Objective:
Isoniazid (INH) preventive therapy is recommended to prevent tuberculosis (TB) disease for persons with HIV (PWH), except for those with regular and heavy alcohol consumption, due to hepatotoxicity concerns. We aimed to quantify the incidence of severe INH-related toxicity among PWH with and without recent alcohol consumption.
Design:
A prospective study of PWH receiving INH.
Methods:
We included PWH in southwest Uganda with recent (prior 3 months) (n = 200) or no (prior year) self-reported alcohol consumption (n = 101), on antiretroviral therapy, TB infected (≥5 mm on tuberculin skin test), and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) 2× or less the upper limit of normal (ULN). Grade 3+ INH-related toxicity was ALT or AST at least 5× the ULN or severe symptoms; we stopped IPT upon detection. Grade 2 INH-related toxicity was ALT or AST 2–5× the ULN or moderate symptoms.
Results:
The cumulative incidence of Grade 3+ INH-related toxicity was 8.3% [95% confidence interval (95% CI) 5.4–12.0]; all resolved after INH cessation. Incidence was 6.0% (95% CI 3.1–10.2) among those reporting recent alcohol use and 12.9% (95% CI 7.0–21.0) among those reporting no prior year alcohol use. We found no differences by baseline phosphatidylethanol-confirmed alcohol severity. The cumulative incidence of Grade 2 toxicities (without Grade 3+) was 21.7% (95% CI 17.0–27.1); 25.0% (95% CI 19.0–31.8) among those with recent alcohol use and 14.8% (95% CI 8.1–23.9) among those with no prior year alcohol use.
Conclusion:
Alcohol use does not appear to increase risk for serious INH-related toxicity among PWH without significant liver enzyme elevations at baseline (≤2x ULN).
Keywords: alcohol consumption, hepatotoxicity, HIV, isoniazid preventive therapy, phosphatidylethanol, tuberculosis infection
Introduction
Tuberculosis (TB) remains the leading cause of death among persons with HIV (PWH), leading to 187 000 deaths in 2022 [1]. Uganda is one of the 30 countries the WHO lists as having high TB burden overall and among PWH [1]. TB preventive therapy with isoniazid (INH) decreases both all-cause mortality and active TB in PWH by 30–50% above and beyond the benefits of antiretroviral therapy (ART) alone [2–6]. On the basis of these findings, the WHO recommends isoniazid (INH) preventive therapy (IPT) for all PWH [7]. Accordingly, the Uganda Ministry of Health (MOH) launched a wide-scale program to provide 6 months of INH to 300 000 PWH in 2019 [8,9].
The HIV/TB problem is exacerbated by alcohol use, which is very prevalent among PWH worldwide and in sub-Saharan Africa, where 22–25% of PWH report high-risk alcohol use [10,11]. Meta-analyses have found that the rate of active TB disease among those consuming any or high-risk alcohol use is two to three-fold higher than among those not consuming alcohol [12–14]; this result is consistent when restricted to studies of PWH [14]. In addition to the higher risk of disease, the prognosis of TB disease is worse for those who consume alcohol compared with those who do not; those who consume alcohol have higher morbidity from TB, treatment failure, and loss to follow up [15]. Thus, TB disease prevention is extremely important for PWH who consume alcohol.
Despite the high risk of TB disease and poor outcomes for PWH who consume alcohol, the WHO recommends the deferral of IPT for persons with ‘regular and heavy alcohol consumption’ [7]. This deferral is due to hepatotoxicity concerns, because there is a small but real risk of serious outcomes. In the general population, INH causes aminotransferase elevations of greater than five times the upper limit of normal (ULN) in 3–5% of persons, and acute liver injury with jaundice in 0.5–1.0%, and fatality for 0.05–0.10% [16]. However, the risk for persons consuming alcohol has not been systematically quantified; thus, there is a lack of data to determine whether the benefits of preventing active TB outweigh the risks of possible increased risk for toxicity among persons consuming alcohol.
To fill this knowledge gap, the primary aim of this study was to determine the incidence of Grade 3+ INH-related toxicity among PWH who consume alcohol. The secondary aim was to examine whether the rate of INH-related toxicity varies by how much alcohol is consumed. Lastly, we aimed to describe the rate of Grade 2 toxicity and whether this toxicity varies by level of alcohol use.
Materials and methods
This study was a prospective single-arm longitudinal cohort study of PWH conducted in southwestern Uganda. The study was entitled the ‘Alcohol Drinkers Exposure to Preventive Therapy for TB’ (ADEPTT).
Eligibility
Participants were recruited from the Mbarara Regional Referral Hospital (MRRH) Immune Suppression Syndrome (ISS) Clinic from April 2017 through December 2019. Study eligibility included being an adult (age ≥18 years) ISS clinic patient, fluent in English or Runyankole, residing within 2 h travel from the clinic, no prior history of TB disease, treatment, or IPT, alanine aminotransferase (ALT) or aspartate aminotransferase (AST) elevations two times or less the ULN, prescribed ART for at least 6 months, and infected with TB infection, as evidenced by a tuberculin skin test (TST). The sample was designed to include 200 persons reporting any current (prior 3 months) alcohol use and 100 persons reporting no alcohol use for at least 1 year, to have a clearly abstinent group for comparison with those currently drinking alcohol. We excluded persons on anticonvulsants, nevirapine, persons planning to move out of the catchment area, and pregnant women. Eligibility was determined via a three-stage process as follows. First, potential participants were screened for age, language, ART, plans to move, prior TB or TB medications, anticonvulsant use, and alcohol use. Those meeting eligibility criteria were tested for liver enzyme elevations and pregnancy and screened for TB disease. Those meeting the criteria were then screened for TB infection using the Mantoux TST and were asked to return 2–3 days later for TST reading. TST indurations at least 5 mm were considered positive and were read by trained medical personnel.
