Abstract
During anaerobic growth of Klebsiella pneumoniae on citrate, 9.4 mmol of H2/mol of citrate (4-kPa partial pressure) was formed at the end of growth besides acetate, formate, and CO2. Upon addition of NiCl2 (36 μM) to the growth medium, hydrogen formation increased about 36% to 14.8 mmol/mol of citrate (6 kPa), and the cell yield increased about 15%. Cells that had been harvested and washed under anoxic conditions exhibited an H2-dependent formation of NAD(P)H in vivo. The reduction of internal NAD(P)+ was also achieved by the addition of formate. In crude extracts, the H2:NAD+ oxidoreductase activity was 0.13 μmol min−1 mg−1, and 76% of this activity was found in the washed membrane fraction. The highest specific activities of the membrane fraction were observed in 50 mM potassium phosphate, with 1.6 μmol of NADPH formed min−1 mg−1 at pH 7.0 and 1.7 μmol of NADH formed min−1 mg−1 at pH 9.5. In the presence of the protonophore carbonyl cyanide m-chlorophenylhydrazone and the Na+/H+ antiporter monensin, the H2-dependent reduction of NAD+ by membrane vesicles decreased only slightly (about 16%). The NADP+- or NAD+-reducing hydrogenases were solubilized from the membranes with the detergent lauryldimethylamine-N-oxide or Triton X-100. NAD(P)H formation with H2 as electron donor, therefore, does not depend on an energized state of the membrane. It is proposed that hydrogen which is formed by K. pneumoniae during citrate fermentation is recaptured by a novel membrane-bound, oxygen-sensitive H2:NAD(P)+ oxidoreductase that provides reducing equivalents for the synthesis of cell material.
In many microorganisms, H2 is oxidized by hydrogenases that provide reducing equivalents for energy conservation, CO2 fixation, and synthesis of cell material. H2-evolving hydrogenases are usually required during the fermentation of organic substrates for the regeneration of electron acceptors with concomitant reduction of protons (1, 34). In enterobacteria like Escherichia coli or Klebsiella spp., the major source of reducing equivalents for H2 production is pyruvate which is cleaved to acetyl coenzyme A (acetyl-CoA) and formate. Subsequently, formate is converted to H2 and CO2 by the formate hydrogen lyase complex (4). The same route could lead to H2 formation during the fermentation of citrate by Klebsiella pneumoniae (Fig. 1). The pathway begins with the cleavage of citrate to acetate and oxaloacetate, which is subsequently decarboxylated by the oxaloacetate decarboxylase Na+ pump (5, 10). Part of the energy stored in the Δμ̃ Na+ thus established is utilized for citrate uptake (11, 24). Pyruvate is further degraded by pyruvate formate lyase to yield acetyl-CoA, which is converted to acetyl-phosphate by phosphotransacetylase. Finally, acetate kinase is used to form ATP from ADP and acetyl-phosphate (4). Whereas most bacteria growing fermentatively must oxidize reduced electron carriers like NADH to ensure the continuous conversion of the substrate, the fermentation of citrate by K. pneumoniae does not involve redox reactions that are coupled to the formation of NADH. The cells gain some NADPH from the oxidation of isocitrate (23), but there is no further NADH formation via the tricarboxylic acid cycle, since the 2-oxoglutarate dehydrogenase is repressed under anaerobic conditions (21). These bacteria are therefore confronted with the opposite problem: they have to synthesize NAD(P)H from NAD(P)+ for biosynthetic pathways.
FIG. 1.
Generation of Δμ̃ Na+, ATP, and NAD(P)H during citrate fermentation by K. pneumoniae. 1, citrate lyase; 2, oxaloacetate decarboxylase; 3, pyruvate formate lyase; 4, phosphotransacetylase; 5, formate hydrogen lyase; 6, acetate kinase; 7, NAD(P)+-reducing hydrogenase.
K. pneumoniae forms 2 mol of acetate, 0.5 mol of formate, and 1.3 mol of CO2 per mol of citrate, indicating that part of the formate obtained from pyruvate is converted to CO2. It was suggested that formate was oxidized to CO2 by an ubiquinone-dependent formate dehydrogenase (23). In a previous report (12), a membrane-bound NADH:ubiquinone oxidoreductase which was stimulated by Na+ was described. This primary, redox-driven Na+ pump oxidized NADH with concomitant translocation of sodium ions into membrane vesicles of K. pneumoniae (12). The enzyme was proposed to catalyze the reverse reaction in vivo, i.e., the Δμ̃ Na+-driven reduction of NAD+ by ubiquinol derived from the oxidation of formate (23).
