Abstract
The idea that human males are most strongly attracted to traits that peak in women in the nubile age group raises the question of how well women in that age group contend with the potential hazards of a first pregnancy. Using data for 1.7 million first births from 1990 U.S. natality and mortality records, we compared outcomes for women with first births (primiparas) aged 16–20 years (when first births typically occur in forager and subsistence groups) with those aged 21–25 years. The younger primiparas had a much lower risk of potentially life-threatening complications of labor and delivery and, when evolutionarily novel risk factors were controlled, fetuses which were significantly more likely to survive despite lower birth weights. Thus, nubile primiparas were more likely to have a successful reproductive outcome defined in an evolutionarily relevant way (an infant of normal birth weight and gestation, surviving to one year, and delivered without a medically necessary cesarean delivery). This suggests that prior to the widespread availability of surgical deliveries, men who mated with women in the nubile age group would have reaped the benefit of having a reproductive partner more likely to have a successful first pregnancy.
Keywords: first pregnancy, female attractiveness, nubility, primiparas, pregnancy complications, mating preferences, reproductive timing
Introduction
Men’s Preferences for Youthful Female Partners
Because evolution depends on reproductive success, mate preferences that increase the likelihood of such success should be under significant selection. Given this potential impact on fitness, human mating preferences have been of considerable interest to evolutionary psychologists (e.g., Buss, 1989; Geary et al., 2004; Sugiyama, 2005). The frequently repeated view that men are attracted to women with low waist–hip ratios (WHRs) and low body mass indices (BMI) (in well-nourished populations) because these traits indicate health and fertility (Grammer et al., 2003; Marlowe 2005; Pawlowski & Dunbar, 2005; Singh, 1993a, 1993b, 2002; Singh & Singh, 2006, 2011; Weeden & Sabini, 2005) does not appear to be well supported (Bovet, 2019; Lassek & Gaulin, 2018a, 2018b). Indeed, a low WHR and BMI are most likely to occur in young women in their late teens who have never been pregnant (nulligravidas) (Andrews et al., 2017; Butovskaya et al., 2017; Lassek & Gaulin, 2019), women who have demonstrably lower fertility and greater liability to infection than women in their 20s (Lassek & Gaulin, 2018a, 2018b).
As an alternative to the “health-and-fertility hypothesis,” the hypothesis first advocated by Symons (1979, 1995) that men are unconsciously drawn to indicators of nubility (see also Fessler et al., 2005; Marlowe, 1998) and its associated high reproductive value (sensuFisher, 1930) rather than high current fertility, has received renewed support (Andrews et al., 2017; Butovskaya et al., 2017; Lassek & Gaulin, 2019; Prokop et al., 2020; Röder et al., 2013). A nubile woman is a nulligravida who has recently completed physical growth, puberty, and sexual development (Symons, 1979). This is usually accomplished 3–4 years after menarche in the mid to late teens when female reproductive value is maximal (Bowles & Posel, 2005; Fisher, 1930; Keyfitz & Flieger, 1971).
As noted, women in the nubile age group have the lowest WHRs and, in well-nourished populations, have lower BMIs than women in their 20s, traits strongly associated with attractiveness. In nutritionally stressed populations where BMI tends to decrease with age and parity, young nulligravidas) have the higher BMIs which males in such populations may prefer (Lassek & Gaulin, 2006; 2019; Sugiyama, 2005). Other traits associated with human female attractiveness are also maximal in the nubile age group, including the most attractive complexions, faces, lips, feet, and voices (reviewed in Lassek and Gaulin, 2019). In addition, recent studies have emphasized the importance of nubility for breast attractiveness (Garza et al., 2021; Koscinksi, 2019; Pazhoohi et al., 2020) as proposed earlier (Marlowe, 1998)
Nubility and Reproductive Onset
Developmental trajectories and life-histories have presumably been shaped by selection in ways that maximize fitness, with female reproduction scheduled to begin at the completion of growth and maturation. In a cross-cultural sample of 11 hunter-gatherers and 18 subsistence agriculturalists, 91% and 83% (respectively) of first marriages were to women aged 12–21 years (Apostolou, 2010). In Binford’s (2001) sample of 339 hunter-gatherer groups, females married at an average age of 14 years, and the mean was also 14 in another sample of 124 (Marlowe & Berbesque, 2012). Even in a recent study of young men in 32 state-level societies (Buss, 1989), men’s mean preferred age for a bride was 20.8 ± 3.0 years.
As would be anticipated from the ages of marriage, the average age at first birth is also in the nubile age group in forager and subsistence populations. In one sample of 18 forager groups the average was 18.6 ± 1.6 years (Marlowe, 2005); and in another sample of 18 forager and subsistence populations (Walker, 2019), the average age of menarche was 14.3 ± 1.5 years (range, 12–17) and for first births was 18.2 ± 1.3 (range, 16–20). In a US sample of women ≤30 years interviewed in 1988–1992, the average age of first birth was 19.6 ± 3.4 years (Lassek & Gaulin, 2019). First pregnancies typically occur in the nubile age group in natural fertility populations despite younger women having documented lower fertility (Lassek & Gaulin, 2018b).
The young ages for first births are particularly worthy of attention because first parturitions are unusually hazardous for human females. Because the fetal head is larger relative to the mother’s size than in most other mammals and primates and because the bipedal pelvis constricts the space through which the fetal head must pass, human mothers—especially those with their first pregnancies—have a high risk of obstructed labor due to cephalopelvic disproportion (CPD), dysfunctional labor, and other complications of labor and delivery that can adversely affect both mother and newborn (Abitbol, 1996; Grunstra et al., 2019; Pavlicev et al., 2020; Rosenberg, 1992; Rosenberg & Trevathan, 1996, 2002; Weiner et al., 2008; Wittman & Wall, 2007). These risks to labor and delivery are much higher for first births, (Kaur & Kaur, 2012; Khunpradit et al., 2005; Surapanthapisit et al., 2006).
In populations without access to sterile surgical delivery by cesarian section (C-section), labor-and-delivery complications account for most maternal deaths in childbirth and are a major cause of maternal morbidity and infant mortality (Alauddin, 1986; Frost, 1984; Gaym, 2002; Nkata, 1997; Rosenfield, 1989; Vork et al., 1997). In 1990, the lifetime risk of a woman dying in childbirth in seven countries with limited access to surgical deliveries was 8%–14% (WHO, 2015a). A review conducted by the World Health Organization (Betran et al., 2015) concluded that 9%–16% of births have a high risk of infant and/or maternal mortality or morbidity without surgical intervention (but did not consider parity).
Although it is more difficult to determine the impact of these challenges on parturition in the past (Wells et al., 2012), available data suggest that there has been a substantial risk of death in childbirth for human mothers. The risk was estimated at 14% in a pre-Columbian sample from Chile (Arriaza et al., 1988), and a study of maternal deaths in England in the 16th–18th centuries indicated maternal death rates of 2.4%–2.9% per pregnancy (Dobbie, 1982). In 19th-century Sweden, 7% of married women died in childbirth (Hogberg & Brostrom, 1985), and in the United States as late as 1900, the rate was 0.9% per pregnancy (CDC, 1999).
Maternal mortality has also been high in 20th-century hunter-gatherers with limited access to modern medicine. In the Hiwi, 1%–2% of mothers die per pregnancy (Hill et al., 2007); the comparable figure is 1% in the Hadza (Blurton Jones, 2016), 0.7% in the Ache (Hill & Hurtado, 1996), and 2.2% in the Agta, accounting for 12%–14% of all adult female deaths (Headland, 1989). Among the fisher-horticulturalist Cuna Amerindians of the San Blas Islands, one-third of adult women were reported to have died in childbirth (Keeler, 1956). Deaths in childbirth have also been linked to skeletal evidence that paleolithic women died on average 5 years earlier than men (Angel, 1984), the reverse of what we see in most contemporary human populations, and to evidence of a higher ratio of females to males among younger adult skeletons (Pfeiffer et al., 2014).
Thus, there was likely a substantial risk of maternal and infant death from complications of the first labor and delivery prior to the availability of modern surgical deliveries. However, maternal age seems to be an important mitigating variable—a fact highly relevant to the nubility hypothesis. Prior studies suggest that women whose first pregnancies occur soon after they attain physical and sexual maturity have a lower risk of complications requiring a surgical delivery, as indicated by a lower frequency of C-sections in mothers aged 15–19 years (Azevedo et al., 2015; Gilbert et al., 2004; Sagili et al., 2012).
Of particular relevance are three other large-scale studies in countries in Africa, Latin America, and Asia which all found a lower risk of C-sections for mothers aged 16–19 years versus 20–24 years, controlling for maternal social and behavioral factors (and parity, where relevant). The risk was 25% lower in two different samples of 120,000 primiparas in 23 countries and 314,000 births in 29 countries (Ganchimeg et al., 2013, 2014), but was higher in those ≤15 years. In a study of 854,000 births in 18 Latin American countries (Conde-Agudelo et al., 2005) with similar adjustments, the risk of a C-section was 17% lower in mothers aged 18–19 years and 20% lower in those 16–17. These studies suggest that, compared with older mothers, women in the nubile age group may have an enhanced ability to successfully deliver their first child.
One reason that younger primiparas may be more successful giving birth without surgical assistance is that they tend to have smaller newborns that can pass more easily through the bipedal pelvis, as shown by the three multicountry studies cited above with controls for social and behavioral risk factors. In the 2013 study by Ganchimeg et al., primiparas aged 16–19 years were 16% more likely to have a low-birth-weight infant (<2.5 kg) than those who are aged 20–24 years, whereas, in the 2014 study, the risk was 15% higher for those aged 16–17 years and 10% for those aged 18–19 years. In the Latin American study (Conde-Agudelo et al., 2005), the risk was 27% higher for 16–17-year olds and 20% for 18–19 versus 20–24-year olds. These studies found similar increases in the risk of preterm births.
However, although neonatal mortality is usually higher in low-birth-weight infants (Eshete, 2019), in all three studies, the risk of neonatal mortality adjusted for social and behavioral factors was not significantly greater in mothers 16–19 versus 20–24 years. Another relevant study of >48,000 births in Germany in the 18th and 19th centuries found that overall infant and child mortalities (before the age of 5 years) were lower for mothers having a first child before the age of 20 years compared with mothers aged 20–24 years (Knodel & Hermalin, 1984). Despite having lower birth weights, the smaller newborns of younger mothers do not seem to be at a significantly greater risk of dying.
