mesa Junior CO, Miasaki F, da Paz Filho GJ, de Carvalho GA. TSH levels are associated with increased risk of thyroid carcinoma in patients with nodular disease. Endokrynol Pol. 2015;66(6):480–485. doi: 10.5603/EP.a2015.0059. [DOI] [PubMed] [Google Scholar]
- 25.Grani G, Lamartina L, Durante C, Filetti S, Cooper DS. Follicular thyroid cancer and Hürthle cell carcinoma: challenges in diagnosis, treatment, and clinical management. Lancet Diabetes Endocrinol. 2018;6(6):500–514. doi: 10.1016/S2213-8587(17)30325-X. [DOI] [PubMed] [Google Scholar]
- 26.Sciacchitano S, Lavra L, Ulivieri A, Magi F, De Francesco GP, Bellotti C, Salehi LB, Trovato M, Drago C, Bartolazzi A. Comparative analysis of diagnostic performance, feasibility and cost of different test-methods for thyroid nodules with indeterminate cytology. Oncotarget. 2017;8(30):49421–49442. doi: 10.18632/oncotarget.17220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McIver B, Castro MR, Morris JC, Bernet V, Smallridge R, Henry M, Kosok L, Reddi H. An independent study of a gene expression classifier (Afirma) in the evaluation of cytologically indeterminate thyroid nodules. J Clin Endocrinol Metab. 2014;99(11):4069–4077. doi: 10.1210/jc.2013-3584. [DOI] [PubMed] [Google Scholar]
- 28.Nikiforov YE, Yip L, Nikiforova MN. New strategies in diagnosing cancer in thyroid nodules: impact of molecular markers. Clin Cancer Res. 2013;19(9):2283–2288. doi: 10.1158/1078-0432.CCR-12-1253. [DOI] [PubMed] [Google Scholar]
- 29.Wu JX, Lam R, Levin M, Rao J, Sullivan PS, Yeh MW. Effect of malignancy rates on cost-effectiveness of routine gene expression classifier testing for indeterminate thyroid nodules. Surgery. 2016;159(1):118–126. doi: 10.1016/j.surg.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 30.Noureldine SI, Olson MT, Agrawal N, Prescott JD, Zeiger MA, Tufano RP. Effect of gene expression classifier molecular testing on the surgical decision-making process for patients with thyroid nodules. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1082–1088. doi: 10.1001/jamaoto.2015.2708. [DOI] [PubMed] [Google Scholar]
- 31.Davis PJ, Goglia LJL. Nongenomic actions of thyroid hormone. Nat Rev Endocrinol. 2016;12(2):111–121. doi: 10.1038/nrendo.2015.205. [DOI] [PubMed] [Google Scholar]
- 32.Cho YA, Kong SY, Shin A, Lee J, Lee EK, Lee YJ, Kim J. Biomarkers of thyroid function and autoimmunity for predicting high-risk groups of thyroid cancer: a nested case–control study. BMC Cancer. 2014;14:873. doi: 10.1186/1471-2407-14-873. [DOI] [PMC free article] [PubMed] [Google Scholar]


