Abstract
PrtP is a major cysteine proteinase of Porphyromonas gingivalis. The gene encoding this proteinase, prtP, was cloned into the Escherichia coli-Bacteroides shuttle vectors pFD288 and pFD340 and was expressed in Bacteroides cells, apparently under the control of its own promoter, when in pFD288, or a Bacteroides promoter present on pFD340. Proteolytically active PrtP was detected by fibrinogen zymography in cells or spent growth medium of several Bacteroides species harboring the recombinant plasmids. The proteinase was recovered from Bacteroides fragilis ATCC 25285(pFD340-prtP) cells by 3-[(3-cholamidopropyl)-dimethyl-ammonio]-1-propanesulfonate (CHAPS) extraction and characterized with regard to exopeptidase specificity and sensitivity to proteinase inhibitors. Lys-amidolytic activity, but not Arg-amidolytic activity, was detected. PrtP was activated by cysteine and, to a lesser extent, dithiothreitol, and it was stimulated by glycine-containing compounds. It also was inhibited by Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK) and, to a lesser extent, H-d-Tyr-l-Pro-l-arginyl chloromethyl ketone (YPRCK) and was relatively insensitive to EDTA and leupeptin. Neither B. fragilis ATCC 25285(pFD340-prtP) cells nor the CHAPS extract effected hemagglutination of sheep red blood cells or collagen cleavage, but the cells did cleave gelatin. Furthermore, P. gingivalis W12, ATCC 33277, KDP110, and HG66 with knockout mutations in prtP were constructed by allelic replacement. Unlike the parent strains, the mutant strains produced beige colonies on plates containing sheep blood. These strains also were affected in their ability to effect hemagglutination, cleave collagen, and cleave a Lys-specific peptide substrate. This report presents the results of the first characterization of the PrtP proteinase clearly in the absence of any influence by other P. gingivalis proteins and describes the properties of P. gingivalis cells defective in the production of PrtP.
Periodontitis is a major cause of tooth loss in the adult population, and several recent studies suggest that it may be a significant risk factor for both cardiovascular disease and preterm labor in humans (for a review, see reference 32). The potential medical importance of these oral infections justifies an intensified effort to develop effective strategies to prevent periodontitis as well as to interfere with disease progression. Accomplishing this will require identification of the major causative agents of periodontitis, as well as an understanding of the mechanisms by which these bacteria contribute to the destruction of connective tissue and bone that characterizes periodontitis lesions. Those virulence factors which render periodontal pathogens uniquely capable of contributing to the destruction of the supporting structures of the teeth, including periodontal connective tissues, periodontal ligaments, and alveolar bone, would be logical targets for the development of novel, highly specific antimicrobial agents (20).
Porphyromonas gingivalis is one of a small number of bacteria implicated in the etiology and pathogenesis of human periodontitis, and its proteinases appear to be among its most important virulence factors (16, 26, 27). These enzymes, which can be recovered from P. gingivalis cells and vesicles as well as from spent growth medium (2), are capable of degrading a variety of substrates, including gelatin, fibrinogen, fibronectin, C3, C5, and C5a receptor (14, 17–19, 51). Moreover, they clearly have the potential to contribute directly, as well as indirectly (via dysregulation of host proteinase cascade systems and the inflammatory response [16]), to destruction of periodontal tissues. Results of early studies suggested that P. gingivalis elaborates a bewildering number of distinct proteolytic enzymes, including at least one which is a collagenase (4). They also suggested that some of these proteinases can function as hemagglutinins (31) as well as adhesins for several host connective tissue matrix and plasma proteins (17, 19). The proteinase that we called porphypain was isolated from P. gingivalis W12 cells as sodium dodecyl sulfate (SDS)-stable conformers (150 and 120 kDa) of a 180-kDa proteinase (6). A proteinase called Lys-gingipain (also gingipain-K) was purified from spent culture medium, following growth of P. gingivalis HG66, as a 105-kDa complex containing a 60-kDa catalytic moiety (35, 37). While these proteinases have properties in common, significant differences in some of their properties were reported. Porphypain appears to contain two types of active sites, one with Lys-X activity and one with Arg-X activity. The amidolytic activity of both types of sites is greatly stimulated by derivatives of glycine and is inhibited by EDTA (6). Lys-gingipain, on the other hand, has been reported to have amidolytic activity exclusively for Lys-X but not Arg-X; it is inhibited by derivatives of glycine and is unaffected by EDTA (37). Since these proteinases were thought to be products of the same gene (2, 35), the difference between the reported activities of the purified enzymes was difficult to explain. Moreover, in spite of the fact that results of inhibitor studies have suggested that the collagenase of P. gingivalis has Lys-X and Arg-X activity (4), it is still unclear what, if any, relationship exists between porphypain, Lys-gingipain, and the collagenase of P. gingivalis.
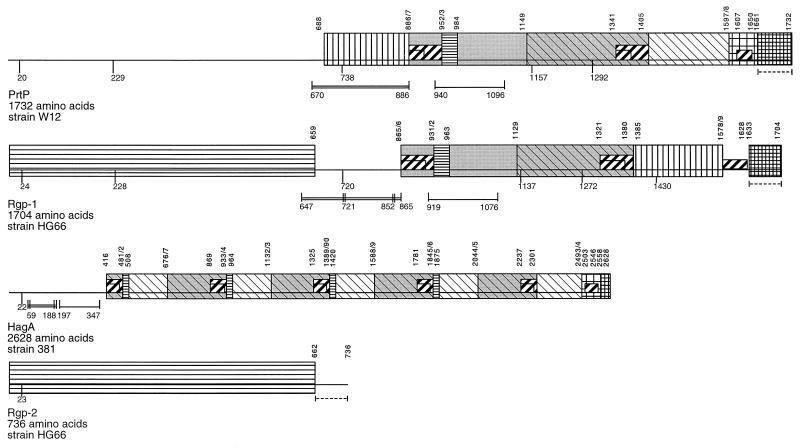
Results of more-recent biochemical and genetic studies (2, 11, 29, 39, 42) have suggested that 85 to 95% of the total proteolytic activity of P. gingivalis is attributable to three proteinases, PrtP (also called Kgp), Rgp-1, and Rgp-2. These enzymes are the products of three related genes (prtP [kgp], rgp-1, and rgp-2, respectively), and they appear to represent a unique family of cysteine proteinases (35). The gene referred to as rgp-1 (36) has also been designated prpRI (1), prtR (45), agpA (34), and rgpA (29) and encodes a 180-kDa protein. A second gene, which we refer to as rgp-2 (GenBank accession no. U85038), is also called prR2 (42), prtRII (GenBank accession no. AF007124), agpB (28), and rgpB (29), and it encodes an 80-kDa protein that is almost identical to the catalytically active region of the product of rgp-1 (42). Finally, prtP (2), like rgp-1 (33), encodes an approximately 180-kDa proteinase. Comparisons of the deduced amino acid sequences of PrtP, Rgp-1, Rgp-2, and a fourth, related P. gingivalis protein, the hemagglutinin HagA, are shown in Fig. 1. The N-terminal two-fifths of the PrtP and Rgp-1 molecules is thought to contain the active sites of the enzymes, and the catalytic Cys residues have been mapped to these domains (35). The C-terminal three-fifths of these two proteinases is composed of regions that are highly homologous to each other and to much of HagA (2). No reports suggesting that HagA has cysteine proteinase activity have appeared.
FIG. 1.
Comparison of PrtP with Rgp-1, Rgp-2, and HagA. Each protein is represented in a linear fashion; HagA is shown at half-scale. Putative cleavage sites in PrtP and Rgp-1 are shown below each protein. Regions in PrtP, Rgp-1, Rgp-2, and HagA with 90% or greater identity are indicated by identical boxes; regions with 50 to 60% identity are similarly underlined. (Modified from reference 2).
It is unclear exactly which regions of HagA mediate hemagglutination. It appeared that a peptide (G-907 to T-919) derived from the C-terminal three-fifths of Rgp-1 inhibited hemagglutination by spent culture medium from P. gingivalis W50 (8), and a monoclonal antibody capable of blocking hemagglutination mapped to an epitope containing the same amino acid residues (7). This same peptide is located in the C-terminal three-fifths of PrtP and can also be found in HagA (2). It is unclear whether other peptides shared by these three gene products can mediate hemagglutination, and it is also unclear what contribution each of these gene products makes to the total hemagglutinating activity of P. gingivalis. Finally, regions of these proteins that mediate binding of P. gingivalis to host connective tissue and plasma proteins have not yet been identified.