It is important to note that when social and behavioral factors are not controlled, studies consistently show that the risk of low birth weight, preterm birth, and neonatal mortality are substantially higher in mothers 16–19 years. Importantly, these risks—and the risk of maternal death (Ujah et al., 2005)—are significantly greater in mothers ≤15 years old, and in this youger age group, statistical controls for social and behavior factors do not mitigate these serious risks.
The Current Study
We explore the effect of maternal age on the outcome of 1.7 million first births in American women in 1990. Because many features of contemporary life diverge from those when women's life-history traits were first evolving, we perform parallel analyses with and without controls for social and behavioral risk factors that adversely affect reproductive outcomes today but which would have been less relevant in the past. Moreover, unlike most previous studies, we (a) assess a large set of maternal, fetal, and infant outcomes and complications of labor and delivery, including CPD; (b) consider only medically necessary C-sections; (c) use age groups structured to reflect the average ages of first births in forager populations, comparing the age of 16–20 years with older and younger mothers; (d) include fetal deaths as well as infant deaths; and (e) consider the evolutionary implications of the findings. We then attempt to determine the maternal age with the greatest likelihood of a positive reproductive outcome by simultaneously considering infant outcomes together with the risk of surgical deliveries required by complications of labor and delivery.
Methods
We used 1990 U.S. data from birth certificates and from fetal and infant death certificates, including 1.691 million primiparas, 17,508 women with fetal deaths and no previous births (nulliparas), and 2.510 million women with one or more previous births (multiparas). The 1990 birth cohort linked birth-infant death data and fetal death data are available at https://www.cdc.gov/nchs/data_access/vitalstatsonline.htm.
Outcomes for primiparas and multiparas were first compared to determine the relative risks associated with a first pregnancy versus subsequent pregnancies. The same data set was then used to explore the relationship between maternal age (and other risk factors) to pregnancy-related outcomes for the 1.691 million primiparas. Because first births in forager and subsistence groups are reliably in the 16–20 years age range (see above), we have selected age groups of ≤15, 16–20, 21–25, 26–30, and ≥31 years. All age groups were included in the logistic regressions.
Using this 1990 sample, rather than more recent U.S. data, has four advantages: (a) the 1989 revision of birth certificate launched a relatively brief period when the diagnosis of CPD was recorded; (b) the overall frequency of C-sections was considerably lower than it is currently and thus more indicative of the true risk of adverse outcomes (see below); (c) there were more mothers in the younger age groups, thus increasing the power of age comparisons; and (d) the U.S. female population had lower levels of obesity, a condition which could be confounded with age.
Variables from birth and death certificates related to maternal, fetal, and infant outcomes are listed in the tables. We distinguish preterm infants (<37 weeks gestation) with normal birth weights (≥2.5 kg) from preterm infants with low birth weight (<2.5 kg) and determined the risk of infant mortality and its relationship to parity and age for each birth-weight group. Newborns were considered small for gestational age if they were below the 10th percentile for birth weight for all newborns of the same gender and gestational age. Relative risks of CPD, C-sections, and infant mortality in relation to different ages and birth weights were determined for various parities and maternal age groups.
Control Variables
To assess how selection may have acted on the timing of first births in natural-fertility populations without access to surgical deliveries, we need to screen out significant disadvantages accruing to younger mothers in the developed world if they would not have been relevant when evolution was shaping women’s life history. Of the available variables on the birth and fetal death certificate, we selected six to use as control variables in logistic regressions predicting pregnancy outcomes:
Cigarete Smoking. Tobacco is a New World plant and so was not available to any humans until ancestral Americans crossed the Bering land bridge, and not available in the Old World until 1492. The earliest archaeological evidence of tobacco use dates to 860 AD in the Pacific Northwest (Tushingham et al., 2013) and to ∼300 BC in the Northeast, where tobacco use apparently originated (Rafferty, 2006). Even after tobacco spread around the world, it was used much more by men than women (WHO, 2007). A careful study of Aka hunter-gatherers in the Congo Basin (Roulette et al., 2016) found that tobacco use was 2.6 times more common among men than women and explicitly suggested that the sex difference was partly the result of women's evolved aversion to plant toxins that might harm a fetus. Other plants in the same botanical family may contain some nicotine but are unlikely to produce intake levels comparable to those supplied by commercially produced and mass-marketed cigaretes.
Education. Any kind of formal schooling is evolutionary novel and hence individual differences in the effects of such education (knowledge, wealth, social capital) are as well.
Race/Ethnicity. Heterogenous populations comprising multiple racial/ethnic groups, with power and resource-access differences among them (what race/ethnicity monitors in most studies), were unlikely before the origins of agriculture.
Marital Status. In traditional cultures where first births regularly occur in women 16–20 years old most mothers are married and, moreover, are supported by a dense web of kin such that these young mothers are socially embedded rather than stigmatized and isolated (Kramer & Lancaster, 2010). “Single, teen mothers” are a modern phenomenon.
Diabetes. The risk of gestational diabetes is strongly related to maternal prepregnancy BMI and is most likely to occur in women who are overweight or obese (2009), weights which, by analogy with contemporary hunter-gatherers, were relatively rare in the past. Because BMI was not available in this data set, controlling for diabetes helps to take this novel factor into account. Because younger mothers appear to have a lower risk for gestational diabetes (Xiong et al., 2001), controlling for this removes a potential disadvantage to older mothers and thus cuts against the hypothesis that nubile mothers have better outcomes.
Prenatal Visits. Modern prenatal care with sophisticated monitoring of mother’s physiology and fetal development—and individual differences in its availability—are 20th-century phenomena.
There were 1.126 million primiparas with data on all variables in the logistic regression analyses. For some variables, odds ratios may indicate higher risk while mean values show a lower risk; this is due to differences in the values of control variables in the two groups.
Optimal Outcome Measures
To help determine what age groups are more likely to achieve optimal reproductive outcomes, we defined three categories:
An optimal newborn is an infant: (a) of normal birth weight (≥2.5 kg), (b) with normal gestation (>36 weeks), and (c) who survived to 1 year.
An optimal delivery is one without a “critical C-section”—one associated with complications of labor and delivery that would pose significant risks to mother and infant (rather than being merely elective). More precisely, we defined a “critical C-section” as one associated with one or more of the following conditions: CPD, fetal distress, dysfunctional labor, placenta previa, placental abruption, prolapsed cord, abnormal fetal position, and maternal eclampsia or hemorrhage. For our sample of primiparas, the percentage of critical C-sections, so defined, was 13.5%, comparable to the 10%–15% rate for C-sections considered medically necessary by the World Health Organization (WHO, 2015b). (The rate for all C-sections for primiparas was 21.0%.)
An optimal pregnancy satisfies all the criteria of both an optimal newborn and an optimal delivery.
Results
Effect of Parity on Perinatal Complications and Infant Deaths
First births entail greater risks (Table 1). Compared to multiparas, primiparas were at much greater risk of all complications of labor and delivery, including CPD, operative delivery (forceps or vacuum extraction), and prolonged and dysfunctional labor, with a five-fold risk of a critical C-section (see Methods). Relative risks for primiparas versus multiparas were comparable with and without controls for six social and behavioral risk factors (see Methods). With controls, the newborns of primiparas were more likely to have experienced fetal distress, to have low birth weights (<2.5 kg), to be preterm, and to die in their first 28 days (neonatal mortality) and the first year, although postneonatal, fetal, and combined fetal/infant mortality were lower.
Table 1.
Characteristics and Perinatal Outcomes in 1.691 Million Primiparas (Para 1) Versus 2.510 Million Multiparas (Para 2+; the Reference Category), United States, 1990.
| Without controls | With controls Para 1 vs. para 2+ odds ratio | ||||
|---|---|---|---|---|---|
| Primipara para 1 | Multipara para 2+ | ||||
| Mean | SE | Mean | SE | ||
| Characteristics | |||||
| Age | 24.23 | 0.004 | 27.89 | 0.003 | |
| Parity | 1.00 | 0.000 | 2.76 | 0.001 | |
| Birth weight, kg | 3.31 | 0.000 | 3.37 | 0.000 | |
| Labor and delivery | |||||
| CPD, % | 6.68 | 0.020 | 1.68 | 0.009 | 4.74 (4.67, 4.81) |
| Dysfunctional labor, % | 4.88 | 0.017 | 1.64 | 0.006 | 3.31 (3.27, 3.38) |
| Prolonged labor, % | 1.95 | 0.011 | 0.46 | 0.004 | 4.67 (4.53, 4.80) |
| Primary C-section, % | 23.79 | 0.033 | 6.98 | 0.016 | 5.36 (5.32, 5.41) |
| Critical C-section a , % | 15.13 | 0.028 | 4.17 | 0.013 | 5.13 (5.08, 5.19) |
| Operative delivery, % | 14.50 | 0.028 | 5.25 | 0.014 | 3.10 (3.07, 3.12) |
| Fetal distress, % | 6.05 | 0.019 | 3.07 | 0.012 | 2.31 (2.29, 2.35) |
| Fetus and neonate | |||||
| Male, % | 51.37 | 0.038 | 51.11 | 0.032 | 1.01 (1.00, 1.03) |
| Low birth weight, % | 7.18 | 0.020 | 6.78 | 0.016 | 1.32 (1.31, 1.34) |
| Preterm birth, % | 10.27 | 0.023 | 10.84 | 0.020 | 1.08 (1.07, 1.09) |
| Birth weight <2.5 kg | 4.32 | 0.016 | 4.21 | 0.013 | 1.20 (1.19, 1.22) |
| Birth weight ≥2.5 kg | 5.92 | 0.018 | 6.58 | 0.016 | 0.96 (0.95, 0.97) |
| Infant deaths | 8.24 | 0.070 | 9.23 | 0.061 | 1.05 (1.01, 1.08) |
| Neonatal deaths | 5.69 | 0.058 | 5.54 | 0.047 | 1.39 (1.34, 1.44) |
| Postneonatal deaths | 2.56 | 0.039 | 3.37 | 0.039 | 0.64 (0.61, 0.68) |
| Fetal deaths | 10.25 | 0.077 | 18.80 | 0.086 | 0.84 (0.81, 0.87) |
| Infant and fetal deaths | 18.41 | 0.102 | 24.01 | 0.075 | 0.97 (0.95, 0.99) |
Raw means are tested for parity-related differences without controls (all significantly different, p < .001). Odds ratios with 95% confidence intervals are established via logistic regression, controlling for maternal age, tobacco use, education, marital status, prenatal visits, diabetes, and race/ethnicity. Deaths are per 1,000 births. CPD = cephalopelvic disproportion; C-section = cesarian section.