The present study demonstrates that H2 which is found as a product during citrate fermentation by K. pneumoniae is oxidized with concomitant reduction of NAD(P)+ by a membrane-bound hydrogenase. We describe the reduction of endogenous NAD(P)+ by H2 in cell suspensions as well as some properties of the NAD(P)+-reducing hydrogenase from K. pneumoniae. A modified pathway of NADH formation during citrate fermentation by K. pneumoniae which does not proceed via reversed electron transfer is proposed.
MATERIALS AND METHODS
Organism and materials.
K. pneumoniae was from laboratory stock (9). The chemicals (Fluka Chemika) contained less than 0.005% (by mass) Ni, Fe, Co, Cu, and Zn.
Growth of K. pneumoniae.
K. pneumoniae was grown in batch culture at 37°C in tubes or serum bottles sealed with rubber septa by the method described by Dimroth (9), modified as follows. The medium (pH 6.9 to 7.0) contained 38 mM Na2HPO4, 20 mM KH2PO4, 17 mM NH4Cl, 7 mM NaCl, 1 mM MgSO4, 0.1 mM CaCl2, and 23 mM trisodium citrate. Due to the formation of CO2, the pH dropped to 6.0 at the end of growth. In one set of experiments, glass tubes (15-ml volume) and septa were washed extensively with dilute nitric acid and distilled H2O which had been passed over a Chelex 100 ion-exchange resin (Bio-Rad) to remove divalent metal ions. Media were prepared with H2O purified with Chelex ion-exchange resin and contained less than 0.25 μM Ni2+, as determined by atomic absorption spectroscopy. Growth was monitored without nickel added or in the presence of 1.0 μM NiCl2. In another set of experiments, K. pneumoniae was grown in 1.1-liter serum bottles with a 0.14-liter headspace, and contaminating metals were not removed from the glassware. In these experiments, the growth and the formation of H2 were monitored without nickel added or in the presence of 36 μM NiCl2. The availability of transition metal cations in the medium is reduced by the citrate, which acts as a chelator (13, 15). After autoclaving, the medium was cooled in an atmosphere of N2, and inoculum (10 ml) from a stationary-phase culture of K. pneumoniae grown anaerobically on 50 mM citrate was added with sterile syringes against an overpressure of N2. Prior to the determination of dry weight, cells were washed once in 50 mM ammonium formate. For experiments with cell suspensions and cell fractions, 1 liter of medium containing 50 mM trisodium citrate was inoculated with 1 ml of a stationary-phase culture of K. pneumoniae grown aerobically on Luria-Bertani medium (26).
Preparation of cell suspensions and cell fractions.
If not indicated otherwise, all manipulations were performed in the absence of oxygen in an anaerobic chamber (Coy Laboratory Products, Ann Arbor, Mich.) with 1 to 2 kPa of H2 in N2 as gas phase. Two 1-liter cultures in the late stationary phase were harvested by centrifugation. For experiments with cell suspensions, the cells were washed five times in buffer A (10 mM Tris-HCl [pH 7.5], 50 mM K2SO4, 5% [by volume] glycerol). For preparation of cell extracts, cells (approximately 2 g) were suspended in 20 ml of buffer A containing 1 mM dithiothreitol, 0.1 mM diisopropyl fluorophosphate, and a trace of DNase and were broken by a single passage through a French press at 80 MPa in 100% N2. Cell debris were removed by centrifugation (35,000 × g, 30 min), and the supernatant was ultracentrifuged (150,000 × g, 90 min). The pellet containing the membrane fraction was washed once, twice, or three times with buffer A.
For solubilization experiments, membranes were washed once in buffer A and resuspended in the same buffer. Aliquots (8 mg of protein in 0.25 ml) were mixed with 0.75 ml of 1% (by mass) Triton X-100, lauryldimethylamine-N-oxide, or dodecyl-d-maltoside in buffer (9 mM Tris-HCl [pH 7.5], 44 mM K2SO4, 10% [by mass] glycerol) and ultracentrifuged immediately (200,000 × g, 30 min). The NAD+- and NADP+-reducing hydrogenase activities of the clear supernatant (solubilized membranes) and the residual pellet (partially extracted membranes) were determined immediately.
Enzyme assays.