The PrtP and Rgp proteinases have been challenging to deal with biochemically. The full-length forms of Rgp-1 and PrtP are the same size (molecular weight, ∼180,000; PrtP is 1,732 amino acids in length, and Rgp-1 is 1,704 amino acids in length); they share regions of amino acid identity with each other and with HagA; and they autodegrade and possibly process each other during purification procedures (2, 6). Much of the confusion regarding the number, sizes, and properties of the proteinases of P. gingivalis can be attributed to these properties. Furthermore, their proteolytic degradation products, some of which are similar in size and amino acid sequence, autoaggregate and tend to stay tightly, though noncovalently, associated in solution, even in the presence of SDS (6). For these reasons, they copurify even when many differently based methods are applied for their separation (1, 6, 11, 38). In addition, the catalytic domains of Rgp-1 and Rgp-2, which are virtually indistinguishable biochemically (42), copurify from the P. gingivalis background. The existence of Rgp-2 as a gene product distinct from Rgp-1 was revealed only after identification of a second genomic locus encoding an arginine-specific proteinase (1, 29).
Absolute separation of Rgp-1, Rgp-2, PrtP, HagA, and their proteolytically processed products from wild-type P. gingivalis cultures may well prove impossible to achieve, which would seriously hamper structure-function studies of these proteins. Expression of these genes in a heterologous host could provide a means for examining the functions of each of these proteins in the clear absence of the others. Coupling the results of these studies with the results of characterizations of corresponding P. gingivalis knockout mutants could provide a means of elucidating the functions of these proteins in P. gingivalis. No reports describing the expression of catalytically active proteinase from the rgp-1, rgp-2, or prtP gene cloned in Escherichia coli or any other prokaryotic heterologous host have appeared, although we (1a) and others (1) have expressed in E. coli, from cloned rgp-1 and prtP genes, proteins immunoreactive with antibodies raised against these proteinases. To date, the expression using a Baculovirus system of only a portion of kgp (prtP), containing just the purported catalytic domain, has been reported (35). The authors stated that supernatants from infected Sf9 cells contained a low level of Lys-specific amidolytic activity, but apparently they conducted no further analysis of the recombinant enzyme. Furthermore, while P. gingivalis strains with inactivated rgp genes have been previously constructed and partially characterized (15, 29, 30, 50, 52), P. gingivalis cells lacking a functional prtP gene have not been described. The purpose of this study was to develop a system for expression of prtP in a heterologous prokaryotic host and to characterize the recombinant enzyme in terms of its specificity, behavior in the presence of stimulators and inhibitors of proteolysis, ability to cleave type I collagen, and ability to function as a hemagglutinin. In addition, we sought to determine the effect of deletion of PrtP functions on P. gingivalis cells.
MATERIALS AND METHODS
Reagents.
Cysteine, N-p-tosyl-Gly-Pro-Arg p-nitroanilide (GPR-pNA), N-p-tosyl-Gly-Pro-Lys p-nitroanilide (GPK-pNA), leupeptin, N-α-p-tosyl-l-lysyl chloromethyl ketone (TLCK), glycylglycine, glycinamide, human fibrinogen, gelatin, bovine pancreatic trypsin, Triton X-100, and 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) were purchased from Sigma Chemical Company (St. Louis, Mo.). H-d-Tyr-l-Pro-l-arginyl chloromethyl ketone (YPRCK) was purchased from Bachem Bioscience Inc. (King of Prussia, Pa.). Clostridial collagenase was purchased from Worthington Biochemical Corp. (Freehold, N.J.). Chemically competent E. coli cells, restriction enzymes, and T4 DNA ligase were obtained from Life Technologies (Grand Island, N.Y.). Mung bean nuclease was obtained from New England Biolabs (Beverly, Mass.). Plasmid DNA was prepared by using either BioExpress matrix and spin columns (Bio101, Vista, Calif.) or Big Prep (5′-3′, Boulder, Colo.). DNA fragments were isolated from agarose gels by using GeneClean (Bio101). Sheep blood was obtained from Colorado Serum Company (Denver, Colo.).
Bacterial strains, plasmids, and growth conditions.
P. gingivalis W12, HG66 (a gift from R. Arnold, University of North Carolina School of Dentistry, Chapel Hill), and ATCC 33277 were maintained on enriched trypticase soy agar plates containing 3% sheep blood (ETSA) as previously described (19). Broth cultures were grown in brain heart infusion (BHI) broth containing 1 mg of cysteine, 5 μg of hemin, and 0.5 μg of menadione/ml (supplemented BHI broth [46]). Cultures were incubated in an anaerobic chamber (Coy Laboratories, Ann Arbor, Mich.) with an atmosphere of 85% nitrogen, 10% hydrogen, and 5% carbon dioxide. Strain KDP110, a derivative of strain ATCC 33277 with an insertion in the rgp-1 gene, was maintained in the presence of 10 μg of erythromycin/ml (29). Derivatives of P. gingivalis W12, HG66, ATCC 33277, and KDP110 with insertion mutations in the prtP gene that were constructed during this study were maintained in the presence of 1 μg of tetracycline/ml. P. gingivalis cells were collected from ETSA plates, placed in 0.5-ml aliquots of sheep blood, and stored frozen at −70°C.
Cloning, subcloning, and mating experiments involving E. coli were conducted with strain DH5α (3), which was grown in Luria-Bertani (LB) broth or on LB agar (24). Bacteroides fragilis IB101 (Rifr Genr) and ATCC 25285 (Rifs Genr), Bacteroides ovatus IB103 (Rifs Genr), Bacteroides thetaiotaomicron BT5482 (Rifr Genr), and Bacteroides uniformis BU1001 (Rifr Genr) were gifts from C. Jeffrey Smith (East Carolina University School of Medicine, Greenville, N.C.). These cultures were maintained under anaerobic conditions on ETSA and supplemented BHI broth or agar as described for P. gingivalis. Rifampicin (Rif; 20 μg/ml) and/or gentamicin (Gen; 25 μg/ml) was incorporated into media for selection of Bacteroides species during triparental mating experiments (43).
Plasmids pFD288 (47) and pFD340 (48), gifts from C. Jeffrey Smith, are E. coli-Bacteroides shuttle vectors. These plasmids and their derivatives were maintained in E. coli in the presence of 25 μg of spectinomycin/ml or 100 μg of carbenicillin/ml, respectively, and they were maintained in Bacteroides species in the presence of 10 μg of erythromycin/ml. Plasmid RK231 (12) (also a gift from C. Jeffrey Smith) was used to provide mobilization functions in triparental matings (43). It cannot replicate in Bacteroides strains; hence, it was maintained in E. coli in the presence of 25 μg of kanamycin/ml. Plasmid pMJF3 (9), a gift from Koji Nakayama, carries the tetA(Q) gene. Plasmid pNH5 is a derivative of pBlueScript SK(+) containing prtP (2). The last two plasmids were maintained in E. coli in the presence of 100 μg of carbenicillin/ml.
Construction of Bacteroides strains containing recombinant prtP.
Initially, prtP from pNH5 was subcloned onto the E. coli-Bacteroides shuttle vector pFD288 (47). Subsequently, the prtP gene also was subcloned downstream of the IS4315-derived Bacteroides promoter present on the shuttle vector pFD340 (48). To make these constructs, pNH5 was digested with BsrBI and SstI and plasmids pFD288 and pFD340 were digested with SmaI and SstI. A 6.6-kb fragment of pNH5, containing the prtP gene and 471 bp of upstream and 995 bp of downstream P. gingivalis chromosomal DNA, was isolated and ligated to the isolated, linearized vectors. Subclones were obtained after transformation of E. coli with the ligation mixtures, and the plasmid structures were confirmed by restriction endonuclease analyses. The resulting plasmids, pFD288-prtP and pFD340-prtP, were mobilized into Bacteroides cells by a triparental filter mating procedure (43), with RK231 as a helper plasmid (12). Transconjugant colonies were streaked for isolation on erythromycin-supplemented ETSA and stored as described above.
Construction of P. gingivalis prtP-defective mutants.