See the Methods section.
As anticipated, preterm infants with normal birth weights did much better than those with low birth weights. In the whole sample (4.201 million births) using logistic regression with the same control variables, infants that were both preterm (≤36 weeks) and low birth weight (<2.5 kg) were much more likely to die in their first year (odds ratio 19.2, confidence interval [CI]: 18.7–19.8), whereas preterm infants with normal birth weights were less likely to die than other infants (odds ratio: 0.82, CI: 0.78–0.85). Primiparas were less likely than multiparas to have preterm births with normal birth weight and more likely to have preterm infants with low birth weights (Table 1).
Relationship of CPD, Critical C-Sections, and Infant Mortality to Birth Weight
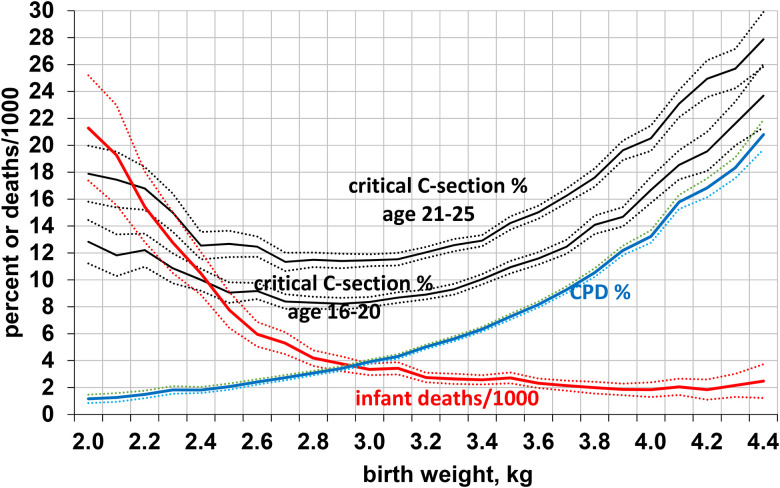
For all primiparas, the risk of CPD rose sharply with birth weight, whereas the risk of infant mortality decreased, reaching a low at 4 kg (Figure 1). The risk of a critical C-section increased for birth weights >2.9 kg. Smaller newborns were better able to successfully pass through their mother’s pelvis but were at greater risk of dying. It is noteworthy that the optimal birth weights for successful birth (without critical C-section) and for infant survival were more than 1 kg apart.
Figure 1.
Risks of CPD and infant deaths in relation to birth weight in 1.691 million primiparas, and percentage with critical C-section in 0.486 million primiparas aged 16–20 and 0.496 million aged 21–25, United States, 1990, (all with 99% confidence intervals). CPD = cephalopelvic disproportion; C-section = cesarian section.
Effect of Age on Maternal Risk Factors and Gestational Complications in Primiparas
Compared to primiparas aged 21–25 years, those aged 16–20 years and especially those <16 years had more social and behavioral risk factors for negative outcomes (Table 2): They were much less likely to be married, had less education and prenatal care, and were more likely to smoke and to belong to racial and ethnic groups with poorer pregnancy outcomes. During pregnancy, they were more likely to have anemia and eclampsia (though not with controls), but less likely to have gestational diabetes, hypertension, or bleeding. The fathers of infants born to younger mothers were younger and had less education than the fathers of infants born to older primiparas
Table 2.
Maternal and Paternal Characteristics and Maternal Gestational Complications for Primiparas Aged 10–25 years, United States, 1990.
| N | Age groups (years) | |||||
|---|---|---|---|---|---|---|
| ≤15 | 16–20 | 21–25 | ||||
| 37,320 | 491,474 | 500,367 | ||||
| Mean | SE | Mean | SE | Mean | SE | |
| Demographic and behavioral factors | ||||||
| Age, years | 14.62 | 0.003 | 18.39 | 0.002 | 22.97 | 0.002 |
| Education, years | 8.39 | 0.007 | 10.99 | 0.003 | 12.57 | 0.003 |
| Married, % | 11.41 | 0.166 | 37.83 | 0.070 | 71.56 | 0.064 |
| Race/ethnicity | ||||||
| Non-Hispanic White, % | 30.08 | 0.239 | 54.58 | 0.071 | 75.07 | 0.066 |
| Non-Hispanic Black, % | 44.93 | 0.260 | 22.52 | 0.060 | 8.40 | 0.042 |
| Hispanic, % | 20.32 | 0.210 | 17.71 | 0.055 | 8.75 | 0.043 |
| Asian, % | 1.48 a | 0.063 | 1.60 a | 0.018 | 5.13 | 0.034 |
| Native American, % | 1.30 | 0.059 | 1.08 | 0.015 | 0.30 | 0.008 |
| Other, % | 1.87 | 0.071 | 2.52 | 0.022 | 2.35 | 0.023 |
| Cigaretes/day | 0.96 | 0.022 | 2.14 | 0.009 | 1.75 | 0.008 |
| Drinks/week | 0.01 | 0.002 | 0.03 | 0.001 | 0.04 | 0.001 |
| Month prenatal care began | 4.31 | 0.012 | 3.50 | 0.003 | 2.73 | 0.002 |
| Prenatal visits | 8.79 | 0.025 | 10.23 | 0.006 | 12.12 | 0.006 |
| Weight gain, lb | 30.39 | 0.085 | 32.31 | 0.022 | 32.49 | 0.020 |
| Father’s age, years | 18.95 | 0.026 | 21.82 | 0.007 | 26.08 | 0.007 |
| Father’s education, years | 9.85 | 0.024 | 11.20 | 0.005 | 12.54 | 0.004 |
| Gestational complications | ||||||
| Anemia, % | 3.11 | 0.093 | 2.30 | 0.022 | 1.52 | 0.018 |
| Eclampsia, % | 1.03 | 0.054 | 0.71 | 0.012 | 0.59 | 0.011 |
| Gestational hypertension, % | 4.15 | 0.106 | 3.84 | 0.028 | 4.18 | 0.029 |
| Diabetes, % | 0.44 | 0.035 | 0.90 | 0.014 | 1.72 | 0.019 |
| Pregnancy bleeding, % | 0.49 | 0.039 | 0.62 | 0.012 | 0.71 | 0.013 |
Raw means are tested for age-related differences without controls. For gestational complications, bold text denotes whether the 16–20 years or 21–25 years group had significantly better outcomes.
All are significantly different, p < .001, except where indicated by the same superscript “a.”
Effect of Maternal Age on Infant Outcomes in Primiparas
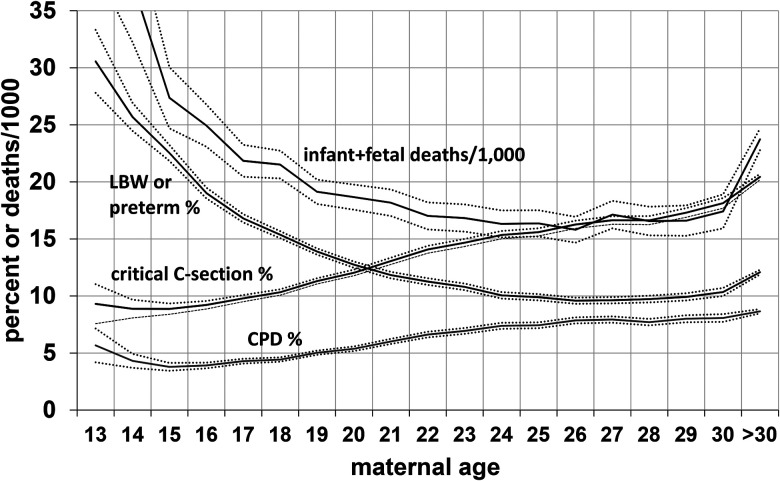
As expected from the higher incidence of social and behavioral risk factors in younger primiparas, when risk factors were not controlled, the newborns of primiparas aged 16–20 years did less well than newborns of those aged 21–25 years, and the infants of primiparas ≤15 generally fared even worse (Table 3). Newborns of younger primiparas had lower mean birth weights and were more likely to weigh <2.5 kg, to be preterm (<37 weeks gestation), to be small for gestational age, and to have low Apgar scores, congenital anomalies, and abnormal newborn conditions (see Table 3 for list). They were more likely to die within 28 days of birth (neonatal mortality) and between 29 days and 1 year (postneonatal mortality), and the risk of fetal death (>20 weeks) was also higher. Figure 2 shows the risk for low birth weight or preterm birth and infant/fetal mortality in relation to age. Mothers in their mid to late 20s had the lowest risks.
Table 3.
