Abstract
Membrane overexpression of ErbB-2 (MErbB-2), a member of the ErbB family of receptor tyrosine kinases, occurs in 15–20% of breast cancers (BC) and constitutes a therapeutic target in this BC subtype (ErbB-2-positive). Although MErbB-2-targeted therapies have significantly improved patients’ clinical outcome, resistance to available drugs is still a major issue in the clinic. Lack of accurate biomarkers for predicting responses to anti-ErbB-2 drugs at the time of diagnosis is also an important unresolved issue. Hence, a better understanding of the ErbB-2 signaling pathway constitutes a critical task in the battle against BC. In its canonical mechanism of action, MErbB-2 activates downstream signaling pathways, which transduce its proliferative effects in BC. The dogma of ErbB-2 mechanism of action has been challenged by the demonstration that MErbB-2 migrates to the nucleus, where it acts as a transcriptional regulator. Accumulating findings demonstrate that nuclear ErbB-2 (NErbB-2) is involved in BC growth and metastasis. Emerging evidence also reveal a role of NErbB-2 in the response to available anti-MErbB-2 agents. Here, we will review NErbB-2 function in BC and will particularly discuss the role of NErbB-2 as a novel target for therapy in ErbB-2-positive BC.
Keywords: Nuclear ErbB-2, Breast cancer, Metastasis, Response to ErbB-2-targeted therapies, Transcriptional coactivator, ErbB-2 signaling pathway
Introduction
ErbB-2, a member of the ErbB family of receptor tyrosine kinases (EGF-R/ErbB-1, ErbB-2, ErbB-3, and ErbB-4), is a major player in the breast cancer (BC) scenario. Approximately 15–20% of diagnosed BC show either membrane overexpression of ErbB-2 (MErbB-2) or ERBB2 gene amplification [1]. In the molecular classification of BC, this subtype is called ErbB-2-positive [1]. Until the development of anti-ErbB-2 agents, this BC subtype was associated with higher metastatic potential and poor prognosis [1]. Notably, the overall survival and cure rates were improved by said agents [2, 3]. Current therapeutic options for patients with ErbB-2-positive BC include monoclonal antibodies (trastuzumab and pertuzumab), tyrosine kinase inhibitors (TKI, lapatinib and neratinib), and trastuzumab-DM1, an antibody-drug conjugate. Despite a significant clinical response to trastuzumab (TZ), the first approved anti-ErbB-2 therapy, nearly 40–60% of patients with ErbB-2-positive metastatic BC do not respond to TZ, showing either intrinsic or acquired resistance [2, 4]. Although lapatinib provides clinical benefit to patients progressing on TZ, less than 25% achieve an objective response, and the majority develop lapatinib resistance [5]. Phase 3 TH3RESA trial, the study performed in metastatic BC patients who had previously received regimens with both trastuzumab and lapatinib, demonstrated that although trastuzumab-DM1 improved the progression-free survival in this cohort, only 31% of the patients achieved an objective response [6]. These data reveal that resistance to ErbB-2-targeted therapy remains a major clinical issue. Multiple mechanisms were found to be involved in TZ resistance, such as hyperactivation of the PI3K/AKT pathway, presence of ligand-induced ErbB-2/ErbB-3 heterodimers or of ErbB-2/insulin-like growth factor receptor I (IGF1-R) dimerization, and expression of constitutively active truncated ErbB-2 forms (reviewed in [7]). Many clinical trials are assessing the efficacy of new treatments with PI3K/AKT inhibitors, anti-IGF-1R antibodies, and TKI afatinib [8]. A better understanding of the ErbB-2 pathway constitutes a critical task in the battle against BC. A major contribution to the understanding of ErbB-2 biology was the demonstration that MErbB-2 migrates to the nucleus (nuclear ErbB-2 (NErbB-2)), where it modulates gene expression and biological responses in BC [9]. Here, we will review NErbB-2 function in BC and will particularly discuss the role of NErbB-2 as a novel target for therapy in ErbB-2-positive BC.