If not indicated otherwise, the assays were performed with stirring at 25°C without oxygen immediately after the harvesting and washing of the cells or preparation of cell fractions. Cells in 2 ml of 50 mM potassium phosphate buffer (pH 7.0) (absorbance at 600 nm of approximately 25, corresponding to 3 to 4 mg of protein ml−1) were placed in quartz cuvettes sealed with septa, and the gas phase (1 to 2 kPa of H2 in N2 from the anaerobic chamber) was exchanged with 100% N2. The formation of NAD(P)H was initiated by the addition of 100 μl of H2-saturated buffer containing 74 nmol of H2 (19) or sodium formate (1 μmol) in N2-saturated buffer (20 μl) with gastight syringes. The reduction of intracellular NAD(P)+ was monitored in the dual-wavelength mode of a Shimadzu UV-3000 spectrophotometer at the wavelength pair 340 and 370 nm (7). There was a linear increase in signal intensity from 0 to 200 nM NADH.
The activity of cell fractions was determined in quartz cuvettes filled with buffer (50 mM potassium phosphate [pH 6.0 to 10.0]) to a final volume of 1 ml. The cuvettes were sealed with septa in the anaerobic chamber (1 to 2 kPa of H2 in N2), and the reaction was started by the addition of NAD(P)+ with gastight syringes. The concentration of pyridine nucleotides in the assays was 100 or 200 μM. Stock solutions of NAD+ and NADP+ in H2O were freshly prepared in the anaerobic chamber. Assays were also performed in Good buffers (20 mM) or in glycine (150 mM), titrated with NaOH. Rates were recorded on an HP 8462A diode array spectrophotometer (Hewlett-Packard). The formation of NAD(P)H was monitored at 340 nm (ɛ340 = 6.22 mM−1 cm−1). The reduction of benzyl viologen with H2 was initiated by the addition of oxidized benzyl viologen to a final concentration of 70 μM. The reduction of benzyl viologen (70 μM) with NADH (0.9 mM final concentration) was determined in sealed cuvettes from the anaerobic chamber which had been evacuated and flushed with 100% N2 prior to the addition of substrates. The NADH dehydrogenase and hydrogenase activities (28) were calculated from the formation of reduced benzyl viologen (ɛ600 = 7.4 mM−1 cm−1).
Other analytical methods.
Protein was determined by the bicinchoninic acid method (31), using the reagent obtained from Pierce and bovine serum albumin as the standard. H2 was determined with a Perkin-Elmer 8700 gas chromatograph. Samples (50 to 300 μl) were injected onto a Porapak Q column (80/100 mesh; 150°C) connected to a thermal conductivity detector (250°C).
The elemental analysis of nickel was carried out on a graphite tube atomic absorption spectrometer (ETV-AAS) with Zeeman background correction (SpectrAA-400; Varian) at 232 nm. A standard solution of 0.34 μM Ni2+ was prepared in 2% HNO3 (Suprapur; Merck). The samples were measured by standard addition (10-μl sample; addition of 10, 20, and 30 μl of standard solution) in a total volume of 50 μl. The detection limit was 0.25 μM Ni2+. Ashing and atomization temperatures were 400 and 2,400°C, respectively.
RESULTS
Formation of H2 and beneficial effect of nickel ions during anaerobic growth of K. pneumoniae on citrate.
K. pneumoniae formed H2 during anaerobic growth on 23 mM citrate. H2 formation commenced during the lag phase (approximately 0.05 mmol) and increased to 0.24 mmol (4 kPa of H2) after 14 h. The effect of Ni2+ on H2 formation and growth was determined because this metal ion is an essential component of the active site of most hydrogenases (1, 14, 32). In a representative experiment, the cell yield increased from 8.3 g of cells/mol of citrate without nickel added to 9.6 g of cells/mol of citrate in the presence of 36 μM NiCl2, corresponding to optical densities (600 nm) of 0.60 and 0.72, respectively. There was a concomitant increase in H2 formation from 9.4 to 14.8 mmol of H2/mol of citrate. In another set of experiments, the contamination of media by nickel ions was reduced (<0.25 μM Ni2+). Under these conditions, the optical density (600 nm) at the end of growth was 0.26. Upon addition of NiCl2 (1.0 μM), the final cell density increased about 28%, to 0.36. The beneficial effect of Ni2+ on growth could indicate an important role of H2 in anabolism, most likely as an electron donor for the reduction of pyridine nucleotides. This inference is supported by the observation that H2 formation increased significantly when the culture had entered the stationary phase.
Reduction of NAD(P)+ by H2 or formate in cell suspensions of K. pneumoniae.
