Abstract
The study aimed to determine whether 25-OH vitamin D3 deficiency is present in patients with diagnosed BCC, and the effect of vitamin D replacement on the rates of BCC recurrence in patients with 25-OH vitamin D3 deficiency. In this prospective study, between 2012 and 2017, in the first stage, 25-OH vitamin D3 levels of all patients diagnosed with BCC between 2012 and 2013 were evaluated. In the second stage between 2014 and 2015, we evaluated the 25-OH vitamin D3 level of patients who had 25-OH vitamin D3 level < 25 ng/mL. All the patients included in the second stage had BCC recurrence. In the third stage, the patients who were diagnosed 25-OH vitamin D3 deficiency with BCC, between 2015 and 2017, were studied. The mean 25-OH vitamin D3 level of the patients in the second stage was 10.12 ng/mL. Recurrence was observed in 9.64% of the patients in the second stage. The mean level of serum 25-OH vitamin D3 in the third stage was 40.1 ng/mL, and 3.49% of these patients presented with recurrence. In all the patients as the initial diagnosis and following the 25-OH vitamin D3 level in all the patients with BCC recurrence, maintaining 25-OH vitamin D3 levels above 25 ng/mL can significantly reduce the recurrence rate.
Keywords: Basal cell carcinoma, Vitamin D, Skin cancers, Vitamin D deficiency
Introduction
The ozone layer has been getting thinner due to the use of chlorofluorocarbon (CFC) and hydrochlorofluorocarbon (HCFC). As a result, the amount of ultraviolet rays reaching earth has increased, thus affecting the incidence of non-melanoma skin cancers (NMSCs). The incidence of basal cell carcinoma (BCC), the most common subtype of NMSC, increases 10% annually [1]. Conversely, sunlight, one of the major risk factors for these cancers, is also the main source of synthesis of 25-hydroxyvitamin D3 (25-OH D3), which provides essential functions for the human body. Vitamin D has pleiotropic effects such as cell growth, differentiation, apoptosis, and regulation of tumor-immune system through its cancer-associated vitamin D receptor (VDR) [2, 3]. Furthermore, vitamin D has a protective role against colon, breast, and prostate cancers [4].
Ultraviolet (UV) radiation has opposing effects on BCC carcinogenesis, a stimulatory effect via mutagenesis, and an inhibitory effect via production of Hedgehog-inhibiting vitamin D3; thus, vitamin D3 could inhibit BCC carcinogenesis [5]. In other studies, it was mentioned that high vitamin D3 level reduces the risk of NMSC [6, 7]. However, we could not find any study investigating the frequency of BCC recurrence in patients with deficiency and patients receiving vitamin D3 replacement treatment.
This study aimed to determine whether 25-OH vitamin D3 deficiency is present in patients with diagnosed BCC and to assess the effect of vitamin D3 replacement on the rates of BCC recurrence in patients with vitamin D deficiency.
Patients and Methods
In this prospective study, between January 2012 and January 2017, we included patients who were admitted with skin lesions and diagnosed with BCC. We excluded patients diagnosed with BCC in the pathology report but having a surgical margin of < 0.5 cm, gastrointestinal absorption problems, severe hypertension, parathyroid nodules, liver or kidney disease, or multiple skin lesions as well as those who did not come to the routine controls and did not use sunscreen protection. Furthermore, patients with BCC in dangerous areas, such as the nose or lower eyelid, wherein facial esthetic could be severely damaged, were excluded. Additionally, patients who followed a strict vegan diet were excluded, because most of the natural sources of vitamin D are animal-based, including fish, fish oils, egg yolks, fortified milk, and beef liver. The Necmettin Erbakan University, Meram Faculty of Medicine Clinical Research Ethics Committee, approved the study. Written informed consent and verbal approval were obtained from the patients. A single surgeon performed all procedures. All Serum 25-OH vitamin D3 levels have been investigated on the Agilent 1100 using Chromsystems’ high-performance liquid chromatography kit.
