Abstract
Many patients with multiple myeloma (MM) have comorbidities and are treated with PPAR agonists. Immunomodulatory agents (IMiDs) are the cornerstones for MM therapy. Currently, little is known about how co-administration of PPAR agonists impacts lenalidomide treatment in patients with MM. Here, we determined the effects of PPAR agonists on anti-myeloma activities of lenalidomide in vitro and in a myeloma xenograft mouse model. Genetic overexpression and CRISPR/cas9 knockout experiments were performed to determine the role of CRBN in the PPAR-mediated pathway. A retrospective cohort study was performed to determine the correlation of PPAR expression with the outcomes of patients with MM. PPAR agonists down-regulated CRBN expression and reduced the anti-myeloma efficacy of lenalidomide in vitro and in vivo. Co-treatment with PPAR antagonists increased CRBN expression and improved sensitivity to lenalidomide. PPAR expression was higher in bone marrow cells of patients with newly diagnosed MM than in normal control bone marrow samples. High PPAR expression was correlated with poor clinical outcomes. Our study provides the first evidence that PPARs transcriptionally regulate CRBN and that drug-drug interactions between PPAR agonists and IMiDs may impact myeloma treatment outcomes.
Keywords: gene regulation, drug-drug interaction, immunomodulatory agent, survival, CRBN
1. Introduction
Multiple myeloma (MM) is a plasma cell malignancy, with approximately 5–7 new cases per 100,000 individuals, that is the second most common hematological malignancy in the United States [1, 2]. Despite improvements in treatment and overall outcomes of patients with MM over the last few decades [3, 4], MM remains incurable, and nearly all patients continuously undergo cycles of treatment, response, and relapse. Almost all patients with MM require life-long treatment, including risk-adapted combination regimens and single-agent maintenance therapy.
Immunomodulatory agents (IMiDs), thalidomide, lenalidomide, and pomalidomide, have been approved for the treatment of patients with newly diagnosed and relapsed MM [5, 6]. Lenalidomide is the most commonly used IMiD, and nearly all patients with MM receive it during the course of their treatment, especially during post-autologous stem cell transplant maintenance therapy. IMiDs bind to a shallow hydrophobic pocket of cereblon (CRBN), leading to the recruitment and targeted ubiquitination and degradation of IKAROS family zinc finger (IKZF)-1 and IKZF3 [7, 8]. CRBN is critical for the anti-myeloma activity of IMiDs, and the expression level of CRBN is one of the major determining factors of drug sensitivity or resistance to IMiDs [9, 10]. High expression of CRBN is associated with improved clinical response and better survival, while low CRBN expression correlates with poor disease outcome in patients treated with IMiDs [11].
In the United States, the incidence of MM has increased over the years, primarily observed in the elderly population with a median age of 69 years at diagnosis. These patients often have coexisting medical problems, including diabetes and/or dyslipidemia. Approximately 26% of patients with myeloma have diabetes and 29.3% have dyslipidemia [12, 13]. Peroxisome proliferator-activated receptor (PPAR) agonists are the U.S. Food and Drug Administration (FDA)-approved drugs for the treatment of diabetes and dyslipidemia [14, 15]. PPARs are nuclear receptor proteins with three main isoforms: PPARα, PPARβ/δ, and PPARγ. PPARs heterodimerize with the retinoid X receptor and function as transcription factors that control the expression of genes involved in lipid and carbohydrate metabolism. PPARα and PPARγ are the molecular targets of several FDA-approved drugs. PPARα agonists are fibrate drugs (fenofibrate, clofibrate, gemfibrozil, ciprofibrate, and bezafibrate) that are used for the treatment of hypercholesterolemia and dyslipidemia [16]. PPARγ agonists constitute a class of thiazolidinediones (pioglitazone, rosiglitazone, and troglitazone) that are used for the treatment of patients with diabetes and insulin resistance [17].
Kim et al. reported that fenofibrate reduced CRBN transcription and attenuated CRBN expression in a mouse model of alcoholic liver disease [18, 19]. Since MM predominantly affects older adults, many patients with MM are expected to have diabetes and/or dyslipidemia and are treated with PPAR agonists. We hypothesized that PPAR agonists would inhibit the transcription and expression of CRBN in myeloma cells, similar to that in hepatocytes. Furthermore, we postulated that owing to the reduced expression of CRBN, PPAR agonists used for the treatment of diabetes and/or dyslipidemia might attenuate the anti-myeloma activity of IMiDs. In the current study, we determined the effects and underlying mechanisms of PPAR agonists on the anti-myeloma activities of lenalidomide in vitro and in vivo in a myeloma xenograft mouse model. Additionally, PPAR expression in the bone marrow (BM) samples of newly diagnosed patients with MM was measured, and its correlation with the clinical outcomes of patients was examined. Our study is the first to investigate the drug-drug interactions between IMiDs and PPAR agonists in patients with MM. Our findings will aid in the effective treatment of patients with MM who also suffer from dyslipidemia and/or diabetes.
2. Materials and methods
2.1. Ethics approval
Studies involving human patient samples were performed in accordance with the ethical standards of the Duke University Institutional Review Board Committee on Human Experimentation. Animal model studies were performed in accordance with Duke University Institutional Animal Care and Use Committee approved- procedures.
2.2. Patient population and retrospective studies
A retrospective study was conducted. PPAR expression was measured via immunohistochemical (IHC) staining of the BM biopsy samples of patients newly diagnosed with MM and PPAR expression levels were correlated with clinical outcomes. One hundred and ninety-five patients with newly diagnosed MM, who were seen at the Duke University Medical Center between 2005 and 2015, were included in the study. Patient characteristics are summarized in Table S1.
The treatment response was characterized using the International Myeloma Working Group (IMWG) treatment response criteria and classified as complete remission, very good partial response, partial response, stable disease, or progressive disease [20]. The International Staging System (ISS) stage and cytogenetic risk were defined using IMWG criteria [21]. PFS was defined as the duration from treatment initiation to the first progression or death, whichever occurred earlier. OS was defined as the duration from the date of diagnosis of MM to the date of death or date of the last follow-up at which the patient was known to be alive, with those alive censored at the date of last contact.
2.3. PPAR IHC staining
Archived BM paraffin blocks obtained at the time of diagnosis were cut into 5 μm sections and fixed on slides. Microwave antigen retrieval was performed in the presence of 1 mM ethylenediaminetetraacetic acid (EDTA) buffer (pH 8.0). Slides were then incubated with anti-CD138 (1:20 dilution, Cat# ms-1793-3; Thermo Fisher, Waltham, MA), PPARα (1:400 dilution, Cat# SC-398394; Santa Cruz, Dallas, TX), PPARβ/δ (1:400 dilution, NBP2-22468; Novus, St. Louis, MO), or PPARγ (1:100 dilution, NBP2-22106; Novus) antibodies for 30 min at room temperature, followed by incubation with horseradish peroxidase (HRP)-labeled secondary antibody (Cat# K4001; DAKO, Santa Clara, CA). Thyroid tissue was used as the positive control for PPAR staining and for the optimization of antibody dilution and staining conditions. For comparison, 11 normal control BM samples and 19 BM biopsy samples from patients with MM, who were treated and in remission, were included as controls. Normal control BM samples were obtained from patients who underwent BM biopsy for lymphoma staging or other evaluations and were found to have a healthy BM.
PPAR expression levels were quantified by incorporating the percentage of cells expressing PPAR and the intensity of PPAR expression, as described previously [8]. The percentage of PPAR-positive cells was calculated by dividing the CD138+ myeloma or CD138− non-myeloma cells with positive PPAR staining by total CD138+ myeloma or CD138− non-myeloma cells, respectively, and were assigned a score ranging from 0–4 (score 0: no PPAR-positive cells; score 1: 1–25% PPAR-positive cells; score 2: 26–50% PPAR-positive cells; score 3: 51–75% PPAR-positive cells; and score 4: 76–100% PPAR-positive cells). Expression intensity was graded as follows: 0 = absent staining, 1 = weakly positive, or 2 = strongly positive. The IHC score for the expression level of PPAR was represented as the sum of the score for the percentage of expression and the score for the intensity of expression and grouped as follows: low = IHC score of 0–1, medium = IHC score of 2–4, or high = IHC score of 5–6.
2.4. Cell lines
The following MM cell lines were used in this study: MM1R, MM1S, RPMI8226, NCIH929, OPM1, OPM2, INA6, L363, JJN3, and U266. Short tandem repeat profiling was performed for cell line authentication. All cell lines were cultured at 37 °C under 5% CO2 in Roswell Park Memorial Institute 1640 medium supplemented with 2 mM GlutaMAX and 10% fetal calf serum (Mediatech). MM cell lines stably co-expressing enhanced green fluorescent protein (eGFP) and luciferase were generated by transducing MM cell lines with lentiviruses encoding eGFP and luciferase as previously reported [22]. eGFP+ MM cells were sorted by flow cytometry, expanded, and used for in vivo study.
2.5. Microarray analysis
The MM1R and MM1S cells were treated with dimethyl sulfoxide (DMSO), SGI-1776 (SGI), or CX-6258 (CX) for 12 h. Total RNA was extracted from individual cell samples using the RNeasy plus mini kit (Qiagen, Valencia, CA, USA). RNA samples were checked for quality using gel electrophoresis, and their concentrations were measured using spectrophotometry. RNA quality (RIN>7) was further checked using a bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Microarray experiments were performed using an Agilent SurePrint G3 Human Gene Expression V3 8×60k microarray kit (Cat# G4851C; Agilent) and an Agilent LowInput QuickAmp labeling kit (Cat# 51902305; Agilent) in the Genomics Core of the University of North Carolina at Chapel Hill. Data preprocessing was carried out via the Duke Center for Genomic and Computational Biology (Duke GCB) for quality filtering and data normalization, as previously described [23]. The retrieved matrix data were collapsed onto the gene symbol as the gene expression value [24]. Differentially expressed genes were analyzed using Partek® Genomics Suite® software Genomics (https://www.partek.com/partek-genomics-suite/) and R software (R x64 3.5.1) (https//www.R-project.org/).