ErbB-2 canonical action
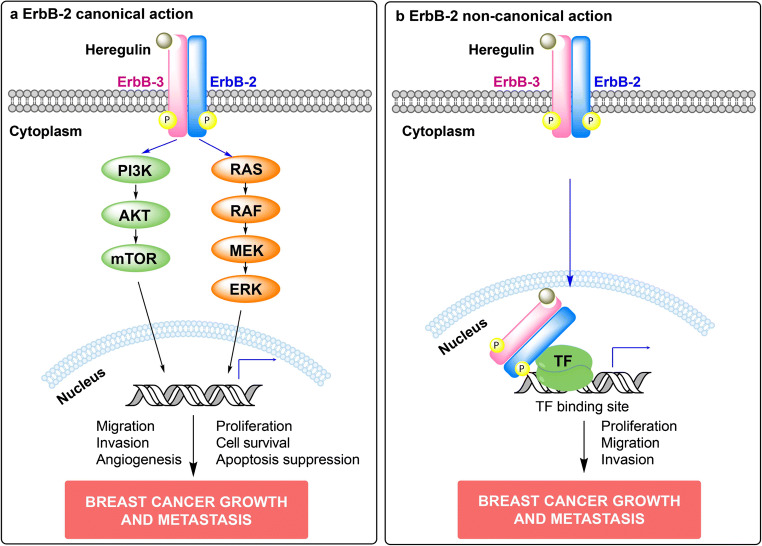
ErbBs ligands include all isoforms of heregulins (HRG), which bind to ErbB-3 and ErbB-4 and recognize EGF-R and ErbB-2 as co-receptors [10]. Upon HRG binding, ErbBs form homo- and heterodimers. This stimulates their tyrosine kinase activity and results in the activation of signaling pathways which transduce ErbB’s biological effects. Although ErbB-2 is an orphan receptor, it dimerizes with other ErbBs [11]. In MErbB-2-overexpressing BC cells, ErbB-2 also forms homo- and heterodimers in the absence of ligand [12]. ErbB-3 critical role as ErbB-2 coreceptor mediating ligand-independent and -dependent ErbB-2 signaling has been reported [13–16]. Among ErbB-2-activated cascades, the best characterized are the p42/p44 mitogen-activated protein kinases (MAPKs) and the phosphatidylinositol-3-kinase (PI3K)/AKT pathways [17]. It has also been reported that ErbB-2 activates the signal transducer and activator of transcription 3 (Stat3) pathway. Indeed, Stat3 activation by ErbB-2 signaling drives BC growth and metastasis [16, 18]. Figure 1a illustrates the signaling pathways activated by ErbB-2 canonical action.
Fig. 1.
Canonical and non-canonical actions of ErbB-2 in breast cancer (BC). a ErbB-2 canonical signaling pathway. Upon ligand binding, ErbBs form homodimers or heterodimers, which activate downstream signaling cascades and transduce ErbBs effects. Heregulin-induced ErbB-2/ErbB-3 heterodimers are illustrated. The two key signaling pathways activated are p42/p44 mitogen-activated protein kinases (MAPKs) and phosphatidylinositol 3-kinase (PI3K)/AKT. Activation of the MAPK pathway leads to the transcription of genes that drive cellular proliferation, migration, and angiogenesis, while activation of the PI3K/AKT pathway leads to downstream cellular endpoints including cell survival and apoptosis suppression (reviewed in [17]). Altogether, activation of these signaling cascades promotes BC growth and metastasis. b ErbB-2 non-canonical action. ErbB-2 non-canonical action involves nuclear translocation of membrane ErbB-2. Heregulin-induced ErbB-2/ErbB-3 heterodimerization and nuclear translocation is depicted [19]. Once in the nucleus, ErbB-2 may directly bind to its response elements and function as a transcription factor (TF), or may tether to other TFs and act as a transcriptional coactivator. An example of ErbB-2 acting as a transcriptional coactivator is shown. NErbB-2 actions also drive BC growth and metastasis
ErbB-2 non-canonical mechanism of action
Multiple pieces of evidence indicate that ErbB-2 is present in the nucleus, where it acts as a transcriptional regulator to modulate biological responses in BC [9, 19–27] (Fig. 1b). Moreover, clinical studies identified NErbB-2 positivity as an independent prognostic factor of poor clinical outcome in patients bearing MErbB-2-positive tumors [28]. The molecular mechanisms underlying ErbB-2 nuclear trafficking have been extensively investigated and reviewed elsewhere [29–31]. ErbB-2 contains a nuclear localization sequence (NLS, 676-KRRQQKIRKYTMRR-689) located adjacent to the transmembrane domain, which is crucial for NErbB-2 trafficking [32]. Indeed, during ErbB-2 nuclear transport, it associates with importin β1 (also called karyopherin β1: Kpnβ1) through the ErbB-2-NLS. Thereafter, the interaction of this ErbB-2/importin β1 complex with the nuclear pore protein Nup358 leads to ErbB-2 nuclear translocation [32]. In spite of multiple findings highlighting NErbB-2’s role in BC and providing insights into the mechanisms of ErbB-2 nuclear trafficking, there is no drug designed to specifically block nuclear ErbB-2 expression and/or function. Interestingly, a recent study showed that Kpnβ1 knockdown inhibited NErbB-2 presence and in vitro proliferation of MErbB-2-overexpressing BC cells [33]. Moreover, the authors demonstrated that Kpnβ1 expression was associated with poor prognosis and identified Kpnβ1 as a possible novel therapeutic target [33]. Further investigations are required to test the effects of Kpnβ1 knockdown in BC growth. Since Kpnβ1 is involved in the nuclear transport of other proteins such as the ribosomal proteins [34], it also remains to be explored if Kpnβ1 knockdown could affect nuclear transportation of other proteins in non-malignant cells.
