Abstract
Pregnancy has a dual effect on the risk of breast cancer. On one hand, pregnancy at a young age is known to be protective. However, pregnancy is also associated with a transient increased risk of breast cancer. For women that have children after the age of 30, the risk remains higher than women who never had children for decades. Involution of the breast has been identified as a window of mammary development associated with the adverse effect of pregnancy. In this review, we summarize the current understanding of the role of involution and describe the role of collagen in this setting. We also discuss the role of a collagen-dependent protease, pappalysin-1, in postpartum breast cancer and its role in activating both insulin-like growth factor signaling and discoidin domain collagen receptor 2, DDR2. Together, these novel advances in our understanding of postpartum breast cancer open the way to targeted therapies against this aggressive breast cancer sub-type.
Keywords: Pregnancy-associated breast cancer, Postpartum breast cancer, Collagen, DDR2, PAPP-A, Involution
Pregnancy as a Risk Factor
Pregnancy induces one of the most drastic remodeling phases to the breast, both molecularly and anatomically [1]. These changes can create a pro-tumorigenic environment [2]. Breast cancer is the most prevalent type of cancer associated with pregnancy, with 1 in 3000 women diagnosed with breast cancer during pregnancy or within the first two postpartum years [3].
Numerous epidemiological studies analyzed the effects of gestation on risk of breast cancer including how a woman’s age, number of pregnancies, or breastfeeding history may modulate risk of breast cancer [4–6]. It is widely accepted that pregnancy incurs a protective effect against breast cancer, as evidenced by a reduction in risk associated with pregnancy at a young age [4]. In fact, full-term pregnancy at a young age is the strongest known risk-reducing factor [7, 8]. However, this protective effect does not begin until several years following pregnancy and can vary depending on the age of the mother at time of pregnancy [4].
What is less well recognized is that in all women there is a transient, but significant, increase in risk of developing breast cancer that peaks at 6 years post-pregnancy. Following this peak, risk of breast cancer gradually declines, however, the risk still remains higher for several years compared to women who have never been pregnant. Even in women under age 25, increased risk in breast cancer persists for a decade post-pregnancy. Further, women over the age of 30 at time of pregnancy remain at a higher risk for developing breast cancer for up to 30 years following childbirth [4]. This is of particular significance considering that women throughout the developed world are steadily delaying childbirth, therefore, the number of breast cancer diagnoses following pregnancy is likely to increase considerably [4, 9].
Compared to the 98% 5-year survival rate in nulliparous patients, women diagnosed with breast cancer even up to 5 years postpartum experience a significant decrease in 5-year survival rate (65.8%) [10–12]. These same women exhibit increased rates of 5-year distant metastasis (31.1% vs. 14.8%) compared to age-, stage-, sub-type-matched nulliparous patients [10]. Collectively, these studies suggest that postpartum-associated breast cancer (PPBC) is a more aggressive form of breast cancer and that pregnancy generates an environment that favors disease progression [13, 14]. Understanding the unique factors driving PPBC would allow the development of better therapies [4].
Involution
Involution refers to the phase of the breast development following cessation of lactation and in which the gross morphology of the breast tissue is restored to that seen in a pre-pregnancy state [1]. Further, involution is a significant risk window for developing PPBC due to the complex cell signaling activation, the pro-inflammatory microenvironment, and rapid extracellular matrix (ECM) remodeling associated with this phase of breast development [2, 4, 15]. During involution, the milk-producing epithelia and lobuloalveolar structures are eliminated and replaced with adipocytes [16]. Involution can be divided into two main phases: an apoptotic phase and a remodeling and adipocyte differentiation phase. The apoptotic phase is reversible and is triggered by an accumulation of milk in the lobules which produces an accumulation of secreted factors that initiates cell death of the secretory epithelial cells [17–19]. Several key regulators of the apoptotic phase of involution have been identified, including IGFBP-5, which is one of the focuses of this review [20, 21].
The second phase of involution is the irreversible ECM remodeling phase. As the epithelial tissue undergoes cell death, the remodeling phase reshapes the ductal tree while simultaneously differentiating tissues into adipocytes [16]. This phase is marked by the activation of proteases, however, apoptosis continues throughout involution [22]. Hormonal factors activate expression of two main classes of proteases: matrix metalloproteinases (MMPs) and serine proteases [22]. The main MMPs involved are MMP-2, MMP-3, and MMP-11, whose main roles are to digest the ECM and cleave substrates such as E-cadherin and collagen [16, 22]. The actions of the MMPs result in cell shedding and subsequent collapse of the alveoli and anoikis of adjacent cells [22–25]. Thus, MMPs are implicated in both ECM remodeling and apoptotic roles during involution [16, 22].
The “involution hypothesis” suggests that the microenvironment during pregnancy plays a significant role in the increased risk of breast cancer associated with pregnancy [4, 26]. Some of the pioneer studies regarding the role of involution compared the effects of ECM isolated from nulliparous or involuting mammary glands of parous rats. Co-incubating breast cancer cells with the involuting matrix was found to significantly increase cell motility, invasion, and micrometastases when injected into mammary fat pads compared to their controls co-incubated with nulliparous matrix [15, 27, 28]. Additionally, using a DCIS xenograft model of breast cancer, the same group showed that involuting parous rats developed larger tumors at a faster rate compared to those injected into the mammary glands of nulliparous rats [15].
Mechanistically, the combination of a pro-inflammatory wound healing–like environment, increased collagen deposition, and MMP activity observed during involution provides an environment that drives breast cancer progression [26]. First, wound healing and pro-inflammatory environments have consistently been reported to promote tumor growth and progression, therefore, as involution mimics such microenvironment, logically these characteristics of involution also drive its tumorigenic effect [19, 29]. Second, the ECM of involution is characterized by increased deposition of fibrillar collagen, MMP activity, immune cell infiltrate, influx of cytokines, and increased presence of bioactive fragments of proteolytic targets [30, 31]. The fibrillar collagen serves as an ECM scaffold that recruits tumor-associated macrophages, a sub-type of macrophages specifically implicated in mammary cell metastasis [32, 33]. Further, tenascin C, an ECM glycoprotein that is frequently overexpressed in breast cancers and which facilitates an epithelial to mesenchymal transition, accumulates during involution and colocalizes with fibrillar collagen present at the metastatic front, suggesting that it contributes to the pro-tumorigenic effect of involution [34–37].
