Abstract
Background:
Data supporting dementia as a risk factor for coronavirus disease 2019 (COVID-19) mortality relied on ICD-10 codes, yet nearly 40% of individuals with probable dementia lack a formal diagnosis. Dementia coding is not well established for people with HIV (PWH), and its reliance may affect risk assessment.
Methods:
This retrospective cohort analysis of PWH with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) PCR positivity includes comparisons to people without HIV (PWoH), matched by age, sex, race, and zipcode. Primary exposures were dementia diagnosis, by International Classification of Diseases (ICD)-10 codes, and cognitive concerns, defined as possible cognitive impairment up to 12 months before COVID-19 diagnosis after clinical review of notes from the electronic health record. Logistic regression models assessed the effect of dementia and cognitive concerns on odds of death [odds ratio (OR); 95% CI (95% confidence interval)]; models adjusted for VACS Index 2.0.
Results:
Sixty-four PWH were identified out of 14 129 patients with SARS-CoV-2 infection and matched to 463 PWoH. Compared with PWoH, PWH had a higher prevalence of dementia (15.6% vs. 6%, P = 0.01) and cognitive concerns (21.9% vs. 15.8%, P = 0.04). Death was more frequent in PWH (P < 0.01). Adjusted for VACS Index 2.0, dementia [2.4 (1.0–5.8), P = 0.05] and cognitive concerns [2.4 (1.1–5.3), P = 0.03] were associated with increased odds of death. In PWH, the association between cognitive concern and death trended towards statistical significance [3.92 (0.81–20.19), P = 0.09]; there was no association with dementia.
Conclusion:
Cognitive status assessments are important for care in COVID-19, especially among PWH. Larger studies should validate findings and determine long-term COVID-19 consequences in PWH with preexisting cognitive deficits.
Keywords: cognitive, coronavirus disease 2019, HIV, mortality, outcomes
Introduction
Risk factors for adverse outcomes after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection include impaired immunity, medical comorbidities, and adverse social determinants of health [1–4], which are disproportionately found in people with HIV (PWH) compared with people without HIV (PWoH) [5]. Although several studies early in the pandemic suggested no difference in outcomes following COVID-19 in PWH compared with PWoH [6–8], recent epidemiological studies suggested that PWH are at higher risk of death and severe illness because of COVID-19 globally [9–12].
We and others showed that neurological comorbidities, including dementia, predict adverse outcomes in COVID-19 [1,13–15]. Although published studies relying on electronic health record (EHR) extraction and International Classification of Diseases (ICD) billing codes allow for assessments of disease risk in large datasets, approximately 40% of individuals with probable dementia in the United States lack a formal diagnosis [16]. Estimates of dementia may be problematic in marginalized subpopulations such as PWH and overlooked in risk prediction. Although the prevalence of HIV-associated dementia has substantially declined, other forms of cognitive impairment, including milder forms of HIV-associated neurocognitive disorders and age-associated cognitive disorders, are common in PWH [17–21]. We hypothesized that impaired cognition could partially explain the higher risk for severe disease in COVID-19, and assessing cognitive concerns as opposed to ICD-based dementia diagnosis is a more useful means of classification, especially among PWH.
This retrospective study analyzed the relationship between preexisting impaired cognition (cognitive concerns or dementia diagnosis) and death after SARS-CoV-2 infection in PWH and PWoH. We employed a natural language processing (NLP) Annotator Tool (NAT) [22], which allowed for efficient evaluation of clinical notes for assessment of cognitive concerns, calculated the Veterans Aging Cohort Study Index 2.0 (VACS Index 2.0) and Veterans Health Administration COVID-19 (VACO) Index for COVID-19 Mortality [23,24], and assessed the relationship between odds of death after SARS-CoV-2 infection and preexisting dementia or cognitive concern in the total cohort, and in analyses restricted to PWH.