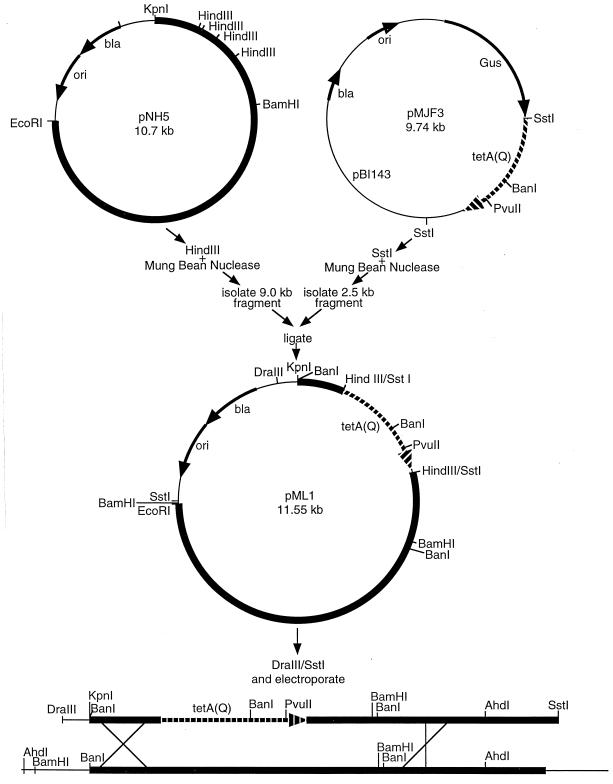
Mutants of P. gingivalis in which the wild-type prtP was replaced by a defective allele were constructed as follows. The first step involved construction of pML1 (Fig. 2), a plasmid that contained prtP into which tetA(Q) had been inserted in place of the region encoding the putative catalytic domain of PrtP. Recombinant plasmid pNH5, containing prtP, was digested with HindIII, yielding 9.0-kb and smaller fragments. The 9.0-kb fragment contained all of the original plasmid except for 1.7 kb of prtP derived from a unique region of the gene (2). Plasmid pMJF3, encoding tetA(Q), was digested with SstI, yielding a 7.2-kb and a 2.5-kb fragment, the latter containing the tetA(Q) gene. Each plasmid digest was treated with mung bean nuclease to create blunt ends, and the 9.0- and 2.5-kb fragments from pNH5 and pMJF3, respectively, were isolated from agarose gels following electrophoresis. Aliquots of the two fragments were mixed, and the mixture was treated with T4 DNA ligase and then used to transform E. coli, with selection for resistance to carbenicillin (encoded by the bla gene from pNH5). The tetA(Q) gene is expressed in Bacteroides spp. (44) and in P. gingivalis (23) but not in E. coli. The resulting constructs were checked for insertion and orientation of the tetA(Q) gene by restriction endonuclease analyses, and a representative plasmid was chosen and designated pML1. This construct was then cleaved with DraIII and SstI to linearize the DNA encoding prtP::tetA(Q) and to separate it from most of the vector sequences. The restriction endonucleases were heat inactivated, and the entire digestion reaction mixture was introduced into P. gingivalis W12, HG66, ATCC 33277, and KDP110 by electroporation as previously described (10). Cells in which prtP had been replaced with prtP::tetA(Q) due to a double-crossover event were recovered by selection for tetracycline resistance.
FIG. 2.
Construction of pML1 and allelic replacement in the P. gingivalis chromosome. Plasmid pNH5 consists of pBlueScript SK(+) with 6.8 kb of DNA, including the prtP gene, from P. gingivalis W12 (2). The P. gingivalis DNA is indicated by a very thick line. Plasmid pMJF3 harbors the tetA(Q) gene (8), which is indicated by the broken line. These plasmids were treated with HindIII or SstI, respectively, and mung bean nuclease. The 9.0-kb fragment from pNH5 and the 2.5-kb fragment from pMJF3 were isolated, purified, and ligated to one another to form pML1. This plasmid was linearized by digestion with DraIII and SstI, and the digestion mixture was introduced into P. gingivalis cells by electroporation. Recovery of tetracycline-resistant cells indicated that a double-crossover event had occurred in prtP.
Southern blot hybridizations.
Southern blot hybridizations were performed as previously described (2). Probes were labeled by using the ECL random primer labeling kit (Amersham, Arlington Heights, Ill.).
Zymography.
Proteinase activity in Bacteroides strains harboring plasmid derivatives was detected by using fibrinogen-containing gels as previously described (19). Human fibrinogen was added at 1 mg/ml to an 8% polyacrylamide resolving gel prior to polymerization. The upper, 4.8% polyacrylamide stacking gel did not contain fibrinogen. Overnight broth cultures were prepared, and cells from 1.5 ml of culture were collected by centrifugation and resuspended in 150 μl of 50 mM Tris-Cl, pH 7.5. Cells were subjected to a total of four 20-s bursts from a Sonifier 250 with microtip at the maximum setting (Branson Ultrasonics Corp., Danbury, Conn.). In addition, 10 ml of spent medium from each of the cultures was concentrated to a 1-ml final volume in a Centriplus filtration unit (Amicon, Beverly, Mass.). Three microliters of the sonicate with 2 M urea added or 12 μl of concentrated medium was loaded per lane. Samples were prepared at room temperature in the absence of reducing agents, and electrophoresis was carried out at 180 V and 4°C. After electrophoresis, the gel was washed twice, for 15 min each, with 2.5% Triton X-100 in distilled water, twice with 2.5% Triton X-100 in 50 mM Tris-Cl (pH 7.5), and twice with 50 mM Tris-Cl (pH 7.5). The proteinases were activated by washing the gel at 37°C for 1.5 h in 50 mM Tris-Cl (pH 7.5) containing 50 mM cysteine. The gels were then stained with Coomassie brilliant blue R-250. Proteolytic activity was visualized as a clear area in the blue background.
Gelatin zymography was used to demonstrate proteolytic activity of P. gingivalis strains. Optical densities at 550 nm (OD550) were determined for overnight cultures of cells grown in supplemented BHI. Cells were pelleted, resuspended to an OD550 of 4 in 50 mM Tris-Cl (pH 7.5), and sonicated as described above. One microliter of the sonicate was diluted to 30 μl, and 10 μl of that sample were used in SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Gels were treated as described for fibrinogen-containing gels, except that each of the washes contained 10 mg of gelatin/ml and the development process was allowed to proceed for 15 min (instead of 1.5 h).
Preparation of crude CHAPS extracts.
Extractions with CHAPS were performed to partially purify PrtP from B. fragilis ATCC 25285 harboring pFD340-prtP. Cells from overnight cultures grown in 1 liter of supplemented BHI broth were pelleted at 3,000 × g, washed in one-half volume of 60 mM Tris-Cl (pH 7.9), resuspended in 200 ml of 60 mM Tris-Cl (pH 7.9)–0.1% CHAPS, and rotated on a platform shaker at 250 rpm for 2 h at room temperature. Cells and large debris were pelleted by centrifugation at 3,000 × g for 20 min. The supernatant was concentrated in an Amicon stirred cell with a YM100 membrane and clarified by centrifugation and passage through a sterile 0.22-μm-pore-size filter. This material was further concentrated to a volume of 1 to 2 ml by using a Centricon-30 filtration device (Amicon). This procedure was developed to eliminate problems associated with cell interference with absorbance readings during peptide substrate analyses and to concentrate the recombinant PrtP, and it was not further optimized.
Peptide substrate hydrolysis.
The initial rates of GPK-pNA and GPR-pNA hydrolysis were determined by measuring the increase in A405 as a function of time at 22°C as previously described (6). Enzyme mixes consisted of 100 μl of the concentrated CHAPS extract described above, 50 mM Tris-Cl (pH 7.9), and 50 mM cysteine in a total volume of 150 μl. The enzyme mix was incubated for 5 min at 22°C, and then 100 μl was added to a prewarmed 1-ml substrate cocktail consisting of 50 mM Tris-Cl (pH 7.9), 100 mM cysteine, 0.1% Triton X-100, and 200 nM substrate.
Cleavage of gelatin.
Gelatin was prepared by heating rat tail collagen (prepared by L. Jack Windsor, University of Alabama, Birmingham) at 65°C for 30 min. B. fragilis cells harboring either the vector pFD340 or its derivate pFD340-prtP were grown to late log phase in supplemented BHI. Cells were recovered by centrifugation and resuspended in assay buffer at a concentration of 1 g (wet weight)/ml; 100 μl of cell suspension was added to a tube containing 250 μl of 5 mM dithiothreitol (DTT) in phosphate-buffered saline (24) plus 15 μg of gelatin. Reaction mixtures were incubated overnight at 37°C on a revolving mixer, and then 15 μl of each sample was assayed by SDS-PAGE after being heated at 100°C for 3 min. The gels were stained with Coomassie brilliant blue R-250 to visualize protein bands.
Hemagglutination assays.
Assays to measure the hemagglutinating activity of P. gingivalis cells were performed essentially as described previously (41). Briefly, 100 μl of cells resuspended in phosphate-buffered saline (24) at an OD550 of 2 were serially diluted in twofold increments. An equal volume of 1% washed sheep blood was added to each dilution, and the reaction mixtures were incubated overnight at room temperature. Hemagglutination was detected as cloudiness, hemolysis resulted in a clear well, and no reaction was evidenced by a dot of erythrocytes in the bottom of the well.
