Abstract
Over centuries of plant morphological research, biologists have enthusiastically explored how distinct vascular arrangements have diversified. These investigations have focused on the evolution of steles and secondary growth and examined the diversity of vascular tissues (xylem and phloem), including atypical developmental pathways generated through modifications to the typical development of ancestral ontogenies. A shared vernacular has evolved for communicating on the diversity of alternative ontogenies in seed plants. Botanists have traditionally used the term ‘anomalous secondary growth’ which was later renamed to ‘cambial variants’ by late Dr. Sherwin Carlquist (1988). However, the term ‘cambial variants’ can be vague in meaning since it is applied for developmental pathways that do not necessarily originate from cambial activity. Here, we review the ‘cambial variants’ concept and propose the term ‘vascular variants’ as a more inclusive overarching framework to interpret alternative vascular ontogenies in plants. In this framework, vascular variants are defined by their developmental origin (instead of anatomical patterns), allowing the classification of alternative vascular ontogenies into three categories: (i) procambial variants, (ii) cambial variants and (iii) ectopic cambia. Each category includes several anatomical patterns. Vascular variants, which represent broader developmental based groups, can be applied to both extant and fossil plants, and thereby offer a more adequate term from an evolutionary perspective. An overview of the developmental diversity and phylogenetic distribution of vascular variants across selected seed plants is provided. Finally, this viewpoint discusses the evolutionary implications of vascular variants.
Keywords: cambium, development, ectopic cambia, evolution, meristems, plant anatomy, procambium, vascular tissue
‘Vascular variant’ is the developmental process by which atypical vascular growth emerges in a lineage in relation to their putative ancestor. These atypical ontogenies emerge from atypical procambium, cambium and de novo cambium patterning, outlining three developmental based categories: procambial variants, cambial variants and ectopic cambia. In turn, each category of vascular variants includes several anatomical patterns that are predictably conserved within a given taxon and, therefore, useful in the species description. These atypical—and frequently complex ontogenies—develop in stems and roots of all major lineages of seed plants, outlining a striking case of convergent evolution. The vascular variants' framework illuminates the evolution of the development of the massive diversity of vascular ontogenies in distantly related plants and promises to facilitate comparative studies that may be informative at various biological scales with meaningful evolutionary implications.
Background
There are more than 300 000 living species of tracheophytes (vascular plants) and the occurrence of secondary growth through a single bifacial vascular cambium is evidenced in thousands of species, particularly in seed plants (Spicer and Groover 2010). However, modifications of this ‘typical’ vascular development arise in many ways (Haberlandt 1914; Esau 1965), generating a myriad of ‘alternative patterns of vascular growth’ (Carlquist 1991). These alternative anatomies are seen particularly via the presentation of distinct stem ontogenies in comparison to the putative ancestral development of seed plants. The ancestor of seed plants likely had a stem ontogeny with a combination of a ‘typical’ eustele (although distinct stelar configurations are also recognized in early seed plants: Wang and Liu 2015), and homogeneous amounts of wood (secondary xylem) and inner bark (secondary phloem) produced by a single bifacial cambium (Simpson 2019; Onyenedum and Pace 2021). Although most extant gymnosperms and angiosperms display that conserved trajectory (= typical growth), some lineages have lost the eustele and/or the bifacial cambium, and others have evolved ‘alternative patterns of vascular growth’. These vascular modifications have been reported in multiple lineages of fossil and extant lignophytes, predominantly among angiosperms (Carlquist 1991; Bodnar and Coturel 2012; Decombeix et al. 2014; Angyalossy et al. 2015). In these alternative anatomies, the timing, spatial distribution, and relative abundance of xylem and phloem formed by vascular meristems are drastically altered, generating stems and roots with unusual shapes and arrangements of vascular tissues.
For many years, ‘anomalous secondary growth’ (Esau 1965) has been the most prevalent term in English to describe alternative vascular ontogenies in plants, though many other terms have existed [see Supporting Information—Table S1]. In 1988, Dr. Sherwin Carlquist coined the term ‘cambial variants’ while pointing out the misleading impression of a disorderly action in the antecedent term ‘anomalous secondary growth’ (Carlquist 1991, 2001). Surprisingly, Carlquist’s definition—and those that post-dated him (e.g. Spicer and Groover 2010; Angyalossy et al. 2012, 2015)—focusses on cambial activity and modifications during secondary growth, with no reference to procambial patterning (primary growth), despite the multiple reports on modifications in procambial organization in both fossil and extant plant lineages (Worsdell 1906; Esau 1965; Beck 2010). Because alternative vascular ontogenies may originate from modifications in procambium patterning, the term ‘cambial variant’ is not the most appropriate to broadly describe these phenomena, confusing our scientific understanding of this concept.
An important evolutionary perspective can be gained by devising morphological concepts that reflect how organisms evolve through changes in ontogeny. In an effort to better define ontogenies originating in an unusual procambial organization, as opposed to the cambial origin, the developmental stem anatomy of species across various families of extant angiosperms will be explored. The term ‘vascular variants’ is proposed to supplant ‘cambial variants’, as it more broadly describes alternative ontogenies within the context of development and evolution.
What Are Vascular Variants?
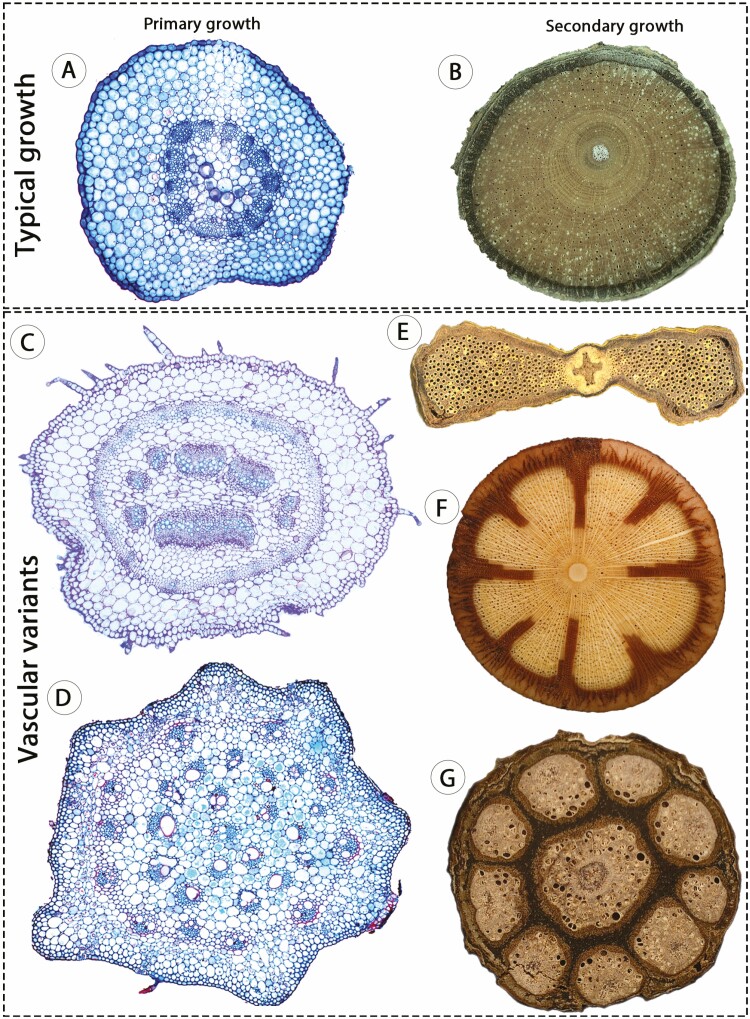
Vascular variants are alternative vascular ontogenies deviating from an ancestral development, achieved through modifications in the organization, activity, position, timing and/or number of vascular meristems. Specifically in stems of seed plants, vascular variants are alternative trajectories that deviate from the typical growth (i.e. eustele + bifacial single cambium; Fig. 1A and B), thereby generating circular or non-cylindrical conformations and altered arrangements of vascular tissues (Fig. 1C–G). These developmental events may originate during primary and/or secondary growth. Therefore, a review of vascular variants originating from procambial origin, and which collectively corroborate a broader developmental based classification of this phenomenon follows.
Figure 1.
Stem vascular arrangements in angiosperms illustrating the typical growth and vascular variants. (A) Typical eustele; Portulaca halimoides, Portulacaceae; courtesy of Thaíla V. A. Santos. (B) Regular secondary growth; Paullinia coriacea, Sapindaceae; courtesy of Robson G. Silva. (C) Eustele with medullary bundles (in the centre of the pith) and continuous procambium forming additional vascular bundles in the periphery; Allionia incarnata, Nyctaginaceae. (D) Atactostele; Commelina platyphylla, Commelinaceae; courtesy of Ricardo S. B. Vita. (E) Non-cylindrical stem resulting from atypical cambial activity (single cambium); Schnella sp. Fabaceae; courtesy of Caian S. Gerolamo. (F) Phloem wedges resulting from atypical cambial activity (single cambium); Mansoa difficilis, Bignoniaceae; courtesy of Caian S. Gerolamo. (G) Compound stem originating from atypical procambial patterning, generating a central cylinder and nine peripheral vascular cylinders, each with its own cambium (multiple cambia); Serjania fuscifolia, Sapindaceae; courtesy of Robson G. Silva. Images not to scale.
