Abstract
Topic
We provide global estimates of the prevalence of corneal blindness and vision impairment in adults 40 years of age and older and examine the burden by age, sex, and geographic region from 1984 through 2020.
Clinical Relevance
Corneal opacities (COs) are among the top 5 causes of blindness worldwide, yet the global prevalence, regional differences, and risk factors are unclear.
Methods
Abstracted data from the published literature and surveys were obtained from the Global Burden of Disease Vision Loss Expert Group. We supplemented this by an independent systematic literature search of several databases. Studies that provided CO vision impairment data based on population-based surveys for those 40 years of age or older were included, for a total of 244. For each of the 4 outcomes of blindness and moderate to severe vision impairment (MSVI) caused by trachomatous and nontrachomatous CO (NTCO), time trends and differences in prevalence by region, age, and sex were evaluated using a Poisson log-linear model with a generalized estimating equation method. Age-standardized estimates of global prevalence of blindness and MSVI were calculated using the 2015 United Nations standard populations.
Results
The global prevalence of blindness resulting from NTCO in those 40 years and older was 0.081% (95% confidence interval [CI], 0.049%–0.315%); that of MSVI was 0.130% (95% CI, 0.087%–0.372%). A significant increase with age was found (prevalence rate ratio, 2.15; 95% CI, 1.99–2.32). Latin America and Europe showed the lowest rates, with 2- to 8-fold higher rates of blindness or MSVI in other regions. The global prevalence of blindness resulting from trachomatous CO in those 50 years and older was 0.0094% (95% CI, 0%-0.0693%); that from MSVI was 0.012% (95% CI, 0%–0.0761%). Blindness resulting from trachomatous CO and MSVI increased with age and female sex, and rates were significantly higher in the African regions. A decrease in trachomatous blindness rates over time was found (prevalence rate ratio, 0.91; 95% CI, 0.86–0.96).
Discussion
An estimated 5.5 million people worldwide are bilaterally blind or have MSVI resulting from CO, with an additional 6.2 million unilaterally blind. Blindness resulting from trachomatous CO is declining over time, likely because of the massive scaleup of the global trachoma elimination program and overall socioeconomic development.
Financial Disclosure(s)
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Keywords: Blindness, Corneal opacity, Global, Trachoma, Vision impairment
Corneal opacity (CO) is estimated to be the cause of 3.2% of all cases of blindness and 1.3% of all cases of moderate to severe vision impairment (MSVI)1 and is among the top 5 causes of blindness worldwide.2 The lifetime burden of corneal blindness is significant because it tends to affect younger people compared with other conditions such as cataract and glaucoma.3
Major causes of corneal opacification include trachoma, infectious keratitis, xerophthalmia, use of traditional eye medicines, and ocular trauma.4,5 Overall, in low- and middle-income countries, infectious keratitis is reported to be the most common problem.3 However, other conditions, such as trachoma or onchocerciasis, may dominate in other areas.
Global trends in vision loss resulting from CO remain largely unknown, but regions that have the least capacity to manage CO are hypothesized to bear the greatest burden. Poor rural communities have limited access to treatment and higher prevalence of communicable diseases such as trachoma.3 The epidemiologic features of vision loss resulting from CO have important implications for the global distribution of eye care provision, especially preventive public health interventions, treatments for infectious keratitis, and corneal transplantation. The purpose of this study was to use global data from published literature and surveys to provide global estimates of the prevalence of corneal blindness and vision impairment and to examine the burden by age, sex, and geographic region from 1984 through 2020.
Methods
Search Strategy and Selection Criteria
We included CO vision impairment data provided by the Global Burden of Disease Vision Loss Expert Group from 1984 through 2020. Their search strategy and data extraction methods have been well described.6 The data included the numbers of cases of blindness and MSVI resulting from NTCO and, if included, from trachomatous CO, and total numbers surveyed by age group and sex, the latter if available. The Vision Loss Expert Group provided 276 studies, of which we used 239, including Rapid Assessment of Avoidable Blindness (RAAB) surveys.7
The Institutional Review Boards approved the study and waived the requirement for informed consent because of the retrospective nature of the study. All research adhered to the tenets of the Declaration of Helsinki. Following their data extraction methods, we performed a parallel literature search to identify additional published studies containing data on vision loss resulting from CO that were published between 1990 and 2020 by searching the following online databases: PubMed, Embase, Web of Science, Scopus, and Cochrane (Appendix A, available at www.aaojournal.org). Studies that provided CO vision impairment data (blindness, MSVI, or both) based on population-based surveys for those 40 years of age or older were included. Studies that were based on school surveys, case reports, or key informants were not eligible. The complete inclusion and exclusion criteria and data sources are given in Appendix B (available at www.aaojournal.org). We included 5 additional studies based on these criteria (Appendix C, available at www.aaojournal.org).8, 9, 10, 11, 12
Definitions
Moderate to severe vision impairment was defined as presenting visual acuity of less than 6/18 but 3/60 or more in the better eye (presenting refers to “as the patient presents, which can be with or without corrective eyewear”13). Blindness was defined as presenting visual acuity of less than 3/60 in the better eye. For both outcomes, best-corrected visual acuity was used if presenting visual acuity was not reported. Unilateral blindness or MSVI was defined as presenting acuity in the worse eye of less than 6/18 but 3/60 or more, where the better-eye acuity was not blindness or MSVI.
