Abstract
Activated platelets may contribute to the metastatic behavior of tumor cells when the cancer cells and platelets interact. The interaction requires cell and platelet surface integrin. Thyroid hormone as L-thyroxine (T4) is the principal ligand for a hormone receptor on integrin αvβ3 on tumor cells and platelets. T4 activates the integrin, promoting platelet aggregation and degranulation (local ATP release) and stimulating tumor cell proliferation. By a variety of molecular mechanisms reviewed here, extracellular ATP promotes tumor cell invasiveness and metastasis and supports a role for T4 as a pro-metastatic factor.
Keywords: 3,5,3′-Triiodo-L-thyronine (T3); Degranulation; Integrin; L-Thyroxine (T4); Metastasis; Thyroid hormone receptors; Tumor cell invasiveness
Introduction
A plasma membrane receptor for thyroid hormone analogues on the extracellular domain of integrin αvβ3 appears to contribute to the metastatic process [1]. The primary thyroid hormone ligand of this receptor is L-thyroxine (T4), a hormone analogue thought to be inactive, except as a source of 3,5,3′-triiodo-L-thyronine (T3). T3 is the active intracellular form of thyroid hormone and initiates its actions at intracellular thyroid hormone receptors (nuclear TRs) [2]. The molecular mechanisms by which unmodified T4 appears to contribute to cancer cell metastasis include cell surface-initiated actions on expression of matrix metalloproteinase genes, of angiogenesis support genes, of certain receptor tyrosine kinase genes and actions on the epithelial-to-mesenchymal transition (EMT) process [1].
It is apparent that platelets can participate in the local process of metastasis [3–5], and we have reported that platelet function is modulated by T4 [6, 7]. Tumor cell surface integrin αvβ3 has also been implicated in metastasis [4]. These observations have caused us to add this perspective on thyroid hormone-platelet interaction to our recent review of the contributions of the hormone to metastasis [1].
Thyroid Hormone-Directed Platelet Aggregation, Degranulation (ATP Release) and Mechanisms of Tumor Cell Invasiveness and Metastasis
Signal Transduction, T4, and Metastasis
Our in vitro studies of platelet aggregation that is caused by T4, but not T3, were expectedly associated with platelet degranulation (= ATP release) [6]. An extensive panel of consequences in tumor cells is promoted by extracellular ATP [8]. A number of these are summarized in Fig. 1. Relevant to pancreatic cancer cells, for example, extracellular ATP invokes cell migration essential to metastasis and this is promoted by protease-activated receptor 2 (PAR-2) [9]. PAR-2 activation appears to be dependent upon mitogen-activated protein kinase (MAPK), the activity of which is enhanced by T4 [10], and by the epidermal growth factor (EGF)–driven signaling pathway [9]. Thyroid hormone analogues are known to regulate transcription of the EGF receptor (EGFR) via integrin αvβ3 [1].
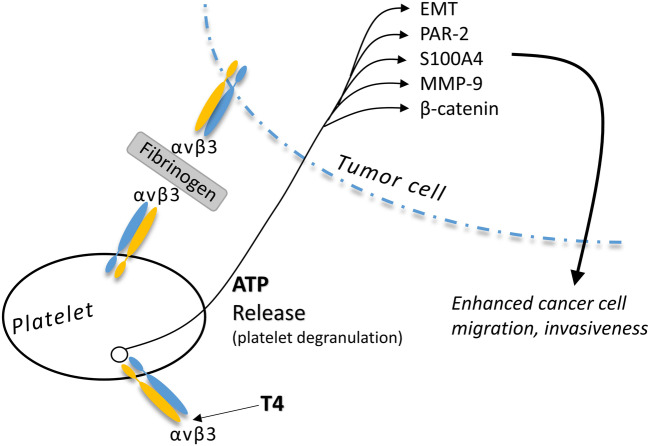
Fig. 1.
Platelet degranulation (ATP release) induced by T4 at the site of tumor cell-platelet interaction. Degranulation is stimulated by the binding of T4 to the thyroid hormone analogue receptor site on platelet membrane integrin αvβ3 [6]. Extracellular ATP and cancer cell ATP uptake results in activation of a set of discrete pathways linked to enhanced tumor cell migration and invasiveness, as discussed in this review. ATP-stimulated factors related to invasiveness include protease-activated receptor 2 (PAR-2), S100 calcium-binding protein A4 (S100A4), matrix metalloproteinase-9 (MMP-9), and β-catenin. Activation of cellular epithelial-to-mesenchymal transition (EMT) also results from increased extracellular ATP about cancer cells. It is not clear that whether the platelet αvβ3-tumor cell plasma membrane αvβ3 is fibrinogen-stimulated [5], as shown in this figure, is directly activated by T4. Thyroid hormone may increase circulating levels of fibrinogen [7] and activates various functions of integrin αvβ3 [10], but the tumor cell-platelet interaction via αvβ3 has not yet been studied
ATP has also been reported to increase renal cell carcinoma (RCC) migration and invasiveness [11]. The process is at least in part dependent upon Ca2+-activated MAPK and matrix metalloproteinase-9 (MMP-9) signaling, as is the breast cancer effect cited below. We have shown that MAPK is activated by T4 and that MMP-9 gene expression is stimulated by thyroid hormone [10].