Determination of collagenase activity.
Assays for collagenase activity were performed by a modification of a previously described method (4). [3H]Collagen (rat tail tendon, type I) was mixed with unlabeled collagen (rat tail tendon, type I) to give a specific activity of 66,667 cpm/mg at a concentration of 3 mg/ml in 13 mM HCl. Immediately prior to use, the solution was neutralized with one-fifth volume of neutralizing phosphate buffer, which was prepared by combining 1 volume each of 200 mM sodium phosphate buffer (pH 7.4) and 0.1 N NaOH with one-tenth volume of 5 M NaCl. Aliquots (35 μl) were dispensed into microcentrifuge tubes, and fibrils were allowed to form at 35°C for 2 h. Assay buffer (50 mM Tris-Cl [pH 7.5], 200 mM NaCl, 5 mM CaCl2) was then layered over the surface of the gel, and the tubes were kept at 35°C overnight. Cells (50 μg [wet weight]) in 100 μl of assay buffer containing 1 mM DTT were added to each tube after the equilibration buffer was removed. Samples were assayed in duplicate. After overnight incubation at 37°C, the level of 3H released into the supernatant buffer was determined. Trypsin and clostridial collagenase controls were included in each experiment. The amount of 3H released by clostridial collagenase was considered to be the result of 100% collagen digestion.
RESULTS
Expression of prtP in Bacteroides species.
The prtP gene and surrounding DNA from P. gingivalis W12 has been cloned into pBlueScript SK(+) (Stratagene, La Jolla, Calif.) in E. coli DH5α (2). Catalytically active proteinase was not expressed from this construct, pNH5, and subsequent attempts to produce catalytically active PrtP in E. coli were unsuccessful. It was reasoned that active PrtP might be produced in Bacteroides species, since this genus is closely related to Porphyromonas. Therefore, prtP was cloned onto two E. coli-Bacteroides shuttle vectors (see Materials and Methods). The vector pFD340 supplied a promoter known to be active in Bacteroides species, whereas pFD288 did not. Initially, pFD288-prtP was introduced into several Bacteroides species capable of supporting the replication of this plasmid to assess the possibility of expression of prtP in these hosts. Plasmid-containing Bacteroides species cells and spent medium were analyzed by fibrinogen zymography for the presence of active PrtP (Fig. 3). We previously demonstrated that porphypain purified from P. gingivalis W12 cells cleaves fibrinogen and, when subjected to SDS-PAGE, migrates as two conformational variants of a 180-kDa protein, appearing as 120- and 150-kDa proteins in the gels (6). When the Bacteroides cells harbored pFD288-prtP, low levels of proteolytic activity were detected in bands of these sizes from the culture supernatants of B. fragilis IB101 and from cells of B. fragilis ATCC 25285, B. ovatus IB103, and B. thetaiotaomicron BT5482 (Fig. 3). Catalytically active enzyme was not detected in either cells or spent medium from B. uniformis BU1001 (data not shown). In an attempt to increase expression of prtP in Bacteroides species, the construct pFD340-prtP was introduced into B. fragilis ATCC 25285 and B. ovatus IB103. Cells containing pFD340-prtP produced a higher level of active enzyme, as determined by fibrinogen zymography, than cells harboring pFD288-prtP (Fig. 4). For this reason, B. fragilis ATCC 25285(pFD340-prtP) cells were used for the experiments described below.
FIG. 3.
Zymogram showing PrtP activity in Bacteroides strains harboring pFD288-prtP. Solubilized cells (lanes A) and concentrated, spent media (lanes B) were analyzed for cleavage of fibrinogen after SDS-PAGE. The gel was washed in solutions containing Triton X-100 to sequester the SDS, and then the proteinase was activated by washing the gel in 50 mM cysteine. After Coomassie blue staining, the digestion of fibrinogen was detected as clear spaces in the gel. The positions of molecular mass markers (in kilodaltons) are indicated to the left. Lanes: 1, B. fragilis ATCC 25285; 2, B. ovatus IB103; 3, B. thetaiotaomicron BT5482; 4, B. fragilis IB101.
FIG. 4.
Zymogram showing PrtP activity in Bacteroides strains harboring pFD340-prtP or pFD288-prtP. Solubilized cells harboring pFD288-prtP (lanes A) or pFD340-prtP (lanes B) were analyzed for cleavage of fibrinogen after SDS-PAGE. The gel was treated as described in the legend to Fig. 3. The positions of molecular mass markers (in kilodaltons) are indicated to the left. Lanes: 1, B. ovatus IB103; 2, B. fragilis ATCC 25285.
Characterization of recombinant PrtP.
Porphypain isolated from P. gingivalis W12 cells cleaved gelatin (21). B. fragilis ATCC 25285 cells harboring the vector pFD340 or the recombinant plasmid pFD340-prtP were tested for their ability to cleave gelatin in solution. Heat denaturation of collagen to gelatin in the presence of DTT was evidenced by the appearance of two bands in the 120- to 130-kDa range in SDS-polyacrylamide gels (Fig. 5, lane 1), and higher-molecular-mass forms detected were incompletely denatured chains. The same pattern and intensity of bands were detected when the gelatin was incubated in the presence of cells harboring the vector alone (Fig. 5, lane 2). No gelatin bands were detected after incubation with B. fragilis ATCC 25285(pFD340-prtP (Fig. 5, lane 3) indicating that the PrtP proteinase degraded the gelatin substrate.
FIG. 5.
Gelatinolytic activity of B. fragilis ATCC 25285(pFD340-prtP). Cells were incubated with gelatin for 16 h at 37°C in the presence of 5 mM DTT, and the samples were analyzed by SDS-PAGE. The gel was stained with Coomassie blue. Lanes 1, no cells; 2, B. fragilis 25285 harboring plasmid pFD340; 3, B. fragilis 25285(pFD340-prtP).
To determine the exopeptidase specificity of PrtP, B. fragilis ATCC 25285 cells harboring the pFD340 vector or pFD340-prtP were tested for their ability to hydrolyze GPR-pNA or GPK-pNA in the presence of cysteine. After overnight incubation, no hydrolysis of either substrate by cells harboring the vector alone could be detected; however, cells harboring pFD340-prtP hydrolyzed the Lys-specific substrate but not the Arg-specific substrate (data not shown). The PrtP proteinase was detected at a dramatically lower level in B. fragilis ATCC 25285(pFD340-prtP) than in wild-type P. gingivalis. Based on the initial rate of GPK-pNA hydrolysis by sonicated cells from overnight cultures resuspended to comparable ODs, B. fragilis ATCC 25285(pFD340-prtP) had less than 0.5% of the Lys-amidolytic activity of P. gingivalis W12.
Extraction with CHAPS previously was used as a preliminary step in the isolation of porphypain (6). Crude CHAPS extracts were used to concentrate PrtP from B. fragilis ATCC 25285(pFD340-prtP) and then further examine the sensitivity of the exopeptidase to inhibitors and a stimulator (Table 1). Recombinant PrtP amidolytic activity was inhibited substantially by TLCK and was less inhibited by the related compound YPRCK at the same concentration. It was only slightly affected by leupeptin or EDTA and was remarkably stimulated by glycine-containing compounds. On a molar basis, cysteine was a more efficient activator of Lys-amidolytic activity than was DTT.
TABLE 1.
Activity on GPK-pNA of crude CHAPS extracts from B. fragilis ATCC 25285(pFD340-prtP)
| Added compound | Concn (mM)a | Relative activityb |
|---|---|---|
| YPRCK | 0.1 | 84.4 |
| 1.0 | 23.6 | |
| TLCK | 0.1 | 26.9 |
| 1.0 | 16.4 | |
| Leupeptin | 0.1 | 102.1 |
| 1.0 | 84.6 | |
| EDTA | 1.0 | 84.4 |
| Glycylglycinec | 200 | 378.3 |
| Glycinamidec | 200 | 207 |
Concentration of compound added to 50 mM cysteine preincubation mixture with enzyme. After 5 min, 0.1 ml of this mixture was added to 1 ml of a 100 mM cysteine substrate cocktail and the reaction rate was measured immediately. Leupeptin and EDTA were present at the indicated concentrations in both the enzyme and substrate cocktails.
The activity in the presence of each inhibitor is reported relative to the activity of the enzyme alone, which was set at 100.
These assays were performed with 1/10 the original level of cysteine, and the data reported are relative to the activity of the enzyme alone under the same conditions.