Nontrachomatous CO (NTCO) was determined in each study by examination of the cornea by trained personnel and observation of an opacity deemed not resulting from trachoma and sufficiently dense and extensive to produce vision loss as severe as MSVI or blindness. If scarring and trichiasis or trichiasis surgery and other signs of trachoma such as Herbert’s pits were present and the survey was in a known endemic trachoma region, the CO was attributable to trachoma.
Data on blindness and MSVI resulting from CO were sparse, and we could not use regions as constructed previously.1 We required at least 3 surveys per region, which resulted in 7 regions: Europe and Central Asia, East Asia, Latin America and the Caribbean, North Africa and the Middle East, South Asia, Southeast Asia and Oceania, and Sub-Saharan Africa. No data on CO from North America were available. Figure 1 shows the makeup of each region.
Figure 1.
Map showing the countries within each region.
Forty-five studies only provided data on the total number of individuals with blindness or MSVI resulting from CO (no age or sex data) but did provide an age breakdown of the total study population. For each of these studies, we calculated age group-specific prevalence risk ratios derived from all other data in the region of the study using the youngest age group as the reference. Then, using the risk ratios, the observed total number of patients in the study, and the population studied in each age group, we were able to impute the fraction of number of patients we expected for each age group such that the sum would equal the total observed in the study.
Data Analysis
Duplicate data sources were cleaned up. These were largely RAAB surveys that were sent as raw data but that also were used in an article in the published literature. If the country or region, year of the survey, and population matched, the published article was excluded, and the RAAB data were used.
For each of the 4 outcomes of blindness and MSVI caused by NTCO and blindness and MSVI caused by trachomatous CO, we assessed whether a significant time trend existed in the prevalence using a univariable Poisson log-linear model. The analysis unit was each survey study. The total number of people surveyed was modeled as an offset in the Poisson model, and year of the survey was the primary exposure variable. Because the same country may have multiple surveys, a generalized estimating equation (GEE) method was applied with the Poisson regression model to account for potential correlation between surveys conducted in the same country. Univariable Poisson log-linear models with GEE also were used to assess whether prevalence of an outcome varied by risk variables including region, age group, and sex (where data on sex were available). The unit of analysis in the models for age group and sex was age group- and sex-specific survey results. A multivariable Poisson model with GEE was used to estimate adjusted associations with an outcome including risk variables that had P values of less than 0.05 from their univariable regression models with the outcome. The 95% confidence intervals (CIs) were calculated from the Poisson log-linear models with GEE. Too few trachoma surveys were from the individual regions to examine the effect of each region independently, so a comparison was made of the high-risk regions (North Africa and the Middle East and Sub-Saharan Africa) versus all others. All analyses were implemented in SAS software (SAS Institute).
A sensitivity analysis to evaluate whether there was a time trend for each outcome was conducted by restricting the analysis to countries that had data from at least 2 surveys conducted in different years. Age–standardized estimates of prevalence of blindness and MSVI resulting from CO were calculated using the United Nations standard population by region in 2015.14 Because most of the studies were from 2000 to 2020, we chose 2015 as the midpoint year. We applied the region- and age-specific rates to the standard population of the region to derive the expected numbers of patients in that region, which then were summed and divided by the estimate of the global population (minus the population of North America because no data were available for North America). We used the same approach for trachomatous CO but with the inclusion of the population of North America and Europe, presuming no individuals with CO resulting from trachoma were from these two regions. The 95% CIs around the estimated prevalences were calculated as follows: using the age- and region-specific lower bound of the 95% CI for the prevalence rate, we calculated the expected minimum number of cases globally; these cases divided by the global population as above became the lower 95% CI. Similarly, using the age- and region-specific upper bound of the 95% CI, we derived the expected maximum number of cases and thus the upper bound of the 95% CI. By using the age-specific rates within the region applied to the regional population distribution, we account for potential differences in the sampled population age structure of the studies within the region.
