ABSTRACT
Background and Aims:
Following induction of general anaesthesia, direct laryngoscopy and endotracheal intubation usually cause tachycardia and hypertension due to increased sympathetic activity. This response is generally exaggerated in hypertensive patients. This study aimed to evaluate the effectiveness of preoperative lignocaine nebulisation in attenuating the pressor response to laryngoscopy and endotracheal intubation in patients with severe preeclampsia undergoing caesarean delivery.
Methods:
After ethical approval, we conducted this randomised, double-blind study, which included 110 patients with severe preeclampsia who underwent caesarean delivery under general anaesthesia. These patients were randomly allocated into two groups to receive either preoperative nebulisation of lignocaine 2% in a dose of 4.5 mg/kg (not exceeding 400 mg) in the lignocaine group or nebulisation of an equivalent volume of 0.9% NaCl in the saline group. The primary objective was the systolic blood pressure after tracheal intubation. The secondary objectives included heart rate, maternal serum cortisol and blood glucose levels, grade of cough during emergence, postoperative sore throat and hoarseness of voice, neonatal Apgar score and umbilical blood gas.
Results:
The systolic blood pressure was significantly lower in the lignocaine group compared to the saline group at 1, 3 and 5 min after endotracheal intubation and after delivery of the foetus (P = 0.001, 0.003, 0.002 and 0.019, respectively). Similarly, the heart rate was significantly lower in the lignocaine group versus saline group at 1 and 3 min after endotracheal intubation (P = 0.041 and 0.042, respectively).
Conclusion:
Preoperative lignocaine nebulisation in a dose of 4.5 mg/kg effectively attenuated the pressor response to laryngoscopy and endotracheal intubation in patients with severe preeclampsia undergoing caesarean delivery.
Keywords: Laryngoscopy, lignocaine, preeclampsia, nebulisation, pressor response, tracheal intubation, caesarean section, general anaesthesia
INTRODUCTION
Preeclampsia is associated with higher risks of maternal and perinatal morbidity and mortality.[1] Although neuraxial anaesthesia is an appropriate option for caesarean delivery, it may be unsuitable for certain patients, such as those with coagulopathy or thrombocytopenia, or in case of patient refusal. In these situations, general anaesthesia is unavoidable.
Following induction of general anaesthesia, direct laryngoscopy and endotracheal intubation usually causes tachycardia and hypertension due to increased sympathetic activity.[2] This pressor response is usually exaggerated in patients with preeclampsia.[3] Therefore, it increases the risks of maternal intracranial haemorrhage, cerebral oedema, pulmonary oedema and heart failure.[3,4] Ameliorating these haemodynamic changes is necessary to ensure maternal and neonatal well-being. Therefore, various drugs are used, including opioids, labetalol, magnesium sulphate, nitroglycerine, hydralazine, nifedipine and lignocaine, with varying degrees of effectiveness.[5,6] Nebulised lignocaine has been used before to anaesthetise the upper airway in different dosages and concentrations with variable degrees of effectiveness.[7-9] This study aimed to evaluate the effectiveness of preoperative lignocaine nebulisation in attenuating the pressor response to laryngoscopy and endotracheal intubation in patients with severe preeclampsia undergoing caesarean delivery.
METHODS
This randomised, double-blind controlled trial was conducted between February 2021 and January 2022 after obtaining approval from the Institutional Review Board (Faculty of Medicine, Assiut University, Egypt) in June 2020 (IRB 17101090). A written informed consent was signed by each patient and her husband after they were explained the technique used in the study and informed for publication of the study for academic purposes. This research was registered in clinicalTrials.gov in June 2020 (ID NCT04441073), and was performed following the ethical principles for medical research involving human subjects of the 1964 Declaration of Helsinki and all of its subsequent revisions (revised 2013).