The study was planned in three stages. In the first stage, the 25-OH vitamin D3 levels of all patients diagnosed with BCC between January 2012 and December 2013 were evaluated. We investigated whether there was a correlation between BCC and 25-OH vitamin D3 deficiency. In the second stage, between January 2014 and January 2015, we evaluated the 25-OH vitamin D3 levels of patients who had 25-OH vitamin D3 levels < 25 ng/mL. The surgical margin of the pathology material examined after primary surgery was 0.5 cm negative. All patients included in the second stage had BCC recurrence. Then, we investigated whether there was a correlation between BCC recurrence and 25-OH vitamin D3 deficiency or not. In the third stage, 50,000 IU of oral vitamin D3 was given per week for 6 weeks as a loading dose, totaling 300,000 IU, to patients who were diagnosed with BCC between February 2015 and January 2017 and who had a negative surgical margin of > 0.5 cm. Twenty days after the last dose, we checked the serum 25-OH vitamin D3 level. This level ranging 25–100 ng/mL was considered normal in the posttreatment controls. For those having serum 25-OH vitamin D3 levels < 25 ng/mL, 800-IU oral treatment was continued as a daily maintenance dose following the loading dose. Patients in whom the serum 25-OH vitamin D3 levels did not increase following replacement therapy were excluded from the study. Patients with BCC who underwent vitamin D3 replacement treatment were followed up by regular controls.
While recording patients’ medical histories, patients were asked about their ages, professions, religion, whether they took a summer vacation or not, and the frequency of sunbathing. In addition, the number of many female patients wearing headscarves because of their religious beliefs was also recorded in addition to the places in which patients lived, which were marked on Google maps.
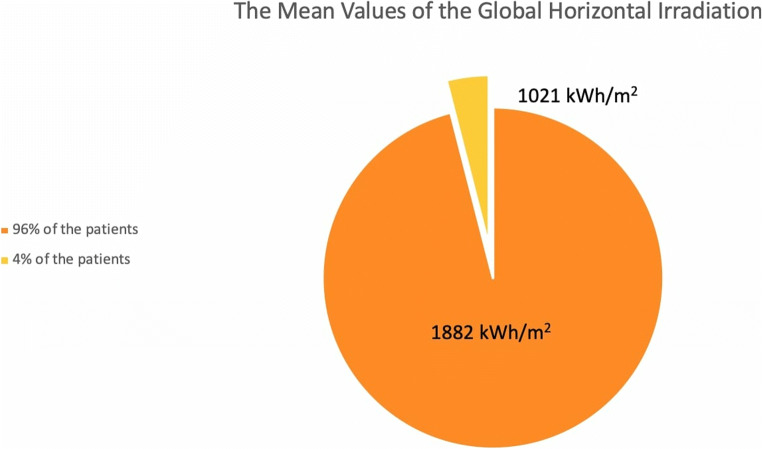
Additionally, in the literature, there is knowledge to compare between different locations and equivalent systems to be used, and global irradiance should be measured, which is defined as solar irradiance on the horizontal plane [8]. Thus, we evaluated the global horizontal irradiation (GHI) of the location data from the Global Solar Atlas (Fig. 1).
Fig. 1.
The mean values of the global horizontal irradiation (GHI)
Recurrence rates in these patients were compared with the recurrence rates in patients who were diagnosed with BCC between January 2012 and December 2013 and who did not receive vitamin D3 replacement treatment (first-stage patients). All surgical procedures were performed under local anesthesia, and a single pathologist reevaluated all the pathological preparations. At the second pathologic examination, patients with < 0.5-cm surgical margins were excluded from the study. All patients were advised to use sun protection cream, avoid sun exposure, and attend the monthly controls.
Statistical Evaluation
Results were analyzed with SPSS 24.0 (IBM, Armonk, NY, USA). The χ2 test was used to evaluate the relationship between dependent variables and independent variables. The level of significance was set at P < 0.05.
Results
A total of 496 patients were included in the study, 239 (141 male, 93 female) in the first stage, 114 (60 male, 54 female) in the second stage, and 143 (84 male, 59 female) in the third stage. The mean age of the patients was 69 years, and the mean follow-up period was 32 (range, 24–36) months. In the first stage, 88% of the patients had skin lesions in the head and neck, 6% in the upper extremities, 2% in the trunk, and 4% in the lower extremities. In the second stage, 79% of the patients had skin lesions in the head and neck, 14% in the extremities, and 7% in the trunk. In the third stage, 97.4% of the patients had the skin lesion in the head and neck and 2.6% in the extremities (Table 1).