Role of the Funding Sources
The National Eye Institute and the benefactor of the El Maghraby Chair had no role in the study design, data collection, data analyses, data interpretation, or the writing of this report. The corresponding author had full access to the data in the study and final responsibility for the decision to submit the manuscript for publication.
Results
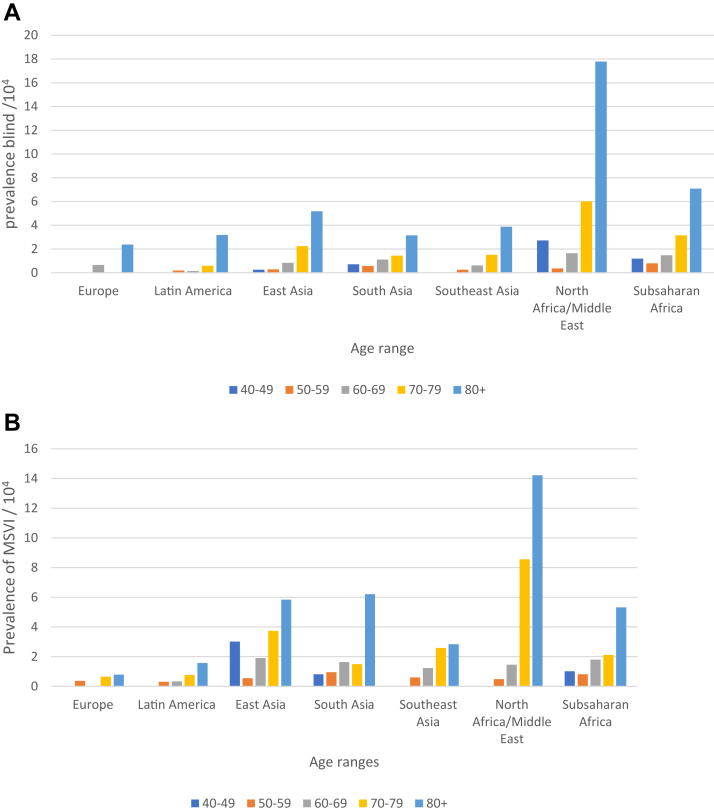
A total of 244 unique data sets, representing 73 countries and close to 2 million people, were used for analyses (Table 1). The age-specific prevalence of blindness resulting from NTCO in each region is shown in Figure 2. An increase with age was found in each region, especially in the oldest groups. Few patients were 90 years of age or older, so they were combined with those 80 to 89 years of age.
Table 1.
Regions Included in the Study and Total Populations Studied in Each Region
| Region | No. of Studies | Population Included |
|---|---|---|
| North Africa/Middle East | 18 | 42 435 |
| Sub-Saharan Africa | 45 | 138 958 |
| East Asia | 31 | 1 286 378 |
| Southeast Asia/Oceania | 75 | 187 723 |
| South Asia | 40 | 186 605 |
| Latin America, Caribbean | 26 | 95 609 |
| Europe/Central Asia | 9 | 22 193 |
| Total | 244 | 1 959 901 |
Figure 2.
Bar graphs showing age-specific prevalences of (A) blindness and (B) moderate to severe vision impairment (MSVI) resulting from nontrachomatous corneal opacity (rates are per 10 000 people).
No statistically significant increase or decrease over time was found in prevalence of blindness or MSVI resulting from NTCO (data not shown). Women tended to have slightly less blindness resulting from NTCO and slightly more MSVI resulting from NTCO compared with men, but the differences were not statistically significant, adjusted by age (Table 2).
Table 2.
Prevalence Rate Ratios for Age and Sex with Blindness and Moderate to Severe Visual Impairment Resulting from Nontrachomatous Corneal Opacity
| Variable | Blindness |
Moderate to Severe Visual Impairment |
||||
|---|---|---|---|---|---|---|
| Rate Ratio Estimate | 95% Confidence Interval | P Value | Rate Ratio Estimate | 95% Confidence Interval | P Value | |
| Increase with each 10 yrs in age | 2.15 | 1.99–2.32 | < 0.0001 | 1.86 | 1.73–2.00 | < 0.0001 |
| Female vs. male sex | 0.92 | 0.77–1.098 | 0.33 | 1.04 | 0.83–1.31 | 0.71 |
When adjusted by age using as a population standard the United Nations population estimates for 2015, the global prevalence of blindness resulting from NTCO in those 40 years of age and older was 0.081% (95% CI, 0.049%–0.315%). The age-adjusted global prevalence of NTCO MSVI was 0.130% (95% CI, 0.087%–0.372%).