Study visits
Following enrollment, all participants were prescribed a 300 mg INH oral tablet and a 25 mg pyridoxine oral tablet daily for 6 months. Refills were given after symptom and transaminase checks at weeks 2, 4, and monthly thereafter, continuing until 1 month after INH was completed (allowing for the 6-month course to be taken over 9 months if necessary). At the baseline visit, INH was distributed in Medication Event Monitoring Systems (MEMS) bottles (AARDEX Group) and the caps were read electronically at the refill visits, to measure adherence. Participants were instructed on their use at the baseline visit. We paused study visits from mid-March to June 2020 and asked participants who were on INH to pause their pill-taking (n = 15) because the COVID-19 movement restrictions prohibited our ability to conduct safety monitoring.
We conducted interviewer-administered structured surveys at baseline, 3 months, and 6 months. Blood was drawn and CD4+ cell count, HIV viral load, and hepatitis B surface antigen (HBsAg) testing was performed at baseline; these tests were performed at the Mbarara University of Science and Technology (MUST) Clinical and Research Laboratory. ALT and AST testing were performed at eligibility screening, and all visits while on INH and 1 month following. Dried blood spots were prepared from venous blood draws at baseline, 3, and 6 months, and were tested for phosphatidylethanol (PEth) analog 16 : 0/18 : 1 with a limit of quantification of 8 ng/ml at the United States Drug Testing Laboratory (USDTL) using previously published procedures [17].
Safety monitoring
We conducted safety monitoring at weeks 2, 4, and monthly thereafter until 1 month after INH completion or termination. These checks included aminotransferase level tests and checks for symptoms of TB and potential adverse symptoms, with severity graded if present. Moderate symptoms were defined as those in which minimal, local, or noninvasive intervention was indicated and that limited age-appropriate instrumental activities of daily life. Severe symptoms were defined as medically significant, with hospitalization or prolongation of hospitalization indicated and disabling, limiting self-care activities of daily life. We defined severe INH-related toxicities (Grade 3+) as events that were considered possibly, probably, or definitely related to INH, with ALT and/or AST at least 5× ULN and/or severe symptoms (Grade 3), and ALT and/or AST at least 10× ULN and/or life-threatening symptoms (Grade 4). We stopped INH and conducted increased monitoring (weekly for Grade 3, every 3–4 days for Grade 4) after these events were detected. Grade 2 INH-related toxicities were defined as adverse events possibly, probably, or definitely related to INH with ALT and/or AST more than 2× ULN, and/or moderate symptoms, and we conducted increased monitoring (bi-weekly) when Grade 2 events were detected. Participants were also given a study card with a toll-free phone number to call in case of adverse symptoms between study visits. The phone line was staffed by medical officers who discussed symptoms and advised on coming to the study site, with a medical doctor available to respond to adverse events.
Study variables
Primary outcome
The primary outcome INH-related toxicity was the occurrence of a Grade 3 or Grade 4 (referred to as Grade 3+) ALT or AST elevation or severe symptoms in the prior 3 months as defined above possibly, probably, or definitely related to INH, at 3 and 6 months.
Secondary outcome
We examined Grade 2 INH-related toxicities among those who did not have Grade 3 or 4 INH-related toxicities as a secondary outcome.
Alcohol consumption
The primary alcohol measure was self-reported recent alcohol use at screening. We further examined the level of alcohol use at baseline and follow up using a composite variable of self-report [the Alcohol Use Disorders Test – Consumption (AUDIT-C), modified to measure prior three months alcohol use], and PEth, hereafter referred to as self-report/PEth combined. We used this composite measure to increase the sensitivity beyond using self-report or the biomarker alone. The cutoffs for this variable are summarized in Table 1, based on previously defined AUDIT-C [18,19], and PEth cutoffs [20,21]. We also examined self-report alone using the AUDIT-C and time-updated self-report/PEth during IPT (i.e. at 3 and 6 months) as the alcohol measures in additional analyses.
Table 1.
Participant characteristics at baseline, overall and by INH-related toxicity during IPT.