Table 1.
The results in all stages
| 1st stage | 2nd stage | 3rd stage | |
|---|---|---|---|
| Mean age (year) | 68 | 71 | 70 |
| Sex | 141 M, 93 F | 60 M, 54 F | 84 M, 59 F |
| Localization | 88% H.N., 10% E., 2% T. | 79% H.N., 14% E., 7% T. | 97.4% H.N., 2.6% E. |
| Serum vitamin D3 (ng/mL) | 12.23 | 10.12 | 11.81 ➔ 40.1a |
| Recurrence (%) | – | 9.64 | 3.49 |
B.B., head-neck; E., extremity; T., trunk; F., female; M., male
aSerum vitamin D3 level after the replacement therapy
During the initial diagnosis, the mean size of the lesions was 2.1 × 1.6 × 0.8 cm. In the second stage, the mean size of the lesions was 1.9 × 1.4 × 0.7, and in the third stage, the mean size was 2.3 × 1.5 × 0.8.
From the patients’ medical histories, 92% of the patients did not sunbathe during the summer, 4% sunbathed 0–5 min/day, 3% sunbathed 5–10 min/day, and 1% sunbathed 10–15 min/day. During the summer months, 89% did not take a holiday, 6% went on vacation 2–5 days/year, 4% went 5–7 days/year, and 1% went 7–10 days/year. Eighty-four percent of female patients were veiled because of their Islamic beliefs. In total, 91% of the patients were Muslim, 7% did not declare their religion, and 2% were Christians. Eighty-one percent of the patients were working in low-income professional groups (earning < 1000 USD/month). Recurrence was observed in 8.7% of the veiled female patients in the second stage, in 2% of the veiled female patients in the third stage.
Ninety-six percent of the patients were living in high-solar areas according to the solar heat map (Fig. 1). The mean value of the global horizontal irradiation of the patients where live is 1894 kWh/m2 (min. 1702–max. 2018 kWh/m2) per year.
The mean serum 25-OH vitamin D3 level of the patients who were diagnosed with BCC in the first stage was 12.2 (range, 7.4–34.4; male, 12.4; female, 11.9) ng/mL. The mean 25-OH vitamin D3 level of the patients with BCC recurrence in the second stage was 10.1 (range, 4.1–23.8; male, 10.2; female, 10.1) ng/mL. Recurrence was observed in 9.64% (male, 10%; female, 9.26) of the patients in the second stage, and skin lesions were found in the head and neck in eight patients, in the upper extremities in one patient, in the upper back in one patient, and in the lower extremities in one patient. The mean serum 25-OH vitamin D3 level in the third stage was 11.8 (range, 5.4–20.1; male, 11.7; female, 12) ng/mL. The mean serum 25-OH vitamin D3 level after replacement therapy was 40.1 (range, 28.9–65.2; male, 42.3; female, 37) ng/mL, and 3.49% (male, 3.57%; female, 3.38%) of these patients presented with recurrence (Table 1). The recurrent lesions were found in the head and neck in five patients. Statistically significant relationships were determined between 25-OH vitamin D3 deficiency and BCC frequency at the initial diagnosis and between 25-OH vitamin D3 deficiency and BCC recurrence (P < 0.05). Recurrence rates in patients in the second stage were statistically higher than those in patients in the third stage (P < 0.05). There were no statistically significant differences on 25-OH vitamin D3 levels and recurrence rates in the second and third stages between male and female patients in all groups.