Methods
Study design and definitions
The present study included 527 adult patients with laboratory-confirmed SARS-CoV-2 infection by positive reverse-transcriptase PCR (RT-PCR), and is a subset of 14 127 individuals evaluated in a respiratory outpatient clinic, the emergency department, or during admission to any Mass General Brigham facility between 27 March 2020 and 13 March 2021. The 527 patients included 64 patients with known HIV based on ICD-10 code (B20) and confirmed by chart review. We performed a 10 : 1 match of PWoH to PWH based on age, sex, race (black vs. other), and zip code of residence to facilitate cognitive classification on a smaller, unbiased subset; replacement was allowed, such that each PWoH could be reused to match any number of PWH, to improve balance (MatchIT v. 3.6.1, Vienna, Austria) [25]. The institutional review board at Mass General Brigham Healthcare approved this study (Protocol #2019P003215) with a waiver of consent for retrospective analyses. Data analysts collected data from EHR using the Mass General Brigham Electronic Data Warehouse, and healthcare providers collected additional information, including HIV characteristics, not available from automated extraction.
The VACS Index 2.0 and VACO index were calculated based on the methods previously reported [23,24]. Lab values collected 14 days before the first positive SARS-CoV-2 result or after diagnosis were used. In PWoH without an available CD4+ T-cell count, the count was imputed as 500 cells/μl. If a lab value was indicated with a greater-than or less-than sign, the maximum or minimum possible value was assumed, respectively; ICD-10 codes used are published [26].
To determine cognitive status in the year before SARS-CoV-2 infection, we used the NAT software tool developed by the MIND Data Science Lab as described [22]. After review of EHR notes, patients were annotated as either: ‘no cognitive concerns’, ‘cognitive concern’, or ‘undetermined’. Patients were annotated ‘cognitive concern’ if they had documented concern or suspicion of cognitive decline, cognitive symptoms, memory impairment, or were prescribed medications primarily for cognitive symptoms, including donepezil and memantine. An ‘undetermined’ cognitive status was applied if there was no note in the year before diagnosis or insufficient evidence to assess cognitive functioning; ‘undetermined’ cognitive status was included as a separate category to minimize biases in analyses.
Statistical analysis
Descriptive statistics summarized patient data. Continuous and categorical variables were presented as median [interquartile range, (IQR)] and n (%), respectively. Missing patient values were not imputed. Independent t tests with Bonferroni correction were used to compare VACS Index 2.0 and VACO Index scores between outpatients and patients with hospitalization, ICU, and death as outcomes. Logistic regressions were used to compute the odds ratio of dementia or cognitive concern with respect to death; exploratory mediation analyses assessed the extent to which impaired cognition and HIV contributed to death. Python3 and R were used for analysis and data visualization [27].
Results
By design, there were no statistically significant differences in the baseline age, gender, or race between PWH and PWoH (Supplemental Table 1). The age distribution was similar across patients with and without HIV (Supplemental Figure 1). PWH were more likely to have ICD-10 codes for dementia, depression or anxiety, or seizure disorders than PWoH before SARS-CoV-2 infection (Supplemental Table 1). Laboratory studies did not differ between groups, except for a lower glomerular filtration rate in PWH.
We investigated baseline HIV-specific characteristics stratified by setting (outpatient or hospital) among PWH at SARS-CoV-2 infection. Younger PWH were more likely to be SARS-CoV-2 PCR-positive in the outpatient setting than those admitted to the hospital (P = 0.02, Supplemental Table 2). Among those hospitalized, 12% had a viral load greater than 200 copies/ml, and 24% had a CD4+ T-cell count less than 200 cells/ml; reported ART usage was not statistically different in the outpatient setting compared with hospitalized individuals. The proportions of patients hospitalized or admitted to the ICU were not statistically different between PWH and PWoH (Supplemental Table 3). Overall, in-hospital deaths were higher (P < 0.01), whereas the age at death was lower in PWH (58 ± 14 years) compared with PWoH (66 ± 9 years, P < 0.05).
