Change history
4/20/2020
The Editors-in-Chief are currently investigating this article [Vahedpoor, Z., Jamilian, M., Bahmani, F. et al. Effects of Long-Term Vitamin D Supplementation on Regression and Metabolic Status of Cervical Intraepithelial Neoplasia: a Randomized, Double-Blind, Placebo-Controlled Trial. HORM CANC 8, 58–67 (2017). https://doi.org/10.1007/s12672-016-0278-x] as concerns have been raised about integrity of the clinical trial reported here. There is also an ongoing investigation by the Iranian National Committee for Ethics in Biomedical Researches. Further editorial action will be taken as appropriate once the investigation into the concerns is complete and all parties have been given an opportunity to respond in full.
Abstract
We are not aware of any study examining the effects of long term vitamin D administration on regression and metabolic status of patients with cervical intraepithelial neoplasia grade 1 (CIN1). This study was performed to evaluate the effects of long-term vitamin D administration on regression and metabolic status of patients with CIN1. This randomized, double-blind, placebo-controlled trial was performed among 58 women diagnosed with CIN1. CIN1 diagnosis was performed based on specific diagnostic procedures of biopsy, pathological diagnosis, and colposcopy. Patients were randomly allocated into two groups to take 50,000 IU vitamin D3 supplements (n = 29) or placebo (n = 29) every 2 weeks for 6 months. Fasting blood samples were taken at the beginning of the study and end-of-trial to measure related markers. After 6 months of vitamin D administration, greater percentage of women in the vitamin D group had regressed CIN1 (84.6 vs. 53.8%, P = 0.01) than those in the placebo group. Long-term vitamin D supplementation increased serum-25(OH) vitamin D levels in the intervention group compared to the placebo group (+12.3 ± 11.4 vs. -0.1 ± 3.7 ng/mL, P < 0.001). In addition, vitamin D intake led to significant decreases in serum insulin levels (−5.3 ± 7.3 vs. +2.4 ± 5.9 μIU/mL, P < 0.001), homeostasis model of assessment-insulin resistance (−1.2 ± 1.6 vs. +0.5 ± 1.2, P < 0.001), homeostatic model assessment-Beta cell function (P = 0.005) and a significant elevation in quantitative insulin sensitivity check index (+0.03 ± 0.04 vs. -0.007 ± 0.02, P < 0.001) compared with the placebo group. Additionally, significant increases in plasma nitric oxide (NO) (+15.5 ± 10.3 vs. +4.0 ± 13.4 μmol/L, P = 0.001), total antioxidant capacity (TAC) (P = 0.04), total glutathione (GSH) (+11.8 ± 153.5 vs. -294.2 ± 595.1 μmol/L, P = 0.01) and a significant reduction in plasma malondialdehyde (MDA) levels (−0.8 ± 1.0 vs. -0.03 ± 1.4 μmol/L, P = 0.03) were observed following the administration of vitamin D supplements compared with the placebo group. In conclusion, vitamin D3 administration for 6 months among women with CIN1 resulted in its regression and had beneficial effects on markers of insulin metabolism, plasma NO, TAC, GSH and MDA levels.
Clinical trial registration number www.irct.ir: IRCT201412065623N30.
Keywords: Vitamin D, Supplementation, CIN1, Regression, Metabolic profiles
Introduction
Human papillomavirus (HPV) is a primary cause for cervical cancer [1] and previous studies have proven HPV vaccine efficacy in preventing cervical intraepithelial neoplasia (CIN) [2]. HPV-induced CIN is a pre-cancerous condition before formation of cervical cancer, usually cervical squamous cell carcinoma (SCC), which is the second most common cancer in women worldwide [3]. A link between metabolic profiles, biomarkers of inflammation, oxidative stress, and CIN has been documented. Increased circulating levels of VLDL-cholesterol in patients with CIN supports the probability of abnormal lipid metabolism in response to tissue injury in CIN [4]. Clinical studies have also shown that the genital tract concentrations of inflammatory factors would result in higher cervicovaginal HPV-1 RNA concentrations in CIN [5].
Data on the relation between nutritional factors especially vitamin D and cervical neoplasia risk are sparse and inconsistent. An inverse association between dietary calcium and vitamin D intake and cervical neoplasia risk was seen among a group of Japanese women [6]. In addition, in a study by Tworoger et al. [7] was reported that vitamin D deficiency is associated with increased risk of ovarian cancer in overweight and obese women. However, in another study conducted by Arslan et al. [8] was not seen any direct association between vitamin D deficiency and risk of ovarian cancer. Moreover, no significant association between vitamin D deficiency and cervical HPV was reported in women with systemic lupus erythematosus [9].
Vitamin D intake may reduce cancer risk through modulating calcium metabolism, inhibiting cellular proliferation, inducing differentiation and apoptosis [10, 11]. However, these findings might suggest the role of vitamin D in the process of malignant transformation and proliferation in cervical carcinogenesis. Therefore, we hypothesized that metabolic profile of CIN patients might be improved by vitamin D supplementation. We are not aware of any study evaluating the effects of vitamin D administration on regression, glucose homeostasis parameters, lipid concentrations, biomarkers of inflammation and oxidative stress in patients with CIN grade 1 (CIN1). The aim of current study was to evaluate the effects of long-term vitamin D supplementation (6 months) on regression and metabolic status of women diagnosed with CIN1.
Materials and Methods
Participants
The study is a randomized double-blind clinical trial conducted in Kashan, Iran, from August 2014 to February 2015. The inclusion criteria were women aged 18–55 years with CIN1 diagnosed by colposcopy, biopsy, and pathological assessment [12]. Women who had abnormal Pap smear test, abnormal cervical cytology, abnormal cervical appearance, postcoital bleeding, intermenstrual bleeding, and chronic vaginal discharge, or were high risk HPV positive were invited for a colposcopy. Exclusion criteria were: a history of cervical cancer or other cancer of the lower genital tract; a history of hysterectomy; and a destructive therapy of the cervix. Pregnant women were also excluded from the study. The current study was conducted according to the guidelines laid down in the Declaration of Helsinki. Written informed consent was obtained from all participants. This trial was approved by the ethics committee of Kashan University of Medical Sciences and has been registered in the Iranian Registry of Clinical Trial (www.irct.ir: IRCT201412065623N30).
