Abstract
Cyclical fluctuations of the ovarian hormones estrogen (E2) and progesterone (PROG) have multiple effects on reproduction and development. However, little is known about the roles of E2 and PROG in women’s social behaviors. Here, based on evolutionary theory suggesting social sensitivity and inhibition ability are conductive to maintaining social relationships, we provide evidence for the association between menstrual phases and social orientation. In Study 1, 78 women provided saliva samples and reported their intensity of behavioral activation/inhibition system (BAS/BIS) and interpersonal sensitivity at either of two phases of the menstrual cycle: late follicular phase (FP), and mid-luteal phase (LP). A significant between-subject association emerged, revealing that women with higher PROG levels reported higher levels of social feedback sensitivity, and women with relatively high PROG levels showed a positive association between their E2 levels and inhibitory response. In Study 2, 30 women reported their interpersonal anxiety and finished the social value orientation (SVO) measures at both late FP and mid-LP. A significant within-person effect emerged: women in the mid-LP, which is characterized by higher PROG levels, reported higher levels of interpersonal anxiety and SVO. In sum, these findings revealed that women’s social orientation could fluctuate naturally with ovarian hormones across the menstrual cycle.
Keywords: menstrual cycle, progesterone, social orientation, interpersonal anxiety, inhibition system
Introduction
Considerable evidence indicates that, beyond the physiological effects on reproduction and development, steroid hormones have broader influences on human and non-human social behaviors. For example, testosterone is reported to rapidly potentiate impulsive and aggressive behaviors (Justin et al., 2017). Endogenous increases in testosterone in human males has been suggested to facilitate aggression in competitive contexts with the function of maintain social dominance and establishing access to mating opportunities (Archer, 2006). Compared to the established effects of testosterone, little attention has been devoted to the involvement of estrogen (E2) and progesterone (PROG) in social processes.
E2 and PROG are two mainly ovarian hormones that fluctuate naturally across the menstrual cycle. Generally, the menstrual cycle can be divided into two main phases: the follicular phase (FP), between onset of menses and ovulation with rising E2 levels and low PROG levels; the luteal phase (LP), starting after ovulation until the onset of the next menses characterized by high levels of PROG and second rising levels of E2 in the mid-LP (Sakaki & Mather, 2012). From an evolutionary perspective, rising in PROG levels during the LP prepares female body for possible pregnancy (Conway et al., 2007), whereas elevated E2 levels favor reproductive goals during the late FP (Thornhill & Gangestad, 2008). Over the past decade, a growing body of research has indicated women’s preferences and behaviors could shift by reproductive goals in the late FP (Durante et al., 2015). During this high fertility phase, researchers find women seek high-status products, increase intra-sexual competition (Durante et al., 2011), prefer appearance-enhancing products (Zhuang & Wang, 2014), and exhibit more impulsivity to high-calorie food and nicotine (Sakaki & Mather, 2012). In contrast, pregnancy is a particularly vulnerable state for women as their mobility is limited (Dufour & Sauther, 2002), thereby intensifying women’s need to avoid disease (Jones et al., 2005) and depend on social alliances (Taylor et al., 2000). In line with this theorizing, women in their LP are found to exhibit greater sensitivity to social stimuli (Maner & Miller, 2014) and to be more strongly committed to their romantic partner (Jones et al., 2005). Recently, a study reported significant covariations between PROG and the affiliation motivation, which was defined as a need to have close, friendly relationships with others (Schultheiss et al., 2004). In another study, researchers found that women with higher average PROG levels reported higher levels of anxiety about the relationship with partner, which the authors speculated was because of heightened interpersonal sensitivity during women’s mid-LP (Reynolds et al., 2018). These indirect lines of evidence warrant closer examination of the involvement of PROG in affiliation motivation.
Bjorklund and Harnishfeger (1995) proposed that for the purposes of cooperation and group cohesion, people evolved inhibition mechanisms that required behavioral and emotional responses. They hypothesized it was important for individuals to control their sexual and aggressive behaviors in small group (Bjorklund & Kipp, 1995). PROG has been used to decrease sexual motivation (Cooper, 1986). Meanwhile, higher E2 and PROG have been generally linked to enhanced cognitive function (Hatta & Nagaya, 2009), which also appears to be improved near the mid-LP characterized by elevated E2 and P4 (Solís-Ortiz & Corsi-Cabrera, 2008). Eating disorder studies further indicate that females are less able to control impulse eating when E2 and PROG are both low (Klump et al., 2013). These studies suggest a possible relationship between ovarian hormones and the self-inhibition ability.
Thus, in the current study, we investigated whether between-women (Study 1) and within-women (Study 2) cyclical fluctuation in ovarian hormones are associated with changes in sensitivity to social feedback, inhibition responses and social orientation. Specifically, in Study 1, we used the behavioral activation system (BAS) and behavioral inhibition system (BIS) scales to test relationships between ovarian hormones and trait impulsivity and inhibition. In prior personality studies, which the BAS correlated strongly with impulsivity and rewards, while the BIS related to inhibition and anxiety (Carver & White, 1994). Besides, in Study 1, we used a self-awareness scale toward self and social feedback to explore the interactions of E2 and PROG on the interpersonal sensitivity. Additionally, given the evidence for interactive effects of E2 and PROG in prior studies (Klump et al., 2013; Llaneza & Frye, 2009) and the associations of PROG with affiliation motivation, we set PROG as a moderating variable on E2 to associations with BAS/BIS and the self-awareness scale. Meanwhile, we chose late FP (high E2, low PROG) and mid-LP (high E2, high PROG) to further explore moderating effects of PROG between the two menstrual groups.