2.6. Lentivirus production and gene transduction
The production and gene transduction of lentiviruses encoding CRBN, PPARα, PPARβ/δ, PPARγ, and control vectors were performed, as previously described [25-27]. PPARα-, PPARβ/δ-, and PPARγ-overexpressing plasmids were purchased from Dharmacon, Inc., CRBN-overexpressing plasmid was obtained from Addgene. Lentiviruses were produced after transient transfection of HEK 293T cells with an individual lentiviral vector along with packaging plasmids (VSV-G and psPax2), according to the manufacturer’s instructions. Supernatants containing viral particles were collected 48 h after transfection. Cells were transduced with lentiviruses by co-centrifugation at 3,000 × g for 3 h at 37 °C in the presence of 8 μg/mL of polybrene.
2.7. Clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) genome editing
To generate CRBN-knockout cells using the CRISPR/Cas9 technique, single guide RNAs (gRNAs) targeting the human CRBN gene were designed by Integrated DNA Technologies. The gRNA sequences used were 5′-CAGGACGCTGCGCACAACAT-3′ and 5′-CGCACCATACTGACTTCTTG-3′. Cells were transfected with a ribonucleoprotein (RNP) complex using RNAiMAX kit (Cat# 13778150; Thermo Fisher Scientific), according to the manufacturer’s instructions. The gRNA fragment was amplified via polymerase chain reaction (PCR) prior to use for transfection, as described previously [28]. To verify the successful knockout of CRBN, two pairs of primers were used to amplify the genomic DNA and detect mutations at 300 nM each, respectively; pair 1, forward: 5′-TCCTTTGCGGGTAAACAGAC-3′ and reverse: 5′-GGTTGGAATCCTGACTCTGC-3′; pair 2, forward: 5′-TGGCACAATCTCAGCTCACT-3′ and reverse: 5′-ACCACTGCAATTACCCATGA-3′. T7E1 digestion was used to detect the mismatch, and the products were examined via electrophoresis at 100 V for 1.5–2 h in 3% (w/v) agarose gels in 1X TAE buffer to visualize the mismatch results [29].
2.8. CRBN firefly luciferase reporter system
The CRBN firefly luciferase reporter plasmid was constructed by subcloning the human CRBN promoter into a pGL3-basic vector containing the firefly luciferase gene (Promega, GenBank accession number U47295). CRBN promoter sequences, 2000 bp upstream of CRBN, were extracted from the NCBI database, and potential transcription binding sites for PPARs were identified using the JASPAR database (http://jaspar.genereg.net/) [30]. The PPARα binding site was identified as: 5′-TTGAGCTCTTCCCTACTC-3′, PPARβ/δ binding site: 5′-GCGATCTTCAACCTCA-3′, and PPARγ binding site: 5′-TCCCCTGTCACCTTC-3′. The human CRBN promoter was PCR-amplified with primers as follows: for CRBN promoter with PPARα binding site, forward: 5′-AGATAAGGGGCTGAGCTTCC-3′ and reverse: 5′-ATGTTTGACTCATTTGGTTGAAGA-3′; for CRBN promoter with a PPARγ binding site, forward: 5′-CCAACTTAAAGGCGAACCAC-3′ and reverse: 5′-GGAACTCTTGATGTAGCTTTAATGG-3′; and for CRBN promoter with PPARβ/δ binding site, forward: 5′-AACTATAAATAAGCCAAGGTTTTTCTC-3′ and reverse: 5′-TCTTTTGGCCTCATTATTCAAA-3′. The pGL3-basic firefly luciferase reporter vector was used as the negative control.
To generate a PPAR-binding site-mutated/deleted CRBN promoter pGL3 luciferase reporter system, Q5 site-directed mutagenesis was performed using the New England Biolabs (NEB) kit (NEB, E0554), according to the manufacturer’s instructions. Primers were designed using the NEBaseChanger online tool and are listed below.
Mutated PPARα: forward: 5′-CTAGCTAGCTAGGCTAGCACGACAAAACTTACCCCATAAC-3′ and reverse: 5′-CCCAAGCTTGGGAAGCTTAGACAGCTTGTGGTGCTT-3′.
Mutated PPARβ/δ: forward: 5′-CTAGCTAGCTAGGCTAGCAATCCAATTTTGTGACATCATC-3′ and reverse: 5′-CCCAAGCTTGGGAAGCTTAAAAGAGGAGAAAAACCTTG-3′.
Mutated PPARγ: forward: 5′-CTAGCTAGCTAGGCTAGCAAAACCTCTAAAATGTTCTTG-3′ and reverse: 5′-CCCAAGCTTGGG AAGCTTGAGAATAACAATATATTAGTGGTTC-3′.
2.9. Reagents and antibodies
PPARα antibody was obtained from Santa Cruz Biotechnology (Cat# SC-398394). PPARβ/δ (NBP2-22468) and PPARγ (NBP2-22106) antibodies were obtained from Novus Biologicals, LLC (Littleton, CO). IKZF1 antibody was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). IKZF3 antibody was purchased from Novus. CRBN antibody was obtained from Sigma-Aldrich (St. Louis, MO).
2.10. Reverse transcription (RT)-PCR
Total RNA was isolated using the TRIzol reagent (Invitrogen). First-strand cDNA was synthesized using an iScript cDNA synthesis kit (Bio-Rad). RT-PCR was performed using Bio-Rad iQ5. The primers used for each gene were as follows: IKZF1: 5′-CCCCTGTAAGCGATACTC CA-3′ and 5′-CCACGACTCTGTCACTCTTGG-3′; IKZF3: 5′-ATCAACAAGGAAGGG GAGGT-3′ and 5′-CAGGGCTCTGTGTTCTCCTC-3′; β-actin: 5′-ACCTTCTACAATGAGCTG-3′ and 5′-CCTGGATAGCAACGTACAGG-3′; and CRBN: 5′-TCCAGCAAGCTAAAGTGCAA-3′ and 5′-AGCGAGGCCATGAAGTTAGA-3′.
2.11. Cell proliferation and apoptosis assays
For the thiazolyl blue tetrazolium bromide (MTT) cell proliferation assay, myeloma cells were plated in triplicates in a 96-well plate at a final volume of 100 μL that contained 5 × 104 cells/well and various concentrations of lenalidomide and PPAR agonists. The cells were cultured at 37 °C in a 5% CO2 incubator for various durations. Then, 20 μL of the combined MTS/PMS solution (5 mg/mL MTT) was added to each well of the 96-well assay plate, the plate was incubated for 3–4 h at 37 °C in a 5% CO2 incubator and the absorbance at 490 nm was measured using an enzyme-linked immunosorbent assay plate reader (VERSAmax, Molecular Devices, San Jose, CA).
2.12. Western blot analysis
MM cells were harvested, washed with phosphate-buffered saline (PBS) and resuspended in a lysis buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS). Cells were lysed via brief sonication. The lysates were centrifuged at high speed for 15 min to remove the cell debris. Total protein was quantified using a Dc protein estimation kit (Bio-Rad) with bovine serum albumin (BSA) as a standard. Approximately 20 μg of protein was loaded and subjected to SDS-polyacrylamide gel electrophoresis. The proteins were transferred onto a nitrocellulose membrane. The membrane was blocked with 5% BSA in tris-buffered saline containing 0.05% Tween 20 (TBST) and primary antibodies against PPARα (ab227074; Abcam), PPARβ/δ (A5656; ABclonal), PPARγ (16643-1-AP; Thermo Fisher), IKZF1 (NBP1-98314; Novus Biologicals), and IKZF3 (NBP2-46048; Novus Biologicals) were incubated with 5% BSA in TBST overnight at 4 °C with gentle rocking. The membrane was then probed with an HRP-conjugated secondary antibody and developed using a Pierce ECL substrate.
2.13. Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed according to the manufacturer’s protocol (#91820; Cell Signaling Technology, Danvers, MA). Briefly, the cells were collected and cross-linked with 1% formaldehyde. After centrifugation, the resulting pellets were sonicated and the chromatin solution was precleared with 30 μL of CHiP-Grade protein G magnetic beads (#9006; Cell Signaling Technology). The soluble fraction was collected, and the chromatin beads were incubated with positive control histone H3 rabbit (#4620; Cell Signaling), normal rabbit IgG (#2729; Cell Signaling), anti-PPARα (ab227074; Abcam), anti-PPARγ (16643-1-AP; Proteintech Rosemont, IL), anti-FLAG M2 (F1804; Sigma; for PPARβ/δ with a FLAG tag) antibodies at 4 °C overnight. ChIP-enriched DNA was analyzed by quantitative PCR using the CRBN promoter primers as follows: forward: 5′-TCCCCTGTCACCTTCAAAAC-3′ and reverse: 5′-TGCCTTGTGAGTCTGACACC-3′. The enrichment of specific genomic regions was assessed relative to the input DNA, followed by normalization to the respective control IgG values. Percent input = 4% × 2(C[T] 4% input sample-C[T] IP sample).
2.14. Confocal microscopy examination of PPARs in MM cell lines
H929 and RPMI8226 cells were allowed to attach to a glass slide coated with 10 μg/mL of fibronectin (catalog 341635; Sigma-Aldrich, St. Louis, MO) for 1 h at 37 °C. The cells were subsequently fixed with 4% formaldehyde in PBS for 15 min at room temperature. After fixation, the slides were blocked with 10% fetal bovine serum in cell culture medium and subsequently incubated overnight at 4 °C with the CRBN antibody (PA598707; Thermo Fisher Scientific). The slides were subsequently washed thrice with PBS and stained with Alexa Fluor 594 goat anti-rabbit antibody (R37117; Thermo Fisher Scientific) for 1 h. After washing thrice with PBS, the slides were stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; 4083; Cell Signaling) for 5 min and mounted with the antifade mounting medium (Vector, H-1000). Images were acquired using a confocal laser-scanning microscope (Leica SP5 inverted confocal microscope). Sequential scanning of the different channels was performed at a resolution of 512 × 512 pixels. The system was equipped with an HC PLAPO CS2 63×1.1. The oil objective was excited with a diode 594-nm laser. Brightness was optimized and applied to the entire image.