Study Design
At the beginning of the study, participants were stratified according their BMI (<30 and ≥30 kg/m2) and age (<35 and ≥35 y), then patients were randomly allocated to receive 50,000 IU vitamin D3 supplements (n = 29) or placebo (n = 29) every 2 weeks for 6 months. We used the blocked randomization method by a trained midwife at maternity clinic. Vitamin D supplements and its placebos were manufactured by Zahravi Pharmaceutical Company (Tabriz, Iran) and Barij Essence Pharmaceutical Company (Kashan, Iran). The appearance of the placebo pearls, including color, shape, size, and packaging, were identical to vitamin D pearls. Women were advised not to change their diet and physical activity during the study and none of them had taken any other nutritional supplements for at least 2 months prior to (or during) this study. Compliance to the vitamin D administration was evaluated through quantification of serum vitamin D concentrations. The use of vitamin D supplements and placebos throughout the study was also controlled through asking patients to bring the medication containers. All patients provided three dietary records (one weekend day and two week days) and three physical activity records at month 2, 4 and 6 of the intervention to make sure that they maintained their usual diet and physical activity during intervention. The dietary records were based on estimated values in household measurements. To obtain nutrient intakes of the participants based on these 3-day food diaries, we used Nutritionist IV software (First Databank, San Bruno, CA) which is a modified version for Iranian food.
Assessment of Variables
Body weight was measured once at the beginning of the study and after 6 months of the intervention at gynecology clinics in an overnight fasting status without shoes in a minimal clothing state using a digital scale (Seca, Hamburg, Germany) by a trained midwife. Height was determined using a non-stretched tape measure (Seca, Hamburg, Germany). BMI was calculated using the height and weight measurements (weight in kg/[height in meters] 2). Fasting blood samples (10 mL) were taken at the first of the study and end-of-trial at Kashan reference laboratory in an early morning after an overnight fast of at least 8 h. Blood samples were immediately centrifuged (Hettich D-78532, Tuttlingen, Germany) at 3500 rpm for 10 min to separate serum. Then, the samples were stored at −80°C until analysis at the KUMS reference laboratory.
Assessment of Primary Outcomes
In the current study, the primary outcome was CIN1 that were determined through colposcopy, cervical biopsy and pathological diagnosis at the study baseline and 6 months after the intervention. To determine the location and extent of CIN, a colposcopy was performed at enrolment for patients who had abnormal Pap smear test, abnormal cervical cytology, abnormal cervical appearance, postcoital bleeding, intermenstrual bleeding, and chronic vaginal discharge or were HPV positive. Colposcopy (Siemens Co, Germany) was conducted with the patient lying back, legs in stirrups, and buttocks at the lower edge of the table (known as the dorsal lithotomy position). A speculum was placed in the vagina after the vulva is examined for any suspicious lesions. Areas of the cervix, which turn dense white after the application of acetic acid or have an abnormal vascular pattern (mosaicism and punctuation), were considered for the biopsy. Specimens were embedded in formalin solution and then sent for the pathological diagnosis. Assessment of the pathological diagnosis was done as blindness by a single experienced pathologist at baseline and 6 months after the intervention.
Secondary Outcomes
The secondary outcomes of the current study were markers of insulin metabolism, lipid profiles, biomarkers of inflammation, and oxidative stress. Serum 25-hydroxyvitamin D concentrations were assessed using a commercial ELISA kit (IDS, Boldon, UK) with inter- and intra-assay CVs ranged from 4.5 to 6.5%. Commercial kits were used to determine the fasting plasma glucose (FPG), serum triglycerides, VLDL-, total-, LDL-, and HDL-cholesterol concentrations (Pars Azmun, Tehran, Iran). All inter- and intra-assay CVs for FPG and lipid concentrations measurements were lower than 5%. Serum insulin concentrations were determined by ELISA kit (Monobind, CA, USA). The homeostatic model of assessment for insulin resistance (HOMA-IR), homeostatic model assessment for β-cell function (HOMA-B) and the quantitative insulin sensitivity check index (QUICKI) were calculated based on suggested formulas [13]. Serum high sensitivity C-reactive protein (hs-CRP) was measured by ELISA kit (LDN, Nordhorn, Germany) with intra- and inter-assay CVs of 2.9 and 4.5%, respectively. Plasma nitrite/nitrate (NOx), taken as an index of nitric oxide (NO) concentrations, was quantified using the Giess method modified by Tatsh et al. [14]. Plasma total antioxidant capacity (TAC) by the FRAP method developed by Benzie and Strain [15], total glutathione (GSH) using the method of Beutler et al. [16] and malondialdehyde (MDA) concentrations by the thiobarbituric acid reactive substance (TBARs) spectrophotometric test [17] were determined.
Sample Size
To determine the sample size, we used a randomized clinical trial sample size formula where type one (α) error was considered as 5% and the study power as 80%. We considered neoplasia as the main outcome of the study; therefore, based on a previous study [18] and considering 20% as the difference in neoplasia between the two groups and the variability of 5%, we needed 24 persons in each group. Assuming a dropout of 5 subjects per group, the final sample size was determined to be 29 subjects per group.
Statistical Methods
Normal distribution of variables was assessed by visual inspection of histograms. To identify between-group differences for non-normally distributed variables, we used Mann-Whitney test. Results of normally distributed variables as mean ± standard deviations (SDs) and non-normally distributed variables (vitamin D, FPG, HOMA-B, hs-CRP and TAC) as median (IQR) were presented. The analyses were conducted in all randomized subjects according to the intention-to-treat (ITT) principle. Missing values were treated based on Last-Observation-Carried-Forward method (LOCF) [19]. LOCF ignores whether the participant’s condition was improving or deteriorating at the time of dropout but instead freezes outcomes at the value observed before dropout (i.e., last observation). We used independent samples Student’s t test to detect mean differences in baseline measures as well as dietary intakes between the two groups. In addition, paired-samples t-test was used to detect within-group differences. Pearson Chi-square test was used for comparison of categorical variables. To determine the effects of vitamin D supplementation on markers of insulin metabolism, lipid concentrations, biomarkers of inflammation and oxidative stress, we used one-way repeated measures analysis of variance. To assess if the magnitude of the change before and after the intervention depended on the baseline values, we adjusted all analyses for the baseline values, including age and baseline BMI, to avoid the potential bias that might have resulted. These analyses were also performed using analysis of covariance (ANCOVA). P < 0.05 was considered as statistically significant. All statistical analyses were done using the Statistical Package for Social Science version 17 (SPSS Inc., Chicago, Illinois, USA).