Fetal and Infant Outcomes for Primiparas Aged ≤15 years (n = 37,320), 16–20 years (n = 491,474), and 21–25 years (n = 500,367, Reference Category), United States, 1990.
| Without controls | With controls | ||||
|---|---|---|---|---|---|
| Mean values | Odds ratios | ||||
| ≤15 | 16–20 | 21–25 | ≤15 vs. 21–25 | 16–20 vs. 21–25 | |
| Mean birth weight, kg | 3.12 | 3.25 | 3.33 | ||
| Low birth weight: <2.5 kg, % | 11.60 | 8.06 | 6.31 | 0.94 (0.90, 0.99) | 0.95 (0.93, 0.97) |
| Birth weight, 2.5–2.9 kg, % | 17.34 | 14.26 | 11.81 | 1.01 (0.97, 1.05) | 1.03 (1.02, 1.05) |
| Birth weight, 3.0–3.4 kg, % | 40.17 | 39.60 | 38.43 | 1.07 (1.04, 1.10) | 1.05 (1.03, 1.06) |
| Birth weight, 3.5–3.9 kg, % | 20.58 | 25.47 | 29.10 | 0.93 (0.90, 0.96) | 0.98 (0.97, 1.00) |
| Birth weight, 4.0 + kg, % | 4.32 | 7.17 | 9.64 | 0.71 (0.66, 0.76) | 0.91 (0.89, 0.93) |
| Male, % | 51.75 a | 51.42 a | 51.30 a | 1.02 (1.00, 1.05) | 1.01 (1.00, 1.02) |
| Preterm birth, % | 20.24 | 12.09 | 9.06 | 1.34 (1.29, 1.39) | 1.04 (1.02, 1.05) |
| With birth weight <2.5 kg, % | 7.65 | 4.71 | 3.80 | 1.05 (0.99, 1.11) | 0.92 (0.90, 0.95) |
| With birth weight ≥2.5 kg, % | 12.45 | 7.33 | 5.32 | 1.48 (1.42, 1.55) | 1.12 (1.10, 1.15) |
| Small for gestational age, % | 13.11 | 12.41 | 10.15 | 0.87 (0.83, 0.91) | 1.00 (0.99, 1.02) |
| Apgar, 5 min, <7% | 11.78 | 10.37 | 9.73 | 0.88 (0.84, 0.92) | 0.93 (0.91, 0.94) |
| Congenital anomaly, % | 1.95 | 1.85 | 1.75 | 1.07 (0.98, 1.17) | 1.02 (0.98, 1.06) |
| Newborn abnormality, % b | 7.82 | 7.04 | 6.72 | 1.01 (0.96, 1.06) | 0.98 (0.96, 1.00) |
| Total infant mortality | 17.10 | 10.23 | 7.45 | 1.04 (0.93, 1.17) | 0.97 (0.91. 1.02) |
| Neonatal mortality | 11.25 | 6.42 | 5.22 | 0.90 (0.78, 1.04) | 0.84 (0.78, 0.90) |
| Postneonatal mortality | 5.85 | 3.81 | 2.23 | 1.45 (1.19, 1.77) | 1.29 (1.17, 1.43) |
| Sudden infant death | 2.42 | 1.58 | 0.71 | 1.65 (1.19, 2.29) | 1.63 (1.38, 1.93) |
| Fetal mortality (>20 weeks) | 15.92 | 10.47 | 9.13 | 0.80 (0.67, 0.95) | 0.79 (0.73, 0.86) |
| Infant and fetal mortality | 32.74 | 20.59 | 16.51 | 0.95 (0.86, 1.04) | 0.90 (0.86, 0.95) |
| Optimal infant outcome, % | 74.13 | 83.30 | 87.29 | 0.82 (0.80, 0.85) | 0.98 (0.96, 0.99) |
Raw means are tested for age-related differences without controls. Odds ratios with 95% confidence intervals are established via logistic regression in 1.126 million primigravidas (including all age groups), controlling tobacco use, education, marital status, prenatal visits, diabetes, and race/ethnicity. Significantly better outcomes for 16–20 years or 21–25 years are shown in bold. Mortality rates are per 1,000 births.
All are significantly different, p < .001, except where indicated by the same superscript “a.”
Abnormal conditions of the newborn include meconium aspiration, hyaline membrane disease, seizures, anemia, and the need for assisted breathing.
Figure 2.
Relationship of maternal age in 1.691 million primigravidas to delivery by critical C-section, LBW or preterm births, CPD, and fetal and infant deaths per 1000 births; United States, 1990, all with 99% confidence intervals. LBW = low birth weight; CPD = cephalopelvic disproportion; C-section = cesarian section.
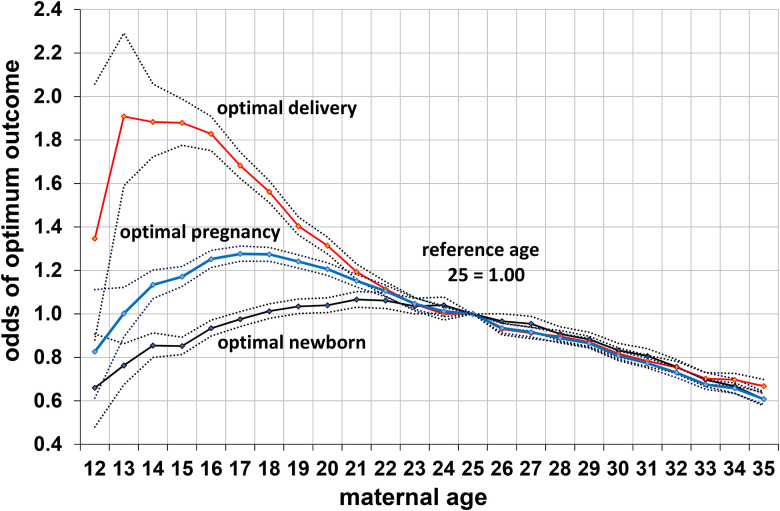
Remarkably, however, when social and behavioral risk factors were controlled, most of the poorer outcomes for fetuses and infants of nubile mothers disappeared or were reversed (Table 3, odds ratios). In fact, mothers aged 16–20 years were now at lower risk than those who were 21–25 years in having infants with low birth weights (<2.5 kg) and low Apgar scores. The newborns of primiparas 16–20 were more likely to have birth weights between 2.5 and 3.4 kg and more likely to survive, with a lower risk of fetal and neonatal deaths, and of total fetal/infant deaths. Although their infants were still more likely to be preterm, their preterm infants were more likely to have normal birth weights—a group with lower infant mortality. The higher risk for postneonatal mortality in infants of younger primiparas was mainly due to an elevated risk of sudden infant death, a condition associated with young maternal age and low socioeconomic status (l’Hoir et al., 1998). With our six controls, primiparas of 16–20 years were almost as likely as those 21–25 years to have an optimal infant outcome (Table 3, Figure 3), whereas those ≤15 years were much less likely to achieve this benchmark.
Figure 3.
Odds of an optimal delivery (delivery without critical cesarian section) and optimal infant (normal birth weight, normal gestation, and survival to 1 year) and an optimal infant and delivery together (optimal pregnancy) in relationship to maternal age in 1.128 million primiparas with age 25 as the reference category, controlling for maternal tobacco use, education, marital status, prenatal care, race/ethnicity, and diabetes, with 95% confidence intervals; United States, 1990.
Effect of Maternal Age on Complications of Labor and Delivery
In marked contrast to their higher risks for newborn problems without controls, primiparas aged 16–20 years had significantly lower risks (p < .001) than those aged 21–25 years for most complications of labor and delivery (Table 4, Figures 1 and 2). They were much less likely to have any C-section, a critical C-section, or an operative delivery (forceps or vacuum extraction), and this lower risk was related to their much lower risks of CPD, maternal fever, premature membrane rupture, and unspecified labor-related complications, and lower risks for most fetal complications including fetal distress, abnormal fetal position, placenta previa, and birth injury.
Table 4.
Labor-and-Delivery Outcomes for Primiparas Aged ≤15 years (n = 37,320), 16–20 years (n = 491,474), and 21–25 years (n = 500,367, Reference Category).
| Without controls | With controls | ||||
|---|---|---|---|---|---|
| Means | Odds ratio, 21–25 = 1 | ||||
| Outcomes | ≤15 | 16–20 | 21–25 | ≤15 vs. 21–25 | 16–20 vs. 21–25 |
| C-section: all, % | 14.55 | 17.21 | 23.07 | 0.55 (0.53, 0.57) | 0.70 (0.69, 0.71) |
| C-section: critical, % a | 9.08 | 10.97 | 17.38 | 0.60 (0.57, 0.63) | 0.73 (0.72, 0.74) |
| Operative delivery, % | 10.78 | 12.15 | 14.63 | 1.06 (1.02, 1.11) | 0.95 (0.93, 0.96) |
| Any complication, % | 33.19 | 34.34 | 38.05 | 0.78 (0.76, 0.81) | 0.84 (0.83, 0.85) |
| CPD, % | 4.00 | 4.69 | 6.80 | 0.65 (0.61, 0.70) | 0.73 (0.71, 0.75) |
| Prolonged labor, % | 1.20 | 1.57 | 2.16 | 0.82 (0.72, 0.92) | 0.91 (0.87, 0.95) |
| Dysfunctional labor, % | 2.75 | 3.69 | 5.55 | 0.69 (0.64, 0.75) | 0.85 (0.83, 0.87) |
| Fetal distress, % | 5.14 | 5.69 | 5.98 | 0.76 (0.72, 0.81) | 0.90 (0.88, 0.92) |
| Abruptio placenta, % | 0.55 b | 0.56 b | 0.54 | 0.78 (0.65, 0.94) | 0.90 (0.84, 0.96) |
| Placenta previa, % | 0.13 b | 0.12 b | 0.17 | 0.67 (0.46, 0.96) | 0.64 (0.56, 0.73) |
| Cord prolapse, % | 0.30 b | 0.28 b | 0.31 b | 0.99 (0.78, 1.26) | 0.93 (0.85, 1.02) |
| Abnormal position, % | 2.60 | 3.13 | 4.04 | 0.72 (0.66, 0.78) | 0.81 (0.79, 0.83) |
| Birth injury, % | 0.16 | 0.21 | 0.25 | 0.92 (0.65, 1.30) | 0.95 (0.85, 1.07) |
| Meconium staining, % | 7.35 | 7.08 | 7.02 | 0.88 (0.84, 0.93) | 0.92 (0.90, 0.94) |
| Membrane rupture, % | 3.34 | 3.57 | 4.08 | 0.71 (0.66, 0.77) | 0.82 (0.80, 0.84) |
| Maternal bleeding, % | 0.52 b | 0.50 b | 0.52 b | 0.98 (0.80, 1.20) | 0.95 (0.88, 1.03) |
| Maternal seizure, % | 0.17 | 0.08 | 0.05 | 1.53 (1.46, 2.24) | 0.99 (0.80, 1.22) |
| Maternal fever, % | 1.92 b | 1.85 b | 1.99 | 0.95 (0.86, 1.04) | 0.93 (0.89, 0.96) |
| Other labor complication, % | 11.89 | 11.70 | 12.45 | 0.94 (0.90, 0.98) | 0.91 (0.90, 0.92) |
| Optimum delivery, % | 90.95 | 88.89 | 85.15 | 1.67 (1.60. 1.75) | 1.36 (1.34, 1.38) |
Raw means are tested for age-related differences without controls. Odds ratios with 95% confidence intervals are established via logistic regression in 1.126 million primigravidas (including all age groups), controlling for tobacco use, education, marital status, prenatal visits, diabetes, and race/ethnicity. Significantly better outcomes for 16–20 years or 21–25 years are shown in bold. CPD = cephalopelvic disproportion; C-section = cesarian section.
