Abstract
Advanced adrenocortical cancer (ACC) is a rare, highly aggressive malignancy, which typically has a poor prognosis. In advanced ACC, the overall trend is toward a short PFS interval following first-line systemic therapy, highlighting a clear need for improved second-/third-line treatment strategies. We conducted a review of the literature and relevant scientific guidelines related to systemic therapy for advanced ACC. Public indexes including PubMed/MEDLINE were searched. Treatment selection in the second-line setting is based on small phase 2 trials, case reports, and pre-clinical evidence. The best data available for initial second-line therapy selection supports the use of gemcitabine and capecitabine (G + C) or streptozotocin (S), both with or without mitotane. G + C is becoming increasingly recommended based on phase 2 clinical trial data in patients of good PS, due to the inferred superior PFS and OS from non-comparative trials. Alternatively, streptozotocin was better tolerated than EDP + M in the FIRM-ACT study and remains an option when warranted. Beyond this, further treatment approaches should be tailored to individual patient characteristics, utilizing a mixture of systemic therapies, local therapies, and enrolment in clinical trials where available. Additionally, the role of molecular stratification, predictive biomarkers, and immune checkpoint inhibitors in specific individuals, such as Lynch syndrome, is evolving and may become increasingly utilized in clinical practice. Advanced ACC necessitates a multidisciplinary approach and is best managed in a specialist center. Although there is no one definitive second-line treatment strategy, there are some favorable approaches, which require further validation in larger clinical trials.
Keywords: Adrenocortical carcinoma, Proteomics, Chemotherapy, Mitotane, Immune checkpoint inhibitors
Introduction
Adrenocortical carcinoma (ACC) has an incidence of ∼ 0.5–2 new cases per million people per year with preponderance in females compared with males (1.5:1) and a bimodal age distribution in childhood and the fourth decade of life [1]. The majority of ACCs are sporadic, while some are associated with hereditary syndromes such as Li-Fraumeni, Beckwith-Wiedeman, multiple endocrine neoplasia (MEN) 1, congenital adrenal hyperplasia (CAH), and Lynch syndrome. The prevalence of Lynch syndrome among patients with ACC is 3.2%, which is similar to that of colorectal and endometrial cancer [2]. Localized ACCs are typically treated curatively with surgical resection, with adjuvant mitotane reserved for those with high risk factors for relapse. Despite adjuvant mitotane, the relapse rate is approximately 50%. Resection of recurrent or metastatic lesions can prolong survival in a proportion of patients. For patients with non-resectable or metastatic ACC, the outcomes are poor despite triple agent chemotherapy with etoposide, doxorubicin, cisplatin, and mitotane (EDP-M) with median overall survival (OS) around 15 months [3]. Therefore, there is a clear need for more effective second-line therapeutic options for advanced ACC and for an improved understanding of the molecular and genetic factors that drive tumor development may be key.
Molecular studies have identified several genes as potential drivers for sporadic adrenocortical tumorigenesis, including insulin-like growth factor 2 (IGF2), β-catenin (CTNNB1), and TP53, with a gain-of-function mutation in β-catenin being demonstrated in approximately 25% of adrenocortical tumors sampled [4, 5]. More recent genomic profiling of ACCs has identified additional candidate driver genes such as ZNRF3 and TERT, and identified molecular subgroups which correlate with variable clinical outcomes. Germline variants of these genes are also associated with many of the previously discussed syndromes which predispose to ACC [6]. Most recently, The Cancer Genome Atlas (TCGA) collected the clinical and pathological features, genomic alterations, DNA methylation profiles, and RNA/proteomic signatures of 91 cases of ACC. The tumor samples were collected from four continents and represented a near-global sampling of this disease. From this dataset, new ACC driver genes were identified, including PRKAR1A, RPL22, TERF2, CCNE1, and NF1. The PRKAR1A mutation was detected in the highest frequency and builds upon the evidence for its role for protein kinase A (PKA) signaling in ACC, in keeping with PRKACA somatic mutations being the founder lesion of benign adrenal tumor-associated endocrinopathies such as Cushing syndrome [6].
Additionally, genome-wide DNA copy number analysis revealed frequent occurrence of massive DNA loss followed by whole genome doubling (WGD) which was associated with rapid disease progression, suggesting WGD is a hallmark of this disease and may predict a poor prognostic group [7]. Further supporting this hypothesis were increased TERT expression, decreased telomere length, and activation of cell cycle programs. Integrated subtype analysis identified three ACC subtypes with distinct clinical outcome and molecular alterations, proposing a strategy for clinical stratification of patients based on molecular markers. These included a “chromosomal” group, which showed the highest frequency of whole chromosome arm gains and losses, a “noisy” group, which was characterized by a significantly higher number of chromosomal breaks as well as frequent loss of 1p with intact 1q, and a “quiet group,” with a few large copy number alterations. Kaplan-Meier analysis demonstrated a significant decrease in survival in the noisy group relative to the chromosomal and quiet subtypes, suggesting that multiple chromosomal breaks with loss of 1p is characteristic of aggressive disease and poorer prognosis [7]. Ultimately, the outcome of both this and previous pan-genomic studies is that disease classification, patient selection, and the development of future therapeutics will likely be driven by the identification of driver genes and genomic subtypes. However, the broad diversity of genomic alterations seen in ACC, with high copy number changes, suggests that combined inhibition of these disease pathways will be required for successful targeted therapy.
The objective of this review is to evaluate and appraise the most up-to-date literature concerning current evidence-based management strategies, following progression after first-line treatment for this challenging malignancy. This article builds upon the previous excellent work on advanced ACC by Megerle et al. [8, 9]. However, this review provides additional in-depth description of the patient-focused outcomes for the relevant clinical trials, including ORR, PFS, and OS data. Furthermore, this review discusses the molecular pathogenic pathways involved in tumorigenesis, how these pathways may effect treatment responses (particularly in the context of immune checkpoint inhibitor therapy), and novel therapeutic approaches for specific patient groups. It also focuses on pre-clinical data and potential future targeted therapies currently in development for advanced ACC.
Search Strategy
We conducted a Medline search (https://www.ncbi.nlm.nih.gov/pubmed; last access: January 3rd, 2020) using the best match advanced combination search function for the search terms “adrenocortical carcinoma” and “second-line,” which yielded 77 articles. A further specific manual search was made by checking the reference lists of relevant studies, thus allowing us to identify an additional 9 articles.
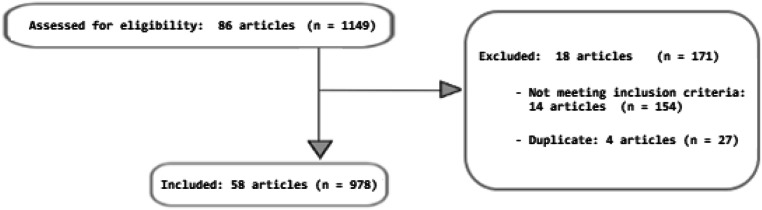
All articles were screened and those which met the eligibility criteria were reviewed. Data from a total of 58 different full articles was extracted. Duplicates and articles not relevant to this review were excluded (Fig. 1). The reports described a total of 978 patients with a diagnosis of ACC, undergoing one or more second-line treatments. Articles included, 10 pre-clinical studies, 3 retrospective analyses, 9 case reports/case series, 1 observational cohort study, 5 phase I trials, 10 phase II trials, and 2 phase III trials.
Fig. 1.
CONSORT diagram demonstrating the article selection process. n = Number of patients
First-Line Therapies in Advanced ACC
Although beyond the scope of this review, both local and systemic therapeutic options are utilized for disease control based on various prognostic factors. Patients with good performance status (PS), high volume disease, and/or rapid tumor progression are treated with EDP + M in the first-line setting based on the FIRM-ACT trial [8]. This randomized, multicenter study in locally advanced, inoperable, or metastatic ACC demonstrated a significantly higher objective response rate (ORR) of 23.2% vs 9.2% and longer median progression-free survival (PFS) of 5.0 months vs 2.1 months when EDP + M was compared with streptozocin + mitotane (S + M), but no difference in survival [10]. Patients with poorer PS, low volume, or indolent disease with long recurrence-free intervals can be treated with mitotane alone, with or without loco-regional therapies for local and or hormonal control if required [11, 12].
There have only been 11 single-arm studies (3 prospective and 8 retrospective) which have reported on a total of 395 patients, investigating mitotane, with or without chemotherapy. ORRs ranged between 7 and 54%, mostly due to variability in the response assessment criteria used in each study. However, the addition of mitotane to chemotherapy was consistently more effective than chemotherapy alone [8, 9]. A more recent study specifically evaluating the efficacy of mitotane monotherapy in 127 patients with advanced inoperable and metastatic ACC showed an ORR of 20.5%, median PFS of 4.1 months, and median OS of 18.5 months [9]. A multivariate analysis identified two favorable prognostic factors: low tumor burden (< 10 lesions) and recurrence more than 1 year after initial definitive treatment; these patients had an ORR over 30% and a median PFS of 8.8 months with median OS of 29.6 months and may especially benefit from mitotane monotherapy [9]. As a consequence, mitotane is still the only systemic therapy for advanced ACC with substantial evidence for improved survival. Nevertheless, unselected patient groups with advanced inoperable and metastatic ACC, unfortunately, remain incurable and patients often develop progressive disease shortly after starting first-line treatment, which highlights the need for improved therapeutic options [11].
