BACKGROUND:
It is unknown whether hypertension plays any role in cerebral myelination. To fill this knowledge gap, we studied 90 cognitively unimpaired adults, age range 40 to 94 years, who are participants in the Baltimore Longitudinal Study of Aging and the Genetic and Epigenetic Signatures of Translational Aging Laboratory Testing to look for potential associations between hypertension and cerebral myelin content across 14 white matter brain regions.
METHODS:
Myelin content was probed using our advanced multicomponent magnetic resonance relaxometry method of myelin water fraction, a direct and specific magnetic resonance imaging measure of myelin content, and longitudinal and transverse relaxation rates (R1 and R2), 2 highly sensitive magnetic resonance imaging metrics of myelin content. We also applied diffusion tensor imaging magnetic resonance imaging to measure fractional anisotropy, mean diffusivity, radial diffusivity, and axial diffusivity values, which are metrics of cerebral microstructural tissue integrity, to provide context with previous magnetic resonance imaging findings.
RESULTS:
After adjustment of age, sex, systolic blood pressure, smoking status, diabetes status, and cholesterol level, our results indicated that participants with hypertension exhibited lower myelin water fraction, fractional anisotropy, R1 and R2 values and higher mean diffusivity, radial diffusivity, and axial diffusivity values, indicating lower myelin content and higher impairment to the brain microstructure. These associations were significant across several white matter regions, particularly in the corpus callosum, fronto-occipital fasciculus, temporal lobes, internal capsules, and corona radiata.
CONCLUSIONS:
These original findings suggest a direct association between myelin content and hypertension and form the basis for further investigations including longitudinal assessments of this relationship.
Keywords: diffusion tensor imaging, hypertension, magnetic resonance imaging, myelin water fraction, neurodegeneration
NOVELTY AND RELEVANCE.
What Is New?
This is the first study, to our knowledge, that explicitly investigated the association between hypertension and cerebral myelin content probed using a direct magnetic resonance imaging measure.
What Is Relevant?
In a healthy adult population, elevated blood pressure is associated with reduced cerebral white matter tissue myelination. Further, patients under hypertension control medication still exhibit significantly lower myelin content as compared with control.
Clinical/Pathophysiological Implications?
This work lay the ground to future efforts to support myelin health in hypertension and examine the effect of hypertensive medications on mitigating myelin damage.
Hypertension is the primary risk factor for stroke, ischemic white matter lesions, infarcts, and atherosclerosis, as well as cardiovascular and microvascular diseases.1–3 Further, hypertension is the most widely accepted risk factor associated with a myriad of neurodegenerative diseases, especially Alzheimer’s disease and related dementias.4 Emerging evidence suggests that hypertension leads to vessel wall remodeling, potentially leading to hypoperfusion and associated hypoxia, as well as reduced glucose transport into the brain with concomitant accelerated cerebral tissue degeneration.5,6 Indeed, this paradigm is further supported by recent longitudinal studies revealing a direct association between hypertension in midlife and reduced cerebral blood flow in later life.7,8 Therefore, examining the extent of any possible association between hypertension and cerebral tissue integrity is paramount for our global understanding of neurodegenerative disease risk factors and progression.
In recent years, magnetic resonance imaging (MRI) studies, based extensively on diffusion tensor imaging (DTI), have revealed an association between hypertension and abnormal cerebral microstructural white matter integrity.8 DTI is an MRI technique sensitive to the underlying microarchitecture of the brain parenchyma and the degree and direction of water molecule mobility. These studies have documented that hypertension, indicated by a blood pressure >140/90 mm Hg or the use of antihypertensive medication, is associated with lower values of fractional anisotropy (FA) and higher values of mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AxD).9–11 Reduced FA concomitant with an increase in RD is associated with demyelination,12 whereas reduced FA in conjunction with increased AxD is believed to be associated with axonal injury or death.13 These changes in cerebral microstructural integrity associated with hypertension have been interpreted as deterioration in axonal myelination. However, although DTI metrics such as FA and MD are sensitive to cerebral microstructural changes, they are not specific. Indeed, there are multiple methodological and biological factors that can affect the DTI-derived eigenvalues from which the DTI indices are calculated14,15; these include, but not limited to, axonal degeneration, flow, temperature, hydration, macromolecular content and architectural features, including fiber fanning or crossing. Therefore, to our knowledge, the association between hypertension and myelination has not yet been established. To address this limitation, multicomponent relaxometry methods provide a greater specificity to quantify myelin content in white matter and probe related changes that occur during brain development and neurodegenerative diseases.16,17 Multicomponent relaxometry separates the measured MR signal in white matter into 2 pools of water, namely the intra- and extracellular water pool and the water trapped between the myelin sheaths calculated as the myelin water fraction (MWF).18,19 MWF is an in vivo specific index of myelin content and is a potential marker for myelin alterations. To the best of our knowledge, no MR studies have employed multicomponent relaxometry analysis, specifically MWF imaging, to investigate the relationship between myelin content and hypertension in aging adults.
In this study, we examined the association between hypertension and myelin content as probed using MWF on a cohort of well-characterized cognitively unimpaired adults (N=90), across the age range of 40 to 94 years. Each participant underwent our Bayesian Monte Carlo-mcDESPOT protocol for MWF as a direct measure of myelin content, as well as mapping of longitudinal and transverse relaxation rates (R1 and R2) as sensitive but nonspecific measures of myelin content.6,20–22 Indeed, R1 and R2 values depend on both water mobility and macromolecular tissue composition, including local lipid and iron content, the main constituents of myelin, and thus are expected to be directly associated with differences in myelin content. To establish a connection with previous MRI findings, participants have undergone our additional DTI protocol for FA, MD, RD, and AxD mapping as well.23
METHODS
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Cohort
Participants are volunteers of the Baltimore Longitudinal Study of Aging (BLSA) and the Genetic and Epigenetic Signatures of Translational Aging Laboratory Testing (GESTALT) studies.24,25 Both BLSA and GESTALT seek to evaluate multiple biomarkers associated with aging, with essentially identical inclusion and exclusion criteria. Participants with metallic implants or major neurological or medical disorders are excluded on first admission. All participants were administered the Mini Mental State Examination (MMSE). Informed consent was obtained from participants before the conduct of the experiments, in compliance with the local Institutional Review Board.
Data Acquisition
MRI scans were performed on a 3T whole body Philips MRI system (Achieva, Best, The Netherlands) using the internal quadrature body coil for transmission and an 8-channel phased-array head coil for reception. Each participant underwent our Bayesian Monte Carlo-mcDESPOT protocol for MWF, R1, and R2 mapping.6,16,26,27 This imaging protocol consisted of 3D spoiled gradient-recalled echo (SPGR) images acquired with flip angles of (2 4 6 8 10 12 14 16 18 20)°, echo time (TE) of 1.37 ms, repetition time (TR) of 5 ms and acquisition time of ~5 minutes, as well as 3D balanced steady-state free precession (bSSFP) images acquired with flip angles of [2 4 7 11 16 24 32 40 50 60]°, TE of 2.8 ms, TR of 5.8 ms, and acquisition time of ~6 minutes. The bSSFP images were acquired with radiofrequency excitation pulse phase increments of 0 or 180° to account for off-resonance effects, with a total scan time of ~12 minutes (~6 minutes for each phase-cycling scan). All SPGR and bSSFP images were acquired with an acquisition matrix of 150×130×94, voxel size 1.6×1.6×1.6 mm. To correct for excitation radiofrequency inhomogeneity,28,29 we used the double-angle method (DAM), which consisted of acquiring 2 fast spin-echo images with flip angles of 45° and 90°, TE of 102 ms, TR of 3000 ms, acquisition voxel size of 2.6×2.6×4 mm, and acquisition time of ~4 minutes. The total acquisition time was ~21 minutes. All images were acquired with field-of-view of 240×208×150 mm, SENSE factor of 2, and reconstructed to a voxel size of 1×1×1 mm. We emphasize that all MRI studies and ancillary measurements were performed with the same MRI system, with the same pulse sequences, and at the same facility for both BLSA and GESTALT participants.