Procambium Patterning—and Not Cambial Activity—Generate Vascular Variants in Some Lineages
In Nyctaginaceae, three out of four stem ontogenies begin with a modified procambium organization (see Cunha Neto et al. 2022). Of these, one ontogeny generates a pattern of ‘interxylary phloem’ (Fig. 2A–F; see Supporting Information—Table S2, Glossary) and two ontogenies produce a pattern called ‘successive cambia’ [see Supporting Information—Fig. S1]. In these species, stems are characterized by medullary bundles—which are derived from procambial traces—combined with a continuous procambium that adds a ring of vascular bundles to the primary vascular system called polycyclic eustele (Fig. 2A–C; Cunha Neto et al. 2020a). Stems with interxylary phloem show evidence not only of polycyclic eusteles, but also the existence of cambium originating from a continuous procambium. This cambium presents a differential cambial activity since its establishment (Fig. 2C–E) generating phloem strands and associated axial parenchyma within the secondary xylem, collectively called ‘phloem islands’ (Fig. 2D–F; Cunha Neto et al. 2021). The phloem of the external vascular bundles of the polycyclic eustele is the first phloem island (Fig. 2C and D), and there is no stage of regular secondary growth (Fig. 2D and F). Therefore, developmental modifications in both procambial and cambial activity are observed in stems with interxylary phloem in Nyctaginaceae. The two other ontogenies that start stem development with polycyclic eusteles develop successive cambia instead of interxylary phloem (see Supporting Information—Fig. S1; Cunha Neto et al. 2022). Successive cambia in these two cases originate either through an ‘extra-fascicular cambium’ as the first cambium (Cunha Neto et al. 2022), or through ectopic cambia—de novo cambium formed in an atypical location—after some time of secondary growth has occurred from a cambium in the usual position (Cunha Neto et al. 2020b).
Figure 2.
Medullary bundles (procambial variant) and interxylary phloem (cambial variant) in stems of Nyctaginaceae. (A, B). Primary growth with medullary bundles and developing vascular bundles (arrows) derived from the continuous procambium (ring of smaller cells). Commicarpus scandens. (C) Early secondary growth showing medullary bundles and cambium developed from the vascular bundles derived from the continuous procambium. Acleisanthes chenopodioides. (D) Note that the phloem of vascular bundles derived from the continuous procambium become the first ‘phloem islands’ and, therefore, there is no formation of a continuous vascular cylinder of secondary xylem and secondary phloem. Guapira pernambucensis. (E) Medullary bundles and atypical cambial activity producing ‘phloem islands’ within the wood. (F) Adult stem showing medullary bundles with secondary growth, absence of continuous vascular cylinder, and multiple ‘phloem islands’. Pisoniella glabrata. (A–F) Light microscopy. (A–D, F) Stained with Safranin and Astra Blue. (E) Stained with Toluidine Blue.
In Piperaceae, stem ontogeny of some species initiates with two or more rings of medullary bundles during primary growth (Fig. 3A; Isnard et al. 2012). Later in development, some of these species (e.g. Manekia) form a bifacial cambium from the middle ring of medullary bundles producing a continuous cylinder of secondary xylem and secondary phloem (Fig. 3B). Each bundle in the external ring experiences secondary growth, whereby each develops into an ‘external vascular cylinder’ (Fig. 3B; Angyalossy et al. 2012, 2015). Medullary bundles from the inner ring continue isolated in the pith, which may also undergo secondary growth (Carlquist 2001). This ontogeny indicates that, in Piperaceae, vascular bundles (from primary vasculature) formed in an unusual position are the main precursor of atypical anatomical patterns observed in mature stems.
Figure 3.
External vascular cylinders in stems of Piperaceae, a case of procambial variant. This ontogeny originates from medullary bundles which undergo secondary growth. (A) Stem during primary growth showing three rings of medullary bundles (three successive coloured rings/green). (B) Mature stem showing medullary bundles in the pith (coloured ring in the center/green), continuous secondary growth derived from a cambium originated from the middle ring of medullary bundles, and isolated ‘external vascular cylinders’ (coloured ring in the periphery/brown) derived from the outer ring of medullary bundles. Small circles indicate vessels in the xylem and dotted area indicates the phloem. Figures not to scale.
In Sapindaceae, the compound vascular cylinder (= compound stem) is the emblematic example of unusual procambial patterning. In general, species with compound stems initiate stem development with lobed stem outlines (Fig. 4A and B). However, two developmental pathways generate similar stems at maturity, both characterized by a central cylinder surrounded by peripheral vascular cylinders (Figs. 1G and 4C). Of these, one ontogeny has two or more vascular bundles organized in a ring-like arrangement (i.e. some bundles have inverted polarity) in each lobe, and from these bundles, a cambium develops and generates the peripheral vascular cylinder (Fig. 4A). Concomitantly, a cambium is formed from vascular bundles in the usual position (delimiting the pith) generating a central vascular cylinder (Fig. 4A). This ontogeny is typical of Serjania species, which may produce 3–11 peripheral vascular cylinders (Fig. 1G; Tamaio and Angyalossy 2009). The other ontogeny is typical of Paullinia species (Van der Walt et al. 1973; Chery et al. 2020), whereby peripheral vascular cylinders from a single isolated bundle (instead of various bundles) are formed in the distal portion of the lobes (Fig. 4B). Mature stems of this ontogeny also have a central vascular cylinder produced in the usual way, and generally, only three peripheral vascular cylinders are produced (Fig. 4C).
Figure 4.
Compound stems in Sapindaceae, a case of procambial variant. (A) Illustration based on stems of many Serjania species during transition from primary to secondary growth; each lobe has multiple vascular bundles from which a vascular cambium develops generating a peripheral vascular cylinder (coloured circles in the periphery/pink); a central vascular cylinder is also produced from vascular bundles in the usual position (coloured circle in the center/blue; see also Fig. 1G). (B) Illustration based on stems of many Paullinia species during transition from primary to secondary growth; a central vascular cylinder is formed in the usual way (coloured circle in the center/blue), and peripheral vascular cylinders derive from a cambium initiating from a single isolated vascular bundle (coloured circles in the periphery/pink); in this case, three isolated bundles will generate three peripheral vascular cylinders. (C) Mature stem with a central vascular cylinder and three peripheral vascular cylinders. Paullinia spicata. Small circles indicate vessels in the xylem and dotted area indicates the phloem. Figures not to scale.
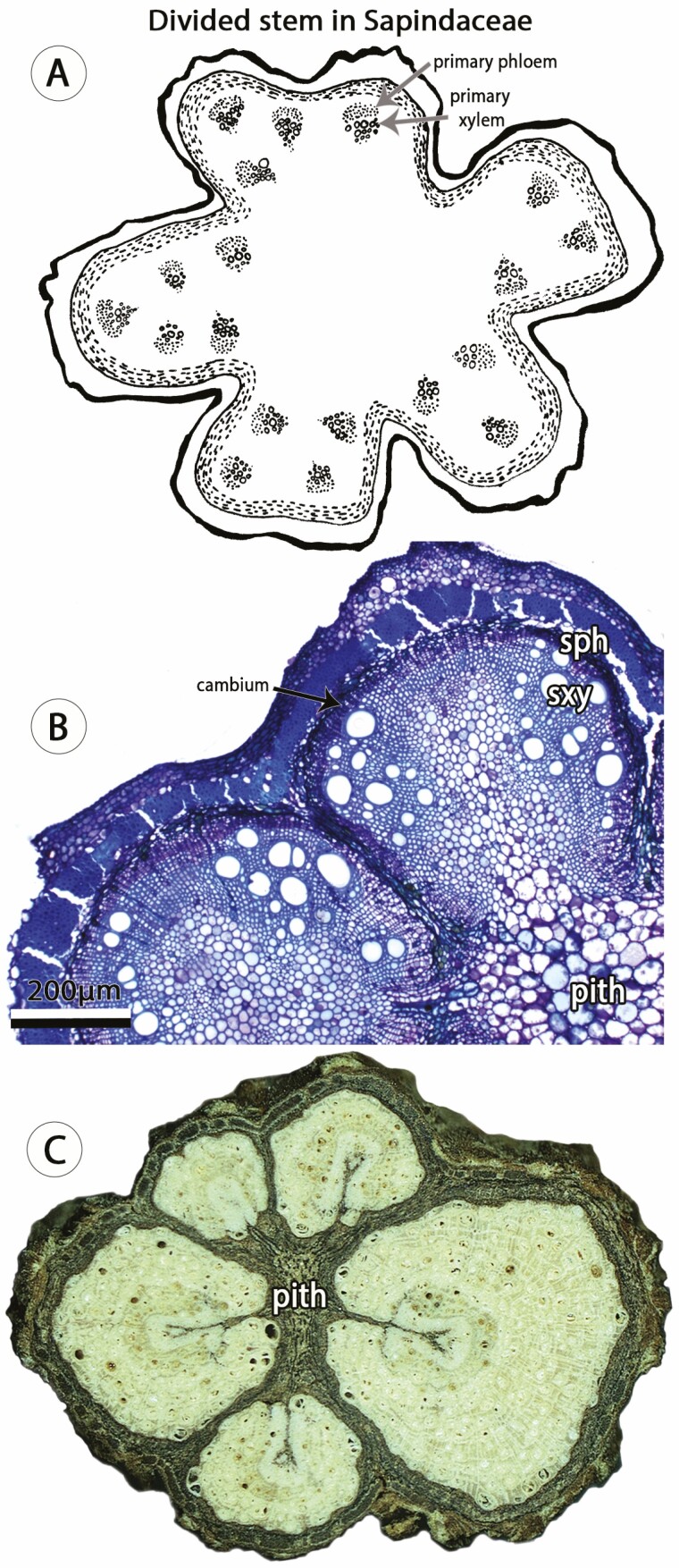
Another example of vascular variants originating from unusual procambial patterning in Sapindaceae is the divided vascular cylinder (= divided stem) (Fig. 5A–C). In this type of vascular variant, the eustele is spatially modified through vascular bundles of usual polarity distributed along the lobes and virtually no vascular bundles in the furrows (Fig. 5A). Research suggests that a single cambium may be formed connecting all bundles during the transition from primary to secondary growth (Rizzieri et al. 2021), shortly thereafter, the continuous cambial activity is interrupted, generating five circular cambia (one in each lobe; Fig. 5B). In the lobes, each cambium forms a peripheral vascular cylinder (Fig. 5C), and, in some cases, a central cylinder is also formed in late developmental stages (Rizzieri et al. 2021).