With the intention of examining within-subject covariations of E2 and PROG on social orientation across the menstrual cycle in Study 2, we asked women to finish a more detailed scale about sensitivity toward interpersonal feelings and a social value orientation (SVO) task both in their late FP and mid-LP. This interpersonal scale reflects individual sensitivity and anxiety to interpersonal communication (Norasakkunkit et al., 2012; Reynolds et al., 2018). The SVO measures willingness to share resources between self and different others (Lange & Paul, 1999). It is a highly efficient and reliable measure, and a good predictor of diverse forms of cooperative and prosocial behaviors both in laboratory and real-life settings (Pirita & Sabine, 2013). In addition, Schultheiss et al. (2003) suggested a possible explanation for the positive relationship between PROG and affiliation motive under the reproductive goal. They proposed that PROG might facilitate behaviors aimed at solidifying social bonds with a potential father of the child (toward a male) or establishing one’s extended social group to ensure adequate support throughout a pregnancy (toward females) (Schultheiss et al., 2003). With parental investment theory, other researchers proposed that, because of the needing for pregnancy and involvement in rearing of offspring, females should show more intrasexual cooperation during these activities. To evaluate the validity of these assumptions, in the SVO task, we distinguished the recipient gender as male and female to further investigate the prosocial preferences toward different genders across menstrual cycle.
Based on prior work on ovarian hormones and menstrual cycle associations with affiliation motivation and social behaviors, we hypothesized that 1) between-subjects, women with higher PROG levels would exhibit higher interpersonal sensitivity and activated more behavioral inhibition system, 2) within-subject, women in their mid-LP had higher interpersonal anxiety and divide more resources (i.e. money) to female recipients than during their late FP, and 3) PROG had a moderating effects on E2’s relationships with inhibitory response and social orientation, with higher PROG and E2 levels were more related to increased inhibitory response and cooperation preference.
Study 1
Some researchers proposed that in order to cooperate, individuals need to suppress their impulsivity and improve cognitive inhibition (Bjorklund & Kipp, 1995). Other studies demonstrate that heightened PROG levels are associated with increasing sensitivity to the social information (Maner & Miller, 2014), mid-LP and higher E2 and PROG levels are linked to enhanced cognitive function (Hatta & Nagaya, 2009). Thus, Study 1 tested the potential positively association between PROG and interpersonal sensitivity, and whether PROG could modulate E2’s relationship with interpersonal sensitivity and inhibitory response.
Materials and Methods
Participants
Seventy-eight healthy, right-handed female undergraduates at East China Normal University, Shanghai, China [mean age ± standard deviation (SD), 22.78 ± 2.30 (age range, 19–28) years] participated in this study. Potential participants completed a questionnaire about their menstrual cycle regularity and length within the previous 3 months, the date of last three menstrual onsets, and the use of hormones in any form. Only participants who reported having 26–34-day menstrual cycles, and had not used hormones in the previous 3 months were included. We then predicted the next menstrual onset based on each participant’s last menstrual onset and the average cycle length of the previous 3 months. We used the backward counting method to predict the timing of each participant’s late FP and mid-LP (DeBruine et al., 2005; Gangestad & Thornhill, 1998; Haselton & Gangestad, 2006; Jones et al., 2005; Penton-Voak et al., 1999; Zhuang & Wang, 2014). Forty-one participants finished the session in their late FP (14–16 days prior to the next predicted menstrual onset; 22.78 ± 2.15 years), 37 participants finished the session in the mid-LP (6–8 days prior to the predicted onset; 22.78 ± 2.49 years). After finishing the session, we continued to track the participant’s next menstrual onset to ensure the date was consistent with our prediction. In addition, prior to the experiment, to test the relationship between PROG and other factors, we calculated the required sample size using G*Power 3.1 (Faul et al., 2007). When we set effect size ρ = 0.3, α error probability = 0.05, and 1 − β error probability = 0.8, to obtain a minimum required sample size of 64.
The Ethics Committee of East China Normal University approved the study protocol, and the study was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent and were compensated with 50 RMB per hour.
Salivary Samples
To control for circadian influences on hormone levels, all experimental sessions were performed between 12:00 pm and 7:00 pm (Ycaza et al., 2016). Participants were asked to abstain from eating 1 hour prior and drinking 30 minutes prior to their sessions. Saliva samples were taken immediately when participants came to the laboratory, via passive drool of ∼2 mL of saliva into a collection tube, and each saliva sample was preserved in a refrigerator (−20 °C). All samples were processed for E2 and PROG levels using DRG International, ELISA kits and measured using Thermo Devices, Multiskan MK3. The inter-assay variations for E2 (6.47%) and PROG (13.40%) were acceptable according to prior research (Ycaza et al., 2016).
BIS/BAS Scales
After collecting saliva, participants completed a Chinese version of the BIS/BAS Scale (Carver & White, 1994). The BIS subscale relates to the anticipation of a punishment and avoidance of cues to negative outcomes (five items, e.g. “I worry about making mistakes”). The BAS is divided into three subscales: a positive response to the occurrence or anticipation of reward (reward responsiveness, four items, e.g. “When I get something I want, I feel excited and energized”), a persistent pursuit of desired goals (drive, four items, e.g. “When I want something, I go all-out to get it”), and a desire for new rewards and willingness to approach a potentially rewarding event on the spur of the moment (fun-seeking, five items, e.g. “I crave excitement and new sensations”). Responses were made on a 4-point scale from 1 (strong disagreement) to 4 (strong agreement). Responses on items were summed to calculate scores for each of the four subscales. A higher score indicates a greater level of the particular trait on each subscale.
Self-Awareness Scale
A Chinese version of nine-item Self-Awareness Scale (SAS) was used to measure the public, private, and surrounding aspects of self-awareness (Govern & Marsch, 2001). Three items each measured self-focus (Private, e.g. “Right now, I am conscious of my inner feelings”), other-focus (Public, e.g. “Right now, I am concerned about what other people think of me”), and non-self-focus (Surroundings, e.g. “Right now, I am conscious of all objects around me”). Each item was accompanied by a 7-point scale, which ranged from 1 (strongly disagree) to 7 (strongly agree). Items were summed to provide one score for each subscale.
Differences between the late FP and mid-LP groups on ovarian hormones, BIS/BAS and SAS scales were analysed via one-way ANOVA. Regression and moderating effect analyses were further used to examine the direction of relationship between ovarian hormones and each subscale. Significance for all analyses providing p-values were set at p < .05. Moderating effect was tested using Model 1 in PROCESS for SPSS (Hayes, 2013), which tested moderating effect via calculation of 5,000 bias-corrected bootstrap 95% confidence intervals (CI).