The DTI protocol consisted of diffusion-weighted images (DWIs) acquired with single-shot EPI, TR of 10 s, TE of 70 ms, 2 b-values of 0 and 700 s/mm2, with the latter encoded in 32 directions, acquisition matrix of 120×104×75, and acquisition voxel size of 2×2×2 mm. Two images at b=0 s/mm2 were acquired and then averaged. All images were acquired with field-of-view of 240×208×150 mm.
Data Processing
For each participant, using the FLIRT analysis as implemented in the The FMRIB Software Library (FSL) software,30 all SPGR, bSSFP, or DAM images were linearly registered to the SPGR image obtained at FA of 8o and the respective derived transformation matrices were then applied to the original SPGR, bSSFP, or DAM images. Then, a whole-brain MWF map was generated using Bayesian Monte Carlo-mcDESPOT from these co-registered SPGR, bSSFP, and DAM datasets.6,16,20 Bayesian Monte Carlo-mcDESPOT assumes a 2-relaxation time components system consisting of a short component, attributed to myelin water, and a long component, attributed to intra- and extracellular water. We used the signal model explicitly accounting for nonzero TE.6,16,20 This emerging method offers rapid and reliable whole-brain MWF map within feasible clinical time6,16,20,31–34 and has been used in assessing myelin loss in mild cognitive impairment and dementias, as well as examining factors influencing cerebral myelination in normative aging.16,22,23,26,32–45 A whole-brain R1 map was also generated from the co-registered SPGR and DAM datasets using DESPOT1,21 and a whole-brain R2 map was generated from the co-registered bSSFP and DAM datasets using DESPOT2.21 The DW images were corrected for eddy current using the function eddy in FSL and for motion effects using the affine registration tools as implemented in FSL30 and registered to the DW image obtained with b=0 s/mm2 using FNIRT. We used the DTIfit tool implemented in FSL to calculate the eigenvalue maps, which were used to calculate FA, RD, MD, and AxD.46
Further, using FSL software,30 the averaged SPGR image over flip angles underwent nonlinear registration to the Montreal Neurological Institute standard space, and the computed transformation matrix was then applied to the corresponding DTI indices, MWF, R1, and R2 maps. Fourteen white matter regions of interest (ROIs) were defined from the Montreal Neurological Institute structural atlas corresponding to the whole brain, the frontal, parietal, temporal, and occipital lobes, cerebellum, corpus callosum, internal capsule, cerebral peduncle, corona radiata, thalamic radiation, fronto-occipital fasciculus, longitudinal fasciculus, and forceps. ROIs were eroded to reduce partial volume effect. Within each ROI, the mean FA, RD, MD, AxD, MWF, R1, and R2 values were calculated.
Systolic and Diastolic Blood Pressures
Systolic blood pressure and diastolic blood pressure were recorded 3 times in both arms in a seated position using a mercury sphygmomanometer sized to the arm of each participant, and the mean of the systolic and diastolic measurements were used in the subsequent analyses.47 Hypertension was defined as a systolic blood pressure greater than or equal to 140 mm Hg, a diastolic blood pressure greater than or equal to 90 mm Hg, or the use of prescription hypertension medications.
Statistical Analysis
To investigate the effect of hypertension on relaxometry (MWF, R1, R2) and diffusion (FA, MD, RD, AxD) MRI metrics, a multiple linear regression analysis was applied using MWF, R1, R2, FA, MD, RD, or AxD within each ROI as the dependent variable and hypertension status, smoking status, systolic blood pressure, diabetes, cholesterol, age, and sex as independent variables. In all cases, the threshold for statistical significance was P<0.05, while for close-to-significance was taken as P<0.1 after correction for multiple ROI comparisons using the false discovery rate method.48,49 All calculations were performed with MATLAB software (MathWorks, Natick, MA).
RESULTS
Participants Demographic Characteristics
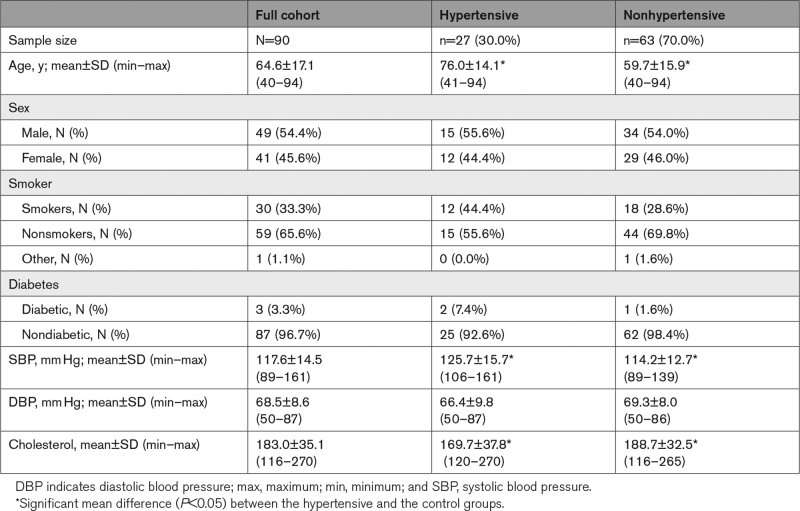
Demographic characteristics of the participants are shown in Table 1. After restricting the age range to participants of 40+ years and excluding 6 participants with either cognitive impairment, missing data or bad quality images due to severe motion artifacts, the final cohort consisted of 90 cognitively unimpaired volunteers (mean±SD MMSE=28.8±1.3) ranging in age from 40 to 94 years (64.6±17.1 years). Of this cohort, 49 (54.4%) were men, 30 (33.3%) were identified as cigarette smokers while 59 (65.6%) as nonsmokers. Among the cohort, 27 were hypertensive (30.0%), 23 of which taking antihypertensive medication. This cohort also included 3 participants (3.3%) with diabetes (2 of them were hypertensive), while 87 participants were nondiabetic (96.7%). The mean±SD values of the systolic blood pressure and diastolic blood pressure were 117.6±14.5 and 68.5±8.6, respectively, and the mean±SD values of cholesterol level were 183.0±35.1. Stratification of this cohort into hypertensive and control groups showed that, compared to the control group, the hypertensive group exhibits significantly (P<0.05) higher mean values of age and systolic blood pressure but a significantly (P<0.05) lower mean value of cholesterol level. The mean diastolic blood pressure was not significantly (P>0.1) different between the 2 groups.
Table 1.