Significant differences by region were found, with the highest age-adjusted rates of blindness resulting from NTCO in the Sub-Saharan Africa region and the North Africa and in Middle East region (0.14% and 0.27%, respectively) and the lowest in Europe and in Latin America and the Caribbean (0.035% and 0.030%, respectively). Except for Europe, all other regions showed higher blindness rates compared with Latin America, although the Southeast Asia rate was not statistically significantly different (Table 3). For MSVI resulting from NTCO, Latin America and Europe showed the lowest rates (0.032% and 0.026%, respectively), and all other regions showed significantly higher rates compared with Latin America (Table 3).
Table 3.
Generalized Estimating Equation Models of Age-Adjusted Prevalence Rate Ratios Comparing Blindness and Moderate to Severe Visual Impairment Resulting from Nontrachomatous Corneal Opacity in Each Region with the Rate in Latin America
| Region | Blindness |
Moderate to Severe Visual Impairment |
||||
|---|---|---|---|---|---|---|
| Rate Ratio Estimate | 95% Confidence Interval | P Value | Rate Ratio Estimate | 95% Confidence Interval | P Value | |
| Europe | 0.80 | 0.27–2.38 | 0.69 | 0.67 | 0.31–1.46 | 0.31 |
| East Asia | 2.25 | 1.38–3.68 | 0.001 | 3.48 | 1.43–8.47 | 0.006 |
| North Africa and Middle East | 6.89 | 2.81–16.85 | < 0.0001 | 8.21 | 2.89–23.37 | < 0.0001 |
| South Asia | 2.44 | 1.04–5.73 | 0.04 | 4.65 | 1.83–11.83 | 0.001 |
| Southeast Asia | 1.70 | 0.81–3.56 | 0.157 | 4.09 | 1.76–9.54 | 0.001 |
| Sub-Saharan Africa | 4.05 | 2.24–7.32 | < 0.0001 | 3.72 | 2.02–6.86 | < 0.0001 |
Rates of blindness and MSVI resulting from trachoma increased with increasing age and were significantly higher in women, adjusting for age (Table 4). When combining Sub-Saharan Africa with North Africa and the Middle East, a 14-fold increased risk of blindness and an 8-fold increased risk of MSVI resulting from trachomatous CO were found compared with all the other regions combined. Evidence was found for a decrease in rate of blindness resulting from trachoma over time (rate ratio estimate for increasing date of the survey, 0.91; 95% CI, 0.86–0.96). This decrease was corroborated by the sensitivity analyses of restricting analyses to countries with 2 or more surveys over time. The rate of MSVI resulting from trachomatous CO also tended to decrease over time, but the decrease was not statistically significant (rate ratio estimate for increasing date of survey, 0.94, 95% CI, 0.82–1.09).
Table 4.
Generalized Estimating Equation Models of Age, Sex, and Region Rate Ratios of Blindness and Moderate to Severe Visual Impairment Resulting from Trachomatous Corneal Opacity
| Variable | Blindness |
Moderate to Severe Visual Impairment |
||||
|---|---|---|---|---|---|---|
| Rate Ratio Estimate | 95% Confidence Interval | P Value | Rate Ratio Estimate | 95% Confidence Interval | P Value | |
| Increase with each 10 yrs in age | 2.39 | 1.83–3.12 | < 0.0001 | 1.88 | 1.63–2.16 | < 0.0001 |
| Female vs. male sex | 3.52 | 2.22–5.56 | < 0.0001 | 2.47 | 1.54–3.94 | 0.0002 |
| Sub-Saharan Africa plus North Africa and Middle East vs all other regions | 14.37 | 6.29–32.79 | < 0.0001 | 8.48 | 3.54–20.33 | < 0.0001 |
Blindness and MSVI resulting from trachomatous CO prevalence rates increased with age within the 6 regions that have some data on trachomatous vision loss (Fig 3). Europe was excluded because no cases of trachomatous CO were reported. The prevalence of trachomatous corneal blindness was greatest in Sub-Saharan Africa and in the oldest age groups in South and Southeast Asia. Trachomatous CO MSVI rates were greatest in the North Africa and Middle East region and in the oldest age groups in South and Southeast Asia. Rates were calculated for those 50 years of age and older because only 1 region had data for those 40 to 49 years of age. When adjusted by age using as a population standard the United Nations population estimates for 2015 and including North America and Europe with 0 cases, the global prevalence of blindness resulting from trachomatous CO in those 50 years of age and older was 0.0094% (95% CI, 0%–0.0693%). The age-adjusted global prevalence of MSVI was 0.012% (95% CI, 0%–0.0761%).