T4 and S100A4, HIF-1/2α, MMP-9, β-Catenin, and Metastasis: EMT
Liu et al. [12] reported that extracellular ATP is a pro-invasive factor for breast cancer cells. The process involves interactions between fibroblasts and cancer cells that are promoted by S100 calcium-binding protein A4 (S100A4), which is known to promote cancer cell proliferation and invasion. Additional information is available regarding extracellular ATP and breast carcinoma invasiveness. Upregulation of expression of hypoxia-inducible factor (HIF)-1/2α may be part of the molecular mechanisms, as may be a contribution of MMP-9 [13]. These factors are also relevant to the angiogenesis required by metastatic lesions.
Extracellular ATP may act in conjunction with the β-catenin axis to induce or support existing breast cancer invasiveness/metastasis [14]. We have shown that T4 acts at the thyroid hormone analogue receptor on integrin αvβ3 to activate β-catenin in colorectal cancer cells [15], possibly enhancing the ATP effect on metastasis.
The EMT process contributes to metastasis and is activated by extracellular ATP [13]. T4 stimulates EMT [1, 14]. Thus, platelet delivery of ATP to cancer cells under the direction of thyroid hormone may be one of the mechanisms involved in the contribution of EMT to metastasis.
Inflammation, T4, and Metastasis
Elevated extracellular ATP levels about tumor cells may be associated with an inflammatory process that can be associated with relapse at or metastasis from the site of surgical tumor removal, as with melanoma [16]. The ATP may be generated by aggregating platelets, but, regardless of microenvironmental ATP levels, it is also important to note that T4 may act synergistically at a site of inflammation, stimulating cytokine [17] and chemokine gene transcription [18].
Cytoskeleton, T4, and ATP
Zanotelli and coworkers [19] have described in breast cancer cells the regulation of the intracellular ATP:ADP ratio by the density of the collagen microenvironment. The amount of ATP and collagen networking act in concert to regulate cell migration and invasion. It is also likely that the state of intracellular actin (fibrous [F] vs. soluble actin) is involved in organizing collagen architecture. T4, but not T3, generates F-actin [10, 20], but it has not been determined whether integrin αvβ3 participates in this process.
The cytoskeleton and intracellular structural organization is essential to the integrity of the signal transduction pathways identified above. Metastasis also requires cell motility and the latter depends upon an intact actin cytoskeleton [21, 22].
Comments
We have recently summarized a number of molecular mechanisms by which thyroid hormone as T4 may act via a cell surface receptor on integrin αvβ3 to support metastatic activity of cancer cells [1]. In a recent review, Weber et al. described the contributions of platelets to metastatic behavior of cancer cells [4]. The latter publication prompted the current review in which we have examined mechanisms by which T4 action on platelets may support tumor cell metastatic behavior.
A major activity of the T4-directed platelet is aggregation and ATP release (degranulation) [6]. Degranulation is a mechanism for increasing extracellular ATP levels at the site of thrombosis-tumor cell interaction. The likelihood of cancer cell-platelet interaction is a function of the cancer cell surface population of αvβ3 protein molecules and platelet αvβ3, the remnant of the plasma membrane of megakaryocytes. As the current review indicates, extracellular ATP may contribute via a number of mechanisms to the invasive/metastatic activities of cancer cells. A set of these mechanisms depend upon signaling molecules, such as those of MAPK, β-catenin, S100A4, and MMP-9. Of particular interest is that in addition to support of ATP release from platelets at the site of tumor cell-activated platelet interaction, thyroid hormone (T4) may via its receptor on cancer cell αvβ3 specifically direct downstream the transcription of genes for signaling molecules that also contribute to invasive cell behavior. The metastasis literature we review here is a rationale for developing anti-metastasis drugs that act at the integrin to block the effects of T4 on tumor cells [10].
Among the specific chemokine genes whose transcription is regulated from the thyroid hormone analogue receptor on integrin αvβ3 are CCL20 and CXCL2 [18]. Both of these chemokines confer chemoresistance on cancer cells [23, 24] by an ATP-dependent process that involves ABCB1 (P-glycoprotein, P-gp) that has been shown to be controlled by thyroid hormone [25]. Local delivery of ATP via the tumor cell-platelet interaction that T4 supports may thus relate to chemoresistance in the tumor cell as well as to the process of metastasis.