Molecular and biological functions of NErbB-2 in breast cancer
The dogma of ErbB-2 mechanism of action has been challenged by the demonstration that MErbB-2 migrates to the nucleus where it binds DNA at specific sequences, called HER-2-associated sequences (HASs) [9]. In this pioneering work, ErbB-2 tyrosine phosphorylation was required for the nuclear localization of the full-length ErbB-2 protein. Cyclooxygenase-2 (COX-2) expression was modulated via NErbB-2’s role as a transcription factor (TF) [9]. Inhibition of COX-2 activity suppressed the invasive activity of ErbB-2-overexpressing BC cells [9], suggesting that the role of NErbB-2 as a TF may induce BC metastasis. Since ErbB-2 lacks a putative DNA-binding domain, it was proposed that NErbB-2 acts as a coactivator of other TFs. NErbB-2’s function as a transcriptional coactivator was identified in BC models that express estrogen (ER) and progesterone receptors (PR) and overexpress MErbB-2 [20]. Bidirectional interactions between PR and MErbB-2 classical signaling pathways had long been investigated in those BC models [15, 35]. Stat3 was revealed as the convergence point between said pathways [16, 36]. A novel class of bidirectional interaction between PR and ErbB-2 was described at the nucleus. Indeed, PR induced the nuclear translocation of full-length ErbB-2 which acts as a coactivator of the TF Stat3 bound to its response elements (GAS sites) at the cyclin D1 (CCND1) promoter [20]. Interestingly, the PR was also assembled along with Stat3 and ErbB-2 in this transcriptional complex. The biological action of NErbB-2 in ER-, PR-, and ErbB-2-positive BC models was investigated by transfection of cells with hErbB-2ΔNLS, a human ErbB-2 nuclear localization domain mutant engineered by Hung and co-workers, which is unable to translocate to the nucleus and functions as a dominant negative inhibitor of endogenous ErbB-2 nuclear migration [20, 32]. The hErbB-2ΔNLS mutant retains ErbB-2 intrinsic tyrosine kinase activity [9, 20, 32]. Notably, blockade of NErbB-2 action by transfection with hErbB-2ΔNLS disrupted the assembly of nuclear Stat3/ErbB-2/PR complexes at the CCND1 promoter and inhibited progestin-induced BC growth [20]. Progestins also induced the assembly of nuclear Stat3/ErbB-2/PR complexes to the GAS sites at the p21CIP1 promoter, thus inducing p21CIP1 expression and growth of PR-positive, MErbB-2-overexpressing BC cells [27]. Another study provided further evidence of the role of NErbB-2 as coactivator of transcription factors in BC growth. Indeed, it was demonstrated that progestins induce the assembly of a transcriptional complex among activator protein 1 (AP-1), Stat3, PR, and ErbB-2 at a region of the CCND1 promoter containing AP-1 response elements (TRE) and GAS sites [26]. It was also reported that NErbB-2 activates transcription of ribosomal RNA genes through its association with RNA polymerase-I and β-actin at the ribosomal DNA (rDNA), leading to ribosome biosynthesis [21]. In this article, the authors demonstrated that NErbB-2 function increases total protein synthesis and cell size, indicating that it may play a role in BC growth [21]. A recent study revealed that NErbB-2, in its role as a Stat3 coactivator and also in its direct role as TF, upregulates microRNA-21, a microRNA regulated by membrane ErbB-2 action [37], and promotes BC metastasis [23]. This reveals a novel NErbB-2 function as a regulator of microRNAs expression. Enhanced levels of microRNA-21 inhibited the expression of programmed cell death 4 (PDCD4) [23], which is a metastasis-suppressor protein and also a well acknowledged microRNA-21 target gene [37]. Consistent with a startling study [9], these findings support NErbB-2’s role in BC metastasis. Table 1 summarizes NErbB-2’s molecular and biological functions described. Although most of the reports indicate that NErbB-2 acts as a transcriptional regulator, ErbB-2 may also exert its tyrosine kinase activity in the nucleus. Indeed, ErbB-2 phosphorylates the cyclin-dependent kinase Cdc2, leading to inhibition of Cdc2 activation and resistance to taxol-induced apoptosis in MErbB-2-overexpressing BC cells [24]. Notably, NErbB-2 colocalized with the Cdc2/cyclin B complexes, raising the possibility that NErbB-2 function as a protein kinase may contribute to Cdc2 inhibition and to taxol resistance [24, 38].
