Abstract
Objectives:
To describe the prevalence of acute retroviral syndrome (ARS) and associated findings during primary HIV, and explore the relationship of ARS to clinical, virological, and immunological outcomes within a longitudinal screen, retest and treat study that minimized ascertainment bias.
Design
We evaluated ARS symptoms among 216 persons with acute and early incident HIV within the Sabes study of timing of antiretroviral therapy (ART) initiation during primary HIV in Peru.
Methods
We evaluated patient reported symptoms and exam findings during primary HIV and used logistic regression and generalized linear models to evaluate associations with CD4+ and CD8+ T cell counts, HIV viral load, and a panel of 23 soluble markers of immune activation.
Results
61% of participants had at least one ARS finding and 35% had at least 3. More ARS findings were reported in those enrolled within a month of estimated date of detectable infection (EDDI). Having more ARS signs/symptoms was associated with increased risk of CD4 decrease below 350 cells/mL within the first 24 weeks, failure to suppress HIV viral load, and was most strongly associated with elevated IP-10. Immediate ART blunted effects on symptoms, CD4 and viral load, as associations were strongest in the arm that started ART after 24 weeks. Detrimental associations of ARS with CD4 counts, and CD4/CD8 ratio were not maintained at 2 or 4 years.
Conclusions
ARS has marked associations with short-term immunologic function and virologic suppression, which were mitigated in participants randomized to initiate ART immediately during primary infection.
INTRODUCTION
Rapid antiretroviral treatment (ART) initiation during early HIV infection has been shown to improve long-term individual clinical outcomes and decreases onward HIV transmission1–4. The clinical recognition of acute retroviral syndrome (ARS), a constellation of non-specific symptoms experienced by 65–95% of persons shortly after HIV acquisition5, could provide a unique opportunity for interventions, including HIV testing and rapid treatment initiation. Importantly, the presence and severity of ARS has been associated with higher initial HIV viral load (VL) and lower CD4 count6–8 and adverse long-term clinical outcomes, primarily before early ART initiation was recommended6–8. Recent studies have highlighted the association of ARS with heightened innate immune responses and cytokine levels. Persistent elevations of some cytokines, such as TNF-α and IL-6, have been demonstrated with ARS even after ART initiation, and are a potential contributor to the increased morbidity in people who experience ARS8.
Characterization of ARS is often limited by ascertainment bias due to its non-specific nature and lack of recognition of its presentation. Outside of highly surveilled populations, most HIV diagnoses are made months or years after acquisition and recall of distant non-specific symptoms is subject to bias. We describe the prevalence of ARS-associated symptoms and physical findings during primary HIV among men who have sex with men (MSM) and transgender women (TW) in Peru, minimizing ascertainment and recall biases and explore the relationship of ARS to virological and immunological outcomes in a prospective study.
METHODS
Trial Design and Study Participants
This analysis includes the 216 MSM and TW diagnosed with acute (seronegative, HIV RNA+) or early (seropositive with a negative HIV result within three months) HIV infection in the Sabes study, whose design has been previously reported9 (details in Supplemental Methods). In brief, the Sabes study assessed an HIV test-and-treat strategy in Lima, Peru from 2013 to 2017, screening over 3000 MSM and TW at risk for HIV acquisition, conducting monthly HIV testing with third generation HIV serology tests and HIV-1 RNA tests (pooled NAAT testing) for over 2,100 participants who were HIV-negative at baseline. Persons with incident primary HIV were enrolled into the treatment phase of Sabes which included randomization to immediate vs. deferred (by six months) ART initiation (Figure 1A). ART was either efavirenz or elvitegravir/cobicistat co-formulated with tenofovir disoproxil fumarate and emtricitabine (EFV/FTC/TDF or EFV/cobi/FTC/TDF, depending on year of entry).3 Individuals in the deferred arm were offered ART before 24 weeks if they reached contemporary Peruvian criteria for ART initiation or by clinician discretion, most often for severe ARS symptoms or low CD4 counts. Peru adopted WHO “test and treat” guidelines after enrollment was completed (2016); PrEP was not yet approved10,11. Participants were followed for at least 48 weeks; a subset of virally-suppressed participants (N=105) remained in a follow-on study for at least 4 years from ART initiation.
FIGURE 1. Number and type of Acute Retroviral Syndrome signs and symptoms, and proprotion achieving viral load suppression over 48 weeks, among Sabes Study participants.