The importance of the tumorigenic role of inflammation during involution is supported by the observation that treatment of breast cancer cells and involuting mammary glands in rats with anti-inflammatory NSAID drugs, such as ibuprofen, reduces fibrillar collagen deposition, tumor growth, and cell migration [15]. This study also suggests a role for COX2, an inflammatory target of NSAIDs, in involution-driven tumorigenesis and metastasis [15].
The increased activity of MMPs, specifically MMP-2, MMP-3, and MMP-9, during involution contributes to the development and progression of PPBC by breaking down the basement membrane barrier, promoting angiogenesis, and producing an influx of bioactive fragments of fibronectin and laminin-5 [38–40]. While collagen deposition is correlated with increased breast cancer development and progression, high collagen abundance can also act as a physical barrier against tumor cells invading the ECM. Therefore, collagen degradation by MMP protease activity is a necessary early step to tumor cell invasion and metastasis [41].
The matrix MMPs however represent only one of the four families of metalloproteases found in both prokaryotes and eukaryotes. Of specific interest to this review is the metalloprotease pappalysin-1 (PAPP-A) that defines a distinct subfamily of metalloproteases due to its unique structure [42]. As described in details below, recent studies have established that PAPP-A also alters the ECM during involution through an entirely different mechanism from the matrix MMPs.
Collagen Orientation Drives PPBC During Involution
In addition to its role in inflammation, the increased deposition of collagen during involution promotes breast cancer development through the stiffening of the ECM producing tension within the microenvironment. A certain amount of tensile force by ECM is required for all normal epithelial development by promoting tissue organization and regulating cell growth, death, survival, and migration [43]. However, as the tension rises due to an increase in collagen deposition, epithelial cell proliferation increases and an epithelial to mesenchymal transition (EMT) is activated, ultimately promoting cell migration and invasion [44]. Further, a spike in ECM stiffness disrupts cell adhesion and polarity, and the loss of both are key hallmarks of an EMT [43]. As the tumor mass expands further resulting in increasing pressure on the surrounding collagen, the collagen is forced to adopt alternative architectures [45].
The array of collagen architectures during tumor development was first described by the Keely group, who characterized the tumor-associated collagen signatures, or TACS. TACS 1 and 2 are characterized as curly (TACS-1) or linearized (TACS-2) dense collagen that remains parallel to the tumor border. This is in contrast to TACS-3 in which linearized dense collagen fibers align perpendicular or protruding from tumor border [45]. Variations in TACS orientation drastically alters breast cancer progression. Notably, TACS-1 is associated with benign or non-invasive breast tumors, while TACS-3 is associated with the more aggressive breast tumors [45–47]. Further, TACS-3 is specifically correlated with worst prognosis in breast cancer patients and is a stronger predictor for metastasis than tumor stage [47].
Specifically, TACS-3 collagen has been implicated in the pro-metastatic effect of involution. This was first described using xenografts in involuting, nulliparous, and parous mammary glands by the Schedin group. In their model, MCF10A-DCIS cells injected into the mammary fat pad of involuting mice resulted in significantly larger tumor growth, tumor number, and micrometastases. The authors also observed increased fibrillar collagen deposition in the mammary glands and tumors of involuting rats as well as a colocalization between COX-2 and fibrillar collagen. More importantly, they noted that the collagen was radially aligned, indicative of a TACS3 phenotype. When cultured ex vivo with collagen, the cells were more invasive but reduction in TACS-3 using a COX-2 inhibitor reduced invasive capacity as well as overall tumor growth in vivo. Taken together, these observations highlight the requirement for TACS3 fibrillar collagen and COX-2 in driving PPBC during involution [15].
Subsequently, the Schedin group performed a follow-up study in which they injected cells into the mammary fat pad of parous rats during the postpartum stage well after the completion of involution. They reported that the postpartum mammary glands also have a higher deposition of collagen than nulliparous mice, yet tumor growth was reduced. Therefore, these observations indicate that postpartum glands provide a tumor suppressive microenvironment. Ex vivo, cells cultured with the isolated ECM of parous and nulliparous rats indicated that the parous ECM was antiproliferative. This finding was initially surprising as the postpartum ECM remains rich in collagen I, however, it was later found that in contrast to the involution-associated collagen, the postpartum collagen adopts a non-fibrillar architecture with reduced stiffness [48]. These studies indicate that collagen abundance plays a dual role in breast cancer progression, and differences in orientation and organization of collagen alters its effect on tumor invasion.
PAPP-A as a Collagen-Dependent Oncogene
The protective effect of postpartum collagen is at odds with the observation that women remain at a higher risk of developing breast cancer for decades after birth [4, 48]. These contradictory observations suggest that there are potential factors that may convert the antiproliferative effect of postpartum collagen into an involution-like pro-tumorigenic collagen.
Our group has identified the protease pappalysin-1 (PAPP-A) as one such factor. The human PAPP-A gene is located on chromosome 9q33.1, where it spans 200 kb, 22 exons, and 21 introns. The PAPP-A precursor protein encodes 1626 amino acids containing a signal peptide of 22 amino acids and a pro-protein sequence of 58 amino acids. The mature and secreted form of the protein contains 1546 amino acids. PAPP-A is highly conserved among species with 91% homology between the human, mouse, and rat protein and contains 5 domains; [1] the N-terminus of 243 amino acids encodes laminin-G domain, [2] the proteolytic domain, which spans approximately 350 amino acids, [3] two lin-12 domain/Notch domains, which are required for its activity as deletion of these domains leads to inactivation of PAPP-A, [4] five consecutive complement control protein (CCP) modules that were found to be required form the cell surface binding of PAPP-A, and [5] a large central region of 500 amino acids of unknown function (Fig. 1) [42, 49–51].