Second-Line Therapies in Advanced Inoperable and Metastatic ACC
Systemic Chemotherapies
Streptozotocin
The default second-line treatment after progression on EDP + M has often been S + M. The latter has demonstrated modest activity with an ORR of 9.2%, a few complete responses, and a median PFS of 2.1 months [8]. FIRM-ACT permitted cross-over to the other arm at the time of progression. Of those who crossed over from the EDP + M group to the S + M group, the median ORR was 7.6%, with a median PFS of 2.2 months [8, 10]. S + M had fewer grade 1 and 2 adverse events than EDP + M, although rates of serious grade 3/4 toxicities were similar. Additionally, streptozotocin can be safely given concurrently with radiotherapy, as bone marrow toxicity is minimal. However, accessing streptozotocin can be challenging due to its limited and variable availability [10].
Gemcitabine + 5-Fluorouracil or Capecitabine
An alternative second-line treatment after progression on EDP + M is the combination of gemcitabine and capecitabine (G + C). A phase II study followed 28 patients with heavily pre-treated advanced ACC who had progressive disease following mitotane together with at least one cytotoxic chemotherapy [12]. On further review, all patients in this study were previously treated with EDP + M and all received ongoing concurrent mitotane, maintained for the duration of the trial. Patients received second-line gemcitabine (800 mg/m2 on days 1 and 8, every 21 days) and intravenous protracted infusion 5-fluorouracil (200 mg/m2 daily without interruption until progression) in the first 6 patients, and capecitabine (1500 mg/daily) in the subsequent 22 patients. After 4 months of treatment, 46% of patients had not progressed. There was 1 CR; 1 PR and 11 patients with SD (39%), with 15 patients (54%) having PD. Median PFS was 5.3 months and OS was 9.8 months. Treatment was well tolerated with 8 patients experiencing grade 3/4 toxicities such as neutropenia in 6 of 8 cases [12]. Due to the paucity of other comparative trials, and that all patients in this trial had received previous EDP + M, which has become the standard first-line therapy in advanced ACC, the combination of G + C has become increasingly recommended as a second-line therapy in this setting. Following this trial, a retrospective multi-study analysis was performed in 2017 to assess the efficacy of G + C and the predictive role of hENT1 and RRM1 [14]. A total of 145 patients with advanced ACC were treated with gemcitabine-based chemotherapy, of which 132 received concomitant capecitabine. The tumor tissue of 70 patients was suitable for hENT1 and RRM1 molecular testing. The median PFS for the patient population was 12 weeks (range, 1 to 94). An ORR of 4.9% was achieved, with 4.9% of patients achieving a PR and 25% having SD. Treatment was generally well tolerated, with adverse events of grade 3 or 4 toxicity occurring in 11.0% of cases. No substantial effect of hENT1 and/or RRM1 expression was observed in response to gemcitabine-based chemotherapy. Ultimately, G + C appears to be well tolerated, but only modestly active, against advanced ACC. No reliable molecular predictive factors have been identified and due to the limited alternative established therapeutic options, G + C remains an important second-line treatment for advanced ACC [14].
Thalidomide
The precise mechanism of action for thalidomide is not known. Postulated mechanisms include inhibition of angiogenesis, inhibition of the ubiquitin ligase cereblon, and generation of reactive oxygen species leading to tumor cell kill. In 2005, Chacón et al. published a case report of an impressive objective response in advanced ACC with liver metastasis after several months of therapy with thalidomide [15]. A further retrospective cohort study of participants in the European Networks for the Study of Adrenal Tumors (ENSAT) registry performed in 2018 evaluated 27 patients who had progressed after mitotane and a median of 4 further systemic treatments. Thalidomide was administered at a starting dose of 50 mg and escalated to a target dose of 200 mg/day. The best treatment response was SD in 2 patients and PD in 25 patients. A median PFS of 11.2 weeks and a median OS of 36.4 weeks were achieved. Overall, thalidomide was well tolerated but resulted in disease control in only 2 of the 27 patients (7.4%). With low therapeutic activity, the absence of predictive biomarkers, and poor efficacy, the role of thalidomide in ACC is very limited and requires further research [16].
Temozolomide
Temozolomide (TMZ) has been demonstrated to show anti-tumor activity for human ACC cells in vitro [17]. In this 2016 study, TMZ was able to induce apoptosis and provoke cytotoxic and cytostatic effects reducing the surviving fraction of ACC colonies and induced cell cycle arrest in 7 of 8 ACC cell lines. This suggested a potential for TMZ in patients not responding to mitotane alone or with etoposide, doxorubicin, and cisplatin [17]. On the basis of these results, TMZ was prescribed as second-/third-line therapy in advanced metastatic ACC patients across 4 referral centers in Italy. A 2019 retrospective analysis collected clinical and pathological data of these 28 patients, who were treated with TMZ at the dose of 200 mg/m2 given for 5 consecutive days every 28 days. Ten patients (35.8%) obtained disease control, with one CR, 5 PRs, and 4 patients had SD. Median PFS was 3.5 months and median OS 7.2 months. Additionally during this trial, the potential predictive role of the DNA repair gene O6-methylguanine-DNA methyltransferase (MGMT) was assessed and disease response was more frequently observed in patients with methylation of the MGMT gene. TMZ therapy was on the whole well tolerated and most toxicities were limited to grade G1–2 according to the WHO criteria [18]. TMZ appears active in the management of advanced ACC patients, although disease control was short-lived and prognosis remained poor. This may be reflective of this study population being heavily pre-treated and there may be a potential role for TMZ to be utilized earlier in the treatment paradigm for advanced ACC, especially in patients with poorer PS given its favorable toxicity profile. However, further trials are required to explore an earlier role of TMZ, possibly in molecular subtypes of ACC, and to better stratify treatment sequencing.
Trofosfamide
Trofosfamide is an alkylating agent structurally related to cyclophosphamide and ifosfamide. In a small observational cohort study, 13 patients with advanced inoperable or metastatic ACC, previously treated with mitotane with or without systemic chemotherapy, were treated with trofosfamide 150 mg/day [19]. A best response of SD occurred in only 3 patients (23%), with 10 patients developing PD (77%). Median PFS was 84 days and median OS 198 days. Trofosfamide was well tolerated with only mild G1/2 adverse events reported. With good overall tolerance, it may be considered in patients of poorer PS who are keen to pursue further systemic therapy [19] although it is currently only licensed for use in Germany (Table 1).
Table 1.
Published clinical trials of systemic chemotherapy in ACC
| Drug | Mechanism | Study phase | Patients | Results | Reference |
|---|---|---|---|---|---|
| Streptozotocin | Alkylating agent | III | 84 |
PFS 2.2 m OS 7.4 m |
[10] |
| Gemcitabine + capecitabine | DNA synthesis inhibitors | II | 28 |
ORR 46.3% PFS 5.3 m OS 9.8 m |
[12] |
| Gemcitabine + capecitabine | DNA synthesis inhibitors | Retrospective analysis | 145 |
ORR 4.9% PR 4.9% OSD 25% |
[13] |
| Thalidomide | Unknown | Case report | 1 | Prolonged objective response | [15] |
| Thalidomide | Unknown | Retrospective Analysis | 27 |
ORR 7.4% PFS 2.6 m OS 8.5 m |
[15] |
| Temozolomide | Alkylating agent | Retrospective analysis | 28 |
SD 14% PR 18% CR 4% |
[17] |
| Trofosfamide | Nitrogen mustard alkylating agent | Observational cohort study | 13 |
ORR 0% PFS 2.8 m OS 6.6 m |
[18] |
PFS progression-free survival, OS overall survival, ORR objective response rate, OSD overall survival difference, PD progressive disease, SD stable disease, CR complete response, m months, MMRD mismatch repair deficiency, NPR non-progression rate
Targeted Therapies
Several molecularly targeted therapies have been investigated for the treatment of advanced ACC, in most cases following PD after mitotane or EDP + M. To date, none of these agents have been approved for use in advanced ACC.
Insulin-Like Growth Factor-1 Receptor Inhibitors
Insulin-like growth factor type-2 (IGF-2) ligand is overexpressed on ∼ 80% of ACCs and generates activation of downstream effector pathways via the insulin-like growth factor-1 receptor (IGF-1R). A pre-clinical study investigated ACC cell lines with activated IGF signaling in tumors to assess the effects of IGF-1R antagonists ± mitotane, in cultured cells and ACC xenograft tumors [20]. The IGF-1R antagonists caused significant dose-dependent growth inhibition in ACC cell lines. Additionally, when used in combination with mitotane, there was enhanced growth inhibition [20].