Results
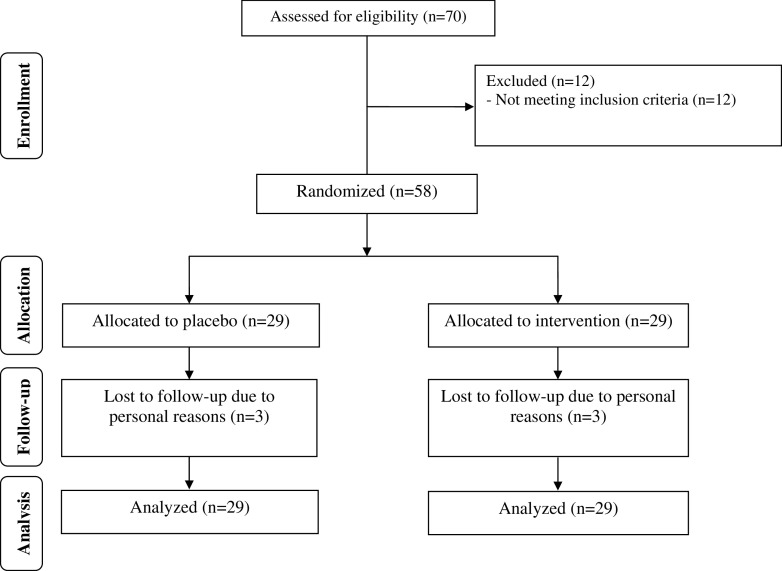
Six patients did not complete the trial [vitamin D (n = 3) and placebo (n = 3)]. Therefore, a total of 52 participants [vitamin D (n = 26) and placebo (n = 26)] completed the trial (Fig. 1). However, as the analysis was done based on ITT principle, all 58 patients were included in the final analysis. On average, the rate of compliance in the current study was high, such that >90% of tablets were taken throughout the study in both groups. No side effects were reported following the administration of vitamin D supplements or placebo throughout the study.
Fig. 1.
Summary of patient flow diagram
Mean age and height of participants were not statistically different between vitamin D and placebo groups. Baseline weight and BMI as well as their means before and after 6 months of intervention were not significantly different between the two groups (Table 1). In addition, after 6 months of vitamin D supplementation, greater percentage of women in the vitamin D group had regressed CIN1 than those in the placebo group (84.6 vs. 53.8%, P = 0.01). Meanwhile, one patient in the placebo group converted to CIN-П.
Table 1.
General characteristics of study participants1
| Placebo group (n = 29) | Vitamin D group (n = 29) | P 2 | |
|---|---|---|---|
| Age (years) | 38.5 ± 8.3 | 36.9 ± 7.4 | 0.43 |
| Height (cm) | 159.5 ± 6.6 | 158.6 ± 4.3 | 0.55 |
| Weight at study baseline (kg) | 75.0 ± 15.9 | 68.1 ± 9.5 | 0.05 |
| Weight at end-of-trial (kg) | 74.9 ± 16.1 | 68.9 ± 9.5 | 0.11 |
| Weight change (kg) | -0.1 ± 1.6 | 0.8 ± 1.4 | 0.02 |
| BMI at study baseline (kg/m2) | 29.5 ± 6.5 | 27.2 ± 4.2 | 0.10 |
| BMI at end-of-trial (kg/m2) | 29.4 ± 6.4 | 27.5 ± 4.2 | 0.16 |
| BMI change (kg/m2) | -0.1 ± 0.6 | 0.3 ± 0.5 | 0.02 |
| CIN1 regression (%) | 14 (53.8) † | 22 (84.6)† | 0.01†† |
1Data are means ± SDs
2Obtained from independent t test
†Analysis was done on 26 patients. Three persons due to withdrawn were excluded
††Obtained from Pearson Chi-square test
Based on the 3-day dietary records obtained throughout the intervention, no significant differences were observed between the two groups in intakes of energy, carbohydrate, protein, fat, saturated fatty acids (SFA), polyunsaturated fatty acids (PUFA), monounsaturated fatty acids (MUFA), cholesterol, total dietary fiber (TDF), vitamin D, calcium and phosphors (Table 2).
Table 2.
Dietary intakes of study participants throughout the study1
| Placebo group (n = 29) | Vitamin D group (n = 29) | P 2 | |
|---|---|---|---|
| Energy (kcal/d) | 2394 ± 227 | 2352 ± 299 | 0.55 |
| Carbohydrates (g/d) | 329.7 ± 39.1 | 328.5 ± 60.6 | 0.92 |
| Protein (g/d) | 85.9 ± 14.7 | 86.4 ± 18.5 | 0.90 |
| Fat (g/d) | 85.0 ± 12.7 | 80.7 ± 16.8 | 0.27 |
| SFA (g/d) | 24.7 ± 4.8 | 25.0 ± 5.6 | 0.81 |
| PUFA (g/d) | 25.5 ± 6.4 | 25.5 ± 7.1 | 0.98 |
| MUFA (g/d) | 23.7 ± 6.9 | 22.6 ± 6.8 | 0.55 |
| Cholesterol (mg/d) | 224.8 ± 121.7 | 194.7 ± 105.1 | 0.31 |
| TDF (g/d) | 19.7 ± 5.2 | 19.2 ± 4.8 | 0.68 |
| Vitamin D (μg/d) | 2.8 ± 0.9 | 2.8 ± 0.8 | 0.93 |
| Calcium (mg/d) | 1141.3 ± 213.6 | 1117.1 ± 175.3 | 0.64 |
| Phosphors (mg/d) | 1159.8 ± 199.2 | 1159.8 ± 207.4 | 0.65 |
1Data are means ± SDs
2Obtained from independent t test
SFA, saturated fatty acid; PUFA, polyunsaturated fatty acid; MUFA, monounsaturated fatty acid; TDF, total dietary fiber
Long-term vitamin D supplementation increased serum-25(OH) vitamin D levels in the intervention group compared to the placebo group (+12.3 ± 11.4 vs. -0.1 ± 3.7 ng/mL, P < 0.001) (Table 3). Compared with the placebo group, women in vitamin D supplementation group had a significant decrease in serum insulin levels (−5.3 ± 7.3 vs. +2.4 ± 5.9 μIU/mL, P < 0.001), HOMA-IR (−1.2 ± 1.6 vs. +0.5 ± 1.2, P < 0.001), HOMA-B (P = 0.005) and a significant elevation in QUICKI score (+0.03 ± 0.04 vs. -0.007 ± 0.02, P < 0.001). Additionally, significant increases in plasma NO (+15.5 ± 10.3 vs. +4.0 ± 13.4 μmol/L, P = 0.001), TAC (P = 0.04), GSH (+11.8 ± 153.5 vs. -294.2 ± 595.1 μmol/L, P = 0.01) and a significant reduction in MDA levels (−0.8 ± 1.0 vs. -0.03 ± 1.4 μmol/L, P = 0.03) were observed following the administration of vitamin D supplements compared with the placebo group. However, analysis was done without ITT approach and no significant change was seen in our findings.