As defined in methods: C-section with CPD, fetal distress, dysfunctional labor, placenta previa, abruptio placenta, cord prolapse, abnormal fetal position, maternal eclampsia, or maternal hemorrhage.
All are significantly different, p < .001, except where indicated by the same superscript “b.”
As shown in Figure 2, the risk of a critical C-section (see Methods section) reached a minimum of 8.9% at the age of 14–15 years, increased by more than one-third to 12.0% by the age of 20 years, and continued to rise steeply for women in their 20s, reaching 18.1% by the age of 30 years, twice the minimum. The risk of CPD followed a similar pattern, doubling from 4.1% at the age of 15 years to 8.4% at the age of 30 years.
For newborns of any given birth weight, mothers aged 16–20 years were much less likely to require a critical C-section than were older mothers (Figure 1), with odds of 0.73 (CI: 0.72–0.74) overall and significantly lower risks for all of the complications associated with critical C-sections except eclampsia.
With the same set of six social and behavioral controls, younger mothers continued to have significantly lower risks for most complications of labor and delivery and a much lower risks of a critical C-section than older primiparas, but the risk of an operative delivery (forceps or vacuum extraction) was higher in primiparas ≤15. Primiparas aged 16–20 years were much more likely to have an optimum delivery than those 21–25, with odds of 1.36 (CI: 1.34–1.38) (Table 4, Figure 3).
Optimal Pregnancy Outcomes
To help determine what would have maximized reproductive success over most of human evolution, we defined an optimal pregnancy outcome as comprising both an optimal newborn and an optimal delivery (see Methods section). Without controls for novel risk factors, the percentage of mothers with an optimal pregnancy outcome was similar in mothers aged 16–20 years (74.7%) and those aged 21–25 years (75.6%). However, in the controlled analysis—which attempts to screen out risk factors that would not have been relevant in the past—those aged 16–20 years were more likely to have optimal pregnancy outcomes than those aged 21–25 years (odds ratio: 1.16, CI: 1.15–1.18), with a peak probability at age 17–18 years (using age of 25 years as the reference category; Figure 3). (The optimum was also slightly higher for those ≤15 because their lower risk of labor-and-delivery complications marginally offset their higher risk of newborn complications.) If an optimum pregnancy outcome is defined as having an optimal newborn plus a vaginal delivery (excluding all C-sections, both critical and otherwise), the odds for primiparas 16–20 increase to 1.28 (CI: 1.27–1.30) (Table 5).
Table 5.
Odds Ratios (with 95% Confidence Intervals) are Established via Logistic Regression Predicting an Optimal Pregnancy Outcome (Normal Birth Weight and Gestation Length, Survival to one Year, and Delivery Without a Critical C-Section) in 1.126 Million Primigravidas, United States, 1990.
| Variable | Odds ratio |
|---|---|
| Age | |
| ≤15 | 1.05 (1.02, 1.09) |
| 16–20 | 1.16 (1.15, 1.17) |
| 21–25 a | 1.00 |
| 26–30 | 0.84 (0.83, 0.85) |
| 30+ | 0.63 (0.62, 0.64) |
| Education, years | 1.03 (1.03, 1.04) |
| Married | 1.01 (1.00, 1.02) |
| Cigaretes/day | 0.99 (0.99, 0.99) |
| Prenatal visits | 1.04 (1.04, 1.04) |
| Diabetes | 0.62 (0.60, 0.63) |
| Race/ethnicity | |
| Non-Hispanic White a | 1.00 |
| Non-Hispanic Black | 0.68 (0.67, 0.69) |
| Hispanic | 1.05 (1.03, 1.07) |
| Asian | 1.16 (1.14, 1.18) |
| Native American | 0.84 (0.83, 0.85) |
| Other | 1.01 (0.97, 1.05) |
Although a maternal age of 16–20 years was optimal for a first birth, the optimal age for a second birth was 21–25 years. Compared with mothers 21–25, the odds of an optimal pregnancy outcome were 0.57 (0.51–0.63) for mothers <16 with a second birth and 0.88 (0.86–0.90) for those aged 16–20 years. For a second birth, mothers 21–25 years had the best outcome of any age group.
Discussion
Nubile Mothers Have Better First-Pregnancy Outcomes
As noted in the Introduction section, recent evidence suggests that heterosexual men are attracted to attributes characteristic of physically and sexually mature women between 15 and 19 years of age, closely corresponding to the 16–20 years age group when first births typically occur in natural-fertility populations (Kramer & Lancaster, 2010; Lassek & Gaulin, 2019; Symons, 1979; Walker, 2019). Relevant to that preference, the current study is consistent with others in a variety of populations indicating that, when surgical deliveries are not available, this is also the age group with the best first-pregnancy outcomes.
In our large sample, primiparas were at much higher risk than multiparas for CPD, critical C-sections, and other complications of labor and delivery that increase the risk of maternal mortality, but primiparas aged 16–20 years had substantially lower risks than those 21–25, experiencing a 30% lower risk of any C-section, a 27% lower risk of a critical C-section, and much lower risks for serious complications of labor and delivery, including CPD, abnormal labor, and fetal distress (Table 4). These findings are consistent with many other studies that show a reduced risk of C-sections in primiparas aged 16–19 years versus older mothers, including studies in many non-Western countries (Conde-Agudelo et al., 2000; Ganchimeg et al., 2013, 2014).
The reduced risk of complications of labor and delivery in primiparas aged 16–20 years is of special importance in a species where the conjunction of bipedalism and very large brains has made vaginal births difficult. Only very recently have these conflicting selection pressures been relieved by the surgical innovation of C-section, an intervention still not available everywhere. Where and when such surgical births are unavailable, it is essential for a first-time mother to produce a child who can successfully pass through her birth canal so that mother and child can survive and continue to augment her fitness.
Our study does not present data on maternal survival, but the lower risk of labor-and-delivery complications for mothers in the nubile age group would likely decrease the risk of maternal deaths in childbirth. In a recent study of maternal mortality in 144 countries (Nove et al., 2014), in a third of countries mortality was lower for mothers aged 15–19 years than those aged 20–24 years; and this included most of the countries with the highest maternal mortality rates.
There may also be survival benefits for the infants of younger mothers. Our study is consistent with others showing comparable survival in the newborns of mothers aged 16–19 years with those aged 20–24 years when social and behavioral risk factors are controlled (Bradford & Giles, 1989; Conde-Agudelo et al., 2000; Gallais et al., 1996; Ganchimeg et al., 2013, 2014; Geist et al., 2006; Geronimus & Korenman, 1992; Phipps-Yonas, 1980; Scholl et al., 1984; Smith & Pell, 2001).
When evolutionarily novel risk factors were controlled, the fetuses and newborns of primiparas aged 16–20 years did as well or better as those of primiparas aged 21–25 years and were more likely to survive to 1 year. Although the 16–20-year old primiparas had significantly more preterm births with normal birth weights (which have good survival rates), their risks for preterm births with low birth weight, overall low birth weight, and neonatal mortality were significantly lower.
These results are also consistent with the finding that in 18th–19th century Germany, when infant and child mortality rates were much higher, the children of mothers aged 15–19 years were more likely to survive to reproductive age than those of older mothers (Knodel & Hermalin, 1984). Such high infant and child mortality rates were also likely in the environment of evolutionary adaptedness (EEA; Tooby and Cosmides, 1992), with almost half of children dying before reaching puberty (Volk & Atkinson, 2013).
Because until quite recently most women gave birth at home with assistance from their relatives, another potential advantage for younger mothers is their greater likelihood of having mothers, grandmothers, and aunts to help with pregnancy and childbirth, especially in groups with shorter life expectancies, where senior kin may have been less common.
Thus, it is not surprising that women's evolved life history seems to schedule first reproduction soon after the attainment of adult size and sexual maturity, as revealed by the demography of subsistence populations. Primiparas aged 16–20 years were likely to have a better newborn outcome than older primiparas and much better labor-and-delivery outcomes, which combine to yield a substantially greater chance for a successful pregnancy outcome (Figure 3).
Evolution of Male Preferences for Nubility
Because first pregnancies are most likely to be successful in women in the 16–20 years age group, we would expect positive selection on male preferences that targeted any reliable phenotypic correlates of this female life stage. Thus, this study, together with others showing the advantages of first births in this age range, when first births typically occur in subsistence populations, supports nubility as a key criterion of female attractiveness.
Men’s preferences for certain female traits associated with nubility—such as low WHRs, low BMIs (in well-nourished populations), and low waist–stature ratios (which may be the best predictor of attractiveness)—have recently been explained in terms of their correlation with female reproductive value (Andrews et al., 2017; Butovskaya et al., 2017; Fessler et al., 2005; Lassek & Gaulin, 2019; Marlowe, 1998; Prokop et al., 2020; Röder et al., 2013; Sugiyama, 2005; Symons, 1979, 1995), an inherently future-oriented parameter (Fisher, 1930). Complementing those findings, this study suggests an additional, more immediate, benefit to preferences for nubility.
If, as our findings suggest, nubile women have had more successful first-pregnancy outcomes than older women (thus enhancing their own reproductive success), men with preferences for traits correlated with nubility would have experienced a parallel fitness advantage. Conscious awareness would not have been required for this preference to evolve (Gaulin & McBurney, 2001; Kenrick, 1995); genetic variance in the preference and a reliable correlation between the preferred trait (e.g., any sign of nubility) and a positive fitness outcome (e.g., a successful first birth) would be sufficient.