Discussion
Skin is the only organ which is known to produce every component involved in the vitamin D3 production pathway in response to ultraviolet B (UVB, 290–320 nm) exposure [9]. As a result of the interaction of 7-dehydrocholesterol with UV, vitamin D3 is formed, and then, it is converted to 25-hydroxyvitamin D3 by hydroxylation reaction in the liver. In the kidneys, 1-alpha, 25-dihydroxyvitamin D3, which is the metabolically active form, is formed [10]. Although this metabolic process occurs in the liver and kidneys, animal studies have shown that the hydroxylases in the epidermis of skin can also actively transform vitamin D3 to 25-hydroxyvitamin D3 without the involvement of the liver [11]. VDR, an intracellular receptor and transcription factor, belongs to a family of proteins that includes some steroid hormone receptors, retinoids, isoprenoids, eicosanoids, and cholesterol metabolites [12]. This receptor is present in > 60 cell types in the human body, and when the activity of this receptor is abnormal, even if the vitamin D level is normal, the vitamin may not act correctly [13]. Activated vitamin D functions with the genomic and non-genomic pathways. The genomic pathway results in the activation of vitamin D response element, which affects over 900 gene expressions [14], whereas the non-genomic pathway is responsible for the intracrine effects of the activated vitamin D [15]. It has been claimed that this pathway may have a protective effect against DNA damage [16]. In patients with vitamin D deficiency or disorders of vitamin D metabolism, the non-genomic pathway failure may result in DNA damage and may lead to the development of NMSCs and recurrence after treatment. High-intensity UVB exposure has been reported to contribute to the development of skin cancer carcinogenesis associated with cutaneous inflammation and DNA damage [17, 18]. Sun creams with sun protection factor absorb 95–98% of the UVB radiation. However, sun protection creams can reduce the synthesis of activated vitamin D in the skin [19, 20]. All 80 patients with recurrence and low 25-OH vitamin D3 level had been using sunscreen. All patients in this study ranged from 1 to 4 according to the Fitzpatrick classification.
Vitamin D is known to have a protective effect against some types of diseases and cancers via signaling mechanisms that include VDR. It has been claimed that the incidence of breast cancer in women with high UVB exposure is 50% less than that in women with low UVB exposure [21]. In another study, it has been claimed that the incidence of prostate cancer in men with high UVB exposure is 50% less than that in men with low UVB exposure [22]. Lappe et al. mentioned that vitamin D3 and calcium supplementation significantly reduce all cancer risks in postmenopausal women [23]. Although there is a relationship between vitamin D and some types of cancer in the literature, there is no study showing a significant relationship between vitamin D supplementation and the prevention treatment of NMSC in large patient populations. In a case-control study in senior men, it was proposed that NMSC development was lower in patients with high serum previtamin D metabolite than in those with low serum previtamin D metabolite [6]. Moreover, increased plasma previtamin D levels have been shown to reduce metastasis and increase survival [16, 24]. Besides, it was claimed that vitamin D3 and 1,25 D3 levels might help to determine survival prognosis and metastasis in skin cancer as well as lung, breast, and prostate cancers [25–27]. In the third stage of our study, patients who underwent vitamin D3 replacement treatment were found to have significantly lesser incidence of BCC recurrence than the non-replacement group. Vitamin D is primarily synthesized in summer due to higher sun exposure, and its synthesis decreases in winter when the sun exposure is lower. Although higher and lower serum levels are expected in summer and winter, respectively, it is recommended that the 25-OH vitamin D3 level should be > 25 ng/mL even in winter when the sun exposure is lower. Therefore, we overlooked the seasonal differences in 25-OH vitamin D3 measurements of the patients at the time of admission.
Although female patients live in areas with intense sun, they have low serum 25-OH vitamin D3 levels. We think that the cause of these low levels was due to the veils that were worn in accordance with their religious beliefs (84%). In men, the low serum 25-OH vitamin D3 levels may have been due to lack of vacations and sunbathing habits in summer months since 92% of the patients did not sunbathe, and 89% did not take vacation during those months. Additionally, a large part of patients live in similar geographic region. Since 96% of the patients live in high solar areas, it may not be true to compare statistically between patients in high solar areas and the other 4% of patients.
A comparison of the recurrence rates of patients diagnosed with BCC between February 2015 and January 2017 with those of patients diagnosed with BCC between January 2012 and February 2013 can be shown as a disadvantage of our study. However, we believe it is not ethically correct not to make the necessary replacement treatment. Therefore, the control and study group consisted of patients who presented in different periods.