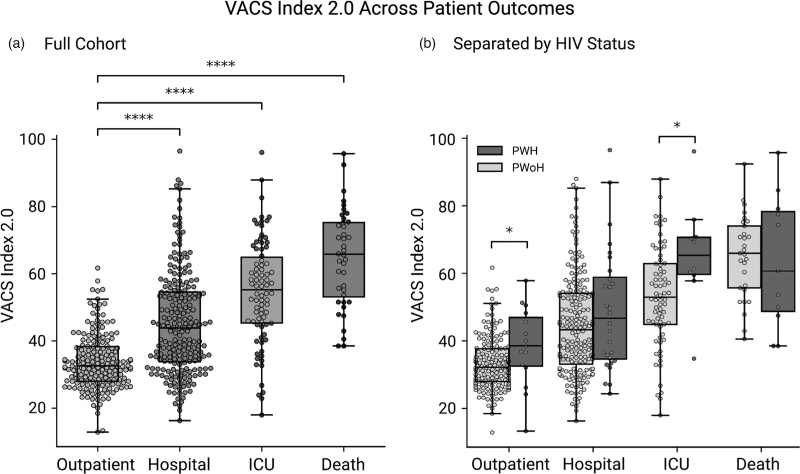
The VACS Index 2.0 score at the time of SARS-CoV-2 positivity reliably predicted the patient's level of care and death (Fig. 1a), with increasing scores predictive of worse outcomes. PWH had higher average scores than PWoH at all levels of care, with statistical significance at outpatient evaluation and ICU admission (Fig. 1b). Older age and measures of reduced liver function (AST, ALT, and FIB4 score) were the primary contributors to increased odds of death in all patients (Supplemental Figure 3). The VACS Index 2.0 showed similar trends to the VACO Index, a validated index to predict 30-day mortality from COVID-19 (data not shown). Given that the VACO Index relies on billing codes and does not incorporate HIV-specific lab values, the VACO Index 2.0 was used as a covariate in subsequent regression models.
Fig. 1.
VACS Index 2.0 correlates with level of care and mortality from coronavirus disease 2019 both in the full cohort (a) and separated by HIV status (b).
Boxplot and swarm plots of VACS Index 2.0 score for outpatients (n = 82), hospitalized patients (n = 220), ICU patients (n = 108), and patients who died (n = 40). Independent t tests demonstrated all patients with hospitalization, ICU, and death as outcomes had significantly higher VACS Index 2.0 scores (P < 0.0001) compared with outpatients (a). Boxplot and swarm plots of VACS 2.0 Index score, separated by HIV status, for outpatients (PWoH, n = 170; PWH, n = 15), hospitalized patients (PWoH, n = 192; PWH, n = 28), ICU patients (PWoH, n = 72; PWH, n = 10), and patients who died (PWoH, n = 29; PWH, n = 11). Independent t tests demonstrated that PWH at the outpatient level of care and ICU had significantly higher VACS Index 2.0 scores (P < 0.05) compared with PWoH (b). PWH, people with HIV; PWOH, people without HIV.
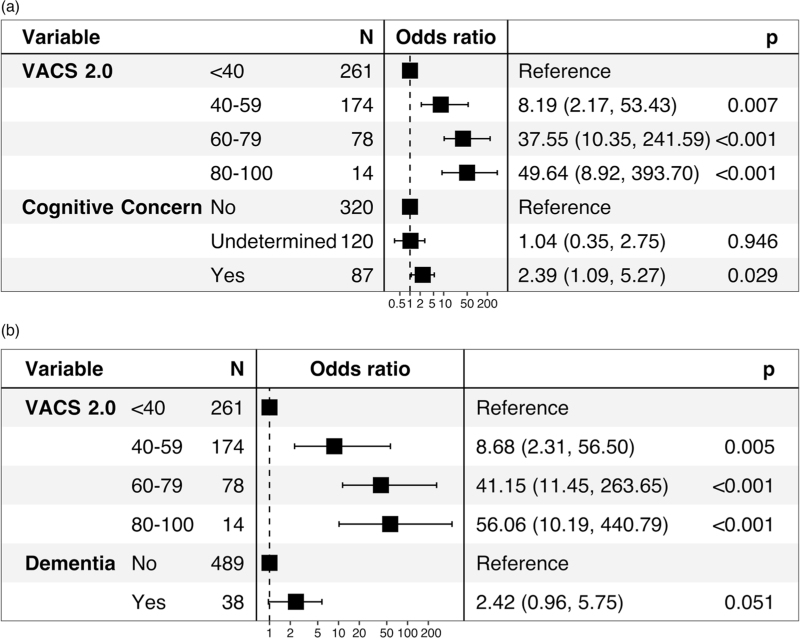
We investigated neurologic variables in PWH and PWoH relative to odds of death following SARS-CoV-2 infection. Among PWoH and PWH without cognitive concern or dementia, 3.9% (n = 15/385) and 10% (n = 5/49) died following SARS-CoV-2 infection, respectively. This contrasts with the 18% (n = 14/78) of PWoH and 40% (n = 6/15) of PWH who died when impaired cognition was present. Both cerebrovascular disease and dementia contributed significantly to odds of death after COVID-19 (Supplemental Figure 2). A preexisting cognitive concern in PWH and PWoH also demonstrated increased odds of death after SARS-CoV-2 infection [odds ratio (OR) 4.96; P < 0.001]. In primary analyses assessing the relationship between impaired cognition and death, adjusted for VACS Index 2.0, there was a marked effect size for dementia and cognitive concern among all people with SARS-CoV-2 infection (Fig. 2). In an exploratory mediation analysis, HIV did not significantly influence the association between dementia, cognitive concerns, and death (P = 0.076 and P = 0.59), respectively. Given that the Centers for Disease Control lists cardiovascular disease and diabetes as underlying conditions that increase the risk of severe disease from SARS-CoV-2 infection, we adjusted for cardiovascular disease and diabetes diagnosis in models. Although effect size was attenuated for dementia (OR 1.99; P = 0.14) and cognitive concerns (OR 2.34; P = 0.04), the trend of increased odds of death with preexisting impaired cognition remained. In secondary analyses among the PWH subset, ICD-10-coded dementia was not associated with adjusted odds of death [OR 1.31; CI (0.21–6.73); P = 0.76], while cognitive concern had a large effect size and a trend towards significance [OR 3.92; CI (0.81–20.19); P = 0.09].