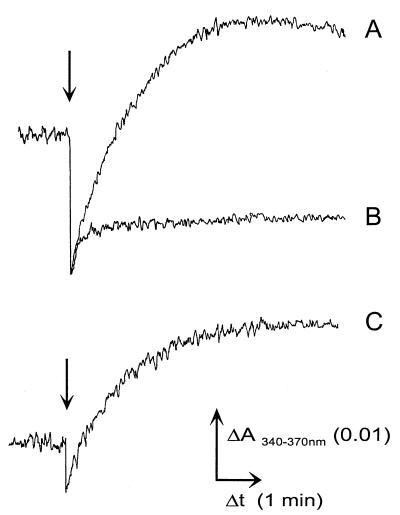
To test whether the endogenous pool of oxidized pyridine nucleotides is reduced by H2, NAD(P)H formation in cell suspensions of K. pneumoniae was determined by dual-wavelength spectroscopy. The addition of 74 nmol of H2 in 100 μl of buffer to fresh, anaerobically prepared cells led to a rise in the intracellular concentration of NAD(P)H (96 pmol min−1 [Fig. 2, trace A]). The H2-induced formation of NAD(P)H was abolished by the addition of oxygen (not shown). A very similar rise in the NAD(P)H concentration was achieved by adding 1 μmol of formate in 20 μl of N2-saturated buffer (78 pmol min−1 [Fig. 2, trace C]). In a control experiment with 100 μl of N2-saturated buffer, the signal decreased due to the dilution of the cell suspension and then remained stable (Fig. 2, trace B). Since the reductants were added in assay buffer, a change in the absorbance of the cell suspension due to osmotic swelling or shrinkage was excluded (2). Only half of the amount of NAD(P)H was formed from H2 or formate in fresh cells that had been harvested and washed under air, and no NAD(P)H formation occurred if the cells were frozen and thawed in the presence of oxygen.
FIG. 2.
Reduction of endogenous NAD(P)+ by H2 or formate in cell suspensions of K. pneumoniae. Arrows indicate the addition either of 74 nmol of H2 in 100 μl of buffer (trace A) or 100 μl of N2-saturated buffer (trace B) or of 1 μmol of formate (trace C) in 20 μl of buffer to cell suspensions of K. pneumoniae (3 to 4 mg of protein ml−1). The formation of NAD(P)H was monitored at the wavelength pair 340 and 370 nm.
Localization and properties of the H2:NAD(P)+ oxidoreductase from K. pneumoniae.
After cell rupture in the absence of oxygen, 76% of the total H2:NAD+ oxidoreductase activity in K. pneumoniae was found in the membrane fraction, which exhibited a specific activity threefold higher than that of the soluble fraction. After three washing steps, the specific activity of the membrane vesicles decreased only marginally, from 0.23 to 0.18 μmol min−1 mg−1 (Table 1). We therefore concluded that K. pneumoniae contains a membrane-bound H2:NAD+ oxidoreductase. The enzyme was sensitive to oxygen (50% loss of activity after 20 min; complete loss of activity after 2 h). Storage in the anaerobic chamber under 1 to 2 kPa of H2 at room temperature led to 20% loss of activity after 30 min and to 50 to 70% loss of activity after 2 h.
TABLE 1.
Localization of the NAD+-dependent hydrogenase from K. pneumoniae
| Cell fraction | Protein (mg) | Total activitya (μmol min−1) | Sp acta (μmol min−1 mg−1) |
|---|---|---|---|
| Crude extract | 315 | 41 | 0.13 |
| Soluble fraction | 164 | 12 | 0.07 |
| Washed membranes | 134 | 31 | 0.23 or 0.18b |
The assay buffer contained 150 mM glycine-NaOH (pH 9.5) and 1 mM dithiothreitol.
Determined with membranes that had been washed once or three times, respectively.
The pH optimum for the reduction of pyridine nucleotides with H2 by the membrane fraction of K. pneumoniae was pH 7.0 with NADP+ and around pH 8.5 to 10.0 with NAD+ as an electron acceptor (Fig. 3). The highest specific activities of the membrane fraction were observed in 50 mM potassium phosphate buffer, with 1.6 μmol of NADPH formed min−1 mg−1 at pH 7.0 and 1.7 μmol of NADH formed min−1 mg−1 at pH 9.5 (Fig. 3A). Dithiothreitol (1 mM) or NiCl2 (1 mM) had no effect on the hydrogenase activity. Note that the assay mixture (membranes added) contained 1 to 2 μM Ni2+, which might be sufficient for the reactivation of hydrogenase without addition of NiCl2. The pH optima for the oxidation of NADH or H2 with benzyl viologen as the electron acceptor were determined with membranes that had been prepared in the absence of oxygen and stored in liquid N2. The NADH dehydrogenase activity increased from 0.03 μmol min−1 mg−1 at pH 7.0 to 0.07 μmol min−1 mg−1 at pH 8.0 and 0.17 μmol min−1 mg−1 at pH 9.5. The H2 uptake activity with benzyl viologen as the electron acceptor was 0.02 to 0.07 μmol min−1 mg−1 below pH 7.0 and showed a broad maximum from pH 7.0 (0.28 μmol min−1 mg−1) to pH 10.6 (0.26 μmol min−1 mg−1).