Fig. 1.
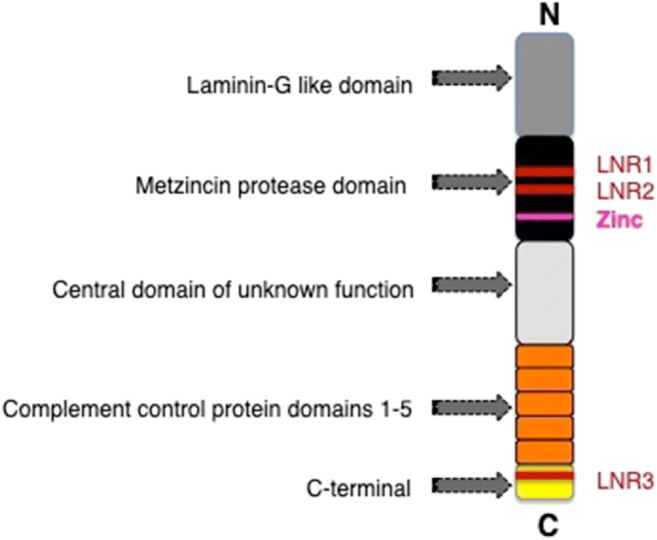
Domains of pappalysin-1 protein: Schematic of the 5 domains of PAPP-A; laminin-G like domain, metzincin protease domain, central domain of unknown function, complement control protein domains 1-5, and C-terminal. The three Lin-Notch repeats (LNR1-3) and putative zinc binding sites are indicated
PAPP-A is a secreted protease that targets insulin-like growth factor binding proteins (IGFBPs), IGFBP-4 and IGFBP-5, for degradation. IGFBP-4 and IGFBP-5 act as inhibitors of IGF signaling and proliferation and, therefore, their degradation by PAPP-A results in both activated IGF signaling and cellular proliferation [52]. IGFBP-5 is especially important as it is a key mediator of involution [53]. Further, a genome-wide association study (GWAS) has identified small polymorphisms that lower IGFBP-5 as a risk factor of developing breast cancer [54]. While PAPP-A is overexpressed in most breast cancers, its role as an oncogene had not been previously investigated [55]. In a study assessing invasive breast cancers, 45 of 46 patient breast tumor samples overexpressed PAPP-A [55]. This finding was further corroborated by a study that indicated PAPP-A to be overexpressed in 79% of premenopausal breast cancers [56]. Additionally, the overexpression of PAPP-A correlates with aggressive breast cancer and acts as an independent predictor for early recurrence [57, 58]. While the mechanisms underlying its overexpression remain poorly defined, PAPP-A was found to be a transcriptional target of mutant p53 [59]. However, since the rate of overexpression of PAPP-A is much more frequent than mutation of p53, other mechanisms must take place. As PAPP-A promoter is regulated by cytokines, the altered immune environment of cancer represents a likely source of overexpression of PAPP-A although this remains to be tested [60].
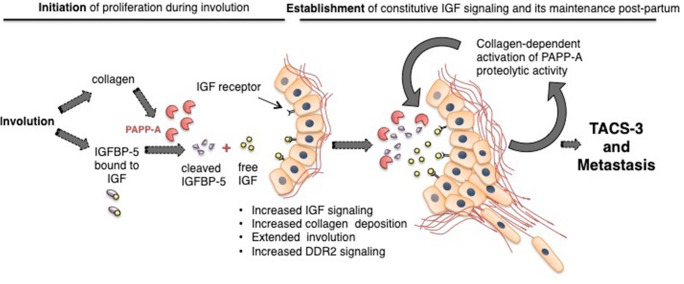
By degrading IGFBP-5, PAPP-A promotes cell proliferation, cell survival, and ultimately a delayed involution (Fig. 2) [53, 56]. We reported that the deposition of collagen during involution is necessary for the activation of PAPP-A and its overexpression in virgin mammary glands in vivo does not promote tumor formation. More specifically in this study, since PAPP-A is active during involution but is not active in a mammary gland from virgin mice despite PAPP-A being expressed at the same level in both cases, this observation suggests that a factor present in involution but absent in virgin mammary glands must be required for its activation. As collagen is maybe such a factor, we incubated recombinant PAPP-A and IGFBP-5 with and without collagen and found that addition of collagen increases significantly the cleavage of IGFBP-5 by PAPP-A in vitro. We further reported that the PPBC-driven by PAPP-A are characterized by constitutive IGF signaling and TACS-3 collagen architecture; therefore, we proposed that PAPP-A is an involution-dependent oncogene [56].
Fig. 2.
The relationship between collagen and PAPP-A in PPBC establishment and progression: The increased collagen deposition that occurs during pregnancy and involution drives PPBC by establishing constitutive IGF signaling. While increased IGFBP-5 during involution normally restricts overt IGF signaling, the collagen deposition during involution promotes PAPP-A’s proteolytic activity which degrades IGFBP-5, resulting in high IGF signaling. This initiation phase establishes a feedforward loop as IGF signaling promotes collagen deposition. Additionally, through various mechanisms, PAPP-A enhances DDR2 activation and TACS3 maintenance to increase the aggressive nature of PPBC. PAPP-A may also sustain DDR2 activation and TACS3 architecture during late postpartum which may therefore extend a woman’s risk of developing PPBC past the window of involution
PAPP-A During Postpartum
Since overexpression of PAPP-A occurs through sporadic mutations, such as mutation in p53 [59], we reasoned that PAPP-A overexpression may arise at any time point after pregnancy. Having established that PAPP-A is activated by collagen, we initiated a study to test whether PAPP-A is able to convert postpartum collagen into a pro-tumorigenic involution–like collagen [61]. If so, this would provide an explanation as to why women remain at higher risk of PPBC for decades after birth [4].