Figure 5.
Divided stem in Sapindaceae initiate with atypical procambial patterning. (A) Drawing illustrating young stem with lobed conformation and vascular bundles distributed along the lobes. Figures not to scale. (B) Light microscopy of Serjania deflexa showing two cambia generating two peripheral vascular cylinders; each cambium and peripheral vascular cylinder is derived from vascular bundles in one lobe. Stained with Toluidine Blue. Courtesy of Neusa Tamaio. (D) Macroscopic image of adult stem of Serjania corrugata showing five peripheral vascular cylinders and no central cylinder. Stem diameter: 15 mm. Courtesy of Robson G. Silva. Small circles indicate vessels in the xylem and dotted area indicates the phloem. sph, secondary phloem; sxy, secondary xylem.
Taken together, the ontogenies described above (and others discussed below, e.g. ‘intraxylary phloem’) represent developmental modifications that derive from unusual procambial organization, suggesting a distinct origin for alternative vascular ontogenies, which also arise from atypical cambial activity and de novo vascular meristems.
The Three Categories of Vascular Variants
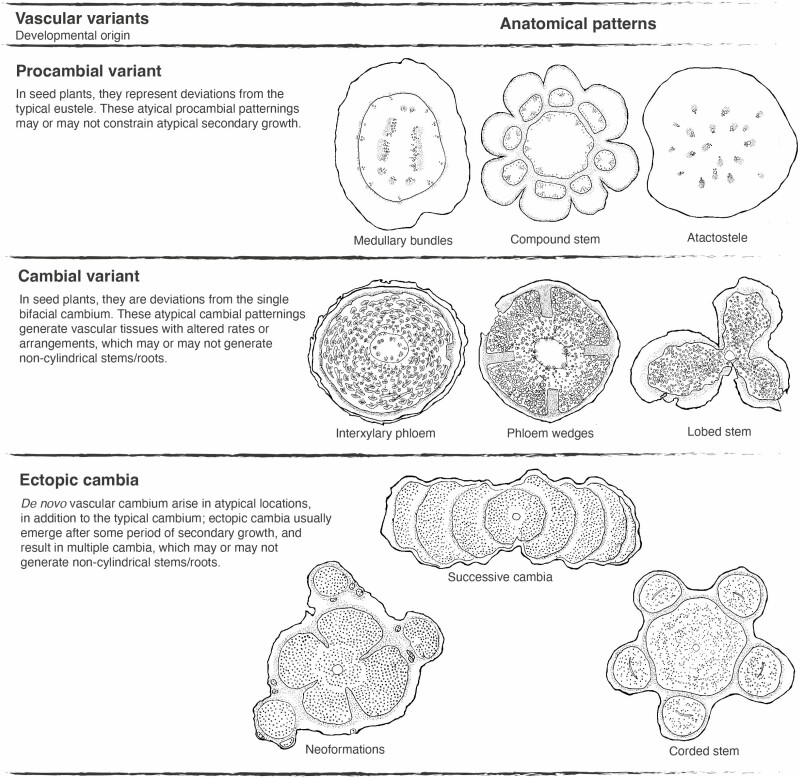
Following this developmental approach, three categories of vascular variants are considered: (i) procambial variants (inspired by Lopes et al. 2017), (ii) cambial variants (following Carlquist 2001) and (iii) ectopic cambia (following Spicer and Groover 2010). Each of these three developmental based categories generates numerous anatomical patterns (Fig. 6; see Supporting Information—Table S3).
Figure 6.
Synthesis of vascular variants categories and their respective anatomical patterns based on the known diversity across seed plants. Only some anatomical patterns are represented in the right side for each category—a complete list of anatomical patterns associated to each category can be found in Supporting Information—Table S1. Drawings of anatomical patterns are inspired from cross sections of species of the following families: medullary bundles: Nyctaginaceae, compound stem: Sapindaceae, atactostele: Commelinaceae, interxylary phloem: Nyctaginaceae, phloem wedges: Bignoniaceae, lobed stem: Sapindaceae, neoformations: Rubiaceae, successive cambia: Fabaceae, corded stem: Sapindaceae. Small circles indicate vessels in the xylem and dotted area indicates the phloem.
Since cambial activity is not the only the source of atypical vascular ontogenies, the term ‘cambial variants’ fails to accurately describe the breadth of alternative vascular development in plants. This terminological inadequacy has been noted by other researchers for some time, including Dr. Sherwin Carlquist, who expressed the inadequacy of the term ‘cambial variants’ when discussing some Sapindaceae (pers. comm. to Dr. Neusa Tamaio). When describing compound stems of the Sapindacee family, three terms have previously been utilized in place of ‘cambial variants’: (i) ‘polystelic stem’ (Acevedo-Rodríguez 1993), (ii) ‘multistelar stem’ (Klaassen 1999) and (iii) ‘procambial variant’ (Lopes et al. 2017). The first two terms were disputed by Tamaio and Angyalossy (2009) on the basis that a single (and not multiple) stele ise produced, whilst the term ‘procambial variant’, arguably a more adequate term to describe compound stems, has not flourished and has only been repeated in related literature once (Pace et al. 2022). Interestingly, besides compound stems in Sapindaceae (Lopes et al. 2017), previous studies have also considered medullary bundles a type of ‘cambial variants’ (de Bary 1884; Esau 1965; Costea and DeMason 2001; Beck 2010), emphasizing the contribution of procambial modifications to the interpretation of alternative vascular ontogenies in seed plants.
Understanding the origin of complex ontogenies is critical since similar mature stems may develop through distinct ontogenetic processes (Cabanillas 2016). For instance, in Sapindaceae, three major stem patterns are known to produce a ‘cable-like’ structure (e.g. compound, divided and corded), revealing the possession of multiple vascular cylinders (Tamaio et al. 2011). Although generating similar adult forms, these anatomical patterns derive from both procambial variants (e.g. compound stems, Fig. 4) and ectopic cambia (e.g. corded stem, Fig. 6). Another example involves ‘external vascular cylinders’ described for distantly related angiosperms families, including Bignoniaceae, Combretaceae, Euphorbiaceae, Piperaceae, Rubiaceae and Sapindaceae (Angyalossy et al., 2012, 2015) and in which the ‘external vascular cylinders’ derived from the activity of both primary and secondary meristems. In Piperaceae, this pattern originates as a procambial variant, in which isolated medullary bundles undergo secondary growth generating the ‘external vascular cylinders’ (Fig. 3). This developmental pathway, although somewhat similar, is not comparable to the development of most anatomical patterns of Sapindaceae or the phenomenon observed in Rubiaceae (Fig. 7; Leal et al. 2020) or Euphorbiaceae (Fig. 7), which are derived from ectopic cambia. These observations indicate that developmental based categories are fundamental for a better understanding of vascular diversity in seed plants.
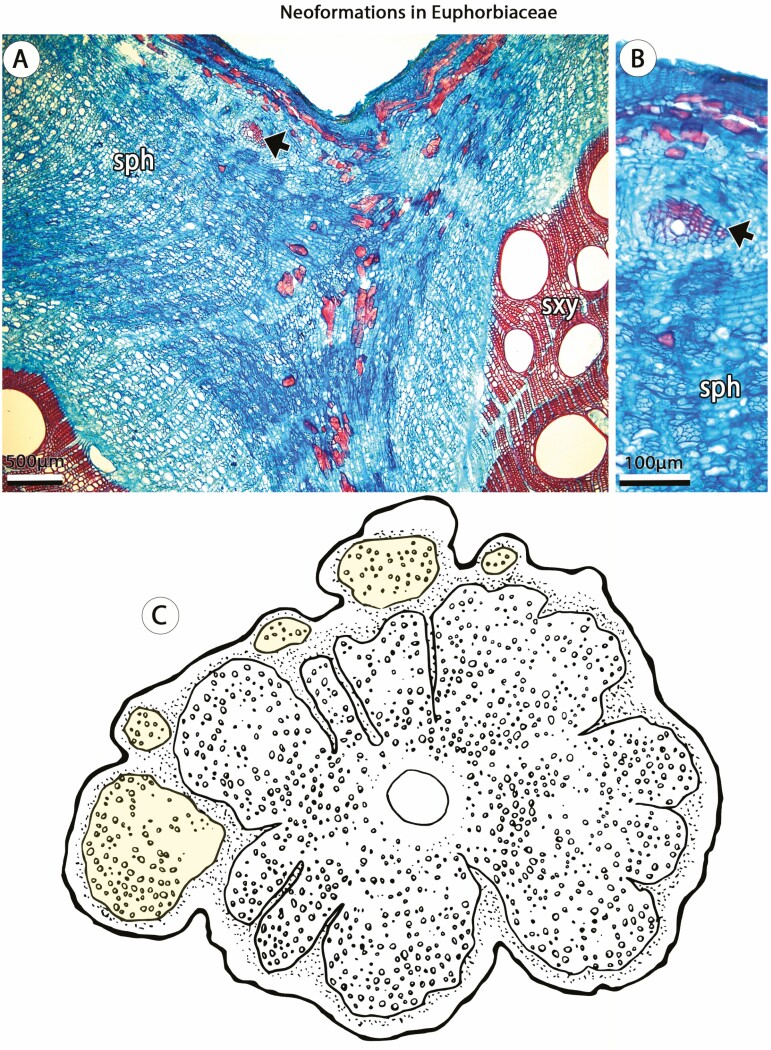
Figure 7.