2.15. In vivo experiment
NOD/SCID/IL-2γ null (NSG mice) were sub-lethally irradiated with 1.5 gray (Gy) and injected with MM.1R transduced with luciferase at the dorsal flank of the mice. Nine days later, when a tumor had formed, the mice received daily treatment with PBS buffer, fenofibrate (100 mg/kg orally) [31, 32], lenalidomide (50 mg/kg orally), or a combination of fenofibrate and lenalidomide for a total of 10 weeks. Tumor size was quantified weekly by measuring the bioluminescence intensity and tumor volume. Tumor volume was calculated using the equation (length × width2)/2. Mice were euthanized when the tumors reached a size of >2000 mm3, became ulcerated, or were >1% of the baseline body weight; the mice paid undue attention to or chewed on the lesion, or the lesion interfered with the “normal” mouse function (such as eating or drinking). All mice receiving PBS or fenofibrate alone were euthanized by week 8 due to extensive tumor burden. The survival of the animals was monitored daily. The tumors were harvested at the end of the experiments, weighed, and used for immunoblotting analysis.
2.16. Statistical analysis
A summary of patient characteristics and treatment has been tabulated (Table S1). Kaplan–Meier estimation was used to determine the median OS and PFS. Differences in OS and PFS among patients with different PPAR IHC scores were determined. The chi-square test and logistic regression analysis were used to study variables predictive of response.
For in vitro and in vivo mouse studies, values reported and shown in graphical displays represent the mean ± standard error of the mean, unless stated otherwise. Graphical displays were used to determine appropriate transformations, and in some cases, data were analyzed on a log scale to adhere to the assumptions of parametric tests. Comparisons of the mean expression across groups were performed using two-sample t-tests. Based on the distribution within groups, we used a t-test with equal or unequal variances. For all comparisons, an alpha level of 0.05 was used to denote significance.
3. Results
3.1. Treatment with PPAR agonists or overexpression of PPARs downregulated the mRNA and protein expression levels of CRBN
We previously reported that pan-PIM (Proviral insertion in murine lymphoma) kinase inhibitors increased CRBN mRNA and protein expression and enhanced lenalidomide-mediated anti-myeloma activity [22]. In an effort to understand the mechanism through which PIM inhibitors (SGI-176 and CX-6258) increase CRBN expression, we performed microarray analysis on myeloma cell lines treated with PIM inhibitors. Pathway enrichment analysis suggested that, in addition to their known effects on apoptosis and the mTOR pathway [33, 34], PIM inhibitors downregulated PPAR pathways (Fig. 1A, red arrows). Western blot analysis confirmed that PIM kinase inhibitors reduced PPAR expression (Fig. S1A). These data suggest a potential role for PPAR in the regulation of CRBN expression in myeloma cells.
Fig. 1. Treatment with PPAR agonists or overexpression of PPARs downregulated the mRNA and protein expression levels of CRBN.
(A). Pathway enrichment analysis based on the microarray assay in MM cells treated with PIM kinase inhibitors; red arrows indicated PPAR pathway. (B). MM1.R, NCIH929 and RPMI8226 cells lines were treated with 5 μM or the EC50 concentration of individual PPAR agonist for 48h, and levels of CRBN mRNA were measured by qRT-PCR. (C). MM1.R, NCIH929 and RPMI8226 cells were treated with 5 μM or the EC50 concentration of individual PPAR agonist for 48h, and CRBN and GAPDH protein expression levels were assessed by Western blot. Bar graph represents the ratio of CRBN/GAPDH protein. One of 3 independent experiments was shown. (D). Representative images of DAPI and CRBN staining in NCIH929 cells treated with 5 μM of PPAR agonists for 48h. (E). NCIH929 and MM1.R cells were transfected with a Lenti-PPARα, Lenti-PPARβ/δ, or Lenti-PPARγ expressing plasmid or control vector for 48h. CRBN protein expression level was measured by Western blot.
There are three isoforms of PPAR: PPARα, PPARβ/δ, and PPARγ. PPAR isoforms are not functionally redundant. In hepatocytes, PPARα was found to be a negative transcriptional regulator of CRBN and a PPARα agonist (fenofibrate) reduced CRBN expression [18, 19]. To determine the effects of PPAR agonists on CRBN expression in myeloma cells, we treated six myeloma cell lines, MM1.R, NCIH929, RPMI8226, OPM2, JJN3, and MM1.S, with fenofibrate (PPARα agonist), GW501516 (PPARβ/δ agonist), or troglitazone (PPARγ agonist) at 5 μM, 1 μM, and at half maximal effective concentration (EC50). EC50 values of fenofibrate, GW501516 and troglitazone were reported at 30μM [35], 2.6nM [36, 37], and 500nM [35, 38], respectively. CRBN mRNA and protein expression levels were then measured (Fig. 1B and C; Fig. S1B and C; Fig. S2). PPAR agonists down-regulated CRBN expression in a dose dependent manner (Fig. S2) and treatment with all three PPAR isoform agonists at EC50 concentrations significantly reduced CRBN mRNA and protein expression levels (Fig. 1B and C). 5 μM or EC50 concentration was used in our subsequent experiments. Similarly, immunofluorescence analysis revealed that CRBN expression was significantly reduced after treatment with PPAR agonists (Fig. 1D; Fig. S1D). Additionally, overexpression of PPARα, PPARβ/δ, or PPARγ by lentiviral gene transduction resulted in reduced CRBN expression in MM cells (Fig. 1E).
3.2. PPAR agonists reduced the anti-myeloma activity of lenalidomides in vitro and in vivo
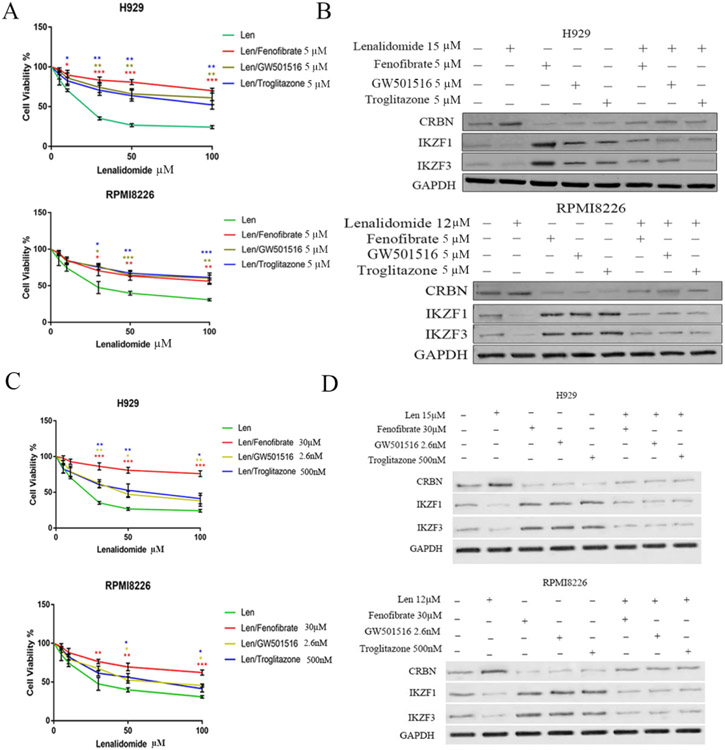
CRBN plays a critical role in the anti-myeloma activity of IMiD. To determine whether the expression of PPAR correlates with sensitivity to lenalidomide, we screened eight MM cell lines and found that PPAR levels negatively correlated with lenalidomide sensitivity. Specifically, the cell lines with the lowest expression of all PPAR isoforms (NCIH929 and RPMI8226) were the most sensitive to lenalidomides (Fig. S3A and B) (IC50 for NCIH929 and RPMI8226 was 15.39 μM and 17.55 μM, respectively). In contrast, the other six MM cell lines, which had higher levels of PPARs, were all relatively resistant to lenalidomide (Fig. S3A and B) (IC50 for these cell lines was approx. 40 μM). We next determined whether the co-administration of PPAR agonists affects the anti-myeloma activity of lenalidomide. The NCIH929, RPMI8226, and MM1.R cell lines were treated with various concentrations of lenalidomide along with 5 μM (Fig. 2A and B) or EC50 concentration (Fig. 2C and D) of the PPAR agonists fenofibrate, GW501516, or troglitazone. The addition of PPAR agonists attenuated the anti-myeloma activity of lenalidomide in vitro (Fig. 2A and C; Fig. S4A). PPAR agonists enhanced IKZF1 and IKZF3 expression levels, and in the presence of the PPAR agonist, lenalidomide treatment failed to induce the degradation of IKZF1 and IKZF3 as effectively as lenalidomide alone (Fig. 2B and D).
Fig. 2. PPAR agonists reduce the anti-myeloma activity of lenalidomide in vitro.
(A). NCIH929 and RPMI8226 were treated with 5 μM of PPAR agonists for 24h, along with indicated concentration of lenalidomide (Len) for 48h. Cell viability was measured by MTT assay. (B). NCIH929 and RPMI8226 cells were treated with DMSO control buffer, lenalidomide, PPAR agonists or the combination of lenalidomide and a PPAR agonist for 48h. Protein lysate was subjected to CRBN, IKZF1 and IKZF3 measurement by Western blot analysis. (C). NCIH929 and RPMI8226 were treated with EC50 concentration of individual PPAR agonist for 24h, along with indicated concentration of lenalidomide (Len) for 48h. Cell viability was measured by MTT assay. (D) NCIH929 and RPMI8226 cells were treated with DMSO control buffer, lenalidomide and/or PPAR agonists at EC50 concentration for 48h. Protein lysate was subjected to CRBN, IKZF1 and IKZF3 measurement by Western blot analysis.