We recently reported that PAPP-A is also activated by collagen during postpartum and in turn is able to convert postpartum collagen into an involution-like pro-tumorigenic collagen and promote cell invasion. This finding suggests the provocative notion that the passage through a single pregnancy is sufficient to predispose a breast to the oncogenic action of PAPP-A, thus potentially extending the risk window for PPBC well beyond involution alone in women with PAPP-A overexpressing breast cancers [61]. Further, it expands the current understanding of PAPP-A as an oncogene and indicates that PAPP-A can act as an oncogene beyond the context of involution.
Collagen Signaling in PPBC
In addition to the structural role that collagen plays during postpartum tumorigenesis, collagen promotes proliferation through binding to collagen receptors [62]. One of the main families of collagen receptors are the discoidin domain receptor (DDR) family of receptor tyrosine kinases that specifically bind to and are activated by collagen. The DDR family is comprised of DDR1 and DDR2 and both have been implicated in breast cancer development [63]. The emerging role for collagen-induced cell signaling through DDRs in mammary oncogenesis has been attributed to the promotion of cell survival, proliferation, activation of EMT, and changes in cell migration and invasion [64]. Notably, DDR2 has recently emerged as a key player in breast cancer metastasis and DDR2 is overexpressed in breast cancer patients associated with poor outcomes [65, 66]. Of note, activation of EMT coincides with a “DDR” switch from DDR1 to DDR2, and new evidence also suggests that DDR2 phosphorylation may actually facilitate an EMT, rather than just act as a marker of EMT [67, 68]. DDR2 maintains the EMT transcription factor Snail stability, promoting its nuclear localization, and expression in breast cancer cells [68]. Further, inhibition of DDR2 by siRNA is sufficient to prevent a TGF-β-driven EMT and cell migration in cancer cells [68, 69].
We recently reported that DDR2 contributes to cell invasion and progression of PAPP-A-driven PPBC by taking advantage of the increased collagen deposition observed during involution and in postpartum mammary glands (Fig. 2) [61]. The recent report of a between IGF and DDR1 leading to increased their signaling raises the possibility that the increased IGF signaling that results from the PAPP-A-mediated degradation of IGFBP-4 and IGFBP-5 may also promote DDR2 signaling in PPBC, however, this possibility remains to be tested [56, 70, 71].
We also reported that the activation of DDR2 leads to an increase in Snail and cell invasive capacity [61, 68]. This finding may provide a mechanism to explain why PPBC is typically a more aggressive form of breast cancer, characterized by higher rates of recurrence, metastasis, and poorer patient survival [4, 10, 72, 73]. This finding is also consistent with the observation that PPBC include triple negative breast cancers, which have the highest rate of mutation in p53 and we have previously shown mutant p53 activates the transcription of PAPP-A [4, 59, 74, 75]. Our recent study also highlights a mechanism by which PAPP-A promotes TACS-3, since inhibition of DDR2 by CRISPR in our model abolished TACS-3 [61]. However, how TACS-3 is formed remains unknown and therefore represents an important line of investigation in the future.
Finally, it is possible that PPBC that arises within a collagen-rich or postpartum microenvironment adopts a distinct genetic landscape that contributes to the aggressive characteristics of PPBC. More importantly, a previous study analyzed the genetic changes associated with parous breast tissue. Of significance, genes related to invasion and migration were upregulated in the parous tissue [76, 77]. To validate our findings, we generated a PAPP-A-driven PPBC signature using PAPP-A/COL1A1/SNAI1 to screen a human breast cancer patient dataset. Our results indicate that this signature identifies patients with higher rate of metastasis and shorter overall survival [61]. We found that the PAPP-A/COL1A1/SNAI1 signature is also associated with a similar genetic landscape to that seen in the parous breast tissue, suggesting that this gene signature is an accurate reflection of the genetic landscape of PAPP-A-driven PPBC [61, 76, 77].
Additionally, a previous study analyzing adjacent normal breast tissue of the microenvironment surrounding invasive breast cancers or DCIS and identified two distinct genetic landscapes: an “active” and “inactive” gene signature [78, 79]. Of significance, EMT factors such as Snail and Twist, LOX, DDR2, TGF-β, ECM proteases, and collagen genes were shared and upregulated in the both the “active” stroma and the PAPP-A/COL1A1/SNAI1-high patients [61, 78, 79]. Therefore, these findings validate the significance of collagen dynamics and DDR2/Snail signaling by PAPP-A in the progression and metastasis of PPBC. Taken together, these studies highlight the similarity between a stroma that drives aggressive breast cancer, the stroma of parous patients, and characteristics of PAPP-A-driven PPBC, thus leading us closer to understanding the molecular underpinnings of PPBC and toward reliable diagnostic and prognostic factors [61, 77, 78].
Conclusions
The recent findings regarding the role of collagen deposition and altered orientation in PPBC have significant implications for our current understanding and definition of PPBC. This provides insight into the epidemiological phenomenon that women are at an increased risk for developing breast cancer up to 3 decades post-pregnancy [4]. Because PAPP-A overexpression is sufficient to convert the collagen-rich environment of the postpartum breast to a pro-proliferative state, it begs the question of what other proteins are able to take advantage of this environment. For instance, LOX has been shown to promote an invasive collagen signature akin to a TACS3 phenotype in the promotion of breast cancer metastasis and has additionally been shown to be overexpressed in PAPP-A/COL1A1/SNAI1-high patients [80]. Additionally, these findings raise the intriguing possibility that PAPP-A may become oncogenic independently of pregnancy in other collagen-rich environments, such as in women with high mammographic density [81].
The existence of PPBC as a distinct breast cancer sub-type remains controversial, in part due to the lack of reliable biomarkers in the diagnosis of PPBC. Clearly distinguishing a PPBC from a sporadic cancer that is unrelated to pregnancy in women diagnosed several years after their last pregnancy is a challenge [4]. The findings described in this review support the mounting evidence that PPBC is an aggressive sub-type characterized by a distinct genetic signature and that PPBC may affect women well beyond their last pregnancy [61, 77, 78]. Clearly, these results strongly argue that a detailed history of pregnancy should be included in medical charts of breast cancer patients and considered as a risk factor. Lastly, further understanding the complexity of the ECM as a critical component to PPBC tumorigenesis represents an important line of investigation in the future [82].