Parturients with severe preeclampsia who underwent caesarean delivery under general anaesthesia were included in this study. A patient with preeclampsia was considered to have severe features if the systolic blood pressure (SBP) was ≥160 mmHg or the diastolic blood pressure (DBP) was ≥110 mmHg, with readings obtained on two occasions at least 4 h apart (unless antihypertensive medications had been started), or if she had visual disturbances or new-onset headache unresponsive to medications.[10] Exclusion criteria included patient refusal, body mass index (BMI) ≥40 kg/m2, cardiac diseases, diabetic mellitus, serum creatinine ≥2 mg/dl, elevated liver enzymes (three times or more of the normal values) or known foetal anomalies. Also, any patient undergoing emergency caesarean section was excluded from the study.
Using computer-generated random numbers, patients who met the inclusion criteria were randomised into two groups (55 patients in each). Patients in the lignocaine group received nebulisation of lignocaine 2% in a dose of 4.5 mg/kg (not exceeding 400 mg), while patients in the saline group received nebulisation of an equivalent volume of 0.9% NaCl. Allocations of patients in groups of the study were kept in serially numbered sealed opaque envelopes. Before nebulisation, the corresponding envelope was opened by a trained nurse, who was also responsible for preparing and administering the nebulised drug to the patient. This nurse did not further participate in any assessment of the patient. Both the patient and the main anaesthesiologist were blinded to the groups.
All patients in this study were managed according to the standard protocol of preeclampsia used in our institution, in which intravenous infusion of magnesium sulphate was given for seizure prophylaxis, initially in a loading dose of 4 g over 20–30 min followed by 1 g/h as a maintenance. Nifedipine was used as the first line of treatment of hypertension.
Preoperatively, routine airway assessment was done by the main anaesthesiologist using the modified Mallampati classification,[11] thyromental distance, mouth opening and range of neck mobility. In the holding area, a blood sample was taken for baseline serum cortisol and blood glucose levels, and baseline SBP and heart rate were measured and recorded before starting nebulisation with either lignocaine or 0.9% NaCl according to patient allocation in the study groups.
Upon arrival at the operating theatre, routine monitoring devices were connected to the patient, including non-invasive blood pressure, electrocardiography, pulse oximetry and temperature probe. All patients were positioned supine with left lateral tilt. After adequate pre-oxygenation for 3–5 min, rapid sequence induction was achieved with intravenous propofol (2 mg/kg) and succinylcholine (1.5 mg/kg) followed by direct laryngoscopy to achieve endotracheal intubation with a cuffed endotracheal tube, with assessment of the grade of direct laryngoscopic view using Cormack–Lehane classification.[12] After that, the patient was connected to a closed circuit with ventilatory parameters adjusted to maintain end-tidal carbon dioxide (EtCO2) of 30–35 mmHg. The study was discontinued for the patients who required more than one attempt of laryngoscopy or more than 40 s to achieve endotracheal intubation. Anaesthesia was maintained with isoflurane (0.8%–1.2%) in oxygen and air (50%:50%) until the time of delivery. Immediately after delivery, a bolus of oxytocin 5 IU was given intravenously followed by an infusion of 20 IU in 500 ml of 0.9% NaCl solution. Also, intravenous fentanyl (100 μg) was given. Neuromuscular blockade was maintained with boluses intravenous of cisatracurium.
Intraoperative hypotension (SBP <100 mmHg) was treated initially by increasing the rate of the infused crystalloid solutions. In comparison, bolus doses of intravenous ephedrine (6 mg each) were given whenever the SBP was <90 mmHg. If heart rate was <50 beats/minute, intravenous atropine (0.5 mg) was given. Intravenous paracetamol (1 g) was given as analgesia.
As a part of this study, another blood sample was taken 10 min after tracheal intubation to measure maternal serum cortisol and blood glucose levels. The neonatal well-being was assessed using Apgar score and an umbilical blood gas sample.
At the end of surgery, isoflurane was discontinued, and neuromuscular blockade was antagonised with intravenous neostigmine (0.05 mg/kg) and atropine (0.02 mg/kg). Tracheal extubation was done once the patient was able to follow a verbal command to open her eyes.