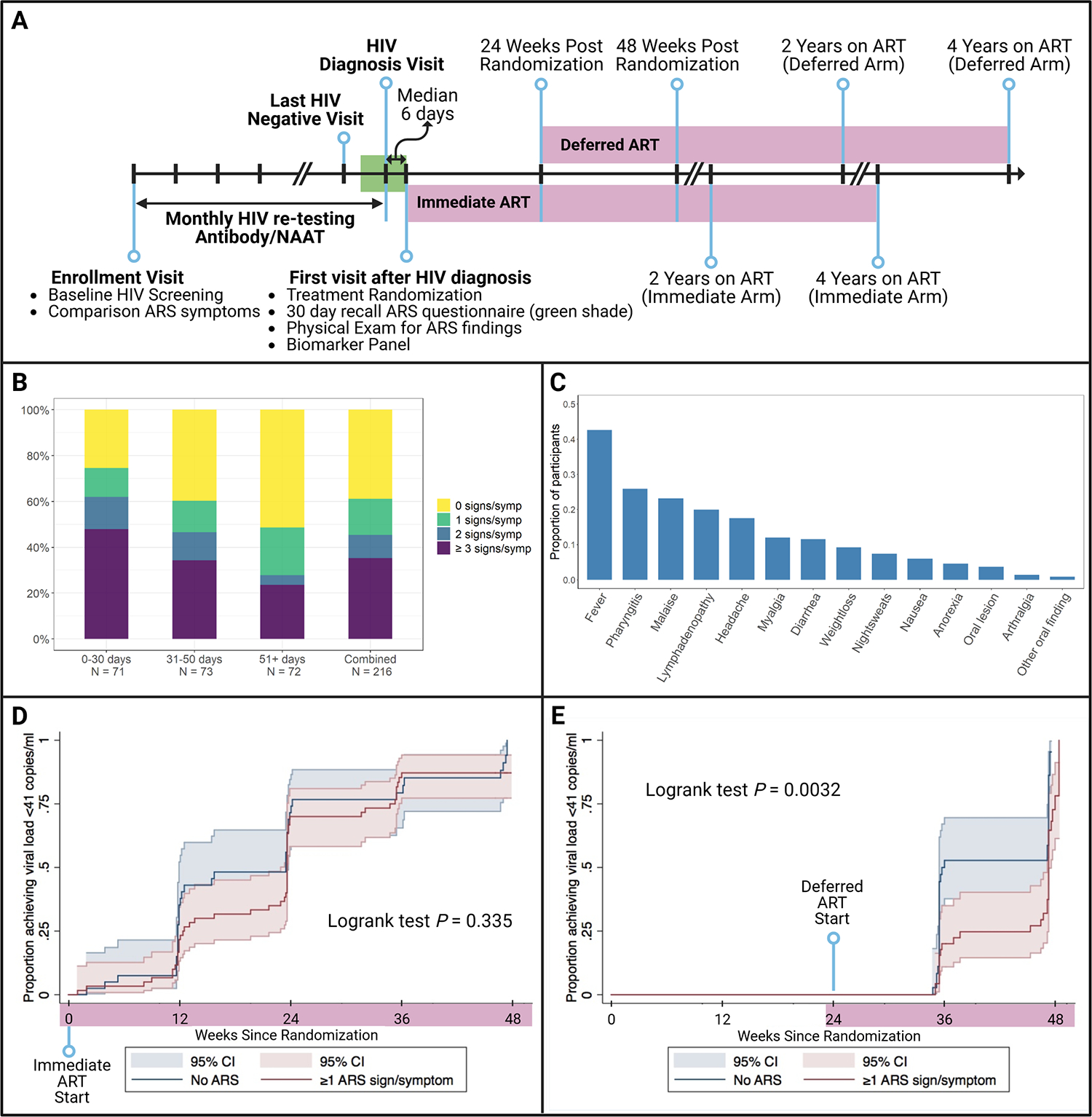
1A. Sabes Study Schema. Participants were screend for HIV at enrollment, and only those testing negative by Ab and RNA were enrolled in the follow-on monthly rescreening step. Those who tested positive for either acute (HIV RNA+ve/Ab -ve) or recent HIV (Ab +ve with negative test within 3 months) infection were enrolled in the treatment step (n=216) and randomized to begin ART Immediately or defer ART initiation by 24 weeks. At the first visit after HIV diagnosis, participants answered questionnaires about acute retroviral syndrome (ARS) symptoms during the past 30 days, to cover the time interval between the Estimated Date of Detectable Infection (EDDI) and the first post-HIV diagnosis visit (green shading). Randomization to immediate vs. deferred ART intiation occurred at this visit (first virsit after HIV diagnosis). Participants started ART per randomization and continued in the Sabes study for a total of 48 weeks. A subset of participants (n=105) continued under research follow-up and continued to receive ART for at least 4 years after ART initiation (lavender shaded intervals).