| Overall | Any Grade 3+ INH-related toxicity | Any Grade 2 INH-related toxicity (among those without Grade 3+) | |||
| (n = 301) | No (n = 276) | Yes (n = 25) | No (n = 216) | Yes (n = 60) | |
| N (%) or median [IQR] | N (%) or median [IQR] | N (%) or median [IQR] | N (%) or median [IQR] | N (%) or median [IQR] | |
| Sex | |||||
| Female | 154 (51.2) | 140 (90.9) | 14 (9.1) | 121 (86.4) | 19 (13.6) |
| Male | 147 (48.8) | 136 (92.5) | 11 (7.5) | 95 (69.9) | 41 (30.2) |
| Age (median [IQR]) | 40 [33–47] | 40 [34–47] | 43 [31–47] | 40 [34–49] | 41 [35–45] |
| BMI | |||||
| Low (<18.5) | 31 (10.3) | 26 (83.9) | 5 (16.1) | 22 (84.6) | 4 (15.4) |
| Middle (18.5 to <25) | 185 (61.5) | 171 (92.4) | 14 (7.6) | 127 (74.3) | 44 (25.7) |
| High (≥25) | 85 (28.2) | 79 (92.9) | 6 (7.1) | 67 (84.8) | 12 (15.2) |
| Alcohol use | |||||
| Any self-reported alcohol use at screening | |||||
| No | 101 (33.6) | 88 (87.1) | 13 (12.9) | 75 (85.2) | 13 (14.8) |
| Yes | 200 (66.5) | 188 (94.0) | 12 (6.0) | 141 (75.0) | 47 (25.0) |
| Self-report at baseline (AUDIT-C)a | |||||
| None | 100 (33.2) | 87 (87.0) | 13 (13.0) | 74 (85.1) | 13 (14.9) |
| Low | 85 (28.2) | 80 (94.1) | 5 (5.9) | 66 (82.5) | 14 (17.5) |
| Medium | 66 (21.9) | 63 (95.5) | 3 (4.6) | 50 (79.4) | 13 (20.6) |
| High/very high | 50 (16.6) | 46 (92.0) | 4 (8.0) | 26 (56.5) | 20 (43.5) |
| PEth (median [IQR]) | 14.0 [1.0–181.0] | 15.5 [1.0–187.5] | 1.0 [1.0–147.0] | 5.0 [1.0–133.0] | 99.5 [1.0–459.5] |
| PEth at baselineb | |||||
| None | 140 (46.5) | 125 (89.3) | 15 (10.7) | 108 (86.4) | 17 (13.6) |
| Low | 44 (14.6) | 43 (97.7) | 1 (2.3) | 33 (76.7) | 10 (23.3) |
| Medium | 45 (15.0) | 41 (91.1) | 4 (8.9) | 33 (80.5) | 8 (19.5) |
| High/very high | 72 (23.9) | 67 (93.1) | 5 (6.9) | 42 (62.7) | 25 (37.3) |
| Self-report /PEth combinedc | |||||
| None | 86 (28.6) | 75 (87.2) | 11 (12.8) | 65 (86.7) | 10 (13.3) |
| Low | 66 (21.9) | 62 (93.9) | 4 (6.1) | 52 (83.9) | 10 (16.1) |
| Medium | 56 (18.6) | 53 (94.6) | 3 (5.4) | 47 (88.7) | 6 (11.3) |
| High/very high | 93 (30.9) | 86 (92.5) | 7 (7.5) | 52 (60.5) | 34 (39.5) |
| Health status | |||||
| Self-reported health status | |||||
| Good/fair/poor | 162 (53.8) | 147 (90.7) | 15 (9.3) | 115 (78.2) | 32 (21.8) |
| Excellent/very good | 139 (46.2) | 129 (92.8) | 10 (7.2) | 101 (78.3) | 28 (21.7) |
| Fib-4 score (median [IQR]) | 0.96 [0.71–1.30] | 0.97 [0.71–1.33] | 0.85 [0.68–1.16] | 0.93 [0.70–1.26] | 1.08 [0.73–1.46] |
| Liver injury due to alcohol use (AST/ALT ratio ≥2) | |||||
| No | 286 (95.0) | 263 (92.0) | 23 (8.0) | 207 (78.7) | 56 (21.3) |
| Yes | 15 (5.0) | 13 (86.7) | 2 (13.3) | 9 (69.2) | 4 (30.8) |
| Liver injury (ALT >1.25× ULN) | |||||
| No | 219 (72.8) | 200 (91.3) | 19 (8.7) | 159 (79.5) | 41 (20.5) |
| Yes | 82 (27.2) | 76 (92.7) | 6 (7.3) | 57 (75.0) | 19 (25.0) |
| Virally suppressed | |||||
| No | 23 (7.9) | 22 (95.7) | 1 (4.4) | 19 (86.4) | 3 (13.6) |
| Yes | 269 (92.1) | 246 (91.5) | 23 (8.6) | 190 (77.2) | 56 (22.8) |
| CD4+ cell count (median [IQR]) | 673 [506–866] | 676 [509–870] | 606 [459–746] | 692 [526–887] | 631 [447–854.5] |
| Hepatitis B status | |||||
| Negative | 296 (98.3) | 271 (91.6) | 25 (8.5) | 4 (80.0) | 1 (20.0) |
| Positive | 5 (1.7) | 5 (100.0) | 0 (0.0) | 212 (78.2) | 59 (21.8) |
| ART regimen | |||||
| Dolutegravir | 55 (18.3) | 51 (92.7) | 4 (7.3) | 40 (78.4) | 11 (21.6) |
| Efavirenz-based | 232 (77.1) | 211 (91.0) | 21 (9.1) | 164 (77.7) | 47 (22.3) |
| PI-based | 14 (4.7) | 14 (100.0) | 0 (0.0) | 12 (85.7) | 2 (14.3) |
| ART adherence (self-reported) | |||||
| Good/fair/poor/very poor | 56 (18.6) | 54 (96.4) | 2 (3.6) | 48 (88.9) | 6 (11.1) |
| Excellent/very good | 245 (81.4) | 222 (90.6) | 23 (9.4) | 168 (75.7) | 54 (24.3) |
| Years since ART initiation (median [IQR]) (n = 283) | 5.1 [3.3–7.6] | 5.1 [3.2–7.7] | 5.4 [3.6–7.4] | 5.1 [3.2–8.1] | 5.1 [3.4–6.6] |
Self-report categories: None: AUDIT-C = 0; Low: AUDIT-C 1-2 for women and 1–3 for men; Medium: AUDIT-C 3–5 for women and 4--5 for men; High/very high: AUDIT-C ≥6.
PEth categories: None: PEth <8 ng/ml; Low: PEth 8–<50 ng/ml; Medium: PEth 50–200 ng/ml, High: PEth ≥200 ng/ml.
Self-report/PEth combined categories assigns the higher of the self-report and PEth categories.