Demographic Characteristics of Participants of the Study Cohort
Associations Between Hypertension and Cerebral Microstructure
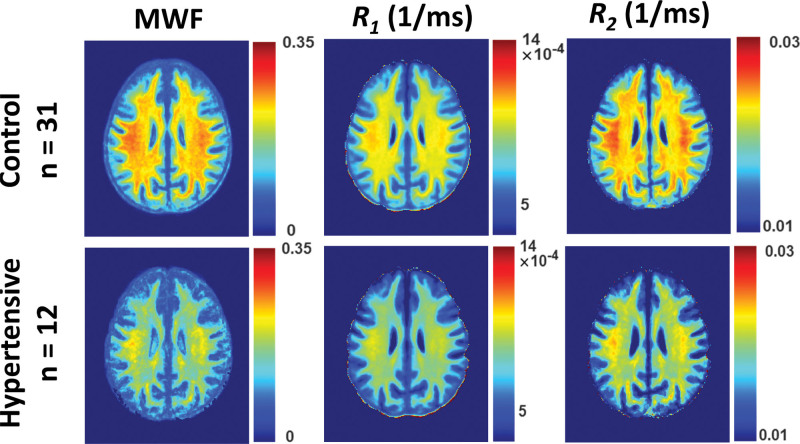
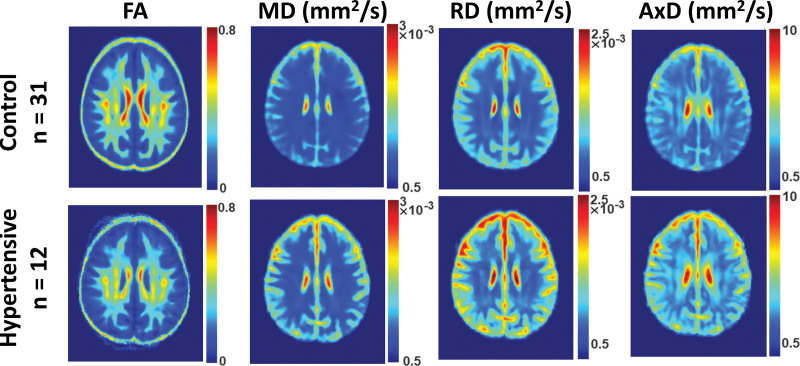
Figure 1 shows the MWF, R1 and R2 relaxometry parameter maps of either hypertensive or nonhypertensive participants within an age range of 70 to 94 years. This limited age range minimizes the potential effect of age on derived MR parameter maps for this qualitative analysis (statistical quantification of the effect of age as a covariate will be presented below [Tables 2 and 3]). Visual inspection indicates that, overall, hypertensive participants exhibit lower regional MWF, R1 and R2 values as compared with nonhypertensive participants. These qualitative results suggest a potentially strong association between hypertension and myelin content.
Figure 1.
Examples of myelin water fraction (MWF), R1 and R2 parameter maps averaged across participants drawn from a limited age range (70–94 years) either hypertensive (n=12) or nonhypertensive (n=31) to mitigate the effect of age. Results are shown for a representative slice. Visual inspection indicates that overall hypertensive patients exhibit lower regional MWF, R1 and R2 values, as compared with controls. R1 indicates longitudinal relaxation rate; and R2, transverse relaxation rate.
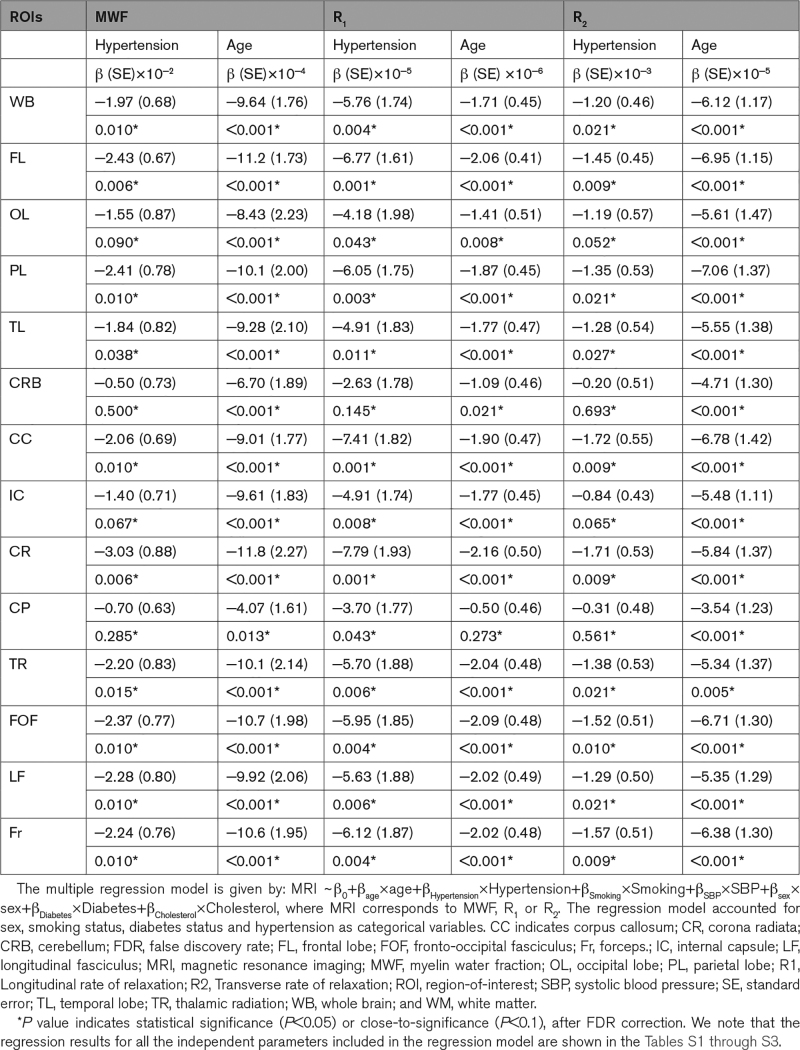
Table 2.
Regression Coefficient (β), Including SE, and Significance (P Value After FDR) of Relaxometry Metrics (MWF, R1, and R2) Versus Hypertension and Age Across 14 WM ROIs
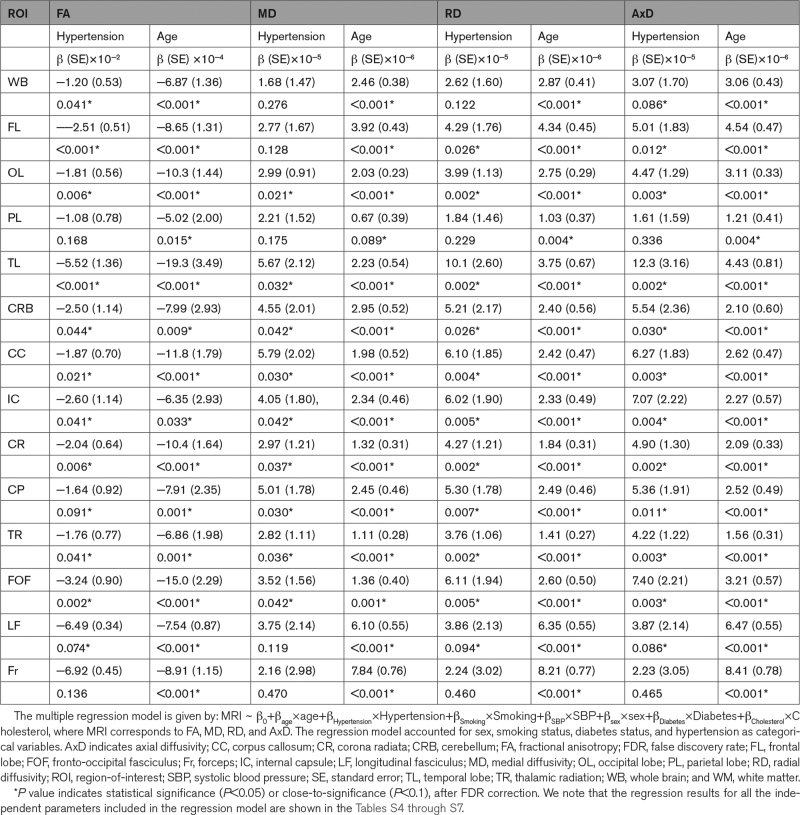
Table 3.