A further phase 1 trial investigating the role of figitumumab, a monoclonal antibody (MAb) directed against the IGF-1R, demonstrated a favorable toxicity profile and a potential efficacy signal. Fourteen patients with heavily pre-treated and treatment-refractory metastatic ACC received a median of 4 (range 1–7) 3-weekly cycles of figitumumab. Six patients (43%) had concomitant mitotane. No confirmed responses were seen; however, 8 out of 14 patients achieved SD (43%) and treatment-related toxicities were generally mild [21]. Additionally, a phase I/II trial investigated cixutumumab, a fully human IgG1 monoclonal antibody directed at IGF-1R, given with mitotane in the first-line setting for metastatic ACC. This study was initially intended to have two treatment arms, one with cixutumumab + mitotane and one with mitotane monotherapy, but was terminated prior to randomization due to slow accrual and limited efficacy. Ultimately, only 20 patients were enrolled in a single arm receiving cixutumumab and achieved a median PFS of only 6 weeks with multiple grade 4/5 toxicities experienced. However, partial responses and disease stabilization were observed in a small subset of patients, with one achieving a PFS of 48 weeks [22]. Linsitinib is an oral small molecule inhibitor (SMI) which targets both the IGF-1R and insulin receptor. Following the previously described phase I trials assessing other IFGR receptors, a placebo-controlled phase III trial was performed in 139 patients, investigating the efficacy of linsitinib in advanced ACC. This trial failed to demonstrate any benefit in PFS or OS with linsitinib compared with placebo [23]. Similarly to cixutumumab, PR and SD were seen in only a small subset of the 90 linsitinib-treated patients and in 3 of these this lasted > 2 years. However, based on this data, neither linsitinib nor other IGF-1R inhibitors can be recommended as treatment for ACC in a molecularly unselected population. Ongoing efforts to identify predictive biomarkers explaining why certain subsets of patients respond favorably may allow for improved future patient selection [21]. IGF-1R inhibitors in combination with other drugs have also been tested, including cixutumumab with temsirolimus, an mTOR inhibitor. In a phase I dose escalation study, this combination was tested in heavily pre-treated patients with metastatic ACC and ~ 50% of patients achieved prolonged SD suggesting potential activity of this combination [24].
Overall, drugs targeting the IGF-1R signaling pathway have shown mixed results, ranging from highly toxic and ineffective, to well tolerated with some activity in a small subsets of patients. Ultimately, future studies should focus on identifying predictive biomarkers to better select for therapeutic benefit. Based on this literature search, potential predictive biomarkers warranting further studies include β-catenin and IGF2. Additionally, although evidence is currently limited and studies are ongoing, the expression levels of various molecules involved in DNA repair and the cell cycle, such as ZWINT, PRC1, CDKN3, CDK1, and CCNA2, may represent reliable prognostic markers for poor prognosis and early recurrence of ACC.
Tyrosine Kinase Inhibitors
Sunitinib is an oral, small molecule, multi-targeted receptor tyrosine kinase inhibitor (TKI), which works by inhibiting cellular signaling. A phase II study investigating sunitinib in 38 patients with heavily pre-treated, refractory ACC (SIRAC trial) reported SD as the best outcome to therapy in only 5 patients (13%) [25]. The remainder of patients all had PD with 6 patients dying prior to the first evaluation. The median PFS was 2.8 months and for those with SD this ranged from 5.6 to 11.2 months and OS ranged between 14.0 and 35.5 months. At best sunitinib demonstrated modest efficacy in this cohort. However, on further analysis, sunitinib serum levels might have been profoundly reduced by mitotane-induced cytochrome P450-3A4 activity, thus attenuating its anti-tumor activity and adverse effects, and accounting for the heterogeneous response [25].
An alternative is sorafenib, a protein kinase inhibitor with established anti-neoplastic properties and known activity against many protein kinases, including VEGFR, PDGFR, and RAF kinases. A phase II study of sorafenib, given with weekly paclitaxel chemotherapy in patients with advanced, treatment-refractory ACC, failed to show any evidence of anti-tumor activity despite its previously demonstrated in vitro activity [26]. Across these two trials, 48 patients received either sorafenib or sunitinib without an objective response being demonstrated [25, 26]. Therefore, TKIs have to date shown very limited evidence for use in advanced refractory ACC. One exception to this is cabozantinib, a small molecule inhibitor of the tyrosine kinase, c-Met, VEGFR2, and RET receptors, which is typically used to treat medullary thyroid cancer and as a second-line treatment for renal cell carcinoma (RCC). In a case series of 15 patients with advanced ACC refractory to a median of 4 (2–8) prior lines of therapy, single-agent treatment with cabozantinib as an off-label therapy resulted in an ORR of 20%, with 3 partial responses and a further 5 patients experiencing SD. A mean PFS of 3 months and OS of 9.5 months was demonstrated, with two cases of long-term disease stabilization [27]. Further studies are awaited.
Pre-clinical evidence has demonstrated the potential utility of nilotinib in the treatment of ACC. Nilotinib is a small molecule TKI targeting the ABL kinase but with broader activity against PDGFR and C-KiT that is commonly used to treat chronic myeloid leukemia. A 2018 study investigated the in vitro effects of different chemotherapy drugs, alone or in combination with mitotane, on the viability of the H295R cell line in monolayers or as spheroids [28]. The agents tested included everolimus, sunitinib, zoledronic acid, imatinib, and nilotinib. A combination of sunitinib and mitotane was individually the most effective treatment, resulting in only 24% of cells in the monolayer preparation remaining viable. However, this was not duplicated in the spheroid preparations. Overall, in both cell line preparations, nilotinib either alone or in combination with mitotane induced the most significant reduction in cell viability. Nilotinib induced significant ERK1/2 phosphorylation which was inhibited by a MEK inhibitor, with concomitant substantial reduction in H295R monolayer viability. A recommendation from this trial was for further investigation of nilotinib as a cytotoxic drug in combination with a MEK inhibitor as a potential novel combination therapy for ACC [28].
Epidermal Growth Factor Receptor Inhibitors
Approximately 80% of ACCs express epidermal growth factor receptor (EGFR), making it a potential binding site for targeted treatments. A small case series of 10 patients with heavily pre-treated advanced ACC investigated salvage chemotherapy with the small molecular EGFR inhibitor erlotinib in combination with gemcitabine. Only one in 10 patients experienced a minor response (PFS of 8 months) and 8 patients had PD at the first evaluation. The authors concluded that salvage chemotherapy with erlotinib and gemcitabine has insignificant activity in patients with advanced ACC and cannot be recommended without further study [29].
TKIs and Monoclonal Antibodies (MAB) Targeting the Vascular Endothelial Growth Factor Receptor
Vascular endothelial growth factor (VEGF) receptor is upregulated in ACC tumor tissue, which has led to further studies investigating molecules which target VEGF signaling in ACC. However, a study investigating bevacizumab (an anti-VEGF humanized monoclonal antibody) in combination with capecitabine in 10 patients with heavily pre-treated ACC showed no benefit and the regimen is not recommended as a salvage therapy for advanced ACC [30]. Additionally, a recent phase II trial of 13 patients with advanced ACC following first-line treatment with mitotane or EDP + M demonstrated very limited single-agent efficacy for the selective VEGF receptor (VEGFR) inhibitor axitinib. The median PFS was only 5.5 months and OS 13.7 months [31]. Unless additional pre-clinical data suggests a role for vascular targeting agents, this class does not appear to warrant further clinical investigation in ACC.
Peptide Receptor Radionuclide Therapy
Iodine-131-Metomidate
A case series studied 11 patients with refractory/advanced ACC exhibiting high uptake of [123I] iodine metomidate (IMTO) in their tumor lesions. Patients were treated with [131I] IMTO (1.6–20 GBq) in 1 to 3 cycles. Only one patient achieved a PR (with a 51% reduction in size of target lesions from baseline) and long-term SD was achieved in a further 5 patients, resulting in an ORR of 9%. Of the 6 patients who achieved either a PR or SD, the median PFS was 14 months [32]. It is likely that peptide receptor radionuclide therapy (PRRT) with [131I] IMTO represents a potential treatment option for selected patients with ACC. However, only 1 in 3 patients with ACC will be eligible for treatment based on their tumor 123I uptake, and ongoing prospective trial data is required.
Radiolabelled DOTATOC
Previous studies have demonstrated heterogeneous intensity and distribution of somatostatin receptor (SSTR) expression in the majority of ACC cells [33, 34]. Until recently, no trials have evaluated the therapeutic role of targeting SSTRs in advanced ACC patients. A recent prospective case series evaluated both the degree of SSTR expression demonstrated on immunohistochemistry (IHC) in ACC cells, as well as on the effect of targeted 90Y/177Lu-DOTATOC PRRT. Here, 19 heavily pre-treated patients were enrolled across two sites, of which all patients who underwent imaging (14/19) demonstrated highly FDG-PET-avid disease [35]. However, on 68Ga-DOTATOC PET/CT imaging, SSTR2A and SSTR5 receptor avidity was only demonstrated in 10 patients (54%). Additionally, the median avidity was overall weak and the pattern of 68Ga distribution among neoplastic lesions was often focal and heterogeneous. It is likely that intratumoral heterogeneity accounted for the irregular distribution of SSTR 2A and -5 within tumor lesions despite maintaining FDG uptake. In only 2 patients (11%) was the uptake of 68Ga sufficient enough to suggest benefit from PRRT: both subsequently received it, with an overall disease control lasting 4 and 12 months, respectively. Parallel IHC analysis of SSTR2A and SSTR5 on primary tumor tissue from the 19 patients demonstrated IHC expression in 43% and 57% of patients, respectively. Of note, SSTR2A tissue expression was scored 3 + in the two patients with strong and diffuse uptake on 68Ga-DOTATOC PET/CT [35]. In conclusion, ACC SSTR expression can be effectively detected on both IHC and 68Ga-DOTATOC PET, with SSTR-targeted PRRT representing a potential treatment option in a highly selected group of advanced ACC patients with high IHC SSTR expression and/or high 68Ga-DOTATOC PET/CT avidity. Given the favorable safety profile of 90Y/177Lu-DOTATOC PRRT and the durable disease stabilization demonstrated in selected patients, its utility likely warrants further investigation (Table 2).