1B. Number of Acute Retroviral Syndrome Signs/Symptoms by interval from Estimated Date of Detectable Infection (EDDI) to treatment study Enrollment, and overall. The average number of symptoms among those who had at least one was 3.25, and the highest number of symptoms reported by a single individual was 13. ARS prevalence differed by time from EDDI to enrollment in the randomized treatment step of the Sabes study. Among those who enrolled/randomized within a month of EDDI, only 25% had no ARS symptoms, and 48% had three or more. The lowest prevalence of ARS was seen among the population enrolling at least 50 days after EDDI, in which 51% of individuals reported no symptoms and 24% reported three or more.
1C. Distribution of signs and symptoms among Sabes participants (n=216). Fever was the most common ARS finding, experienced by 43% all study participants, followed by pharyngitis (26%), malaise (23%), enlarged lymph nodes (20%), and headache (18%). Myalgia, diarrhea, and weight loss were also common.
1D and 1E. Proportion of Sabes participants achieving viral load suppression over 48 weeks by presence or absence of acute retroviral syndrome findings, stratified by immediate (1D) or deferred (1E) antiretroviral therapy (ART) initiation. Kaplan-Meier analysis with log-rank tests were performed. In participants treated with ART immediately during primary HIV infection, there was no difference in viral load suppression by presence of acute retroviral syndrome (1D, p=0.335), while in those randomized to defer ART for 24 weeks after primary HIV diagnosis, persons with at least 1 sign/symptom of acute retroviral syndrome were less likely to achieve viral load suppression over 48 weeks (p=0.0032).
The estimated date of detectable infection (EDDI) was calculated using a published algorithm incorporating all HIV testing data including assay-specific uncertainty windows12.
This study was approved by the Institutional Review Board at the Fred Hutchinson Cancer Center and the Institutional Bioethics Committee at Asociación Civil Impacta Salud y Educación and authorized by the Peruvian National Institute of Health. All participants provided written consent in Spanish.
Data Collection
Structured histories and physicals were performed for all participants at the baseline HIV screening visit, and onward monthly visits at clinician discretion. At the first post-HIV diagnosis visit (a median of 6 days later) (ART randomization visit), participants had a physical exam and completed a questionnaire about ARS signs and symptoms (ARS findings) collecting data on the prior 30 days (Figure 1A). In addition, any ARS findings present at HIV diagnosis were noted.
We evaluated CD4+ and CD8+ T cell counts and quantitative HIV-1 VL (Abbott Laboratories, Lake Bluff, IL; lower limit of quantitation, 40 copies/mL) at treatment randomization, every 12 weeks for the first year, and semiannually thereafter. A panel of 20 markers of immune activation was performed using Meso Scale Discovery U-PLEX chemiluminescent immunoassay panels (Meso Scale Diagnostics, Rockville, MD, USA) on plasma from trial entry on a subset of participants. EIA assays for soluble CD14 (sCD14), C-reactive protein (CRP), and D-dimer were also performed (full biomarker list in Table 1) 3,13
Table 1. Relationship of Acute Retroviral Syndrome findings to concurrent cytokine concentration at time of HIV diagnosis.