Covariates
We considered other factors as covariates that may impact INH-related toxicity such as sex, age, self-reported health status (excellent/very good versus good/fair/poor), BMI (18.5, 18.5 to <25, ≥25), baseline liver injury [22,23], and viral hepatitis (HBsAg) [23], and explored ART regimen (dolutegravir versus efavirenz versus protease inhibitor-based), ART adherence (prior month self-report, excellent/very good versus good/fair/poor/very poor), duration on ART (years), IPT adherence, duration on INH (3 or 6 months), and HIV viral suppression (<40 copies/ml). We measured baseline liver injury using the Fib-4 score, elevated ALT (ALT > 1.25× the ULN), and AST/ALT ratio at least 2. Adherence to INH was considered as a time-updated variable and was assessed by the percentage of days with at least one pill bottle opening in the 3 months prior to the 3 and 6-month interviews, or until INH discontinuation (if applicable), dichotomized as at least 90% of pills taken, versus less than 90%.
Statistical analyses
We conducted descriptive analyses of the baseline characteristics of the study sample, and report proportions for categorical variables, and medians (interquartile range [IQR]) for continuous variables, overall and by both Grade 3+ INH-related toxicity and Grade 2 INH-related toxicity (among those without a Grade 3+ INH-related toxicity). We calculated the cumulative incidence of INH-related toxicities, overall and by alcohol use at screening.
We conducted multivariable analyses to examine the association between the level of alcohol use at baseline and the occurrence of a Grade 3+ INH-related toxicity in the prior 3 months. We used generalized estimating equation (GEE) models with the logit link, using robust standard errors and an exchangeable working correlation structure. Our initial primary model (Model 1) examined the association of self-report/PEth combined alcohol use at baseline on Grade 3+ INH related toxicity, controlling for a priori covariates (age, sex, health status, and study visit), and added additional covariates as follows. We took a stepwise modeling approach, by first fitting separate GEE models for each potential additional covariate (baseline BMI, HBsAg, HIV viral suppression, CD4+ cell count, ART regimen, years on ART, ART adherence, Fib-4 score, elevated ALT, AST/ALT, and time-updated INH adherence), adding each variable to the above model, one by one. We included any covariates with a P value less than 0.10 together in a multivariable model and then removed covariates one at a time if the P value was more than 0.10, removing the variable with the largest P value first, and then re-assessing. Finally, we evaluated factors not previously included, one at a time, and retained them in the model if the P value was less than 0.10. We considered the last multivariable model the ‘final’ Model 1. We also used this approach to construct exploratory models evaluating the alternative measures of alcohol consumption, that is, baseline alcohol use by self-report (Model 2) and time-updated self-report/PEth (Model 3).
Additional sensitivity and exploratory analyses
To determine whether our findings on the relationship between alcohol use and INH-related toxicity were impacted by ‘sick-quitters’ who quit drinking due to health reasons, we re-ran the final Models 1–3, excluding participants who were in the no-alcohol use group but who had reported ever drinking. We also conducted analyses of Grade 2 INH-related toxicities in the prior 3 months, among those who did not have a Grade 3+ INH-related toxicity, using the same steps as above, to create models 4–6. All analyses were conducted using Stata Statistica Software, Release 14, 2015 (StataCorp. LP, College Station, Texas, USA).
Ethical review
All participants gave written informed consent for the study procedures, which were approved by the Uganda Council on Science and Technology, and the Institutional Review Boards of the Mbarara University of Science and Technology, the University of California, San Francisco, Boston University, and Boston Medical Center, and was registered at ClinicalTrials.gov (NCT03302299).
Results
Screening and enrollment
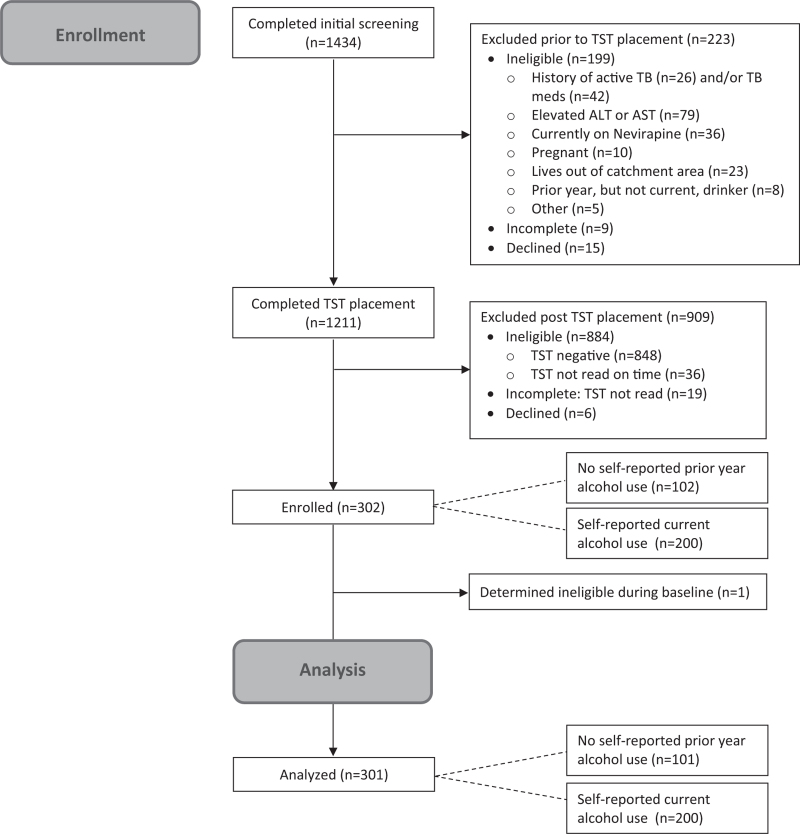
One thousand four hundred thirty-four participants completed the first stage of screening (Fig. 1). Of those, 1327 (92.5%) met the initial eligibility criterion and 1211 (84.4%) were eligible for TST. Exclusions at the second stage were mostly due to elevated transaminases (n = 79/1327, 6.0%). Of the 1192 (98.4%) who had TSTs placed, 308 (25.8%) were positive, 848 were negative (71.1%), and 36 (3.0%) were not read on time because the participant did not return within 72 h. Three hundred and two persons were enrolled, 200 who reported current alcohol use and 102 (2 more than the planned 100 due to an error) reported no alcohol use in the past year. One participant was found to be ineligible during their baseline interview and was subsequently disenrolled, leaving 301 participants for analysis.