During emergence, which was defined as the duration from the end of surgery to 5 min after extubation, cough as a sudden contraction of the abdomen was assessed by the following cough grading system: grade 0, no cough; grade 1, single cough with mild severity; grade 2, moderately severe cough for less than 5 s; grade 3, severe cough or bucking for more than 5 s.[13]
Patients were kept in the post-anaesthesia care unit (PACU) for at least 2 h. The incidence of postoperative sore throat (POST) and hoarseness of voice was assessed 1 and 2 h after admission to the PACU by asking the patients direct questions. Severity of POST was graded according to the answers of the patients using a 4-point score as follows: grade 0, no POST; grade 1, minimal POST; grade 2, moderate POST; grade 3, severe POST.[14] Severity of hoarseness of voice was graded using a 4-point score as follows: grade 0, no hoarseness; grade 1, hoarseness at the time of interview but noted only by the patient; grade 2, mild, readily apparent hoarseness; grade 3, severe, readily apparent hoarseness.[14]
Swallowing and gag reflex were assessed before shifting the patient from the PACU to the ward. The gag reflex was assessed by asking the patient to open her mouth to gently touch the posterior pharynx with a cotton applicator, where elevation of the uvula indicates proper gag reflex.
The primary outcome was the SBP after tracheal intubation. The secondary outcomes included heart rate, maternal serum cortisol and blood glucose levels, grade of cough during emergence from general anaesthesia, neonatal Apgar score, umbilical blood gas, POST and postoperative hoarseness of voice.
Using G*Power 3 software (University of Kiel, Germany) for calculation of the sample size, based on the mean ± standard deviation (SD) of SBP in a previous study,[15] a minimum of 44 patients in each group were needed to detect an effect size of 0.5 in the mean level of pressor response (SBP) with an α-error of 0.05 and a power of 80% on a two-tailed test. The sample was increased to 55 patients in each group to compensate for dropouts.
IBM-Statistical Package for the Social Sciences (SPSS) Version 24 (International Business Machines, Armonk, New York, United States). was used for data analysis. Data were expressed as mean ± SD, median and interquartile range, or number. Chi-square/Fisher’s exact/Monte Carlo exact test was used to compare the categorical data as appropriate. Student’s t-test was used to compare the means of normally distributed variables. P value was considered significant if <0.05.
RESULTS
Out of 144 patients with severe preeclampsia who were screened, 110 were ultimately included in this study and randomly allocated as 55 patients in each group. The study was discontinued in nine patients: four patients with difficult intubation (grade IV Cormack–Lehane) who required more than one attempt of laryngoscopy or more than 40 s to achieve endotracheal intubation, three patients who suffered from severe bleeding affecting their haemodynamics and two patients who required postoperative mechanical ventilation. So, the final analysis included 51 patients in the lignocaine group versus 50 patients in the saline group [Figure 1].
Figure 1.
Consolidated standards of reporting trials (CONSORT) flow diagram of the participants
Patients’ characteristics including age, weight, BMI and parity were comparable in both groups [Table 1]. Airway assessment of the patients preoperatively using the modified Mallampati classification and during direct laryngoscopy using the Cormack–Lehane classification showed no significant differences between both groups [Table 1].
Table 1.
Patient characteristics and airway assessment
| Parameters | Lignocaine group (n=51) | Saline group (n=50) | P |
|---|---|---|---|
| Age (years) | 28.5±5.3 | 27.6±5.4 | 0.413 |
| Weight (kg) | 77.6±12.5 | 74.8±12.7 | 0.272 |
| BMI (kg/m2) | 30.0±4.1 | 29.8±4.7 | 0.799 |
| Parity | |||
| • Primigravida | 11 | 12 | 0.771 |
| • Multipara | 40 | 3 | |
| Modified Mallampati classification | |||
| • Class I | 29 | 21 | 0.263 |
| • Class II | 19 | 22 | |
| • Class III | 3 | 5 | |
| • Class IV | 0 | 2 | |
| Cormack–Lehane classification | |||
| • Grade I | 37 | 25 | 0.056 |
| • Grade II | 12 | 23 | |
| • Grade III | 2 | 2 |
BMI=body mass index, SD=standard deviation, n-number. Data are presented as mean±SD or number
The duration of laryngoscopy, anaesthesia and surgery were comparable between both groups. Also, there were no significant differences between both groups in serum cortisol level or blood glucose concentration before nebulisation or 10 min after intubation. Moreover, the incidence and grade of cough during emergence from general anaesthesia did not significantly differ between both groups [Table 2].