Table 1.
Molecular and biological functions of Nuclear ErbB-2 in breast cancer
| Biological function | Molecular function | Transcription factor | Transcriptional complex components | Target gene | Transcription factor binding site | Breast cancer model | Reference |
|---|---|---|---|---|---|---|---|
| Breast cancer invasion and metastasis | Transcription factor | ErbB-2 | Not described | COX-2 | HASa | MErbB-2-positived | [9] |
| Transcription factor | ErbB-2 | Not described | microRNA-21 | HAS | MErbB-2-positive | [23] | |
| Transcriptional coactivator | Stat3 | ErbB-2/Stat3 | microRNA-21 | GASb | MErbB-2-positive | [23] | |
| Breast cancer growth and cell proliferation | Transcriptional coactivator | Stat3 | Stat3/ErbB-2/PR | Cyclin D1 | GAS | ER-, PR-, MErbB-2-positive e | [20] |
| Transcriptional coactivator | Stat3 | Stat3/ErbB-2/PR | p21CIP1 | GAS | ER-, PR-, MErbB-2-positive | [27] | |
| Transcriptional coactivator | Stat3 | AP-1/Stat3/ErbB-2/PR | Cyclin D1 | TREc/GAS | ER-, PR-, MErbB-2-positive | [26] | |
| Transcriptional coactivator | Stat3 | ErbB-2/ErbB-3/Stat3 | Cyclin D1 | GAS | MErbB-2-positive BC both sensitive and resistant to trastuzumab | [19] | |
| Ribosomal RNA synthesis and protein translation | Transcriptional coactivator | Not described | ErbB-2/β-actin/RNA Polymerase I | ribosome DNA | Not described | MErbB-2 positive | [21] |
aHAS (HER-2-associated sequence) sites: ErbB-2 response elements
bGAS (gamma interferon-activated sequence) sites: Stat3 response elements
cTRE (cis-tetradecanoyl phorbol acetate-responsive element) sites: activator protein 1 (AP-1) response elements
dMErbB-2-positive breast cancer (BC) models overexpress membrane ErbB-2 (MErbB-2)
eER-, PR-, MErbB-2- positive breast cancer models express estrogen receptor (ER) and progesterone receptor (PR) and also overexpress membrane ErbB-2 (MErbB-2)
NErbB-2 role in response to anti-MErbB-2 therapies
Full-length nuclear ErbB-2 was found to be involved in the mechanisms of BC resistance to anti-MErbB-2 agents. Basal levels of nuclear ErbB-2, ErbB-3, and Stat3, as well as of nuclear ErbB-2/ErbB-3 and nuclear ErbB-2/Stat3 complexes were detected in trastuzumab (TZ)-sensitive and -resistant ErbB-2-positive BC lines [19]. Heregulin treatment further enhanced nuclear migration and colocalization of all three proteins. Interestingly, basal MErbB-2/MErbB-3 dimers were more numerous in TZ-responsive than in -resistant cells, whereas nuclear dimers were more abundant in resistant cells, highlighting a role of nuclear ErbB dimers in the response to anti-ErbB-2 agent TZ [19]. Moreover, heregulin induced the assembly of a Stat3/ErbB-2/ErbB-3 transcriptional complex at the GAS sites of the CCND1 promoter, where both ErbB-2 and ErbB-3 act as Stat3 coactivators [19] (Table 1). This study identifies the first nuclear function of ErbB-2/ErbB-3 dimers. Furthermore, blockade of NErbB-2 action by transfection with the hErbB-2ΔNLS mutant abrogated growth of BC cells, sensitive and resistant to TZ, in a scenario in which ErbB-2/ErbB-3 dimers are formed and the PI3K/AKT pathway is activated [19], conditions where TZ is inefficient [39–42]. Disruption of the Stat3/ErbB-2/ErbB-3 nuclear complex driving CCND1 expression was revealed as the differential molecular signature underlying hErbB-2ΔNLS growth inhibitory effects in TZ-resistant cells [19]. All these findings identified NErbB-2 as a major proliferation driver in TZ-resistant BC and highlighted NErbB-2 as a novel target to overcome