P. gingivalis clearly cleaves type I collagen (4). The CHAPS extract and cells of B. fragilis ATCC 25285(pFD340-prtP) were tested for collagenolytic activity, but none was detected. In addition, PrtP may contribute to the hemagglutinin activity of P. gingivalis (see the introduction). However, no hemagglutination was detected in assays using the CHAPS extract or cells of B. fragilis ATCC 25285(pFD340-prtP).
Characterization of P. gingivalis prtP::tetA(Q) strains.
P. gingivalis strains containing a knockout mutation in prtP were constructed as described in Materials and Methods. Strains W12, HG66, and ATCC 33777 were chosen for this experiment because much of the work regarding P. gingivalis cysteine proteinases has been done with these strains. Strain KDP110, a derivative of ATCC 33777 with an insertion in rgp-1 (29), was used to construct a double-knockout strain. The genomic structures of the prtP mutant strains were confirmed by Southern blot hybridization (Fig. 6). DNA fragments detected by using the 2.5-kb, tetA(Q)-containing SstI fragment of pMJF3 as a probe were of the size predicted for a double-crossover event at the prtP locus (Fig. 6A and data not shown), e.g., a 4.5-kb BamHI fragment (lane 6). An 8.2-kb fragment would have been detected if circular, uncut pML1 was present, and the probe did not hybridize to genomic DNA of the parent P. gingivalis strains (Fig. 6A, lane 1, and data not shown). Furthermore, a probe prepared from a 1.16-kb HindIII fragment of prtP that was removed during construction of pML1 did not hybridize to genomic DNA from the mutant strains but readily detected a 3.5-kb BamHI fragment in genomic DNA of the parent strains (Fig. 6B and data not shown).
FIG. 6.
Evidence for allelic replacement of prtP in P. gingivalis W12 by a double-crossover event. Digested fragments of P. gingivalis genomic DNA were detected with a probe prepared from the 2.5-kb fragment of pMJF3 containing tetA(Q) (A) or a 1.16-kb HindIII fragment from pNH5 containing DNA absent in pML1 (B). (A) Lanes: 1, genomic DNA from strain W12 digested with BamHI; 2 to 6, genomic DNA from strain W12 prtP::tetA(Q) digested with BanI (lane 2), AhdI plus PvuII (lane 3), AhdI (lane 4), BamHI plus PvuII (lane 5), or BamHI (lane 6). (B) Lanes 1 and 2, BamHI-digested genomic DNA from strain W12 (lane 1) or strain W12 prtP::tetA(Q) (lane 2). The sizes (in kilobases) and locations of DNA standards are indicated.
P. gingivalis W12, HG66, ATCC 33277, and KDP110 all produced black-pigmented colonies on ETSA plates containing 3% sheep blood. After incubation for 10 to 14 days, the plates became clear due to hemolysis and appeared greenish yellow. Fortuitously, we noted that when the corresponding strains harboring the knockout mutation in prtP were similarly grown, the colonies remained beige and the plates became clear but retained their red color. The knockout mutation in the rgp-1 gene of strain KDP110 did not cause the same phenotypic effect; on ETSA plates, colonies of strain KDP110 appeared identical to those of the parent strain, ATCC 33277.
P. gingivalis W12, HG66, ATCC 33277, and KDP110, and the corresponding strains harboring the prtP knockout mutation, were examined by gelatin zymography (Fig. 7). Since the product of the rgp-1 gene is approximately the same size as PrtP (roughly 180 kDa), P. gingivalis W12 (lane 2), HG66 (lane 4), and ATCC 33277 (lane 6) with the prtP::tetA(Q) mutation are not clearly distinguishable from their nonmutant counterparts (lanes 1, 3, and 5, respectively) in this assay. However, when the rgp-1 gene is inactivated as in strain KDP110, the knockout of prtP corresponded to a loss of gelatinolytic activity in the 180-kDa region of the zymogram (Fig. 7, lanes 7 and 8). The activity seen in the 80-kDa range of the zymogram is probably due to the product of the rgp-2 gene, which has a predicted size of 81 kDa when unprocessed (28, 42). It is interesting that the loss of prtP in strain HG66 may have affected the level of rgp-2 produced (Fig. 7, lanes 3 and 4).
FIG. 7.
Zymogram of P. gingivalis cells. Solubilized cells were analyzed for cleavage of gelatin after SDS-PAGE. The gel was washed in gelatin solutions containing Triton X-100 to sequester the SDS, and then the proteinase was activated by washing the gel in 50 mM cysteine. After Coomassie blue staining, the digestion of gelatin was detected by clear spaces in the gel. The positions of molecular mass markers (in kilodaltons) are indicated to the left. Lanes: 1, W12; 2, W12 prtP::tetA(Q); 3, HG66; 4, HG66 prtP::tetA(Q); 5, ATCC 33277; 6, ATCC 33277 prtP::tetA(Q); 7, KDP110; 8, KDP110 prtP::tetA(Q).
The wild-type and mutant strains of P. gingivalis were also tested for their ability to cleave type I collagen in a radiofibril assay and to hemagglutinate sheep erythromyctes (Table 2). A substantial decrease in the ability of P. gingivalis to degrade collagen was detected when the prtP gene was insertionally inactivated. In clear contrast, the collagenase activity of strain KDP110, which lacks Rgp-1, was indistinguishable from that of the wild-type parent strain, ATCC 33277. Keeping in mind that hemagglutination assays are based on 1:2 dilutions, only strain KDP110 showed a dramatic difference in hemagglutinin activity in the absence of PrtP. Surprisingly, residual Lys-specific amidolytic activity was detected for each of the strains lacking a functional prtP gene. Based on the initial rate of GPK-pNA hydrolysis when using cells adjusted to the same OD, this activity in the mutant strains ranged from 5 to 10% of the activity in the parent strains (data not shown). Since the strains were constructed such that DNA encoding part of the catalytically active moiety of PrtP was lost, the residual activity must be attributable to another proteinase.
TABLE 2.
Activities of P. gingivalis strains with and without PrtP
| Strain | Hemagglutination titera | Collagenase activityb |
|---|---|---|
| W12 | 32 | 65 |
| W12 prtP::tetA(Q) | 16 | 42 |
| HG66 | 64 | 75 |
| HG66 prtP::tetA(Q) | 16 | 50 |
| ATCC 33277 | 32 | 97 |
| ATCC 33277 prtP::tetA(Q) | 16 | 35 |
| KDP110 | 32 | 100 |
| KDP110 prtP::tetA(Q) | 2 | 57 |
Titers were determined using cells adjusted to an OD550 of 1 and are the inverse of the highest cell dilution still causing hemagglutination.
Values are relative to the amount of collagen cleavage by clostridial collagenase at 0.5 mg/ml, which was set at 100. Cleavage activity by a trypsin control at 0.5 mg/ml was 17.
DISCUSSION
The ability to express catalytically active PrtP in a heterologous prokaryotic host in the clear absence of contamination by Rgp-1 and Rgp-2 has allowed us to compare the properties of the prtP gene product with those of porphypain isolated in our laboratory from P. gingivalis W12 cells (6) and Lys-gingipain (gingipain-K; Kgp) isolated by Pike et al. (37) from culture medium following growth of P. gingivalis HG66. PrtP differs in one major way from porphypain: it has only Lys-amidolytic activity, whereas our porphypain preparation had both Lys- and Arg-amidolytic activities. These results suggest that our original porphypain preparation (6) was contaminated with Rgp-1 and/or Rgp-2. PrtP clearly has the unusual property of being stimulated by derivatives of glycine, a property which we reported for both the Arg- and Lys-amidolytic activities of porphypain (6). While Pike et al. reported this property for Rgp proteinases of strain HG66, they reported that the activity of Kgp (isolated as a 105-kDa complex, which they suggest contains a 60-kDa Lys-specific proteinase complexed with hemagglutinins) from the same strain was inhibited by derivatives of glycine (37). Since kgp and prtP are strain-specific alleles of the same gene (2, 35), it may be that the behavior of the active site in the 105-kDa proteinase complex purified by Pike et al. (37) is altered so that it is inhibited rather than stimulated by glycylglycine. Alternatively, failure to adjust the pH of the glycylglycine solutions used for the characterization of Kgp would also account for their findings. Since it has been reported that stimulation of Rgp-1 activity by glycylglycine is unique among the known proteinases, and since we have now shown that this property is also shared by PrtP, we propose that these proteinase genes evolved from a common ancestral gene. This hypothesis also is supported by the considerable conservation seen over short stretches in the deduced amino acid sequences immediately amino terminal to the catalytic Cys residues of Rgp-1, Rgp-2, and PrtP (2, 36).