Regression Coefficient (β), Including SE, and Significance (P value After FDR) of Diffusion Tensor Imaging metrics (FA, MD, RD, and AxD) Versus Hypertension and Age Across 14 WM ROIs
Similarly, Figure 2 shows the FA, MD, RD, and AxD DTI parameter maps of either hypertensive or nonhypertensive participants within an age range of 70 to 94 years. Again, this limited age range is used to minimize the potential effect of age on derived DTI parameter maps for this qualitative analysis. Visual inspection indicates that, overall, hypertensive participants exhibit lower FA and higher MD, RD, and AxD values. In other words, hypertension is associated with higher diffusivities and a lower level of water diffusion. These qualitative results provide further support that hypertension is associated with reduced microstructural white matter integrity.
Figure 2.
Examples of fractional anisotropy (FA), medial diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AxD) parameter maps averaged across participants drawn from a limited age range (70–94 years) either hypertensive (n=12) or nonhypertensive (n=31) to mitigate the effect of age. Results are shown for a representative slice. Visual inspection indicates that, overall, hypertensive participants exhibit lower FA and higher MD, RD, and AxD.
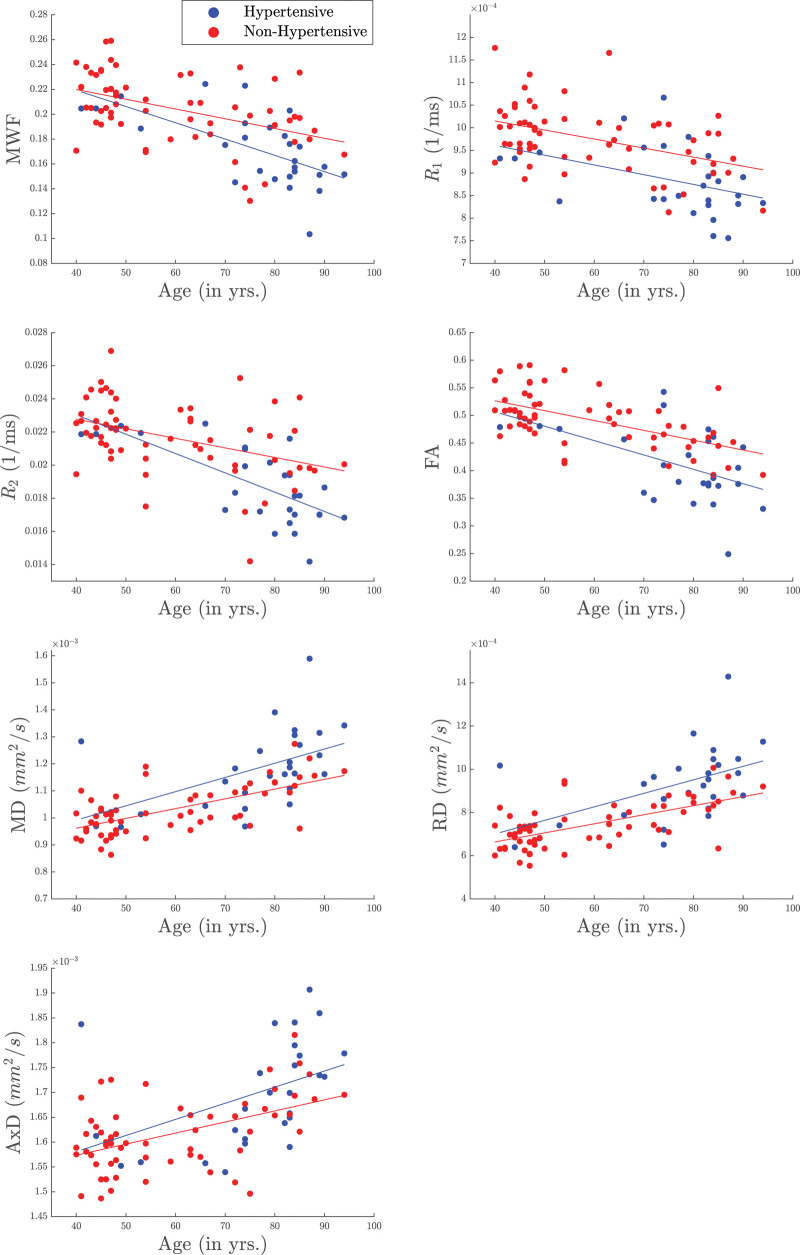
Table 2 summarizes the results of the multiple regression analysis of MWF, R1 and R2 versus hypertension and age in 14 white matter ROIs. In agreement with Figure 1, there are significant (P<0.05), or close-to-significant (P<0.1), negative correlations after false discovery rate correction, between hypertension and MWF or R2 in all regions but the cerebellum and cerebral peduncles, and between hypertension and R1 in all regions but the cerebellum. This is clearly seen in Figure 3 indicating that the hypertensive patients exhibit lower parameter values as compared with the control group. It was also found that the corpus callosum and the corona radiata exhibited the steepest slopes in the correlations between hypertension and MWF, R1 and R2. Furthermore, as expected, age was found to be a significant covariate with hypertension and exhibited negative slopes with respect to all metrics except for R1 in the cerebral peduncles (Table 2; Figure 3). Finally, the regression results for all the independent parameters included in the regression model are shown in the Supplemental Material.
Figure 3.
Examples of scatter plots of myelin water fraction (MWF), R1, R2, fractional anisotropy (FA), medial diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AxD) as a function of age stratified by groups. The hypertensive group is indicated by the blue color while the control group is indicated by the red color. Results were obtained from the corpus callosum ROI for MWF, R1 and R2, while from the temporal lobes ROIs for FA, MD, RD, and AxD. It is readily seen that derived MWF, R1, R2, and FA parameter values decrease with age while the diffusivity values increase with age, as expected. Importantly, the hypertensive patients exhibit lower MWF, R1, R2, and FA parameter values or higher diffusivity values as compared to control. R1 indicates longitudinal relaxation rate; and R2, transverse relaxation rate.