Neoformations in stems of Dalechampia (Euphorbiaceae), a case of ectopic cambia. (A, B) Dalechampia alata. (A) Adult stem showing the initiation of an ectopic cambia (arrow) from vascular parenchyma at the periphery of the non-conducting phloem. (B) Detail of ectopic cambia (arrow) initiation. (C) Line drawing of mature stem showing multiple ectopic cambia generating neoformations (yellow) at the periphery of the vascular cylinder. Small circles indicate vessels in the xylem and dotted area indicates the phloem. sph, secondary phloem; sxy, secondary xylem. (B, C) Stained with Safranin and Astra Blue.
Undoubtedly, vascular variants are complex anatomies, and like the boundaries between procambium and cambium (Esau 1965), their ontogenetic stages cannot be sharply distinguished in some cases. This fuzziness has culminated in contrasting interpretations. For instance, the distinctive stem anatomy of Aristolochia (Piperales)—with wide, large rays greatly dividing the axial elements of the vascular system into discrete portions—was considered a typical growth by Esau (1965) but is a pattern of ‘cambial variants’ in several recent studies (i.e. axial vascular elements in segments, sensu Angyalossy et al. 2015). Other patterns with atypical ray development, as well as raylessness (reviewed by Frankiewicz and Oskolski 2023), may represent additional examples. Moreover, vascular variants can be constrained by the activity of other primary meristems. This is observed, for instance, in stems that develop as non-cylindrical organs since early stages, as observed in Malvaceae (Luna-Márquez et al. 2021) or Sapindaceae (Chery et al. 2020). However, non-cylindrical stems are frequently determined by cambial variants (e.g. lobed stem) or ectopic cambia (e.g. successive cambia), generating different categories (e.g. angular, flattened, lobed; Luna-Márquez et al. 2021).
Revisiting Alternative Ontogenies in Seed Plants
An investigation of vascular ontogenies following the present developmental framework reveals distinct categories of vascular variants in both stems and roots of major lineages of seed plants. For instance, there is a striking stele variation in stems of some Nymphaeales (ANA grade) and Gunnerales (core eudicots), including polycyclic eusteles and siphonostele (Seago 2020; Seago et al. 2021). Mature stems of Apocynaceae (asterids, eudicot) have been reported with ‘intraxylary phloem’, interxylary phloem, successive cambia and non-cylindrical stems (Acevedo-Rodríguez 2015, onwards; Angyalossy et al. 2015), while young stems are described with siphonosteles, bicollateral bundles and/or medullary bundles (Salas et al. 2018; Sathya et al. 2022). This indicates that not only cambial variants occur in this family, but also procambial variants and ectopic cambia, which may or may not be developmentally constrained.
The typical stem development of monocots could also be described as vascular variants since they deviate from the putative ontogeny in the ancestor of seed plants (Fig. 6). Monocots are characterized by the well-known ‘atactostele’ (Fig. 1C), which is considered as a highly modified eustele (Beck et al. 1982), closed vascular bundles (absence of procambial remnants) and by the lack of a ‘typical cambium’. However, some lineages produce secondary growth through the Secondary Thickening Meristem (STM), also called monocot cambium (Carlquist 2012). In these lineages (e.g. some Asparagales: Jura-Morawiec et al. 2015; and Arecales, also through the STM and not diffuse growth: Botânico and Angyalossy 2013), the monocot cambium produces vascular bundles centripetally and parenchyma centrifugally. Although considered analogous meristems, the monocot cambium and the typical cambium of seed plants show a considerable overlap of gene expression (Zinkgraf et al. 2017; Roodt et al. 2019; Povilus et al. 2020). These observations indicate that similarities in molecular programmes between the two lineages were likely achieved through the co-optation of key genes during the evolution of the monocot cambium (Zinkgraf et al. 2017). It also highlights the advantage of broad developmental based categories allowing the inclusion of monocots in the context of alternative vascular ontogenies, which may facilitate integrative research exploring vascular diversity in flowering plants.
In roots, vascular variants are understood to be the cause for the concentric rings in sugar beets (Chenopodiaceae) due to successive cambia—ectopic cambia (Artschwager 1926). Vascular variants are also observed in roots of species from other families of gymnosperms (e.g. successive cambia in Cycas revoluta: Avetta 1887) and angiosperms (Schenck 1893), including Nyctaginaceae (Cunha Neto et al. 2020b) and Polygonaceae (Rajput 2015), also with successive cambia, Bignoniaceae with phloem wedges (Victorio 2016) or Apiaceae forming strands of vascular tissues called ‘multisteles’ (Chuang 1970). Procambial variants in roots of seed plant lineages appear to be less common than those found in their respective stems, though the variants may be present in parasitic plants (Kuijt and Bruns 1987; Carlquist 1988; Wilson and Calvin 1996; Pellissari et al. 2022). As emphasized by Wilson and Calvin (1996: 104), in parasitic plants, alternative ontogenies ‘are problematic as they may refer to anomalous primary growth’ instead of cambial modifications. The vascular variant concept would also be appropriate to describe these alternative ontogenies.
Phylogenetic Distribution of Vascular Variants
Vascular variants have evolved independently multiple times across the evolution of seed plants. Procambial variants, cambial variants and ectopic cambia are reported in at least 24, 21 and 22 orders, respectively (Fig. 8). In total, vascular variants occur in 4 orders of gymnosperms and 31 orders of angiosperms (Fig. 8; see Supporting Information—Table S4). Vascular variants are disproportionately distributed, occurring more abundantly in angiosperms in comparison to gymnosperms, and more frequently in eudicots when compared to other flowering plants (Fig. 8). Outside the core eudicots, Piperales are distinct for having all three categories represented in the order (Fig. 8). Monocots produce both procambial variants and species with the monocot cambium. Within gymnosperms, vascular variants are characterized predominantly by successive cambia (ectopic cambia). Nevertheless, procambial variants (i.e. medullary bundles) and cambial variants (e.g. non-cylindrical) are also observed in Cycadales and Ephedrales, respectively [see Supporting Information—Table S4].
Figure 8.
Distribution of vascular variants in the phylogeny of seed plants. Tree topology follows Stevens (2001 onwards) for angiosperms and Li et al. (2021) for gymnosperms. All types of ‘cambial variants’ presented by Angyalossy et al. (2015) are distributed in one of the three categories of vascular variants [see Supporting Information—Table S2 for additional details]. The ‘monocot cambium’ is indicated for their respective orders and the atactostele is indicated in the branch leading to the monocot lineage.
Developmental Implications of the Vascular Variants’ Framework
Within seed plants, over 10 anatomical patterns of vascular variants have been described as types of ‘cambial variants’ (Bodnar and Coturel 2012; Yang et al. 2014; Acevedo-Rodríguez 2015 onwards; Angyalossy et al. 2015). Here, these anatomical patterns are further grouped into one of three vascular variants categories, which emphasizes their developmental origin (Fig. 6; see Supporting Information—Table S3). Although naming conventions previously employed to describe anatomical patterns vary and are not developmentally accurate, they nonetheless should remain in certain instances, as they carry important taxonomic meaning (e.g. Sapindaceae).
In a sense, procambial variants and ectopic cambia encompass changes in the location and spatial organization of vascular meristems (and their products), while cambial variants are comprised of changes primarily in the rate and spatial distribution of cambial derivatives (Fig. 6). Multiple anatomical patterns may also be described in a single stem, which result from a combination of two or more vascular variants categories. In Nyctaginaceae, for instance, stems with procambial variants (i.e. medullary bundles) independently produce distinct vascular variants during secondary growth, for example, interxylary phloem (cambial variants) or successive cambia (ectopic cambia). In other cases, such as the ‘external vascular cylinders’ in Piperaceae (Fig. 3) and the compound stems in Sapindaceae, the modifications during primary growth (procambial variants) developmentally constrain the atypical anatomical pattern observed during secondary growth (Fig. 4).
Acceptance of the term vascular variants as an overarching developmental based framework for interpreting alternative vascular ontogenies affords the opportunity to review fundamental phenomena in plant biology, such as ectopic cambia. Ectopic cambia, which comprise the formation of a de novo cambium in addition to the typical cambium, arise from parenchymatous tissues derived from the ground meristem (i.e. the cortex—Leal et al. 2020; Nejapa et al. 2021) or from vascular tissue, including the pericycle (Tamaio et al. 2009; Cunha Neto et al. 2018), phloem axial parenchyma (Leme et al. 2020), phloem rays (Cunha Neto et al. 2018), non-lignified axial parenchyma in the xylem (Pace et al. 2018) and pith cells (Carlquist 2001; Patil and Rajput 2008). At the anatomical level, ectopic cambia include patterns delimiting concentric rings or discrete fragments, that is, successive cambia (Tamaio et al. 2009; Cunha Neto et al. 2018), and circular vascular units, that is, neoformations or neo-formed vascular strands (Bastos et al. 2016; Leal et al. 2020), which includes the typical peripheral cylinders in corded stems of Thinouia, Sapindaceae (Tamaio and Somner 2010). Independent of their anatomical organization, these patterns derive from the same developmental mechanism, thus, constituting examples of the same vascular variant category (Fig. 6). In the context of vascular variants, ‘neoformations’ are used particularly to describe additional vascular tissue produced in late developmental stages of woody vines containing other vascular variants (e.g. Bignoniaceae: Angyalossy et al. 2015; Malpighiaceae: Cabanillas et al. 2017; Sapindaceae: Bastos et al. 2016). Because ‘neoformations’ normally occur in late developmental stages, this phenomenon was initially characterized as ‘tertiary growth’ (Van Tieghem 1884), also called ‘tertiary thickening’—used to characterize anatomical modifications in storage organs such as in potato or beets (Hayward 1938; Wilson and Lowe 1973; Forbes 1985). ‘Tertiary growth’ is, therefore, ambiguous as it includes vascular (e.g. ectopic cambia in beets) and non-vascular processes (e.g. parenchyma proliferation in potato; Tribble et al. 2021).