Results
Menstrual Phase Effects on Hormones, BIS/BAS, and SAS
One-way ANOVA revealed significantly higher PROG levels for the mid-LP than the late FP (F1, 76 = 8.368, p = 0.005, η2 = 0.099). No other significant differences were found for E2 levels, BIS/BAS scales, and SAS between the late FP and mid-LP, see Table 1 for mean (M) and standard deviation (SD).
Table 1.
Descriptive Statistics and One-Way ANOVA Results for Each Menstrual Phase Group in Study 1.
| Groups | M | SD | df | F | Sig. | η2 | |
|---|---|---|---|---|---|---|---|
| E2 (pg/ml) | late FP | 5.45 | 3.25 | 1,76 | 0.916 | 0.341 | 0.012 |
| mid-LP | 4.81 | 2.56 | |||||
| PROG (pg/ml) | late FP | 58.67 | 78.84 | 1,76 | 8.368* | 0.005 | 0.099 |
| mid-LP | 125.84 | 123.36 | |||||
| BAS rewards | late FP | 14.02 | 1.53 | 1,76 | 0.059 | 0.809 | 0.001 |
| mid-LP | 13.95 | 1.31 | |||||
| BAS drive | late FP | 12.02 | 1.85 | 1,76 | 0.364 | 0.548 | 0.005 |
| mid-LP | 12.30 | 2.15 | |||||
| BAS-fun seeking | late FP | 14.90 | 2.47 | 1,76 | 0.845 | 0.361 | 0.011 |
| mid-LP | 15.38 | 2.10 | |||||
| BIS | late FP | 16.85 | 2.29 | 1,76 | 0.518 | 0.474 | 0.007 |
| mid-LP | 16.49 | 2.21 | |||||
| SAS public | late FP | 13.88 | 2.79 | 1,76 | 0.489 | 0.486 | 0.006 |
| mid-LP | 14.35 | 3.19 | |||||
| SAS private | late FP | 13.39 | 2.87 | 1,76 | 0.316 | 0.576 | 0.004 |
| mid-LP | 13.00 | 3.26 | |||||
| SAS surroundings | late FP | 14.49 | 2.79 | 1,76 | 0.598 | 0.442 | 0.008 |
| mid-LP | 13.97 | 3.09 |
* indicated the significant of effects with uncorrected p < 0.05.
Further, we ran moderating effect analyses to determine whether the menstrual cycle groups had different effects on the relationship between PROG and BIS/BAS, controlling for E2 levels. However, we did not find any interaction effects (βint) between menstrual cycle groups and PROG on BIS/BAS (BAS reward, βint = −0.004, p = 0.269, 95%CI = [−0.012, 0.003]; BAS drive, βint = 0.002, p = 0.706, 95%CI = [−0.009, 0.013]; BAS-fun seeking, βint = −0.000, p = 0.967, 95%CI = [−0.013, 0.012]; BIS, βint = 0.009, p = 0.149, 95%CI = [−0.003, 0.021]), or SAS (Public, βint = 0.011, p = 0.156, 95%CI = [−0.004, 0.027]; Private, βint = 0.012, p = 0.144, 95%CI = [−0.004, 0.028]; Surroundings, βint = 0.007, p = 0.4769, 95%CI = [−0.010, 0.021]).
Regression Analysis Between Ovarian Hormones and BIS/BAS, SAS
Using Pearson correlations, we found that PROG levels were positively correlated with SAS public subscale (ignoring menstrual groups), r = 0.225, uncorrected p = 0.047, suggesting that as PROG levels increased, women had higher scores on public items. However, this correlation was no longer significant after Bonferroni correction. No other correlation was found related to ovarian hormones, see Table 2. Next, we used regression analyses to further set E2 as a covariable to analyze the relationship between PROG and SAS public subscale. Under this condition, the regression model was no longer significant (R2 = 0.068, p = 0.108, Table 3). This change might be due to a potential interaction between E2 and PROG, which was analyzed further in the following part.
Table 2.
Pearson Correlations Between BIS/BAS and SAS Subscales in Study 1.
| BASr | BASd | BASf | BIS | SASpu | SASpr | SASs | E2 | PROG | |
|---|---|---|---|---|---|---|---|---|---|
| BASr | — | r = .295* | r = .438* | r = .354* | r = .130 | r = .070 | r = .016 | r = −.010 | r = .070 |
| p = .009 | p = .000 | p = .001 | p = .258 | p = .544 | p = .887 | p = .928 | p = .544 | ||
| BASd | r = .295* | — | r = .288* | r = −.088 | r = .158 | r = .012 | r = −.073 | r = −.002 | r = .016 |
| p = .009 | p = .011 | p = .444 | p = .168 | p = .918 | p = .523 | p = .983 | p = .887 | ||
| BASf | r = .438* | r = .288* | — | r = .115 | r = .201 | r = .176 | r = .044 | r = .077 | r = −.073 |
| p = .000 | p = .011 | p = .317 | p = .078 | p = .124 | p = .703 | p = .505 | p = .523 | ||
| BIS | r = .354* | r = −.088 | r = .115 | — | r = .056 | r = .206 | r = .063 | r = −.034 | r = .044 |
| p = .001 | p = .444 | p = .317 | p = .629 | p = .071 | p = .581 | p = .766 | p = .703 | ||
| SASpu | r = .130 | r = .158 | r = .201 | r = .056 | — | r = .561* | r = .316* | r = .161 | r = .225 |
| p = .258 | p = .168 | p = .078 | p = .629 | p = .000 | p = .005 | p = .159 | p = .047* | ||
| SASpr | r = .070 | r = .012 | r = .176 | r = .206 | r = .561* | — | r = .533* | r = .079 | r = .038 |
| p = .544 | p = .918 | p = .124 | p = .071 | p = .000 | p = .000 | p = .492 | p = .738 | ||
| SASs | r = .016 | r = −.073 | r = .044 | r = .063 | r = .316* | r = .533* | — | r = .009 | r = .084 |
| p = .887 | p = .523 | p = .703 | p = .581 | p = .005 | p = .000 | p = .940 | p = .462 | ||
| E2 | r = −.010 | r = −.002 | r = .077 | r = −.034 | r = .161 | r = .079 | r = .009 | — | r = .375* |
| p = .928 | p = .983 | p = .505 | p = .766 | p = .159 | p = .492 | p = .940 | p = .001 | ||
| PROG | r = .070 | r = .016 | r = −.073 | r = .044 | r = .225 | r = .038 | r = .084 | r = .375* | — |
| p = .544 | p = .887 | p = .523 | p = .703 | p = .047* | p = .738 | p = .462 | p = .001 |
* indicated the significant of effects with uncorrected p < 0.05. BASr = BAS rewarding, BASd = BAS driving, BASf = BAS-fun seeking, SASpu = SAS public, SASpr = SAS private.