Table 2.
The study parameters in the two groups
| Parameters | Lignocaine group (n=51) | Saline group (n=50) | P |
|---|---|---|---|
| Duration of laryngoscopy (s) | 26.9±7.9 (24.7–29.1) | 26.4±7.7 (24.2–28.6) | 0.756 |
| Duration of surgery (min) | 40.1±9.3 (37.5–42.7) | 40.6±7.7 (38.4–42.8) | 0.769 |
| Duration of anaesthesia (min) | 47.9±9.5 (45.3–50.6) | 48.6±8.1 (46.3–50.9) | 0.718 |
| Intraoperative fluids (ml) | 1480.4±386.8 (1371.6–1589.2) | 1460±362 (1357.1–1562.9) | 0.785 |
| Serum cortisol (nmol/l) | |||
| • Before nebulisation | 1156.0±75.1 (1134.9–1177.2) | 1135.5±57.1 (1119.3–1151.8) | 0.126 |
| • 10 min after intubation | 1056.2±77.9 (1034.3–1078.1) | 1037.6±67.9 (1018.3–1056.9) | 0.205 |
| Blood glucose (mg/dl) | |||
| • Before nebulisation | 125.8±38.2 (115.1–136.6) | 120.2±37.5 (109.6–130.9) | 0.460 |
| • 10 min after intubation | 124.5±37.8 (113.9–135.1) | 117.4±33.3 (108.0–126.9) | 0.323 |
| • Incidence of cough during emergence | 19 | 18 | 0.896 |
| Grade of cough during emergence | 0.554 | ||
| • Grade 0 | 32 | 32 | |
| • Grade 1 | 16 | 13 | |
| • Grade 2 | 2 | 5 | |
| • Grade 3 | 1 | 0 |
SD=standard deviation. Values are presented as mean±SD (95% confidence interval) or number
The baseline SBP and that before induction of anaesthesia did not significantly differ between both groups. However, the SBP was significantly lower in the lignocaine group compared to the saline group 1, 3 and 5 min after endotracheal intubation and after delivery of the foetus (P = 0.001, 0.003, 0.002 and 0.019, respectively). By the end of surgery, the SBP was again comparable in both groups [Figure 2a].
Figure 2.
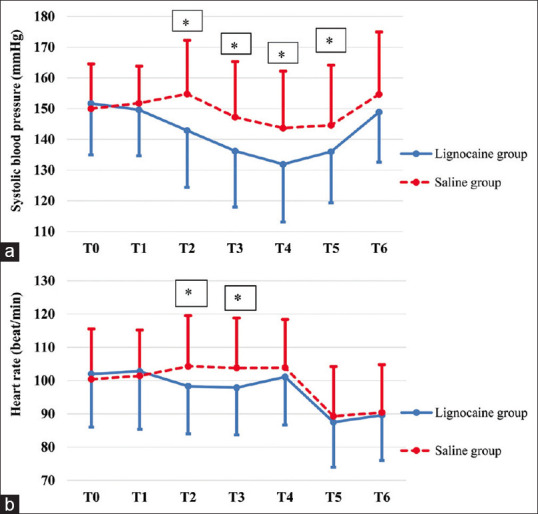
(a and b): Changes in systolic blood pressure and heart rate. T0: baseline (prior to nebulisation), T1: before induction of anaesthesia, T2: one minute after intubation, T3: three minutes after intubation, T4: five minutes after intubation, T5: after delivery of the foetus, T6: at the end of surgery. Values are presented as mean±SD. *Indicates significant difference between both groups (P<0.05)
The baseline heart rate and that before induction of anaesthesia did not significantly differ between both groups. However, 1 and 3 min after endotracheal intubation, the heart rate was significantly lower in the lignocaine group compared to the saline group (P = 0.041 and 0.042, respectively). There were no significant differences between both groups regarding the heart rate 5 min after endotracheal intubation, after delivery of the foetus or at the end of surgery [Figure 2b].