Generalized Linear Models (GLM) were used to evaluate ordinal categories of ARS findings (0, 1–2, 3+ signs or symptoms) with biomarker concentrations in unadjusted models, as well as models adjusted by interval in days between Estimated Date of Detectable Infection to enrollment/randomization to ART step (EDDI to ENR, treated as a continuous variable). Regression results only presented if unadjusted p <0.20; biomarkers analyzed but not shown due to lack of effect include: SDF-1α, MIP-1β, IL-2, IL-4, IL-6, IL-7, IL-8, IL-12p70, IL-1α, IL-1β, IL-16, TNF-α, MIP-1α, and CRP.
| ARS Category (Unadjusted) | ARS Category (adjusted for EDDI to ENR) | ||||||
|---|---|---|---|---|---|---|---|
| Biomarkera | Coeff | P | p adj (BH)b | Coeff | P | p adj (BH) | |
| TNF-β | −0.249 | 0.096 | 0.42 | TNF-β | −0.260 | 0.062 | 0.45 |
| MCP-1 | 0.969 | 0.039 | 0.21 | MCP-1 | 0.507 | 0.28 | 0.87 |
| IL-23p40 | 0.499 | 0.019 | 0.14 | IL-23p40 | 0.420 | 0.037 | 0.41 |
| IFN-α2a | 0.105 | 0.158 | 0.48 | IFN-α2a | 0.044 | 0.548 | 0.93 |
| IP-10 | 0.704 | 0.004 | 0.088 | IP-10 | 0.521 | 0.031 | 0.41 |
| IFN-γ | 0.463 | 0.011 | 0.12 | IFN-γ | 0.245 | 0.199 | 0.73 |
| IL-10 | 0.347 | 0.159 | 0.48 | IL-10 | −0.019 | 0.940 | 0.98 |
| D-dimer | −0.101 | 0.173 | 0.48 | D-dimer | −0.114 | 0.111 | 0.61 |
All biomarkers were log transformed, with exception of CRP and D-dimer, which were analyzed untransformed per standard usage.
Unadjusted p values are presented in the table. P values were also adjusted using the Benjamini-Hochberg method, none retained significance at the level of <0.05. One relationship trended towards significance after BH adjustment (IP-10, p=0.088).
Analysis Methods
All ARS sign and symptom data collected after EDDI up to and including the first post-diagnosis (randomization) visit were included in this analysis.
The percentage of individuals with 0, 1, 2, or ≥3 findings at these visits was used to describe the overall prevalence of presumptive ARS. We analyzed the prevalence of ARS findings stratified by time from EDDI to enrollment into the treatment phase (ENR), using three EDDI to ENR intervals: ≤30 days, 31–50 days, or >50 days. We used logistic regression to examine the relationship of number of ARS findings to subsequent CD4 counts and HIV VL. These analyses considered number of ARS findings (0, 1–2, or ≥3) as predictors of CD4 count drop below 350 cells/mL in the first 24 weeks following enrollment and failure to suppress viral load to ≤40 copies/mL within 12 weeks after ART initiation. Analyses evaluating associations with CD4 counts and CD4/CD8 ratio were performed out to 4 years after ART initiation. Additional analyses were adjusted for days from EDDI to ENR and ART regimen (EFV vs EVG/cobi-based regimen). Kaplan-Meier analysis with log-rank tests evaluated the association between ARS and time to VL suppression (≤40 copies/mL) after starting ART. We performed exploratory analyses of the relationship between number of ARS findings and inflammatory markers using general linear models (GLM) and biomarker concentrations (log10 transformed except CRP and D-dimer), and with adjustment for EDDI-to-ENR interval. The Benjamini-Hochberg (BH) method was used to correct for multiple comparisons of biomarkers14.
Analyses were performed in STATA version 17.0 (College Station, TX) and R studio (Version 1.3.1093, Open Source)
Results
Of 216 persons with acute or early incident HIV, 84 individuals (39%) had no signs or symptoms of ARS, while 132 (61%) had documentation of at least one and 35% had three or more (Figure 1B). In contrast, only 9.9% of 162 participants with available data from baseline (first HIV-negative screen) (Figure 1A) reported any ‘ARS’ signs/symptoms in the past 30 days. ARS findings were most common in those enrolled within a month of EDDI and fever, pharyngitis and malaise were most reported (Figure 1C). Demographics of this population has been previously described3, and was similar among those with or without ARS (Table S1). At enrollment, CD4 counts and CD4/CD8 ratios trended higher, but not significantly so, in those without ARS findings, and the viral load was higher in those with at least one ARS finding (Table S1).