Table 3.
Metabolic profiles, biomarkers of inflammation and oxidative stress at study baseline and after 6 months intervention in women with CIN1 that received either vitamin D supplements or placebo1
| Placebo group (n = 29) | Vitamin D group (n = 29) | P 2 | |||||
|---|---|---|---|---|---|---|---|
| Baseline | End-of-trial | Change | Baseline | End-of-trial | Change | ||
| Vitamin D (ng/mL) | 11.2 (14.7) | 10.5 (13.3) | -0.8 (2.7) | 10.8 (13.5) | 26.8 (29.1)* | 14.3 (17.3) | <0.001† |
| FPG (mg/dL) | 85.0 (88.5) | 84.0 (100.0) | 0 (13.0) | 85.0 (91.0) | 80.0 (84.0) | -4.0 (0) | 0.08† |
| Insulin (μIU/mL) | 12.2 ± 4.1 | 14.6 ± 7.0* | 2.4 ± 5.9 | 13.5 ± 6.5 | 8.2 ± 4.6* | -5.3 ± 7.3 | <0.001 |
| HOMA-IR | 2.6 ± 1.1 | 3.1 ± 1.5* | 0.5 ± 1.2 | 2.9 ± 1.4 | 1.7 ± 1.0* | -1.2 ± 1.6 | <0.001 |
| HOMA-B | 48.9 (58.1) | 47.8 (81.1) | 0 (36.4) | 50.3 (66.0) | 30.7 (51.2)* | -13.2 (0.6) | 0.005† |
| QUICKI | 0.33 ± 0.02 | 0.32 ± 0.02 | -0.007 ± 0.02 | 0.33 ± 0.02 | 0.36 ± 0.04* | 0.03 ± 0.04 | <0.001 |
| Triglycerides (mg/dL) | 106.2 ± 59.0 | 118.5 ± 52.8 | 12.3 ± 41.7 | 115.6 ± 50.3 | 108.6 ± 51.6 | -7.0 ± 32.1 | 0.05 |
| VLDL-cholesterol (mg/dL) | 21.2 ± 11.8 | 23.7 ± 10.5 | 2.5 ± 8.3 | 23.1 ± 10.1 | 21.7 ± 10.3 | -1.4 ± 6.4 | 0.05 |
| Total cholesterol (mg/dL) | 174.7 ± 30.3 | 174.3 ± 22.9 | -0.4 ± 21.4 | 170.1 ± 34.6 | 173.2 ± 31.9 | 3.1 ± 20.4 | 0.52 |
| LDL-cholesterol (mg/dL) | 95.0 ± 19.6 | 92.9 ± 19.2 | -2.1 ± 16.1 | 91.3 ± 25.8 | 95.8 ± 27.3 | 4.5 ± 18.3 | 0.14 |
| HDL-cholesterol (mg/dL) | 58.4 ± 9.6 | 57.7 ± 8.0 | -0.7 ± 6.9 | 55.7 ± 7.6 | 55.7 ± 7.0 | 0.0 ± 5.3 | 0.66 |
| hs-CRP (ng/mL) | 1587.0 (4290.0) | 1641.0 (4290.0) | 0 (618.5) | 1534.0 (3507.0) | 1957.0 (3716.0) | 320.1 (864.8) | 0.28† |
| NO (μmol/L) | 52.2 ± 7.2 | 56.2 ± 12.6 | 4.0 ± 13.4 | 43.5 ± 9.4 | 59.0 ± 10.7* | 15.5 ± 10.3 | 0.001 |
| TAC (mmol/L) | 768.6 (883.9) | 788.7 (956.6) | 6.2 (44.1) | 879.4 (1177.1) | 1150.0 (1320.7)* | 50.0 (339.5) | 0.04† |
| GSH (μmol/L) | 827.7 ± 598.9 | 533.5 ± 83.9* | -294.2 ± 595.1 | 769.4 ± 246.2 | 781.2 ± 229.1 | 11.8 ± 153.5 | 0.01 |
| MDA (μmol/L) | 6.0 ± 2.1 | 6.0 ± 2.3 | -0.03 ± 1.4 | 4.8 ± 1.4 | 4.0 ± 1.1* | -0.8 ± 1.0 | 0.03 |
1Values are means ± SDs for normally distributed variables and median (IQR) for non-normally distributed variables
2Obtained from repeated measures ANOVA test
†Obtained from Mann-Whitney test
FPG, fasting plasma glucose; GSH, total glutathione; HOMA-IR, homeostasis model of assessment-estimated insulin resistance; HOMA-B, homeostasis model of assessment-estimated B cell function; hs-CRP, high-sensitivity C-reactive protein; MDA, malondialdehyde; NO, nitric oxide; QUICKI, quantitative insulin sensitivity check index; TAC, total antioxidant capacity
*Different from study baseline, P < 0.05
There was a significant difference in the baseline levels of MDA (P = 0.01) between the two groups. Therefore, we adjusted the analysis for baseline values of biochemical parameters, age, baseline BMI, and baseline levels of insulin, VLDL-cholesterol, hs-CRP and TAC. When we adjusted the analyses for baseline values of biochemical variables, there was no significant change in our findings (data not shown). In addition, when we adjusted the analysis for baseline values of biochemical parameters, age, and baseline BMI, findings did not alter (Table 4). When we adjusted the analysis for baseline values of biochemical parameters, age and baseline BMI, and baseline levels of insulin, VLDL-cholesterol, hs-CRP, and TAC, plasma NO (P = 0.17) and MDA levels (P = 0.13) became non-significant, while FPG (P = 0.04), triglycerides (P = 0.04), and VLDL-cholesterol (P = 0.04) became statistically significant, and other findings did not alter.
Table 4.