Acknowledgments
We thank the members of the Germain lab for their support.
Funding Information
This work was supported by grant to D.G. from the Chemotherapy Foundation and the Breast Cancer Research Foundation.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Macias Hector, Hinck Lindsay. Mammary gland development. Wiley Interdisciplinary Reviews: Developmental Biology. 2012;1(4):533–557. doi: 10.1002/wdev.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDaniel SM, Rumer KK, Biroc SL, et al. Remodeling of the mammary microenvironment after lactation promotes breast tumor cell metastasis. Am J Pathol. 2006;168(2):608–620. doi: 10.2353/ajpath.2006.050677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keyser EA, Staat BC, Fausett MB, Shields AD. Pregnancy-associated breast cancer. Rev Obstet Gynecol. 2012;5(2):94–99. doi: 10.3909/riog0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. 2006;6(4):281–291. doi: 10.1038/nrc1839. [DOI] [PubMed] [Google Scholar]
- 5.Partridge A, Schapira L (2005) Pregnancy and breast cancer: epidemiology, treatment, and safety issues. Oncol (willist Park) [PubMed]
- 6.Ruiz Rossana, Herrero Carmen, Strasser-Weippl Kathrin, Touya Diego, St. Louis Jessica, Bukowski Alexandra, Goss Paul E. Epidemiology and pathophysiology of pregnancy-associated breast cancer: A review. The Breast. 2017;35:136–141. doi: 10.1016/j.breast.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Russo J, Moral R, Balogh GA, Mailo D, Russo IH (2005) The protective role of pregnancy in breast cancer. Breast Cancer Res. 10.1186/bcr1029 [DOI] [PMC free article] [PubMed]
- 8.Husby A, Wohlfahrt J, Øyen N, Melbye M. Pregnancy duration and breast cancer risk. Nat Commun. 2018;9(1). 10.1038/s41467-018-06748-3 [DOI] [PMC free article] [PubMed]
- 9.Bellieni C (2016) The best age for pregnancy and undue pressures. J Family Reprod Health [PMC free article] [PubMed]
- 10.Callihan EB, Gao D, Jindal S, et al. Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast Cancer Res Treat. 2013;138(2):549–559. doi: 10.1007/s10549-013-2437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali Sheikh Asim, Gupta Sameer, Sehgal Rajesh, Vogel Victor. Survival Outcomes in Pregnancy Associated Breast Cancer: A Retrospective Case Control Study. The Breast Journal. 2012;18(2):139–144. doi: 10.1111/j.1524-4741.2011.01201.x. [DOI] [PubMed] [Google Scholar]
- 12.Johansson A. L. V., Andersson T. M.-L., Hsieh C.-C., Cnattingius S., Lambe M. Increased Mortality in Women with Breast Cancer Detected during Pregnancy and Different Periods Postpartum. Cancer Epidemiology Biomarkers & Prevention. 2011;20(9):1865–1872. doi: 10.1158/1055-9965.EPI-11-0515. [DOI] [PubMed] [Google Scholar]
- 13.Borges Virginia F., Schedin Pepper J. Pregnancy-associated breast cancer. Cancer. 2011;118(13):3226–3228. doi: 10.1002/cncr.26643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichols Hazel B., Schoemaker Minouk J., Cai Jianwen, Xu Jiawei, Wright Lauren B., Brook Mark N., Jones Michael E., Adami Hans-Olov, Baglietto Laura, Bertrand Kimberly A., Blot William J., Boutron-Ruault Marie-Christine, Dorronsoro Miren, Dossus Laure, Eliassen A. Heather, Giles Graham G., Gram Inger T., Hankinson Susan E., Hoffman-Bolton Judy, Kaaks Rudolf, Key Timothy J., Kitahara Cari M., Larsson Susanna C., Linet Martha, Merritt Melissa A., Milne Roger L., Pala Valeria, Palmer Julie R., Peeters Petra H., Riboli Elio, Sund Malin, Tamimi Rulla M., Tjønneland Anne, Trichopoulou Antonia, Ursin Giske, Vatten Lars, Visvanathan Kala, Weiderpass Elisabete, Wolk Alicja, Zheng Wei, Weinberg Clarice R., Swerdlow Anthony J., Sandler Dale P. Breast Cancer Risk After Recent Childbirth. Annals of Internal Medicine. 2018;170(1):22. doi: 10.7326/M18-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyons Traci R, O'Brien Jenean, Borges Virginia F, Conklin Matthew W, Keely Patricia J, Eliceiri Kevin W, Marusyk Andriy, Tan Aik-Choon, Schedin Pepper. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nature Medicine. 2011;17(9):1109–1115. doi: 10.1038/nm.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson CJ (2006) Key stages in mammary gland development involution: apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ. Breast Cancer Res. 10.1186/bcr1401 [DOI] [PMC free article] [PubMed]
- 17.Li M., Liu X., Robinson G., Bar-Peled U., Wagner K.-U., Young W. S., Hennighausen L., Furth P. A. Mammary-derived signals activate programmed cell death during the first stage of mammary gland involution. Proceedings of the National Academy of Sciences. 1997;94(7):3425–3430. doi: 10.1073/pnas.94.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quaglino Ana, Salierno Marcelo, Pellegrotti Jesica, Rubinstein Natalia, Kordon Edith C. Mechanical strain induces involution-associated events in mammary epithelial cells. BMC Cell Biology. 2009;10(1):55. doi: 10.1186/1471-2121-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lund LR, Rømer J, Thomasset N et al (1996) Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development [DOI] [PMC free article] [PubMed]
- 20.Ning Yun, Hoang Bao, Schuller Alwin G. P., Cominski Tara P., Hsu Ming-Sing, Wood Teresa L., Pintar John E. Delayed Mammary Gland Involution in Mice with Mutation of the Insulin-Like Growth Factor Binding Protein 5 Gene. Endocrinology. 2007;148(5):2138–2147. doi: 10.1210/en.2006-0041. [DOI] [PubMed] [Google Scholar]