Fig. 2.
Cognitive concerns in people with HIV and people without HIV increase the odds ratios for death after coronavirus disease 2019.
(a) Forest plot with odds ratio for multivariate logistic regression model using VACS Index 2.0 score and cognitive concern to predict death following COVID-19 for all patients. Model fit as assessed by Akaike information criterion (AIC) for VACS and VACS with cognitive concern was 227.9 and 226.8, respectively. (b) Forest plot with odds ratio for multivariate logistic regression model using VACS Index 2.0 score and dementia to predict death for all patients. AIC for a model with VACS with dementia was 226.4.
Discussion
This retrospective study details the clinical characteristics, neurologic risk factors, and outcomes data in PWH and age-matched, sex-matched, and zipcode-matched PWoH following SARS-CoV-2 infection. PWH had a significantly increased risk of death from COVID-19 compared with PWoH, and on average, died at a younger age, despite most being virally suppressed on ART. VACS Index 2.0 reliably predicted the risk of hospitalization, ICU admission, and death and may have utility in predicting severe disease, particularly among PWH with SARS-CoV-2 infection. Finally, preexisting dementia or cognitive concerns were associated with higher odds of death following COVID-19 in all patients. In contrast, cognitive concern had a large effect size and trended to higher odds of death in PWH, a factor not evident when relying on ICD-10 coding of dementia alone, and a distinction that may have implications for HIV care and risk-assessment during SARS-CoV-2 infection.
Neurologic comorbidities can influence clinical outcomes after COVID-19 [28–30], but the extent to which preexisting neurological disorders contribute to poor outcomes in PWH is relatively unknown. This is despite contemporary data suggesting that PWH have a higher prevalence of preexisting cognitive disorders compared with PWoH [31]. Documentation of cognitive symptoms before infection was associated with an approximately three-fold increased odds of death after SARS-CoV-2 infection. Although the exact causal link to death is unclear, we hypothesize that preexisting cognitive impairment are associated with delirium or COVID-19-associated encephalopathy, a condition that may contribute to adverse outcomes after SARS-CoV-2 infection [32]. Emerging evidence also suggests a role for myeloid cell dysregulation in dementia and COVID-19 [33], which may provide a link between dementia and increased risk of death following SARS-CoV-2 infection [34,35]. Importantly, confounding factors, not easily assessed in small cohorts, such as substance use, polypharmacy, and mental health conditions in PWH may significantly contribute to disease severity.