In addition to induction of chemoresistance as another contribution of thyroid hormone-stimulated platelets to the aggressiveness of cancer cells, it is apparent that the thyroid hormone receptor on αvβ3 may also confer radioresistance on cancer cells. It is apparent that increased intracellular ATP levels are associated with radioresistance [26], and Leith and coworkers have shown that the activation state of tumor cell plasma membrane αvβ3 is correlated with resistance to X-ray and that thyroid hormone analogues control the conformation/activation state of the integrin [27, 28]. Thus, the process of metastasis in tumor cells is subject to regulation by thyroid hormone-induced, platelet-dependent delivery of ATP, but the integrin receptor for the hormone may also be linked to chemo- and radioresistance.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mousa SA, Glinsky GV, Lin HY, Ashur-Fabian O, Hercbergs A, Keating KA, Davis PJ. Contributions of thyroid hormone to cancer metastasis. Biomedicines. 2018;6(3):89. doi: 10.3390/biomedicines6030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31(2):139–170. doi: 10.1210/er.2009-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber MR, Zuka M, Lorger M, Tschan M, Torbett BE, Zijlstra A, Quigley JP, Staflin K, Eliceiri BP, Krueger JS, Marchese P, Ruggeri ZM, Felding BH. Activated tumor cell integrin αvβ3 cooperates with platelets to promote extravasation and metastasis from the blood stream. Thromb Res. 2016;140(Suppl 1):S27–S36. doi: 10.1016/S0049-3848(16)30095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavergne Marion, Janus-Bell Emily, Schaff Mathieu, Gachet Christian, Mangin Pierre. Platelet Integrins in Tumor Metastasis: Do They Represent a Therapeutic Target? Cancers. 2017;9(10):133. doi: 10.3390/cancers9100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mousa SS, Davis FB, Davis PJ, Mousa SA. Human platelet aggregation and degranulation is induced in vitro by L-thyroxine, but not by 3,5,3′-triiodo-L-thyronine or diiodothyropropionic acid (DITPA) Clin Appl Thromb Hemost. 2010;16(3):288–293. doi: 10.1177/1076029609348315. [DOI] [PubMed] [Google Scholar]
- 7.Davis PJ, Mousa SA, Schechter GP. New interfaces of thyroid hormone actions with blood coagulation and thrombosis. Clin Appl Thromb Hemost. 2018;24(7):1014–1019. doi: 10.1177/1076029618774150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider G, Glaser T, Lameu C, Abdelbaset-Ismail A, Sellers ZP, Moniuszko M, Ulrich H, Ratajczak MZ. Extracellular nucleotides as novel, underappreciated pro-metastatic factors that stimulate purinergic signaling in human lung cancer cells. Mol Cancer. 2015;14:201. doi: 10.1186/s12943-015-0469-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi K, Queiroz KC, Stap J, Richel DJ, Spek CA (2013) Protease-activated receptor-2 induces migration of pancreatic cancer cells in an extracellular ATP-dependent manner. J Thromb Haemost 11(10):1892–1902. 10.1111/jth.12361 [DOI] [PubMed]
- 10.Davis Paul J., Goglia Fernando, Leonard Jack L. Nongenomic actions of thyroid hormone. Nature Reviews Endocrinology. 2015;12(2):111–121. doi: 10.1038/nrendo.2015.205. [DOI] [PubMed] [Google Scholar]
- 11.Gong D, Zhang J, Chen Y, Xu Y, Ma J, Hu G, Huang Y, Zheng J, Zhai W, Xue W. The m6a-suppressed P2RX6 activation promotes renal cancer cells migration and invasion through ATP-induced Ca2+ influx modulating ERK1/2 phosphorylation and MMP9 signaling pathway. J Exp Clin Cancer Res. 2019;38(1):233. doi: 10.1186/s13046-019-1223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Geng YH, Yang H, Yang H, Zhou YT, Zhang HQ, Tian XX, Fang WG. Extracellular ATP drives breast cancer cell migration and metastasis via S100A4 production by cancer cells and fibroblasts. Cancer Lett. 2018;430:1–10. doi: 10.1016/j.canlet.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 13.Yang H, Geng YH, Wang P, Zhou YT, Yang H, Huo YF, Zhang HQ, Li Y, He HY, Tian XX, Fang WG. Extracellular ATP promotes breast cancer invasion and epithelial-mesenchymal transition via hypoxia-inducible factor 2α signaling. Cancer Sci. 2019;110(8):2456–2470. doi: 10.1111/cas.14086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang JL, Liu Y, Yang H, Zhang HQ, Tian XX, Fang WG. ATP-P2Y2-β-catenin axis promotes cell invasion in breast cancer cells. Cancer Sci. 2017;108(7):1318–1327. doi: 10.1111/cas.