Body weights of the neonates were comparable in both groups. Regarding neonatal status after delivery, there were no significant differences in Apgar scores at 1, 5 or 10 min between both groups. Moreover, umbilical blood gas showed no significant differences between both groups in pH, PaO2, PaCO2, HCO3 or lactate level. Two neonates in the lignocaine group and one neonate in the saline group required admission to neonatal intensive care (NICU) for mechanical ventilation [Table 3].
Table 3.
Neonatal study parameters in the two groups
| Parameters | Lignocaine group (n=51) | Saline group (n=50) | P |
|---|---|---|---|
| Body weight at birth (kg) | 2.9±0.7 (2.7–3.1) | 2.8±0.6 (2.6–2.9) | 0.210 |
| Apgar score | |||
| • Apgar (1 min) | 7 (5-7) | 7 (5.8-7.3) | 0.294 |
| • Apgar (5 min) | 9 (7-9) | 9 (7-9) | 0.192 |
| • Apgar (10 min) | 10 (9-10) | 9 (9-10) | 0.430 |
| Umbilical blood gas | |||
| • pH | 7.29±0.05 (7.27–7.30) | 7.29±0.06 (7.27–7.31) | 0.631 |
| • PaO2 (mmHg) | 29.0±15.1 (24.7–33.3) | 32.5±30.4 (23.9–41.2) | 0.463 |
| • PaCO2 (mmHg) | 49.5±21.9 (43.2–55.7) | 43.8±8.7 (41.3–46.3) | 0.092 |
| • HCO3 (mmol/l) | 20.9±2.6 (20.2–21.7) | 21.6±2.2 (20.9–22.2) | 0.187 |
| • Lactate (mmol/l) | 2.3±0.8 (2.1–2.5) | 2.2±0.7 (2.0–2.4) | 0.441 |
| Admission to NICU for MV | 2 | 1 | 0.508 |
MV=mechanical ventilation, NICU=neonatal intensive care unit, SD=standard deviation. Values are presented as mean±SD (95% confidence interval), median (interquartile range) or number
There were no significant differences between both groups in the incidence or severity of POST 1 or 2 h after admission to the PACU. Similarly, there were no significant differences between both groups in the incidence or severity of hoarseness of voice [Table 4].
Table 4.
Postoperative sore throat and hoarseness of voice
| Parameters | Lignocaine group (n=51) | Saline group (n=50) | P |
|---|---|---|---|
| Incidence of POST (T1) | 20 | 18 | 0.739 |
| Severity of POST (T1) | |||
| • Grade 0 | 31 | 32 | 0.514 |
| • Grade 1 | 12 | 13 | |
| • Grade 2 | 7 | 5 | |
| • Grade 3 | 1 | 0 | |
| Incidence of POST (T2) | 9 | 9 | 0.963 |
| Severity of POST (T2) | |||
| • Grade 0 | 42 | 41 | |
| • Grade 1 | 7 | 6 | 0.739 |
| • Grade 2 | 2 | 2 | |
| • Grade 3 | 0 | 1 | |
| Incidence of hoarseness of voice (T1) | 13 | 14 | 0.776 |
| Severity of hoarseness of voice (T1) | |||
| • Grade 0 | 38 | 36 | 0.536 |
| • Grade 1 | 8 | 11 | |
| • Grade 2 | 5 | 3 | |
| • Grade 3 | 0 | 0 | |
| Incidence of hoarseness of voice (T2) | 4 | 5 | 0.741 |
| Severity of hoarseness of voice (T2) | |||
| • Grade 0 | 47 | 45 | 0.754 |
| • Grade 1 | 4 | 5 | |
| • Grade 2 | 0 | 0 | |
| • Grade 3 | 0 | 0 |
POST=postoperative sore throat, T1 and T2: 1 and 2 h after admission to the post-anaesthesia care unit. Values are presented as numbers
DISCUSSION
In this study, results demonstrated that preoperative lignocaine nebulisation in a dose of 4.5 mg/kg (not exceeding 400 mg) was effective in attenuating the pressor response as evident by the lower SBP and heart rate in the first few minutes after intubation in lignocaine group versus saline group.