Table 3 summarizes the results of the multiple regression analysis of FA, MD, RD, and AxD versus hypertension and age in the 14 white matter ROIs studied after controlling for relevant covariates. There are significant (P<0.05), or close-to-significant (P<0.1), negative correlations after false discovery rate correction, between FA and hypertension and positive correlations with MD, RD, and AxD in most ROIs investigated. Examples of these association are illustrated shown in Figure 3. Here, we found that the steepest slopes in the correlation between hypertension and FA, MD, RD, and AxD were found in the temporal lobe, fronto-occipital fasciculus, and the internal capsules. We note that in contrast to the results of the multicomponent relaxometry analysis, less ROIs were found to be statistically significant between hypertensive and control groups, with the parietal lobe and forceps regions found to be insignificant in the correlation between hypertension and diffusivity metrics. However, the overall trend of the data follows the paradigm of impaired white matter microstructural integrity. Furthermore, in all ROIs investigated, the effect of age was found to be significant with respect to all metrics (Table 3; Figure 3). Finally, the regression results for all the independent parameters included in the regression model are shown in the Supplemental Material.
DISCUSSION
In this MRI study, using advanced multicomponent relaxometry and DTI analyses for both direct and indirect measurements of myelin content, we found that the hypertension status is associated with lower regional myelin content as measured by the MWF, R1, R2, and DTI metrics (FA, MD, RD, and AxD). These regional associations were observed in a cohort of well-characterized, cognitively unimpaired, adults. These results provide further evidence of the association between a well-known cardiovascular risk factor, specifically hypertension, and cerebral white matter tissue integrity in the absence of cognitive impairment. Furthermore, to our knowledge, this is the first investigation to indicate a direct association between hypertension and myelin deterioration, as measured by a specific proxy of myelin content (ie, MWF). In our analysis, we found that hypertension was associated with higher MD, RD, and AxD values and lower MWF, FA, R1, and R2 values. Our DTI results agree with previous DTI studies that have also shown a connection between cardiovascular risk factors, especially hypertension, and decreased cerebral microstructural integrity.9–12,50–52
While our relaxometry results, in conjunction with our DTI results, do not prove causality, they support the paradigm that hypertension impairs white matter microstructural integrity, especially the myelination process.5,53 Indeed, studies have revealed association between increased arterial stiffness and hypertension during the aging process.54–56 One of these paradigms suggests that vascular dysregulation due to potential synergetic effects of arterial remodeling and blood pressure may lead to transient reductions in cerebral blood flow, consequently resulting in transient decreased glucose transport into brain and hypoxia, and concomitant myelin injury.35 Indeed, recent works have demonstrated that deficits in cerebral blood flow are directly linked to reductions in cerebral tissue integrity. This association is present to a greater extent with white matter tissue damage.26,41,57 In fact, myelin maintenance through oligodendrocyte metabolism is an energy-demanding process, and therefore myelin homeostasis is particularly sensitive to hypoperfusion and consequent hypoxia or lack of essential nutrients for myelin synthesis.58,59 Recent in vitro studies have shown that oligodendrocytes are substantially more vulnerable to hypoxia and reduced supply of substrates that provide energy as can occur from hypoperfusion, when compared with other glial cells such as microglia and astroglia.60 Furthermore, it has been shown that hypertension also interferes with perivascular glymphatic drainage and blood-brain permeability. This would result in reduced drainage of toxic metabolites that adversely impact oligodendrocytes, the main cells synthesizing and maintaining myelin in the brain.61 Finally, interruption in the myelination process could result from chronic inflammation. Indeed, animal studies have demonstrated that chronically elevated blood pressure leads to adverse glial activation and increased brain inflammatory mediators that can be harmful to the myelin sheets and the normal functioning of oligodendrocyte cells.62 Nevertheless, despite these potential plausible mechanisms, further studies, especially of a longitudinal nature, are still required to shed light on the association between blood pressure and myelination.
We found the steepest slopes in the correlations between hypertension and MWF, R1 and R2, in the corpus callosum, fronto-occipital fasciculus and corona radiata cerebral regions (Table 2). Numerous studies have found that these brain regions are particularly susceptible to microstructural damages due to elevated blood pressure.63–65 Interestingly, these brain structures have also been shown to exhibit higher sensitivity to the cerebral blood supply.12,20,66,67 For example, the corpus callosum has an especially high level of metabolic demand, receiving blood from the anterior communicating, anterior pericallosal, and posterior cerebral arteries.68 Although ischemia in this region is rare due to the trifurcated nature of the vascular pathway, the energy-demanding process of myelination could be impeded from even minor changes in blood flow, such as those that occur from hypertension.69,70 However, it should be emphasized that the corpus callosum and the fronto-occipital fasciculus and corona radiata cerebral regions exhibit uniform myelination throughout their structures which may have led to better detection of the association between hypertension and myelination.
Longitudinal studies have found that antihypertensive medication has a protective effect on the brain and helps to reduce the rate of cognitive decline and neurodegeneration, including in Alzheimer’s disease.71,72 These studies consistently find that elevated blood pressure in midlife is more closely associated with cognitive decline when compared with elevated blood pressure in late life.73,74 This could possibly be due to the slow progression of hypertensive arterial remodeling eventually leading to reduced blood flow post-ischemia.5 Interestingly, among the 27 hypertensive subjects in our study, 23 subjects were taking antihypertensive medication at the time of the scan. Although it is unclear whether the antihypertensive medication had some level of a protective effect on these participants, it is interesting to note that participants undergoing treatment still had significantly lower myelin content or higher microstructural damage in many of the regions analyzed (Table 1). We conjecture that this may be due to either microstructural damage being done before the treatment of the antihypertensive medication or as a demonstration of the possible limitations of the antihypertensive medication on protecting the overall cerebral microstructure long term (Figures 1 and 2). Unfortunately, the information regarding the duration under medication was not available to further explore these interesting aspects including the effect of medication duration on myelination.
Although our investigation examined a relatively large cohort and used advanced MRI methodology to probe myelin content and obtain diffusion metrics, our work has certain limitations. The cross-sectional nature of the study precludes us from drawing any causal link between hypertension and demyelination; future longitudinal studies are needed to further support this potential association. We also note that the causal relationship between hypertension and myelination is difficult to determine as hypertension commonly occurs concomitant with many other cardiovascular risk factors, and we cannot control for all of them in our limited multiple linear regression model given the cohort size. Finally, determination of MR parameters can be biased due to several biological and methodological factors. These include, but are not limited to, the effects of magnetization transfer between macromolecules and free water protons, exchange between water pools, J-coupling, off-resonance, spin locking effects, water diffusion within different compartments, flow, temperature, hydration, internal gradients, and architectural features, including fiber fanning or crossing.57 Moreover, while the local distortions and motion artefacts in the DW images were corrected, we used the original b-vectors used in the acquisition. This could have introduced some bias in derived DTI indices. Finally, we did not control for white matter hyperintensity. Our inspection of this dataset revealed that white matter hyperintensities were limited to only a few subjects. This was expected given the very healthy nature of the BLSA and GESTALT cohorts. Further, the white matter hyperintensities were limited to small areas in the brain so that their impact in derived parameter values is negligible given the very large ROIs used in this study.
Perspectives
This study provides new insights into the association between hypertension and axonal demyelination among cognitively normal individuals spanning a wide age range. This work motivates further investigations to elucidate the extent to which hypertension and myelination are related in the pathological progression of neurodegenerative diseases, including in Alzheimer’s disease and dementias. This study will provide guidance towards new targets for intervention through re-enforcing myelination and controlling blood pressure.