Fig. 1.
Consort diagram for ADEPTT study.
Half (51.2%) of the participants were women, median age was 40 years (IQR 33–47), and 92.1% [95% confidence interval (95% CI) 88.4–94.7] were virally suppressed at baseline (Table 1). Using combined self-report/PEth, 28.6% engaged in no alcohol use, 21.9% low alcohol use, 18.6% medium alcohol use group, and 30.9% in high/very high alcohol use at baseline. These levels were higher than by study screening and baseline self-report alone (Table 1). About half (46.2%) reported excellent/very good health, and 81.4% self-reported excellent/very good ART adherence.
Grade 3+ INH-related toxicities
Overall, the cumulative incidence of Grade 3+ INH-related toxicity was 8.3% (95% CI 5.4–12.0), with 19 persons reaching Grade 3 and six reaching Grade 4. The toxicities occurred a median of 55 days after starting the INH course (IQR 31–97), and the majority (n = 22) were due to elevated ALT or AST only (i.e., without symptoms or mild symptoms only). Almost all (n = 24) toxicities completely resolved after stopping INH while one remained a Grade 1 toxicity. The cumulative incidence was 6.0% (95% CI 3.1–10.2) among those reporting current alcohol use at screening and 12.9% (95% CI 7.0–21.0) among those reporting no prior year alcohol use at screening.
In multivariable modeling of Grade 3+ INH-related toxicities in Model 1, we found no evidence of an association between self-report/PEth alcohol category at baseline and experiencing a Grade 3+ INH-related toxicity in the prior 3 months (P = 0.32; Table 2). All levels of alcohol use were at lower odds of Grade 3+ INH-related toxicity compared with the no-alcohol use group, although these comparisons were not statistically significant. Model 2, which used baseline self-reported alcohol use (alone), also showed no significant association between alcohol use and Grade 3+ INH-related toxicity. Model 3, which used a time-updated self-report/PEth to measure alcohol use, showed an association between alcohol use and Grade 3+ INH-related toxicity (P = 0.04). The adjusted odds ratio (aOR) was 0.10 (95% CI 0.01–0.76) for those in the low-alcohol use group, 0.23 (95% CI 0.06–0.93) for those in the medium-alcohol use group, and 0.73 (95% CI 0.23–2.26) in the high/very high alcohol use group, each compared with the no-alcohol use group. Different from models 1 and 2, model 3 retained INH adherence. In sensitivity analyses that excluded 48 participants in the lowest alcohol group, who had reported ever drinking, the results were similar for Models 1–3 (data not shown).
Table 2.
Final multivariable models of Grade 3+ INH-related toxicity in the prior 3 months.
| Model 1 Baseline alcohol use by self-report/PEth combineda (N = 298) |
Model 2 Baseline alcohol use by self-report aloneb (N = 298) |
Model 3 Time-updated alcohol use by self-report/PEtha (N = 297) |
||||
| aOR (95% CI) | P | aOR (95% CI) | P | aOR (95% CI) | P | |
| Alcohol use | 0.32 | 0.14 | 0.04 | |||
| None | 1.00 | 1.00 | 1.00 | |||
| Low | 0.43 (0.13–1.44) | 0.38 (0.13–1.10) | 0.10 (0.01–0.76) | |||
| Medium | 0.36 (0.10–1.29) | 0.30 (0.09–1.08) | 0.23 (0.06–0.93) | |||
| High/very high | 0.56 (0.16–1.93) | 0.50 (0.12–2.04) | 0.73 (0.23–2.26) | |||
| Age | 0.98 (0.93–1.03) | 0.47 | 0.98 (0.93–1.03) | 0.43 | 0.96 (0.91–1.01) | 0.15 |
| Sex | 0.83 | 0.69 | 0.58 | |||
| Female | 1.00 | 1.00 | 1.00 | |||
| Male | 1.11 (0.41–3.05) | 1.21 (0.47–3.16) | 1.32 (0.49–3.57) | |||
| Health status | 0.39 | 0.41 | 0.24 | |||
| Good/fair/poor | 1.00 | 1.00 | 1.00 | |||
| Excellent/very good | 0.70 (0.31–1.59) | 0.70 (0.31–1.61) | 0.60 (0.26–1.40) | |||
| Study visit | 0.02 | 0.02 | 0.04 | |||
| 3 months | 1.00 | 1.00 | 1.00 | |||
| 6 months | 0.33 (0.13–0.86) | 0.33 (0.13–0.86) | 0.37 (0.15–0.94) | |||
| INH adherence, prior 3 months | 0.08 | |||||
| < 90% | – | – | 1.00 | |||
| ≥ 90% | – | – | 2.79 (0.90–8.61) | |||
Self-report/PEth combined categories assigns the higher of the self-report and PEth categories. Self-report as below. PEth categories: None: PEth <8 ng/ml; Low: PEth 8–<50 ng/ml; Medium: PEth 50–200 ng/ml, High: PEth ≥200 ng/ml.
