Abstract
20-Hydroxyeicosatetraenoic acid (20-HETE) is generated intracellularly through the ω-hydroxylation of arachidonic acid by the cytochrome P450 (in humans, CYP4A11 and CYP4F2). 20-HETE induces mitogenic responses in different cancer cells. The aim of this study was to analyze how 20-HETE impacts cell survival, proliferation, and apoptosis in prostate cancer cells. Incubation of the human androgen-sensitive cells (LNCaP) with 1–10 μM HET0016 (a selective inhibitor of 20-HETE synthesis) reduced cell viability by 49*–64%* (*p < 0.05 vs. control). This was explained by a reduction in cell proliferation (vehicle, 46 ± 3%; 1 μM, 23 ± 3%*; 10 μM, 28 ± 3%*) and by an increase in apoptosis (vehicle, 2.1 ± 0%; 1 μM, 16 ± 4%*; 10 μM, 31 ± 3%*). Furthermore, the increase in LNCaP cell viability induced by dihydrotestosterone (DHT, 0.1 nM) was abrogated by 30*–42%* by 1–10 μM HET0016. Incubation with 20-HETE (5–1000 nM) increased LNCaP cell viability up to 50%*, together with a 70%* reduction in apoptosis. PC-3 (androgen-insensitive) cell viability was not affected by either HET0016 or 20-HETE. In LNCaP cells, HET0016 (10 μM) diminished the expression of androgen receptors (AR): messenger RNA (mRNA) (40%*) and protein (50%*). DHT (10 nM) augmented CYP4F2 protein expression (1.9-fold*) and 20-HETE levels (50%*). Oppositely, enzalutamide (AR antagonist) reduced CYP4F2 mRNA and protein expressions by 30 and 25%, respectively. Thus, intracellular availability of 20-HETE is necessary to sustain LNCaP cell viability. 20-HETE may act as a signaling molecule in the pathways involved in LNCaP cell viability upon stimulation of the AR. This effect may be partially attributed to its role on securing normal AR expression levels that in turn contribute to maintain intracellular levels of 20-HETE.
Keywords: LNCaP Cell Viability, Androgen Receptor (AR), Enzalutamide, LNCaP Cells, Time-related Control
Introduction
It is well known that n-6 fatty acids, such as arachidonic acid (AA), promote cell proliferation in prostate cancer cell lines, whereas long-chain polyunsaturated n-3 fatty acids, which are abundant, for example, in fish oil, inhibit cell proliferation [1, 2]. These promotional and inhibitory effects of n-6 and n-3 fatty acids, respectively, have also been demonstrated in prostate carcinogenesis and progression in vivo [3]. Thus, dietary fat composition may greatly influence the risk of prostate cancer [4].
Phospholipases A2 are phospholipid hydrolase enzymes that mediate the release of biologically active fatty acids such as AA, which is the precursor of eicosanoids. Previous studies have demonstrated increased expression levels of the secreted form of phospholipase A2 (sPLA2-IIA) in patients with prostate cancer, which was specifically upregulated in the highest-grade prostate cancer samples and was related to the increased proliferative index that characterizes advanced prostate cancer [5–7].
AA metabolites fall into two general groups: prostaglandins (PGs) and leukotrienes (LTs) which are associated with the classical enzymatic pathways of the cyclooxygenases (COX-1 and COX-2) and lipoxygenases (LOXs) [8]. Also, a least explored pathway of AA includes its metabolism through cytochrome P450 (CYP) enzymes. Thus, the profile of compounds produced is complex and abundant.
Several CYP4A and CYP4F enzymes generate the active signaling compound, 20-hydroxyeicosatetraenoic acid (20-HETE), by ω-hydroxylation of AA. CYP4F2 is the most active isoform that produces 20-HETE in humans, followed by CY4A11 [9–11].
There are increasing reports suggesting that 20-HETE can play an important role in cell growth and cancer development. In vitro studies show that 20-HETE induces mitogenic and angiogenic responses in several types of cancer cells, and inhibitors of the 20-HETE pathway have been shown to reduce the growth of brain, breast, and kidney tumors [12–14]. Moreover, the observation that the expression of CYP4A-CYP4F genes was markedly elevated in thyroid, breast, colon, and ovarian cancers [15] further highlights the significance of 20-HETE-producing enzymes in the progression of different types of human cancer.