Another phenomenon worth considering is conducting vascular tissue in the pith. There are distinct patterns that originate from developmentally diverse processes and which are described with several names, for example, ‘internal phloem’ (Patil and Rajput 2008; Rajput and Gondaliya 2017), bicollateral bundles (Hayden and Hayden 1994; Patil et al. 2011), perimedullary phloem (Araújo and Costa 2006) and medullary cambia (Philipson and Ward 1965). In the context of vascular variants, these overlapping yet disparate phenomena (Carlquist 2012) have been placed under the term ‘intraxylary phloem’ (Carlquist 2013a; Angyalossy et al. 2015; Rajput et al. 2022), and considered a synapomorphy of some lineages such as Myrtales and core Convolvulaceae (Stevens 2001 onwards; Stefanovic et al. 2002). Since many patterns deviate from the typical eustele, for instance, through the formation of bicollateral bundles (e.g. Cucurbitaceae) or siphonosteles (e.g. Apocynaceae), they are better described as cases of procambial variants. In some cases, perimedullary pith cells may differentiate into phloem followed by a de novo cambium, which may produce large amounts of vascular tissue in the pith (Rajput et al. 2018, 2022). Yet, in some cases, it may be difficult to distinguish whether perimedullary phloem originates from procambial-derived cells or from a de novo cambium, in which cases even vascular variants categories may be difficult to apply. Fossil plants may also pose a difficult scenario for determining if conducting vascular tissue in the pith originated from procambial remnants or ectopic cambia. This difficulty may further explain the use of the term ‘medullary vascular system’ (Bodnar and Coturel 2012).
Remarkably, ectopic cambia generate enormous vascular diversity representing a striking developmental potential that may be present in many but not all plant lineages. While their structural diversity and functional significance have begun to be elucidated (Schmitz et al. 2008; Carlquist 2013b; Robert et al. 2014), and possible mechanisms for the origin have been proposed (Robischon et al. 2011; Smetana et al. 2019), the molecular underpinnings of this phenomenon await thorough clarification.
Vascular Variants Beyond Seed Plants
It is believed that the fossil record likely encompasses a much broader diversity of vascular ontogenies if compared to extant flora (Decombeix et al. 2019). Since alternative vascular ontogenies also exist in other extant and fossil tracheophytes, it may be possible to apply a broader context to the vascular variants’ framework. For example, in free-sporing plants, two instances of deviations from typical to atypical ontogenies in relation to their putative ancestors can be found in the independent evolution of the unusual bifacial cambium in Isoëtes (Lycophyta; Spicer and Groover 2010; Onyenedum and Pace 2021), and the unifacial cambium in some Ophioglossales (ferns; Esau 1965; Stevenson 1980). Of note, the instance of unifacial cambium in Ophioglossales is debated (Rothwell and Karrfalt 2008). Undeniably, most cases of vascular variants in stems of fossil and extant ferns should be related to procambial modifications as they display an enormous diversity of stele types including protosteles and siphonosteles, which allowed for their rapid radiation during the Carboniferous period (Suissa and Friedman 2022). Debate in this field has promoted significant advances in stelar theory (Tomescu 2021).
Deviations from typical to atypical ontogenies are also prolific in the fossil record (Artabe and Brea 2003; Bodnar and Coturel 2012; Crepet and Niklas 2019; D'Antonio and Boyce 2021). In fossil seed plants, all three categories of vascular variants can be recognized. ‘Medullary vascular systems’ (Corystopermales: Bodnar 2012) likely include cases of procambial variants, as in the atypical medullosan eustele (Dunn et al. 2003). Stems with differential cambial activity that generate ‘axial vascular elements in segments’ (Corystopermales: Bodnar and Coturel 2012) represent one type of cambial variant, while successive cambia (e.g. fossil Cycadales: Artabe and Stevenson 1999; Bodnar and Coturel 2012) and ‘neoformations’ (e.g. Sapindaceae: Jud et al. 2021) indicate the existence of ectopic cambia. This evidence suggests an enduring use of vascular variants, and highlights their systematics, functional and ecological significance in the evolution of plants. A greater understanding of the diversity beyond that of seed plants and fossil plants is needed, but beyond the scope of this study. Additional research is necessary, with respect to fossil and non-seed plant lineages, in order to expand the framework towards a more robust inclusion of all vascular plants.
Evolutionary Implications of Vascular Variants
Like other complex traits, the convergent evolution of vascular variants across seed plants is a striking phenomenon worth considering. In the past few years, research on this topic has focused on understanding not only vascular variants’ developmental anatomy, but also their evolutionary history (e.g. Bignoniaceae: Pace et al. 2009; Malpighiaceae: Quintanar-Castillo and Pace 2022; Malvaceae: Luna-Márquez et al. 2021; Sapindaceae: Chery et al. 2020), their impact to lineages diversification (e.g. Nyctaginaceae: Cunha Neto et al. 2022), structure–function relationships (Feild and Isnard 2013; Rowe and Speck 2015; Gerolamo et al. 2020; Hu and Lin 2022) and molecular regulation (Lima 2020), which remains largely unknown.
Since vascular variants are abundantly distributed in lineages containing climbing plants (Angyalossy et al. 2015), their ecological and evolutionary significances have been tested more frequently. Studies suggest that vascular variants evolved in association with the evolution of the climbing habit in several families (e.g. Bignoniaceae: Pace et al. 2009; Malpighiaceae: Quintanar-Castillo and Pace 2022). Along with their disparate climbing mechanisms (Isnard and Silk 2009; Gianoli 2015), the evolution of vascular variants in climbing plants likely facilitated their ecological diversification and physiological performance. Such complex anatomies are believed to increase stem flexibility, conductivity and mechanical resistance allowing them to climb without breaking apart (Feild and Isnard 2013; Isnard and Feild 2015). Furthermore, the evolution of vascular variants has been correlated with lobed conformation in primary growth (e.g. Sapindaceae: Chery et al. 2020), the arrangement of the primary vascular system and the formation of dermal appendices, such as prickles (e.g. Malvaceae: Luna-Márquez et al. 2021). Complex anatomies are further observed in fossil climbing plants dating from the Carboniferous period, such as the seed fern Medullosa (Dunn et al. 2003), and they are also recorded in early Miocene Ampelorhiza, the oldest fossil evidence of Paullinieae, Sapindaceae (Jud et al. 2021).
By contrast, vascular variants are widespread in some lineages regardless of growth forms (e.g. Menispermaceae: Jacques and de Franceschi 2007; Nyctaginaceae: Cunha Neto et al. 2022). In Nyctaginaceae, which have diverse growth forms and whose most common ancestor is reconstructed with vascular variants, increased diversification rates have been associated with the acquisition of medullary bundles, but not with transitions from self-supporting to climbing plants (Cunha Neto et al. 2022). The evolution of the climbing habit has been postulated as a key innovation in angiosperms (Gianoli 2004). Nyctaginaceae is likely one of a few examples where Gianoli’s (2004) hypothesis did not sustain. Among other evidence, support for Gianoli’s hypothesis can be found in Piperales, which is also diverse in growth forms and cambial modifications (Trueba et al. 2015), including vascular variants (Angyalossy et al. 2015). The ancestor of the perianth-bearing Piperales was reconstructed with a herb- or shrub-like habit, and the climbing habit was proposed as a derived growth form, which might have been a key feature in the diversification of Aristolochia, the most speciose lineage and the only genus with climbing plants (Wagner et al. 2014).
In self-supporting plants (non-climbing), functional properties of vascular variants may include adaptations for storage (e.g. beets: Artschwager 1926) or strategies for enhancing survival in conditions of extreme physiological drought such as arid soils or in the mangrove environment (e.g. Avicennia, the mangrove tree: Robert et al. 2014). Alternatively, vascular variants have been interpreted as evolutionary constraints (phylogenetic inertia), which means that self-supporting plants inherited these complex morphologies from their climbing ancestors in which these features could have been originally selected for the climbing habit (Jacques and de Franceschi 2007; Pace 2015; Gerolamo and Angyalossy 2017). Characterizing the vasculature of predominantly herbaceous plants, the atactostele in monocots is a remarkable deviation with enormous implications that explain some of the growth habit differences between monocots and eudicots (DeMason 1994). Remarkably, radial growth through the monocot cambium evolved solely in two lineages where arborescent monocotyledons are conspicuous. However, similar radial growth has also been reported in rhizomes and corms of some geophytes, especially in the Asparagales (Tribble et al. 2021).
In evolutionary developmental biology, any genetic change resulting in altered phenotypes may be considered a shift in developmental programmes which can modify the descendant’s morphology relative to the putative ancestor (Bateman 1994). In vascular development, evidence suggests that distinct developmental genetic modules can be protracted, prolonged, change location or acquire new functions resulting in modifications in cambial development (Onyenedum and Pace 2021). These re-patterning mechanisms likely occur through modifications in expression patterns of homeotic genes that determine shared molecular regulatory programmes across vascular plant lineages (Tomescu and Groover 2019). Enhancing our understanding of gene function in stem development of plants with and without vascular variants will provide insights into lineage-specific regulators, as well as conserved molecular programmes in vascular meristem formation (Tomescu and Groover 2019). In other words, the convergent evolution of vascular variants may be due to modifications in the tempo and mode of gene regulatory programmes generating the multitude of anatomical patterns observed in plants. Therefore, within a developmental approach, the identification of developmental origins and processes determining vascular variants may be informative at various biological scales with meaningful evolutionary implications.