On average, individuals with more ARS findings were more likely to experience a CD4 decline to ≤350 in the first 24 weeks of the study. Among those who began ART immediately, 45% with ≥3 findings experienced CD4 ≤350, vs. 38% of those with none. In the deferred arm, 62% of those with ≥3 ARS findings experienced CD4 counts ≤350 before 24 weeks, vs. 39% among those without ARS findings. In logistic regression models limited to the deferred arm, a CD4 count decline to ≤350 was associated with ≥3, but not 1–2, ARS findings vs. none (adjusted OR (aOR) =2.96, p=0.03, adjusted for EDDI to enrollment interval and ART type). However, 2 years after ART initiation, CD4 counts averaged 63 cells/mL higher per each ARS category (0, 1–2, or ≥3; p=0.042, adjusted). Among participants who maintained viral suppression, there were no associations between ARS and CD8 count or CD4/CD8 ratio at 2 (N=100) or 4 (N=105) years after ART initiation, nor with CD4 count at year 4 (Table S2).
Number of ARS findings also predicted failure to suppress VL. Among those with no ARS findings, 62% and 47% failed to achieve viral suppression within 12 weeks of ART initiation in the Immediate and Deferred arms, respectively. Among those with three or more symptoms, 81% and 80%, failed to achieve suppression within 3 months. In models controlling for time from EDDI to enrollment and ART regimen, ≥3, but not 1–2, ARS findings were associated with increased risk of failure to suppress VL in the Immediate arm (aOR=4.61, p=0.016). Among Deferred ART participants, those with 1–2 or ≥3 ARS findings were at increased risk of failure to suppress VL compared to those with none (aOR=4.78, P<0.01 and aOR=4.77, p=0.02, respectively). Kaplan-Meier (KM) analysis comparing viral suppression over 48 weeks in participants with at least one ARS finding to those with none found no difference among Immediate arm participants (log rank p=0.335, Figure 1D). The KM curves differed significantly among individuals with ≥1 ARS finding vs. none in the Deferred arm (p=0.0032, Figure 1E) with higher likelihood of suppression by week 12 on ART in those without ARS.
There was a trend between number of ARS findings and clinical trial enrollment levels of TNF-β, MCP-1, IL-23p40, IFN-γ, and IP-10 (Table 1). All coefficients were positive, except for TNF-β, indicating that biomarker levels increased on average with increasing number of ARS findings. IP-10 had the strongest association with ARS (0.70 log10 increase per ARS category, p=0.004, slightly attenuated to 0.52 log10 per category when adjusted for time from EDDI to ENR). After BH correction, a trend remained for association with IP-10 (p=0.088); no other significant associations were seen. Among the 40 individuals with available pre-HIV-acquisition data (collected at monthly HIV screening visits (Figure 1A), there was no association between ARS category and change in IP-10 levels from pre-infection baseline.
Discussion
Our results confirm the observations of others that the signs and symptoms of ARS are largely non-specific5 and occur early after HIV acquisition (near peak VL)15 or around time of seroconversion8. Our analysis showed that not only was VL higher in those with ARS, but persons with ARS were less likely to suppress their VL, with a greater effect in the deferred group, suggesting that the effect was not confounded by timing of diagnosis or viral load at diagnosis. Deferred ART participants with ≥3 ARS findings were more likely to experience CD4 declines to ≤350 than those with none. Importantly, immediate ART initiation blunted both the ARS-associated VL and CD4 effects compared with what was seen in those randomized to defer ART by 24 weeks; CD4 counts at 2 years recovered to higher levels in participants with ARS, more prominently in those treated immediately. A randomized study like that reported here could no longer be undertaken given the clear personal and population health benefits of immediate ART initiation11,16,17. There were no long-term associations of ARS with CD8 counts or CD4/CD8 ratios overall, or by treatment allocation.
Our analysis, conducted in a subtype B cohort, confirmed a prior report of a strong association between IP-10 with ARS in predominantly African participants with Fiebig I and Fiebig II infection18, although we did not observe an association after subtracting pre-infection IP-10 levels as they described. We saw weaker associations of ARS with other markers (e.g. IFN-γ, TNF-α, CRP and IL-6) reported previously from cohorts in Africa and Thailand8,18.
Strengths of this analysis include assessment of ARS findings shortly after identification of incident HIV infection during follow-up, pre-infection data on findings associated with ARS and immune activation markers, randomized allocation to time of ART initiation. Limitations include small sample size and limited follow-up for some participants.
Our analyses suggest that presence of ARS is a predictor of short-term CD4 count decline and delayed VL suppression, both in persons who were treated or untreated during primary infection. The initiation of ART immediately during early primary infection was shown to blunt these deleterious effects.