Self-report categories: None: AUDIT-C = 0; Low: AUDIT-C 1--2 for women and 1–3 for men; Medium: AUDIT-C 3–5 for women and 4--5 for men; High/very high: AUDIT-C ≥6.
Grade 2 INH-related toxicities
The cumulative incidence of a Grade 2 INH-related toxicity (among those without Grade 3 toxicities, n = 276) was 21.7% (60/276, 95% CI 17.0–27.1). Overall, there were 71 Grade 2 INH-related toxicities among 60 persons; 65 of these resolved to normal, while four were Grade 1 and two were Grade 2 at the end of follow-up. The toxicities occurred a median of 55 days after starting the INH course (IQR 29–107). The cumulative incidence was 25.0% (95% CI 19.0–31.8) among those reporting current alcohol use at screening and 14.8% (95% CI 8.1–23.9) among those reporting no prior year alcohol use at screening.
In multivariable regressions, we found evidence of an association between self-report/PEth alcohol use at baseline and experiencing a Grade 2 INH-related toxicity (in the absence of Grade 3+ INH-related toxicities) (Model 4, P < 0.01; Table 3). The aOR of a Grade 2 toxicity was 1.11 (95% CI 0.44–2.76) for those in the low-alcohol use group, 0.69 (95% CI 0.25–1.91) in the medium-alcohol use group, and 3.63 (95% CI: 1.48, 8.93) in the high/very high alcohol use group, compared with those in the no-alcohol use group. There was some attenuation of the alcohol effect when using self-reported alcohol alone in Model 5; the results using time-updated self-report/PEth measured alcohol use (Model 6) were similar to those of Model 4.
Table 3.
Final multivariable models of Grade 2 INH-related toxicity in the prior 3 months (n = 273 participants with no Grade 3+ INH-related toxicities).
| Model 4 Baseline alcohol use by self-report and PEth combineda |
Model 5 Baseline alcohol use by self-report aloneb |
Model 6 Time-updated alcohol use by self-report and PEth combineda |
||||
| aOR (95% CI) | P | aOR (95% CI) | P | aOR (95% CI) | P | |
| Alcohol use | <0.01 | 0.07 | <0.01 | |||
| None | 1.00 | 1.00 | 1.00 | |||
| Low | 1.11 (0.44–2.76) | 1.00 (0.45–2.21) | 1.98 (0.84–4.65) | |||
| Medium | 0.69 (0.25–1.91) | 1.44 (0.60–3.44) | 0.97 (0.36–2.66) | |||
| High/very high | 3.63 (1.48–8.93) | 2.70 (1.12–6.49) | 3.58 (1.51–8.53) | |||
| Age | 0.98 (0.95–1.01) | 0.14 | 0.98 (0.95–1.01) | 0.17 | 0.97 (0.95–1.00) | 0.09 |
| Sex | 0.17 | 0.07 | 0.07 | |||
| Female | 1.00 | 1.00 | 1.00 | |||
| Male | 1.63 (0.81–3.25) | 1.83 (0.96–3.51) | 1.88 (0.96–3.70) | |||
| Health status | <0.01 | 0.07 | 0.03 | |||
| Good/fair/poor | 1.00 | 1.00 | 1.00 | |||
| Excellent/very good | 0.43 (0.23–0.79) | 0.59 (0.33–1.04) | 0.50 (0.27–0.92) | |||
| Study visit | <0.01 | <0.01 | <0.01 | |||
| 3 months | 1.00 | 1.00 | 1.00 | |||
| 6 months | 0.37 (0.20–0.68) | 0.38 (0.21–0.68) | 0.38 (0.21–0.70) | |||
| ART adherence | 0.04 | 0.07 | 0.07 | |||
| Good/fair/poor/very poor | 1.00 | 1.00 | 1.00 | |||
| Excellent/very good | 3.13 (1.06–9.25) | 2.74 (0.93–8.12) | 2.76 (0.94–8.13) | |||
Self-report/PEth combined categories assigns the higher of the self-report and PEth categories. Self-report as below. PEth categories: None: PEth <8 ng/ml; Low: PEth 8–<50 ng/ml; Medium: PEth 50–200 ng/ml, High: PEth ≥200 ng/ml.
Self-report categories: None: AUDIT-C = 0; Low: AUDIT-C 1–2 for women and 1–3 for men; Medium: AUDIT-C 3–5 for women and 4–5 for men; High/very high: AUDIT-C ≥6.
Discussion
We found that the incidence of Grade 3+ INH-related toxicity among PWH with TB infection engaging in current alcohol use, including some with high/very high-risk alcohol use, was 6%. This incidence was similar to that reported in a study of severely immunosuppressed PWH receiving INH (7.3%) [23], and the 7.0% incidence of yellow urine, jaundice, or elevated liver enzyme tests reported in a study of PWH receiving IPT at referral hospitals in Uganda [24]. We did not find that the proportion of Grade 3+ INH-related toxicity increased with the level of alcohol use, and the toxicities resolved with the cessation of INH. These results suggest that concurrent alcohol use, per se, is not a major factor contributing to severe INH-related toxicity. We found lower IPT adherence among those with the highest level of alcohol use in an analysis published elsewhere [25]; however, IPT adherence did not change our findings when considered for inclusion in multivariable models. Taken together with the higher risk of poor TB disease outcomes for persons who consume alcohol [12–14], we suggest that current guidelines be amended to recommend TB preventive therapy for PWH who consume alcohol at any level in the absence of baseline liver enzyme elevations (<2× the ULN). Recent modeling studies show that the benefits of IPT outweigh the risks in this population [26], and updated models of this prior work using these data confirm this is true, even when baseline liver enzyme monitoring is not possible (A. Savinkina, personal communication).