- 21.Humphreys Robin C., Bierie Brian, Zhao Ling, Raz Regina, Levy David, Hennighausen Lothar. Deletion of Stat3 Blocks Mammary Gland Involution and Extends Functional Competence of the Secretory Epithelium in the Absence of Lactogenic Stimuli. Endocrinology. 2002;143(9):3641–3650. doi: 10.1210/en.2002-220224. [DOI] [PubMed] [Google Scholar]
- 22.Green Kirsty A., Lund Leif R. ECM degrading proteases and tissue remodelling in the mammary gland. BioEssays. 2005;27(9):894–903. doi: 10.1002/bies.20281. [DOI] [PubMed] [Google Scholar]
- 23.Pullan S, Wilson J, Metcalfe A et al (1996) Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J Cell Sci [DOI] [PubMed]
- 24.Vallorosi Christopher J., Day Kathleen C., Zhao Xin, Rashid Michael G., Rubin Mark A., Johnson Keith R., Wheelock Margaret J., Day Mark L. Truncation of the β-Catenin Binding Domain of E-cadherin Precedes Epithelial Apoptosis during Prostate and Mammary Involution. Journal of Biological Chemistry. 2000;275(5):3328–3334. doi: 10.1074/jbc.275.5.3328. [DOI] [PubMed] [Google Scholar]
- 25.Watson Christine J., Kreuzaler Peter A. Remodeling mechanisms of the mammary gland during involution. The International Journal of Developmental Biology. 2011;55(7-8-9):757–762. doi: 10.1387/ijdb.113414cw. [DOI] [PubMed] [Google Scholar]
- 26.Lyons Traci R., Schedin Pepper J., Borges Virginia F. Pregnancy and Breast Cancer: when They Collide. Journal of Mammary Gland Biology and Neoplasia. 2009;14(2):87–98. doi: 10.1007/s10911-009-9119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schedin P, Strange R, Mitrenga T, Wolfe P, Kaeck M (2000) Fibronectin fragments induce MMP activity in mouse mammary epithelial cells: evidence for a role in mammary tissue remodeling. J Cell Sci [DOI] [PubMed]
- 28.Bemis LT, Schedin P (2000) Reproductive state of rat mammary gland stroma modulates human breast cancer cell migration and invasion. Cancer Res [PubMed]
- 29.Strange R, Li F, Saurer S, Burkhardt A, Friis RR (1992) Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development [DOI] [PubMed]
- 30.Clarkson Richard WE, Wayland Matthew T, Lee Jennifer, Freeman Tom, Watson Christine J. Breast Cancer Research. 2004;6(2):R92. doi: 10.1186/bcr754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gusterson BA, Stein T, Morris JS, et al (2004) Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res BCR [DOI] [PMC free article] [PubMed]
- 32.Condeelis John, Pollard Jeffrey W. Macrophages: Obligate Partners for Tumor Cell Migration, Invasion, and Metastasis. Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Wyckoff J. B., Wang Y., Lin E. Y., Li J.-f., Goswami S., Stanley E. R., Segall J. E., Pollard J. W., Condeelis J. Direct Visualization of Macrophage-Assisted Tumor Cell Intravasation in Mammary Tumors. Cancer Research. 2007;67(6):2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 34.Lloyd Jones P, Boudreau N, Myers CA, Erickson HP, Bissell MJ (1995) Tenascin-C inhibits extracellular matrix-dependent gene expression in mammary epithelial cells. Localization of active regions using recombinant tenascin fragments. J Cell Sci [DOI] [PubMed]
- 35.Nagaharu Keiki, Zhang Xinhui, Yoshida Toshimichi, Katoh Daisuke, Hanamura Noriko, Kozuka Yuji, Ogawa Tomoko, Shiraishi Taizo, Imanaka-Yoshida Kyoko. Tenascin C Induces Epithelial-Mesenchymal Transition–Like Change Accompanied by SRC Activation and Focal Adhesion Kinase Phosphorylation in Human Breast Cancer Cells. The American Journal of Pathology. 2011;178(2):754–763. doi: 10.1016/j.ajpath.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hancox RA, Allen MD, Holliday DL et al (2009) Tumour-associated tenascin-C isoforms promote breast cancer cell invasion and growth by matrix metalloproteinase-dependent and independent mechanisms. Breast Cancer Res. 10.1186/bcr2251 [DOI] [PMC free article] [PubMed]
- 37.Oskarsson Thordur, Acharyya Swarnali, Zhang Xiang H-F, Vanharanta Sakari, Tavazoie Sohail F, Morris Patrick G, Downey Robert J, Manova-Todorova Katia, Brogi Edi, Massagué Joan. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nature Medicine. 2011;17(7):867–874. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giannelli Gianluigi, Pozzi Ambra, Stetler-Stevenson William G., Gardner Humphrey A., Quaranta Vito. Expression of Matrix Metalloprotease-2-Cleaved Laminin-5 in Breast Remodeling Stimulated by Sex Steroids. The American Journal of Pathology. 1999;154(4):1193–1201. doi: 10.1016/S0002-9440(10)65371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zutter Mary M., Sun Hong, Santoro Samuel A. Journal of Mammary Gland Biology and Neoplasia. 1998;3(2):191–200. doi: 10.1023/a:1018798907544. [DOI] [PubMed] [Google Scholar]
- 40.Saharinen Pipsa, Tammela Tuomas, Karkkainen Marika J, Alitalo Kari. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends in Immunology. 2004;25(7):387–395. doi: 10.1016/j.it.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Page-McCaw Andrea, Ewald Andrew J., Werb Zena. Matrix metalloproteinases and the regulation of tissue remodelling. Nature Reviews Molecular Cell Biology. 2007;8(3):221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boldt HB, Overgaard MT, Laursen LS, Weyer K, Sottrup-Jensen L, Oxvig C. Mutational analysis of the proteolytic domain of pregnancy-associated plasma protein-A (PAPP-A): classification as a metzincin. Biochem J. 2001;367:359–367. doi: 10.1042/0264-6021:3580359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paszek Matthew J., Zahir Nastaran, Johnson Kandice R., Lakins Johnathon N., Rozenberg Gabriela I., Gefen Amit, Reinhart-King Cynthia A., Margulies Susan S., Dembo Micah, Boettiger David, Hammer Daniel A., Weaver Valerie M. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK (2000) Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res [PubMed]