Table 3.
Regression Model of Ovarian Hormones on SAS Public in Study 1.
| Model | R2 | df | F | Sig. | Factors | t | Sig. | Cohen’ d |
|---|---|---|---|---|---|---|---|---|
| 1 | .051 | 1,76 | 4.060 | 0.047a | PROG | 2.015 | 0.047* | 0.462 |
| 2 | .058 | 2,75 | 2.290 | .108b | PROG | 1.586 | 0.117 | 0.366 |
| E2 | 0.738 | 0.463 | 0.170 |
* indicated the significant of effects with uncorrected p < 0.05. a. predictive variable: PROG; b. predictive variable: PROG, E2.
Moderating Effects of PROG on the Association Between E2 and BIS/BAS, SAS
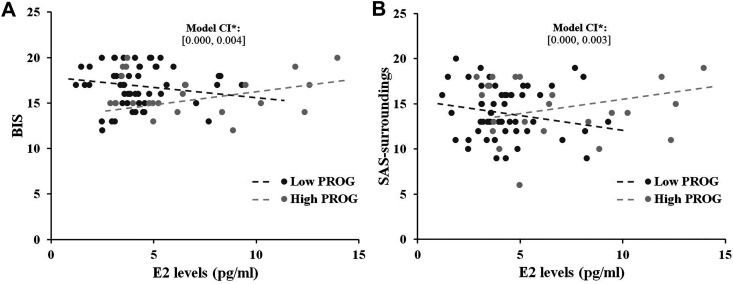
With ignoring participants’ menstrual phases, a significant moderating effect of PROG was revealed on the association between E2 and BIS subscale across whole samples. PROG had a positive moderating effect on the association between E2 and BIS (βint = 0.002, p = 0.024, 95%CI = [0.000, 0.003]). Specifically, with higher PROG levels, E2 showed a positive relationship with BIS scores (MPROG +2SD, βint = 0.379, p = 0.050, 95%CI = [0.000, 0.752]; MPROG − SD, βint = −0.227, p = 0.094, 95%CI = [−0.494, 0.040]). A similar moderating effect of PROG was found between E2 and SAS-surrounding subscale (βint = 0.002, p = 0.038, 95%CI = [0.000, 0.004]); with lower PROG levels, we found a negative tendency between E2 and SAS-surrounding scores (MPROG − 1SD, βint = −0.297, p = 0.095, 95%CI = [−0.648, 0.531]; MPROG +1SD, βint = 0.074, p = 0.567, 95%CI = [−0.185, 0.332]). Figure 1 illustrates this moderating effect graphically, setting the moderator PROG to its sample mean.
Figure 1.
Moderating effects model for ovarian hormones. A. When PROG was higher than mean levels, E2 tended to positively correlation with BIS scores; when PROG was lower than mean levels, E2 tended to correlate negatively with BIS scores. B. Similar moderating effect was found for the PROG on the association between E2 and SAS-surroundings subscale.
No other moderating effects were found on BAS (reward, βint = −0.000, p = 0.818, 95%CI = [−0.001, 0.001]; drive, βint = −0.001, p = 0.064, 95%CI = [−0.002, 0.000]; fun seeking, βint = −0.000, p = 0.717, 95%CI = [−0.002, 0.001]) or SAS (Public, βint = 0.001, p = 0.395, 95%CI = [−0.001, 0.003]; Private, βint = 0.002, p = 0.070, 95%CI = [−0.000, 0.004]).
Discussion
In Study 1, we found that women with higher PROG levels exhibited greater sensitivity to public social feedback. PROG may also modulate E2’s association with BIS: When women had relatively high PROG levels, higher E2 levels were associated with greater activation of the inhibitory system. However, there were limitations to Study1. First, although SAS was related to social feedback sensitivity, there was no direct evidence that such sensitivity was related to prosocial behaviors. Second, we did not find higher public scores in the mid-LP, even though this phase had higher PROG levels. This might be due to the few public items in SAS, and the between-person design for the late FP and mid-LP (Maner & Miller, 2014). Third, we ran the regression and moderating effect analyses by directly using PROG and E2 levels with ignoring women’s menstrual phases. Even though the women in the mid-LP had higher PROG levels in Study 1, it was unclear whether the association was due to women who had higher average PROG across their cycles or heightened PROG during the mid-LP. Consequently, we cannot come to the conclusion that, women showed prosocial tendency during LP. We addressed these limitations in Study 2.
Study 2
In Study 2, we continued to examine the within-subject covariations of E2 and PROG on social orientation across the menstrual cycle. Prior research indicates that increased social sensitivity is always accompanied by enhanced interpersonal anxiety, as well as cooperative preferences (Mcpeek & Cialdini, 1977; Velasquez, 2011). Thus, based on the limitations in Study 1, in Study 2, female participants first completed a pathological scale of self-awareness in private and public in their late FP and mi-LP. This scale has been found to be positively associated with interpersonal sensitivity and symptoms of interpersonal anxiety (Zucker et al., 2015). Then participants finished a SVO task that ask participants to divide money between self and an anonymous female or male at each menstrual phase (Lange & Paul, 1999). We predicted that, within-subjects, women in their mid-LP would have higher interpersonal anxiety and tend to allocate more money to an anonymous recipient than when in their late FP.