The present study has some limitations. First, its retrospective nature and reliance upon EHR data limits a comprehensive capture of full medical histories and outcomes; thus, some misclassification of prior medical diagnoses and incomplete capture of deaths is possible. To minimize misclassification bias, our group validated data relying on clinicians with domain expertise to review cognitive concerns, and manually extracted HIV-associated variables. Complete evaluations with cognitive testing were not performed; thus, assessment of preexisting cognitive concerns was limited, and we relied upon providers signaling concerns in clinical notes. Long-term follow-up that includes cognitive assessments for PWH are critical to understanding the contribution of cognitive function to COVID-19 severity and longitudinal impact. In this study, a smaller portion of PWoH had dementia compared with the PWH group, and power to detect an association between dementia and mortality was lower in PWoH; a larger study of PWoH, likely in older age categories, is required. We imputed CD4+ cell counts as 500 cells/μl when data was unavailable; however, recent studies suggest that CD4+ cell counts above 500 cells/μl may also contribute to outcomes after SARS-CoV-2 infection [36]. Although a minority of patients were vaccinated by the conclusion of this study in March 2021 and may influence outcomes, vaccination status was not consistently reported in the EHR [37]. Finally, we used SARS-CoV-2 RT-PCR-positive results to indicate COVID-19; however, PCR results do not reflect symptomatic disease, and this study cannot differentiate between symptomatic and asymptomatic outpatients. Similarly, this study does not include people who tested positive for SARS-CoV-2 by home-based testing alone and may not generalize to persons who did not access medical care.
In conclusion, this study demonstrates a significantly increased death from COVID-19 among PWH, and that accurate assessment of cognitive baseline is an important consideration when risk-stratifying both PWH and PWoH for death after SARS-CoV-2 infection. Additional studies in larger cohorts are needed to validate findings and further explore the contribution of baseline cognitive symptoms to COVID-19 disease risk, and the influence of preexisting cognitive concerns to the development of postacute COVID-19 cognitive syndromes.
Acknowledgements
We wish to acknowledge all colleagues who care for people with COVID-19 for their dedication to patient care. The authors also thank Kathleen A. McGinnis and co-creators of the VACS Index 2.0, Marco Loggia, and Eva Ratai for their support of the study, Diara Canton and Gretchen Westover for assistance in data organization, and PWH for their feedback on ongoing studies related to neurological outcomes in COVID-19.
Contributions: D.R.W. participated in study design and data acquisition, and lead analyses, data interpretation, and manuscript drafting. E.A.R. participated in data acquisition, executed data cleaning and analyses, prepared all tables, and participated in manuscript drafting. E.Y. performed automated data extraction from the electronic health record, executed analyses, created figures, and provided statistical guidance. A.N., C.M, and A.J. created the NLP-powered semiautomated annotation tool (NAT) used for acquisition of cognitive phenotype data and modified it for use in this study. H.A. participated in data acquisition. V.A.T. and G.K.R. provided clinical guidance, assisted with data interpretation and analyses. M.B.W. and S.D. conceptualized and supervised the creation of NAT. S.S.M. conceptualized and supervised the study, designed the cohort, led and participated in data acquisition, analysis, and interpretation, and participated in manuscript drafting. All authors read and participated in editing manuscript drafts and approved the final manuscript.
Funding: this work was supported by R01DA047088 and K23MH115812. S.S.M. is supported by the National Institute of Mental Health at the National Institutes of Health (grant number K23MH115812, R01MH131194, and R01DA047088), James S. McDonnell Foundation, and Rappaport Fellowship. D.R.W. is supported by the National Institute of Neurological Disorders and Stroke R25 Research Program (R25NS065743).
Conflicts of interest
H.A. is current employed at Biogen. G.K.R. has received trial support from Leonard Meron Biosciences, been a consultant to Teradyne Inc and SEED Inc, is a member of the DHHS OI guideline review panel. M.B.W. is the co-founder of Beacon Biosignals.
Supplementary Material
D.R.W., E.A.R., and E.Y. contributed equally.