Table 2.
Published clinical trials of targeted therapy and PRRT in ACC
| Drug | Mechanism | Study phase | Patients | Results | Reference |
|---|---|---|---|---|---|
| Figitumumab | IgG2 monoclonal antibody directed at IGF-1R | I | 14 |
ORR 0% SD 43% |
[20] |
| Cixutumumab | IgG1 monoclonal antibody directed at IGF-1R | I/II | 12 |
ORR 16.6% PFS 1.5 m |
[21] |
| Linsitinib | SMI of IGF-1R | III | 90 | No benefit | [22] |
| Temsirolimus + cixutumumab | mTOR Inhibitor | I | 18 | ∼ 50% achieved SD | [23] |
| Sunitinib | Small molecule multi-kinase inhibitor | II | 38 |
SD 13% PFS 2.8 m OS 14–35.5 m |
[24] |
| Sorafenib + paclitaxel | Protein kinase inhibitor | II | 10 | ORR 0% | [25] |
| Cabozantinib | Protein kinase inhibitor | Case series | 15 |
ORR 53% PFS 3.0 m OS 9.1 m |
[26] |
| Nilotinib | SM-TKI against PGFR and C-Kit | Pre-clinical/in vitro | 0 | N/A | [27] |
| Erlotinib + gemcitabine | EGFR inhibitor | Case series | 10 | ORR 10% | [28] |
| Bevacizumab + capecitabine | Humanized anti-VEGF monoclonal antibody | I | 10 |
ORR 0% SD 0% PD 100% |
[29] |
| Axitinib |
Selective VEGF-I inhibitor |
II | 13 |
PFS 5.5 m OS 13.7 m |
[30] |
| Iodine-131-metomidate | Peptide receptor radionucleotide therapy (PRRT) | Case series | 11 |
ORR 9% SD 45% PD 57% |
[31] |
| Radiolabelled 90Y/177Lu-DOTATOC | Peptide receptor radionucleotide therapy (PRRT) | Case series | 2 (selected from 14 based on SSTR2A/SSTR5 avidity) | ORR 100% of treated patients (14% of enrolled patients) | [34] |
PFS progression-free survival, OS overall survival, ORR objective response rate, OSD overall survival difference, PD progressive disease, SD stable disease, CR complete response, m months, MMRD mismatch repair deficiency, NPR non-progression rate
Immune Checkpoint Inhibitors
Currently available immune checkpoint inhibitors for the management of solid organ malignancies are those which target and inhibit the programmed cell death 1 (PD-1) receptor and its ligand PDL-1, and the cytotoxic T lymphocyte–associated protein 4 (CTLA-4) receptor. There are currently multiple ongoing trials investigating the efficacy of these drugs in ACC. To date, only one trial has reported mature data and the initial findings indicate that ACC is not a particularly responsive tumor to immune checkpoint inhibition and further investigation for potential drivers of immunotherapy failure in ACC are ongoing [36].
A recent study retrospectively analyzed PDL-1 mRNA expression in 146 clinical ACC samples and assessed for correlations between PDL expression and multiple clinical and pathological data points. PDL-1 mRNA expression was heterogeneous across the included samples and showed a small positive correlation with PDL-1 DNA copy number as seen in the TCGA data set. Tumor samples were categorized into either PDL-1 expression high or low, using a median expression level as a cut off. Tumors with high PDL-1 mRNA expression were associated with greater T cell response and longer 5-year DFS in both univariate and multivariate analyses compared with the low PDL-1 expressing group (76% vs 29%) [37]. Thus, improved responsiveness to checkpoint blockade immunotherapy likely requires the presence of CD8+ T cells within the local tumor microenvironment. Based on this data, reactivation of dormant tumor–infiltrating lymphocytes by PDL-1-inhibitors could represent a promising strategy in “PDL-1-high” ACCs, supporting the ongoing clinical trials. Additionally, the development of correlative studies or new trials investigating the relationship between treatment response and the degree of PD-1/PDL-1 expression, using a validated mRNA expression or protein/immunohistochemical method, are recommended.
However, recent molecular analyses have shown that the activation of specific oncogenic pathways in ACC cells can alter the local infiltration of CD8+ lymphocytes resulting in impaired local anti-tumor immune responses. Oncogenic pathways such as the Wnt/β-catenin signaling pathway and the acquisition of TP53 mutations in tumor cells are involved in the pathogenesis of ACC, but may also impair CD8+ infiltration. Both the regulation of β-catenin and TP53 mutation in ACC cells reduces production of different chemokines, leading to defective dendritic cell recruitment and reduced effector T cell infiltration [38]. These pathways may therefore result in immune evasion and impaired efficacy of immune checkpoint inhibitor therapies targeting the PD-1 receptor, despite high PDL-1 expression in ACC tumor cells. Therefore, caution should be used regarding treatment stratification based solely on the level of PDL-1 expression alone in ACC tumor cells. On these grounds, an effective strategy to overcome this may be the administration of immune checkpoint inhibitors in association with drugs targeting the WNT/β-catenin and p53 pathways. Ultimately, current biomarkers for determining which tumors will respond to checkpoint inhibitor immunotherapy are an evolving landscape and future advances in this field could allow for stratification of ACC tumors, with subsets of patients showing substantial anti-tumor benefits from selected checkpoint inhibitors..
The JAVELIN trial was an international, multicenter phase Ib expansion cohort trial, investigating the anti-PDL-1 monoclonal antibody avelumab in patients with different metastatic solid tumors including 50 patients with ACC, who had been heavily pre-treated with mitotane- or platinum-based chemotherapy [36]. To our knowledge, this is the largest prospective trial of a checkpoint inhibitor in advanced ACC. Patients received avelumab 10 mg/kg IV, every 2 weeks until PD or unacceptable toxicity. Only a modest clinical benefit was observed, with just 3 of 50 (6%) patients achieving a PR. However, 42% of patients achieved SD, with an overall median PFS of 2.6 months and OS of 10.6 months. Results demonstrated an acceptable safety profile in keeping with previous studies. Immunotherapy alone did not appear to be able to improve the current standard therapy in ACC [11, 36]. Additionally, the first 7 evaluable patients enrolled on a separate ongoing phase II study, assessing nivolumab for patients with metastatic ACC, has demonstrated similarly unexciting results. The median PFS of these patients was only 8 weeks and the best ORR so far has been PD in 5 patients with 2 patients pending evaluation [39] (Table 3).
Table 3.
Published clinical trials of immune checkpoint inhibitors and novel therapy in ACC
| Drug | Mechanism | Study phase | Patients | Outcomes | Reference |
|---|---|---|---|---|---|
| Avelumab | PDL-1 inhibitor | I | 50 |
ORR 6% PFS 2.6 m OS10.6 m |
[37] |
| Pembrolizumab | PDL-1 inhibitor | II | 16 |
NPR 36% SD 50% PR 14% |
[39] |
| Pembrolizumab | PDL-1 inhibitor | II | 39 |
ORR 23.1% PFS 2.1 m OS 24.9 m |
[40] |
| Pembrolizumab | PDL-1 inhibitor | Case report | 2 | Prolonged CR in patient with MSH2 mutation (MMRD) | [41] |
| Pembrolizumab | PDL-1 inhibitor | Case report | 1 | Sustained PR | [42] |
| Pembrolizumab/ipilimumab | PDL-1 inhibitor/CTLA-4 inhibitor | II | 18 |
ORR 8% PR 8% SD 36% |
[43] |
| Mebendazole | Anti-helminthic/microtubule inhibitor | Case report | 1 | PR with SD for 24 months | [45] |
| Zoledronic acid | Osteoclast inhibition | Case report | 1 | CR with SD for 7 years | [47] |
PFS progression-free survival, OS overall survival, ORR objective response rate, OSD overall survival difference, PD progressive disease, SD stable disease, CR complete response, m months, MMRD mismatch repair deficiency, NPR non-progression rate
Two more recent phase II single-center studies have been conducted, evaluating the efficacy and safety of the PD-1 inhibitor pembrolizumab, in patients with advanced ACC. One study included a total of 16 patients with advanced ACC, who had all progressed through at least one line of prior systemic therapy within the prior 6 months. In this trial, the primary endpoint was non-progression rate (NPR) at 27 weeks and interestingly ten patients (63%) had functional tumors (seven with a cortisol-producing ACC). Non-progression rate at 27 weeks was evaluable in 14 patients; 5 of 14 patients (36%) were alive and progression free at 27 weeks, meeting the pre-specified primary endpoint. Based on RECIST 1.1 criteria, two patients (14%) achieved a partial response (including one with cortisol-producing ACC), seven patients (50%) had stable disease (including three with cortisol-producing ACC), and five patients (36%) had progressive disease, representing an ORR of 64%. Of those who achieved stable disease, six had disease stabilization that lasted ≥ 4 months. Severe treatment-related adverse events (≥ grade 3) were seen in 2 of 16 patients (13%) and resulted in one patient discontinuing study participation. All studied tumor specimens (14/14) were negative for programmed cell death ligand-1 (PDL-1) expression and thirteen of 14 tumor specimens (93%) were microsatellite stable [40]. The second and most recent prospective phase II study enrolled 39 patients, treated with 200 mg pembrolizumab every 3 weeks, with a median follow-up of 17.8 months. An objective response occurred in 9 patients (23%) and disease control was seen in 16 patients (52%). Six patients had MSI-H/MMR-D tumors, of which two patients had a treatment response. The other seven patients with objective responses had microsatellite stable tumors. The median PFS was 2.1 months and the median OS was 24.9 months. Five patients (13%) had treatment-related grade 3 or 4 adverse events and again, tumor PDL-1 expression and MSI-H/MMR-D status were not associated with objective response [41].