TZ resistance. A series of truncated ErbB-2 variants known as p95ErbB-2 were both found at the cytoplasm and the nucleus of BC cells [43, 44]. Although the exact molecular mechanism by which these NErbB-2 fragments exert their action remains to be established, it has been shown that nuclear p95ErbB-2 variants induce BC growth [43, 44] and are also involved in the response to anti-MErbB-2 therapies. Indeed, treatment of BC cells with lapatinib increased the expression of a tyrosine phosphorylated p95ErbB-2 form (p95L) located in the nucleus, which rendered these cells resistant to lapatinib [45]. Furthermore, enhanced expression of nuclear p95L was detected in biopsies from metastatic BC sites that had developed while patients were on lapatinib therapy [45], indicating a role of NErbB-2 fragments in acquired resistance to lapatinib. However, these findings need to be reconciled with other reports which found that the antiproliferative effects of lapatinib are mediated by its ability to inhibit phosphorylation of membrane ErbB-2 and its nuclear migration [25, 46]. ErbB-2 activating mutation at leucine 755 (L755), which is located at the ErbB-2 kinase domain and is near a putative nuclear export signal, has been associated with lapatinib resistance [47]. Interestingly, ectopic expression of ErbB-2 L755 mutants (L755P and L755S) enhanced NErbB-2 localization in ErbB-2-positive BC cells and increased their ability to form mammospheres in vitro, a key feature of cancer stem cells, highlighting NErbB-2 function in cancer stemness [48]. Although the mechanistic relationship among NErbB-2, lapatinib resistance, and cancer stemness needs to be elucidated, this work further supports the importance of targeting NErbB-2 presence or function to overcome resistance to anti-ErbB-2 therapies.
Conclusions
Accumulating findings have proven ErbB-2 localization and function at the nucleus of ErbB-2-positive BC cells. NErbB-2 is involved in BC growth and metastasis, both key features of this disease. NErbB-2 is also involved in BC de novo and acquired resistance to ErbB-2-targeted therapies. Most importantly, blockade of NErbB-2 presence abrogates BC growth in ErbB-2-positive BC, revealing NErbB-2 as a novel therapeutic target. Although both full-length ErbB-2 molecules and fragments were identified in the nucleus of BC cells, the exact molecular mechanisms by which NErbB-2 fragments exert their role remains to be established. An open question that also needs to be confirmed is whether ErbB-2 exerts its tyrosine kinase activity in the nucleus. In light of the key role of NErbB-2 in the resistance to anti-MErbB-2 agents, the clinical significance of NErbB-2 in response to these therapies should be explored. The identification of NErbB-2 as a novel biomarker for predicting responses to anti-ErbB-2 therapies at diagnosis, allowing the identification of patients who will fail to respond to these therapies, will be extremely important to modify the current therapeutic protocols and include NErbB-2-targeted therapy. Finally, research efforts should be focused on developing drugs designed to target NErbB-2 function.
Acknowledgements
We thank Mien-Chie Hung (MD Anderson Cancer Center, Houston, TX, USA) for his generous help and support during the course of our studies of ErbB-2 nuclear function. We are grateful to Valerie Paul Roux for her assistance in the preparation of the manuscript. We thank René Barón Foundation and Willliams Foundation for their institutional support.