Suppressing the pressor response to laryngoscopy and endotracheal intubation in patients with severe preeclampsia undergoing caesarean delivery is a challenge. Although opioids are effective in attenuating this cardiovascular response,[16] many anaesthesiologists prefer to postpone their administration till delivery of the foetus, to avoid respiratory depression in an already compromised foetus. Even remifentanil, an extremely short-acting opioid, was reported to cause neonatal respiratory depression when given during induction.[15]
Topical anaesthesia of the upper airway with lignocaine spray is another effective method.[17] However, it requires two laryngoscopies, which are not suitable with the rapid-sequence induction that is commonly used in those patients.
Venus et al.[18] found that the pressor response was successfully abolished in nine adult cancer patients who received 6 ml of aerosolised 4% lignocaine for 5 min during pre-oxygenation, when compared to 10 patients who received aerosolised saline. Later on, various studies had investigated the effects of nebulised lignocaine versus, the commonly used, intravenous lignocaine on pressor response. Ganesan et al.,[8] in their study on 100 adult patients, reported that nebulised lignocaine (8 ml of 2%) for 20 min may be more effective than intravenous lignocaine (1.5 mg/kg) in suppressing the pressor response. This result was consistent with another study that included 40 adult patients and used 5 ml of lignocaine 2% in both routes of administration.[19]
Contrary to these findings, Raj et al.[9] found that nebulised lignocaine (2 mg/kg of 2%) was less effective in attenuating the pressor response compared to intravenous lignocaine in the same dose and concentration, while Laurito et al.,[7] in their randomised, double-blind study, reported that the maximum pressor response elicited by intubation did not differ significantly among four different groups (aerosolised lignocaine, intravenous lignocaine, combination of both or placebo); however, their study included only 10 patients in each group.
In comparison to the previously mentioned studies, we used a relatively large dose of lignocaine to compensate for the anticipated drug loss during the process of nebulisation. Chinn et al.[20] reported that up to 50% of the local anaesthetic could be lost around the patient’s mouth if the process of nebulisation is continuous during both inspiration and expiration.
In the present study, the maternal serum cortisol and blood glucose levels showed no significant differences between both groups as measured 10 min after intubation. A previous study of Kaba et al.[21] demonstrated that intravenous lignocaine had no significant effect on serum cortisol level in patients undergoing laparoscopic colectomy. Contrary to these findings, El-Tahan et al.[22] found that the maternal cortisol level was significantly lower in the group of patients who received intravenous lignocaine versus the control group at 5 min after intubation and 1 h after caesarean delivery.
Since the neonates born to patients with pregnancy-induced hypertension are at greater risk of respiratory distress syndrome and may have low birth weights,[23,24] it was worthy to study the effect of any pharmacological intervention on neonatal well-being. In the present study, the Apgar scores at 1, 5 and 10 min were comparable between both groups, and so were the umbilical blood gas parameters. Also, the number of neonates who required admission to the NICU for mechanical ventilation did not significantly differ between the studied groups. Accordingly, lignocaine nebulisation had no obvious hazardous effects on neonatal outcome.