Supplemental digital content is available for this article.
References
- 1.Izurieta HS, Graham DJ, Jiao Y, Hu M, Lu Y, Wu Y, et al. Natural history of coronavirus disease 2019: risk factors for hospitalizations and deaths among >26 million US Medicare Beneficiaries. J Infect Dis 2021; 223:945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qeadan F, VanSant-Webb E, Tingey B, Rogers TN, Brooks E, Mensah NA, et al. Racial disparities in COVID-19 outcomes exist despite comparable Elixhauser comorbidity indices between Blacks, Hispanics, Native Americans, and Whites. Sci Rep 2021; 11:8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magesh S, John D, Li WT, Li Y, Mattingly-App A, Jain S, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis. JAMA Netw Open 2021; 4:e2134147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jing Sun M, Zheng Q, Madhira V, Olex AL, Anzalone AJ, Vinson A, et al. for the National COVID Cohort Collaborative (N3C) Consortium. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med 2022; 182:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pellowski JA, Kalichman SC, Matthews KA, Adler N. A pandemic of the poor: social disadvantage and the U.S. HIV epidemic. Am Psychol 2013; 68:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett CL, Ogele E, Pettit NR, Bischof JJ, Meng T, Govindarajan P, et al. RECOVER Investigators. Multicenter study of outcomes among persons with HIV who presented to US Emergency Departments with suspected SARS-CoV-2. JAIDS J Acquir Immune Defic Syndr 2021; 88:406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Härter G, Spinner CD, Roider J, Bickel M, Krznaric I, Grunwald S, et al. COVID-19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection 2020; 48:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gervasoni C, Meraviglia P, Riva A, Giacomelli A, Oreni L, Minisci D, et al. Clinical features and outcomes of patients with human immunodeficiency virus with COVID-19. Clin Infect Dis 2020; 71:2276–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James MT, Pierce JL, Zamboni L, Wu M, Holtgrave DR, Gonzalez CJ, et al. COVID-19 outcomes among persons living with or without diagnosed HIV infection in New York state. JAMA Netw Open 2021; 4:e2037069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhaskaran K, Rentsch CT, MacKenna B, Schultze A, Mehrkar A, Bates CJ, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV 2021; 8:e24–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silvia Bertagnolio M, Thwin SS, Silva R, Diaz J. Clinical features and prognostic factors of COVID-19 in people living with HIV hospitalized with suspected or confirmed SARS-CoV-2 infection. WHO Global Clinical Platform for COVID-19 2021; (Data for public health response):20. [Google Scholar]
- 12.Ssentongo P, Heilbrunn ES, Ssentongo AE, Advani S, Chinchilli VM, Nunez JJ, Du P. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: a systematic review and meta-analysis. Scientific Rep 2021; 11:6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Q, Davis PB, Gurney ME, Xu R. COVID-19 and dementia: analyses of risk, disparity, and outcomes from electronic health records in the US. Alzheimers Dement 2021; 17:1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukerji SS, Das S, Alabsi H, Brenner LN, Jain A, Magdamo C, et al. Prolonged intubation in patients with prior cerebrovascular disease and COVID-19. Front Neurol 2021; 12:642912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin L, Al-Faraj A, Ayub N, Bravo P, Das S, Ferlini L, et al. Electroencephalographic abnormalities are common in COVID-19 and are associated with outcomes. Ann Neurol 2021; 89:872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amjad H, Roth DL, Sheehan OC, Lyketsos CG, Wolff JL, Samus QM. Underdiagnosis of dementia: an observational study of patterns in diagnosis and awareness in US older adults. J Gen Intern Med 2018; 33:1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin LH, Sundermann EE, Moore DJ. The current understanding of overlap between characteristics of HIV-associated neurocognitive disorders and Alzheimer's disease. J Neurovirol 2019; 25:661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saylor D. Neurologic complications of human immunodeficiency virus infection. Continuum (Minneap Minn) 2018; 24 (5 Neuroinfectious Disease):1397–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Francesco D, Underwood J, Post FA, Vera JH, Williams I, Boffito M, et al. POPPY study group. Defining cognitive impairment in people-living-with-HIV: the POPPY study. BMC Infect Dis 2016; 16:617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winston A, Spudich S. Cognitive disorders in people living with HIV. Lancet HIV 2020; 7:e504–e513. [DOI] [PubMed] [Google Scholar]