Figure 3.
Bar graphs showing age-specific prevalences of (A) blindness and (B) moderate to severe vision impairment (MSVI) resulting from trachomatous corneal opacity (CO; rates are per 10 000 people). E = East; N = North; S = South; SE = Southeast.
Most surveys did not include unilateral blindness resulting from CO, so the estimate of the ratio of unilateral to bilateral blindness is based on data from 17 studies (Appendix D, available at www.aaojournal.org). The average ratio of unilateral to bilateral blindness was 2.96, suggesting that the burden of blindness in at least 1 eye resulting from CO is much greater than the bilateral estimates. For trachoma, the ratio was 1.38 but with the caveat that only 3 studies provided data on unilateral and bilateral blindness.
Discussion
Corneal opacity is considered one of the top 5 causes of global blindness and MSVI.1 We report a global age-standardized rate of blindness among those 40 years of age and older resulting from NTCO of 0.08% and rate of MSVI of 0.13%; an estimated 2.096 million people older than 40 years are blind, and 3.372 million people have MSVI resulting from NTCO. A 2017 meta-analysis with data to 2014 estimated a rate of CO blindness in those older than 50 years of 0.06% and a rate of MSVI of 0.12%.1 In addition, we found no evidence of a change in blindness or MSVI resulting from NTCO over time, which differed from the 2017 meta-analysis.1 The differences may result from our restricting the analyses to adults 40 years of age and older because few articles met criteria on children. We had data to 2020, which may have changed the previous finding of a decrease over time. We also performed a sensitivity analysis using only data from countries with 2 or more surveys over time and corroborated our findings, suggesting no significant change in CO vision loss over time.
We found a strong increase in risk of CO blindness and MSVI with age, which others have also found,1,15,16 with a striking increase in those 80 years of age and older. This may be a cohort effect, because those who are 80 years or older lived their young lives in an environment where trachoma, vitamin A deficiency, and onchocerciasis were more prevalent and may have experienced cataract surgery at a time when complications leading to corneal edema were more common. In addition, they had little to no access to eye care services to prevent or treat corneal blindness. As conditions improved, succeeding generations benefited. Also, the denominators for those 80 years of age and older are smaller than in other age groups, and cases of blindness or MSVI have a bigger impact on the rates. This is likely the case in the rate for North Africa and the Middle East, where all the surveys yielded 39 patients with a denominator of only 2193 people total in the studies.
No significant difference by gender was found in blindness or MSVI resulting from NTCO. Studies report mixed findings, some with an excess of corneal disease in women and others in men.15, 16, 17 Flaxman et al1 found an excess risk of CO blindness in men. Others, studying CO in all ages, found no difference by sex.18 These differences likely relate to disparate local population structures and gendered environmental exposures. We found a slight excess risk of NTCO blindness in men with a rate ratio of 1.09, but the increase was not statistically significant.
The risks of blindness and MSVI resulting from NTCO were not equal across regions, with lower rates in Europe and Latin America compared with Asia and the North Africa and the Middle East region. It is noteworthy that blindness and MSVI prevalence rate ratios were 4- to 8-fold higher in these regions compared with Latin America. This disparity may result in part from diseases that affect the cornea that are rarer elsewhere in the world, like trachoma, onchocerciasis, and vitamin A deficiency. It also may represent lack of access to care for corneal diseases. A recent survey estimated that 53% of the world’s population did not have access to corneal transplantation services, with an estimated 1 cornea available for 70 people who need it.19 Supply and demand for services reflect the imbalance in Africa and in East Asia and are reflected in blindness and MSVI rates resulting from CO.19
Blindness and MSVI resulting from trachoma have an even greater disparity comparing the Africa and Middle East regions with all the others, with a more than 8- to 14-fold increase in prevalence rate ratios. This is predictable because most countries with trachoma are now confined to Africa,20 and most affected individuals live in countries where trachoma is still endemic. Cases of trachomatous trichiasis (scarring on the inside of the upper eyelid that causes the eyelid to retract and the eyelashes to turn inwards) leading to CO and vision loss are the result of exposure to active trachoma long ago in childhood, which is why corneal blindness resulting from trachoma still occurs in some regions where trachoma has largely been eliminated.