Similar to PPAR agonists, genetic overexpression of PPAR isoforms reduced the anti-myeloma activity of lenalidomide (Fig. 3A; Fig. S4B). To confirm that PPAR agonists affect lenalidomide anti-myeloma activity through CRBN, we genetically overexpressed CRBN in MM cell lines and treated them with lenalidomide in the presence or absence of PPAR agonists. CRBN overexpression increased the sensitivity of MM cell lines to lenalidomide compared to the empty vector control. Additionally, CRBN overexpression enhanced the anti-myeloma activity of lenalidomide in the presence of PPAR agonists (Fig. 3B; Fig. S4C low panel). Next, we knocked out CRBN expression using CRISPR/Cas9 technology in NCIH929 and RPMI8226 cell lines and confirmed CRBN deletion by western blot analysis (Fig. S4D). Knockout of CRBN rendered MM cells resistant to lenalidomide treatment, which was reversed by transfection with a CRBN-overexpressing plasmid (Fig. 3C; Fig. S4E). These data demonstrate the critical role of CRBN in PPAR agonist-mediated inhibition of the anti-myeloma activity of lenalidomide.
Fig 3. Overexpression of PPAR resulted in reduced activity of lenalidomide in vitro.
(A). NCIH929 and RPMI8226 cell lines were transfected with control vector or a PPAR over-expressing plasmid for 24h and then treated with lenalidomide for additional 48h. Cell viability was measured by MTT assay. (B). NCIH929 and RPMI8226 cell lines were transfected with control vector or a CRBN over-expressing plasmid for 24h and then treat with lenalidomide alone or in combination with PPAR agonists for 48h. Cell viability was measured by MTT assay. (C). NCIH929 and RPMI8226 cells were transduced with CRBN specific CRISP/cas9 knockout plasmid for 24h and then treated with lenalidomide, PPAR agonists alone, or in combination for additional 48h. Cell viability was measured by MTT assay. Results are presented as mean ± SD from at least three separate experiments. *: p<0.05; **: p<0.01; ***: p<0.001.
We then examined the in vivo effects of PPAR agonists on the sensitivity of myeloma cells to IMiDs using the PPARα agonist fenofibrate. NSG mice were sub-lethally irradiated with 1.5 Gy and injected with MM1.R cells transduced with luciferase. Nine days later, when a tumor had formed, the mice received daily treatments with PBS buffer, fenofibrate (100 mg/kg orally), lenalidomide (50 mg/kg orally), or a combination of fenofibrate and lenalidomide for a total of 10 weeks. Tumor size was quantified weekly by measuring bioluminescence intensity. All mice receiving PBS or fenofibrate alone were euthanized by week 8 because of extensive tumor burden. As expected, lenalidomide monotherapy was effective in controlling tumor growth; however, mice treated with the combination of lenalidomide and fenofibrate showed tumor growth beginning at approximately week 5. By week 10, when all remaining animals were euthanized, tumor burden and tumor volume were significantly higher in mice treated with the combination of lenalidomide and fenofibrate (Fig. 4A and B), demonstrating that co-administration of a PPAR agonist significantly reduced the anti-myeloma activity of IMiDs in vivo. Tumors were harvested at the end of the experiments, and CRBN mRNA expression was measured. Tumor CRBN mRNA levels were significantly lower in mice receiving lenalidomide and fenofibrate combination than in mice treated with lenalidomide alone (Fig. 4C).
Fig. 4. PPAR agonists reduce the anti-myeloma activity of lenalidomide in vivo.
(A). Bioluminescence intensity showing the different tumor burdens in NSG mice implanted with MM1.R myeloma cells and treated with control PBS (CTL), fenofibrate (Fen), lenalidomide (Len), or the combination (Len and Fen) (7 mice per group) from week 1 to week 10. (B). Left panel: the bioluminescence activity was quantified by determining the total flux (photons/sec) in each mouse from week 1 to week 10. Right panel: representative images of tumors harvested from the control group (CTL) and the fenofibrate group (Fen) at week 8 and tumors harvested from the lenalidomide group (Len) and the combination group (Len and Fen) at week 10. (C). CRBN mRNA expression of the harvested tumors. qRT-PCR was used to detect the CRBN mRNA expression in the harvested tumors.
3.3. PPARs directly regulate the transcription of CRBN in MM cells
Next, we determined the molecular mechanism by which PPAR regulates CRBN expression. PPAR agonists downregulated both mRNA and protein expression of CRBN, suggesting regulation at the transcriptional level. The JASPAR database predicted that PPAR could potentially bind to the CRBN promoter region (Fig. S5A). In silico analysis of the GSE31161 database revealed a strong negative correlation between CRBN expression and PPARα and PPARβ/δ (Fig. S5B). CHiP assay demonstrated that all three PPAR isoforms could bind to the CRBN promoter in NCIH929 and MM1.R, whereas IgG control showed no signal (Fig. 5A; Fig. S5C). Fenofibrate, GW501516, and troglitazone significantly promoted PPAR occupancy of the CRBN promoter (Fig. 5B; Fig. S5D for 4% of input). To confirm the inhibitory effects of PPAR agonists on the CRBN promoter, we subcloned the CRBN promoter into a PGL3 firefly luciferase reporter vector and transfected the construct into NCIH929 and MM1.R cells. CRBN promoter activity was suppressed after treatment of these cell lines with PPAR agonists (Fig. 5C; Fig. S6A). To validate the specificity of PPAR binding to the CRBN promoter, we performed site-specific mutagenesis of CRBN’s PPAR-binding region of CRBN in H929 and MM1.R cells. Deletion of the PPAR-binding site within the CRBN promoter abrogated the inhibitory effect of PPAR on CRBN promoter activity (Fig. 5D; Fig. S6B). These data demonstrated that PPARs are direct transcriptional repressors of CRBN in MM cells.
Fig. 5. PPARs directly regulate transcription of CRBN in MM cells.
(A). Representative images of ChiP-PCR products amplified by primers (100bp) on 2% agarose gel in NCIH929 cells. (B). Bar graph of ChiP DNA shows fold change of CRBN promoter binding after treatment with PPAR agonists. (C). NCIH929 cells were transfected with CRBN/PGL3 firefly luciferase reporter vector construct. The cells were then treated with PPAR agonists and luciferase bio-luminate activity was measured. (D). NCIH929 cells were transfected with mutated CRBN/PGL3 firefly luciferase reporter vector construct. The cells were then treated with PPAR agonists and luciferase bio-luminate activity was measured.
3.4. Co-treatment with PPAR antagonists increases CRBN expression levels and improves the sensitivity to lenalidomide
To take advantage of the effects of PPARs on CRBN to improve the response to IMiDs, we examined PPAR antagonists in MM cell lines. GW6471 (PPARα antagonist), GSK3787 (PPARβ/δ antagonist), and GW9662 (PPARγ antagonist) were used in this study. We first determined the effects of these PPAR antagonists on the CRBN promoter by using the PGL3-CRBN firefly/Renilla reporter system. NCIH929 cells were transduced using the PGL3-CRBN reporter system and treated with PPAR antagonists. Treatment with PPAR antagonists increased firefly/Renilla intensity, indicating that these compounds increased CRBN transcription (Fig. 6A). Western blot analysis confirmed that PPAR antagonists upregulated CRBN expression (Fig. 6B). Co-administration of PPARs antagonists (GW6471, GSK3787, or GW9662) with lenalidomide increased MM cell killing compared with lenalidomide alone (Fig. 6C). These data suggest that PPAR antagonists enhance the anti-myeloma activity of IMiD.
Fig. 6. Co-administration of PPAR antagonists increase CRBN expression and improve the sensitivity to lenalidomide.
(A) NCIH929 cells were transfected with CRBN/PGL3 firefly luciferase reporter vector construct. The cells were then treated with PPAR antagonists and luciferase bio-luminate activity was measured. (B) MM1.R and NCIH929 cells were treated with PPAR antagonists (GW6471 10 μM, GSK3787 10 μM and GW9662 10 μM) or DMSO control for 48h. CRBN expression was then measured by Western blot analysis. (C). MM1.R and NCIH929 were treated DMSO, lenalidomide, PPAR antagonists, or in combination for 48h. Cell viability was measured by MTT assays. Results are presented as mean ± SD from at least three separate experiments. *: p<0.05; **: p<0.01; ***: p<0.001.
3.5. PPAR expression levels are upregulated in patients newly diagnosed with MM and correlate with poor clinical outcomes
We determined PPAR expression in BM samples from patients with newly diagnosed MM (NDMM) and its association with clinical outcomes. IHC staining for PPARα, PPARβ/δ, and PPARγ was performed on BM biopsies of 195 patients with NDMM. The patient characteristics are summarized in Table S1. We quantified PPAR expression using the methodology we recently published that incorporated measurements of the percentage of PPAR-positive cells and the intensity of PPAR expression [8]. All three PPAR isoforms (PPARα, PPARβ/δ, and PPARγ) were upregulated in the BM CD138+ plasma cells and BM CD138− microenvironment cells in patients with NDMM compared to those in normal BM controls (Fig. 7A and B). PPARs were detected in both the cytoplasm and the nucleus. Furthermore, high IHC scores for total PPAR, PPARβ/δ, and PPARγ expression levels in BM biopsy samples were associated with worse PFS and OS in patients with NDMM (Fig. 7C, D, and E).
Fig. 7. PPAR expression is upregulated in MM patients and is associated with poorer clinical outcomes.