In summary, single-agent pembrolizumab has modest efficacy as a salvage therapy in ACC regardless of the tumor’s hormonal function, microsatellite instability status, or PDL-1 expression. Treatment was well tolerated in most study participants in keeping with the established toxicity profile for immune checkpoint inhibitors. Further studies are ongoing and it is still too early to speculate on the ultimate role of immune check point inhibitors in the treatment of advanced ACC. However, case studies have demonstrated very positive outcomes in specific groups of patients, most notably those with Lynch syndrome and somatic mismatch repair (MMR)–deficient (MSI high) tumors [42]. A case study published in 2019 reviewed 2 patients with heavily pre-treated metastatic ACC treated with pembrolizumab. Next-generation sequencing detected high mutational burden (> 10 mutations/megabase) in both patients and one of them had an MSH2 mutation. The patient with the MSH2 mutation experienced a long-term CR, while the patient with high mutational burden and absence of MMR deficiency did not have any response [42]. This is the first evidence of a durable CR with immune checkpoint inhibitors in metastatic ACC. Another case study demonstrated a sustained PR in a 59-year-old female with metastatic ACC treated with pembrolizumab after having progressed through mitotane and subsequent EDP + M. Unfortunately, tumor genomic analysis was not performed so it remains unclear if MMR deficiency was present [43].
Most recently, at the 2019 European Society of Medical Oncology (ESMO) annual meeting, the initial results of a phase II study of combination nivolumab and ipilimumab in metastatic adrenal tumors were presented. This multicenter, single-arm study recruited patients with rare genitourinary tumors, of which a cohort of 18 patients had metastatic ACC and had received at least one prior systemic therapy. The primary endpoint was overall response rate by RECIST 1.1 criteria. After a median follow-up of 3.6 months (range 1.4–10.2), 7 patients (39%) remained on treatment and 11 patients (61%) had discontinued due to either PD (6 patients) or unacceptable toxicity (5 patients). The ORR was 8%, with only one patient achieving a PR. However, the disease control rate (DCR) was 44%, with a further 6 patients achieving SD (36%). Additionally, the toxicity profile appears higher than that seen with single-agent PD1 inhibitors, but remains similar to the established toxicity profile seen in metastatic RCC patients treated with combination ipilimumab and nivolumab [44].
In our view, outside of clinical trials, ACC patients should not be treated with checkpoint inhibitors. However, MMR-deficient patients and those with Lynch syndrome, who represent 3.2% of all ACCs, should be the subject of further checkpoint inhibitor therapy studies, especially those targeted against the PD-1 receptor (Table 3).
Novel Therapy
Mebendazole
Mebendazole is a synthetic broad-spectrum anti-helmintic, which selectively inhibits the synthesis of microtubules thereby blocking polymerization of tubulin dimers in intestinal cells of parasites. This disruption blocks uptake of glucose and other nutrients, resulting in the gradual immobilization and eventual death of worms. However, mebendazole also inhibits mammalian tubulin polymerization, thereby disrupting essential microtubule structures such as the mitotic spindle, which results in apoptosis and programmed cell death [45].
Evidence to support an anti-tumor effect of mebendazole in human malignancies includes a small study in 2007, which assessed its role specifically in ACC. In human ACC cells studied both in vitro and following implantation into nude mice, mebendazole appeared to inhibit ACC cell growth via induction of apoptosis in vitro, and metastasis formation in vivo [45]. In 2011, a case report demonstrated successful long-term tumor control in a 48-year-old man with metastatic ACC, whose disease had progressed despite multiple lines of systemic therapy, including mitotane, 5-fluorouracil, streptozotocin, and bevacizumab. All chemotherapeutic drugs were ceased, and he was prescribed mebendazole, 100 mg twice daily, as a single agent. An initial PR was seen, with a reduction in size of his metastases, which subsequently remained stable on continued mebendazole alone for 19 months. He had good quality of life without significant adverse effects, but disease subsequently progressed after 24 months of mebendazole monotherapy [46]. While promising, further testing is required to confirm these results before any recommendations can be made.
Zoledronic Acid
Zoledronic acid is a potent inhibitor of bone resorption. It inhibits osteoclast proliferation and induces apoptotic cell death. Its potency results from its high affinity for mineralized bone and especially for sites of high bone turnover. Zoledronic acid has also been shown to exhibit both in vitro and in vivo anti-tumor effects through direct tumor cell adhesion, induction of apoptosis and inhibition of angiogenesis, and immunomodulatory mechanisms [47].
A case study published in 2009 demonstrated successful long-term tumor control in a 48-year-old man with heavily pre-treated, refractory metastatic ACC. Following 3 lines of systemic therapy, rapid PD occurred in the lung, peritoneum, and liver, and zoledronic acid was initiated as a monthly 4 mg intravenous infusion. After 5 months, CT scans demonstrated a complete disappearance of lung metastases and necrosis of the liver metastases. A hepatectomy confirmed massive tumor necrosis with negative margins. The patient continued zoledronic acid for 3 years and continued with SD until June 2009, 6 years after the diagnosis of liver metastases and 3 years after ceasing zoledronic acid [48].
There is supporting evidence in the literature for the anti-neoplastic effect of bisphosphonates in multiple tumor types, such as breast, prostate, and cervical tumors [49, 50]. This example is encouraging, albeit unusual for the natural history of refractory ACC and further prospective evaluation of zoledronic acid is warranted prior to making recommendations for its use in advanced ACC.
Local Therapies
While not the focus of this review, a trans-arterial chemo-embolization (TACE) procedure offers the capacity to deliver high concentrations of chemotherapy to specific metastatic deposits predominantly in the liver. There is no established or gold standard cytotoxic drug used for TACE. Additionally, although a well-established treatment modality in many solid organ tumors, there is a paucity of evidence for its use in ACC. Most centers use either cisplatin, doxorubicin, and/or mitomycin C, often with lipiodol. The largest study to date investigated the use of TACE in 29 patients with metastatic ACC and progressive liver metastases. Three months following administration of TACE, 6 patients (21%) demonstrated a PR with reduction in the size of liver metastases. SD was seen in 18 patients (62%) and PD in 5 (17%). A per-lesion analysis (n = 103) showed a morphologic response in 23 lesions (22%), stabilization in 67 (65%), and progression in 13 (13%). Predictive markers associated with a favorable response were high lipiodol uptake prior to treatment and lesion size of < 3 cm. The median PFS was 9.0 months and OS 11.0 months, with no evidence to suggest that either of these parameters were improved with treatment [51]. Another more recent single-center retrospective review of 65 patients treated with systemic therapy for advanced ACC identified 23 patients with liver metastases, of which 6 patients received liver-directed therapies. Two patients received TACE, 3 patients received selective internal radiation therapy (SIRT), with y90-labeled microsphere, and 1 patient received microwave ablation.
These 6 cases had significantly longer median OS at 32 months, compared with 10 months in those patients with liver metastases who did not receive liver-directed therapies (p = 0.011). Although the results of this analysis are favorable and suggest a possible role for incorporating liver-directed therapies for patients with hepatic metastases, the sample number was small and bias in case selection may have contributed to perceived better outcomes [52]. Beyond this, only single case reports are available, which often describe extremely prolonged PFS or OS following TACE and SIRT [53, 54]. In summary, TACE represents a potentially effective treatment strategy for managing liver metastases from ACC. It is perhaps most strongly recommended for those patients with liver metastases involving only a single lobe, measuring < 3 cm and with high levels of lipiodol uptake, as they are most likely to benefit. However, as TACE is well tolerated, it can comfortably be used in most patients with ACC and liver metastases, as either monotherapy, or in combination if there is a strong desire for aggressive treatment in fit patients. Additionally, SIRT is promising in the management of liver metastases, but needs more data prior to drawing any conclusions about the role of SIR sphere–based therapy in the management of liver metastases from ACC [54].
Mitotane in Refractory Disease
Mitotane is the only approved drug for ACC and has been the foundation of ACC treatment for many years. It is often continued alongside multiple lines of subsequent systemic therapy following disease progression. Toxicity can include nausea and vomiting, adrenal insufficiency, hepatic transaminitis without chronic hepatitis or liver failure, and encephalopathy [55].
Despite this, the long-term use of mitotane is not without its downsides and therefore, its role should be subject to ongoing review. Precision with mitotane dosing is complicated by a very long and highly variable half-life (18 to 159 days, median 53 days). This can result in unpredictable effects, challenges in therapeutic drug monitoring, and even exclusion from phase 1 trials, even after mitotane has been ceased. Furthermore, mitotane metabolites are also problematic, with both their true anti-tumor effect and contribution to toxicity with mitotane being unknown. Mitotane metabolites, DDA (1,1-(o,p′-dichlorodiphenyl) acetic acid) and DDE (1,1-(o,p′-dichlorodiphenyl)-2,2 dichloroethene), have been demonstrated to be potent inducers of cytochrome P450-3A4, with potential to decrease the effectiveness of many other drugs given concurrently with mitotane [55]. As discussed previously, the SIRAC trial demonstrated this, where sunitinib, a TKI which is rapidly metabolized by cytochrome P450-3A4, when used concurrently with mitotane, resulted in an attenuated anti-tumor affect and heterogeneous response. Therefore, the ongoing use of mitotane needs vigilance and patient preference and tolerance taken into consideration. However, some reasons to consider ceasing mitotane include the following: severe or intractable mitotane-associated toxicities; disease progression after two lines of systemic therapy alongside mitotane; and disease progression after continuous mitotane for a period of over 12 months [8, 56].