Adjusted changes in metabolic variables in women with CIN1 that received either vitamin D supplements or placebo1
| Placebo group (n = 29) | Vitamin D group (n = 29) | P 2 | |
|---|---|---|---|
| Vitamin D (ng/mL) | |||
| Model 1† | -0.3 ± 1.1 | 12.5 ± 1.1 | <0.001 |
| Model 2†† | -0.6 ± 1.2 | 12.8 ± 1.2 | <0.001 |
| FPG (mg/dL) | |||
| Model 1 | 1.8 ± 1.9 | -3.5 ± 1.9 | 0.06 |
| Model 2 | 2.2 ± 1.9 | -3.9 ± 1.9 | 0.04 |
| Insulin (μIU/mL) | |||
| Model 1 | 2.1 ± 1.1 | -5.0 ± 1.1 | <0.001 |
| Model 2 | 2.0 ± 1.2 | -5.0 ± 1.2 | <0.001 |
| HOMA-IR | |||
| Model 1 | 0.4 ± 0.2 | -1.1 ± 0.2 | <0.001 |
| Model 2 | 0.5 ± 0.2 | -1.1 ± 0.2 | <0.001 |
| HOMA-B | |||
| Model 1 | 8.8 ± 5.0 | -20.6 ± 5.0 | <0.001 |
| Model 2 | 8.5 ± 5.3 | -20.3 ± 5.3 | 0.001 |
| QUICKI | |||
| Model 1 | -0.007 ± 0.006 | 0.03 ± 0.006 | <0.001 |
| Model 2 | -0.009 ± 0.007 | 0.03 ± 0.007 | <0.001 |
| Triglycerides (mg/dL) | |||
| Model 1 | 10.9 ± 6.7 | -5.6 ± 6.7 | 0.09 |
| Model 2 | 13.7 ± 7.0 | -8.5 ± 7.0 | 0.04 |
| VLDL-cholesterol (mg/dL) | |||
| Model 1 | 2.2 ± 1.3 | -1.1 ± 1.3 | 0.09 |
| Model 2 | 2.7 ± 1.4 | -1.7 ± 1.4 | 0.04 |
| Total cholesterol (mg/dL) | |||
| Model 1 | 0.1 ± 3.4 | 2.7 ± 3.4 | 0.58 |
| Model 2 | 1.1 ± 3.6 | 1.7 ± 3.6 | 0.91 |
| LDL-cholesterol (mg/dL) | |||
| Model 1 | -1.9 ± 3.1 | 4.4 ± 3.1 | 0.16 |
| Model 2 | -1.2 ± 3.3 | 3.7 ± 3.3 | 0.33 |
| HDL-cholesterol (mg/dL) | |||
| Model 1 | -0.006 ± 1.0 | -0.7 ± 1.0 | 0.62 |
| Model 2 | -0.5 ± 1.0 | -0.2 ± 1.0 | 0.88 |
| hs-CRP (ng/mL) | |||
| Model 1 | -143.2 ± 328.5 | 261.7 ± 328.5 | 0.39 |
| Model 2 | -61.5 ± 356.5 | 179.9 ± 356.5 | 0.65 |
| NO (μmol/L) | |||
| Model 1 | 6.1 ± 2.2 | 13.4 ± 2.2 | 0.03 |
| Model 2 | 7.1 ± 2.4 | 12.4 ± 2.4 | 0.17 |
| TAC (mmol/L) | |||
| Model 1 | 16.8 ± 35.9 | 183.6 ± 35.9 | 0.003 |
| Model 2 | 3.7 ± 35.0 | 204.1 ± 35.1 | <0.001 |
| GSH (μmol/L) | |||
| Model 1 | -272.9 ± 31.3 | -9.5 ± 31.3 | <0.001 |
| Model 2 | -265.2 ± 32.4 | -17.1 ± 32.4 | <0.001 |
| MDA (μmol/L) | |||
| Model 1 | 0.003 ± 0.2 | -0.8 ± 0.2 | 0.01 |
| Model 2 | -0.1 ± 0.2 | -0.7 ± 0.2 | 0.13 |
1All values are means ± SEs
2Obtained from ANCOVA test
†Adjusted for baseline values of biochemical variables, age and baseline BMI
††Adjusted for baseline values of biochemical variables, age, baseline BMI, and baseline levels of insulin, VLDL-cholesterol, hs-CRP and TAC
FPG, fasting plasma glucose; GSH, total glutathione; HOMA-IR, homeostasis model of assessment-estimated insulin resistance; HOMA-B, homeostasis model of assessment-estimated B cell function; hs-CRP, high-sensitivity C-reactive protein; MDA, malondialdehyde; NO, nitric oxide; QUICKI, quantitative insulin sensitivity check index; TAC, total antioxidant capacity
Discussion
To our knowledge, the effects of vitamin D supplementation on CIN have not been evaluated previously. The present study for the first time demonstrated that in patients with CIN1 taking one dose of 50,000 IU vitamin D supplement every 2 weeks for 6 months compared with the placebo group resulted in (1) a higher regression; (2) decreased markers of insulin metabolism; and (3) improved biomarkers of inflammation and oxidative stress such as NO, TAC, GSH, and MDA.
Patients with CIN are susceptible to cervical cancer in a linear fashion [20]. Previous epidemiological studies have shown that vitamin D has various anticancer effects [21, 22]; however, the biological mechanism of vitamin D in cancer cells remains poorly understood. Vitamin D is metabolized to 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], which regulates cell growth and differentiation, and immune system in various tissues [23, 24]. Moreover, in two studies by Friedrich et al. [25, 26], it was demonstrated that messenger RNA and protein expression of vitamin D receptor and vitamin D activating enzyme were increased in cervical cancer tissue compared with normal tissues. In another study by Schulte-Uebbing et al. [27] was seen that treatment with vitamin D vaginal suppositories (12,500 IU, three nights a week, for 6 weeks) resulted in antidysplastic effects in the CIN 1 group, but did not affect in the CIN 2 group.
We found that vitamin D administration in women with CIN led to significant reductions in serum insulin concentrations, HOMA-IR, HOMA-B and a significant rise in QUICKI score, but had no effects on FPG and lipid concentrations. Supporting our findings, significant decrease in markers of insulin metabolism were seen with oral vitamin D supplementation at 1250 μg/week for 8 weeks among adult males with obesity or normal weight [28] and following the intake of 100,000 IU vitamin D3 among women with gestational diabetes mellitus (GDM) for 6 weeks [29]. Similarly, our previous study among pregnant women at risk of pre-eclampsia showed a significant difference in markers of insulin metabolism following the administration of 50,000 IU vitamin D supplements every 2 weeks for 12 weeks [30]. In another study supplementation with 150,000 IU vitamin D every 3 months for 24 weeks did not alter lipid profiles in overweight and obese youth [31]. However, some other studies did not report such favorable effects of vitamin D supplementation on glucose homeostasis parameters. For example, vitamin D supplementation with 400 IU or 2000 IU per day for 12 weeks did not affect β-cell function or insulin function in obese nondiabetic adolescents with relatively good vitamin D status [32]. Furthermore, similar findings were observed among patients with type 2 diabetes mellitus (T2DM) after 24 weeks of intervention [33] and after 6 months of [34] vitamin D supplementation. The anti-inflammatory activity of vitamin D [35] and increased expression of the insulin receptor and/or proteins of the insulin-signaling cascade by vitamin D [36] may result in an important role in insulin secretion and sensitivity. The absent of significant change of vitamin D supplementation on lipid concentrations in our study might be mediated by distinct trial designs, various dosages of vitamin D supplements, and characteristics of the participants.