(A). Bone marrow biopsy sections from newly diagnosed MM patients and normal controls were stained with PPAR antibodies. Representative images of PPAR staining were shown. (B). Box plot shows the IHC scores of PPARs expression in CD138+ and CD138− cells in bone marrow biopsy samples of newly diagnosed myeloma patients and in normal control bone marrows. (C) Progression free survival (left) and overall survival (right) between newly diagnosed myeloma patients with high total PPAR IHC score (IHC score >8) and newly diagnosed myeloma patients with low total PPAR IHC score (IHC score ≤ 4). (D). Progression free survival and overall survival between patients with high PPARβ/δ expression (IHC >4) and patients with low PPARβ/δ expression (IHC ≤4). (E). Progression free survival and overall survival between patients with high PPARγ expression (IHC >4) and patients with low PPARγ expression (IHC ≤ 4). IHC scores represented the sum of score for CD138+ cells and score for CD138− cells.
4. Discussion
High expression of CRBN is associated with an improved clinical response in patients with MM treated with IMiDs, such as lenalidomide. Modulation of CRBN expression could potentially improve the efficacy of IMiDs in patients with low endogenous CRBN levels. Many studies have investigated CRBN genetic variants and their association with lenalidomide responses [39-41]. However, it remains unclear how CRBN is regulated and whether different genomic approaches or drugs could affect sensitivity to IMiDs through regulation of CRBN expression. We have previously shown that the pan-PIM kinase inhibitors SGI-1776 and CX-6528 enhanced the anti-myeloma activity of lenalidomide through the upregulation of CRBN expression [22]. Pathway enrichment analysis based on microarray data suggested that PPAR pathways were downregulated following treatment with PIM kinase inhibitors. In the present study, the negative link between PPARs and CRBN activation was further elucidated using genetic and pharmacological approaches. Overexpression of PPARs or treatment with PPAR agonists in MM cells results in decreased CRBN expression. Importantly, data from our CHiP and firefly/Renilla reporter analyses demonstrated that PPARs bind to and repress the CRBN promoter, providing the first direct evidence of the mechanism of PPAR-mediated CRBN repression in MM cells. In addition, by investigating the expression of PPARs in BM cells from newly diagnosed MM patients, we provided evidence that the activation of PPARs correlated with poor clinical outcomes. Furthermore, we demonstrated that co-administration of PPAR agonists with lenalidomide significantly reduced the anti-myeloma activity of IMiDs in vitro and in vivo in a xenograft myeloma mouse model.
Our study is significant in several respects, providing the first evidence that PPARs regulate CRBN expression and that PPAR agonists may affect MM patient outcomes when treated with IMiDs. This finding is particularly relevant because MM is predominately a disease of the older population, and many patients are taking PPAR agonists for medical comorbidities concurrently with IMiD treatment. Our study has potential implications in clinical practice and may alter the treatment of a substantial proportion of myeloma patients who also suffer from dyslipidemia and/or type II diabetes. Our data provide rationale for prospectively testing the impact of PPAR agonists on the anti-myeloma activity of IMiDs. Our study could also serve as a proof of concept for further testing and development of PPAR partial agonist/partial antagonists or selective retinoid X receptor (RXR) modulators that retain the lipid-lowering and anti-diabetic effects while enhancing CRBN expression [42, 43]. These PPAR partial agonist/partial antagonists and selective RXR modulators will be particularly useful in patients with myeloma with dyslipidemia and/or type II diabetes.
CRBN plays a critical role in IMiD-mediated anti-myeloma activity. IMiDs bind to CRBN and redirect CUL4–RBX1–DDB1–CRBN (CRL4CRBN) E3 ligase complexes to target numerous neo-substrates, such as IKZF1 and IKZF3, for ubiquitylation and proteasome-dependent degradation [44, 45]. IKZF1 and IKZF3 are important transcription factors for myeloma cell growth, and degradation of IKZF1 and IKZF3 leads to downregulation of IRF4 and MYC, causing myeloma cell cycle arrest and apoptosis. Although CRBN is required for the anti-myeloma activity of IMiDs [11], several studies have indicated that CRBN expression is not the sole determinant of IMiD sensitivity/resistance. For instance, the BM microenvironment and angiogenesis also affect the response to IMiDs, and IMiDs exert their anti-myeloma function in part by inhibiting angiogenesis through downregulation of tumor necrosis factor-α, vascular endothelial growth factor (VEGF), interleukin (IL)-6, and phosphorylated AKT serine/threonine kinase [46]. Extracellular vesicle secretion and cell adhesion are linked to lenalidomide resistance in MM [47]. SUMOylation has recently been identified as a potential mechanism for regulating lenalidomide resistance in MM cells [48]. Inhibition of SUMOylation induces the degradation of IRF4 and enhances sensitivity to lenalidomide [48]. Similarly, DNA methylation and chromatin accessibility regulate sensitivity and resistance to IMiDs [49]. The capacity to decompose H2O2 was found to correlate with lenalidomide sensitivity/resistance in MM cells [50]. MM cells with lower H2O2 decomposition capacity are more sensitive to lenalidomide [50]. Using an in vitro model of acquired resistance, Jakobsen et al. found that genome-wide circular RNA expression patterns were correlated with IMiD sensitivity [51]. Additional mechanisms, such as the Wnt/β-catenin pathway, CDK6 activation, mitogen-activated protein kinase pathway, and mitogen-activated protein kinase–extracellular signal-regulated kinase overactivation, have been linked to resistance to IMiDs [52-54].
In addition to inducing myeloma cell death, IMiDs possess immunostimulatory effects, promoting IL-2 and interferon (IFN)-γ secretion, which enhances T cell and subsequent NK cell activation, improves immune checkpoint-based immune response, and reduces regulatory T cell suppressor function [55]. IMiDs also augment TCR signaling via CD28, leading to upregulation of the nuclear factor (NF)-κB [56]. However, further investigation revealed an inhibitory effect of PPARα on pro-inflammatory NF-κB, a possible mechanism of its anti-inflammatory action [57]. Interestingly, studies have also demonstrated dysregulation of cytokine production in T lymphocytes from PPARα-deficient (PPARα−/−) mice, whereby deficient cells produce significantly larger quantities of IFN-γ in response to anti-CD3/anti-CD28 activation [58]. These data would suggest that PPARα is capable of modulating the function and immunological response of a variety of immune cells and may therefore play a significant role in the determination of T cell responsiveness in vivo. The impact of PPARs on immune cells in patients with MM should be considered in future research and clinical approaches.
The effect of IMiDs on PPAR expression is largely uncharacterized, and there are reports that showed opposite effects of thalidomide on PPARγ expression, depending on the tissue/cell line. In orbital adipose tissue of Graves’ ophthalmopathy, thalidomide was found to downregulate the expression of PPARγ in a dose-dependent manner and inhibit adipogenesis [59]. In contrast, in non-small cell lung cancer, thalidomide was found to dose-dependently increase PPAR protein and inhibit cancer cell growth [60].
PPARs are critical lipid sensors and regulators because of their indispensable roles in various lipid-related bioactivities, such as lipid transport, adipocyte differentiation, and metabolism of various lipid components, such as fatty acids, ketone bodies, and cholesterols [61, 62]. Several PPAR-directed processes have been linked to either pro-or anti-tumorigenesis [63-65]. PPAR isoforms are not functionally redundant and may have different biological effects. For instance, PPARα inhibits angiogenesis by inhibiting endothelial cell proliferation, increasing the expression of angiogenic inhibitors, such as endostatin and thrombospondin 1, and downregulating VEGF and cytochrome P450 CYP2C [66]. The interaction between PPARα and NADPH oxidase 1 also regulates angiogenesis [67]. In contrast, ligand-activated PPARγ facilitates terminal differentiation and promotes cell cycle arrest and apoptosis in cancer cells [68, 69]. The PPARγ agonist pioglitazone enhances the cytotoxic effects of histone deacetylase inhibitors and valproic acid on MM cells [70, 71]. In addition, PPARγ overexpression suppresses growth and induces apoptosis in MM cells [72]. Unlike PPARα and PPARγ, the relationship between PPARβ/δ and cancer is complex [73], and a better clarification of the specific contribution of PPARβ/δ to the tumorigenic process is warranted. Our study suggests that, in MM cells, all three PPAR isoforms exert similar effects on the regulation of CRBN. This redundancy could be in part due to the fact that the CRBN promoter region contains binding sequences for all 3 PPAR isoforms. We have observed that PPAR agonists were effective in down-regulating CRBN expression and attenuating lenalidomide anti-myeloma activity across a wide range of concentrations. The effects of PPAR agonists ultimately depend on the availability and occupancy of CRBN gene promoter. The copy number of CRBN gene promoter is the major limiting factor and is expected to remain relatively constant, which could explain that the effects of PPAR agonists were similar between EC50 and concentration at 5μM. It is also noteworthy that the reported EC50 values were determined using cell-based PPAR-GAL4 transactivation assay [35]. EC50 values may change in difference cell types or different target/reporter genes.
Several PPAR agonists, including fenofibrate (NCT01965834), efatutazone (NCT01504490), and pioglitazone (EUCTR 2008-002768032, NCT0101243), have also been tested in patients with MM. The first two treatments were terminated early for unclear reasons, whereas those involving pioglitazone are ongoing. Combining a PPAR subtype agonist with other antitumor compounds has been reported, but the data have not been consistent. Treatment with PPARγ ligands significantly alters the response and cell viability when co-treated with imatinib in chronic myeloid leukemia [74]. PPARγ agonists and antagonists have differential effects on organic cation transporter-1 activity, which is associated with the response to imatinib [74], probably due to the different target populations (mononuclear vs. CD34+ cells) [75, 76]. Drug-drug interactions between PPAR agonists and anti-tumor agents remain largely uncharacterized. Although PPAR agonists have been widely studied, no clinical evidence is available for PPAR antagonists. Preclinical studies have described the roles of PPARγ and PPARβ/δ antagonists in anti-obesity and anti-cancer therapies, respectively [77, 78]. We believe that exploring the clinical feasibility of PPAR antagonists in MM treatment may result in fruitful outcomes considering the involvement of PPARs in tumorigenic processes, such as oxidative stress homeostasis, tumor-associated metabolism, and drug response [79-81]. Taken together, targeting PPARs may be an effective strategy for cancer treatment. We believe that PPAR modulators will continue to be a main theme in basic science and clinical research in the future.