Potential Future Targets
Wnt
The Wnt/β-catenin signaling pathway is important for the development of many organs, including the adrenal gland [57]. β-catenin mutations are seen in multiple cancers and when overexpressed are generally associated with more aggressive tumors [58]. Consequently, there is significant interest in developing drugs capable of inhibiting this pathway, such as inhibitors of the PORCN membrane protein (porcupine), required for secretion of Wnt ligands [59]. LGK974 and ETC-1922159, which target this protein, are currently in early phase clinical trials, although no results have been reported to date. Additionally, a chimeric antibody antagonist for extracellular Wnt ligands has performed well in an early phase I study and is currently being tested in combination with other drugs for non-adrenal cancers [60, 61].
PRI724 is another novel compound which blocks the interaction of β-catenin with transcriptional partners. Phase I studies have showed acceptable toxicity, and further studies are ongoing [62]. Phase II studies in ACC may be considered. CWP232291 suppresses Wnt signaling by promoting β-catenin degradation, and is currently in early phase I trials for leukemia [63]. However, as discussed earlier in this review, many benign adrenocortical tumors also harbor β-catenin mutations, rendering the specificity of this potentially drugable target unclear and its role in ACC remains to be determined [4, 5].
ACAT1
Acetyl-coenzyme A: cholesterol O-acetyltransferase 1 (ACAT1) is an enzyme typically located in the endoplasmic reticulum (ER), where it catalyzes the esterification of intracellular free cholesterol into cholesterol esters. ACAT1 inhibitors were initially developed as potential cholesterol-lowering agents for cardiovascular disease. However, animal studies demonstrated marked adrenal toxicity from the drug, similar to mitotane, and establishing a potential role in adrenal tumors [64]. The role of ACAT inhibitors in ACC awaits further studies.
SF1
SF1 is a transcription factor essential for adrenal development and amplification of this protein has been implicated in ACC [65]. Additionally, agonists of SF1 have been successful at reducing the proliferation of ACC cell lines in vitro. Therefore, compounds affecting SF1 transcription are currently in pre-clinical development, with clinical trials yet to be commenced [66].
Conclusion
Advanced ACC is a rare, but highly aggressive malignancy, with a heterogeneous, but predominantly very poor prognosis. Its management is complex, necessitating a multidisciplinary approach and is best managed in specialized centers familiar with ACC and therapeutic drug monitoring processes. Based on the FIRM-ACT trial, there is a clearly established first-line treatment approach for advanced ACC with mitotane either as monotherapy or in combination with EDP. Unfortunately, the majority of patients achieve only a short PFS following first-line therapy and there remains a clear need for improved second- and third-line treatment strategies, as well as biomarkers to identify tumors likely to recur and to identify the best second-line therapy in those that do recur. Although there is no one definitive initial second-line treatment strategy, there are options such as gemcitabine plus capecitabine, or streptozotocin given either with or without concurrent mitotane. G + C is becoming increasingly recommended in patients of good PS, due to the demonstrated clinical activity. In patients with poor PS still requesting treatment, if available, streptozotocin may be an option. Beyond this, there are some favorable approaches, which require further validation in larger clinical trials and these should be tailored to individual patient characteristics, utilizing a mixture of systemic therapies, local therapies, and enrolment in clinical trials where available. Table 4 summarizes clinical trials that are ongoing or recruiting, that are investigating second-line therapies in advanced ACC. Additionally, the role of predictive biomarkers and use of immune checkpoint inhibitors in specific individuals, such as those with Lynch syndrome, is evolving and likely to become increasingly utilized in clinical practice.
Table 4.
Summary of ongoing and recruiting clinical trials of therapies in ACC
| Drug | Mechanism/target | Study phase | Status | Intended sample size | Reference |
|---|---|---|---|---|---|
| Cabazitaxel | Tubulin depolymerization inhibitor | II | Recruiting | 25 | NCT03257891 |
| Cabozantinib | Protein kinase inhibitor | II | Recruiting | 37 | NCT03612232 |
| Gefitinib | EGFR inhibitor | II | Active, not recruiting | 16 | NCT00215202 |
| Interleukin-12/trastuzumab | Anti HER-2 | I | Recruiting | 15 | NCT00004074 |
| Nivolumab + ipilimumab | PDL-1/CTLA-4 | II | Recruiting | 707 | NCT02834013 |
| Nivolumab + ipilimumab | PDL-1/CTLA-4 | II | Recruiting | 57 | NCT033333616 |
| Ipilimumab + radiotherapy | CTLA-4 | I/II | Active, not recruiting | 143 | NCT02239900 |
| Pembrolizumab | PDL-1 | II | Active, not recruiting | 39 | NCT02673333 |
| Pembrolizumab | PDL-1 | II | Recruiting | 275 | NCT02721732 |
| Dovitinib | Multi-kinase inhibitor | II | Active, not recruiting | 30 | 2011–002873-47 |
| ARQ087 | FGFR inhibitor | I/II | Recruiting patients with solid tumors with FGFR genetic alterations | 121 | 2015-001443-36 |
| Lisitinib | SMI of IGF-1R | III | Active, not recruiting | 135 | 2009-012820-97 |
PFS progression-free survival, OS overall survival, ORR objective response rate, OSD overall survival difference, PD progressive disease, SD stable disease, CR complete response, m months, MMRD mismatch repair deficiency, NPR non-progression rate
Compliance with Ethical Standards
Ethical and Professional Principles
The accepted principles of compliance with ethical and professional standards have been followed in this review article.
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fassnacht M, Libé R, Kroiss M, Allolio B. Adrenocortical carcinoma: a clinician’s update. Nat Rev Endocrinol. 2011;7(6):323–335. doi: 10.1038/nrendo.2010.235. [DOI] [PubMed] [Google Scholar]
- 2.Terzolo M, Angeli A, Fassnacht M, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356(23):72–2380. doi: 10.1056/NEJMoa063360. [DOI] [PubMed] [Google Scholar]
- 3.Raymond VM, Everett JN, Furtado LV, Gustafson SL, Jungbluth CR, Gruber SB, Hammer GD, Stoffel EM, Greenson JK, Giordano TJ, Else T. Adrenocortical carcinoma is a Lynch syndrome-associated cancer. J Clin Oncol. 2013;31(24):3012–3018. doi: 10.1200/JCO.2012.48.0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giordano TJ, Thomas DG, Kuick R, Lizyness M, Misek DE, Smith AL, Sanders D, Aljundi RT, Gauger PG, Thompson NW, Taylor JM, Hanash SM. Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. Am J Pathol. 2003;162(2):521–531. doi: 10.1016/S0002-9440(10)63846-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tissier F, Cavard C, Groussin L, Perlemoine K, et al. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. J Cancer Res Ther. 2005;65(17):7622–7627. doi: 10.1158/0008-5472.CAN-05-0593. [DOI] [PubMed] [Google Scholar]
- 6.Assié G, Letouzé E, Fassnacht M, Jouinot A, Luscap W, et al. Integrated genomic characterization of adrenocortical carcinoma. J Nat Genet. 2014;46(6):607–612. doi: 10.1038/ng.2953. [DOI] [PubMed] [Google Scholar]
- 7.Zheng S, Cherniack AD, Dewal N et al. Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell 29(5):723–736. DOI: 10.1016/j.ccell.2016.04.002 [DOI] [PMC free article] [PubMed]
- 8.Megerle F, Kroiss M, Hahner S, Fassnacht M. Advanced adrenocortical carcinoma – what to do when first-line therapy fails? Exp Clin Endocrinol Diabetes. 2019;127(02/03):109–116. doi: 10.1055/a-0715-1946. [DOI] [PubMed] [Google Scholar]
- 9.Megerle F, Herrmann W, Fassnacht M, et al. German ACC study group, mitotane monotherapy in patients with advanced adrenocortical carcinoma. J Clin Endocrinol Metab. 2018;103(4):1686–1695. doi: 10.1210/jc.2017-02591. [DOI] [PubMed] [Google Scholar]