We previously reported that the Lys-specific activity of porphypain was inhibited by EDTA (6), and we have now shown that PrtP activity is unaffected by EDTA. Again, as suggested above, the EDTA sensitivity of porphypain may have resulted from destabilization of the active site as a result of complex interactions between PrtP and peptides derived from Rgp-1, Rgp-2, and/or HagA in the porphypain preparation. It may be that in these tight, noncovalently bonded complexes the PrtP active site requires stabilization by Ca2+ and that when PrtP is removed from these complexes this stabilization is no longer required. The ability to express each of these proteinases free from contamination by the other two and HagA, so that the effects of one on the other can be tested, will be invaluable in sorting out the nature of these complex interactions and their effects on proteinase activity. While our results indicate that the exopeptidase activity of PrtP is specific for hydrolysis of Lys-X bonds, further studies will be required to determine whether this strict specificity is maintained in its endoproteinase activity.
The major collagenase of P. gingivalis has not yet been purified. It has been suggested in several reports that this purification has been achieved; however, in each of these studies, flawed collagenase assays were employed and gelatinase activity was incorrectly interpreted as collagenase activity (for a review, see reference 16). We have attempted to purify a collagenase from P. gingivalis and have found that the activity is detectable after some initial steps have been taken to extract the enzyme from cells, such as sonication, but that it is lost when further attempts are made to purify it from resultant extracts (15a). It may be that specific conformational constraints must be met for the proteinase to remain collagenolytic. The collagenase of P. gingivalis is activated by reducing agents and is inhibited by -SH blocking agents, leupeptin, and TLCK, suggesting that, like the Rgp proteinases and PrtP, it has Arg-X and Lys-X activity (4, 15a). In this study, we examined the relationship between PrtP and the collagenase of P. gingivalis in two ways: first, by determining whether PrtP can function as a collagenase; and second, by assessing the ability of P. gingivalis mutants lacking a functional PrtP to mediate cleavage of type I collagen. Neither B. fragilis 25285 cells harboring pFD340-prtP nor PrtP-containing CHAPS extracts prepared from these cells cleaved type I collagen. However, the interruption of prtP, but not rgp-1, significantly decreased (30 to 60%), but did not eliminate, the ability of P. gingivalis strains to cleave collagen.
The failure of PrtP to mediate collagenolysis in this system does not necessarily mean that this enzyme is not a collagenase. It is clear from the results of the present study (Fig. 5 and 7) and others (40) that the Rgp proteinases and PrtP are potent gelatinases, indicating that these enzymes can rapidly degrade the α chains of type I collagen. Furthermore, based on the small amount of collagenase activity released from P. gingivalis cells, Birkedal-Hansen et al. (4) suggested that either the P. gingivalis collagenase has a much lower specific activity than vertebrate and clostridial collagenases or the enzyme is a minor component of P. gingivalis. Hence, the failure to detect collagenase activity (relative to clostridial collagenase) in B. fragilis 25285(pFD340-prtP) or PrtP-containing CHAPS extracts derived from these cells may have been due to the relatively small amount of enzyme present, particularly if its relative specific activity is very low. Alternatively, in addition to the possible requirement for specific conformational constraints on the enzyme(s), collagenolysis by P. gingivalis might require the concerted action of more than one proteinase. Results of the inhibitor studies cited above suggest that both Rgp-proteinases and PrtP could be involved in collagenolysis. Significantly, an rgp-1 knockout mutant, strain KDP110, did not show any decrease in collagenolytic activity, whereas all prtP single-knockout mutants as well as the rgp-1 prtP double-knockout mutant showed significant decreases in collagenolytic activity. It may be that KDP110 does not show a decrease in collagenolytic activity because it has functional Rgp-2 and PrtP and that in order for P. gingivalis to express full collagenase activity it must have one functional Rgp as well as functional PrtP. Testing of this hypothesis will require construction and analysis of an rgp-1 rgp-2 double-knockout mutant strain as well as an rgp-1 rgp-2 prtP triple-knockout mutant strain; the latter should be devoid of the activity. Finally, it may be that the Rgp proteinases and PrtP are not directly involved in collagenolysis but that at least PrtP is required for complete processing of a collagenase precursor.
We have examined the role of PrtP in hemagglutination in two ways: first, by determining whether PrtP can function as a hemagglutinin; and second, by assessing the hemagglutinin phenotypes of P. gingivalis mutants lacking a functional PrtP. It was reasoned that PrtP might function as a hemagglutinin because large regions within it exhibit a high degree of identity to regions repeated in the HagA hemagglutinin of P. gingivalis (2) and because the 105-kDa Lys-specific proteinase, as purified by Pike et al. (38), causes hemagglutination. The results of our experiments are inconclusive. Neither B. fragilis ATCC 25285(pFD340-prtP) nor the PrtP-containing CHAPS extract derived from these cells caused hemagglutination. Also, when either the prtP or rgp-1 gene was inactivated in P. gingivalis, the hemagglutination titer was only slightly affected. While these results suggest that PrtP does not play a major role in mediating hemagglutination, the dramatic reduction in hemagglutinin titer in cells in which both rgp-1 and prtP are inactivated suggests otherwise. Interestingly, Nakayama et al. (29) found that disrupting either rgp-1 or rgp-2 alone had only a small effect on the hemagglutination activity of P. gingivalis ATCC 33277, whereas strains inactivated in both rgp-1 and rgp-2 showed a more dramatic loss of hemagglutination activity. It is possible that cells require the function of at least two of the three major cysteine proteinases to hemagglutinate erythrocytes and that these enzymes can compensate for one another. For example, these gene products may function directly as hemagglutinins or proteolytically process hemagglutinin precursors, or both. Further studies will be needed to sort out these possibilities. Finally, results of studies such as those conducted by Pike et al. (38), in which various forms of gingipains-Rs and -K were purified from P. gingivalis and tested for hemagglutinin activity, should be interpreted with caution because of possible cross-contamination of the proteinase preparations.
When the tetA(Q) gene was substituted in several P. gingivalis strains for a part of the prtP gene thought to encode the catalytically active moiety, the appearance of the P. gingivalis colonies on ETSA was drastically altered and suggested that the mutant cells were unable to recover iron from the erythrocytes. We did not detect a pigmentation defect in strain KDP110, a derivative of P. gingivalis ATCC 33277 which lacks a functional rgp-1 gene. P. gingivalis strains with pigmentation defects have been described previously (13, 27). The loss of pigmentation correlated with hemagglutination defects and a decrease in Arg-amidolytic activity, but Lys-specific activity was not examined.
Okamoto et al. (33) reported that knockout mutations in the rgp genes affected the level of P. gingivalis Lys-specific amidolytic activity in culture supernatants, but we detected no difference in the initial rates of GPK-pNA hydrolysis of cells of strains ATCC 33277 and KDP110 (data not shown). Since PrtP apparently does not have Arg-specific exopeptidase activity, and pigmentation defects were detected in P. gingivalis strains lacking prtP but not in those lacking rgp-1, it is possible that expression of rgp-1 and prtP is coregulated, perhaps by a hemin-responsive element (5, 25). Preliminary data also suggest that the initial rate of GPR-pNA hydrolysis may be affected in the cells containing prtP::tetA(Q) (data not shown). While it is possible that the tetA(Q) insertion has polar effects on downstream genes, there is no evidence that prtP is transcribed as part of an operon. Moreover, in P. gingivalis W12, an insertion element highly homologous to IS1126, but lacking a 451-bp central region, is located immediately downstream of prtP (2), so that at least in this strain there are no downstream genes in the operon that could be affected by the tetA(Q) insertion in prtP.
Cell-associated PrtP was produced by three Bacteroides strains harboring a plasmid expressing prtP but not by B. uniformis BU101 harboring the same plasmid. Since only one clone from each strain was examined, it is possible that prtP can be expressed in B. uniformis and that a nonfunctional clone was analyzed inadvertently. Inactive clones have been recovered multiple times from B. fragilis ATCC 25285(pFD340-prtP), particularly upon passage on ETSA after mating (data not shown). These nonfunctional clones have not been further analyzed. Recovery of PrtP from B. fragilis ATCC 25285(pFD340-prtP) by CHAPS extraction suggested that the proteinase was present at the surface of the cells. In contrast, B. fragilis IB101 released PrtP into the medium. By comparison, the proteinase has been recovered from spent medium and vesicles of P. gingivalis, as well as from CHAPS extracts of these cells.