Materials and Methods
Participants
Thirty healthy, heterosexual, right-handed female undergraduates at East China Normal University, Shanghai, China [age, 20.00 ± 2.20 (age range, 18–25) years] participated in this study. Potential participants completed the same recruitment questionnaire used in Study 1. Only participants who were heterosexual, reported having 26–34-day menstrual cycles, and had not used hormones in the previous 3 months were included. Participants were asked to come to the laboratory on two separate sessions (late FP and mid-LP) to complete a pathological self-awareness scale and a SVO task. We used different versions of the SVO task for the two testing sessions, but did not mention this explicitly to participants. As in Study 1, we used the G*Power 3.1 to calculate the sample size. Under the within-subject design for Study 2, we set effect size F = 0.25, α error probability = 0.05, 1 − β error probability = 0.8. The minimum required sample size was calculated as 24.
We again used the backward counting method to predict the timing of each participant’s late FP, mid-LP, and next menstrual onset. This method had been successfully used to predict menstrual phase in Study 1. If a woman’s next predicted menstrual onset was 8–14 days away, she was scheduled to complete the session in the mid-LP first (6–8 days prior to the predicted onset, N = 15); otherwise, she was scheduled in the late FP first (14–16 days prior to the predicted onset, N = 15).
Before each session began, participants used the one-step urinary test kits (luteinizing hormone kits, LH kits) to confirm being in the late FP or in the mid-LP. One-step kits detect the LH surge and report results using color intensity in reference areas (Nielsen et al., 2001). Participants tested once during the late FP first (kits color: dark pink) were tested again 5–6 days later during their mid-LP (kits color: gray). If the participant was assigned to finish the first session during the mid-LP, then she need to test again in the next late FP.
The Ethics Committee of East China Normal University approved the study protocol, and the study was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent and were compensated with 50 RMB per hour.
Self-Absorption Scale
The self-absorption scale (SABS) is a 17-item self-report measure of maladjustment self-awareness toward self and others. Ingram (1990) proposed the “pathological equivalent” of self-awareness, self-absorption, and defined it as excessive, sustained, and rigid attention to information emanating from internal sources. The other-focused subscale of SABS was identified to be related to the social anxiety and primarily concerns interpersonal processes (Mckenzie & Hoyle, 2008).
The SABS distinguishes self-focus (private) from other-focus (public) and was scaled on a 5-point response format (1 = not at all like me and 5 = very much like me). The private self-absorption subscale (eight items) assesses three aspects of excessive attention in relation to thoughts about the self, (e.g., “I think about myself more than anything else.” [Excessive]; “My mind never focuses on things other than myself for very long.” [Sustained]; and “When I try to think of something other than myself, I cannot.” [Rigidity]) The public self-absorption subscale (nine items) measured excessive focus and difficulty disengaging thoughts about what others are thinking about oneself (e.g., “I am very aware of what others think of me, and it bothers me.” [Excessive]; “I feel like others are constantly evaluating me when I’m with them” [Sustained]; and “I find myself wondering what others think of me even when I don’t want to” [Rigidity]). The coefficient alpha was 0.83 for private subscale and 0.90 for public subscale (McKenzie & Hoyle, 2008). Items were averaged to provide one score for private or public subscales.
SVO Slider Measures
Original version of the SVO Slider Measure has six primary items (Murphy et al., 2011); available at http://journal.sjdm.org/11/m25/m25.html). For each item, the task is to indicate the preferred distribution of fictitious monetary amounts between oneself and an anonymous person with joint outcomes ranging between 15 and 100 dollars (in the Chinese version, it was 15–100 yuan). Based on these choices, the inverse tangent of the ratio between the mean allocation for themselves minus 50 and the mean allocation for the other minus 50is computed to obtain each participant’s individual SVO index following Murphy et al.’s (2011) instructions. This results in angles between −16.26° and 61.39° (SVO angles), with larger angles reflecting more prosocial and smaller angles more individualistic preferences.
Previous studies indicated that women show prosocial preference toward females and males under different affiliation motivations (Schultheiss et al., 2003). Thus, in Study 2, participants were asked to imagine that they would complete the following SVO task with an anonymous female recipient and an anonymous male recipient. Finally, each participant finished 12 SVO items in each menstrual phase session, six items for a female recipient and another six for a male recipient. Four versions of SVO tests were prepared, and test orders of the female/male recipient and menstrual phase sessions were counterbalanced.
Results
Menstrual Phase Effects on Urinary Test, SABS, and SVO
All participants were successful using the one-step urinary LH kits to confirm the late FP and mid-LP following the instructions of color intensity in the test.
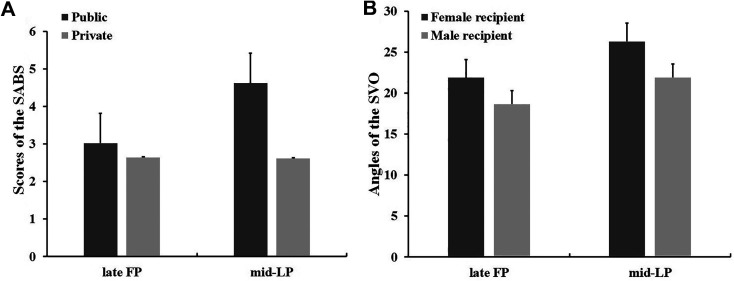
The paired-samples t-test revealed significantly higher scores on the public SABS in the mid-LP than the late FP (t1, 29 = 6.794, p < 0.001, Cohen’s d = 2.523). No such menstrual phase effects on the private SABS scores were observed (t1, 29 = 0.270, p = 0.789, Cohen’s d = 0.100, Figure 2, Table 4).
Figure 2.
Menstrual phase effects on the SABS and SVO tasks. A. The public SABS scores were significantly. A. The public SABS scores were significantly higher during the mid-LP than the late FP (error bars indicate 1 SEM). B. SVO angles were significantly higher during the mid-LP than the late FP (error bars indicate 1 SEM), which meant more prosocial in the mid-LP, no matter anonymous recipient’s gender was female or male.
Table 4.