The main cost to males who prefer nubile women is the lower frequency of ovulation in younger women (see Lassek and Gaulin, 2018a). However, this can be largely overcome by an increased frequency of coitus, which is usual for this age group (Weinstein et al., 1990). The fact that the typical age at first birth falls in the nubile period in traditional populations suggests that copulatory effort is normally sufficient to overcome the lower probability of conception with women in this age group.
Because of the lower fertility of nubile women, Symons (1979) suggested that males seeking short-term mating with minimal commitment might prefer older women who would have a greater chance of conceiving, as fertility is maximal in the late 20s. However, Symons (1995) changed his view because of studies in modern hunter-gatherers showing most potentially fertile women over 20 are either pregnant or nursing, with small windows of time when conception is possible. In this context, nubile women are likely to have a greater chance of conceiving despite their reduced frequency of ovulation.
Why do Nubile Primiparas Have Better Obstetric Outcomes?
Three factors help to explain the lower risk of critical surgical deliveries in the 16–20-year-old mothers: (a) Younger primiparas had smaller newborns than older primiparas, with more neonates weighing 2.5–3.4 kg, in the lower two-thirds of the normal range (2.5–3.9 kg), and fewer weighing 3.5 kg or more (Table 3). As shown in Figure 1, smaller neonates are less likely to have CPD or require a critical C-section. (b) For newborns of the same birth weight, nubile primiparas had a much lower risk of a critical C-section (Figure 1), and all but one of its associated complications, than those over 20. (c) The fetuses of younger primiparas were less likely to experience complications during labor and delivery, including fetal distress, cord prolapse, placenta previa, and abnormal position (Table 4). In total, younger mothers seem to have an enhanced ability to move their fetus through the birth canal and their fetuses also seem to be more tolerant of the stresses of labor and delivery.
Negative Consequences of Teen Pregnancy Today
It is important to stress that our analysis also documents the disadvantages of teen pregnancy in WEIRD (as defined by Henrich et al., 2010) populations, such as the United States in 1990, especially in younger teens. Where both surgical births and birth control are widely available and where teen pregnancy is usually associated with social and behavioral risk factors, teen pregnancies are very likely to have many negative long-term consequences for the mother and infant (Black et al., 2012). Pregnancies in teens younger than 16 years had much poorer infant outcomes and were more likely to have an operative delivery (forceps or vacuum extraction). Thus, efforts to prevent teen pregnancies are highly desirable and are not in any way contradicted by any of our conclusions concerning past selection on female life history.
Despite the disadvantages of teen pregnancy in contemporary WEIRD societies, we suggest that our findings are relevant to understanding human evolution, in particular women’s life history and men’s mating preferences. Given the obvious evolutionary importance of a successful vaginal delivery, early first pregnancies and a male preference for nubility were probably advantageous in the premodern era. They would have produced the best odds of a successful reproductive outcome—a benefit to mother, father, and infant.
Limitations
The use of data from a modern North American population to gauge probable reproductive outcomes in evolutionarily relevant populations is not ideal; but because of the extremely recent nature of shifts in human reproductive ecology, the relevant underlying biology may be largely unchanged. By controlling for evolutionarily novel risk factors which make “teen pregnancy” disadvantageous in contemporary populations, the results should have some validity for natural-fertility populations where first pregnancies in 16–20 year-olds are normative. Our findings of a much lower risk of critically necessary surgical births and a slightly lower risk of neonatal deaths for mothers in the 16–20 years age group (with controls for social and behavioral risk factors) are consistent with findings from a large variety of non-Western countries (Conde-Agudelo et al., 2000; Ganchimeg et al., 2013, 2014) and from 18th–19th century Germany (Knodel & Hermalin, 1984).
It might be argued that modern American obstetric practices are quite different from those in traditional societies where experienced midwives play a crucial role, but the uniformity of findings in the US and in three different samples of 23, 29, and 18 non-Western countries points to common underlying factors in reproductive biology and suggests that similar biological factors are likely to have been operating in the EEA.
Because all of the many available studies uniformly show a decrease in obstetric complications requiring a C-section in the 16–19 years age group, this is more likely to be species-typical. Limiting the analysis to C-sections associated with complications that would be likely to cause significant harm to the mother or fetus without surgical intervention may give some indication of the risk in natural-fertility populations without access to surgical deliveries.
Traditional populations are also likely to have much higher infant and child mortality: Infant mortality has been estimated at 27% in the EEA (Volk & Atkinson, 2013), compared with 0.8% in the 1990 data set. However, our analysis controlling for contemporary maternal risk factors suggests that the infants of nubile mothers in the past would likely have done as well or better than those of older mothers, as also indicated by findings from 18th to19th century Germany, where the overall infant mortality rate was 23% (Knodel & Hermalin, 1984).
When comparable infant outcomes are combined with the much lower risk of death in childbirth from complications of labor and delivery, it seems likely that nubile women would have been the most successful primiparas, thus suggesting an adaptive explanation for the timing of first births in a wide range of forager and subsistence populations (Kramer & Lancaster, 2010; Lassek & Gaulin, 2019; Symons, 1979; Walker, 2019).
Conclusion
When surgical deliveries are not available—the normal case during human evolution—women who first become pregnant in the nubile age group of 16–20 years, when first births typically occur in forager and subsistence populations, are the most likely to survive childbirth and deliver a viable infant. Thus, men who preferred traits more strongly manifested in this age group would not only have targeted mates of high reproductive value but would also have selected the partners most likely to have a successful first pregnancy.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article except for the publication fee paid for by the University of California at Santa Barbara.
ORCID iD: William D. Lassek https://orcid.org/0000-0003-0519-6615
References
- CDC. (1999). Achievements in public health, 1900–1999: Healthier mothers and babies. Morbidity & Mortality Weekly Report, 48(38), 849–858. [PubMed] [Google Scholar]
- WHO. (2007). Sifting the evidence: Gender and tobacco control. World Health Organization. [Google Scholar]
- WHO. (2015a). Trends in maternal mortality, 1990–2015. World Health Organization. [Google Scholar]
- WHO. (2015b). WHO Statement on caesarean section rates. World Health Organization. [Google Scholar]
- Abitbol M. M. (1996). Birth and human evolution: Anatomical and obstetrical mechanics in primates. Bergin & Garvey. [Google Scholar]
- Alauddin M. (1986). Maternal mortality in rural Bangladesh: The Tangail district. Studies in Family Planning, 17(1), 13–21. 10.2307/1966951 [DOI] [PubMed] [Google Scholar]
- Andrews T. M., Lukaszweski A. W., Simmons Z. L., Bleske-Recheck A. (2017). Cue-based estimates of reproductive value explain women's Body attractiveness. Evolution and Human Behavior, 38(4), 461–467. 10.1016/j.evolhumbehav.2017.04.002 [DOI] [Google Scholar]
- Angel J. L. (1984). Health as a crucial factor in the changes from hunting to developed farming in the Eastern Mediterranean. In Cohen M. N., Armelagos G. J. (Eds.), Paleopathology at the origins of agriculture (pp. 51–73). Academic Press. [Google Scholar]
- Apostolou M. (2010). Parental choice: What parents want in a son-in-law and a daughter-in-law across 67 pre-industrial societies. British Journal of Psychology, 101(4), 695–704 [DOI] [PubMed] [Google Scholar]
- Arriaza B., Allison M., Gerszten E. (1988). Maternal mortality in pre-Columbian Indians of Arica, Chile. American Journal of Physical Anthropology, 77(1), 35–41. 10.1002/ajpa.1330770107 [DOI] [PubMed] [Google Scholar]
- Azevedo W. F., Diniz M. B., Fonseca E. S., Azevedo L. M., Evangelista C. B. (2015). Complications in adolescent pregnancy: Systematic review of the literature. Einstein, 13(4), 618–626. 10.1590/S1679-45082015RW3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betran A. P. Torloni M. R. Zhang J. Ye J. Mikolajczyk R. Deneux-Tharaux C. Oladapo OT Tuncalp O, , Souza JP, Vogel JP. (2015). What is the optimal rate of caesarean section at population level? A systematic review of ecologic studies. Reproductive Health, 12(1), 57. 10.1186/s12978-015-0043-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binford L. R. (2001). Constructing frames of reference: An analytical method for archaeological theory building using ethnographic and environmental data sets. University of California Press. [Google Scholar]