Regulation of the expression of ω-hydroxylases is isoform-, species-, and tissue-dependent. Former studies showed that treatment with an agonist for the androgen receptor, 5α-dihydrotestosterone (DHT), upregulates the expression of ω-hydroxylases in rat prostate [16], as well as in rat and mouse kidneys [17, 18]. Furthermore, inhibition of 20-HETE synthesis by HET0016 attenuated androgen-mediated blood pressure elevation, thus demonstrating the role of 20-HETE as an intracellular signaling molecule in androgen-dependent hypertension [19].
Prostate cancer tumor growth is initially dependent on androgens as documented by Huggins as early as in 1941 [20]. Eventually, advanced prostate cancer reaches the stage of castration-resistant prostate cancer (CRPC) but remains dependent on androgen receptors [21].
Thus far, there is lack of knowledge regarding the involvement of 20-HETE in prostate cancer progression. Interestingly, urinary concentrations of 20-HETE were significantly higher in benign prostatic hypertrophy and prostate cancer patients than in normal subjects. The removal of the prostate gland in cancer patients decreased 20-HETE urinary excretion to normal levels [22]. Furthermore, the expression of CYP4Z1, another ω-hydroxylase first described in normal mammary gland [23], has been suggested as one reliable marker of prostate cancer prognosis tested on biopsy specimens [24].
Taking into account the pro-tumorigenic effects of 20-HETE described in a number of tumor models and the involvement of 20-HETE in androgen-dependent effects in animal models, as well as the higher expression of CYP4Z1 in prostate cancer biopsies and the increased urinary levels of 20-HETE in prostate tumor patients, we hypothesized the participation of 20-HETE in the androgen-dependent prostate tumor growth.
The aim of this study was to analyze the role of 20-HETE on cell survival, proliferation, and apoptosis in two prostate cancer cell lines, the androgen-dependent LNCaP cells and the androgen-independent PC-3 cells, and to investigate the possible interaction between the expression of the most active CYP isoform in 20-HETE synthesis, CYP4F2, and the androgen receptor in androgen-dependent prostate cancer cells.
Methods
Drugs and Reagents
20-HETE (Cayman Chemical Company, Ann Arbor, MI, USA) and N-hydroxy-N′-(4-n-butyl-2-methylphenyl)formamidine (HET0016), a selective inhibitor of 20-HETE synthesis [25] that was kindly provided by Dr. Richard Roman (University of Mississippi Medical Center, USA), were dissolved in absolute ethanol. The storage of stock and working solutions was accomplished under nitrogen atmosphere at −80 °C.
DHT and indomethacin were obtained from Sigma-Aldrich (Saint Louis, MO, USA) and were dissolved in absolute ethanol to prepare stock solutions. Enzalutamide was purchased from Chemscene LLC (Monmouth Junction, NJ, USA), and dimethylsulfoxide (DMSO) was used as a dissolvent to prepare stock solutions. All these stock solutions were stored at −20 °C. In each case, control conditions refer to the treatment with the vehicle used at the highest concentration which never surpassed 0.1%.
Specific antibodies for CYP4A11 (1:1000) and CYP4F2 (1:400) were obtained from Abcam (Cambridge, UK), and androgen receptor antibody (1:200) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). For apoptosis assays, specific antibodies for PARP-1 (1:500), Caspase-3 (1:500), Caspase-9 (1:500), BAX (1:500), and Bcl-xL (1:250) (Santa Cruz Biotechnology) were used. Also, β-tubulin (1:1000) (Sigma-Aldrich) and β-actin (1:1000) antibodies (Cell Signaling Technology, Danvers, MA, USA) were used as loading controls.
Polyclonal anti-rabbit (1:5000) (Cell Signaling Technology), anti-mouse (1:12,000) (Amersham Biosciences, Sweden), or anti-goat (1:2000) (Santa Cruz Biotechnology) antibodies conjugated with horseradish peroxidase (HPR) were used as secondary antibodies, accordingly. For immunocytochemistry studies, anti-Ki-67 antibody, MIB-1 clone (1:100) (Dako Agilent Technologies, Glostrup, Denmark), was used to assess proliferating cells.
For the quantitation of prostate-specific antigen (PSA), Advia Centaur Complex PSA kit was used in a Siemens Advia Centaur Inmunoassay System (Siemens Healthcare GmbH, Erlangen, Germany).