In this study, we demonstrated the existence of a statistically significant relationship between low serum 25-OH vitamin D3 level and both primary BCC and recurrent BCC. Furthermore, we found that vitamin D3 replacement therapy in patients with BCC reduced the recurrence rate. Vitamin D can also be taken with nutrients, but its most important source is the exposure of skin with sunlight [28]. It is known that this synthesis is mediated by UVB (290–320 nm) that reaches earth at its highest level around noon [28]. On the other hand, UVB is known to be a risk factor in the development of BCC, and patients are advised to avoid sunlight at noon, when the sunlight reaches vertically [6]. As a solution to this paradox, we think that patients with BCC may avoid sunlight exposure of dangerous areas, but may expose other body areas without BCC during daylight for 10–15 minutes. In patients who should not be in contact with sunlight, 25-OH vitamin D3 levels should be checked regularly, and vitamin D3 replacement should be considered a solution.
Conclusion
After measuring the 25-OH vitamin D3 levels in all the patients with BCC recurrence, we conclude that maintaining 25-OH vitamin D3 levels > 25 ng/mL in patients with an initial diagnosis of BCC can significantly reduce the recurrence rate after BCC.
Acknowledgments
The authors thank Mehmet Uyar, MD, for his statistical analysis support.
Author Contributions
Study conception and design: Ince. Acquisition of data: Ince, Yildirim. Analysis and interpretation of data: Ince, Dadaci
Compliance with Ethical Standards
Disclaimer
The research was not sponsored by an outside organization. We (all of the authors) have agreed to allow full access to the primary data and to allow the journal to review the data if requested.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Review Committee Statement
This study conformed to the Helsinki Declaration.
Footnotes
Synopsis
We aimed to determine whether vitamin D deficiency is present in patients diagnosed with BCC and the effect of vitamin D replacement on the rates of BCC recurrence in patients with vitamin D deficiency. In this study we determined a relation between low serum vitamin D level and both primary BCC and recurrent BCC. In all the patients as the initial diagnosis and follow-up of vitamin D level in all the patients with BCC recurrence maintaining vitamin D levels above 25 ng/mL can significantly reduce the recurrence rate
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bilsev Ince, Phone: +90 332 223 60 00-7791, Email: bilsevince@yahoo.com.
Mehmet Emin Cem Yildirim, Phone: +90 532 171 1957, Email: dr.cem_yildirim@hotmail.com.
Mehmet Dadaci, Phone: +903322237965, Email: mdadaci@gmail.com.
References
- 1.Goto M, Kai Y, Arakawa S, et al. Analysis of 256 cases of basal cell carcinoma after either one-step or two-step surgery in a Japanese institution. J Dermatol. 2012;39(1):68–71. doi: 10.1111/j.1346-8138.2011.01306.x. [DOI] [PubMed] [Google Scholar]
- 2.Tosetti F, Ferrari N, De Flora S, et al. ‘Angioprevention’: angiogenesis is a common and key target for cancer chemopreventive agents. FASEB J. 2002;16(1):2–14. doi: 10.1096/fj.01-0300rev. [DOI] [PubMed] [Google Scholar]
- 3.Ince B, Sakarya ME, Dadaci M. An assessment of the effects of serum vitamin d levels on snoring in patients who have undergone septorhinoplasty. Turk J Plast Surg. 2018;26(2):50–55. doi: 10.4103/tjps.TJPS_14_18. [DOI] [Google Scholar]
- 4.Giovannucci E. Vitamin D and cancer incidence in the Harvard cohorts. Ann Epidemiol. 2009;19(2):84–88. doi: 10.1016/j.annepidem.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Makarova A, Wang G, Dolorito JA, KC S, Libove E, Epstein EH., Jr Vitamin D3 produced by skin exposure to UVR inhibits murine basal cell carcinoma carcinogenesis. J Invest Dermatol. 2017;137(12):2613–2619. doi: 10.1016/j.jid.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 6.Tang JY, Parimi N, Wu A, et al. Inverse association between serum 25(OH) vitamin D levels and non-melanoma skin cancer in elderly men. Cancer Causes Control. 2010;21:387–391. doi: 10.1007/s10552-009-9470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park MS, Li T, Wu S, et al. Vitamin D intake and risk of skin cancer in US women and men. PLoS One. 2016;11(8):e0160308. doi: 10.1371/journal.pone.0160308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altmeyer P, Hoffmann K, Stücker M, editors. Skin cancer and UV radiation. Berlin: Springer; 1997. [Google Scholar]