- 45.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4(1):38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pavithra V, Sowmya S, Rao RS, et al. Tumor-associated collagen signatures: an insight. World J Dent. 2017;8(3):224–230. doi: 10.5005/jp-journals-10015-1442. [DOI] [Google Scholar]
- 47.Conklin MW, Eickhoff JC, Riching KM, et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 2011;178(3):1221–1232. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maller O, Hansen KC, Lyons TR, et al. Collagen architecture in pregnancy-induced protection from breast cancer. J Cell Sci. 2013;126(Pt 18):4108–4110. doi: 10.1242/jcs.121590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boldt Henning B., Kjaer-Sorensen Kasper, Overgaard Michael T., Weyer Kathrin, Poulsen Christine B., Sottrup-Jensen Lars, Conover Cheryl A., Giudice Linda C., Oxvig Claus. The Lin12-Notch Repeats of Pregnancy-associated Plasma Protein-A Bind Calcium and Determine Its Proteolytic Specificity. Journal of Biological Chemistry. 2004;279(37):38525–38531. doi: 10.1074/jbc.M405222200. [DOI] [PubMed] [Google Scholar]
- 50.Weyer Kathrin, Boldt Henning B., Poulsen Christine B., Kjaer-Sorensen Kasper, Gyrup Claus, Oxvig Claus. A Substrate Specificity-determining Unit of Three Lin12-Notch Repeat Modules Is Formed inTranswithin the Pappalysin-1 Dimer and Requires a Sequence Stretch C-terminal to the Third Module. Journal of Biological Chemistry. 2007;282(15):10988–10999. doi: 10.1074/jbc.M607903200. [DOI] [PubMed] [Google Scholar]
- 51.Conover Cheryl A. Key questions and answers about pregnancy-associated plasma protein-A. Trends in Endocrinology & Metabolism. 2012;23(5):242–249. doi: 10.1016/j.tem.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laursen LS, Overgaard MT, Soe R, et al. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett. 2001;504(1-2):36–40. doi: 10.1016/S0014-5793(01)02760-0. [DOI] [PubMed] [Google Scholar]
- 53.Ning Y, Hoang B, Schuller AGP, et al (2013) Delayed mammary gland involution in mice with mutation of the insulin-like growth factor binding protein 5 gene. Endocrinology 148(5):2138–2147. http://press.endocrine.org/doi/10.1210/en.2006-0041?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3Dpubmed. [DOI] [PubMed]
- 54.Ghoussaini M, Edwards SL, Michailidou K et al (2014) Evidence that breast cancer risk at the 2q35 locus is mediated through IGFBP5 regulation. Nat Commun. 10.1038/ncomms5999 [DOI] [PMC free article] [PubMed]
- 55.Mansfield AS, Visscher DW, Hart SN, et al. Pregnancy-associated plasma protein-A expression in human breast cancer. Growth Hormon IGF Res. 2014;24(6):264–267. doi: 10.1016/j.ghir.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takabatake Y, Oxvig C, Nagi C, et al. Lactation opposes pappalysin-1-driven pregnancy-associated breast cancer. EMBO Mol Med. 2016;8(4):388–406. doi: 10.15252/emmm.201606273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuhajda Francis P., Abeloff Martin D., Eggleston Joseph C. Pregnancy-associated plasma protein A: A clinically significant predictor of early recurrence in stage II breast carcinoma. Human Pathology. 1985;16(3):228–235. doi: 10.1016/s0046-8177(85)80007-1. [DOI] [PubMed] [Google Scholar]
- 58.Kuhajda FP, Eggleston JC (1985) Pregnancy-associated plasma protein A. A clinically significant predictor of early recurrence in stage I breast carcinoma is independent of estrogen receptor status. Am J Pathol [PMC free article] [PubMed]
- 59.Chander Harish, Halpern Max, Resnick-Silverman Lois, Manfredi James J., Germain Doris. Skp2B Overexpression Alters a Prohibitin-p53 Axis and the Transcription of PAPP-A, the Protease of Insulin-Like Growth Factor Binding Protein 4. PLoS ONE. 2011;6(8):e22456. doi: 10.1371/journal.pone.0022456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Resch Zachary T., Chen Bing-Kun, Bale Laurie K., Oxvig Claus, Overgaard Michael T., Conover Cheryl A. Pregnancy-Associated Plasma Protein A Gene Expression as a Target of Inflammatory Cytokines. Endocrinology. 2004;145(3):1124–1129. doi: 10.1210/en.2003-1313. [DOI] [PubMed] [Google Scholar]
- 61.Slocum E, Craig A, Villanueva A, Germain D (2019) Parity predisposes breasts to the oncogenic action of PAPP-A and activation of the collagen receptor DDR2. Breast Cancer Res:1–20 [DOI] [PMC free article] [PubMed]
- 62.Leitinger Birgit. Transmembrane Collagen Receptors. Annual Review of Cell and Developmental Biology. 2011;27(1):265–290. doi: 10.1146/annurev-cellbio-092910-154013. [DOI] [PubMed] [Google Scholar]
- 63.Valiathan RR, Marco M, Leitinger B, Kleer CG, Fridman R. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev. 2012;31(1-2):295–321. doi: 10.1007/s10555-012-9346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rammal H, Saby C, Magnien K et al (2016) Discoidin domain receptors: potential actors and targets in cancer. Front Pharmacol. 10.3389/fphar.2016.00055 [DOI] [PMC free article] [PubMed]