FIG. 3.
pH optima of the NAD(P)+-dependent hydrogenase from K. pneumoniae. The reduction of 200 μM NAD+ (○) or NADP+ (•) by membrane vesicles was determined in 50 mM potassium phosphate (A) or in 20 mM concentrations of each of the following buffers: morpholineethanesulfonic acid (MES)-morpholinepropanesulfonic acid (MOPS)-Tricine (pH 5.5 to 6.5), MOPS-Tricine-glycine (pH 7.0 to 9.5), and glycine (pH 9.5 to 10) (B). The gas phase contained 1 to 2 kPa of H2 in N2.
Since the oxidation of H2 and the subsequent reduction of membrane-bound electron carriers could generate a Δμ̃ H+ (27) which might drive the reduction of NAD+ by H2 in membrane vesicles from K. pneumoniae, we tested whether the reaction was sensitive to an uncoupler and an Na+/H+ antiporter (Fig. 4). The H2-dependent reduction of NAD+ by fresh membrane vesicles at pH 9.5 decreased only slightly, from 0.18 μmol min−1 mg−1 in the absence to 0.15 μmol min−1 mg−1 in the presence of high amounts of the protonophore CCCP (carbonyl cyanide m-chlorophenylhydrazone) and the Na+/H+ antiporter monensin (each 5 μM, or 100 nmol mg of protein−1). At pH 7.0, the specific activity decreased from 0.09 to 0.08 μmol min−1 mg−1 (17% inhibition; not shown). After treatment of membrane vesicles with 1% (by mass) lauryldimethylamine-N-oxide, Triton X-100, or dodecyl-d-maltoside, the H2-dependent reduction of NAD+ or NADP+ by solubilized and partially extracted membranes was determined. Good solubilization was observed for the NAD+-reducing hydrogenase with Triton X-100 (0.4 μmol min−1 mg−1, pH 9.0) or lauryldimethylamine-N-oxide (0.04 μmol min−1 mg−1, pH 9.0); the residual activity in the extracted membranes was 6 or 9 nmol min−1 mg−1, respectively. With dodecyl-d-maltoside, the specific NAD+-reducing hydrogenase activity was 9 nmol min−1 mg−1, and no activity was detected in the partially extracted membrane pellet. The H2-dependent reduction of NADP+ was observed after solubilization of membranes with lauryldimethylamine-N-oxide (0.03 μmol min−1 mg−1, pH 7.5) but not after solubilization with Triton X-100 or dodecyl-d-maltoside. These results thus indicate that a membrane-bound enzyme catalyzes the reduction of NAD(P)+ by H2 in K. pneumoniae and that the catalysis is not dependent on Δμ̃ H+ or Δμ̃ Na+.
FIG. 4.
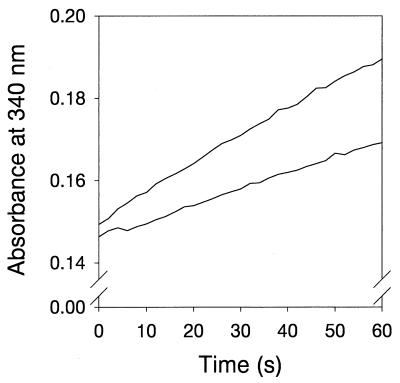
Activity of the NAD+-dependent hydrogenase from K. pneumoniae in the presence of an uncoupler and the Na+/H+ antiporter monensin. The reduction of NAD+ (0.1 mM) by membrane vesicles (0.05 mg of protein) was monitored in the absence (upper trace) and in the presence (lower trace) of 5 μM monensin and 5 μM CCCP. The buffer consisted of 150 mM glycine-NaOH (pH 9.5) and 1 mM dithiothreitol.