- 10.Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S, Schade-Brittinger C, Lacroix A, Jarzab B, Sorbye H, Torpy DJ, Stepan V, Schteingart DE, Arlt W, Kroiss M, Leboulleux S, Sperone P, Sundin A, Hermsen I, Hahner S, Willenberg HS, Tabarin A, Quinkler M, de la Fouchardière C, Schlumberger M, Mantero F, Weismann D, Beuschlein F, Gelderblom H, Wilmink H, Sender M, Edgerly M, Kenn W, Fojo T, Müller HH, Skogseid B, FIRM-ACT Study Group Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366(23):2189–2197. doi: 10.1056/NEJMoa1200966. [DOI] [PubMed] [Google Scholar]
- 11.Fassnacht M, Dekkers O, Else T, et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2018;179(4):1–46. doi: 10.1530/EJE-18-0608. [DOI] [PubMed] [Google Scholar]
- 12.Berruti A, Baudin E, Gelderblom H, Haak HR, et al. On behalf of the ESMO Guidelines Working Group, Adrenal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(7):131–138. doi: 10.1093/annonc/mds231. [DOI] [PubMed] [Google Scholar]
- 13.Sperone P, Ferrero A, Daffara F, Priola A, Zaggia B, Volante M, Santini D, Vincenzi B, Badalamenti G, Intrivici C, del Buono S, de Francia S, Kalomirakis E, Ratti R, Angeli A, Dogliotti L, Papotti M, Terzolo M, Berruti A. Gemcitabine plus metronomic 5-fluorouracil or capecitabine as a second−/third-line chemotherapy in advanced adrenocortical carcinoma: a multicenter phase II study. Endocr Relat Cancer. 2010;17:445–453. doi: 10.1677/ERC-09-0281. [DOI] [PubMed] [Google Scholar]
- 14.Henning JEK, Deutschbein T, Fassnacht M, et al. Gemcitabine-based chemotherapy in adrenocortical carcinoma: a multicenter study of efficacy and predictive factors. J Clin Endocrinol Metab. 2017;102(11):4323–4332. doi: 10.1210/jc.2017-01624. [DOI] [PubMed] [Google Scholar]
- 15.Chacón R, Tossen G, Loria FS, Chacón M. Response in a patient with metastatic adrenal cortical carcinoma with thalidomide. J Clin Oncol. 2005;23(7):1579–1580. doi: 10.1200/JCO.2005.03.195. [DOI] [PubMed] [Google Scholar]
- 16.Kroiss M, Deutschbein T, Schlötelburg W, Ronchi CL, Hescot S, Körbl D, Megerle F, Beuschlein F, Neu B, Quinkler M, Baudin E, Hahner S, Heidemeier A, Fassnacht M. Treatment of refractory adrenocortical carcinoma with thalidomide. Exp Clin Endocrinol Diabetes. 2019;127(9):578–584. doi: 10.1055/a-0747-5571. [DOI] [PubMed] [Google Scholar]
- 17.Creemers SG, Van Koetsveld PM, Van Den Dungen ES, et al. Inhibition of human adrenocortical cancer cell growth by temozolomide in vitro and the role of the MGMT gene. J Clin Endocrinol Metab. 2016;101(12):4574–4584. doi: 10.1210/jc.2016-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosentini D, Badalamenti G, Grisanti S, Basile V, Rapa I, Cerri S, Spallanzani A, Perotti P, Musso E, Laganà M, Ferrari VD, Luppi G, Dalla Volta A, Incorvaia L, Sigala S, Russo A, Volante M, Terzolo M, Berruti A. Activity and safety of temozolomide in advanced adrenocortical carcinoma patients. Eur J Endocrinol. 2019;181(6):681–689. doi: 10.1530/EJE-19-0570. [DOI] [PubMed] [Google Scholar]
- 19.Kroiss M, Deutschbein T, Schlotelburg W, et al. Salvage treatment of adrenocortical carcinoma with trofosfamide. J Horm Cancer. 2016;7(3):211–218. doi: 10.1007/s12672-016-0260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barlaskar FM, Spalding AC, Heaton JH, Kuick R, Kim AC, Thomas DG, Giordano TJ, Ben-Josef E, Hammer GD. Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. J Clin Endocrinol Metab. 2009;94(1):204–212. doi: 10.1007/s12672-016-0260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haluska P, Worden F, Olmos D, Yin D, Schteingart D, Batzel GN, Paccagnella ML, de Bono JS, Gualberto A, Hammer GD. Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer Chemother Pharmacol. 2010;65(4):765–773. doi: 10.1007/s00280-009-1083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lerario AM, Worden FP, Ramm CA, Hesseltine EA, Stadler WM, Else T, Shah MH, Agamah E, Rao K, Hammer GD. The combination of insulin-like growth factor receptor 1 (IGF1R) antibody cixutumumab and mitotane as a first-line therapy for patients with recurrent/metastatic adrenocortical carcinoma: a multi-institutional NCI-sponsored trial. J Horm Cancer. 2014;5(4):232–239. doi: 10.1007/s12672-014-0182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fassnacht M, Berruti A, Baudin E, Demeure MJ, Gilbert J, Haak H, Kroiss M, Quinn DI, Hesseltine E, Ronchi CL, Terzolo M, Choueiri TK, Poondru S, Fleege T, Rorig R, Chen J, Stephens AW, Worden F, Hammer GD. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol. 2015;16(4):426–435. doi: 10.1016/S1470-2045(15)70081-1. [DOI] [PubMed] [Google Scholar]
- 24.Naing A, Lorusso P, Fu S, Hong D, Chen HX, Doyle LA, Phan AT, Habra MA, Kurzrock R. Insulin growth factor receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with metastatic adrenocortical carcinoma. Br J Cancer. 2013;108(4):826–830. doi: 10.1016/S1470-2045(15)70081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroiss M, Quinkler M, Johanssen S, van Erp N, Lankheet N, Pöllinger A, Laubner K, Strasburger CJ, Hahner S, Müller HH, Allolio B, Fassnacht M. Sunitinib in refractory adrenocortical carcinoma: a phase II, single-arm, open-label trial. J Clin Endocrinol Metab. 2012;97(10):3495–3503. doi: 10.1210/jc.2012-1419. [DOI] [PubMed] [Google Scholar]
- 26.Berruti A, Sperone P, Ferrero A, Germano A, Ardito A, Priola AM, de Francia S, Volante M, Daffara F, Generali D, Leboulleux S, Perotti P, Baudin E, Papotti M, Terzolo M. Phase II study of weekly paclitaxel and sorafenib as second/third-line therapy in patients with adrenocortical carcinoma. Eur J Endocrinol. 2012;166(3):451–458. doi: 10.1530/EJE-11-0918. [DOI] [PubMed] [Google Scholar]
- 27.Kroiss M, Megerle F, Kurlbaum M et al (2020). Objective response and prolonged disease control of advanced adrenocortical carcinoma with cabozantinib. J Clin Endocrinol Metab dgz318. 10.1210/clinem/dgz318 [DOI] [PMC free article] [PubMed]
- 28.Silveira E, Cavalcante L, Kremer JL, et al. The tyrosine kinase inhibitor nilotinib is more efficient than mitotane in decreasing cell viability in spheroids prepared from adrenocortical carcinoma cells. Cancer Cell Int. 2018;18(1):29. doi: 10.1186/s12935-018-0527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinkler M, Hahner S, Wortmann S, Johanssen S, Adam P, Ritter C, Strasburger C, Allolio B, Fassnacht M. Treatment of advanced adrenocortical carcinoma with erlotinib plus gemcitabine. J Clin Endocrinol Metab. 2008;93(6):2057–2062. doi: 10.1210/jc.2007-2564. [DOI] [PubMed] [Google Scholar]
- 30.Wortmann S, Quinkler M, Ritter C, Kroiss M, Johanssen S, Hahner S, Allolio B, Fassnacht M. Bevacizumab plus capecitabine as a salvage therapy in advanced adrenocortical carcinoma. Eur J Endocrinol. 2010;162(2):349–356. doi: 10.1530/EJE-09-0804. [DOI] [PubMed] [Google Scholar]
- 31.O’Sullivan C, Edgerly M, Velarde M, Wilkerson J, Venkatesan AM, Pittaluga S, Yang SX, Nguyen D, Balasubramaniam S, Fojo T. The VEGF inhibitor axitinib has limited effectiveness as a therapy for adrenocortical cancer. J Clin Endocrinol Metab. 2014;99(4):1291–1297. doi: 10.1210/jc.2013-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hahner S, Kreissl MC, Fassnacht M, Haenscheid H, Knoedler P, Lang K, Buck AK, Reiners C, Allolio B, Schirbel A. [131I] iodometomidate for targeted radionuclide therapy of advanced adrenocortical carcinoma. J Clin Endocrinol Metab. 2012;97(3):914–922. doi: 10.1210/jc.2011-2765. [DOI] [PubMed] [Google Scholar]
- 33.Mariniello B, Finco I, Sartorato P, Patalano A, Iacobone M, Guzzardo V, Fassina A, Mantero F. Somatostatin receptor expression in adrenocortical tumors and effect of a new somatostatin analogue SOM230 on hormone secretion in vitro and in ex vivo adrenal cells. J Endocrinol Investig. 2011;34(6):131–138. doi: 10.1007/BF03346721. [DOI] [PubMed] [Google Scholar]
- 34.Germano A, Rapa I, Duregon E, Votta A, Giorcelli J, Buttigliero C, Scagliotti GV, Volante M, Terzolo M, Papotti M. Tissue expression and pharmacological in vitro analyses of mTOR and SSTR pathways in adrenocortical carcinoma. Endocr Pathol. 2017;28(2):95–102. doi: 10.1007/s12022-017-9473-8. [DOI] [PubMed] [Google Scholar]
- 35.Grisanti S, Filice A, Basile V, et al. Treatment with 90Y/177Lu DOTATOC in patients with metastatic adrenocortical carcinoma expressing somatostatin receptors. J Clin Endocrinol Metab. 2020;105(3):91. doi: 10.1210/clinem/dgz091. [DOI] [PubMed] [Google Scholar]