Grade 2 INH-related toxicities that did not progress to more severe toxicities were quite common, and most frequent among those with the highest level of alcohol use. The vast majority of these resolved without stopping INH. It is unknown whether these resolved due to decreases in alcohol use after receiving results indicating liver enzyme elevations and receiving increased monitoring, or whether they resolved on their own, which may suggest that increased monitoring may not be needed.
The generalizability of this work was hindered by the ethical need to conduct frequent monitoring that likely found INH-related toxicities that would not have been detected without the monitoring and inflated our incidence estimate. We also limited the sample to those with baseline liver enzyme elevations of two times or less the ULN for ethical reasons, which may have caused us to underestimate the incidence of INH-related toxicities. This restriction was similar to that of another study conducted in Uganda, which limited INH receipt to those with liver enzymes less than 2.5 times the ULN [23]. Because of this exclusion, we are unable to extend our conclusions to PWH consuming alcohol who have liver damage. However, we note that very few of the persons we screened (1.1%) had Grade 3+ liver enzyme elevations [27]. An additional limitation is that we stopped IPT when we detected INH-related toxicities of Grade 3+; thus, we are unable to comment on what would have happened if IPT was continued. It is possible that the toxicities would have resolved on their own, as most settings do not do monthly liver enzyme monitoring. However, there is real risk for a small number of catastrophic outcomes so stopping treatment was appropriate [28]. In addition, we limited the sample to those on ART for at least 6 months. Although this criterion was needed to be able to attribute toxicities observed to INH, it further limited the generalizability of our findings. Our study tested for HBsAg, but not hepatitis C virus (HCV), based on our prior finding of zero prevalence in 81 PWH starting ART in Uganda, but 4% (3/76) prevalence of HBsAg. Although low HCV prevalence was confirmed in one study [29], others have found higher prevalence [30,31], thus we may have failed to identify this possible source of ALT or AST elevations. Another limitation is that this study was conducted at a single site among persons on ART for at least 6 months, and thus, there was a high proportion with viral suppression, meaning this might not generalize to persons not virally suppressed.
A strength of the study was our intentional sampling of persons who consume alcohol, including many persons with high/very high-risk alcohol use; prior research studied more general populations. In addition, we carefully measured alcohol use by self-report and PEth, an objective biomarker. Using PEth, several participants were re-classified from no alcohol use at screening to current alcohol use at baseline (14/100), and several were categorized to a higher level of alcohol use than when using self-report alone, for example, the highest risk group doubled in size when PEth was added to the definition. Despite this, our conclusions using self-report combined with PEth were similar to those when using self-report alone. Another strength of the study was the frequent monitoring to detect liver enzyme changes, although this may have led to discontinuation of IPT that was not necessary as discussed above.
In conclusion, we found that severe INH-related toxicity among PWH with TB infection and liver enzymes less than 2x the ULN did not appear to be elevated among those who engaged in current alcohol use, compared with those not engaging in alcohol use and compared with the literature. We did not observe a dose--response by level of alcohol use. These data support revising guidelines to drop the recommendation of deferring IPT for this group at a high risk for TB and poor TB outcomes, particularly among those without prior liver enzyme elevations.
Acknowledgements
The authors would like to thank the research staff for their work on this research and the study participants for their time in participating in this study.
J.A.H., K.R.J., W.R.M., J.A., D.M.C., N.I.E. designed the research. D.M.C. led the statistical analyses, which were conducted by R.F. C.N., A.T., and N.I.E. led the data collection. J.A.H. wrote the first draft of the manuscript and all authors contributed to revisions.
Funding for this study was provided by the National Institutes of Health Institute of Alcohol Abuse and Alcoholism U01 AA020776 (Hahn), K24 AA022586 (Hahn), U24 AA020779 (Cheng).
Conflicts of interest
There are no conflicts of interest.