DISCUSSION
In one of the classical studies on citrate fermentation by Aerobacter aerogenes and Aerobacter indologenes, now classified as Klebsiella pneumoniae and Klebsiella oxytoca, H2 was found as an end product (6). In accordance with these results, H2 was also formed by our K. pneumoniae strain during anaerobic growth on citrate. We show here that K. pneumoniae has the enzymatic properties to reduce NAD(P)+ with H2. Hence, the NAD(P)H required for biosynthesis could originate from this reaction, provided that thermodynamic demands are fulfilled. The reduction of NAD(P)+ by H2 is an exergonic reaction under standard conditions (ΔG0′ = −18.1 kJ mol−1). During anaerobic growth on glucose, K. pneumoniae exhibits NADH/NAD+ ratios of 1/3 to 1/7 (33), corresponding to an NADH/NAD+ redox potential of approximately −300 mV. Under these conditions, the reduction of NAD(P)+ by H2 is feasible with H2 partial pressures as low as 10 Pa, or 10−4 bar. In the natural environment, these low H2 concentrations (10−4 to 10−5 bar) are maintained by hydrogen-oxidizing, methanogenic bacteria (29). With up to 6 kPa of H2 formed during citrate fermentation by K. pneumoniae under laboratory conditions, the redox potential is sufficiently negative to drive NAD(P)+ reduction. Since the reduction of NAD(P)+ by H2 is catalyzed by membrane vesicles in the presence of an uncoupler plus monensin and by enzyme solubilized from the membranes, we conclude that the reduction of NAD(P)+ by membrane vesicles of K. pneumoniae is independent of Δμ̃ H+ and Δμ̃ Na+. The formation of H2 from formate during citrate fermentation in K. pneumoniae was previously demonstrated by Dagley and Dawes (8). The rise in H2 formation during growth of K. pneumoniae in the presence of Ni2+ might be due to higher cell densities or might reflect an increase in active formate hydrogen lyase. In the related species E. coli, proton reduction is catalyzed by the nickel-dependent hydrogenase 3 of the formate hydrogen lyase complex (25), whereas the nickel-containing hydrogenases 1 and 2 are uptake hydrogenases (27). We propose that during citrate fermentation, K. pneumoniae derives the reducing equivalents necessary for the biosynthesis of cell matter from H2 that is generated by formate hydrogen lyase and recaptured by an NAD(P)+-dependent hydrogenase (Fig. 1). This hypothesis is supported by the increase in cell yield in the presence of Ni2+ and the replacement of H2 by formate as the electron donor for the reduction of NAD(P)+ in cell suspensions of K. pneumoniae.
To our knowledge, this is the first report on an active, membrane-bound NAD(P)+-reducing hydrogenase from a facultatively anaerobic, gram-negative bacterium. A membrane association of NAD(P)+-dependent hydrogenase was reported for the aerobic, non-N2-fixing cyanobacterium Anacystis nidulans (22). In the obligately anaerobic, gram-negative bacterium Acidaminococcus fermentans, a membrane-bound H2:NAD+ oxidoreductase was proposed to generate H2 from NADH during glutamate fermentation (16), but only H2:benzyl viologen and NADH:iodonitrosotetrazolium oxidoreductase activities were detected in membranes (17).
The activity of the NAD(P)+-reducing hydrogenase of K. pneumoniae increases up to 40-fold in the presence of potassium phosphate compared to Good buffers containing Na+. Increased specific activities in the presence of K+ compared to Na+ acting as the inhibitor have also been reported for the cytoplasmic NAD+-dependent hydrogenase from Alcaligenes eutrophus (18). The latter enzyme did not reduce NADP+, although NADH and NADPH were oxidized in the presence of artificial electron acceptors (30). NADP+, but not NAD+, was reduced by the soluble hydrogenase from Desulfovibrio fructosovorans (20). In contrast, both NAD+ and NADP+ are reduced by H2 in the presence of membrane vesicles from K. pneumoniae. Since the pH optima for NAD+ and NADP+ reduction by membrane vesicles differ significantly, it will be of interest to investigate whether there are two different hydrogenases in K. pneumoniae acting on NAD+ and NADP+. So far, little is known about the number and types of hydrogenases present during anaerobic growth of K. pneumoniae on citrate. Recently, a fourth hydrogenase was identified in E. coli based on sequence analysis (3). This hydrogenase is part of a putative formate hydrogen lyase system (hyf operon) and comprises open reading frames which exhibit homology to subunits of the proton-translocating NADH:ubiquinone oxidoreductase. However, no formate- or H2-dependent NAD(P)+ reduction has been demonstrated in E. coli.