Descriptive Statistics for Each Menstrual Phase in Study 2.
| late FP | mid-LP | |||
|---|---|---|---|---|
| M | SD | M | SD | |
| Public | 3.03 | 0.66 | 4.62 | 0.74 |
| Private | 2.65 | 0.56 | 2.62 | 0.69 |
| Female recipient | 21.87 | 16.19 | 26.29 | 13.74 |
| Male recipient | 18.66 | 16.29 | 21.91 | 14.67 |
In a 2 menstrual phases (late FP, mid-LP, within-subject) × 2 anonymous recipient’s gender (female, male, within-subject) repeated-measures ANOVA, there was a significant main effect of menstrual phase: larger SVO angles in the mid-LP than the late FP (F1, 29 = 4.798, p = 0.037, η p 2 = 0.142; late FP, M = 20.53, SD = 16.00, mid-LP, M = 24.46, SD = 14.17). In addition, there was a significant main effect of the anonymous recipient’s gender: larger SVO angles for the female than the male (F1, 29 = 12.368, p = 0.001, η p 2 = 0.299; female, M = 24.41, SD = 14.91, male, M = 20.59, SD = 15.33). No interaction effect was found (F1, 29 = 0.313, p = 0.580, η p 2 = 0.011). These results showed an overall higher cooperation preference in the mid-LP (Figure 2, Table 4).
Public SABS Positive Correlation With SVO Angles
We conducted a series correlation analyses to confirm the relationships of interpersonal anxiety and prosocial preference. We did not find significant correlations between SABS and SVO during each menstrual phase (Table 5), but results showed that the mean scores of public SABS were positively correlated with mean SVO angles on the overall level when ignoring the recipient’s gender and participants’ menstrual phases (r = 0.359, uncorrected p = 0.051). No correlation was found between the mean private SABS scores and SVO angles (r = 0.143, uncorrected p = 0.452).
Table 5.
Pearson Correlations Between SABS and SVO in the Late FP in Study2.
| Public | Private | Female recipient | Male recipient | |
|---|---|---|---|---|
| late FP | ||||
| Public | — | r = .285 | r = .351 | r = .279 |
| p = .127 | p = .057 | p = .135 | ||
| Private | r = .285 | — | r = .264 | r = .082 |
| p = .127 | p = .159 | p = .667 | ||
| Female recipient | r = .351 | r = .264 | — | r = .846* |
| p = .057 | p = .159 | p = .000 | ||
| Male recipient | r = .279 | r = .082 | r = .846* | — |
| p = .135 | p = .667 | p = .000 | ||
| mid-LP | ||||
| Public | — | r = .448* | r = −.114 | r = −.101 |
| p = .013 | p = .550 | p = .595 | ||
| Private | r = .448* | — | r = .039 | r = .044 |
| p = .013 | p = .836 | p = .816 | ||
| Female recipient | r = −.114 | r = .039 | — | r = .867* |
| p = .550 | p = .836 | p = .000 | ||
| Male recipient | r = −.101 | r = .044 | r = .867* | — |
| p = .595 | p = .816 | p = .000 |
* indicated the significant of effects with uncorrected p < 0.05.
Discussion
Extending the findings of Study1, Study 2 demonstrated a positive association between interpersonal anxiety and cooperation preference, and provided evidence for increased interpersonal anxiety and heightened cooperation preference during the mid-LP vs. the late FP. Further, regardless of whether the recipient was female or male, women in the mid-LP had higher SVO angles, which represented a generally higher cooperation preference during the mid-LP.
General Discussion
Across two studies, we provided evidence for the association among menstrual phase, inhibitory responses and social orientation. In Study 1, women’s PROG levels (between-subjects) positively corresponded to their reports of social feedback sensitivity, and women whose PROG was higher than the sample average showed a positive association between E2 and inhibitory responses. In Study 2, women in their mid-LP, who experienced a within-subjects cyclical increase in PROG, had elevated public SABS scores, which indicated a heightened interpersonal anxiety. Importantly, women in the mid-LP showed greater preference in cooperation with others, no matter whether the opponent was female or male (i.e., within-subject). Taken together, these findings suggest that women’s social orientation is guided, in part, by naturally occurring endocrinological changes relevant to the menstrual cycle.
Specifically, as predicted, PROG levels were positively related to social feedback sensitivity. This association was more obvious when comparing the mid-LP and late FP within participants, with women during their mid-LP having higher scores on the public SABS. Meanwhile, when women devoted more self-awareness attention resources to interpersonal information, women during the mid-LP showed more cooperation behaviors in SVO tasks than during their late FP. Although no straightforward causal inference can be made due to the correlational design of the present studies, these results suggest that during women’s LP, interpersonal sensitivity, anxiety, and cooperation preference all increase with PROG levels. Furthermore, we found a moderating effect of PROG on the association between E2 and other social orientation indicators. With relatively higher PROG levels than the sample average, women’s inhibitory responses showed a positive correlation with E2, but no such relationships under low PROG condition. These results further illustrate that women showed heightened self-inhibition responses and exhibited prosocial behaviors during the LP, when they had high levels of both PROG and E2.
Obtaining similar results to ours, some researchers have suggested that women in the mid-LP need to solidify social bonds (Maner & Miller, 2014) and that social closeness increases with salivary PROG levels (Brown et al., 2009). In contrast, during the late FP, compared to the mid-LP, besides the need for harmonious relationships, females may be motivationally primed to engage in a new, or intimate relationship at that time, which could weaken women’s desire to consider others or surroundings (Durante et al., 2011). In order to better explore the prosocial performance of females under different reproductive goals during their menstrual cycles, we divided social orientation into intrasexual and intersexual condition. As mentioned above, female affiliation motivation could be directed toward males to acquire a potential father for children, or toward other females to obtain support in their daily lives (Schultheiss et al., 2003). Interestingly, in the present study, female participants were more likely to allocate resources to a female recipient than to a male recipient in both late FP and mid-LP. Previous studies have indicated an automatic in-group bias (i.e., own gender preference) in women (Reynolds et al., 2020; Rudman & Goodwin, 2004). They suspected that because of early attachment to maternal caregivers, people’s mental machinery may be geared to automatically favor the feminine sex (Rudman & Goodwin, 2004). Our results supported this female in-group bias, but more experiments are needed to further explore whether this in-group bias exists for better rearing offspring.