- 65.Ren T, Zhang J, Zhang J, Liu X, Yao L. Increased expression of discoidin domain receptor 2 (DDR2): a novel independent prognostic marker of worse outcome in breast cancer patients. Med Oncol. 2013;30(1). 10.1007/s12032-012-0397-3 [DOI] [PubMed]
- 66.Quan Jinhua, Yahata Tetsuro, Adachi Sosuke, Yoshihara Kosuke, Tanaka Kenichi. Identification of Receptor Tyrosine Kinase, Discoidin Domain Receptor 1 (DDR1), as a Potential Biomarker for Serous Ovarian Cancer. International Journal of Molecular Sciences. 2011;12(2):971–982. doi: 10.3390/ijms12020971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maeyama Michiko, Koga Hironori, Selvendiran Karuppaiyah, Yanagimoto Chikatoshi, Hanada Shinichiro, Taniguchi Eitaro, Kawaguchi Takumi, Harada Masaru, Ueno Takato, Sata Michio. Switching in discoid domain receptor expressions in SLUG-induced epithelial-mesenchymal transition. Cancer. 2008;113(10):2823–2831. doi: 10.1002/cncr.23900. [DOI] [PubMed] [Google Scholar]
- 68.Zhang K, Corsa CA, Ponik SM, et al. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat Cell Biol. 2013;15(6):677–687. doi: 10.1038/ncb2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walsh Logan A., Nawshad Ali, Medici Damian. Discoidin domain receptor 2 is a critical regulator of epithelial–mesenchymal transition. Matrix Biology. 2011;30(4):243–247. doi: 10.1016/j.matbio.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mata R, Palladino C, Nicolosi ML, et al. IGF-I induces upregulation of DDR1 collagen receptor in breast cancer cells by suppressing MIR-199a-5p through the PI3K/AKT pathway. Oncotarget. 2016;7(7):7683–7700. doi: 10.18632/oncotarget.6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Malaguarnera R, Nicolosi ML, Sacco A, et al. Novel cross talk between IGF-IR and DDR1 regulates IGF-IR trafficking, signaling and biological responses. Oncotarget. 2015;6(18):16084–16105. doi: 10.18632/oncotarget.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goddard Erica T., Bassale Solange, Schedin Troy, Jindal Sonali, Johnston Jeremy, Cabral Ethan, Latour Emile, Lyons Traci R., Mori Motomi, Schedin Pepper J., Borges Virginia F. Association Between Postpartum Breast Cancer Diagnosis and Metastasis and the Clinical Features Underlying Risk. JAMA Network Open. 2019;2(1):e186997. doi: 10.1001/jamanetworkopen.2018.6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo Fangjian, Kuo Yong-fang, Shih Ya Chen Tina, Giordano Sharon H., Berenson Abbey B. Trends in breast cancer mortality by stage at diagnosis among young women in the United States. Cancer. 2018;124(17):3500–3509. doi: 10.1002/cncr.31638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Turner Natalie, Moretti Erica, Siclari Olimpia, Migliaccio Ilenia, Santarpia Libero, D’Incalci Maurizio, Piccolo Stefano, Veronesi Andrea, Zambelli Alberto, Del Sal Gianni, Di Leo Angelo. Targeting triple negative breast cancer: Is p53 the answer? Cancer Treatment Reviews. 2013;39(5):541–550. doi: 10.1016/j.ctrv.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 75.Asztalos S, Pham TN, Gann PH et al (2015) High incidence of triple negative breast cancers following pregnancy and an associated gene expression signature. Springerplus. 10.1186/s40064-015-1512-7 [DOI] [PMC free article] [PubMed]
- 76.Russo J., Balogh G. A., Russo I. H. Full-term Pregnancy Induces a Specific Genomic Signature in the Human Breast. Cancer Epidemiology Biomarkers & Prevention. 2008;17(1):51–66. doi: 10.1158/1055-9965.EPI-07-0678. [DOI] [PubMed] [Google Scholar]
- 77.Harvell Djuana M. E., Kim Jihye, O’Brien Jenean, Tan Aik-Choon, Borges Virginia F., Schedin Pepper, Jacobsen Britta M., Horwitz Kathryn B. Genomic Signatures of Pregnancy-Associated Breast Cancer Epithelia and Stroma and their Regulation by Estrogens and Progesterone. Hormones and Cancer. 2013;4(3):140–153. doi: 10.1007/s12672-013-0136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Román-Pérez E, Casbas-Hernández P, Pirone JR et al (2012) Gene expression in extratumoral microenvironment predicts clinical outcome in breast cancer patients. Breast Cancer Res. 10.1186/bcr3152 [DOI] [PMC free article] [PubMed]
- 79.Sun X., Gierach G. L., Sandhu R., Williams T., Midkiff B. R., Lissowska J., Wesolowska E., Boyd N. F., Johnson N. B., Figueroa J. D., Sherman M. E., Troester M. A. Relationship of Mammographic Density and Gene Expression: Analysis of Normal Breast Tissue Surrounding Breast Cancer. Clinical Cancer Research. 2013;19(18):4972–4982. doi: 10.1158/1078-0432.CCR-13-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cox T. R., Bird D., Baker A.-M., Barker H. E., Ho M. W.-Y., Lang G., Erler J. T. LOX-Mediated Collagen Crosslinking Is Responsible for Fibrosis-Enhanced Metastasis. Cancer Research. 2013;73(6):1721–1732. doi: 10.1158/0008-5472.CAN-12-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huo CW, Chew G, Hill P et al (2015) High mammographic density is associated with an increase in stromal collagen and immune cells within the mammary epithelium. Breast Cancer Res. 10.1186/s13058-015-0592-1 [DOI] [PMC free article] [PubMed]
- 82.Bonnans Caroline, Chou Jonathan, Werb Zena. Remodelling the extracellular matrix in development and disease. Nature Reviews Molecular Cell Biology. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]