The current study demonstrated that the administration of vitamin D supplementation in women with CIN resulted in a significant increase in plasma NO levels compared with the placebo, but did not influence serum hs-CRP levels. In accordance with our study, another study [35] also showed that NO production notably decreased with decreasing 25(OH)D plasma concentration in patients with osteoarthritis. Moreover, vitamin D3 supplementation with doses of 1000, 2000, or 4000 IU/day orally for 3 months did not show any significant change in CRP concentrations [37]. However, a 6-week supplementation with 1000 mg calcium per day and 50,000 IU vitamin D3 pearl two times during the study (at study baseline and day 21 of intervention) did not alter hs-CRP and NO concentrations among patients with GDM [38]. Recent studies have reported that impaired generation and signaling of NO contribute significantly to cardiovascular risk associated with hypertension and hyperlipidemia [39]. Several studies have shown increased inflammatory factors associated with increased risk of cervical cancer development [40, 41]. Therefore, vitamin D due to their anti-inflammatory and anti-oxidative actions may be useful to decrease the progression of CIN to cervical cancer. Accurate explanation to the beneficial effects of vitamin D supplements on NO levels cannot be provided, but it seems that vitamin D may affect NO production through vitamin D receptor (VDR). In a study by Abu El Maaty et al. [42] was reported that VDR mutant mice resulted in lower bioavailability of the vasodilator NO due to reduced expression of the key NO synthesizing enzyme and endothelial NO synthase.
The present study showed that taking vitamin D supplements for 6 months was associated with a significant rise in plasma TAC, GSH and a significant reduction in MDA concentrations in women with CIN compared with the placebo. In our previous study, vitamin D and calcium supplementation for 6 weeks prevented a rise in MDA levels among women with GDM [38]. Another trail l also showed a non-significant reduction in MDA levels after vitamin D supplementation for 6 weeks among patients with T2DM [43]. Supplementation with 50,000 IU vitamin D3 every 14 days for 4 months among adult patients with non-alcoholic fatty liver disease resulted in amelioration in MDA concentrations but did not affect TAC concentrations [44]. Increased bioavailability of reactive oxygen species and free radicals has been implicated in progression of diabetes mellitus and cardiovascular complications [45]. Oxidative stress could have an important key during the progression of neoplasias [46]. Lipid peroxidation and reducing levels of antioxidants were shown to be increased in patients with high-grade squamous intraepithelial lesion or invasive cervical cancer [47]. The increased production of oxidant compounds in CIN may be involved in angiogenesis, leading to tumor growth and dissemination [48]. Vitamin D intake may reduce oxidative stress and lipid peroxidation by induced superoxide dismutase activity [49] and upregulation of antioxidant systems including glutathione peroxidase via its nuclear receptors [50].
The current study had few strengths. First of all, we focused on interesting questions using a randomized, double-blind, placebo-controlled trial. The findings of increased regression in the vitamin D group are intriguing, but need to be confirmed in a larger study. In addition to looking at the primary endpoint (regression of CIN1), we also evaluate the metabolic status of these patients and found that there was an improvement in glucose homeostasis among other metabolic parameters. To interpret our findings, some limitations need to be taken into account. We were unable to assess the effects of vitamin D administration on HPV. Furthermore, due to limited funding, we did not examine the effect of supplementation on signaling pathway involved in CIN. It must be kept in mind that in the current study, at the onset of the study, participants were stratified according their BMI (<30 and ≥30 kg/m2) and age (<35 and ≥35 y). As increased abnormal lipid metabolism may be present in patients with CIN1, stratification of participants based on baseline circulating VLDL-cholesterol levels is required. Therefore, this should be taken into account in the interpretation of our findings. In addition, stratification of participants based on baseline circulating VLDL-cholesterol levels is suggested in future studies.
In conclusion, vitamin D3 supplementation for 6 months among women with CIN1 resulted in its regression and had beneficial effects on glucose homeostasis parameters, plasma levels of NO and MDA.
Acknowledgements
The present study was supported by a grant from the Vice-chancellor for Research, KUMS, and Iran. The authors would like to thank the staff of Naghavi Clinic (Kashan, Iran) for their assistance in this project and all women who were participated in this study.
Authors’ Contributions
ZA contributed in the conception, design, statistical analysis, and drafting of the manuscript. ZV, MJ, FB, EA, and MK contributed in the conception, data collection, and manuscript drafting. All authors approved the final version for submission.
Compliance with Ethical Standards
Conflicts of Interest
None.
References
- 1.Kukimoto I, Mori S, Aoyama S, Wakae K, Muramatsu M, Kondo K. Hypermutation in the E2 gene of human papillomavirus type 16 in cervical intraepithelial neoplasia. J Med Virol. 2015;87:1754–1760. doi: 10.1002/jmv.24215. [DOI] [PubMed] [Google Scholar]
- 2.Kumar S, Biswas M, Jose T. HPV vaccine: current status and future directions. Med J Armed Forces India. 2015;71:171–177. doi: 10.1016/j.mjafi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castellsague X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol. 2008;110:S4–S7. doi: 10.1016/j.ygyno.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 4.Hasim A, Ali M, Mamtimin B, Ma JQ, Li QZ, Abudula A. Metabonomic signature analysis of cervical carcinoma and precancerous lesions in women by (1)H NMR spectroscopy. Exp Ther Med. 2012;3:945–951. doi: 10.3892/etm.2012.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell C, Hitti J, Paul K, Agnew K, Cohn SE, Luque AE, Coombs R. Cervicovaginal shedding of HIV type 1 is related to genital tract inflammation independent of changes in vaginal microbiota. AIDS Res Hum Retrovir. 2011;27:35–39. doi: 10.1089/aid.2010.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosono S, Matsuo K, Kajiyama H, Hirose K, Suzuki T, Kawase T, Kidokoro K, Nakanishi T, Hamajima N, Kikkawa F, Tajima K, Tanaka H. Association between dietary calcium and vitamin D intake and cervical carcinogenesis among Japanese women. Eur J Clin Nutr. 2010;64:400–409. doi: 10.1038/ejcn.2010.28. [DOI] [PubMed] [Google Scholar]