Blindness resulting from trachomatous CO is declining significantly over time, a welcome trend. The rate of MSVI is also decreasing, although the change is not statistically significant. This decline coincides with socioeconomic development in much of the world, as well as massive scale-up in the global trachoma program.21 The latter affects vision loss resulting from trachomatous CO in 2 ways: first, by increasing access to surgery that reverses trichiasis, which prevents the progression to blindness, and second, by reducing the burden of active trachoma infection, so that significantly fewer children and young adults are exposed to repeated bouts of infection that lead to scarring sufficient to cause trichiasis. This is consistent with some of the age-specific patterns of trachomatous CO blindness and MSVI observed, which in the Asian regions are almost exclusively found in the oldest age groups now, with very few patients younger than 70 years.
The increase with age and the preponderance of trachomatous CO blindness and MSVI in women is consistent with the epidemiologic features of trachoma.22,23 Trichiasis is seen largely in older individuals, and resultant CO is also more prevalent in the elderly.22,23 Women experience 2 to 4 times the rate of trichiasis compared with men,23, 24, 25, 26 and we found a rate ratio of 2.5 to 3.5 for MSVI and blindness, respectively, resulting from trachomatous CO in women globally. This gender inequity is likely to persist even in the presence of surgical programs that cover men and women equally.25,27 The inequity speaks to the need to ensure high coverage of services to women by reducing barriers that are specific to them.28,29
This study has several limitations that must be acknowledged. A major limitation is the relatively scarce regional data on blindness and vision impairment resulting from CO by age and sex. To assure some stability of regional estimates, we created larger regions than were used previously in estimating overall blindness and vision loss. This assumes that countries within regions are more homogeneous than countries between regions, which impacts our comparison between regions and may blunt differences. To the extent that a few countries may represent a region also may bias the regional estimates.
An extreme example is the absence of data from North America. For NTCO, we assumed the addition of data from North America would not substantially affect the global prevalence estimate of blindness and MSVI. If anything, we may have overestimated the prevalence rates somewhat because the rates are liable to be at least as low as the estimate for Europe.
Vision loss data from trachomatous CO were even more sparse, rendering a regional comparison of rates unreasonable. The trachoma endemic countries, largely in Africa, continue to conduct large population-based studies periodically to assess progress toward elimination of active trachoma and trachomatous trichiasis but do not include visual acuity and an examination for trachomatous CO. The additional data would be a burden to collect but would add useful information about the decline of vision loss resulting from trachoma in the most affected regions.
Another limitation is the use of studies in which the age distributions of the patients with CO blindness and MSVI were unknown. Rather than discard the studies, the assumption was made that the age distributions of patients would result in rate ratios similar to the rate ratios by age in the region as a whole, and we used the rate ratios to assign the patients to 10-year age categories, where the sum of patients was constrained to equal the total number of patients observed. This approach reinforced the existing rate ratios within the region, which created more homogeneity than might have been the case. However, only 18% of the data sources used had this issue, and most were publications with small numbers of patients, so we believe that the impact was minimal.
The choice of 2015 global and regional populations to standardize our rates was deliberate. We did not find evidence for a change over time in NTCO blindness or MSVI, so we might have used any year, but we were concerned that most of our data came from 2000 onward. Only 5 published studies came from the 1980s, and 23 came from the 1990s. We chose 2015 as the midpoint of the time between 2000 and 2020. The population globally and in most regions not only grew over this period, but also aged, so the increasing rate of blindness and MSVI by age, coupled with a proportionally higher population in group older than 65 years, might have resulted in a higher overall rate had we used global and regional population estimates from a later time point. Conversely, using a population from an earlier time point also could have resulted in a lower overall rate.
The determination of blindness and MSVI resulting from CO relies on trained and standardized observers and likely is prone to some misclassification. In RAAB surveys, if an examiner sees a cataract and a CO in an eye with moderate vision impairment, the rule is to assign the primary cause of vision loss to cataract because it is amenable to having vision restored. This underestimates corneal disease as a cause of vision loss. Also, concern exists for separating trachomatous from nontrachomatous CO, especially in trachoma-endemic areas. A study in The Gambia found a much higher incidence of trachomatous scarring in those older than 45 years with eyes whose disease was called CO, but not trachoma related, than in eyes with other causes of vision loss.30 The reliability of the surveys is likely highly variable as well, depending on the examiner and the representativeness of the surveyed population of the eligible population. The response rate of the eligible population to the survey overall was good, the lowest being one study with a 67% response rate, and most surveys reported a more than 90% response rate.