The promoter and translation start sites of subcloned prtP were functional in Bacteroides species, based on expression of the gene subcloned in pFD288. However, the proteinase activity detected in Bacteroides cells harboring pFD288-prtP was much lower than that observed in P. gingivalis. Transcription and translation start sites have not been characterized for either P. gingivalis or Bacteroides species, so there are many possible explanations for this difference; for example, there may be differences between the preferred ribosome binding sites of the two organisms. Also, there may be a hemin-responsive element upregulating expression of prtP in P. gingivalis that is absent in Bacteroides species (5, 25). In P. gingivalis, an increase in Arg-X exopeptidase activity occurred under conditions of hemin excess, relative to the activity under hemin-limited conditions. This activity cannot be attributed to PrtP, but its expression could be coregulated with prtP expression. To increase expression of prtP in Bacteroides strains, the gene was subcloned onto pFD340, which supplied a promoter that is active in Bacteroides species (48). More-active PrtP was apparently produced, but the proteinase was still detected at dramatically lower levels than in P. gingivalis. However, using this Bacteroides expression system, we were able to readily obtain sufficient active, full-length PrtP to unambiguously characterize some of the properties of this proteinase. Additional characterization likely will require further modifications of this system.
This is the first report of expression of one of the major cysteine proteinases of P. gingivalis, full-length PrtP, in a catalytically active form from a heterologous prokaryotic host. While the minor P. gingivalis proteinases PrtC (49) and PrtT (22) have been expressed in catalytically active forms in E. coli, no reports of successful expression of active Rgp-1, Rgp-2, or PrtP (Kgp) in E. coli have appeared. There are many potential reasons why these enzymes are not readily produced in active forms in E. coli; e.g., E. coli may lack factors required for maturation or processing of these gene products. The Bacteroides expression system for prtP described in this report will permit assignment of specific functions to specific structural domains of the major cysteine proteinases of P. gingivalis and may be useful for the production of other P. gingivalis proteins that cannot be readily expressed in a biologically active form in E. coli.
ACKNOWLEDGMENTS
We gratefully thank D. LeBlanc for many helpful discussions and critical reading of the manuscript and L. Jack Windsor and C. Jeffery Smith for scientific support.
This work was supported by National Institute for Dental Research grant DE07256 from the National Institutes of Health.
ADDENDUM IN PROOF
During the review of this manuscript, a paper was published describing some properties of a KGP-deficient mutant constructed using P. gingivalis ATCC 33277. K. Okamoto, K. Nakayama, T. Kadowaki, N. Abe, D. B. Ratnayake, and K. Yamamoto. J. Biol. Chem. 273:21225-21231, (1998). Notably, this mutant strain did not form black-pigmented colonies on blood agar plates.
REFERENCES
- 1.Aduse-Opoku J, Muir J, Slaney J M, Rangarajan M, Curtis M A. Characterization, genetic analysis, and expression of a protease antigen (PrpRI) of Porphyromonas gingivalis W50. Infect Immun. 1995;63:4744–4754. doi: 10.1128/iai.63.12.4744-4754.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Barkocy-Gallagher, G. A. Unpublished data.
- 2.Barkocy-Gallagher G A, Han N, Patti J M, Whitlock J, Progulske-Fox A, Lantz M S. Analysis of the prtP gene encoding porphypain, a cysteine proteinase of Porphyromonas gingivalis. J Bacteriol. 1996;178:2734–2741. doi: 10.1128/jb.178.10.2734-2741.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bethesda Research Laboratories. BRL pUC host: E. coli DH5αTM competent cells. Bethesda Res Lab Focus. 1986;8:9. [Google Scholar]
- 4.Birkedal-Hansen H, Taylor R E, Zambon J J, Barwa P K, Neiders M E. Characterization of collagenolytic activity from strains of Bacteroides gingivalis. J Periodont Res. 1988;23:258–264. doi: 10.1111/j.1600-0765.1988.tb01369.x. [DOI] [PubMed] [Google Scholar]
- 5.Carman R J, Ramakrishnan M D, Harper F H. Hemin levels in culture medium of Porphyromonas (Bacteroides) gingivalis regulate both hemin binding and trypsinlike protease production. Infect Immun. 1990;58:4016–4019. doi: 10.1128/iai.58.12.4016-4019.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciborowski P, Nishikata M, Allen R D, Lantz M S. Purification and characterization of two forms of a high-molecular-weight cysteine proteinase (porphypain) from Porphyromonas gingivalis. J Bacteriol. 1994;176:4549–4557. doi: 10.1128/jb.176.15.4549-4557.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtis M A, Aduse-Opoku J, Slaney J M, Rangarajan M, Booth V, Cridland J, Shepherd P. Characterization of an adherence and antigenic determinant of the ArgI protease of Porphyromonas gingivalis which is present on multiple gene products. Infect Immun. 1996;64:2532–2539. doi: 10.1128/iai.64.7.2532-2539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtis M A, Aduse-Opoku J, Woodward S K. Baculovirus-mediated expression of the adhesin domain of the ArgI protease of P. gingivalis. J Dent Res. 1995;74:848. [Google Scholar]
- 9.Feldhaus M J, Hwa V, Cheng Q, Salyers A A. Use of an Escherichia coli β-glucuronidase gene as a reporter gene for investigation of Bacteroides promoters. J Bacteriol. 1991;173:4540–4543. doi: 10.1128/jb.173.14.4540-4543.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher H M, Schenkein H A, Morgan R M, Bailey K A, Berry C R, Macrina F L. Virulence of a Porphyromonas gingivalis W83 mutant defective in the prtH gene. Infect Immun. 1995;63:1521–1528. doi: 10.1128/iai.63.4.1521-1528.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gron H, Pike R, Potempa J, Travis J, Thorgersen I B, Enghild J J, Pizzo S V. The potential role of α2-macroglobulin in the control of cysteine proteinases (gingipains) from Porphyromonas gingivalis. J Periodont Res. 1997;32:61–68. doi: 10.1111/j.1600-0765.1997.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 12.Guiney D G, Hasegawa P, Davis C E. Plasmid transfer from Escherichia coli to Bacteroides fragilis: differential expression of antibiotic resistance phenotypes. Proc Natl Acad Sci USA. 1984;81:7203–7206. doi: 10.1073/pnas.81.22.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoover C I, Yoshimura F. Transposon-induced pigment-deficient mutants of Porphyromonas gingivalis. FEMS Microbiol Lett. 1994;124:43–48. doi: 10.1111/j.1574-6968.1994.tb07259.x. [DOI] [PubMed] [Google Scholar]
- 14.Jagels M A, Travis J, Potempa J, Pike R, Hugli T E. Proteolytic inactivation of the leukocyte C5a receptor by proteinases derived from Porphyromonas gingivalis. Infect Immun. 1996;64:1984–1991. doi: 10.1128/iai.64.6.1984-1991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuramitsu H K, Tokuda M, Yoneda M, Duncan M, Cho M-I. Multiple colonization defects in a cysteine protease mutant of Porphyromonas gingivalis. J Periodont Res. 1997;32:140–142. doi: 10.1111/j.1600-0765.1997.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 15a.Lantz, M. Unpublished data.