Cell Culture
Two prostate cancer cell lines were studied: the androgen-sensitive LNCaP and the androgen-insensitive PC-3; both were obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured at 37 °C with 5% CO2 for up to 8 weeks and were used up to passage 50. They were routinely maintained in RPMI media (Microvet, Buenos Aires, Argentina) supplemented with fetal bovine serum (FBS) 10% (Natocor, Córdoba, Argentina), penicillin 100 IU/mL, streptomycin 100 μg/mL, and amphotericin B 2.5 μg/mL (Life Technologies, Carlsbad, CA, USA). For experiments that required steroid-deprived media, FBS was treated with activated charcoal and dextran (2.5 and 0.25%, respectively) for 1 h at 45 °C. This charcoal-stripped serum (CS-FSB) was added to RPMI phenol red-free media (Microvet) at a final concentration of 10%. Steroid-deprived media were used to prevent the action of androgen precursors or other androgen receptor agonists to ensure that the response was due to the added drug.
Viability Assay
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was used to determine cell viability. Cells were seeded in 96-well plates at a density of 4 × 103 for LNCaP or 1 × 103 for PC-3 cells. When 20-HETE was added to the media, indomethacin was used to prevent COX-mediated 20-HETE degradation. Stimuli began 48 h later and were renewed every 24 h for 20-HETE and HET0016 and every 48 h for DHT; media were refreshed every 48 h during the experiments.
Finally, cells were incubated for 2 h at 37 °C with MTT (0.5 mg/mL); DMSO was added, and color intensity was measured at 540 nm using a plate spectrophotometer. Results are expressed as percentage of control (vehicle), except in the time-course experiments for which the values of absorbance are presented. Each condition was assayed using sextuplicates in at least two independent experiments.
Results from the MTT viability assays were confirmed in a smaller subset of experiments by direct counting of cells in a Neubauer chamber after incubation with 0.5% trypan blue solution.
Proliferation Assay: Immunocytochemistry for Ki-67
The expression of Ki-67 antigen was determined by immunocytochemistry to assess the proliferative fraction of the cell culture. Briefly, 5 × 104 LNCaP cells were seeded in 24-well plates; after 24 h, cells were synchronized by FBS deprivation for 16 h. Later, stimuli were performed as detailed before. Cells were washed with phosphate buffer solution (PBS) and fixated with methanol to acetic acid (3:1) for 30 min. DNA was denaturated with ethanol 70% and NaOH 0.2 M for 3 min, followed by 1-min incubation with 70 and 96% ethanol at 4 °C. To block endogenous peroxidase, H2O2, 3%, in methanol was added followed by serum in PBS (1:100) to mask unspecific sites; later, incubation with Ki-67 antibody was performed at 4 °C overnight.
Immunocytochemical staining was performed using a commercial kit (Vectastain ABC Kit, Vector Laboratories, Burlingame, CA, USA). Diaminobenzidine was used as a final substrate for the reaction, and hematoxylin 10% (30 seg) was used to counterstain cells. For each condition, over 2000 nuclei were counted and results are expressed as percentage of Ki-67-positive (Ki-67+) cells. Each condition was performed in triplicates in at least two independent experiments.
TUNEL Assay
Cell samples were processed using the In Situ Cell Death Detection Kit AP (Roche Diagnostics, Indianapolis, IN, USA) following the recommendations of the manufacturer. Briefly, LNCaP cells were seeded in 24-well plates at a density of 6 × 104; stimuli were performed as detailed before. Later, cells were fixed with paraformaldehyde, 4%, in PBS for 1 h at room temperature, followed by incubation with fresh permeabilization solution (Triton X-100, 0.1%, in sodium citrate, 0.1%) for 2 min at 4 °C. Then, the terminal deoxynucleotidyl transferase (TdT) enzyme and fluorescein-12-dUTP were added for 1 h at 37 °C. To counterstain nuclei, DAPI (4′,6-diamidino-2-phenylindole) was used. At least 200 nuclei were counted for each condition using a fluorescence microscope. Results are expressed as percentage of TUNEL-positive nuclei (TUNEL+). Each condition was assayed using duplicates in at least three independent experiments.