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YS, Chin YT, Shih YJ, Nana AW, Chen YR, Wu HC, Yang YSH, Lin HY, Davis PJ. Thyroid hormone promotes β-catenin activation and cell proliferation in colorectal cancer. Horm Cancer. 2018;9(3):156–165. doi: 10.1007/s12672-018-0324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manica A, Da Silva AM, Cardoso AM, Moreno M, Leal DB, Da Silva AD, Schetinger MRC, Morsch VMM, Bagatini MD. High levels of extracellular ATP lead to chronic inflammatory response in melanoma patients. J Cell Biochem. 2018;119(5):3980–3988. doi: 10.1002/jcb.26551. [DOI] [PubMed] [Google Scholar]
- 17.Davis PJ, Glinsky GV, Lin HY, Incerpi S, Davis FB, Mousa SA, Tang HY, Hercbergs A, Luidens MK. Molecular mechanisms of actions of formulations of the thyroid hormone analogue, tetrac, on the inflammatory response. Endocr Res. 2013;38(2):112–118. doi: 10.3109/07435800.2013.778865. [DOI] [PubMed] [Google Scholar]
- 18.Davis PJ, Glinsky GV, Lin HY, Mousa SA. Actions of thyroid hormone analogues on chemokines. J Immunol Res. 2016;2016:3147671. doi: 10.1155/2016/3147671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zanotelli MR, Goldblatt ZE, Miller JP, Bordeleau F, Li J, VanderBurgh JA, Lampi MC, King MR, Reinhart-King CA. Regulation of ATP utilization during metastatic cell migration by collagen architecture. Mol Biol Cell. 2018;29(1):1–9. doi: 10.1091/mbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leonard JL, Farwell AP. Thyroid hormone-regulated actin polymerization in brain. Thyroid. 1997;7(1):147–151. doi: 10.1089/thy.1997.7.147. [DOI] [PubMed] [Google Scholar]
- 21.Nersesian S, Williams R, Newsted D, Shah K, Young S, Evans PA, Allingham JS, Craig AW. Effects of modulating actin dynamics on HER2 cancer cell motility and metastasis. Sci Rep. 2018;8(1):17243. doi: 10.1038/s41598-018-35284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohishi T, Yoshida H, Katori M, Migita T, Muramatsu Y, Miyake M, Ishikawa Y, Saiura A, Iemura SI, Natsume T, Seimiya H. Tankyrase-binding protein TNKS1BP1 regulates actin cytoskeleton rearrangement and cancer cell invasion. Cancer Res. 2017;77(9):2328–2338. doi: 10.1158/0008-5472.CAN-16-1846. [DOI] [PubMed] [Google Scholar]
- 23.Su S, Sun X, Zhang Q, Zhang Z, Chen J. CCL20 promotes ovarian cancer chemotherapy resistance by regulating ABCB1 expression. Cell Struct Funct. 2019;44(1):21–28. doi: 10.1247/csf.18029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W, Qin Y, Wang D, Zhou L, Liu Y, Chen S, Yin L, Xiao Y, Yao XH, Yang X, Ma W, Chen W, He X, Zhang L, Yang Q, Bian X, Shao ZM, Liu S. CCL20 triggered by chemotherapy hinders the therapeutic efficacy of breast cancer. PLoS Biol. 2018;16(7):e2005869. doi: 10.1371/journal.pbio.2005869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis PJ, Incerpi S, Lin HY, Tang HY, Sudha T, Mousa SA. Thyroid hormone and P-glycoprotein in tumor cells. Biomed Res Int. 2015;2015:168427. doi: 10.1155/2015/168427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato K, Azuma R, Imai T, Shimokawa T. Enhancement of mTOR signaling contributes to acquired X-ray and C-ion resistance in mouse squamous carcinoma cell line. Cancer Sci. 2017;108(10):2004–2010. doi: 10.1111/cas.13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leith JT, Mousa SA, Hercbergs A, Lin HY, Davis PJ. Radioresistance of cancer cells, integrin αvβ3 and thyroid hormone. Oncotarget. 2018;9(97):37069–37075. doi: 10.18632/oncotarget.26434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leith JT, Hercbergs A, Kenney S, Mousa SA, Davis PJ. Activation of tumor cell integrin αvβ3 by radiation and reversal of activation by chemically modified tetraiodothyroacetic acid (tetrac) Endocr Res. 2018;43(4):215–219. doi: 10.1080/07435800.2018.1456550. [DOI] [PubMed] [Google Scholar]