ARTICLE INFORMATION
Acknowledgment
We thank Christopher Bergeron and Dr Linda Zukley for their help with data acquisition and logistics. We are also grateful to the participants of this study.
Sources of Funding
This research was supported entirely by the Intramural research Program of the National Institutes of Health, National institute on Aging.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AxD
- axial diffusivity
- bSSFP
- balanced steady-state free precession
- DAM
- double angle method
- DTI
- diffusion tensor imaging
- FA
- fractional anisotropy
- FSL
- The FMRIB Software Library
- MD
- mean diffusivity
- MRI
- magnetic resonance imaging
- MWF
- myelin water fraction
- R1
- longitudinal relaxation rate
- R2
- transverse relaxation rate
- RD
- radial diffusivity
- ROI
- regions of interest
- SPGR
- spoiled gradient-recalled echo
- TE
- echo time
- TR
- repetition time
For Sources of Funding and Disclosures, see page 1736.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.123.21012.
REFERENCES
- 1.Wajngarten M, Silva GS. Hypertension and stroke: update on treatment. Eur Cardiol. 2019;14:111–115. doi: 10.15420/ecr.2019.11.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kokubo Y, Iwashima Y. Higher blood pressure as a risk factor for diseases other than stroke and ischemic heart disease. Hypertension. 2015;66:254–259. doi: 10.1161/HYPERTENSIONAHA.115.03480 [DOI] [PubMed] [Google Scholar]
- 3.Longstreth WT, Jr, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol. 1998;55:1217–1225. doi: 10.1001/archneur.55.9.1217 [DOI] [PubMed] [Google Scholar]
- 4.Skoog I, Gustafson D. Update on hypertension and Alzheimer’s disease. Neurol Res. 2006;28:605–611. doi: 10.1179/016164106X130506 [DOI] [PubMed] [Google Scholar]
- 5.Humphrey JD. Mechanisms of vascular remodeling in hypertension. Am J Hypertens. 2021;34:432–441. doi: 10.1093/ajh/hpaa195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouhrara M, Spencer RG. Incorporation of nonzero echo times in the SPGR and bSSFP signal models used in mcDESPOT. Magn Reson Med. 2015;74:1227–1235. doi: 10.1002/mrm.25984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Dalen JW, Mutsaerts HJ, Petr J, Caan MW, van Charante EPM, MacIntosh BJ, van Gool WA, Nederveen AJ, Richard E. Longitudinal relation between blood pressure, antihypertensive use and cerebral blood flow, using arterial spin labelling MRI. J Cereb Blood Flow Metab. 2021;41:1756–1766. doi: 10.1177/0271678X20966975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabisz A, Naumczyk P, Marcinkowska A, Graff B, Gasecki D, Glinska A, Witkowska M, Jankowska A, Konarzewska A, Kwela J, et al. Aging and hypertension - independent or intertwined white matter impairing factors? Insights from the quantitative diffusion tensor imaging. Front Aging Neurosci. 2019;11:35. doi: 10.3389/fnagi.2019.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gons RA, de Laat KF, van Norden AG, van Oudheusden LJ, van Uden IW, Norris DG, Zwiers MP, de Leeuw F-E. Hypertension and cerebral diffusion tensor imaging in small vessel disease. Stroke. 2010;41:2801–2806. doi: 10.1161/STROKEAHA.110.597237 [DOI] [PubMed] [Google Scholar]
- 10.Nitkunan A, Charlton RA, McIntyre DJ, Barrick TR, Howe FA, Markus HS. Diffusion tensor imaging and MR spectroscopy in hypertension and presumed cerebral small vessel disease. Magn Reson Med. 2008;59:528–534. doi: 10.1002/mrm.21461 [DOI] [PubMed] [Google Scholar]
- 11.Maclullich AM, Ferguson KJ, Reid LM, Deary IJ, Starr JM, Seckl JR, Bastin ME, Wardlaw JM. Higher systolic blood pressure is associated with increased water diffusivity in normal-appearing white matter. Stroke. 2009;40:3869–3871. doi: 10.1161/STROKEAHA.109.547877 [DOI] [PubMed] [Google Scholar]
- 12.Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028 [DOI] [PubMed] [Google Scholar]
- 13.Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13(6 Pt 1):1174–1185. doi: 10.1006/nimg.2001.0765 [DOI] [PubMed] [Google Scholar]
- 14.Mohammadi S, Nagy Z, Moller HE, Symms MR, Carmichael DW, Josephs O, Weiskopf N. The effect of local perturbation fields on human DTI: characterisation, measurement and correction. Neuroimage. 2012;60:562–570. doi: 10.1016/j.neuroimage.2011.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubicki M, Westin CF, Maier SE, Mamata H, Frumin M, Ersner-Hershfield H, Kikinis R, Jolesz FA, McCarley R, Shenton ME. Diffusion tensor imaging and its application to neuropsychiatric disorders. Harv Rev Psychiatry. 2002;10:324–336. doi: 10.1080/10673220216231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouhrara M, Spencer RG. Rapid simultaneous high-resolution mapping of myelin water fraction and relaxation times in human brain using BMC-mcDESPOT. Neuroimage. 2017;147:800–811. doi: 10.1016/j.neuroimage.2016.09.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouhrara M, Cortina LE, Rejimon AC, Khattar N, Bergeron C, Bergeron J, Melvin D, Zukley L, Spencer RG. Quantitative age-dependent differences in human brainstem myelination assessed using high-resolution magnetic resonance mapping. Neuroimage. 2020;206:116307. doi: 10.1016/j.neuroimage.2019.116307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alonso-Ortiz E, Levesque IR, Pike GB. MRI-based myelin water imaging: a technical review. Magn Reson Med. 2015;73:70–81. doi: 10.1002/mrm.25198 [DOI] [PubMed] [Google Scholar]
- 19.MacKay A, Whittall K, Adler J, Li D, Paty D, Graeb D. In vivo visualization of myelin water in brain by magnetic resonance. Magn Reson Med. 1994;31:673–677. doi: 10.1002/mrm.1910310614 [DOI] [PubMed] [Google Scholar]
- 20.Bouhrara M, Spencer RG. Improved determination of the myelin water fraction in human brain using magnetic resonance imaging through Bayesian analysis of mcDESPOT. Neuroimage. 2016;127:456–471. doi: 10.1016/j.neuroimage.2015.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deoni SC, Rutt BK, Peters TM. Rapid combined T1 and T2 mapping using gradient recalled acquisition in the steady state. Magn Reson Med. 2003;49:515–526. doi: 10.1002/mrm.10407 [DOI] [PubMed] [Google Scholar]
- 22.Gong Z, Bilgel M, Kiely M, Triebswetter C, Ferrucci L, Resnick SM, Spencer RG, Bouhrara M. Lower myelin content is associated with more rapid cognitive decline among cognitively unimpaired individuals [published online January 31, 2023]. Alzheimer’s Dement. doi: 10.1002/alz.12968. https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/alz.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiely M, Triebswetter C, Cortina LE, Gong Z, Alsameen MH, Spencer RG, Bouhrara M. Insights into human cerebral white matter maturation and degeneration across the adult lifespan. Neuroimage. 2022;247:118727. doi: 10.1016/j.neuroimage.2021.118727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrucci L. The Baltimore Longitudinal Study of Aging (BLSA): a 50-year-long journey and plans for the future. J Gerontol A Biol Sci Med Sci. 2008;63:1416–1419. doi: 10.1093/gerona/63.12.1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shock NW, Gerontology Research Center (U.S.). Normal human aging: the Baltimore longitudinal study of aging. [Baltimore, Md.] Washington, D.C.: U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Aging For sale by the Supt. of Docs., U.S. G.P.O.; 1984. xix, 399, [34] p. p. [Google Scholar]