The strength of this study is in the large number of databases that were used to determine the prevalence rate of blindness and MSVI resulting from CO and to point out the burden of unilateral vision loss resulting from CO as well. Because this is a relatively rare outcome in individual population-based studies, the large database provides stability for the findings on age, sex, and regional differences.
Conclusions
Evidence for global female excess of trachomatous CO blindness and vision loss is solid, which speaks to the need to ensure attention to gender equity and high coverage in delivery of trachoma programs. New evidence was found for a decline in blindness and MSVI resulting from trachomatous CO over time, although not for other causes of CO. The global burden of NTCO blindness is estimated at 0.08% of those 40 years of age and older and for MSVI, is estimated at 0.13%. This translates to an estimated 5.467 million people 40 years of age and older with bilateral MSVI and blindness resulting from NTCO. An additional estimated 6.2 million people have unilateral blindness resulting from NTCO. The burden falls disproportionally on regions that are least equipped to manage corneal diseases and should be a priority for training and service provision programs.
Manuscript no. OPHTHA-D-22-02144
Footnotes
Supplemental material available at www.aaojournal.org.
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Supported by the El Maghraby Chair in Preventive Ophthalmology (S.W.), and the National Eye Institute, National Institutes of Health, Bethesda, Maryland (grant no.: P30).
HUMAN SUBJECTS: No human subjects were included in this study. The Institutional Review Boards approved the study and waived the requirement for informed consent because of the retrospective nature of the study. All research adhered to the tenets of the Declaration of Helsinki.
No animal subjects were included in this study.
See Appendix E (available at www.aaojournal.org) for complete dataset.
Author Contributions:
Conception and design: Wang, Kong, Wolle, Mariotti, Bourne, West
Analysis and interpretation: Wang, Kong, Wolle, Gasquet, Mariotti, Bourne, Taylor, Resnikoff, West
Data collection: Wang, Kong, Gasquet, Ssekasanvu, Bourne, West
Obtained funding: N/A
Overall responsibility: Wang, Kong, Wolle, Gasquet, Ssekasanvu, Mariotti, Bourne, Taylor, Resnikoff, West
Supplementary Data
References
- 1.Flaxman S.R., Bourne R.R.A., Resnikoff S., et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization World health statistics. https://www.who.int/gho/publications/world_health_statistics/2015/en/ Available at: Accessed Februray 20, 2019.
- 3.Ung L., Acharya N.R., Agarwal T., et al. Infectious corneal ulceration: a proposal for neglected tropical disease status. Bull World Health Organ. 2019;97(12):854–856. doi: 10.2471/BLT.19.232660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeng B.H., Ahmad S. In pursuit of the elimination of corneal blindness: is establishing eye banks and training surgeons enough? Ophthalmology. 2021;128(6):813–815. doi: 10.1016/j.ophtha.2020.06.042. [published correction appears in Ophthalmology. 2021;128(8):1245] [DOI] [PubMed] [Google Scholar]
- 5.Whitcher J.P., Srinivasan M., Upadhyay M.P. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79(3):214–221. [PMC free article] [PubMed] [Google Scholar]
- 6.Global Burden of Disease 2019 Blindness and Vision Impairment Collaborators. Vision Loss Expert Group of the Global Burden of Disease Study Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e130–e143. doi: 10.1016/S2214-109X(20)30425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mactaggart I., Limburg H., Bastawrous A., et al. Rapid assessment of avoidable blindness: looking back, looking forward. Br J Ophthalmol. 2019;103(11):1549–1552. doi: 10.1136/bjophthalmol-2019-314015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He Y., Nie A., Pei J., et al. Prevalence and causes of visual impairment in population more than 50 years old: the Shaanxi Eye Study. Medicine (Baltimore) 2020;99(20) doi: 10.1097/MD.0000000000020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo C., Wang Z., He P., et al. Prevalence, causes and social factors of visual impairment among Chinese adults: based on a national survey. Int J Environ Res Public Health. 2017;14(9):1034. doi: 10.3390/ijerph14091034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tafida A., Kyari F., Abdull M.M., et al. Poverty and blindness in Nigeria: results from the national survey of blindness and visual impairment. Ophthalmic Epidemiol. 2015;22(5):333–341. doi: 10.3109/09286586.2015.1077259. [DOI] [PubMed] [Google Scholar]
- 11.AlSawahli H., McCormick I., Mpyet C.D., et al. Population-based rapid assessment of avoidable blindness survey in Sohag governorate in Egypt. BMJ Open. 2020;10(10) doi: 10.1136/bmjopen-2019-036337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melese M., Alemayehu W., Bayu S., et al. Low vision and blindness in adults in Gurage Zone, central Ethiopia. Br J Ophthalmol. 2003;87(6):677–680. doi: 10.1136/bjo.87.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Global Burden of Disease 2019 Blindness and Vision Impairment Collaborators. Vision Loss Expert Group of the Global Burden of Disease Study Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e144–e160. doi: 10.1016/S2214-109X(20)30489-7. [published correction appears in Lancet Glob Health. 2021;9(4):e408] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Population Division, Department of Economic and Social Affairs, United Nations. World population prospects 2019. Custom data acquired via website: https://population.un.org/wpp/Download/Standard/MostUsed/ Accessed June 1, 2022.