Concluding Remarks
Anatomical patterns resulting from modifications to the origin, development and activity of vascular meristems in comparison to a putative ancestor of a given lineage are here called ‘vascular variants’. This term reflects that vascular ontogenies can be altered through re-patterning of both primary and secondary vascular meristems, as well as through the natural formation of ectopic cambia. In this context, ‘vascular variants’ unequivocally describe the diversity of alternative vascular ontogenies in seed plants, as opposed to historical yet vague terms such as ‘anomalous secondary growth’ and ‘cambial variants’. Rooted in an evolutionary and developmental context, this developmental based framework reveals three categories of vascular variants: procambial variants, cambial variants and ectopic cambia. Each category is defined by the origin of its vascular meristem and comprises multiple anatomical patterns. Each named anatomical pattern may be further formed through relatively distinct ontogenies, yet they are predictably conserved within a given species. Anatomical patterns have important systematic value and, therefore, precisely clarifying the diverse origins of such complex anatomies will facilitate our communication about the evolution of vascular meristems and potentially reduce barriers to research. As we strengthen our understanding of the developmental origins and ontogenetic processes generating vascular variants, future studies are warranted, and may prove essential to our understanding of molecular regulation in these complex anatomies. These works will be necessary not only to shed light on the diversity of vascular variants at the molecular level but will also elucidate the underlying mechanisms shaping the existing diversity of vascular structures in plants.
Supporting Information
The following supporting information is available in the online version of this article –
Table S1. List of terms used to describe vascular variants in different languages.
Table S2. Glossary of terms related to vascular variants.
Table S3. List of anatomical patterns distributed across categories of vascular variants as observed in seed plants.
Table S4. Distribution of vascular variants and anatomical patterns across orders and families of seed plants.
Figure S1. Ontogenies generating procambial variants and successive cambia in Nyctaginaceae.
Sources of Funding
No direct funding is associated with this manuscript. Previously, the author received financial support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Código de Financiamento 001 and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (2017/17107-3). Current research is supported by Startup lab funds from Cornell University.
Contributions by the Authors
I.L.C.N designed and wrote the manuscript.
Acknowledgements
I thank Dr. Joyce G. Onyenedum for providing the scientific environment, supporting my current research and for helpful discussions on vascular variants, and Dr. Marcelo R. Pace for the suggestion to write this paper during the 72º Brazilian Botanical Congress. I also acknowledge their constructive criticism on earlier drafts of this article. This article would not be possible without the mentorship of my former advisors Dr. Fabiano M. Martins, Dr. Neusa Tamaio and Dr. Veronica Angyalossy. I also thank colleagues from Rio de Janeiro Botanical Garden, University of São Paulo, Cornell University and the Plant Anatomy and Vascular Variants community for valuable discussions. I am grateful to two anonymous reviewers and the Associate Editor Juliana Medeiros for their helpful comments. Thanks also to Cora Oberst for proofreading the manuscript and Rubens K. Ito for support with drawings.
Form and Function. Chief Editor: Kate McCulloh
Conflict of Interest Statement
The author declare no conflicts of interest.
Data Availability
The original contributions presented in the study are included in the Supporting Information and in online repositories [Zenodo: 10.5281/zenodo.8003032 and GitHub: github.com/ilcneto/VascularVariants]. Further inquiries can be directed to the corresponding author.
REFERENCES
- Acevedo-Rodríguez P. 1993. Systematics of Serjania (Sapindaceae) part I: a revision of Serjania sect. Platycoccus. Memoirs of the New York Botanical Garden .:2–91. [Google Scholar]
- Acevedo-Rodríguez P. 2015. (onwards). Lianas and climbing plants of the Neotropics. https://naturalhistory.si.edu/research/botany/research/lianas-and-climbing-plants-neotropics.
- Angyalossy V, Angeles G, Pace MR, Lima AC, Dias-Leme CL, Lohmann LG, Madero-Vega C. 2012. An overview of the anatomy, development and evolution of the vascular system of lianas. Plant Ecology and Diversity .:167–182. [Google Scholar]
- Angyalossy V, Angeles G, Pace M, Lima AC. 2015. Liana anatomy: a broad perspective on structural evolution of the vascular system. In: Schnitzer SA, Bongers F, Burnham RJ, eds. Ecology of lianas. Chinchester: JohnWiley & Sons, 253–287. [Google Scholar]
- Araújo GUC, Costa CG. 2006. Cambial variant in the stem of Serjania corrugata (Sapindaceae). IAWA Journal 2.:269–280. [Google Scholar]
- Artabe AE, Brea M. 2003. A new approach to Corystospermales based on Triassic permineralized stems from Argentina. Alcheringa 2.:209–229. [Google Scholar]
- Artabe AE, Stevenson DW. 1999. Fossil Cycadales of Argentina. The Botanical Review 6.:219–238. [Google Scholar]
- Artschwager E. 1926. Anatomy of the vegetative organs of the sugar beet. Journal of Agricultural Research 3.:143–176. [Google Scholar]
- Avetta C. 1887. Contribuzione allo studio delle anomalie di struttura nelle radici delle dicotiledoni. Annuario del R. Istituto botanico di Roma .:3–19. [Google Scholar]
- Bastos CL, Tamaio N, Angyalossy V. 2016. Unravelling roots of lianas: a case study in Sapindaceae. Annals of Botany 11.:733–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RM. 1994. Evolutionary-developmental change in the growth architectures of rhizomorphic lycopsids Scenarios built on cladistic foundations. Biological Reviews 6.:527–597. [Google Scholar]
- Beck CB. 2010. An introduction to plant structure and development. Plant anatomy for the twenty-first century. Cambridge: Cambridge University Press. [Google Scholar]
- Beck CB, Schmid R, Rothwell GW. 1982. Stelar morphology and the primary vascular system of seed plants. The Botanical Review 4.:691–815. [Google Scholar]
- Bodnar J. 2012. Estudios evolutivos-del desarrollo en tallos fósiles de Corystospermaceae (Corystospermales, Spermatopsida). Revista del Museo Argentino de Ciencias Naturales 1.:143–166. [Google Scholar]
- Bodnar J, Coturel EP. 2012. El origen y diversificación del crecimiento cambial atípico en plantas fósiles: procesos del desarrollo involucrados. Boletin de la Sociedad Argentina de Botanica 4.:33–70. [Google Scholar]
- Botânico MP, Angyalossy V. 2013. Is the secondary thickening in palms always diffuse? Anais da Academia Brasileira de Ciencias 8.:1461–1472. [DOI] [PubMed] [Google Scholar]
- Cabanillas PA. 2016. Nuevo método para la interpretación ontogénica de tallos con variantes cambiales. Lilloa 5.:173–185. [Google Scholar]
- Cabanillas PA, Pace MR, Angyalossy V. 2017. Structure and ontogeny of the fissured stems of Callaeum (Malpighiaceae). IAWA Journal 3.:49–66. [Google Scholar]
- Carlquist S. 1988. Comparative wood anatomy. Berlin: Springer Verlag. [Google Scholar]
- Carlquist S. 1991. Anatomy of vine and liana stems: a review and synthesis. In: Putz FE, Mooney HA, eds. The biology of vines. Cambridge : Cambridge University Press, 53–72. [Google Scholar]
- Carlquist S. 2001. Comparative wood anatomy. Systematic, ecological and evolutionary aspects of dicotyledon wood. Berlin: Springer Verlag. [Google Scholar]
- Carlquist S. 2012. Monocot xylem revisited: new information, new paradigms. Botanical Review 7.:87–153. [Google Scholar]
- Carlquist S. 2013a. Interxylary phloem: diversity and functions. Brittonia 6.:477–495. [Google Scholar]
- Carlquist S. 2013b. More woodiness/less woodiness: evolutionary avenues, ontogenetic mechanisms. International Journal of Plant Sciences 17.:964–991. [Google Scholar]
- Chery JG, Pace MR, Acevedo-Rodríguez P, Specht CD, Rothfels CJ. 2020. Modifications during early plant development promote the evolution of nature’s most complex woods. Current Biology 3.:237–244.e2. [DOI] [PubMed] [Google Scholar]
- Chuang T-I. 1970. A systematic anatomical study of the genus Perideridia (Umbelliferae- Apioideae). American Journal of Botany 5.:495–503. [Google Scholar]
- Costea M, DeMason DA. 2001. Stem morphology and anatomy in Amaranthus L. (Amaranthaceae)—taxonomic significance. Journal of the Torrey Botanical Society 12.:254–281. [Google Scholar]
- Crepet WL, Niklas KJ. 2019. The evolution of early vascular plant complexity. International Journal of Plant Sciences 18.:800–810. [Google Scholar]
- Cunha Neto IL, Martins FM, Somner GV, Tamaio N. 2018. Successive cambia in liana stems of Paullinieae and their evolutionary significance in Sapindaceae. Botanical Journal of the Linnean Society 18.:66–88. [Google Scholar]