- 9.Webb AR. Who, what, where and when- influences on cutaneous vitamin D synthesis. Prog Biophys Mol Biol. 2006;92(1):17–25. doi: 10.1016/j.pbiomolbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 10.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 11.Bikle DD, Nemanic MK, Gee E, Elias P. 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kynetic and regulation. J Clin Invest. 1986;78(2):557–566. doi: 10.1172/JCI112609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78(4):1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 13.Walters MR. Newly identified actions of the vitamin D endocrine system. Endocr Rev. 1992;13(4):719–764. doi: 10.1210/edrv-13-4-719. [DOI] [PubMed] [Google Scholar]
- 14.Schuster I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim Biophys Acta. 2011;1814(1):186–199. doi: 10.1016/j.bbapap.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov. 2004;3:27–41. doi: 10.1038/nrd1283. [DOI] [PubMed] [Google Scholar]
- 16.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7(9):684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer. I. Basal cell carcinoma. Arch Dermatol. 1995;131(2):157–163. doi: 10.1001/archderm.1995.01690140041006. [DOI] [PubMed] [Google Scholar]
- 18.Strickland PT, Vitasa BC, West SK, Rosenthal FS, Emmett EA, Taylor HR. Quantitative carcinogenesis in man: solar ultraviolet B dose dependence of skin cancer in Maryland watermen. J Natl Cancer Inst. 1989;81(24):1910–1913. doi: 10.1093/jnci/81.24.1910. [DOI] [PubMed] [Google Scholar]
- 19.Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res. 2007;22(Suppl 2):V28–V33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- 20.Ince B, Uyar I, Dadaci M. Effect of Vitamin D deficiency on hypertrophic scarring. Dermatol Surg. 2019;45(2):274–279. doi: 10.1097/DSS.0000000000001680. [DOI] [PubMed] [Google Scholar]
- 21.Grant WB. Relation between prediagnostic serum 25-hydroxyvitamin D level and incidence of breast, colorectal, and other cancers. J Photochem Photobiol B. 2010;101(2):130–136. doi: 10.1016/j.jphotobiol.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Baeke F, van Etten E, Gysemans C, et al. Vitamin D signaling in immune-mediated disorders: evolving insights and therapeutic opportunities. Mol Asp Med. 2008;29(6):376–387. doi: 10.1016/j.mam.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85(6):1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 24.Godar DE, Landry RJ, Lucas AD. Increased UVA exposures and decreased cutaneous Vitamin D(3) levels may be responsible for the increasing incidence of melanoma. Med Hypotheses. 2009;72(4):434–443. doi: 10.1016/j.mehy.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 25.Ramirez AM, Wongtrakool C, Welch T, Steinmeyer A, Zügel U, Roman J. Vitamin D inhibition of pro-fibrotic effects of transforming growth factor beta1 in lung fibroblasts and epithelial cells. J Steroid Biochem Mol Biol. 2010;118(3):142–150. doi: 10.1016/j.jsbmb.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shannan B, Seifert M, Boothman DA, Tilgen W, Reichrath J. Clusterin over-expression modulates proapoptotic and antiproliferative effects of 1,25(OH)2D3 in prostate cancer cells in vitro. J Steroid Biochem Mol Biol. 2007;103(3-5):721–725. doi: 10.1016/j.jsbmb.2006.12.068. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Lopez FR, Chedraui P, Haya J. Review article: vitamin D acquisition and breast cancer risk. Reprod Sci. 2009;16(1):7–19. doi: 10.1177/1933719108327595. [DOI] [PubMed] [Google Scholar]
- 28.Ince B, Yildirim MEC, Ismayilzade M, Dadaci M. Vitamin D and systemic effects of vitamin D deficiency. Selcuk Med J. 2018;2(34):84–89. [Google Scholar]