- 15.Gupta N., Vashist P., Tandon R., et al. Prevalence of corneal diseases in the rural Indian population: the Corneal Opacity Rural Epidemiological (CORE) study. Br J Ophthalmol. 2015;99(2):147–152. doi: 10.1136/bjophthalmol-2014-305945. [DOI] [PubMed] [Google Scholar]
- 16.Hashemi H., Pakzad R., Yekta A., Khabazkhoob M. The prevalence of corneal opacity in rural areas in Iran: a population-based study. Ophthalmic Epidemiol. 2018;25(1):21–26. doi: 10.1080/09286586.2017.1337912. [DOI] [PubMed] [Google Scholar]
- 17.Hashemi H., Pakzad R., Aghamirsalim M.R., et al. Age and sex standardized prevalence of corneal opacity and its determinants; Tehran Geriatric Eye Study (TGES) Iran J Public Health. 2022;51(3):643–651. doi: 10.18502/ijph.v51i3.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheng X.L., Li H.P., Liu Q.X., et al. Prevalence and associated factors of corneal blindness in Ningxia in northwest China. Int J Ophthalmol. 2014;7(3):557–562. doi: 10.3980/j.issn.2222-3959.2014.03.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gain P., Jullienne R., He Z., et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134(2):167–173. doi: 10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- 20.Trachomadata.org. Map: trachoma atlas. https://atlas.trachomadata.org/ Available at: Accessed July 25, 2022.
- 21.Renneker K.K., Abdala M., Addy J., et al. Global progress toward the elimination of active trachoma: an analysis of 38 countries. Lancet Glob Health. 2022;10(4):e491–e500. doi: 10.1016/S2214-109X(22)00050-X. [DOI] [PubMed] [Google Scholar]
- 22.Taylor H.R., Burton M.J., Haddad D., et al. Trachoma. Lancet. 2014;384(9960):2142–2152. doi: 10.1016/S0140-6736(13)62182-0. [DOI] [PubMed] [Google Scholar]
- 23.West S.K., Munoz B., Turner V.M., et al. The epidemiology of trachoma in central Tanzania. Int J Epidemiol. 1991;20(4):1088–1092. doi: 10.1093/ije/20.4.1088. [DOI] [PubMed] [Google Scholar]
- 24.Courtright P., Sheppard J., Schachter J., et al. Trachoma and blindness in the Nile Delta: current patterns and projections for the future in the rural Egyptian population. Br J Ophthalmol. 1989;73(7):536–540. doi: 10.1136/bjo.73.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Courtright P., West S.K. Contribution of sex-linked biology and gender roles to disparities with trachoma. Emerg Infect Dis. 2004;10(11):2012–2016. doi: 10.3201/eid1011.040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cromwell E.A., Courtright P., King J.D., et al. The excess burden of trachomatous trichiasis in women: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2009;103(10):985–992. doi: 10.1016/j.trstmh.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 27.West S., Nguyen M.P., Mkocha H., et al. Gender equity and trichiasis surgery in the Vietnam and Tanzania national trachoma control programmes. Br J Ophthalmol. 2004;88(11):1368–1371. doi: 10.1136/bjo.2004.041657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bickley R.J., Mkocha H., Munoz B., West S. Identifying patient perceived barriers to trichiasis surgery in Kongwa District, Tanzania. PLoS Negl Trop Dis. 2017;11(1) doi: 10.1371/journal.pntd.0005211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajak S.N., Habtamu E., Weiss H.A., et al. Why do people not attend for treatment for trachomatous trichiasis in Ethiopia? A study of barriers to surgery. PLoS Negl Trop Dis. 2012;6(8) doi: 10.1371/journal.pntd.0001766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowman R.J., Faal H., Dolin P., Johnson G.J. Non-trachomatous corneal opacities in the Gambia—aetiology and visual burden. Eye (Lond) 2002;16(1):27–32. doi: 10.1038/sj.eye.6700027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.