Funding Information
This work was supported by IDB/PICT 2015–1587, IDB/PICT 2012-668, and PID 2012-066 grants from the National Agency of Scientific Promotion of Argentina (ANPCyT); by a grant from the Nelia and Amadeo Barletta Foundation from Switzerland; and by a grant from the National Institute of Cancer from Argentina, all of them awarded to PVE. RICR was awarded with an early career research grant from AJ Roemmers Foundation.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rosalía I. Cordo Russo, Phone: 5411-4783-2869, Email: rcordorusso@gmail.com
Patricia V. Elizalde, Phone: 5411-4783-2869, Email: patriciaelizalde@ibyme.conicet.gov.ar
References
- 1.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 4.Esteva FJ, Valero V, Booser D, Guerra LT, Murray JL, Pusztai L, Cristofanilli M, Arun B, Esmaeli B, Fritsche HA, Sneige N, Smith TL, Hortobagyi GN. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(7):1800–1808. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 5.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 6.Krop IE, Kim SB, Gonzalez-Martin A, LoRusso PM, Ferrero JM, Smitt M, Yu R, Leung AC, Wildiers H. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(7):689–699. doi: 10.1016/S1470-2045(14)70178-0. [DOI] [PubMed] [Google Scholar]
- 7.Esteva FJ, Yu D, Hung MC, Hortobagyi GN. Molecular predictors of response to trastuzumab and lapatinib in breast cancer. Nat Rev Clin Oncol. 2010;7(2):98–107. doi: 10.1038/nrclinonc.2009.216. [DOI] [PubMed] [Google Scholar]
- 8.Singh JC, Jhaveri K, Esteva FJ. HER2-positive advanced breast cancer: optimizing patient outcomes and opportunities for drug development. Br J Cancer. 2014;111(10):1888–1898. doi: 10.1038/bjc.2014.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang SC, Lien HC, Xia W, Chen IF, Lo HW, Wang Z, Ali-Seyed M, Lee DF, Bartholomeusz G, Ou-Yang F, Giri DK, Hung MC. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer Cell. 2004;6(3):251–261. doi: 10.1016/j.ccr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, Ratzkin BJ, Yarden Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16(10):5276–5287. doi: 10.1128/MCB.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16(7):1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao RH, Maruyama IN. All EGF(ErbB) receptors have preformed homo- and heterodimeric structures in living cells. J Cell Sci. 2008;121(Pt 19):3207–3217. doi: 10.1242/jcs.033399. [DOI] [PubMed] [Google Scholar]
- 13.Balana ME, Labriola L, Salatino M, Movsichoff F, Peters G, Charreau EH, Elizalde PV. Activation of ErbB-2 via a hierarchical interaction between ErbB-2 and type I insulin-like growth factor receptor in mammary tumor cells. Oncogene. 2001;20(1):34–47. doi: 10.1038/sj.onc.1204050. [DOI] [PubMed] [Google Scholar]
- 14.Salatino M, Schillaci R, Proietti CJ, Carnevale R, Frahm I, Molinolo AA, Iribarren A, Charreau EH, Elizalde PV. Inhibition of in vivo breast cancer growth by antisense oligodeoxynucleotides to type I insulin-like growth factor receptor mRNA involves inactivation of ErbBs, PI-3K/Akt and p42/p44 MAPK signaling pathways but not modulation of progesterone receptor activity. Oncogene. 2004;23(30):5161–5174. doi: 10.1038/sj.onc.1207659. [DOI] [PubMed] [Google Scholar]
- 15.Labriola L, Salatino M, Proietti CJ, Pecci A, Coso OA, Kornblihtt AR, Charreau EH, Elizalde PV. Heregulin induces transcriptional activation of the progesterone receptor by a mechanism that requires functional ErbB-2 and mitogen-activated protein kinase activation in breast cancer cells. Mol Cell Biol. 2003;23(3):1095–1111. doi: 10.1128/MCB.23.3.1095-1111.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proietti CJ, Rosemblit C, Beguelin W, Rivas MA, Diaz Flaque MC, Charreau EH, Schillaci R, Elizalde PV. Activation of Stat3 by heregulin/ErbB-2 through the co-option of progesterone receptor signaling drives breast cancer growth. Mol Cell Biol. 2009;29(5):1249–1265. doi: 10.1128/MCB.00853-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19(13):3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranger JJ, Levy DE, Shahalizadeh S, Hallett M, Muller WJ. Identification of a Stat3-dependent transcription regulatory network involved in metastatic progression. Cancer Res. 2009;69(17):6823–6830. doi: 10.1158/0008-5472.CAN-09-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cordo Russo RI, Beguelin W, Diaz Flaque MC, Proietti C, Venturutti L, Galigniana NM, Tkach M, Guzman P, Roa JC, O'Brien N, Charreau EH, Schillaci R, Elizalde PV. Targeting ErbB-2 nuclear localization and function inhibits breast cancer growth and overcomes trastuzumab resistance. Oncogene. 2015;34(26):3413–3428. doi: 10.1038/onc.2014.272. [DOI] [PubMed] [Google Scholar]