- Black A. Y., Fleming N. A., Rome E. S. (2012). Pregnancy in adolescents. Adolescent Medicine: State of the Art Reviews, 23(1), 123–138. [PubMed] [Google Scholar]
- Blurton Jones N. (2016). Demography and evolutionary ecology of Hadza Hunter-Gatherers. Cambridge University Press. [Google Scholar]
- Bovet J. (2019). Evolutionary theories and Men's Preferences for Women’s Waist-to-Hip ratio: Which hypotheses remain? A systematic review. Frontiers in Psychology, 10(4), 1221. 10.3389/fpsyg.2019.01221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles S., Posel D. (2005). Genetic relatedness predicts South African migrant workers’ remittances to their families. Nature, 434(7031), 380–383. 10.1038/nature03420 [DOI] [PubMed] [Google Scholar]
- Bradford J. A., Giles W. B. (1989). Teenage pregnancy in western Sydney. Australian and New Zealand Journal of Obstetrics and Gynaecology, 29(1), 1–4. 10.1111/j.1479-828X.1989.tb02865.x [DOI] [PubMed] [Google Scholar]
- Buss D. M. (1989). Sex differences in human mate preferences: Evolutionary hypothesis tested in 37 cultures. Behavioral and Brain Sciences, 12(1), 1–49. 10.1017/S0140525X00023992 [DOI] [Google Scholar]
- Butovskaya M., Sorokowska A., Karwowski M., Sabiniewicz A., Fedenok J., Dronova D., Negasheva, M., Selivanova, E., & Sorokowski, P. (2017). Waist-to-hip ratio, body-mass index, age and number of children in seven traditional societies. Scientific Reports, 7, 1622. 10.1038/s41598-017-01916-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Agudelo A., Belizan J. M., Diaz-Rossello J. L. (2000). Epidemiology of fetal death in Latin America. Acta Obstetricia et Gynecologica Scandinavica, 79(5), 371–378. 10.1034/j.1600-0412.2000.079005371.x [DOI] [PubMed] [Google Scholar]
- Conde-Agudelo A., Belizan J. M., Lammers C. (2005). Maternal-perinatal morbidity and mortality associated with adolescent pregnancy in Latin America: Cross-sectional study. American Journal of Obstetrics and Gynecology, 192(2), 342–349. 10.1016/j.ajog.2004.10.593 [DOI] [PubMed] [Google Scholar]
- Dobbie B. M. W. (1982). An attempt to estimate the true rate of maternal mortality, sixteenth to eighteenth centuries. Medical History, 26(1), 79–90. 10.1017/S0025727300040795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshete A., Alemu A., Zerfu T. A. (2019). Magnitude and risk of dying among low birth weight neonates in rural Ethiopia: A community-based cross-sectional study. International Journal of Pediatrics, 2019. 10.1155/2019/9034952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler D. M. T., Nettle D., Afshar Y., de Pinheiro I., Bolyanatz A., Borgerhoff Mulder M., Cravalho M, Delgado T, Gruzd B, Correia M, Khaltourina D, … (2005). A cross-cultural investigation of the role of foot size in physical attractiveness. Archives of Sexual Behavior, 34(38), 267–276. 10.1007/s10508-005-3115-9 [DOI] [PubMed] [Google Scholar]
- Fisher R. A. (1930). The genetical theory of natural selection. Clarendon Press. [Google Scholar]
- Frost O. (1984). Maternal and perinatal deaths in an Addis Ababa hospital, 1980. Ethiopian Medical Journal, 22(3), 143–146. [PubMed] [Google Scholar]
- Gallais A., Robillard P. Y., Nuissier E., Cuirassier T. (1996). Adolescence and pregnancy in Guadeloupe. 184 cases. Journal de Gynécologie Obstétrique et Biologie de la Reproduction, 25(5), 523–527. [PubMed] [Google Scholar]
- Ganchimeg T. Mori R. Ota E. Koyanagi A. Gilmour S. Shibuya K. Torloni MR, , Souza J. P., Betran AP, Vogel J, Seuc A. (2013). Maternal and perinatal outcomes among nulliparous adolescents in low- and middle-income countries: A multi-country study. BJOG: An International Journal of Obstetrics and Gynaecology, 120(13), 1622–1630. 10.1111/1471-0528.12391 [DOI] [PubMed] [Google Scholar]
- Ganchimeg T., Ota E., Morisaki N., Laopaiboon M., Lumbiganon P., Zhang J., Yamdamsuren B, Say L Tunclap O Vogel JP Souza JP Mori R (2014). Pregnancy and childbirth outcomes among adolescent mothers: A World Health Organization multi-country study. BJOG: An International Journal of Obstetrics and Gynaecology, 1(S1), 40–48. 10.1111/1471-0528.12630 [DOI] [PubMed] [Google Scholar]
- Garza R., Pazhoohi F., Byrd-Craven J. (2021). Does ecological harshness influence men's Perceptions of women's Breast size, ptosis, and intermammary distance? Evolutionary Psychological Sciences, 7(1), 174–193. 10.1007/s40806-020-00262-w [DOI] [Google Scholar]
- Gaulin S. J. C., McBurney D. H. (2001). Psychology: An evolutionary approach. Prentice Hall. [Google Scholar]
- Gaym A. (2002). Obstructed labor at a district hospital. Ethiopian Medical Journal, 40(1), 11–18. [PubMed] [Google Scholar]
- Geary D. C., Vigil J., Byrd-Craven J. (2004). Evolution of human mate choice. Journal of Sex Research, 41(1), 27–42. 10.1080/00224490409552211 [DOI] [PubMed] [Google Scholar]
- Geist R. R., Beyth Y., Shashar D., Beller U., Samueloff A. (2006). Perinatal outcome of teenage pregnancies in a selected group of patients. Journal of Pediatric and Adolescent Gynecology, 19(3), 189–193. 10.1016/j.jpag.2006.02.005 [DOI] [PubMed] [Google Scholar]
- Geronimus A. T., Korenman S. (1992). The socioeconomic consequences of teen childbearing reconsidered. The Quarterly Journal of Economics, 107(4), 1187–1214. 10.2307/2118385 [DOI] [Google Scholar]
- Gilbert W., Jandial D., Field N., Bigelow P., Danielsen B. (2004). Birth outcomes in teenage pregnancies. Journal of Maternal-Fetal and Neonatal Medicine, 16(5), 265–270. 10.1080/jmf.16.5.265.270 [DOI] [PubMed] [Google Scholar]
- Grammer K., Fink B., Moller A. P., Thornhill R. (2003). Darwinian aesthetics: Sexual selection and the biology of beauty. Biological Reviews of the Cambridge Philosophical Society, 78(3), 385–407. 10.1017/S1464793102006085 [DOI] [PubMed] [Google Scholar]
- Grunstra N. D. S., Zachos F. E., Herdina A. N., Fischer B., Pavlicev M., Mitteroecker P. (2019). Humans as inverted bats: A comparative approach to the obstetric conundrum. American Journal of Human Biology, 31(2), e23227. 10.1002/ajhb.23227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headland T. N. (1989). Population decline in a Phillipine Negrito hunter-gatherer society. American Journal of Human Biology, 1(1), 59–72. [DOI] [PubMed] [Google Scholar]
- Henrich J., Heine S. J., Norenzayan A. (2010). The weirdest people in the world? Behavioral and Brain Sciences, 33(2–3), 61–83. 10.1017/S0140525X0999152X [DOI] [PubMed] [Google Scholar]
- Hill K., Hurtado A. M. (1996). Ache life history. Aldine de Gruyter. [Google Scholar]
- Hill K., Hurtado A. M., Walker R. S. (2007). High adult mortality among Hiwi hunter-gatherers: Implications for human evolution. Journal of Human Evolution, 52(4), 443–454. [DOI] [PubMed] [Google Scholar]
- Hogberg U., Brostrom G. (1985). The demography of maternal mortality–seven Swedish parishes in the 19th century. International Journal of Gynaecology and Obstetrics, 23(6), 489–497. 10.1016/0020-7292(85)90074-8 [DOI] [PubMed] [Google Scholar]
- Kaur J., Kaur K. (2012). Obstetric complications: Primiparity vs. Multiparity. European Journal of Experimental Biology, 2(5), 1462–1468. [Google Scholar]
- Keeler C. E. (1956). Land of the moon-children. University of Georgia Press. [Google Scholar]
- Kenrick D. T. (1995). Evolutionary theory versus the confederacy of dunces. Psychological Inquiry, 6(1), 56–62. 10.1207/s15327965pli0601_10 [DOI] [Google Scholar]
- Keyfitz N., Flieger W. (1971). Population facts and methods of demography. W. H. Freeman. [Google Scholar]
- Khunpradit S., Patumanond J., Tawichasri C., Khunpradit S., … (2005). Risk indicators for cesarean section due to cephalopelvic disproportion in Lamphun hospital. Journal of the Medical Association of Thailand, 88(Suppl 2), S63–S68. [PubMed] [Google Scholar]
- Knodel J., Hermalin A. I. (1984). Effects of birth rank, maternal age, birth interval, and sibship size on infant and child mortality: Evidence from 18th and 19th century reproductive histories. American Journal of Public Health, 74(10), 1098–1106. 10.2105/AJPH.74.10.1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koscinksi K. (2019). Breast firmness is of greater importance to women’ attractiveness than breast size. American Journal of Human Biology, 31(4), e23287. https://doi.org/10.1002/ajhb.23287 [DOI] [PubMed] [Google Scholar]
- Kramer K. L., Lancaster J. B. (2010). Teen motherhood in cross-cultural perspective. Annals of Human Biology, 37(5), 613–628. 10.3109/03014460903563434 [DOI] [PubMed] [Google Scholar]
- Lassek W. D., Gaulin S. J. C. (2006). Changes in body fat distribution in relation to parity in American women: A covert form of maternal depletion. American Journal of Physical Anthropology, 131(2), 295–302. 10.1002/ajpa.20394 [DOI] [PubMed] [Google Scholar]
- Lassek W. D., Gaulin S. J. C. (2019). Evidence supporting nubility and reproductive value as the key to human female physical attractiveness. Evolution and Human Behavior, 40(5), 408–419. 10.1016/j.evolhumbehav.2019.05.001 [DOI] [Google Scholar]
- Lassek W. D., Gaulin S. J. C. (2018a). Do the low WHRs and BMIs judged most attractive indicate better health? Evolutionary Psychology, 16(4), 1–13. 10.1177/1474704918803998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassek W. D., Gaulin S. J. C. (2018b). Do the low WHRs and BMIs judged most attractive indicate higher fertility? Evolutionary Psychology, 16(4), 1–16. 10.1177/1474704918800063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- l’Hoir M., Engelberts A., van Well G. T. J., Westers P., Mellenbergh G., Wolters W., Huber J. (1998). Case-control study of current validity of previously described risk factors for SIDS in the Netherlands. Archives of Disease in Childhood, 79(5), 386–393. 10.1136/adc.79.5.386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlowe F. (1998). The nubility hypothesis: The human breast as an honest signal of residual reproductive value. Human Nature, 9(3), 263–271. 10.1007/s12110-998-1005-2 [DOI] [PubMed] [Google Scholar]