Regarding the nebulisation process, it was well tolerated by all patients who accepted to be enrolled in the study. After recovery, the patients were tested for swallowing and gag reflex, and they were all intact. There were no significant differences between both groups in the incidence or severity of POST or hoarseness of voice.
The limitations in this study included the absence of invasive monitoring that could allow better beat to beat assessment of the blood pressure and the lack of measurements of maternal and neonatal serum levels of lignocaine and catecholamines.
We recommend further studies of different doses and/or concentrations of lignocaine to achieve optimum attenuation of the pressor response in these critical patients.
CONCLUSION
Preoperative lignocaine nebulisation in a dose of 4.5 mg/kg was a safe, simple and generally tolerable technique that can be used effectively to attenuate the pressor response to laryngoscopy and endotracheal intubation in patients with severe preeclampsia undergoing caesarean section delivery. Moreover, it had no obvious hazardous effects on neonates.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors thank Ms Asmaa Mostafa and Ms Eman Ramadan, the great nurses, who helped us so much in conducting this study by their great efforts preoperatively in drug preparation and administration.
REFERENCES
- 1.Ives CW, Sinkey R, Rajapreyar I, Tita ATN, Oparil S. Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;76:1690–702. doi: 10.1016/j.jacc.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Hosseini Valami SM, Hosseini Jahromi SA, Masoodi N. The effect of alfentanil on maternal haemodynamic changes due to tracheal intubation in elective caesarean sections under general anaesthesia. Indian J Anaesth. 2015;59:728–32. doi: 10.4103/0019-5049.170033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bansal S, Pawar M. Haemodynamic responses to laryngoscopy and intubation in patients with pregnancy-induced hypertension:effect of intravenous esmolol with or without lidocaine. International Journal of Obstetric Anesthesia. 2002;11:4–8. doi: 10.1054/ijoa.2001.0918. [DOI] [PubMed] [Google Scholar]
- 4.Goddard J, Wee MYK, Vinayakarao L. Update on hypertensive disorders in pregnancy. BJA Educ. 2020;20:411–6. doi: 10.1016/j.bjae.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Upadya M, Rao ST. Hypertensive disorders in pregnancy. Indian J Anaesth. 2018;62:675–81. doi: 10.4103/ija.IJA_475_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon SW, Choi GJ, Seong HK, Lee MJ, Kang H. Pharmacological strategies to prevent haemodynamic changes after intubation in parturient women with hypertensive disorders of pregnancy: A network meta-analysis. Int J Med Sci. 2021;18:1039–50. doi: 10.7150/ijms.54002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laurito CE, Baughman VL, Becker GL, Polek WV, Riegler FX, VadeBoncouer TR. Effects of aerosolized and/or intravenous lidocaine on hemodynamic responses to laryngoscopy and intubation in outpatients. Anesth Analg. 1988;67:389–92. [PubMed] [Google Scholar]
- 8.Ganesan P, Balachander H, Elakkumanan L. Evaluation of nebulized lignocaine versus intravenous lignocaine for attenuation of pressor response to laryngoscopy and intubation. Curr Med Issues. 2020;18:184–8. [Google Scholar]
- 9.Raj SS, Deepti R, Rao S. Comparative study of lignocaine nebulization with intravenous lignocaine for attenuation of hemodynamic response to laryngoscopy and endotracheal intubation. Med Int J Anesth. 2021;18 doi:10.26611/10151821. [Google Scholar]
- 10.Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. 2020;135:e237–60. doi: 10.1097/AOG.0000000000003891. [DOI] [PubMed] [Google Scholar]