Our clinical cohort studies have limitations and drawbacks. These studies are single-center retrospective studies and are thus limited by the nature of retrospective studies. Second, our patient population was not a uniform group of patients. There are heterogeneities in the phase of treatment (induction, maintenance, lines of relapsed/refractory). Prospective studies will be needed to further define the effects of PPAR agonists on IMiDs’ anti-myeloma and immunomodulatory activities.
5. Conclusions
In this study, we observed the opposing effects of PPARs and lenalidomide, which were distinct from the synergistic effects of pan-PIM kinase inhibitors and lenalidomide in MM cells. The findings presented in this study demonstrate that treatment with PPAR ligands significantly alters the response to lenalidomide via direct transcriptional regulation of the CRBN promoter. PPAR transcriptional activity was significantly negatively correlated with CRBN. Furthermore, high PPAR expression was significantly associated with poor OS and PFS. These findings demonstrate the critical role of PPARs in CRBN regulation and highlight the potential to target this interaction to drive MM therapy.
Supplementary Material
Fig. S1. Treatment with PPAR agonists downregulated the mRNA and protein expression levels of CRBN.
Fig. S2. PPAR agonists downregulated the mRNA and protein expression levels of CRBN in a dose dependent manner.
Fig. S3. PPAR expression level negatively correlated with lenalidomide sensitivity.
Fig. S4. Overexpression of CRBN reversed the effect of PPAR agonists on lenalidomide sensitivity in vitro.
Fig. S5. The relationship of PPARs and CRBN in MM human datasets and cell lines.
Fig. S6. PPARs are direct transcriptional regulator of CRBN in MM1.R cells.
Table S1. Newly diagnosed myeloma patient characteristics.
Acknowledgements
The authors thank Drs. Jing Zheng, Dadong Zhang and Kouros Owzar for assistance and technical support. The authors thank Janis Curtis and Curtis Kieler at Duke School of Medicine Clinical Translational Science Institute for querying the database and identifying patients in this study. The project is supported in part by the Biostatistics Shared Resource, Duke Cancer Institute. We would like to thank Editage (www.editage.com) for English language editing.
Funding
This work was supported by the Duke Cancer Institute Fund [to YK] and the National Cancer Institute [grant numbers R44CA199767, R01CA197792, and R21CA234701 to YK]. The sponsors have no roles in study design, the collection, analysis and interpretation of data, the writing of the report or the decision to submit the article for publication.
Abbreviations:
- PPAR
Peroxisome proliferator-activated receptor
- CRBN
Cereblon
- MM
Multiple myeloma
- IMiDs
Immunomodulatory agents
- IKZF1
IKAROS family zinc finger 1
- IKZF3
IKAROS family zinc finger 3
- IHC
Immunohistochemical staining
- Len
Lenalidomide
- OS
Overall survival
- PFS
Progression free survival
- NDMM
Newly diagnosed MM
- RXR
Retinoid X receptor
- PIO
Pioglitazone
- NSG
NOD/SCID/IL-2γnull
- ChIP
Chromatin immunoprecipitation
Footnotes
Declaration of competing interests
Dr. Yubin Kang received research funding from InCyte Corporation and Consultancy fee from Takeda Oncology USA and Sanofi Genzyme Corp. All other authors declare no competing interests.
References
- [1].Wenthe J, Naseri S, Hellstrom AC, Wiklund HJ, Eriksson E, Loskog A, Immunostimulatory oncolytic virotherapy for multiple myeloma targeting 4-1BB and/or CD40, Cancer Gene Ther, 27 (2020) 948–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bonello F, Grasso M, D'Agostino M, Celeghini I, Castellino A, Boccadoro M, Bringhen S, The Role of Monoclonal Antibodies in the First-Line Treatment of Transplant-Ineligible Patients with Newly Diagnosed Multiple Myeloma, Pharmaceuticals (Basel), 14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Harousseau JL, Ten years of improvement in the management of multiple myeloma: 2000-2010, Clin Lymphoma Myeloma Leuk, 10 (2010) 424–442. [DOI] [PubMed] [Google Scholar]
- [4].Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust JA, Greipp PR, Kyle RA, Gertz MA, Improved survival in multiple myeloma and the impact of novel therapies, Blood, 111 (2008) 2516–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Moreau P, Attal M, Facon T, Frontline therapy of multiple myeloma, Blood, 125 (2015) 3076–3084. [DOI] [PubMed] [Google Scholar]
- [6].Nooka AK, Kastritis E, Dimopoulos MA, Lonial S, Treatment options for relapsed and refractory multiple myeloma, Blood, 125 (2015) 3085–3099. [DOI] [PubMed] [Google Scholar]
- [7].Feinberg D, Paul B, Kang Y, The promise of chimeric antigen receptor (CAR) T cell therapy in multiple myeloma, Cell Immunol, 345 (2019) 103964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Paul B, Zhao Y, Loitsch G, Feinberg D, Mathews P, Barak I, Dupuis M, Li Z, Rein L, Wang E, Kang Y, The impact of bone marrow fibrosis and JAK2 expression on clinical outcomes in patients with newly diagnosed multiple myeloma treated with immunomodulatory agents and/or proteasome inhibitors, Cancer Med, 9 (2020) 5869–5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim SA, Go A, Jo SH, Park SJ, Jeon YU, Kim JE, Lee HK, Park CH, Lee CO, Park SG, Kim P, Park BC, Cho SY, Kim S, Ha JD, Kim JH, Hwang JY, A novel cereblon modulator for targeted protein degradation, Eur J Med Chem, 166 (2019) 65–74. [DOI] [PubMed] [Google Scholar]
- [10].Gao S, Wang S, Song Y, Novel immunomodulatory drugs and neo-substrates, Biomark Res, 8 (2020) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhu YX, Braggio E, Shi CX, Bruins LA, Schmidt JE, Van Wier S, Chang XB, Bjorklund CC, Fonseca R, Bergsagel PL, Orlowski RZ, Stewart AK, Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide, Blood, 118 (2011) 4771–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hidalgo JA, Florez A, Agurto C, Pinedo Y, Ayarza R, Rodriguez L, La Rosa A, Gutierrez R, Metabolic and Cardiovascular Comorbidities Among Clinically Stable HIV Patients on Long-Term ARV Therapy in Five Ambulatory Clinics in Lima-Callao, Peru, Open AIDS J, 12 (2018) 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sehgal K, Fadel HJ, Tande AJ, Pardi DS, Khanna S, Outcomes in Patients with SARS-CoV-2 and Clostridioides difficile Coinfection, Infect Drug Resist, 14 (2021) 1645–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Matsuba I, Matsuba R, Ishibashi S, Yamashita S, Arai H, Yokote K, Suganami H, Araki E, Effects of a novel selective peroxisome proliferator-activated receptor-alpha modulator, pemafibrate, on hepatic and peripheral glucose uptake in patients with hypertriglyceridemia and insulin resistance, J Diabetes Investig, 9 (2018) 1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Patel SP, Cox DH, Gollihue JL, Bailey WM, Geldenhuys WJ, Gensel JC, Sullivan PG, Rabchevsky AG, Pioglitazone treatment following spinal cord injury maintains acute mitochondrial integrity and increases chronic tissue sparing and functional recovery, Exp Neurol, 293 (2017) 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].O'Connell TD, Mason RP, Budoff MJ, Navar AM, Shearer GC, Mechanistic insights into cardiovascular protection for omega-3 fatty acids and their bioactive lipid metabolites, Eur Heart J Suppl, 22 (2020) J3–J20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA, An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma), J Biol Chem, 270 (1995) 12953–12956. [DOI] [PubMed] [Google Scholar]
- [18].Kim YD, Lee KM, Hwang SL, Chang HW, Kim KJ, Harris RA, Choi HS, Choi WS, Lee SE, Park CS, Inhibition of cereblon by fenofibrate ameliorates alcoholic liver disease by enhancing AMPK, Biochim Biophys Acta, 1852 (2015) 2662–2670. [DOI] [PubMed] [Google Scholar]
- [19].Kim HK, Ko TH, Nyamaa B, Lee SR, Kim N, Ko KS, Rhee BD, Park CS, Nilius B, Han J, Cereblon in health and disease, Pflugers Arch, 468 (2016) 1299–1309. [DOI] [PubMed] [Google Scholar]
- [20].Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, Munshi N, Lonial S, Blade J, Mateos MV, Dimopoulos M, Kastritis E, Boccadoro M, Orlowski R, Goldschmidt H, Spencer A, Hou J, Chng WJ, Usmani SZ, Zamagni E, Shimizu K, Jagannath S, Johnsen HE, Terpos E, Reiman A, Kyle RA, Sonneveld P, Richardson PG, McCarthy P, Ludwig H, Chen W, Cavo M, Harousseau JL, Lentzsch S, Hillengass J, Palumbo A, Orfao A, Rajkumar SV, Miguel JS, Avet-Loiseau H, International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma, Lancet Oncol, 17 (2016) e328–e346. [DOI] [PubMed] [Google Scholar]
- [21].Kumar SK, Callander NS, Hillengass J, Liedtke M, Baljevic M, Campagnaro E, Castillo JJ, Chandler JC, Cornell RF, Costello C, Efebera Y, Faiman M, Garfall A, Godby K, Holmberg L, Htut M, Huff CA, Kang Y, Landgren O, Malek E, Martin T, Omel J, Raje N, Sborov D, Singhal S, Stockerl-Goldstein K, Tan C, Weber D, Johnson-Chilla A, Keller J, Kumar R, NCCN Guidelines Insights: Multiple Myeloma, Version 1.2020, J Natl Compr Canc Netw, 17 (2019) 1154–1165. [DOI] [PubMed] [Google Scholar]