- Marlowe F., Apicella C., Reed D. (2005). Men's preferences for women’s Profile waist-to-hip ratio in two societies. Evolution and Human Behavior, 26(6), 458–468. 10.1016/j.evolhumbehav.2005.07.005 [DOI] [Google Scholar]
- Marlowe F. W., & Berbesque J. C. (2012). The human operational sex ratio: Effects of marriage, concealed ovulation, and menopause on mate competition. Journal of Human Evolution, 63(6), 834–842. [DOI] [PubMed] [Google Scholar]
- Marlowe F. W. (2005). Hunter-gatherers and human evolution. Evolutionary Anthropology: Issues, News, and Reviews, 14(2), 54–67. [Google Scholar]
- Nkata M. (1997). Maternal mortality due to obstructed labor. International Journal of Gynaecology and Obstetrics, 57(1), 65–66. 10.1016/S0020-7292(97)02834-8 [DOI] [PubMed] [Google Scholar]
- Nove A., Matthews Z., Neal S., Camacho A. V. (2014). Maternal mortality in adolescents compared with women of other ages: Evidence from 144 countries. The Lancet Global Health, 2(3), e155–e164. 10.1016/S2214-109X(13)70179-7 [DOI] [PubMed] [Google Scholar]
- Pavlicev M., Romero R., Mitteroecker P. (2020). Evolution of the human pelvis and obstructed labor: New explanations of an old obstetrical dilemma. American Journal of Obstetrics and Gynecology, 222(1), 3–16. 10.1016/j.ajog.2019.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski B., Dunbar R. I. M. (2005). Waist-to-hip ratio versus body mass index as predictors of fitness in women. Human Nature, 16(2), 164–177. 10.1007/s12110-005-1002-7 [DOI] [PubMed] [Google Scholar]
- Pazhoohi F., Garza R., Kingstone A. (2020). Effects of breast size, intermammary cleft distance (cleavage) and ptosis on perceived attractiveness, health, fertility, and age: Do life history, self-perceived mate value, and sexism attitude play a role. Adaptive Human Behavior and Physiology, 6, 75–92. 10.1007/s40750-020-00129-1 [DOI] [Google Scholar]
- Pfeiffer S., Doyle L. E., Kurki H. K., Harrington L., Ginter J. K., Merritt C. E. (2014). Discernment of mortality risk associated with childbirth in archaeologically derived forager skeletons. International Journal of Paleopathology, 7, 15–24. 10.1016/j.ijpp.2014.05.005 [DOI] [PubMed] [Google Scholar]
- Phipps-Yonas S. (1980). Teenage pregnancy and motherhood: A review of the literature. American Journal of Orthopsychiatry, 50(3), 403–431. 10.1111/j.1939-0025.1980.tb03302.x [DOI] [PubMed] [Google Scholar]
- Prokop P., Zvaríková M., Zvarík M., Fedor P. (2020). Cues of pregnancy decrease female physical attractiveness for males. Current Psychology, 1–8. https://doi.org/10.1007/s12144-020-00608-4 [Google Scholar]
- Rafferty S. M. (2006). Evidence of early tobacco in Northeastern North America? Journal of Archaeological Science, 33(4), 453–458. 10.1016/j.jas.2005.08.006 [DOI] [Google Scholar]
- Röder S., Fink B., Jones B. C. (2013). Facial, olfactory, and vocal cues to female reproductive value. Evolutionary Psychology, 11(2), 392–404. https://doi.org/10.1177/147470491301100209 [PubMed] [Google Scholar]
- Rosenberg K., Trevathan W. (1996). Bipedalism and human birth: The obstetrical dilemma revisited. Evolutionary Anthropology, 4(5), 161–168. 10.1002/evan.1360040506 [DOI] [Google Scholar]
- Rosenberg K., Trevathan W. (2002). Birth, obstetrics and human evolution. BJOG: An International Journal of Obstetrics and Gynaecology, 109(11), 1199–1206. 10.1046/j.1471-0528.2002.00010.x [DOI] [PubMed] [Google Scholar]
- Rosenberg K. R. (1992). The evolution of modern human childbirth. Yearbook of Physical Anthropology, 35(S15), 89–124. 10.1002/ajpa.1330350605 [DOI] [Google Scholar]
- Rosenfield A. (1989). Maternal mortality in developing countries. An ongoing but neglected “epidemic”. JAMA, 262(3), 376–379. 10.1001/jama.1989.03430030064035 [DOI] [PubMed] [Google Scholar]
- Roulette C. J., Hagen E., Hewlett B. S. (2016). A biocultural investigation of gender differences in tobacco use in an egalitarian hunter-gatherer population. Human Nature, 27(2), 105–129. 10.1007/s12110-016-9255-x [DOI] [PubMed] [Google Scholar]
- Sagili H., Pramya N., Prabhu K., Mascarenhas M., Rani P. R. (2012). Are teenage pregnancies at high risk? A comparison study in a developing country. Archives of Gynecology and Obstetrics, 285(3), 573–577. 10.1007/s00404-011-1987-6 [DOI] [PubMed] [Google Scholar]
- Scholl T. O., Decker E., Karp R. J., Greene G., De Sales M. (1984). Early adolescent pregnancy: A comparative study of pregnancy outcome in young adolescents and mature women. Journal of Adolescent Health Care, 5(3), 167–171. 10.1016/S0197-0070(84)80037-6 [DOI] [PubMed] [Google Scholar]
- Singh D. (2002). Female Mate value at a glance: Relationship of Waist to Hip ratio to health. Fecundity and Attractiveness. Neuroendo Letters, 23(Suppl. 4), 81–91. [PubMed] [Google Scholar]
- Singh D. (1993a). Adaptive significance of female physical attractiveness, role of waist-hip ratio. Journal of Personality and Social Psychology, 65(2), 293–307. 10.1037/0022-3514.65.2.293 [DOI] [PubMed] [Google Scholar]
- Singh D. (1993b). Body shape and attractiveness: The critical role of waist to hip ratio. Human Nature, 4(3), 297–321. 10.1007/BF02692203 [DOI] [PubMed] [Google Scholar]
- Singh D., Singh D. (2006). Role of body fat and body shape on judgment of female health and attractiveness: An evolutionary perspective. Psychological Topics, 15(2), 331–350. [Google Scholar]
- Singh D., Singh D. (2011). Shape and significance of feminine beauty: An evolutionary perspective. Sex Roles, 64(9-10), 723–731. 10.1007/s11199-011-9938-z [DOI] [Google Scholar]
- Smith G. C., Pell J. P. (2001). Teenage pregnancy and risk of adverse perinatal outcomes associated with first and second births: Population based retrospective cohort study. BMJ, 323(7311), 476. 10.1136/bmj.323.7311.476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama L. S. (2005). Physical attractiveness in adaptationist perspective. In Buss D. M. (Ed.), Handbook of evolutionary psychology (pp. 292–343). John Wiley. [Google Scholar]
- Surapanthapisit P., Thitadilok W., Surapanthapisit P., Thitadilok W. (2006). Risk factors of caesarean section due to cephalopelvic disproportion. Journal of the Medical Association of Thailand, 89(Suppl. 4), S105–S111. [PubMed] [Google Scholar]
- Symons D. (1979). Evolution of human sexuality. Oxford University Press. [Google Scholar]
- Symons D. (1995). Beauty is in the adaptations of the beholder: The evolutionary psychology of human female sexual attractiveness. In Abramson P. R., Pinkerton S. D. (Eds.), Sexual nature, sexual culture (pp. 80–118). University of Chicago Press. [Google Scholar]
- Tooby J., Cosmides L. (1992). The psychological foundations of culture. In Barkow J., Cosmides L., Tooby J. (Eds.), The adapted mind (pp. 19–136). Oxford University Press. [Google Scholar]
- Torloni M. R. Betran A. P., Daher S. Widmer M. Dolan S. M. Menon R. Bergel E. Allen T. Bergel E. Allen T. Merialdi M. (2009). Maternal BMI and preterm birth: A systematic review of the literature with meta-analysis. Journal of Maternal-Fetal and Neonatal Medicine, 22(11), 957–970 [DOI] [PubMed] [Google Scholar]
- Tushingham S., Ardura D., Eerkens J. W., Palazoglu M., Shahbaz S., Fiehn O. (2013). Hunter-gatherer tobacco smoking: Earliest evidence from the pacific northwest coast of North America. Journal of Archaeological Science, 40(2), 1397–1407. 10.1016/j.jas.2012.09.019 [DOI] [Google Scholar]
- Ujah I. A. O., Aisien O. A., Mutihir J. T., Vanderjagt D. J., Glew R. H., Uguru V. E. (2005). Maternal mortality among adolescent women in Jos, North-Central, Nigeria. Journal of Obstetrics and Gynaecology, 25(1), 3–6. 10.1080/01443610400023395 [DOI] [PubMed] [Google Scholar]
- Volk A. A., Atkinson J. A. (2013). Infant and child death in the human environment of evolutionary adaptation. Evolution and Human Behavior, 34(3), 182–192. 10.1016/j.evolhumbehav.2012.11.007 [DOI] [Google Scholar]
- Vork F. C., Kyanamina S., van Roosmalen J. (1997). Maternal mortality in rural Zambia. Acta Obstetricia et Gynecologica Scandinavica, 76(7), 646–650. 10.3109/00016349709024604 [DOI] [PubMed] [Google Scholar]
- Walker R. (2019). Database for Indigenous Cultural Evolution , http://dice.missouri.edu/.
- Weeden J., Sabini J. (2005). Physical attractiveness and health in western societies: A review. Psychological Bulletin, 131(5), 635–653. 10.1037/0033-2909.131.5.635 [DOI] [PubMed] [Google Scholar]
- Weiner S., Monge J., Mann A., Weiner S., Monge J., Mann A. (2008). Bipedalism and parturition: An evolutionary imperative for cesarean delivery? Clinics in Perinatology, 35(3), 469–478. 10.1016/j.clp.2008.06.003 [DOI] [PubMed] [Google Scholar]
- Weinstein M., Wood J. W., Stoto M. A., Greenfield D. D. (1990). Components of age-specific fecundability. Population Studies, 44(3), 447–467. 10.1080/0032472031000144846 [DOI] [Google Scholar]
- Wells J. C., DeSilva J. M., Stock J. T. (2012). The obstetric dilemma: An ancient game of Russian roulette, or a variable dilemma sensitive to ecology? American Journal of Physical Anthropology, 149(S55), 40–71. 10.1002/ajpa.22160 [DOI] [PubMed] [Google Scholar]
- Wittman A. B., Wall L. L. (2007). The evolutionary origins of obstructed labor: Bipedalism, encephalization, and the human obstetric dilemma. Obstetrical and Gynecological Survey, 62(11), 739–748. 10.1097/01.ogx.0000286584.04310.5c [DOI] [PubMed] [Google Scholar]
- Xiong X., Saunders L. D., Wang F. L., Demianczuk N. N. (2001). Gestational diabetes mellitus: Prevalence, risk factors, maternal and infant outcomes. International Journal of Gynaecology and Obstetrics, 75(3), 221–228. 10.1016/S0020-7292(01)00496-9 [DOI] [PubMed] [Google Scholar]