- 20.Beguelin W, Diaz Flaque MC, Proietti CJ, Cayrol F, Rivas MA, Tkach M, Rosemblit C, Tocci JM, Charreau EH, Schillaci R, Elizalde PV. Progesterone receptor induces ErbB-2 nuclear translocation to promote breast cancer growth via a novel transcriptional effect: ErbB-2 function as a coactivator of Stat3. Mol Cell Biol. 2010;30(23):5456–5472. doi: 10.1128/MCB.00012-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li LY, Chen H, Hsieh YH, Wang YN, Chu HJ, Chen YH, Chen HY, Chien PJ, Ma HT, Tsai HC, Lai CC, Sher YP, Lien HC, Tsai CH, Hung MC. Nuclear ErbB2 enhances translation and cell growth by activating transcription of ribosomal RNA genes. Cancer Res. 2011;71(12):4269–4279. doi: 10.1158/0008-5472.CAN-10-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Kuang J, Shen Y, Majer MM, Nelson CC, Parsawar K, Heichman KA, Kuwada SK. The atypical histone macroH2A1.2 interacts with HER-2 protein in cancer cells. J Biol Chem. 2012;287(27):23171–23183. doi: 10.1074/jbc.M112.379412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venturutti L, Romero LV, Urtreger AJ, Chervo MF, Cordo Russo RI, Mercogliano MF, Inurrigarro G, Pereyra MG, Proietti CJ, Izzo F, Diaz Flaque MC, Sundblad V, Roa JC, Guzman P, Bal de Kier Joffe ED, Charreau EH, Schillaci R, Elizalde PV. Stat3 regulates ErbB-2 expression and co-opts ErbB-2 nuclear function to induce miR-21 expression, PDCD4 downregulation and breast cancer metastasis. Oncogene. 2016;35(17):2208–2222. doi: 10.1038/onc.2015.281. [DOI] [PubMed] [Google Scholar]
- 24.Tan M, Jing T, Lan KH, Neal CL, Li P, Lee S, Fang D, Nagata Y, Liu J, Arlinghaus R, Hung MC, Yu D. Phosphorylation on tyrosine-15 of p34(Cdc2) by ErbB2 inhibits p34(Cdc2) activation and is involved in resistance to taxol-induced apoptosis. Mol Cell. 2002;9(5):993–1004. doi: 10.1016/S1097-2765(02)00510-5. [DOI] [PubMed] [Google Scholar]
- 25.Kim HP, Yoon YK, Kim JW, Han SW, Hur HS, Park J, Lee JH, Oh DY, Im SA, Bang YJ, Kim TY. Lapatinib, a dual EGFR and HER2 tyrosine kinase inhibitor, downregulates thymidylate synthase by inhibiting the nuclear translocation of EGFR and HER2. PLoS One. 2009;4(6):e5933. doi: 10.1371/journal.pone.0005933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diaz Flaque MC, Galigniana NM, Beguelin W, Vicario R, Proietti CJ, Russo RC, Rivas MA, Tkach M, Guzman P, Roa JC, Maronna E, Pineda V, Munoz S, Mercogliano MF, Charreau EH, Yankilevich P, Schillaci R, Elizalde PV. Progesterone receptor assembly of a transcriptional complex along with activator protein 1, signal transducer and activator of transcription 3 and ErbB-2 governs breast cancer growth and predicts response to endocrine therapy. Breast Cancer Res. 2013;15(6):R118. doi: 10.1186/bcr3587. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Diaz Flaque MC, Vicario R, Proietti CJ, Izzo F, Schillaci R, Elizalde PV. Progestin drives breast cancer growth by inducing p21(CIP1) expression through the assembly of a transcriptional complex among Stat3, progesterone receptor and ErbB-2. Steroids. 2013;78(6):559–567. doi: 10.1016/j.steroids.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Schillaci R, Guzman P, Cayrol F, Beguelin W, Diaz Flaque MC, Proietti CJ, Pineda V, Palazzi J, Frahm I, Charreau EH, Maronna E, Roa JC, Elizalde PV. Clinical relevance of ErbB-2/HER2 nuclear expression in breast cancer. BMC Cancer. 2012;12(1):74. doi: 10.1186/1471-2407-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YN, Yamaguchi H, Huo L, Du Y, Lee HJ, Lee HH, Wang H, Hsu JM, Hung MC. The translocon Sec61beta localized in the inner nuclear membrane transports membrane-embedded EGF receptor to the nucleus. J Biol Chem. 2010;285(49):38720–38729. doi: 10.1074/jbc.M110.158659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carpenter G, Liao HJ. Receptor tyrosine kinases in the nucleus. Cold Spring Harb Perspect Biol. 2013;5(10):a008979. doi: 10.1101/cshperspect.a008979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen MK, Hung MC. Proteolytic cleavage, trafficking, and functions of nuclear receptor tyrosine kinases. FEBS J. 2015;282(19):3693–3721. doi: 10.1111/febs.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giri DK, Ali-Seyed M, Li LY, Lee DF, Ling P, Bartholomeusz G, Wang SC, Hung MC. Endosomal transport of ErbB-2: mechanism for nuclear entry of the cell surface receptor. Mol Cell Biol. 2005;25(24):11005–11018. doi: 10.1128/MCB.25.24.11005-11018.