- [22].Zheng J, Sha Y, Roof L, Foreman O, Lazarchick J, Venkta JK, Kozlowski C, Gasparetto C, Chao N, Ebens A, Hu J, Kang Y, Pan-PIM kinase inhibitors enhance Lenalidomide's anti-myeloma activity via cereblon-IKZF1/3 cascade, Cancer Lett, 440-441 (2019) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tseng GC, Oh MK, Rohlin L, Liao JC, Wong WH, Issues in cDNA microarray analysis: quality filtering, channel normalization, models of variations and assessment of gene effects, Nucleic acids research, 29 (2001) 2549–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Miller JA, Cai C, Langfelder P, Geschwind DH, Kurian SM, Salomon DR, Horvath S, Strategies for aggregating gene expression data: the collapseRows R function, BMC Bioinformatics, 12 (2011) 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Song JH, Kraft AS, Pim kinase inhibitors sensitize prostate cancer cells to apoptosis triggered by Bcl-2 family inhibitor ABT-737, Cancer Res, 72 (2012) 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zheng Z, Fan S, Zheng J, Huang W, Gasparetto C, Chao NJ, Hu J, Kang Y, Inhibition of thioredoxin activates mitophagy and overcomes adaptive bortezomib resistance in multiple myeloma, J Hematol Oncol, 11 (2018) 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Green MM, Chao N, Chhabra S, Corbet K, Gasparetto C, Horwitz A, Li Z, Venkata JK, Long G, Mims A, Rizzieri D, Sarantopoulos S, Stuart R, Sung AD, Sullivan KM, Costa L, Horwitz M, Kang Y, Plerixafor (a CXCR4 antagonist) following myeloablative allogeneic hematopoietic stem cell transplantation enhances hematopoietic recovery, J Hematol Oncol, 9 (2016) 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Stathopoulou A, Chhetri JB, Ambrose JC, Esteve PO, Ji L, Erdjument-Bromage H, Zhang G, Neubert TA, Pradhan S, Herrero J, Schmitz RJ, Ooi SKT, A novel requirement for DROSHA in maintenance of mammalian CG methylation, Nucleic Acids Res, 45 (2017) 9398–9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bhattacharya D, Van Meir EG, A simple genotyping method to detect small CRISPR-Cas9 induced indels by agarose gel electrophoresis, Sci Rep, 9 (2019) 4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Castro-Mondragon JA, Riudavets-Puig R, Rauluseviciute I, Lemma RB, Turchi L, Blanc-Mathieu R, Lucas J, Boddie P, Khan A, Manosalva Perez N, Fornes O, Leung TY, Aguirre A, Hammal F, Schmelter D, Baranasic D, Ballester B, Sandelin A, Lenhard B, Vandepoele K, Wasserman WW, Parcy F, Mathelier A, JASPAR 2022: the 9th release of the open-access database of transcription factor binding profiles, Nucleic Acids Res, 50 (2022) D165–D173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chang CJ, Tzeng TF, Liou SS, Chang YS, Liu IM, Regulation of lipid disorders by ethanol extracts from Zingiber zerumbet in high-fat diet-induced rats, Food Chem, 132 (2012) 460–467. [DOI] [PubMed] [Google Scholar]
- [32].Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, Park AS, Tao J, Sharma K, Pullman J, Bottinger EP, Goldberg IJ, Susztak K, Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development, Nat Med, 21 (2015) 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nawijn MC, Alendar A, Berns A, For better or for worse: the role of Pim oncogenes in tumorigenesis, Nat Rev Cancer, 11 (2011) 23–34. [DOI] [PubMed] [Google Scholar]
- [34].Braso-Maristany F, Filosto S, Catchpole S, Marlow R, Quist J, Francesch-Domenech E, Plumb DA, Zakka L, Gazinska P, Liccardi G, Meier P, Gris-Oliver A, Cheang MC, Perdrix-Rosell A, Shafat M, Noel E, Patel N, McEachern K, Scaltriti M, Castel P, Noor F, Buus R, Mathew S, Watkins J, Serra V, Marra P, Grigoriadis A, Tutt AN, PIM1 kinase regulates cell death, tumor growth and chemotherapy response in triple-negative breast cancer, Nat Med, 22 (2016) 1303–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Willson TM, Brown PJ, Sternbach DD, Henke BR, The PPARs: from orphan receptors to drug discovery, J Med Chem, 43 (2000) 527–550. [DOI] [PubMed] [Google Scholar]
- [36].Kim DS, Lee J, Londhe AM, Kadayat TM, Joo J, Hwang H, Kim KH, Pae AN, Chin J, Cho SJ, Kang H, Synthesis and evaluation of an orally available "Y"-shaped biaryl peroxisome proliferator-activated receptor delta agonist, Bioorg Med Chem, 26 (2018) 4382–4389. [DOI] [PubMed] [Google Scholar]
- [37].Kaupang A, Hansen TV, The PPAR Omega Pocket: Renewed Opportunities for Drug Development, PPAR Res, 2020 (2020) 9657380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Camp HS, Li O, Wise SC, Hong YH, Frankowski CL, Shen X, Vanbogelen R, Leff T, Differential activation of peroxisome proliferator-activated receptor-gamma by troglitazone and rosiglitazone, Diabetes, 49 (2000) 539–547. [DOI] [PubMed] [Google Scholar]
- [39].Huang PA, Beedie SL, Chau CH, Venzon DJ, Gere S, Kazandjian D, Korde N, Mailankody S, Landgren O, Figg WD, Cereblon gene variants and clinical outcome in multiple myeloma patients treated with lenalidomide, Sci Rep, 9 (2019) 14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Iskierka-Jazdzewska E, Canzian F, Stepien A, Martino A, Campa D, Stein A, Krawczyk-Kulis M, Rybicka-Ramos M, Kyrcz-Krzemien S, Butrym A, Mazur G, Jurczyszyn A, Zawirska D, Grzasko N, Tomczak W, Subocz E, Watek M, Pasiarski M, Rymko M, Calbecka M, Druzd-Sitek A, Walewski J, Kruszewski M, Razny M, Zaucha JM, Dudzinski M, Gaj P, Robak T, Warzocha K, Jamroziak K, Cereblon (CRBN) gene polymorphisms predict clinical response and progression-free survival in relapsed/refractory multiple myeloma patients treated with lenalidomide: a pharmacogenetic study from the IMMEnSE consortium, Leuk Lymphoma, 61 (2020) 699–706. [DOI] [PubMed] [Google Scholar]
- [41].Gooding S, Ansari-Pour N, Towfic F, Ortiz Estevez M, Chamberlain PP, Tsai KT, Flynt E, Hirst M, Rozelle D, Dhiman P, Neri P, Ramasamy K, Bahlis N, Vyas P, Thakurta A, Multiple cereblon genetic changes are associated with acquired resistance to lenalidomide or pomalidomide in multiple myeloma, Blood, 137 (2021) 232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Leibowitz MD, Ardecky RJ, Boehm MF, Broderick CL, Carfagna MA, Crombie DL, D'Arrigo J, Etgen GJ, Faul MM, Grese TA, Havel H, Hein NI, Heyman RA, Jolley D, Klausing K, Liu S, Mais DE, Mapes CM, Marschke KB, Michellys PY, Montrose-Rafizadeh C, Ogilvie KM, Pascual B, Rungta D, Tyhonas JS, Urcan MS, Wardlow M, Yumibe N, Reifel-Miller A, Biological characterization of a heterodimer-selective retinoid X receptor modulator: potential benefits for the treatment of type 2 diabetes, Endocrinology, 147 (2006) 1044–1053. [DOI] [PubMed] [Google Scholar]
- [43].Chute JP, Muramoto GG, Whitesides J, Colvin M, Safi R, Chao NJ, McDonnell DP, Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells, Proc Natl Acad Sci U S A, 103 (2006) 11707–11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hao BB, Li XJ, Jia XL, Wang YX, Zhai LH, Li DZ, Liu J, Zhang D, Chen YL, Xu YH, Lee SK, Xu GF, Chen XH, Dang YJ, Liu B, Tan MJ, The novel cereblon modulator CC-885 inhibits mitophagy via selective degradation of BNIP3L, Acta Pharmacol Sin, 41 (2020) 1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yang K, Zhao Y, Nie X, Wu H, Wang B, Almodovar-Rivera CM, Xie H, Tang W, A Cell-Based Target Engagement Assay for the Identification of Cereblon E3 Ubiquitin Ligase Ligands and Their Application in HDAC6 Degraders, Cell Chem Biol, 27 (2020) 866–876 e868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Martinez-Høyer S, Karsan A, Mechanisms of lenalidomide sensitivity and resistance, Experimental Hematology, 91 (2020) 22–31. [DOI] [PubMed] [Google Scholar]
- [47].Yamamoto T, Nakayama J, Yamamoto Y, Kuroda M, Hattori Y, Ochiya T, SORT1/LAMP2-mediated extracellular vesicle secretion and cell adhesion are linked to lenalidomide resistance in multiple myeloma, Blood Adv, 6 (2022) 2480–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Du L, Liu W, Pichiorri F, Rosen ST, SUMOylation inhibition enhances multiple myeloma sensitivity to lenalidomide, Cancer Gene Ther, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dimopoulos K, Søgaard Helbo A, Fibiger Munch-Petersen H, Sjö L, Christensen J, Sommer Kristensen L, Asmar F, Hermansen NEU, O'Connel C, Gimsing P, Liang G, Grønbaek K, Dual inhibition of DNMTs and EZH2 can overcome both intrinsic and acquired resistance of myeloma cells to IMiDs in a cereblon-independent manner, Molecular oncology, 12 (2018) 180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sebastian S, Zhu YX, Braggio E, Shi CX, Panchabhai SC, Van Wier SA, Ahmann GJ, Chesi M, Bergsagel PL, Stewart AK, Fonseca R, Multiple myeloma cells' capacity to decompose H(2)O(2) determines lenalidomide sensitivity, Blood, 129 (2017) 991–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jakobsen T, Dahl M, Dimopoulos K, Grønbæk K, Kjems J, Kristensen LS, Genome-Wide Circular RNA Expression Patterns Reflect Resistance to Immunomodulatory Drugs in Multiple Myeloma Cells, Cancers (Basel), 13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ocio EM, Fernández-Lázaro D, San-Segundo L, López-Corral L, Corchete LA, Gutiérrez NC, Garayoa M, Paíno T, García-Gómez A, Delgado M, Montero JC, Díaz-Rodríguez E, Mateos MV, Pandiella A, Couto S, Wang M, Bjorklund CC, San-Miguel JF, In vivo murine model of acquired resistance in myeloma reveals differential mechanisms for lenalidomide and pomalidomide in combination with dexamethasone, Leukemia, 29 (2015) 705–714. [DOI] [PubMed] [Google Scholar]