- 26.Bouhrara M, Alisch JSR, Khattar N, Kim RW, Rejimon AC, Cortina LE, Qian W, Ferrucci L, Resnick SM, Spencer RG. Association of cerebral blood flow with myelin content in cognitively unimpaired adults. BMJ Neurol Open. 2020;2:e000053. doi: 10.1136/bmjno-2020-000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian W, Khattar N, Cortina LE, Spencer RG, Bouhrara M. Nonlinear associations of neurite density and myelin content with age revealed using multicomponent diffusion and relaxometry magnetic resonance imaging. Neuroimage. 2020;223:117369. doi: 10.1016/j.neuroimage.2020.117369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stollberger R, Wach P. Imaging of the active B1 field in vivo. Magn Reson Med. 1996;35:246–251. doi: 10.1002/mrm.1910350217 [DOI] [PubMed] [Google Scholar]
- 29.Bouhrara M, Spencer RG. Steady state double angle method for rapid B1 mapping. Magn Reson Med. 2019;82:189–201. doi: 10.1002/mrm.27708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 31.Bouhrara M, Rejimon AC, Cortina LE, Khattar N, Bergeron CM, Ferrucci L, Resnick SM, Spencer RG. Adult brain aging investigated using BMC-mcDESPOT-based myelin water fraction imaging. Neurobiol Aging. 2020;85:131–139. doi: 10.1016/j.neurobiolaging.2019.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouhrara M, Khattar N, Elango P, Resnick SM, Ferrucci L, Spencer RG. Evidence of association between obesity and lower cerebral myelin content in cognitively unimpaired adults. Int J Obes (Lond). 2021;45:850–859. doi: 10.1038/s41366-021-00749-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouhrara M, Reiter DA, Bergeron CM, Zukley LM, Ferrucci L, Resnick SM, Spencer RG. Evidence of demyelination in mild cognitive impairment and dementia using a direct and specific magnetic resonance imaging measure of myelin content. Alzheimers Dement. 2018;14:998–1004. doi: 10.1016/j.jalz.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker KA, Duggan MR, Gong Z, Dark HE, Laporte JP, Faulkner ME, An Y, Lewis A, Moghekar AR, Resnick SM, et al. MRI and fluid biomarkers reveal determinants of myelin and axonal loss with aging. Ann Clin Transl Neurol. 2023;10:397–407. doi: 10.1002/acn3.51730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knopman DS, Mosley TH, Catellier DJ, Sharrett AR; Atherosclerosis Risk in Communities (ARIC) Study. Cardiovascular risk factors and cerebral atrophy in a middle-aged cohort. Neurology. 2005;65:876–881. doi: 10.1212/01.wnl.0000176074.09733.a8 [DOI] [PubMed] [Google Scholar]
- 36.Bouhrara M, Reiter DA, Celik H, Fishbein KW, Kijowski R, Spencer RG. Analysis of mcDESPOT- and CPMG-derived parameter estimates for two-component nonexchanging systems. Magn Reson Med. 2016;75:2406–2420. doi: 10.1002/mrm.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouhrara M, Kim RW, Khattar N, Qian W, Bergeron CM, Melvin D, Zukley LM, Ferrucci L, Resnick SM, Spencer RG. Age-related estimates of aggregate g-ratio of white matter structures assessed using quantitative magnetic resonance neuroimaging. Hum Brain Mapp. 2021;42:2362–2373. doi: 10.1002/hbm.25372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khattar N, Triebswetter C, Kiely M, Ferrucci L, Resnick SM, Spencer RG, Bouhrara M. Investigation of the association between cerebral iron content and myelin content in normative aging using quantitative magnetic resonance neuroimaging. Neuroimage. 2021;239:118267. doi: 10.1016/j.neuroimage.2021.118267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Triebswetter C, Kiely M, Khattar N, Ferrucci L, Resnick SM, Spencer RG, Bouhrara M. Differential associations between apolipoprotein E alleles and cerebral myelin content in normative aging. Neuroimage. 2022;251:118988. doi: 10.1016/j.neuroimage.2022.118988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouhrara M, Cortina LE, Khattar N, Rejimon AC, Ajamu S, Cezayirli DS, Spencer RG. Maturation and degeneration of the human brainstem across the adult lifespan. Aging (Albany NY). 2021;13:14862–14891. doi: 10.18632/aging.203183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiely M, Triebswetter C, Gong Z, Laporte JP, Faulkner ME, Akhonda M, Alsameen MH, Spencer RG, Bouhrara M. Evidence of an association between cerebral blood flow and microstructural integrity in normative aging using a holistic MRI approach [published online November 3, 2022]. J Magn Reson Imaging. doi: 10.1002/jmri.28508. https://onlinelibrary.wiley.com/doi/10.1002/jmri.28508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortina LE, Kim RW, Kiely M, Triebswetter C, Gong Z, Alsameen MH, Bouhrara M. Cerebral aggregate g-ratio mapping using magnetic resonance relaxometry and diffusion tensor imaging to investigate sex and age-related differences in white matter microstructure. Magn Reson Imaging. 2022;85:87–92. doi: 10.1016/j.mri.2021.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouhrara M, Rejimon AC, Cortina LE, Khattar N, Bergeron CM, Ferrucci L, Resnick SM, Spencer RG. Adult brain aging investigated using BMC-mcDESPOT based myelin water fraction imaging. Neurobiol Aging. 2019. doi: 10.1016/j.neurobiolaging.2019.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouhrara M, Cortina LE, Rejimon AC, Khattar N, Bergeron C, Bergeron J, Bergeron J, Melvin D, Zukley L, Spencer RG. Quantitative age-dependent differences in human brainstem myelination assessed using high-resolution magnetic resonance mapping. Neuroimage. 2020;206:116307 doi: 10.1016/j.neuroimage.2019.116307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faulkner ME, Laporte JP, Gong Z, Akhonda MABS, Triebswetter C, Kiely M, Palchamy E, Spencer RG, Bouhrara M. Lower myelin content is associated with lower gait speed in cognitively unimpaired adults [published online March 5, 2023]. J Gerontol. doi: 10.1093/gerona/glad080. https://academic.oup.com/biomedgerontology/advance-article/doi/10.1093/gerona/glad080/7069863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed. 2002;15:456–467. doi: 10.1002/nbm.783 [DOI] [PubMed] [Google Scholar]