- 7.Tworoger SS, Lee IM, Buring JE, Rosner B, Hollis BW, Hankinson SE. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of incident ovarian cancer. Cancer Epidemiol Biomark Prev. 2007;16:783–788. doi: 10.1158/1055-9965.EPI-06-0981. [DOI] [PubMed] [Google Scholar]
- 8.Arslan AA, Clendenen TV, Koenig KL, Hultdin J, Enquist K, Agren A, Lukanova A, Sjodin H, Zeleniuch-Jacquotte A, Shore RE, Hallmans G, Toniolo P, Lundin E. Circulating vitamin d and risk of epithelial ovarian cancer. J Oncol. 2009;2009:672492. doi: 10.1155/2009/672492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Carrasco M, Mendoza-Pinto C, Munguia-Realpozo P, Rodriguez-Gallegos A, Vallejo-Ruiz V, Munoz-Guarneros M, Mendez-Martinez S, Soto-Santillan P, Pezzat-Said E, Reyes-Leyva J, López-Colombo A, Ruiz-Argüelles A, Cervera R. Lack of association between serum 25-hydroxyvitamin D levels and cervical human papillomavirus infection in systemic lupus erythematosus. Lupus. 2015;24:606–612. doi: 10.1177/0961203314559628. [DOI] [PubMed] [Google Scholar]
- 10.Miettinen S, Ahonen MH, Lou YR, Manninen T, Tuohimaa P, Syvala H, Ylikomi T. Role of 24-hydroxylase in vitamin D3 growth response of OVCAR-3 ovarian cancer cells. Int J Cancer. 2004;108:367–373. doi: 10.1002/ijc.11520. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler RG, Jones CJ, Brinton LA, Norman SA, Mallin K, Levine RS, Lehman HF, Hamman RF, Trumble AC, Rosenthal JF. Diet and the risk of in situ cervical cancer among white women in the United States. Cancer Causes Control. 1991;2:17–29. doi: 10.1007/BF00052357. [DOI] [PubMed] [Google Scholar]
- 12.Jin Y, Li JP, He D, Tang LY, Zee CS, Guo SZ, Zhou J, Chen JN, Shao CK. Clinical significance of human telomerase RNA gene (hTERC) amplification in cervical squamous cell lesions detected by fluorescence in situ hybridization. Asian Pac J Cancer Prev. 2011;12:1167–1171. [PubMed] [Google Scholar]
- 13.Pisprasert V, Ingram KH, Lopez-Davila MF, Munoz AJ, Garvey WT. Limitations in the use of indices using glucose and insulin levels to predict insulin sensitivity: impact of race and gender and superiority of the indices derived from oral glucose tolerance test in African Americans. Diabetes Care. 2013;36:845–853. doi: 10.2337/dc12-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatsch E, Bochi GV, Pereira Rda S, Kober H, Agertt VA, de Campos MM, Gomes P, Duarte MM, Moresco RN. A simple and inexpensive automated technique for measurement of serum nitrite/nitrate. Clin Biochem. 2011;44:348–350. doi: 10.1016/j.clinbiochem.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 16.Beutler E, Gelbart T. Plasma glutathione in health and in patients with malignant disease. J Lab Clin Med. 1985;105:581–584. [PubMed] [Google Scholar]
- 17.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 18.Karamali M, Nourgostar S, Zamani A, Vahedpoor Z, Asemi Z. The favourable effects of long-term selenium supplementation on regression of cervical tissues and metabolic profiles of patients with cervical intraepithelial neoplasia: a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2015;114:2039–2045. doi: 10.1017/S0007114515003852. [DOI] [PubMed] [Google Scholar]
- 19.Lachin JM. Fallacies of last observation carried forward analyses. Clin Trials. 2016;13:161–168. doi: 10.1177/1740774515602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agorastos T, Miliaras D, Lambropoulos AF, Chrisafi S, Kotsis A, Manthos A, Bontis J. Detection and typing of human papillomavirus DNA in uterine cervices with coexistent grade I and grade III intraepithelial neoplasia: biologic progression or independent lesions? Eur J Obstet Gynecol Reprod Biol. 2005;121:99–103. doi: 10.1016/j.ejogrb.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Cui Y, Rohan TE. Vitamin D, calcium, and breast cancer risk: a review. Cancer Epidemiol Biomark Prev. 2006;15:1427–1437. doi: 10.1158/1055-9965.EPI-06-0075. [DOI] [PubMed] [Google Scholar]
- 22.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 23.Holick MF. Evolution and function of vitamin D. Recent Results Cancer Res. 2003;164:3–28. doi: 10.1007/978-3-642-55580-0_1. [DOI] [PubMed] [Google Scholar]
- 24.Reichrath J. Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol. 2007;16:618–625. doi: 10.1111/j.1600-0625.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- 25.Friedrich M, Villena-Heinsen C, Axt-Fliedner R, Meyberg R, Tilgen W, Schmidt W, Reichrath J. Analysis of 25-hydroxyvitamin D3-1alpha-hydroxylase in cervical tissue. Anticancer Res. 2002;22:183–186. [PubMed] [Google Scholar]
- 26.Friedrich M, Rafi L, Mitschele T, Tilgen W, Schmidt W, Reichrath J. Analysis of the vitamin D system in cervical carcinomas, breast cancer and ovarian cancer. Recent Results Cancer Res. 2003;164:239–246. doi: 10.1007/978-3-642-55580-0_17. [DOI] [PubMed] [Google Scholar]
- 27.Schulte-Uebbing C, Schlett S, Craiut I, Antal L, Olah H. Chronical cervical infections and dysplasia (CIN I, CIN II): vaginal vitamin D (high dose) treatment: a new effective method? Dermatoendocrinol. 2014;6:e27791. doi: 10.4161/derm.27791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou JC, Zhu YM, Chen Z, Mo JL, Xie FZ, Wen YH, Guo P, Peng J, Xu J, Wang J, Liu XL. Oral vitamin D supplementation has a lower bioavailability and reduces hypersecretion of parathyroid hormone and insulin resistance in obese Chinese males. Public Health Nutr. 2015;18:2211–2219. doi: 10.1017/S1368980014002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asemi Z, Hashemi T, Karamali M, Samimi M, Esmaillzadeh A. Effects of vitamin D supplementation on glucose metabolism, lipid concentrations, inflammation, and oxidative stress in gestational diabetes: a double-blind randomized controlled clinical trial. Am J Clin Nutr. 2013;98:1425–1432. doi: 10.3945/ajcn.113.072785. [DOI] [PubMed] [Google Scholar]