- [53].Ng YLD, Ramberger E, Bohl SR, Dolnik A, Steinebach C, Conrad T, Müller S, Popp O, Kull M, Haji M, Gütschow M, Döhner H, Walther W, Keller U, Bullinger L, Mertins P, Krönke J, Proteomic profiling reveals CDK6 upregulation as a targetable resistance mechanism for lenalidomide in multiple myeloma, Nature communications, 13 (2022) 1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bjorklund CC, Ma W, Wang ZQ, Davis RE, Kuhn DJ, Kornblau SM, Wang M, Shah JJ, Orlowski RZ, Evidence of a role for activation of Wnt/beta-catenin signaling in the resistance of plasma cells to lenalidomide, J Biol Chem, 286 (2011) 11009–11020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cippitelli M, Stabile H, Kosta A, Petillo S, Gismondi A, Santoni A, Fionda C, Role of Aiolos and Ikaros in the Antitumor and Immunomodulatory Activity of IMiDs in Multiple Myeloma: Better to Lose Than to Find Them, Int J Mol Sci, 22 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sponaas AM, Waage A, Vandsemb EN, Misund K, Borset M, Sundan A, Slordahl TS, Standal T, Bystander Memory T Cells and IMiD/Checkpoint Therapy in Multiple Myeloma: A Dangerous Tango?, Front Immunol, 12 (2021) 636375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hines IN, Kremer M, Moore SM, Wheeler MD, Impaired T cell-mediated hepatitis in peroxisome proliferator activated receptor alpha (PPARalpha)-deficient mice, Biol Res, 51 (2018) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jones DC, Ding X, Zhang TY, Daynes RA, Peroxisome proliferator-activated receptor alpha negatively regulates T-bet transcription through suppression of p38 mitogen-activated protein kinase activation, J Immunol, 171 (2003) 196–203. [DOI] [PubMed] [Google Scholar]
- [59].Zhang C, Zhang X, Ma L, Peng F, Huang J, Han H, Thalidomide inhibits adipogenesis of orbital fibroblasts in Graves’ ophthalmopathy, Endocrine, 41 (2012) 248–255. [DOI] [PubMed] [Google Scholar]
- [60].DeCicco KL, Tanaka T, Andreola F, De Luca LM, The effect of thalidomide on non-small cell lung cancer (NSCLC) cell lines: possible involvement in the PPARgamma pathway, Carcinogenesis, 25 (2004) 1805–1812. [DOI] [PubMed] [Google Scholar]
- [61].Hu J, Cao X, Pang D, Luo Q, Zou Y, Feng B, Li L, Chen Z, Huang C, Tumor grade related expression of neuroglobin is negatively regulated by PPARgamma and confers antioxidant activity in glioma progression, Redox Biol, 12 (2017) 682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Xu J, Zhang Y, Xiao Y, Ma S, Liu Q, Dang S, Jin M, Shi Y, Wan B, Zhang Y, Inhibition of 12/15-lipoxygenase by baicalein induces microglia PPARbeta/delta: a potential therapeutic role for CNS autoimmune disease, Cell Death Dis, 4 (2013) e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cheng HS, Lee JXT, Wahli W, Tan NS, Exploiting vulnerabilities of cancer by targeting nuclear receptors of stromal cells in tumor microenvironment, Mol Cancer, 18 (2019) 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Caioni G, Viscido A, d'Angelo M, Panella G, Castelli V, Merola C, Frieri G, Latella G, Cimini A, Benedetti E, Inflammatory Bowel Disease: New Insights into the Interplay between Environmental Factors and PPARgamma, Int J Mol Sci, 22 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Rashid J, Alobaida A, Al-Hilal TA, Hammouda S, McMurtry IF, Nozik-Grayck E, Stenmark KR, Ahsan F, Repurposing rosiglitazone, a PPAR-gamma agonist and oral antidiabetic, as an inhaled formulation, for the treatment of PAH, J Control Release, 280 (2018) 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Pozzi A, Ibanez MR, Gatica AE, Yang S, Wei S, Mei S, Falck JR, Capdevila JH, Peroxisomal proliferator-activated receptor-alpha-dependent inhibition of endothelial cell proliferation and tumorigenesis, J Biol Chem, 282 (2007) 17685–17695. [DOI] [PubMed] [Google Scholar]
- [67].Wang Y, Xu Y, Zou R, Wu L, Liu P, Yang H, Xie Z, Wang C, Effect of Levocarnitine on the Therapeutic Efficacy of Conventional Therapy in Children with Dilated Cardiomyopathy: Results of a Randomized Trial in 29 Children, Paediatr Drugs, 20 (2018) 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhao Q, Zhong J, Lu P, Feng X, Han Y, Ling C, Guo W, Zhou W, Yu F, DOCK4 Is a Platinum-Chemosensitive and Prognostic-Related Biomarker in Ovarian Cancer, PPAR Res, 2021 (2021) 6629842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Aceto GM, Catalano T, Curia MC, Molecular Aspects of Colorectal Adenomas: The Interplay among Microenvironment, Oxidative Stress, and Predisposition, Biomed Res Int, 2020 (2020) 1726309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Aouali N, Broukou A, Bosseler M, Keunen O, Schlesser V, Janji B, Palissot V, Stordeur P, Berchem G, Epigenetic Activity of Peroxisome Proliferator-Activated Receptor Gamma Agonists Increases the Anticancer Effect of Histone Deacetylase Inhibitors on Multiple Myeloma Cells, PLoS One, 10 (2015) e0130339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Falank C, Fairfield H, Reagan MR, Signaling Interplay between Bone Marrow Adipose Tissue and Multiple Myeloma cells, Front Endocrinol (Lausanne), 7 (2016) 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Garcia-Bates TM, Bernstein SH, Phipps RP, Peroxisome proliferator-activated receptor gamma overexpression suppresses growth and induces apoptosis in human multiple myeloma cells, Clin Cancer Res, 14 (2008) 6414–6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Peters JM, Gonzalez FJ, Muller R, Establishing the Role of PPARbeta/delta in Carcinogenesis, Trends Endocrinol Metab, 26 (2015) 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wang J, Lu L, Kok CH, Saunders VA, Goyne JM, Dang P, Leclercq TM, Hughes TP, White DL, Increased peroxisome proliferator-activated receptor gamma activity reduces imatinib uptake and efficacy in chronic myeloid leukemia mononuclear cells, Haematologica, 102 (2017) 843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Engler JR, Frede A, Saunders VA, Zannettino AC, Hughes TP, White DL, Chronic myeloid leukemia CD34+ cells have reduced uptake of imatinib due to low OCT-1 activity, Leukemia, 24 (2010) 765–770. [DOI] [PubMed] [Google Scholar]
- [76].Nies AT, Schaeffeler E, van der Kuip H, Cascorbi I, Bruhn O, Kneba M, Pott C, Hofmann U, Volk C, Hu S, Baker SD, Sparreboom A, Ruth P, Koepsell H, Schwab M, Cellular uptake of imatinib into leukemic cells is independent of human organic cation transporter 1 (OCT1), Clin Cancer Res, 20 (2014) 985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Gou Q, Gong X, Jin J, Shi J, Hou Y, Peroxisome proliferator-activated receptors (PPARs) are potential drug targets for cancer therapy, Oncotarget, 8 (2017) 60704–60709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bao M, Zhang K, Wei Y, Hua W, Gao Y, Li X, Ye L, Therapeutic potentials and modulatory mechanisms of fatty acids in bone, Cell Prolif, 53 (2020) e12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Tan EHP, Sng MK, How ISB, Chan JSK, Chen J, Tan CK, Wahli W, Tan NS, ROS release by PPARbeta/delta-null fibroblasts reduces tumor load through epithelial antioxidant response, Oncogene, 37 (2018) 2067–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Montagner A, Wahli W, Tan NS, Nuclear receptor peroxisome proliferator activated receptor (PPAR) beta/delta in skin wound healing and cancer, Eur J Dermatol, 25 Suppl 1 (2015) 4–11. [DOI] [PubMed] [Google Scholar]
- [81].Takada I, Makishima M, Peroxisome proliferator-activated receptor agonists and antagonists: a patent review (2014-present), Expert Opin Ther Pat, 30 (2020) 1–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Treatment with PPAR agonists downregulated the mRNA and protein expression levels of CRBN.
Fig. S2. PPAR agonists downregulated the mRNA and protein expression levels of CRBN in a dose dependent manner.
Fig. S3. PPAR expression level negatively correlated with lenalidomide sensitivity.
Fig. S4. Overexpression of CRBN reversed the effect of PPAR agonists on lenalidomide sensitivity in vitro.
Fig. S5. The relationship of PPARs and CRBN in MM human datasets and cell lines.
Fig. S6. PPARs are direct transcriptional regulator of CRBN in MM1.R cells.
Table S1. Newly diagnosed myeloma patient characteristics.