- 47.Brant LJ, Ferrucci L, Sheng SL, Concin H, Zonderman AB, Kelleher CC, Longo DL, Ulmer H, Strasak AM. Gender differences in the accuracy of time-dependent blood pressure indices for predicting coronary heart disease: a random-effects modeling approach. Gend Med. 2010;7:616–627. doi: 10.1016/j.genm.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benjamini Y. Discovering the false discovery rate. J Royal Stat Soc. 2010;72:405–416. doi: 10.1111/j.1467-9868.2010.00746.x [Google Scholar]
- 49.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x [Google Scholar]
- 50.Huang L, Ling XY, Liu SR. Diffusion tensor imaging on white matter in normal adults and elderly patients with hypertension. Chin Med J (Engl). 2006;119:1304–1307. [PubMed] [Google Scholar]
- 51.Pasi M, van Uden IW, Tuladhar AM, de Leeuw FE, Pantoni L. White matter microstructural damage on diffusion tensor imaging in cerebral small vessel disease: clinical consequences. Stroke. 2016;47:1679–1684. doi: 10.1161/STROKEAHA.115.012065 [DOI] [PubMed] [Google Scholar]
- 52.Suri S, Topiwala A, Chappell MA, Okell TW, Zsoldos E, Singh-Manoux A, Kivimäki M, Mackay CE, Ebmeier KP. Association of midlife cardiovascular risk profiles with cerebral perfusion at older ages. JAMA Netw Open. 2019;2:e195776. doi: 10.1001/jamanetworkopen.2019.5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol. 2013;304:H1598–H1614. doi: 10.1152/ajpheart.00490.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scuteri A, Nilsson PM, Tzourio C, Redon J, Laurent S. Microvascular brain damage with aging and hypertension: pathophysiological consideration and clinical implications. J Hypertens. 2011;29:1469–1477. doi: 10.1097/HJH.0b013e328347cc17 [DOI] [PubMed] [Google Scholar]
- 55.Oh YS. Arterial stiffness and hypertension. Clin Hypertens. 2018;24:17. doi: 10.1186/s40885-018-0102-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Safar ME, Asmar R, Benetos A, Blacher J, Boutouyrie P, Lacolley P, Laurent S, London G, Pannier B, Protogerou A, et al. ; French Study Group on Arterial Stiffness. Interaction between hypertension and arterial stiffness. Hypertension. 2018;72:796–805. doi: 10.1161/HYPERTENSIONAHA.118.11212 [DOI] [PubMed] [Google Scholar]
- 57.Bouhrara M, Triebswetter C, Kiely M, Bilgel M, Dolui S, Erus G, Meirelles O, Bryan NR, Detre JA, Launer LJ. Association of cerebral blood flow with longitudinal changes in cerebral microstructural integrity in the coronary artery risk development in young adults (CARDIA) study. JAMA Netw Open. 2022;5:e2231189. doi: 10.1001/jamanetworkopen.2022.31189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Costa-Mattioli M, Walter P. The integrated stress response: from mechanism to disease. Science. 2020;368:6489. doi: 10.1126/science.aat5314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosko L, Smith VN, Yamazaki R, Huang JK. Oligodendrocyte bioenergetics in health and disease. Neuroscientist. 2019;25:334–343. doi: 10.1177/1073858418793077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lyons SA, Kettenmann H. Oligodendrocytes and microglia are selectively vulnerable to combined hypoxia and hypoglycemia injury in vitro. J Cereb Blood Flow Metab. 1998;18:521–530. doi: 10.1097/00004647-199805000-00007 [DOI] [PubMed] [Google Scholar]
- 61.Mohammadi MT, Dehghani GA. Acute hypertension induces brain injury and blood-brain barrier disruption through reduction of claudins mRNA expression in rat. Pathol Res Pract. 2014;210:985–990. doi: 10.1016/j.prp.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 62.Bajwa E, Klegeris A. Neuroinflammation as a mechanism linking hypertension with the increased risk of Alzheimer’s disease. Neural Regen Res. 2022;17:2342–2346. doi: 10.4103/1673-5374.336869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gons RA, van Oudheusden LJ, de Laat KF, van Norden AG, van Uden IW, Norris DG, Zwiers MP, van Dijk E, de Leeuw F-E. Hypertension is related to the microstructure of the corpus callosum: the RUN DMC study. J Alzheimers Dis. 2012;32:623–631. doi: 10.3233/JAD-2012-121006 [DOI] [PubMed] [Google Scholar]
- 64.de Groot M, Ikram MA, Akoudad S, Krestin GP, Hofman A, van der Lugt A, Niessen WJ, Vernooij MW. Tract-specific white matter degeneration in aging: the Rotterdam Study. Alzheimers Dement. 2015;11:321–330. doi: 10.1016/j.jalz.2014.06.011 [DOI] [PubMed] [Google Scholar]
- 65.Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, Carmichael O, Wolf PA, DeCarli C. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet Neurol. 2012;11:1039–1047. doi: 10.1016/S1474-4422(12)70241-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson B, Zhang K, Gay M, Neuberger T, Horovitz S, Hallett M, Sebastianelli W, Slobounov S. Metabolic alterations in corpus callosum may compromise brain functional connectivity in MTBI patients: an 1H-MRS study. Neurosci Lett. 2012;509:5–8. doi: 10.1016/j.neulet.2011.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumral E, Bayulkem G. Spectrum of single and multiple corona radiata infarcts: clinical/MRI correlations. J Stroke Cerebrovasc Dis. 2003;12:66–73. doi: 10.1053/jscd.2003.11 [DOI] [PubMed] [Google Scholar]
- 68.Goldstein A, Covington BP, Mahabadi N, Mesfin FB. Neuroanatomy, Corpus Callosum. StatPearls: Treasure Island (FL); 2022. [PubMed] [Google Scholar]
- 69.McQueen J, Reimer MM, Holland PR, Manso Y, McLaughlin M, Fowler JH, Horsburgh K. Restoration of oligodendrocyte pools in a mouse model of chronic cerebral hypoperfusion. PLoS One. 2014;9:e87227. doi: 10.1371/journal.pone.0087227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow Metab. 2003;23:263–274. doi: 10.1097/01.WCB.0000053472.41007.F9 [DOI] [PubMed] [Google Scholar]
- 71.Beason-Held LL, Moghekar A, Zonderman AB, Kraut MA, Resnick SM. Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke. 2007;38:1766–1773. doi: 10.1161/STROKEAHA.106.477109 [DOI] [PubMed] [Google Scholar]
- 72.Khachaturian AS, Zandi PP, Lyketsos CG, Hayden KM, Skoog I, Norton MC, Tschanz JT, Mayer LS, Welsh-Bohmer KA, Breitner JCS. Antihypertensive medication use and incident Alzheimer disease: the Cache County Study. Arch Neurol. 2006;63:686–692. doi: 10.1001/archneur.63.5.noc60013 [DOI] [PubMed] [Google Scholar]
- 73.Shah NS, Vidal JS, Masaki K, Petrovitch H, Ross GW, Tilley C, DeMattos RB, Tracy RP, White LR, Launer LJ. Midlife blood pressure, plasma beta-amyloid, and the risk for Alzheimer disease: the Honolulu Asia Aging Study. Hypertension. 2012;59:780–786. doi: 10.1161/HYPERTENSIONAHA.111.178962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Power MC, Weuve J, Gagne JJ, McQueen MB, Viswanathan A, Blacker D. The association between blood pressure and incident Alzheimer disease: a systematic review and meta-analysis. Epidemiology. 2011;22:646–659. doi: 10.1097/EDE.0b013e31822708b5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.