- 21.Mukerji SS, Petersen KJ, Pohl KM, Dastgheyb RM, Fox HS, Bilder RM, et al. Machine learning approaches to understand cognitive phenotypes in people with HIV. J Infect Dis 2023; 227: (Suppl 1): S48–S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noori A, Magdamo C, Liu X, Tyagi T, Li Z, Kondepudi A, et al. Development and evaluation of a natural language processing annotation tool to facilitate phenotyping of cognitive status in electronic health records: diagnostic study. J Med Internet Res 2022; 24:e40384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kathleen A. McGinnis ACJ, Richard D. Moore, Michael J. Silverberg, Keri N. Althoff, Maile Karris, Viviane D. Lima, et al. North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD)a of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) and Veterans Aging Cohort Study (VACS). Discrimination and calibration of the Veterans Aging Cohort Study Index 2.0 for predicting mortality among people with human immunodeficiency virus in North America. Clin Infect Dis 2021; 75:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King JT, Jr, Yoon JS, Rentsch CT, Tate JP, Park LS, Kidwai-Khan F, et al. Development and validation of a 30-day mortality index based on preexisting medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One 2020; 15:e0241825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naranbhai V, Chang CC, Beltran WFG, Miller TE, Astudillo MG, Villalba JA, et al. High seroprevalence of anti-SARS-CoV-2 antibodies in Chelsea, Massachusetts. J Infect Dis 2020; 222:1955–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun H, Jain A, Leone MJ, Alabsi HS, Brenner LN, Ye E, et al. CoVA: an acuity score for outpatient screening that predicts coronavirus disease 2019 prognosis. J Infect Dis 2021; 223:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy N. Forestmodel: forest plots from regression models. R Package Version 2020. p. 6. [Google Scholar]
- 28.Bacellar A, Pedreira BB, Costa G, Assis T. Frequency, associated features, and burden of neurological disorders in older adult inpatients in Brazil: a retrospective cross-sectional study. BMC Health Serv Res 2017; 17:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park J, Kwon YS, Kim HA, Kwon DH, Hwang J, Jang SH, et al. Clinical implications of neurological comorbidities and complications in ICU patients with COVID-19. J Clin Med 2021; 10:2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eskandar EN, Altschul DJ, de la Garza Ramos R, Cezayirli P, Unda SR, Benton J, et al. Neurologic syndromes predict higher in-hospital mortality in COVID-19. Neurology 2021; 96:e1527–e1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubin LH, Maki PM. HIV, depression, and cognitive impairment in the era of effective antiretroviral therapy. Curr HIV/AIDS Rep 2019; 16:82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao SC, Lai CC, Chen YH, Chen YC, Hung MJ, Liao SC. Prevalence, incidence and mortality of delirium in patients with COVID-19: a systematic review and meta-analysis. Age Ageing 2021; 50:1445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez Zapata C, Giacomello G, Spruth EJ, Middeldorp J, Gallaccio G, Dehlinger A, et al. Differential compartmentalization of myeloid cell phenotypes and responses towards the CNS in Alzheimer's disease. Nat Commun 2022; 13:7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu R, Mao T, Lu Q, Tianjiao Su T, Wang J. Myeloid dysregulation and therapeutic intervention in COVID-19. Semin Immunol 2021; 55:101524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon IH, Singh A, Folkerth RD, Mukerji SS. What can we still learn from brain autopsies in COVID-19?. Semin Neurol 2021; 742:135528. [DOI] [PubMed] [Google Scholar]
- 36.Lee GC, Restrepo MI, Harper N, Manoharan MS, Smith AM, Meunier JA, et al. Immunologic resilience and COVID-19 survival advantage. J Allergy Clin Immunol 2021; 148:1176–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massachusetts Department of Public Health. Daily COVID-19 Vaccine Report – Saturday, March 13, 2021. In: Commonwealth of Massachusetts; 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.