References
- 1.WHO. Global tuberculosis report. Geneva: World Health Organization; 2022. [Google Scholar]
- 2.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev 2010; CD000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edessa D, Likisa J. A description of mortality associated with IPT plus ART compared to ART alone among HIV-infected individuals in Addis Ababa, Ethiopia: a cohort study. PLoS One 2015; 10:e0137492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Group TAS, Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373:808–822. [DOI] [PubMed] [Google Scholar]
- 5.Ayele HT, Mourik MS, Debray TP, Bonten MJ. Isoniazid prophylactic therapy for the prevention of tuberculosis in HIV infected adults: a systematic review and meta-analysis of randomized trials. PLoS One 2015; 10:e0142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golub JE, Pronyk P, Mohapi L, Thsabangu N, Moshabela M, Struthers H, et al. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS (London, England) 2009; 23:631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. WHO consolidated guidelines on tuberculosis: tuberculosis preventive treatment. Geneva: World Health Organization; 2020. [PubMed] [Google Scholar]
- 8.Hahn JA, Page-Shafer K, Lum PJ, Ochoa K, Moss AR. Hepatitis C virus infection and needle exchange use among young injection drug users in San Francisco. Hepatology 2001; 34:180–187. [DOI] [PubMed] [Google Scholar]
- 9.WHO. Global tuberculosis report. Geneva: World Health Organization; 2020. [Google Scholar]
- 10.Necho M, Belete A, Getachew Y. The prevalence and factors associated with alcohol use disorder among people living with HIV/AIDS in Africa: a systematic review and meta-analysis. Subst Abuse Treat Prev Policy 2020; 15:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duko B, Ayalew M, Ayano G. The prevalence of alcohol use disorders among people living with HIV/AIDS: a systematic review and meta-analysis. Subst Abuse Treat Prev Policy 2019; 14:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehm J, Samokhvalov AV, Neuman MG, Room R, Parry C, Lonnroth K, et al. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health 2009; 9:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imtiaz S, Shield KD, Roerecke M, Samokhvalov AV, Lönnroth K, Rehm J. Alcohol consumption as a risk factor for tuberculosis: meta-analyses and burden of disease. Eur Respir J 2017; 50:1700216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simou E, Britton J, Leonardi-Bee J. Alcohol consumption and risk of tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis 2018; 22:1277–1285. [DOI] [PubMed] [Google Scholar]
- 15.Ragan EJ, Kleinman MB, Sweigart B, Gnatienko N, Parry CD, Horsburgh CR, et al. The impact of alcohol use on tuberculosis treatment outcomes: a systematic review and meta-analysis. Int J Tuberc Lung Dis 2020; 24:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badrinath M, John S. Isoniazid toxicity. Treasure Island, FL: StatPearls Publishing LLC.; 2019. [PubMed] [Google Scholar]
- 17.Jones J, Jones M, Plate C, Lewis D. The detection of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanol in human dried blood spots. Anal Methods 2011; 3:1101. [Google Scholar]
- 18.Williams EC, McGinnis KA, Edelman EJ, Matson TE, Gordon AJ, Marshall BDL, et al. Level of alcohol use associated with HIV care continuum targets in a National U.S. sample of persons living with HIV receiving healthcare. AIDS Behav 2019; 23:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubinsky AD, Dawson DA, Williams EC, Kivlahan DR, Bradley KA. AUDIT-C scores as a scaled marker of mean daily drinking, alcohol use disorder severity, and probability of alcohol dependence in a U.S. general population sample of drinkers. Alcohol Clin Exp Res 2013; 37:1380–1390. [DOI] [PubMed] [Google Scholar]
- 20.Hahn JA, Emenyonu NI, Fatch R, Muyindike WR, Kekiibina A, Carrico AW, et al. Declining and rebounding unhealthy alcohol consumption during the first year of HIV care in rural Uganda, using phosphatidylethanol to augment self-report. Addiction 2016; 111:272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luginbühl M, Wurst FM, Stöth F, Weinmann W, Stove CP, Van Uytfanghe K. Consensus for the use of the alcohol biomarker phosphatidylethanol (PEth) for the assessment of abstinence and alcohol consumption in clinical and forensic practice (2022 Consensus of Basel). Drug Test Anal 2022; 14:1800–1802. [DOI] [PubMed] [Google Scholar]
- 22.Bliven-Sizemore EE, Sterling TR, Shang N, Benator D, Schwartzman K, Reves R, et al. Three months of weekly rifapentine plus isoniazid is less hepatotoxic than nine months of daily isoniazid for LTBI. Int J Tuberc Lung Dis 2015; 19:1039–1044. i-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ngongondo M, Miyahara S, Hughes MD, Sun X, Bisson GP, Gupta A, et al. Hepatotoxicity during isoniazid preventive therapy and antiretroviral therapy in people living with HIV with severe immunosuppression: a secondary analysis of a multi-country open-label randomized controlled clinical trial. J Acquir Immune Defic Syndr 2018; 78:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nanyonga SM, Kitutu FE, Kalyango J, Frank M, Kiguba R. High burden of adverse drug reactions to isoniazid preventive therapy in people living with HIV at 3 tertiary hospitals in Uganda: associated factors. J Acquir Immune Defic Syndr 2022; 89:215–221. [DOI] [PubMed] [Google Scholar]
- 25.Muyindike WR, Fatch R, Cheng DM, Emenyonu NI, Forman L, Ngabirano C, et al. Unhealthy alcohol use is associated with suboptimal adherence to isoniazid preventive therapy in persons with HIV in Southwestern Uganda. J Acquir Immune Defic Syndr 2022; 91:460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freiman JM, Jacobson KR, Muyindike WR, Horsburgh CR, Ellner JJ, Hahn JA, et al. Isoniazid preventive therapy for people with HIV who are heavy alcohol drinkers in high TB-/HIV-burden countries: a risk-benefit analysis. J Acquir Immune Defic Syndr 2018; 77:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freiman JM, Fatch R, Cheng D, Emenyonu N, Ngabirano C, Geadas C, et al. Prevalence of elevated liver transaminases and their relationship with alcohol use in people living with HIV on antiretroviral therapy in Uganda. PLoS One 2021; 16:e0250368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol 2008; 23:192–202. [DOI] [PubMed] [Google Scholar]
- 29.Loarec A, Carnimeo V, Molfino L, Kizito W, Muyindike W, Andrieux-Meyer I, et al. Extremely low hepatitis C prevalence among HIV co-infected individuals in four countries in sub-Saharan Africa. AIDS 2019; 33:353–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baseke J, Musenero M, Mayanja-Kizza H. Prevalence of hepatitis B and C and relationship to liver damage in HIV infected patients attending Joint Clinical Research Centre Clinic (JCRC), Kampala, Uganda. Afr Health Sci 2015; 15:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nankya-Mutyoba J, Apica BS, Otekat G, Kyeyune DB, Nakyagaba L, Nabunje J, et al. Hepatitis C in Uganda: identification of infected blood donors for micro-elimination. J Virus Erad 2021; 7:100041. [DOI] [PMC free article] [PubMed] [Google Scholar]