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheng C, Qiu J, He Z, Wang H, Wang Q, Guo Z, Zhu L, Ni Q. Suppression of Kpnbeta1 expression inhibits human breast cancer cell proliferation by abrogating nuclear transport of Her2. Oncol Rep. 2018;39(2):554–564. doi: 10.3892/or.2017.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marfori M, Mynott A, Ellis JJ, Mehdi AM, Saunders NF, Curmi PM, Forwood JK, Bodén M, Kobe B. Molecular basis for specificity of nuclear import and prediction of nuclear localization. Biochim Biophys Acta. 2011;1813(9):1562–1577. doi: 10.1016/j.bbamcr.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Balana ME, Lupu R, Labriola L, Charreau EH, Elizalde PV. Interactions between progestins and heregulin (HRG) signaling pathways: HRG acts as mediator of progestins proliferative effects in mouse mammary adenocarcinomas. Oncogene. 1999;18(46):6370–6379. doi: 10.1038/sj.onc.1203028. [DOI] [PubMed] [Google Scholar]
- 36.Proietti C, Salatino M, Rosemblit C, Carnevale R, Pecci A, Kornblihtt AR, Molinolo AA, Frahm I, Charreau EH, Schillaci R, Elizalde PV. Progestins induce transcriptional activation of signal transducer and activator of transcription 3 (Stat3) via a Jak- and Src-dependent mechanism in breast cancer cells. Mol Cell Biol. 2005;25(12):4826–4840. doi: 10.1128/MCB.25.12.4826-4840.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang TH, Wu F, Loeb GB, Hsu R, Heidersbach A, Brincat A, Horiuchi D, Lebbink RJ, Mo YY, Goga A, McManus MT. Up-regulation of miR-21 by HER2/neu signaling promotes cell invasion. J Biol Chem. 2009;284(27):18515–18524. doi: 10.1074/jbc.M109.006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang SC, Hung MC. Nuclear translocation of the epidermal growth factor receptor family membrane tyrosine kinase receptors. Clin Cancer Res. 2009;15(21):6484–6489. doi: 10.1158/1078-0432.CCR-08-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, Hortobagyi GN, Hung MC, Yu D. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6(2):117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 40.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, Sampath D, Sliwkowski MX. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15(5):429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh R, Narasanna A, Wang SE, Liu S, Chakrabarty A, Balko JM, Gonzalez-Angulo AM, Mills GB, Penuel E, Winslow J, Sperinde J, Dua R, Pidaparthi S, Mukherjee A, Leitzel K, Kostler WJ, Lipton A, Bates M, Arteaga CL. Trastuzumab has preferential activity against breast cancers driven by HER2 homodimers. Cancer Res. 2011;71(5):1871–1882. doi: 10.1158/0008-5472.CAN-10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62(14):4132–4141. [PubMed] [Google Scholar]
- 43.Anido J, Scaltriti M, Bech Serra JJ, Santiago JB, Todo FR, Baselga J, Arribas J. Biosynthesis of tumorigenic HER2 C-terminal fragments by alternative initiation of translation. EMBO J. 2006;25(13):3234–3244. doi: 10.1038/sj.emboj.7601191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scaltriti M, Rojo F, Ocana A, Anido J, Guzman M, Cortes J, Di Cosimo S, Matias-Guiu X, Cajal S, Arribas J, Baselga J. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99(8):628–638. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 45.Xia W, Liu Z, Zong R, Liu L, Zhao S, Bacus SS, Mao Y, He J, Wulfkuhle JD, Petricoin EF, III, Osada T, Yang XY, Hartman ZC, Clay TM, Blackwell KL, Lyerly HK, Spector NL. Truncated ErbB2 expressed in tumor cell nuclei contributes to acquired therapeutic resistance to ErbB2 kinase inhibitors. Mol Cancer Ther. 2011;10(8):1367–1374. doi: 10.1158/1535-7163.MCT-10-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scaltriti M, Verma C, Guzman M, Jimenez J, Parra JL, Pedersen K, Smith DJ, Landolfi S, Cajal S, Arribas J, Baselga J. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28(6):803–814. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 47.Bose R, Kavuri SM, Searleman AC, Shen W, Shen D, Koboldt DC, Monsey J, Goel N, Aronson AB, Li S, Ma CX, Ding L, Mardis ER, Ellis MJ. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3(2):224–237. doi: 10.1158/2159-8290.CD-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang W-L, Nie L, Huynh K-T, Chen J-Y, Yao J-H, Hung M-C, Huang W-C. Abstract #4018: mutations of HER2 at L755 residue results in HER2 nuclear accumulation and enhances breast cancer stem cell activity. Cancer Res. 2018;78:4018. [Google Scholar]