- Cunha Neto IL, Pace MR, Douglas NA, Nee MH, Sá CFC, Moore MJ, Angyalossy V. 2020a. Diversity, distribution, development, and evolution of medullary bundles in Nyctaginaceae. American Journal of Botany 10.:707–725. [DOI] [PubMed] [Google Scholar]
- Cunha Neto IL, Silva JP, Angyalossy V. 2020b. Anatomy of vegetative organs in Allionia (Nyctaginaceae), with emphasis on the vascular system. Journal of the Botanical Research Institute of Texas 1.:373–394. [Google Scholar]
- Cunha Neto IL, Pace MR, Angyalossy V. 2021. A new interpretation of the successive cambia of some Nyctaginaceae as interxylary phloem. International Journal of Plant Sciences 18.:620–637. [Google Scholar]
- Cunha Neto IL, Pace MR, Hernández-Gutiérrez R, Angyalossy V. 2022. Linking the evolution of development of stem vascular system in Nyctaginaceae and its correlation to habit and species diversification. EvoDevo 1.:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antonio MP, Boyce CK. 2021. Secondary phloem in arborescent lycopsids. New Phytologist 23.:967–972. [DOI] [PubMed] [Google Scholar]
- De Bary A. 1884. Comparative anatomy of the vegetative organs of the phanerogams and ferns. Oxford: Clarendon Press. [Google Scholar]
- Decombeix AL, Galtier J, Meyer-Berthaud B. 2014. Secondary phloem in early carboniferous seed plants: anatomical diversity and evolutionary implications. International Journal of Plant Sciences 17.:891–910. [Google Scholar]
- Decombeix AL, Boura A, Tomescu AMF. 2019. Plant hydraulic architecture through time: Lessons and questions on the evolution of vascular systems. IAWA Journal 4.:387–420. [Google Scholar]
- DeMason DA. 1994. Stem thickening in monocotyledons. In: Iqbal M, ed. Growth patterns in vascular plants. Portland, OR: Dioscorides Press, 288–312. [Google Scholar]
- Dunn MT, Krings M, Mapes G, Rothwell GW, Mapes RH, Keqin S. 2003. Medullosa steinii sp. nov., a seed fern vine from the Upper Mississippian. Review of Palaeobotany and Palynology 12.:307–324. [Google Scholar]
- Esau K. 1965. Plant anatomy. New York: John Wiley & Sons. [Google Scholar]
- Feild TS, Isnard S. 2013. Climbing habit and ecophysiology of Schisandra glabra (Schisandraceae): implications for the early evolution of angiosperm lianescence. International Journal of Plant Sciences 17.:1121–1133. [Google Scholar]
- Forbes JC. 1985. Weed-crop competition studies in swedes: III. The effects of weed competition on the developmental anatomy of swede plants. Annals of Applied Biology 10.:525–540. [Google Scholar]
- Frankiewicz KE, Oskolski AA. 2023. Raylessness and paedomorphosis: losses and gains of xylem rays en route from procambium to vascular cambium. IAWA Journal .:1–15. [Google Scholar]
- Gerolamo CS, Angyalossy V. 2017. Wood anatomy and conductivity in lianas, shrubs and trees of Bignoniaceae. IAWA Journal 3.:412–432. [Google Scholar]
- Gerolamo CS, Nogueira A, Pace MR, Angyalossy V. 2020. Interspecific anatomical differences result in similar highly flexible stems in Bignoniaceae lianas. American Journal of Botany 10.:1622–1634. [DOI] [PubMed] [Google Scholar]
- Gianoli E. 2004. Evolution of a climbing habit promotes diversification in flowering plants. Proceedings of the Royal Society B: Biological Sciences 27.:2011–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoli E. 2015. Evolutionary implications of the climbing habit in plant. In: Schnitzer SA, Bongers F, Burnham RJ, Putz FE, eds. Ecology of Lianas. West Sussex: John Wiley & Sons, 239–250. [Google Scholar]
- Haberlandt GFJ. 1914. Physiological plant anatomy. London: Macmillan Co. [Google Scholar]
- Hayden SM, Hayden WJ. 1994. Stem development, medullary bundles, and wood anatomy of Croton glandulosus var. septentrionalis (Euphorbiaceae). IAWA Journal 1.:51–63. [Google Scholar]
- Hayward HE. 1938. The structure of economic plants. London and New York: Macmillan. [Google Scholar]
- Hu L, Lin Y. 2022. How weak twining lianas adapt to competition with host tree trunks: case of Merremia boisiana. Ecology and Evolution 1.:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isnard S, Feild TS. 2015. The evolution of angiosperm lianescence: a perspective from xylem structure-function In: Schnitzer SA, Bongers F, Burnham RJ, eds. Ecology of lianas. Chinchester: JohnWiley & Sons, 221–250. [Google Scholar]
- Isnard S, Silk WK. 2009. Moving with climbing plants from Charles Darwin’s time into the 21st century. American Journal of Botany 9.:1205–1221. [DOI] [PubMed] [Google Scholar]
- Isnard S, Prosperi J, Wanke S, Wagner ST, Samain M-S, Trueba S, Frenzke L, Neinhuis C, Rowe NP. 2012. Growth form evolution in Piperales and its Relevance for understanding angiosperm diversification: an integrative approach combining plant architecture, anatomy, and biomechanics. International Journal of Plant Sciences 17.:610–639. [Google Scholar]
- Jacques FMB, De Franceschi D. 2007. Menispermaceae wood anatomy and cambial variants. IAWA Journal 2.:139–172. [Google Scholar]
- Jud NA, Allen SE, Nelson CW, Bastos CL, Chery JG. 2021. Climbing since the early Miocene: the fossil record of Paullinieae (Sapindaceae). PLoS One 1.:e0248369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jura-Morawiec J, Tulik M, Iqbal M. 2015. Lateral meristems responsible for secondary growth of the monocotyledons: a survey of the state of the art. Botanical Review 8.:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen R. 1999. Wood anatomy of the Sapindaceae. Leiden: International Association of Wood Anatomist. [Google Scholar]
- Kuijt J, Bruns D. 1987. Roots in Corynaea (Balanophoraceae). Nordic Journal of Botany .:539–542. [Google Scholar]
- Leal MOL, Nascimento LB do, Coutinho AJ, Tamaio N, Brandes AF das N. 2020. Development of external vascular cylinders (neoformations) in stems and roots of Chiococca alba (L.) Hitchc. (Rubiaceae). Flora: Morphology, Distribution, Functional Ecology of Plants 26.:151569. [Google Scholar]
- Leme CLD, Cunha Neto IL, Angyalossy V. 2020. How the neotropical liana Machaerium multifoliolatum (Fabaceae) develop their distinctive flattened stems? Flora: Morphology, Distribution, Functional Ecology of Plants 26.:151629. [Google Scholar]
- Li H-T, Luo Y, Gan L, Ma P-F, Gao L-M, Yang J-B, Cai J, Gitzendanner MA, Fritsch PW, Zhang T, et al. 2021. Plastid phylogenomic insights into relationships of all flowering plant families. BMC Biology 1.:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima AC. 2020. More than supportive: liana attachment to supports lead to profound changes in xylem anatomy, hydraulic conductivity and cambium transcriptional profile. PhD Thesis. Brazil: University of São Paulo. [Google Scholar]
- Lopes WAL, De Souza LA, De Almeida OJG. 2017. Procambial and cambial variants in Serjania and Urvillea species (Sapindaceae: Paullinieae). Journal of the Botanical Research Institute of Texas 1.:421–432. [Google Scholar]
- Luna-Márquez L, Sharber WV, Whitlock BA, Pace MR. 2021. Ontogeny, anatomical structure and function of lobed stems in the evolution of the climbing growth form in Malvaceae (Byttneria Loefl.). Annals of Botany 12.:859–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejapa R, Cabanillas PA, Pace MR. 2021. Cortical origin of the successive cambia in the stems of the charismatic temperate lianescent genus Wisteria (Fabaceae) and its systematic importance. Botanical Journal of the Linnean Society 19.:667–677. [Google Scholar]
- Onyenedum JG, Pace MR. 2021. The role of ontogeny in wood diversity and evolution. American Journal of Botany 10.:2331–2355. [DOI] [PubMed] [Google Scholar]
- Pace MR. 2015. Evolution of the vascular system in lineages that contain lianas. PhD Thesis. Brazil: University of São Paulo. [Google Scholar]
- Pace MR, Lohmann LG, Angyalossy V. 2009. The rise and evolution of the cambial variant in Bignonieae (Bignoniaceae). Evolution and Development 1.:465–479. [DOI] [PubMed] [Google Scholar]
- Pace MR, Acevedo-Rodríguez P, Amorim AM, Angyalossy V. 2018. Ontogeny, structure and occurrence of interxylary cambia in Malpighiaceae. Flora: Morphology, Distribution, Functional Ecology of Plants 24.:46–60. [Google Scholar]
- Pace MR, Gerolamo CS, Onyenedum JG, et al. 2022. The wood anatomy of Sapindales: diversity and evolution of wood characters. Revista Brasileira de Botanica 4.:283–340. [Google Scholar]
- Patil VS, Rajput KS. 2008. Structure and development of inter- and intraxylary phloem in Leptadenia reticulata (Asclepiadaceae). Polish Botanical Journal 5.:5–13. [Google Scholar]
- Patil VS, Marcati CR, Rajput KS. 2011. Development of intra- and interxylary secondary phloem in Coccinia indica (Cucurbitaceae). IAWA Journal 3.:475–491. [Google Scholar]
- Philipson WR, Ward JM. 1965. The ontogeny of the vascular cambium in the stem of seed plants. Biological Reviews 4.:534–579. [Google Scholar]