- 36.Le Tourneau C, Hoimes C, Zarwan C, et al. Avelumab in patients with previously treated metastatic adrenocortical carcinoma: phase 1b results from the JAVELIN solid tumor trial. J Immunother Cancer. 2018;6(1):111–119. doi: 10.1186/s40425-018-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Billon E, Finetti P, Bertucci A, Niccoli P, Birnbaum D, Mamessier E, Bertucci F. PDL1 expression is associated with longer postoperative, survival in adrenocortical carcinoma. J OncoImmunol. 2019;8(11):e1655362. doi: 10.1080/2162402X.2019.1655362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiorentini C, Grisanti S, Cosentini D et al (2019). Molecular drivers of potential immunotherapy failure in adrenocortical carcinoma. J Oncol Special issue 2019(7) 10.1155/2019/6072863 [DOI] [PMC free article] [PubMed]
- 39.Cavalcante L, Carneiro B, Costa R, et al. Preliminary results from a phase II study of nivolumab for patients with metastatic adrenocortical carcinoma (ACC) J Clin Oncol. 2017;35(7):96–96. doi: 10.1200/JCO.2017.35.7. [DOI] [Google Scholar]
- 40.Habra M, Stephen B, Campbell M, et al. Phase II clinical trial of pembrolizumab efficacy and safety in advanced adrenocortical carcinoma. J immunother Cancer. 2019;7(253):1–9. doi: 10.1186/s40425-019-0722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raj N, Zheng Y, Kelly V, Katz SS, Chou J, Do RKG, Capanu M, Zamarin D, Saltz LB, Ariyan CE, Untch BR, O’Reilly EM, Gopalan A, Berger MF, Olino K, Segal NH, Reidy-Lagunes DL. PD-1 blockade in advanced adrenocortical carcinoma. J Clin Oncol. 2020;38(1):71–80. doi: 10.1200/JCO.19.01586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mota J, Sousa L, Braghiroli M, Siqueira LT, Neto JEB, Chapchap P, Hoff AAO, Hoff PM. Pembrolizumab for metastatic adrenocortical carcinoma with high mutational burden: two case reports. Medicine (Baltimore) 2018;97(52):e13517. doi: 10.1097/MD.0000000000013517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Addington L, Thomas H, Hawk NC, et al. Impressive response to immunotherapy in a patient with metastatic adrenocortical carcinoma. Clin Oncol Case Rep. 2019;2(1):110–112. [Google Scholar]
- 44.Campbell M, Xie W, Shah A, et al. Initial results of a phase II study of nivolumab and ipilimumab in metastatic adrenal tumors. Ann Oncol. 2019;30(5):356–402. doi: 10.1093/annonc/mdz249. [DOI] [Google Scholar]
- 45.Martarelli D, Pompei P, Baldi C, Mazzoni G. Mebendazole inhibits growth of human adrenocortical carcinoma cell lines implanted in nude mice. Cancer Chemother Pharmacol. 2008;61(5):809–817. doi: 10.1007/s00280-007-0538-0. [DOI] [PubMed] [Google Scholar]
- 46.Dobrosotskaya I, Hammer G, Schteingart D, Maturen KE, Worden FP. Mebendazole monotherapy and long-term disease control in metastatic adrenocortical carcinoma. Endocr Pract. 2011;17(3):e59–e62. doi: 10.4158/EP10390.CR. [DOI] [PubMed] [Google Scholar]
- 47.Black DM, Kelly M, Gennant H, et al. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med. 2010;362(19):1761–1771. doi: 10.1056/NEJMoa1001086. [DOI] [PubMed] [Google Scholar]
- 48.Boudou-Rouquette P, Alexandre J, Soubrane O, Bertagna X, Goldwasser F. Antitumoral effect of the bisphosphonate zoledronic acid against visceral metastases in an adrenocortical cancer patient. Ann Oncol. 2009;20(10):1747. doi: 10.1093/annonc/mdp378. [DOI] [PubMed] [Google Scholar]
- 49.Clezardin P, Ebetino F, Fournier P. Bisphosphonates and cancer-induced bone disease: beyond their antiresorptive activity. Cancer Res. 2005;65(12):4971–4974. doi: 10.1158/0008-5472.CAN-05-0264. [DOI] [PubMed] [Google Scholar]
- 50.Diel I, Solomayer E, Costa S, Gollan C, Goerner R, Wallwiener D, Kaufmann M, Bastert G. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998;339(6):357–363. doi: 10.1056/NEJM199808063390601. [DOI] [PubMed] [Google Scholar]
- 51.Cazejust J, De Baere T, Auperin A, et al. Transcatheter arterial chemoembolization for liver metastases in patients with adrenocortical carcinoma. J Vasc Interv Radiol. 2010;21(10):1527–1532. doi: 10.1016/j.jvir.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 52.Owen D, Patel S, Wei L, Phay JE, Shirley LA, Kirschner LS, Schmidt C, Abdel-Misih S, Brock P, Shah MH, Konda B. Metastatic adrenocortical carcinoma: a single institutional experience. J Horm Cancer. 2019;10(4–6):161–167. doi: 10.1007/s12672-019-00367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong E, Jacques S, Bennett M, et al. Complete response in a patient with stage IV adrenocortical carcinoma treated with adjuvant trans-catheter arterial chemo-embolization (TACE) Asia Pac J Clin Oncol. 2018;14(3):279–281. doi: 10.1111/ajco.12759. [DOI] [PubMed] [Google Scholar]
- 54.Makary M, Krishner L, Wuthrick E, Bloomston MP, Dowell JD. Yttrium-90 microsphere selective internal radiation therapy for liver metastases following systemic chemotherapy and surgical resection for metastatic adrenocortical carcinoma. World J Clin Oncol. 2018;9(1):20–25. doi: 10.5306/wjco.v9.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bethesda (MD): LiverTox: clinical and research information on drug-induced liver injury [Internet]. National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Mitotane. [Updated 2016 Jun 15] [PubMed]
- 56.Kroiss M, Quinkler M, Lutz W, Allolio B, Fassnacht M. Drug interactions with mitotane by induction of CYP3A4 metabolism in the clinical management of adrenocortical carcinoma. Clin Endocrinol. 2011;75(5):585–591. doi: 10.1111/j.1365-2265.2011.04214.x. [DOI] [PubMed] [Google Scholar]
- 57.Tissier F, Cavard C, Groussin L, Perlemoine K, Fumey G, Hagneré AM, René-Corail F, Jullian E, Gicquel C, Bertagna X, Vacher-Lavenu MC, Perret C, Bertherat J. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005;65(17):7622–7627. doi: 10.1158/0008-5472.CAN-05-0593. [DOI] [PubMed] [Google Scholar]
- 58.Gaujoux S, Grabar S, Fassnacht M, Ragazzon B, Launay P, Libé R, Chokri I, Audebourg A, Royer B, Sbiera S, Vacher-Lavenu MC, Dousset B, Bertagna X, Allolio B, Bertherat J, Tissier F. Beta-catenin activation is associated with specific clinical and pathologic characteristics and a poor outcome in adrenocortical carcinoma. Clin Cancer Res. 2011;17(2):328–336. doi: 10.1158/1078-0432.CCR-10-2006. [DOI] [PubMed] [Google Scholar]
- 59.Madan B, Ke Z, Harmston N, Ho SY, Frois AO, Alam J, Jeyaraj DA, Pendharkar V, Ghosh K, Virshup IH, Manoharan V, Ong EH, Sangthongpitag K, Hill J, Petretto E, Keller TH, Lee MA, Matter A, Virshup DM. Wnt addiction of genetically defined cancers reversed by PORCN inhibition. Oncogene. 2016;35(17):2197–2207. doi: 10.1038/onc.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Le P, McDermott J, Jimeno A. Targeting the Wnt pathway in human cancers: therapeutic targeting with a focus on OMP-54F28. Pharmacol Ther. 2015;146:1–11. doi: 10.1016/j.pharmthera.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jimeno A, Gordon M, Chugh R, et al. A first-in-human phase 1 study of anticancer stem cell agent OMP-54F28 (FZD8-Fc), decoy receptor for WNT ligands, in patients with advanced solid tumors. J Clin Oncol. 2017;23(24):7490–7497. doi: 10.1158/1078-0432.CCR-17-2157. [DOI] [PubMed] [Google Scholar]
- 62.Lenz H, Kahn M. Safely targeting cancer stem cells via selective catenin coactivator antagonism. Cancer Sci. 2014;105(9):1087–1092. doi: 10.1111/cas.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cortes J, Faderl S, Pagel J, et al. Phase 1 study of CWP232291 in relapsed/refractory acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) J Clin Oncol. 2019;38(1):342. doi: 10.1200/jco.2015.33.15_suppl.7044. [DOI] [Google Scholar]
- 64.Floettmann J, Buckett L, Turnbull A, Smith T, Hallberg C, Birch A, Lees D, Jones HB. ACAT-selective and nonselective DGAT1 inhibition: adrenocortical effects--a cross-species comparison . Toxicol Pathol. 2013;41(7):941–950. doi: 10.1177/0192623313477753. [DOI] [PubMed] [Google Scholar]
- 65.Lalli E. Adrenocortical development and cancer: focus on SF-1. J Mol Endocrinol. 2010;44(6):301–307. doi: 10.1677/JME-09-0143. [DOI] [PubMed] [Google Scholar]
- 66.Doghman M, Cazareth J, Douguet D, Madoux F, Hodder P, Lalli E. Inhibition of adrenocortical carcinoma cells proliferation by SF-1 inverse agonists. J Clin Endocrinol Metab. 2009;94(6):2178–2183. doi: 10.1210/jc.2008-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]