Some limitations of the present work should be mentioned. First, our interpretations are based on a correlational design. The observed association among PROG, interpersonal anxiety and cooperation preference cannot demonstrate a causal role of the reproductive costs in shifting women’s social orientation. Moreover, while we found significant correlations between PROG and self-awareness toward others, these correlations were no longer significant after Bonferroni correction. Considering that the present study was motivated by a relatively strong hypothesis about associations between ovarian hormones and social orientation, we believe that it is nevertheless worth reporting these results. Second, in Study 2, we did not examine PROG directly. Although the urine test verified that women were in their mid-LP, assumptions that the emergent pattern was unique to PROG should be interpreted with caution. Third, we used both within- and between-groups to support the hypothesis that women’s social orientation could change across the menstrual cycle. However, research suggests that there is substantial intercycle variability in women’s PROG levels (Eisenlohr-Moul & Owens, 2016). Thus, women assigned to the high PROG condition in Study 1 might not had high mean levels of PROG across menstrual cycles. Future research could track multiple cycles in a woman to determine the effect of average PROG levels on social orientation. Finally, as cortisol plays a key role in stress and anxiety, it would be useful to collect both ovarian hormones and cortisol future research (Reynolds et al., 2018).
In conclusion, our studies suggest that social orientation varies across the menstrual cycle. Consistent with evolutionary theory and prior work suggesting that interpersonal sensitivity and inhibitory responses are conductive to maintaining social relationships, we found that women showed increased public social feedback sensitivity, interpersonal anxiety, inhibition responses, and cooperative behaviors at times in the menstrual phase characterized by higher PROG levels, suggesting the possibility that increased PROG could contribute to these shift.
Footnotes
Authors’ Note: Jin-Ying Zhuang is a Professor in the School of Psychology and Cognitive Science, East China Normal University, Shanghai, China. The research interest includes Decision making, ovarian hormones, social cognition, and evolutionary mechanism on women’s social behaviors and brian connectivity. Recently, our team is also committed to exploring human behaviors using the functional magnetic resonance imaging.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial supportfor the research, authorship, and/or publication of this article: This work was supported by the Fundamental Research Funds for the Central Universities (41300-20101-222128) and National Natural Science Foundation of China (71971084)
ORCID iD: Jin-Ying Zhuang  https://orcid.org/0000-0002-9642-3117
https://orcid.org/0000-0002-9642-3117
References
- Archer J. (2006). Testosterone and human aggression: An evaluation of the challenge hypothesis. Neuroscience & Biobehavioral Reviews, 30(3), 319–345. [DOI] [PubMed] [Google Scholar]
- Bjorklund D., Kipp K. (1995). The role of inhibition mechanisms in the evolution of human cognition and behavior. New Perspectives on Interference and Inhibition in Cognition. 10.1016/B978-012208930-5/50006-4 [DOI]
- Brown S. L., Fredrickson B. L., Wirth M. M., Poulin M. J., Meier E. A., Heaphy E. D., Cohen M. D., Schultheiss O. C. (2009). Social closeness increases salivary progesterone in humans. Hormones & Behavior, 56(1), 108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver C. S., White T. L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology, 67(2), 319–333. [Google Scholar]
- Conway C. A., Jones B. C., Debruine L. M., Welling L. L., Law Smith M. J., Perrett D. I., Sharp M. A., Aldujaili E. A. (2007). Salience of emotional displays of danger and contagion in faces is enhanced when progesterone levels are raised. Hormones and Behavior, 51(2), 202–206. [DOI] [PubMed] [Google Scholar]
- Cooper A. J. (1986). Progestogens in the treatment of male sex offenders: A review. Canadian Journal of Psychiatry/Revue Canadienne De Psychiatrie, 31(1), 73–79. [DOI] [PubMed] [Google Scholar]
- DeBruine L. M., Jones B. C., Perrett D. I. (2005). Women’s attractiveness judgments of self-resembling faces change across the menstrual cycle. Hormones and Behavior, 47(4), 379–383. [DOI] [PubMed] [Google Scholar]
- Dufour D. L., Sauther M. L. (2002). Comparative and evolutionary dimensions of the energetics of human pregnancy and lactation. American Journal of Human Biology, 14, 584–602. [DOI] [PubMed] [Google Scholar]
- Durante K. M., Griskevicius V., Cantú S. M., Simpson J. A. (2015). Money, status, and the ovulatory cycle. Journal of Marketing Research, 51(51), 27–39. [Google Scholar]
- Durante K. M., Griskevicius V., Hill S. E., Perilloux C., Li N. P. (2011). Ovulation, female competition, and product choice: Hormonal influences on consumer behavior. Journal of Consumer Research, 37(6), 921–934. [Google Scholar]
- Eisenlohr-Moul T. A., Owens S. (2016). Hormones and personality. In Zeigler-Hill V.&Shackelford T. K. (Eds.), Encyclopedia of personality and individual Differences. Springer. 10.1007/978-3-319-28099-8_762-1 [DOI] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. [DOI] [PubMed] [Google Scholar]
- Gangestad S. W., Thornhill R. (1998). Menstrual cycle variation in women’s preferences for the scent of symmetrical men. Proceedings of the Royal Society of London. Series B: Biological Sciences, 265(1399), 927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govern J. M., Marsch L. A. (2001). Development and validation of the situational self-awareness scale. Consciousness and Cognition, 10(3), 366–378. [DOI] [PubMed] [Google Scholar]
- Haselton M. G., Gangestad S. W. (2006). Conditional expression of women’s desires and men’s mate guarding across the ovulatory cycle. Hormones and Behavior, 49(4), 509–518. [DOI] [PubMed] [Google Scholar]
- Hatta T., Nagaya K. (2009). Menstrual cycle phase effects on memory and Stroop task performance. Archives of Sexual Behavior, 38(5), 821–827. [DOI] [PubMed] [Google Scholar]