- 11.Wang L-y, Zhang K-d, Zhang Z-h, Zhang D-x, Wang H-l, Qi F. Evaluation of the reliability of the upper lip bite test and the modified mallampati test in predicting difficult intubation under direct laryngoscopy in apparently normal patients: A prospective observational clinical study. BMC Anesthesiology. 2022;22:314. doi: 10.1186/s12871-022-01855-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yemam D, Melese E, Ashebir Z. Comparison of modified mallampati classification with Cormack and Lehane grading in predicting difficult laryngoscopy among elective surgical patients who took general anesthesia in Werabie comprehensive specialized hospital-Cross sectional study. Ethiopia, 2021 Ann Med Surg (Lond) 2022;79:103912. doi: 10.1016/j.amsu.2022.103912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JH, Koo BN, Jeong JJ, Kim HS, Lee JR. Differential effects of lidocaine and remifentanil on response to the tracheal tube during emergence from general anaesthesia. Br J Anaesth. 2011;106:410–5. doi: 10.1093/bja/aeq396. [DOI] [PubMed] [Google Scholar]
- 14.Park SY, Kim SH, Lee AR, Cho SH, Chae WS, Jin HC, et al. Prophylactic effect of dexamethasone in reducing postoperative sore throat. Korean J Anesthesiol. 2010;58:15–9. doi: 10.4097/kjae.2010.58.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo KY, Jeong CW, Park BY, Kim SJ, Jeong ST, Shin MH, et al. Effects of remifentanil on cardiovascular and bispectral index responses to endotracheal intubation in severe pre-eclamptic patients undergoing Caesarean delivery under general anaesthesia. Br J Anaesth. 2009;102:812–9. doi: 10.1093/bja/aep099. [DOI] [PubMed] [Google Scholar]
- 16.Rout CC, Rocke DA. Effects of alfentanil and fentanyl on induction of anaesthesia in patients with severe pregnancy-induced hypertension. Br J Anaesth. 1990;65:468–74. doi: 10.1093/bja/65.4.468. [DOI] [PubMed] [Google Scholar]
- 17.Meng YF, Cui GX, Gao W, Li ZW. Local airway anesthesia attenuates hemodynamic responses to intubation and extubation in hypertensive surgical patients. Med Sci Monit. 2014;20:1518–24. doi: 10.12659/MSM.890703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venus B, Polassani V, Pham CG. Effects of aerosolized lidocaine on circulatory responses to laryngoscopy and tracheal intubation. Crit Care Med. 1984;12:391–4. doi: 10.1097/00003246-198404000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Bhaskar V, Shetty S, Rajaram P, Varmudy M. Comparison of intravenous versus nebulised lignocaine for suppression of haemodynamic responses to tracheal intubation. Airway. 2021;4:175–8. [Google Scholar]
- 20.Chinn WM, Zavala DC, Ambre J. Plasma levels of lidocaine following nebulized aerosol administration. Chest. 1977;71:346–8. doi: 10.1378/chest.71.3.346. [DOI] [PubMed] [Google Scholar]
- 21.Kaba A, Laurent Stanislas R, Detroz Bernard J, Sessler Daniel I, Durieux Marcel E, Lamy Maurice L, et al. Intravenous Lidocaine Infusion Facilitates Acute Rehabilitation after Laparoscopic Colectomy. Anesthesiology. 2007;106:11–8. doi: 10.1097/00000542-200701000-00007. [DOI] [PubMed] [Google Scholar]
- 22.El-Tahan MR, Warda OM, Diab DG, Ramzy EA, Matter MK. A randomized study of the effects of perioperative i.v. lidocaine on hemodynamic and hormonal responses for cesarean section. J Anesth. 2009;23:215–21. doi: 10.1007/s00540-009-0738-3. [DOI] [PubMed] [Google Scholar]
- 23.Lei F, Liu D, Shen Y, Zhang L, Li S, Liu X, et al. Study on the influence of pregnancy-induced hypertension on neonatal birth weight. J Investig Med. 2018;66:1008–14. doi: 10.1136/jim-2017-000626. [DOI] [PubMed] [Google Scholar]
- 24.Tian T, Wang L, Ye R, Liu J, Ren A. Maternal hypertension, preeclampsia, and risk of neonatal respiratory disorders in a large-prospective cohort study. Pregnancy Hypertension. 2020;19:131–7. doi: 10.1016/j.preghy.2020.01.006. [DOI] [PubMed] [Google Scholar]