Supplementary Material
Funding:
This work was supported by the US National Institutes of Health (R01DA032106 and R01DA040532). RBI is supported by the NIAID (K23AI129659). RG received a summer intern fellowship from the American Society of Tropical Medicine and Hygiene. Gilead and Merck provided antiretroviral therapy drugs for the Sabes and MERLIN studies at no cost.
Footnotes
Disclosures: All authors report no conflict of interest with this work
References
- 1.Crowell TA, Phanuphak N, Pinyakorn S, et al. Virologic failure is uncommon after treatment initiation during acute HIV infection. Aids. 2016;30(12):1943–1950. [DOI] [PubMed] [Google Scholar]
- 2.Hogan CM, DeGruttola V, Sun X, et al. The setpoint study (ACTG A5217): effect of immediate versus deferred antiretroviral therapy on virologic set point in recently HIV-1–infected individuals. Journal of Infectious Diseases. 2012;205(1):87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lama JR, Ignacio RAB, Alfaro R, et al. Clinical and immunologic outcomes after immediate or deferred antiretroviral therapy initiation during primary human immunodeficiency virus infection: the Sabes randomized clinical study. Clinical Infectious Diseases. 2021;72(6):1042–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimitrov D, Wood D, Ulrich A, et al. Projected effectiveness of HIV detection during early infection and rapid ART initiation among MSM and transgender women in Peru: a modeling study. Infectious Disease Modelling. 2019;4:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henn A, Flateau C, Gallien S. Primary HIV infection: clinical presentation, testing, and treatment. Current infectious disease reports. 2017;19(10):1–10. [DOI] [PubMed] [Google Scholar]
- 6.Socías ME, Sued O, Laufer N, et al. Acute retroviral syndrome and high baseline viral load are predictors of rapid HIV progression among untreated Argentinean seroconverters. Journal of the International AIDS Society. 2011;14(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinicco A, Fora R, Sciandra M, Lucchini A, Caramello P, Gioannini P. Risk of developing AIDS after primary acute HIV-1 infection. Journal of acquired immune deficiency syndromes. 1993;6(6):575–581. [PubMed] [Google Scholar]
- 8.Crowell TA, Colby DJ, Pinyakorn S, et al. Acute Retroviral Syndrome Is Associated With High Viral Burden, CD4 Depletion, and Immune Activation in Systemic and Tissue Compartments. Clin Infect Dis. 2018;66(10):1540–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lama JR, Brezak A, Dobbins JG, et al. Design strategy of the Sabes Study: diagnosis and treatment of early HIV infection among men who have sex with men and transgender women in Lima, Peru, 2013–2017. American journal of epidemiology. 2018;187(8):1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.AVAC. PrEPWatch Global PrEP Tracker. https://www.prepwatch.org/country/peru/. Accessed 26 June, 2022.
- 11.WHO. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. World Health Organization; 2015. [PubMed] [Google Scholar]
- 12.Grebe E, Facente SN, Bingham J, et al. Interpreting HIV Diagnostic Histories into Infection Time Estimates: Analytical Framework and Online Tool. 2018:323808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MesoScale Diagnostics L MSD U-PLEX Platform Product Insert. 2015; https://www.mesoscale.com/~/media/files/product%20inserts/u-plex%20%20human%20group%201%20custom%20product%20insert.pdf. Accessed 14 April 2016.
- 14.Thissen D, Steinberg L, Kuang D. Quick and easy implementation of the Benjamini-Hochberg procedure for controlling the false positive rate in multiple comparisons. Journal of educational and behavioral statistics. 2002;27(1):77–83. [Google Scholar]
- 15.Robb ML, Eller LA, Kibuuka H, et al. Prospective study of acute HIV-1 infection in adults in East Africa and Thailand. New England Journal of Medicine. 2016;374(22):2120–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundgren JD, Babiker AG, Gordin F, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen MS, McCauley M, Gamble TR. HIV treatment as prevention and HPTN 052. Current Opinion in HIV and AIDS. 2012;7(2):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassan AS, Hare J, Gounder K, et al. A Stronger Innate Immune Response During Hyperacute Human Immunodeficiency Virus Type 1 (HIV-1) Infection Is Associated With Acute Retroviral Syndrome. Clin Infect Dis. 2021;73(5):832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