- Hayes A. (2013). Introduction to mediation, moderation, and conditional process analysis. Journal of Educational Measurement, 51(3), 335–337. [Google Scholar]
- Ingram R. E. (1990). Self-focused attention in clinical disorders: Review and a conceptual model. Psychological Bulletin, 107(2), 156–176. [DOI] [PubMed] [Google Scholar]
- Jones B., Little A., Boothroyd L., DeBruine L., Feinberg D., Smith M., Cornwell R. E., Moore F. R., Perrett D. (2005). Commitment to relationships and preferences for femininity and apparent health in faces are strongest on days of the menstrual cycle when progesterone level is high. Hormones and Behavior, 48(3), 283–290. [DOI] [PubMed] [Google Scholar]
- Justin M. C., Shawn N. G., Triana L. O., Brian M. B., Amber V., Pierre L. B. (2017). Exogenous testosterone rapidly increases aggressive behavior in dominant and impulsive men. Biological Psychiatry, 82(4), 249–256. 10.1016/j.biopsych.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Klump K. L., Keel P. K., Racine S. E., Burt S. A., Neale M., Sisk C. L., Boker S., Hu J. Y. (2013). The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. Journal of Abnormal Psychology, 122(1), 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange V., Paul A. M. (1999). The pursuit of joint outcomes and equality in outcomes: An integrative model of social value orientation. Journal of Personality & Social Psychology, 77(2), 337–349. [Google Scholar]
- Llaneza D. C., Frye C. A. (2009). Progestogens and estrogen influence impulsive burying and avoidant freezing behavior of naturally cycling and ovariectomized rats. Pharmacology Biochemistry and Behavior, 93(3), 337–342. 10.1016/j.pbb.2009.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maner J. K., Miller S. L. (2014). Hormones and social monitoring: Menstrual cycle shifts in progesterone underlie women’s sensitivity to social information. Evolution & Human Behavior, 35(1), 9–16. [Google Scholar]
- McKenzie K. S., Hoyle R. H. (2008). The Self-Absorption Scale: Reliability and validity in non-clinical samples. Personality and Individual Differences, 45(8), 726–731. [Google Scholar]
- Mcpeek R. W., Cialdini R. B. (1977). Social anxiety, emotion, and helping behavior. Motivation & Emotion, 1(3), 225–233. [Google Scholar]
- Murphy R. O., Ackermann K. A., Handgraaf M. J. J. (2011). Measuring social value orientation. Judgment & Decision Making, 6(8), 771–781. [Google Scholar]
- Nielsen M. S., Barton S. D., Hatasaka H. H., Stanford J. B. (2001). Comparison of several one-step home urinary luteinizing hormone detection test kits to OvuQuick (R). Fertility & Sterility, 76(2), 384–387. [DOI] [PubMed] [Google Scholar]
- Norasakkunkit V., Kitayama S., Uchida Y. (2012). Social anxiety and holistic cognition: self-focused social anxiety in the United States and other-focused social anxiety in Japan. Journal of Cross-Cultural Psychology, 43(5), 742–757. [Google Scholar]
- Penton-Voak I. S., Perrett D. I., Castles D. L., Kobayashi T., Burt D. M., Murray L. K., Minamisawa R. (1999). Menstrual cycle alters face preference. Nature, 399(6738), 741–742. [DOI] [PubMed] [Google Scholar]
- Pirita K. S., Sabine W. (2013). Dual-processing altruism. Frontiers in Psychology, 4, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds T. A., Makhanova A., Marcinkowska U. M., Jasienska G., Mcnulty J. K., Eckel L. A., Nikonova L., Maner J. K. (2018). Progesterone and women’s anxiety across the menstrual cycle. Hormones & Behavior, 102, 34–40. [DOI] [PubMed] [Google Scholar]
- Reynolds T., Howard C., Sjstad H., Zhu L., Kim J. H. (2020). Man up and take it: Gender bias in moral typecasting. Organizational Behavior and Human Decision Processes, 161, 120–141. [Google Scholar]
- Rudman L. A., Goodwin S. A. (2004). Gender differences in automatic in-group bias: Why do women like women more than men like men? Journal of Personality & Social Psychology, 87(4), 494. [DOI] [PubMed] [Google Scholar]
- Sakaki M., Mather M. (2012). How reward and emotional stimuli induce different reactions across the menstrual cycle. Social and Personality Psychology Compass, 6(1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss O. C., Dargel A., Rohde W. (2003). Implicit motives and gonadal steroid hormones: Effects of menstrual cycle phase, oral contraceptive use, and relationship status. Hormones and Behavior, 43(2), 293–301. [DOI] [PubMed] [Google Scholar]
- Schultheiss O. C., Wirth M. M., Stanton S. J. (2004). Effects of affiliation and power motivation arousal on salivary progesterone and testosterone. Hormones and Behavior, 46(5), 592–599. [DOI] [PubMed] [Google Scholar]
- Solís-Ortiz S., Corsi-Cabrera M. (2008). Sustained attention is favored by progesterone during early luteal phase and visuo-spatial memory by estrogens during ovulatory phase in young women. Psychoneuroendocrinology, 33(7), 989–998. [DOI] [PubMed] [Google Scholar]
- Taylor S. E., Klein L. C., Lewis B. P., Gruenewald T. L., Gurung R. A., Updegraff J. A. (2000). Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychological Review, 107(3), 411–429. [DOI] [PubMed] [Google Scholar]
- Thornhill R., Gangestad S. W. (2008). The evolutionary biology of human female sexuality. Oxford University Press. [Google Scholar]
- Velasquez L. (2011). The influence of empathy priming and social value orientations on the motivation for prosocial behavior. ProQuest Dissertations and Theses 11(4), 86. [Google Scholar]
- Ycaza H. A., Nielsen S. E., Mather M. (2016). Stress-induced increases in progesterone and cortisol in naturally cycling women. Neurobiology of Stress, 3, 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J.-Y., Wang J.-X. (2014). Women ornament themselves for intrasexual competition near ovulation, but for intersexual attraction in luteal phase. PloS One, 9(9), e106407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker N., Wagner H. R., Merwin R., Bulik C. M., Moskovich A., Keeling L., Hoyle R. (2015). Self-focused attention in anorexia nervosa. International Journal of Eating Disorders, 48(1), 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]