It has previously been demonstrated that K. pneumoniae possesses an Na+-translocating NADH:ubiquinone oxidoreductase (12). This enzyme, by acting in the direction of NAD+ reduction, could therefore provide an alternative route of NADH formation for assimilatory reactions of the cell. This hypothesis was previously investigated with cell suspensions and membrane vesicles, and the data seemed to indicate that NADH could be formed by Na+-dependent, reversed electron transfer from formate (23). The discovery of an energy-independent route of NAD(P)+ reduction in K. pneumoniae reported here has initiated a repetition of the previous experiments, with the clear result that the reported conclusions concerning NADH formation by reversed electron transfer are erroneous. The observed reduction of NAD+ to NADH apparently resulted from dithionite in the assay mixtures. In control experiments, NAD+ (0.1 mM) was completely reduced to NADH by dithionite (1 mM) in 30 s at an apparent rate of 0.18 μmol min−1 in the absence of cellular fractions (not shown).
In summary, we conclude that K. pneumoniae fermenting citrate forms hydrogen which is used by a membrane-bound hydrogenase to reduce NAD(P)+ to NAD(P)H.
ACKNOWLEDGMENTS
This work was supported by a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft to J.S. We thank H. Cousin, Swiss Federal Institute of Technology, Zürich, Switzerland, for the analysis of nickel.
REFERENCES
- 1.Albracht S P J. Nickel hydrogenases: in search of the active site. Biochim Biophys Acta. 1994;1188:167–204. doi: 10.1016/0005-2728(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 2.Alemohammad M M, Knowles C J. Osmotically induced volume and turbidity changes of Escherichia coli due to salts, sucrose and glycerol, with particular reference to the rapid permeation of glycerol into the cell. J Gen Microbiol. 1974;82:125–142. doi: 10.1099/00221287-82-1-125. [DOI] [PubMed] [Google Scholar]
- 3.Andrews S C, Berks B C, McClay J, Ambler A, Quail M A, Golby P, Guest J R. A 12-cistron Escherichia coli operon (hyf) encoding a putative proton-translocating formate hydrogenlyase system. Microbiology. 1997;143:3633–3647. doi: 10.1099/00221287-143-11-3633. [DOI] [PubMed] [Google Scholar]
- 4.Böck A, Sawers G. Fermentation. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 262–282. [Google Scholar]
- 5.Bott M. Anaerobic citrate metabolism and its regulation in enterobacteria. Arch Microbiol. 1997;167:78–88. [PubMed] [Google Scholar]
- 6.Brewer C R, Werkman C H. The anaerobic dissimilation of citric acid by Aerobacter indologenes. Enzymologia. 1939;6:273–281. [Google Scholar]
- 7.Chance B. Spectrophotometry of intracellular respiratory pigments. Science. 1954;120:767–775. doi: 10.1126/science.120.3124.767. [DOI] [PubMed] [Google Scholar]
- 8.Dagley S, Dawes E A. Citric acid metabolism of Aerobacter aerogenes. J Bacteriol. 1953;66:259–265. doi: 10.1128/jb.66.3.259-265.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimroth P. Preparation, characterization, and reconstitution of oxaloacetate decarboxylase from Klebsiella aerogenes, a sodium pump. Methods Enzymol. 1986;125:530–540. doi: 10.1016/s0076-6879(86)25042-9. [DOI] [PubMed] [Google Scholar]
- 10.Dimroth P. The role of vitamins and their carrier proteins in citrate fermentation. In: Kleinkauf H, Von Döhren H, Jaenicke J, editors. The roots of modern biochemistry. Berlin, Germany: De Gruyter; 1988. pp. 191–204. [Google Scholar]
- 11.Dimroth P. Primary sodium ion translocating enzymes. Biochim Biophys Acta. 1997;1318:11–51. doi: 10.1016/s0005-2728(96)00127-2. [DOI] [PubMed] [Google Scholar]
- 12.Dimroth P, Thomer A. A primary respiratory Na+ pump of an anaerobic bacterium: the Na+-dependent NADH:quinone oxidoreductase of Klebsiella pneumoniae. Arch Microbiol. 1989;151:439–444. doi: 10.1007/BF00416604. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich B, Heine E, Finck A, Friedrich C G. Nickel requirement for active hydrogenase formation in Alcaligenes eutrophus. J Bacteriol. 1981;145:1144–1149. doi: 10.1128/jb.145.3.1144-1149.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedrich B, Schwartz E. Molecular biology of hydrogen utilization in aerobic chemolithotrophs. Annu Rev Microbiol. 1993;47:351–383. doi: 10.1146/annurev.mi.47.100193.002031. [DOI] [PubMed] [Google Scholar]