- 30.Karamali M, Beihaghi E, Mohammadi AA, Asemi Z. Effects of high-dose vitamin D supplementation on metabolic status and pregnancy outcomes in pregnant women at risk for pre-eclampsia. Horm Metab Res. 2015;47:867–872. doi: 10.1055/s-0035-1548835. [DOI] [PubMed] [Google Scholar]
- 31.Shah S, Wilson DM, Bachrach LK. Large doses of vitamin D fail to increase 25-hydroxyvitamin D levels or to Alter cardiovascular risk factors in obese adolescents: a pilot study. J Adolesc Health. 2015;57:19–23. doi: 10.1016/j.jadohealth.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Javed A, Vella A, Balagopal PB, Fischer PR, Weaver AL, Piccinini F, Dalla Man C, Cobelli C, Giesler PD, Laugen JM, Kumar S. Cholecalciferol supplementation does not influence beta-cell function and insulin action in obese adolescents: a prospective double-blind randomized trial. J Nutr. 2015;145:284–290. doi: 10.3945/jn.114.202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryu OH, Chung W, Lee S, Hong KS, Choi MG, Yoo HJ. The effect of high-dose vitamin D supplementation on insulin resistance and arterial stiffness in patients with type 2 diabetes. Korean J Intern Med. 2014;29:620–629. doi: 10.3904/kjim.2014.29.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strobel F, Reusch J, Penna-Martinez M, Ramos-Lopez E, Klahold E, Klepzig C, Wehrle J, Kahles H, Badenhoop K. Effect of a randomised controlled vitamin D trial on insulin resistance and glucose metabolism in patients with type 2 diabetes mellitus. Horm Metab Res. 2014;46:54–58. doi: 10.1055/s-0033-1358453. [DOI] [PubMed] [Google Scholar]
- 35.Al-Sofiani ME, Jammah A, Racz M, Khawaja RA, Hasanato R, El-Fawal HA, Mousa SA, Mason DL. Effect of vitamin D supplementation on glucose control and inflammatory response in type II diabetes: a double blind, randomized clinical trial. Int J Endocrinol Metab. 2015;13:e22604. doi: 10.5812/ijem.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in south Asian women living in New Zealand who are insulin resistant and vitamin D deficient—a randomised, placebo-controlled trial. Br J Nutr. 2010;103:549–555. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 37.Chandler PD, Scott JB, Drake BF, Ng K, Manson JE, Rifai N, Chan AT, Bennett GG, Hollis BW, Giovannucci EL, Emmons KM, Fuchs CS. Impact of vitamin D supplementation on inflammatory markers in African Americans: results of a four-arm, randomized, placebo-controlled trial. Cancer Prev Res (Phila) 2014;7:218–225. doi: 10.1158/1940-6207.CAPR-13-0338-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asemi Z, Karamali M, Esmaillzadeh A. Effects of calcium-vitamin D co-supplementation on glycaemic control, inflammation and oxidative stress in gestational diabetes: a randomised placebo-controlled trial. Diabetologia. 2014;57:1798–1806. doi: 10.1007/s00125-014-3293-x. [DOI] [PubMed] [Google Scholar]
- 39.Sverdlov AL, Ngo DT, Chan WP, Chirkov YY, Horowitz JD. Aging of the nitric oxide system: are we as old as our NO? J Am Heart Assoc. 2014;18:3. doi: 10.1161/JAHA.114.000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sousa H, Oliveira S, Santos AM, Catarino R, Moutinho J, Medeiros R. Tumour necrosis factor alpha 308 G/a is a risk marker for the progression from high-grade lesions to invasive cervical cancer. Tumour Biol. 2014;35:2561–2564. doi: 10.1007/s13277-013-1337-3. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Ma K, Wang Z, Mou Y, Ma L, Guo Y. Association between tumor necrosis factor alpha rs1800629 polymorphism and risk of cervical cancer. Int J Clin Exp Med. 2015;8:2108–2117. [PMC free article] [PubMed] [Google Scholar]
- 42.Abu El Maaty MA, Hanafi RS, El-Badawy S, Gad MZ. Interplay of vitamin D and nitric oxide in post-menopausal knee osteoarthritis. Aging Clin Exp Res. 2014;26:363–368. doi: 10.1007/s40520-013-0192-9. [DOI] [PubMed] [Google Scholar]
- 43.Eftekhari MH, Akbarzadeh M, Dabbaghmanesh MH, Hassanzadeh J. The effect of calcitriol on lipid profile and oxidative stress in hyperlipidemic patients with type 2 diabetes mellitus. ARYA Atheroscler. 2014;10:82–88. [PMC free article] [PubMed] [Google Scholar]
- 44.Sharifi N, Amani R, Hajiani E, Cheraghian B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non-alcoholic fatty liver disease? A randomized clinical trial. Endocrine. 2014;47:70–80. doi: 10.1007/s12020-014-0336-5. [DOI] [PubMed] [Google Scholar]
- 45.Sedeek M, Montezano AC, Hebert RL, Gray SP, Di Marco E, Jha JC, Cooper ME, Jandeleit-Dahm K, Schiffrin EL, Wilkinson-Berka JL, Touyz RM. Oxidative stress, Nox isoforms and complications of diabetes--potential targets for novel therapies. J Cardiovasc Transl Res. 2012;5:509–518. doi: 10.1007/s12265-012-9387-2. [DOI] [PubMed] [Google Scholar]
- 46.Roessner A, Kuester D, Malfertheiner P, Schneider-Stock R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol Res Pract. 2008;204:511–524. doi: 10.1016/j.prp.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Kim YT, Kim JW, Choi JS, Kim SH, Choi EK, Cho NH. Relation between deranged antioxidant system and cervical neoplasia. Int J Gynecol Cancer. 2004;14:889–895. doi: 10.1111/j.1048-891X.2004.14526.x. [DOI] [PubMed] [Google Scholar]
- 48.Noonan DM, Benelli R, Albini A. Angiogenesis and cancer prevention: a vision. Recent Results Cancer Res. 2007;174:219–224. doi: 10.1007/978-3-540-37696-5_19. [DOI] [PubMed] [Google Scholar]
- 49.Somjen D, Katzburg S, Grafi-Cohen M, Knoll E, Sharon O, Posner GH. Vitamin D metabolites and analogs induce lipoxygenase mRNA expression and activity as well as reactive oxygen species (ROS) production in human bone cell line. J Steroid Biochem Mol Biol. 2011;123:85–89. doi: 10.1016/j.jsbmb.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Hamden K, Carreau S, Jamoussi K, Miladi S, Lajmi S, Aloulou D, Ayadi F, Elfeki A. 1Alpha,25 dihydroxyvitamin D3: therapeutic and preventive effects against oxidative stress, hepatic, pancreatic and renal injury in alloxan-induced diabetes in rats. J Nutr Sci Vitaminol (Tokyo) 2009;55:215–222. doi: 10.3177/jnsv.55.215. [DOI] [PubMed] [Google Scholar]