- Pellissari LCO, Teixeira-Costa L, Ceccantini G, et al. 2022. Parasitic plant, from inside out: endophytic development in Lathrophytum peckoltii (Balanophoraceae) in host liana roots from tribe Paullineae (Sapindaceae). Annals of Botany 12.:331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povilus RA, DaCosta JM, Grassa C, Satyaki Prasad R V, Moeglein M, Jaenisch J, Xi Z, Mathews S, Gehring M, Davis Charles C, et al. 2020. Water lily (Nymphaea thermarum) genome reveals variable genomic signatures of ancient vascular cambium losses. Proceedings of the National Academy of Sciences of the United States of America 11.:8649–8656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanar-Castillo A, Pace MR. 2022. Phloem wedges in Malpighiaceae: origin, structure, diversification, and systematic relevance. EvoDevo 1.:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput KS. 2015. Comparative study on secondary xylem and formation of successive cambia in stems and roots of Antigonon leptopus Hook. & Arn. (Polygonaceae). Flora: Morphology, Distribution, Functional Ecology of Plants 21.:131–137. [Google Scholar]
- Rajput KS, Gondaliya AD. 2017. Internal cambium and intraxylary phloem development in Ipomoea turbinata Lag. (Convolvulaceae). Flora: Morphology, Distribution, Functional Ecology of Plants 22.:47–54. [Google Scholar]
- Rajput KS, Gondaliya AD, Lekhak MM, Yadav SR. 2018. Structure and ontogeny of intraxylary secondary xylem and phloem development by the internal vascular cambium in Campsis radicans (L.) Seem. (Bignoniaceae). Journal of Plant Growth Regulation 3.:755–767. [Google Scholar]
- Rajput KS, Kapadane KK, Ramoliya DG, Thacker KD, Gondaliya AD. 2022. Inter- and intraxylary phloem in vascular plants: a review of subtypes, occurrences, and development. Forests 1.:2174. [Google Scholar]
- Rizzieri YC, Brandes AFN, Cunha Neto IL, et al. 2021. Ontogeny of divided vascular cylinders in Serjania: the rise of a novel vascular architecture in Sapindaceae. IAWA Journal 4.:121–133. [Google Scholar]
- Robert EMR, Jambia AH, Schmitz N, De Ryck DJR, De Mey J, Kairo JG, Dahdouh-Guebas F, Beeckman H, Koedam N. 2014. How to catch the patch? A dendrometer study of the radial increment through successive cambia in the mangrove Avicennia. Annals of Botany 11.:741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robischon M, Du J, Miura E, Groover A. 2011. The Populus class III HD ZIP, popREVOLUTA, influences cambium initiation and patterning of woody stems. Plant Physiology 15.:1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodt D, Li Z, Van De Peer Y, Mizrachi E, Gaut B. 2019. Loss of wood formation genes in monocot genomes. Genome Biology and Evolution 1.:1986–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell GW, Karrfalt EE. 2008. Growth, development, and systematics of ferns: does Botrychium s.l. (Ophioglossales) really produce secondary xylem? American Journal of Botany 9.:414–423. [DOI] [PubMed] [Google Scholar]
- Rowe NP, Speck T. 2015. Stem biomechanics, strength of attachment, and developmental plasticity of vines and lianas In: Schnitzer SA, Bongers F, Burnham R, Putz FE, eds. Ecology of lianas. Oxford: Wiley–Blackwell, 323–341. [Google Scholar]
- Salas DS, Sinamban EB, Buenavista DP. 2018. Comparative morpho-anatomical studies of Hoya incrassate and Hoya soligamiana (Apocynaceae) from Mount Hamiguitan, Philippines. Ruhuna Journal of Science .:1. [Google Scholar]
- Sathya E, Muthukumar T, Sekar T. 2022. Comparative vegetative anatomy of Wrightia tinctoria R.Br. and the endemic Wrightia indica Ngan (Apocynaceae Juss.) occurring in peninsular India. Flora 29.:152043. [Google Scholar]
- Schenck H. 1893. Beiträge zur Biologie und Anatomie der Lianen im Besonderen der in Brasilien einheimische. Arten. 2 In: Schimper AFW, Fischer G, eds. Botanische Mittheilungen aus der Tropens. Jena: Gustav Fischer, 1–271. [Google Scholar]
- Schmitz N, Robert EMR, Verheyden A, Kairo JG, Beeckman H, Koedam N. 2008. A patchy growth via successive and simultaneous cambia: key to success of the most widespread mangrove species Avicennia marina? Annals of Botany 10.:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seago JL. 2020. Revisiting the occurrence and evidence of endodermis in angiosperm shoots. Flora 27.:151709. [Google Scholar]
- Seago JL, Tylová E, Soukup A, Bona C, Vortubová O. 2021. A new examination of anatomical structures characterizing the genus Gunnera. Flora 28.:151919. [Google Scholar]
- Simpson MG. 2019. Plant systematics. San Diego, CA: Elsevier Academic Press. [Google Scholar]
- Smetana O, Mäkilä R, Lyu M, et al. 2019. High levels of auxin signalling define the stem-cell organizer of the vascular cambium. Nature 56.:485–489. [DOI] [PubMed] [Google Scholar]
- Spicer R, Groover A. 2010. Evolution of development of vascular cambia and secondary growth. New Phytologist 18.:577–592. [DOI] [PubMed] [Google Scholar]
- Stefanovic S, Krueger L, Olmstead RG. 2002. Monophyly of the Convolvulaceae and circumscription of their major lineages based on DNA sequences of multiple chloroplast loci. American Journal of Botany 8.:1510–1522. [DOI] [PubMed] [Google Scholar]
- Stevens P. 2001. (onwards). Angiosperm Phylogeny Website. Version 14, July 2017 [and more or less continuously updated since]. http://www.mobot.org/MOBOT/research/APweb/.
- Stevenson DW. 1980. Ontogeny of the vascular system of Botrychium multifidum (S. G. Gmelin) Rupr. (Ophioglossaceae) and its bearing on stelar theories. Botanical Journal of the Linnean Society 8.:41–52. [Google Scholar]
- Suissa JS, Friedman WE. 2022. Rapid diversification of vascular architecture underlies the Carboniferous fern radiation. Proceedings of the Royal Society B: Biological Sciences 28.:20212209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaio N, Angyalossy V. 2009. Variação cambial em Serjania caracasana (Sapindaceae): enfoque na adequação terminológica. Rodriguésia 6.:651–666. [Google Scholar]
- Tamaio N, Vieira RC, Angyalossy V. 2009. Origin of successive cambia on stem in three species of Menispermaceae. Revista Brasileira de Botânica 3.:839–848. [Google Scholar]
- Tamaio N, Somner GV. 2010. Development of corded vascular cylinder in Thinouia restingae Ferruci & Somner (Sapindaceae: Paullinieae). The Journal of the Torrey Botanical Society 13.:319–326. [Google Scholar]
- Tamaio N, Neves MF, Brandes AFN, Vieira RC. 2011. Quantitative analyses establish the central vascular cylinder as the standard for wood-anatomy studies in lianas having compound stems (Paullinieae: Sapindaceae). Flora: Morphology, Distribution, Functional Ecology of Plants 20.:987–996. [Google Scholar]
- Tomescu AMF. 2021. The stele—a developmental perspective on the diversity and evolution of primary vascular architecture. Biological Reviews 9.:1263–1283. [DOI] [PubMed] [Google Scholar]
- Tomescu AMF, Groover AT. 2019. Mosaic modularity: an updated perspective and research agenda for the evolution of vascular cambial growth. New Phytologist 22.:1719–1735. [DOI] [PubMed] [Google Scholar]
- Tribble CM, Martínez-Gómez J, Howard CC, Males J, Sosa V, Sessa EB, Cellinese N, Specht CD. 2021. Get the shovel: morphological and evolutionary complexities of belowground organs in geophytes. American Journal of Botany 10.:372–387. [DOI] [PubMed] [Google Scholar]
- Trueba S, Rowe NP, Neinhuis C, Wanke S, Wagner ST, Isnard S. 2015. Stem anatomy and the evolution of woodiness in Piperales. International Journal of Plant Sciences 17.:468–485. [Google Scholar]
- Van der Walt JJA, Van der Schijff HP, Schweickerdt HG. 1973. Anomalous secondary growth in the stem of lianas Mikania cordata (Burm. F.) Robins (Compositae) and Paullinia pinnata Linn. (Sapindaceae). Kirkia .:109–138. [Google Scholar]
- Van Tieghem P. 1884. Traité de botanique. Paris: Librairie F. Savy. [Google Scholar]
- Victorio MP. 2016. Roots and stems anatomy of Bignonieae: lianescent syndrome and secondary xylem. Master’s Thesis. Brazil: University of São Paulo. [Google Scholar]
- Wagner ST, Hesse L, Isnard S, Samain M-S, Bolin J, Maass E, Neinhuis C, Rowe NP, Wanke S. 2014. Major trends in stem anatomy and growth forms in the perianth-bearing Piperales, with special focus on Aristolochia. Annals of Botany 11.:1139–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Liu L. 2015. A new Late Devonian genus with seed plant affinities. BMC Evolutionary Biology 1.:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CA, Calvin CL. 1996. Anatomy of the dwarf mistletoe shoot system. In: Hawksworth FG, Wiens D, eds. Dwarf mistletoes: biology, pathology, and systematics. Washington, DC: United States Department of Agriculture, 95–112. [Google Scholar]
- Wilson LA, Lowe SB. 1973. The anatomy of the root system in West Indian sweet potato (Ipomoea batatas (L.) Lam.) cultivars. Annals of Botany 3.:633–643. [Google Scholar]
- Worsdell WC. 1906. The structure and origin of the Cycadaceae. Annals of Botany os-2.:129–159. [Google Scholar]
- Yang SZ, Chen PH, Chen YJ. 2014. Classification on anomalous structure of lianas stem. Classification on Anomalous Structure of Lianas Stem 6.:45–74. [Google Scholar]
- Zinkgraf M, Gerttula S, Groover A. 2017. Transcript profiling of a novel plantmeristem, the monocot cambium. Journal of Integrative Plant Biology 5.:436–449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the Supporting Information and in online repositories [Zenodo: 10.5281/zenodo.8003032 and GitHub: github.com/ilcneto/VascularVariants]. Further inquiries can be directed to the corresponding author.