- 15.Glusker J P. Citrate conformation and chelation: enzymatic implications. Acc Chem Res. 1980;13:345–352. [Google Scholar]
- 16.Härtel U, Buckel W. Sodium-ion dependent hydrogen production in Acidaminococcus fermentans. Arch Microbiol. 1996;166:350–356. doi: 10.1007/s002030050394. [DOI] [PubMed] [Google Scholar]
- 17.Härtel U, Buckel W. Fermentation of trans-aconitate via citrate, oxaloacetate, and pyruvate by Acidaminococcus fermentans. Arch Microbiol. 1996;166:342–349. doi: 10.1007/s002030050393. [DOI] [PubMed] [Google Scholar]
- 18.Keefe R G, Axley M J, Harabin A L. Kinetic studies of the soluble hydrogenase from Alcaligenes eutrophus H16. Arch Biochem Biophys. 1995;317:449–456. doi: 10.1006/abbi.1995.1187. [DOI] [PubMed] [Google Scholar]
- 19.Lide D R. CRC handbook of chemistry and physics. 76th ed. Boca Raton, Fla: CRC Press; 1995. [Google Scholar]
- 20.Malki S, Saimmaime I, de Luca G, Rousset M, Dermoun Z, Belaich J-P. Characterization of an operon encoding an NADP-reducing hydrogenase in Desulfovibrio fructosovorans. J Bacteriol. 1995;177:2628–2636. doi: 10.1128/jb.177.10.2628-2636.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien R W, Stern J R. Requirement for sodium in the anaerobic growth of Aerobacter aerogenes on citrate. J Bacteriol. 1969;98:388–393. doi: 10.1128/jb.98.2.388-393.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peschek G A. Evidence for two functionally distinct hydrogenases in Anacystis nidulans. Arch Microbiol. 1979;123:81–92. [Google Scholar]
- 23.Pfenninger-Li X D, Dimroth P. NADH formation by Na+-coupled reversed electron transfer in Klebsiella pneumoniae. Mol Microbiol. 1992;6:1943–1948. doi: 10.1111/j.1365-2958.1992.tb01367.x. [DOI] [PubMed] [Google Scholar]
- 24.Pos K M, Dimroth P. Functional properties of the purified Na+-dependent citrate carrier of Klebsiella pneumoniae: evidence for asymmetric orientation of the carrier protein in proteoliposomes. Biochemistry. 1996;35:1018–1026. doi: 10.1021/bi951609t. [DOI] [PubMed] [Google Scholar]
- 25.Rossmann R, Sauter M, Lottspeich F, Böck A. Maturation of the large subunit (HYCE) of Escherichia coli hydrogenase 3 requires nickel incorporation followed by C-terminal processing at Arg537. Eur J Biochem. 1994;20:377–384. doi: 10.1111/j.1432-1033.1994.tb18634.x. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 27.Sawers G. The hydrogenases and formate dehydrogenases of Escherichia coli. Antonie Leeuwenhoek. 1994;66:57–88. doi: 10.1007/BF00871633. [DOI] [PubMed] [Google Scholar]
- 28.Sawers R G, Ballantine S P, Boxer D H. Differential expression of hydrogenase isoenzymes in Escherichia coli K-12: evidence for a third isoenzyme. J Bacteriol. 1985;164:1324–1331. doi: 10.1128/jb.164.3.1324-1331.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schink B, Friedrich M. Energetics of syntrophic fatty acid oxidation. FEMS Microbiol Rev. 1994;15:85–94. [Google Scholar]
- 30.Schneider K, Schlegel H G. Purification and properties of soluble hydrogenase from Alcaligenes eutrophus H 16. Biochim Biophys Acta. 1976;452:66–80. doi: 10.1016/0005-2744(76)90058-9. [DOI] [PubMed] [Google Scholar]
- 31.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 32.Volbeda A, Charon M-H, Piras C, Hatchikian E C, Frey M, Fontecilla-Camps J-C. Crystal structure of the nickel-iron hydrogenase from Desulfovibrio gigas. Nature. 1995;373:580–587. doi: 10.1038/373580a0. [DOI] [PubMed] [Google Scholar]
- 33.Wimpenny J W, Firth A. Levels of nicotinamide dinucleotide and reduced nicotinamide dinucleotide in facultative bacteria and the effect of oxygen. J Bacteriol. 1972;111:24–32. doi: 10.1128/jb.111.1.24-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu L F, Mandrand M A. Microbial hydrogenases: primary structure, classification, signatures and phylogeny. FEMS Microbiol Rev. 1993;104:243–270. doi: 10.1111/j.1574-6968.1993.tb05870.x. [DOI] [PubMed] [Google Scholar]