- 16.Lantz M S. Are bacterial proteinases important virulence factors? J Periodont Res. 1997;32:126–132. doi: 10.1111/j.1600-0765.1997.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 17.Lantz M S, Allen R D, Duck L W, Blume J L, Switalski L M, Hook M. Identification of Porphyromonas gingivalis components that mediate its interactions with fibronectin. J Bacteriol. 1991;173:4263–4270. doi: 10.1128/jb.173.14.4263-4270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lantz M S, Allen R D, Duck L W, Switalski L M, Hook M. Porphyromonas gingivalis surface components bind and degrade connective tissue proteins. J Periodont Res. 1991;26:283–285. doi: 10.1111/j.1600-0765.1991.tb01659.x. [DOI] [PubMed] [Google Scholar]
- 19.Lantz M S, Allen R D, Vail T A, Switalski L M, Hook M. Specific cell components of Bacteroides gingivalis mediate binding and degradation of human fibrinogen. J Bacteriol. 1991;173:495–504. doi: 10.1128/jb.173.2.495-504.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lantz M S, Switalski L M. Host-bacterial interactions in the pathogenesis of periodontitis. In: Cohen M M, Baum B J, editors. Studies in stomatology and craniofacial biology. Amsterdam, The Netherlands: IOS Press; 1997. pp. 473–497. [Google Scholar]
- 21.Lantz M S, Vail T A, Allen R D, Switalski L M, Hook M. Characterization of high molecular weight proteases of B. gingivalis W12. J Dent Res. 1989;68:953. [Google Scholar]
- 22.Madden T E, Clark V L, Kuramitsu H K. Revised sequence of the Porphyromonas gingivalis PrtT cysteine protease/hemagglutinin gene: homology with streptococcal pyrogenic exotoxin B/streptococcal proteinase. Infect Immun. 1995;63:238–247. doi: 10.1128/iai.63.1.238-247.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maley J, Shoemaker N B, Roberts I S. The introduction of colonic-Bacteroides shuttle plasmids into Porphyromonas gingivalis: identification of a putative P. gingivalis insertion-sequence element. FEMS Microbiol Lett. 1992;93:75–82. doi: 10.1016/0378-1097(92)90492-7. [DOI] [PubMed] [Google Scholar]
- 24.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 25.Marsh P D, McKee A S, McDermid A S. Effects of haemin on enzyme activity and cytotoxin production by Bacteroides gingivalis W50. FEMS Microbiol Lett. 1988;55:87–92. [Google Scholar]
- 26.Mayrand D, Holt S C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988;52:134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKee A S, McDermid A S, Wait R, Baskerville A, Marsh P D. Isolation of colonial variants of Bacteroides gingivalis W50 with a reduced virulence. J Med Microbiol. 1988;27:59–64. doi: 10.1099/00222615-27-1-59. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama K. Domain-specific rearrangement between the two Arg-gingipain-encoding genes in Porphyromonas gingivalis: possible involvement of nonreciprocal recombination. Microbiol Immunol. 1997;41:185–196. doi: 10.1111/j.1348-0421.1997.tb01189.x. [DOI] [PubMed] [Google Scholar]
- 29.Nakayama K, Kadowaki T, Okamoto K, Yamamoto K. Construction and characterization of arginine-specific cysteine proteinase (Arg-gingipain)-deficient mutants of Porphyromonas gingivalis. J Biol Chem. 1995;270:23619–23626. doi: 10.1074/jbc.270.40.23619. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama K, Yoshimura F, Kadowaki T, Yamamoto K. Involvement of arginine-specific cysteine proteinase (Arg-gingipain) in fimbriation of Porphyromonas gingivalis. J Bacteriol. 1996;178:2818–2824. doi: 10.1128/jb.178.10.2818-2824.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishikata M, Yoshimura F. Characterization of Porphyromonas (Bacteroides) gingivalis hemagglutinin as a protease. Biochem Biophys Res Commun. 1991;178:336–342. doi: 10.1016/0006-291x(91)91819-x. [DOI] [PubMed] [Google Scholar]
- 32.Offenbacher S. Periodontal disease: pathogenesis. Ann Periodontol. 1996;1:821–878. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto K, Kadowaki T, Nakayama K, Yamamoto K. Cloning and sequencing of the gene encoding a novel lysine-specific cysteine proteinase (Lys-gingipain) in Porphyromonas gingivalis: structural relationship with the arginine-specific cysteine proteinase (Arg-gingipain) J Biochem. 1996;120:398–406. doi: 10.1093/oxfordjournals.jbchem.a021426. [DOI] [PubMed] [Google Scholar]
- 34.Okamoto K, Misumi Y, Kadowaki T, Yoneda M, Yamamoto K, Ikehara Y. Structural characterization of argingipain, a novel arginine-specific cysteine proteinase, as a major periodontal pathogenic factor from Porphyromonas gingivalis. Arch Biochem Biophys. 1995;316:917–925. doi: 10.1006/abbi.1995.1123. [DOI] [PubMed] [Google Scholar]
- 35.Pavloff N, Pemberton P A, Potempa J, Chen W-C A, Pike R N, Prochazka V, Kiefer M C, Travis J, Barr P J. Molecular cloning and characterization of Porphyromonas gingivalis lysine-specific gingipain. J Biol Chem. 1997;272:1595–1600. doi: 10.1074/jbc.272.3.1595. [DOI] [PubMed] [Google Scholar]
- 36.Pavloff N, Potempa J, Pike R N, Prochazka V, Kiefer M C, Travis J, Barr P J. Molecular cloning and structural characterization of the Arg-gingipain proteinase of Porphyromonas gingivalis. J Biol Chem. 1995;270:1007–1010. doi: 10.1074/jbc.270.3.1007. [DOI] [PubMed] [Google Scholar]
- 37.Pike R, McGraw W, Potempa J, Travis J. Lysine- and arginine-specific proteinases from Porphyromonas gingivalis. J Biol Chem. 1994;269:406–411. [PubMed] [Google Scholar]
- 38.Pike R N, Potempa J, McGraw W, Coetzer T H T, Travis J. Characterization of the binding activities of proteinase-adhesin complexes from Porphyromonas gingivalis. J Bacteriol. 1996;178:2876–2882. doi: 10.1128/jb.178.10.2876-2882.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Potempa J, Pike R N, Travis J. Titration and mapping of the active site of cysteine proteinases from Porphyromonas gingivalis (gingipains) using peptidyl chloromethanes. Biol Chem. 1997;378:223–230. doi: 10.1515/bchm.1997.378.3-4.223. [DOI] [PubMed] [Google Scholar]
- 40.Potempa J, Pike R, Travis J. The multiple forms of trypsin-like activity present in various strains of Porphyromonas gingivalis are due to the presence of either Arg-gingipain or Lys-gingipain. Infect Immun. 1995;63:1176–1182. doi: 10.1128/iai.63.4.1176-1182.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Progulske-Fox A, Tumwarson S, Holt S C. The expression and function of Bacteroides gingivalis hemagglutinin gene in Escherichia coli. Oral Microbiol Immunol. 1989;4:121–131. doi: 10.1111/j.1399-302x.1989.tb00238.x. [DOI] [PubMed] [Google Scholar]
- 42.Rangarajan M, Aduse-Opoku J, Slaney J M, Young K A, Curtis M A. The prpR1 and prR2 arginine-specific protease genes of Porphyromonas gingivalis W50 produce five biochemically distinct enzymes. Mol Microbiol. 1997;23:955–965. doi: 10.1046/j.1365-2958.1997.2831647.x. [DOI] [PubMed] [Google Scholar]
- 43.Shoemaker N B, Getty C, Gardner J F, Salyers A A. Tn4351 transposes in Bacteroides spp. and mediates the integration of plasmid R751 into the Bacteroides chromosome. J Bacteriol. 1986;165:929–936. doi: 10.1128/jb.165.3.929-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shoemaker N B, Salyers A A. A cryptic 65-kilobase-pair transposonlike element isolated from Bacteroides uniformis has homology with Bacteroides conjugal tetracycline resistance elements. J Bacteriol. 1990;172:1694–1702. doi: 10.1128/jb.172.4.1694-1702.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slakeski N, Cleal S M, Reynolds E C. Characterization of a Porphyromonas gingivalis gene prtR that encodes an arginine-specific thiol proteinase and multiple adhesins. Biochem Biophys Res Commun. 1996;224:605–610. doi: 10.1006/bbrc.1996.1073. [DOI] [PubMed] [Google Scholar]
- 46.Smith C J. Characterization of Bacteroides ovatus plasmid pBI136 and structure of its clindamycin resistance region. J Bacteriol. 1985;161:1069–1073. doi: 10.1128/jb.161.3.1069-1073.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith C J. Clindamycin resistance and the development of genetic systems in the Bacteroides. Dev Ind Microbiol. 1989;30:23–33. [Google Scholar]
- 48.Smith C J, Rogers M B, McKee M L. Heterologous gene expression in Bacteroides fragilis. Plasmid. 1992;27:141–154. doi: 10.1016/0147-619x(92)90014-2. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi N, Kato T, Kuramitsu H K. Isolation and preliminary characterization of the Porphyromonas gingivalis prtC gene expressing collagenase activity. FEMS Microbiol Lett. 1991;84:135–138. doi: 10.1016/0378-1097(91)90116-r. [DOI] [PubMed] [Google Scholar]
- 50.Travis J, Pike R, Imamura T, Potempa J. Porphyromonas gingivalis proteinases as virulence factors in the development of periodontitis. J Periodont Res. 1997;32:120–125. doi: 10.1111/j.1600-0765.1997.tb01392.x. [DOI] [PubMed] [Google Scholar]
- 51.Wingrove J A, DiScipio R G, Chen Z, Potempa J, Travis J, Hugli T E. Activation of complement components C3 and C5 by a cysteine proteinase (gingipain-1) from Porphyromonas (Bacteroides) gingivalis. J Biol Chem. 1992;267:18902–18907. [PubMed] [Google Scholar]
- 52.Yoneda M, Kuramitsu H K. Genetic evidence for the relationship of Porphyromonas gingivalis cysteine protease and hemagglutinin activities. Oral Microbiol Immunol. 1996;11:129–134. doi: 10.1111/j.1399-302x.1996.tb00347.x. [DOI] [PubMed] [Google Scholar]