Quantitative PCR
Total RNA from cell lysates was obtained using commercially available extraction kits (Direct-zol RNA extraction kit, Zymo Research, Irvine, CA, USA). Initial retrotranscription was performed using SuperScript II enzyme (Invitrogen) with OligodT (0.5 μg per reaction) (Invitrogen) using equal amounts of total RNA for each sample (1 μg); cycling conditions were as follows: 42 °C for 50 min and 72°°C for 15 min. Quantitative PCR analysis was performed using SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and StepOne Plus Real-Time PCR System (Applied Biosystems). Primers used for CYP4F2 and HPRT-1 amplification were previously published, and primers for androgen receptors were a gift from Dr. Adriana De Siervi (IBYME-CONICET, Buenos Aires, Argentina) (Table 1). Cycling parameters were as follows: 40 cycles at 95 °C for 15 s and 60 °C for 1 min. The relative expression for each gene was obtained by comparing against a standard curve. The relative quantity of target messenger RNA (mRNA) was calculated using the ratio between the expression of the gene of interest and HPRT-1 (housekeeping gene).
Table 1.
Primer sequences
| Primer | Sequence (5′–3′) | Bibliography |
|---|---|---|
| CYP4F2 forward | GAGGGTAGTGCCTGTTTGGAT | Primer bank: http://pga.mgh.harvard.edu/cgi-bin /primerbank/new_displayDetail2.cgi?primerID=13435390c1 |
| CYP4F2 reverse | CAGGAGGATCTCATGGTGTCTT | |
| AR forward | GGCATGGTGAGCAGAGTG | Gift by Dr. A. De Siervi, Instituto de Biología y Medicina Experimental (IBYME-CONICET), Buenos Aires, Argentina. |
| AR reverse | GGGTGGAAAGTAATAGTCAATGG | |
| HPRT-1 forward | TGACACTGGCAAAACAATGCA | Vandesompele et al. [26] |
| HPRT-1 reverse | GGTCCTTTTCACCAGCAAGCT |
AR androgen receptor, HPRT-1 hypoxanthine phosphoribosyltransferase 1
Western Blot Assays
LNCaP or PC-3 cells were harvested in lysis buffer (Tris, 10 mM; NaCl, 150 mM; Triton X-100, 1%; EDTA, 1 mM; EGTA, 1 mM; Na4P2O7.10(H2O), 10 mM; Na3VO4, 10 mM; and NaF, 100 mM) supplemented with a mix of protease inhibitors (cOmplete™ Mini EDTA-free, Roche Diagnostics, Mannheim Germany), and total protein content was assessed using the Bradford method.
Equal amounts of protein were separated in a polyacrylamide gel and transferred onto a PVDF membrane (GE Healthcare, Buckinghamshire, UK). Membranes were blocked with skimmed milk, 5%, in TBS-T buffer (Tris, 50 mM; NaCl, 150 mM; and Tween 20®, 0.1%) except for when Caspase-3 antibody was used (TBS with Tween 20® ,0.4%; EDTA, 1 mM; and bovine serum albumin (BSA), 0.1%). Next, they were incubated overnight at 4 °C with specific antibodies. After incubation with the secondary antibody, membranes were developed using a chemiluminescent reaction (ECL Blotting Detection Kit, GE Healthcare) and exposed to films (CL-XPosure Films®, Thermo Scientific, Rockford, IL; EEUU). Afterwards, the antibodies were stripped off using a stripping buffer containing glycine, 0.2 M; sodium dodecyl sulfate, 0.1%; and Tween 20®, 1%, at pH 2.2 and were reblotted to detect β-actin or β-tubulin, used as loading controls. The densitometric analysis was performed with ImageJ software [25]. Results are presented as the ratio between the protein of interest and the loading control.
20-HETE Quantitation
The quantitation of 20-HETE was achieved using a commercial ELISA kit (BioTarget, Detroit R&D, Detroit, MI, USA) following the instructions provided by the manufacturer. Briefly, 5 × 105 cells were seeded in six-well plates in complete media. After 24 h, they were incubated with HET0016 in complete media or with DHT, 10 nM, in steroid-deprived media. Cell homogenates were collected with triphenylphosphine (0.03–0.05 mg/mL), and pH was adjusted to 3–4. The lipid fraction was extracted with ethyl acetate which was later evaporated under nitrogen atmosphere. The residue was dissolved with ethanol and seeded in the 96-well ELISA plate. The colorimetric reaction was quantified at 450 nm using a plate spectrophotometer. Results are presented as nanograms of 20-HETE per milligram of protein. Each condition was performed in triplicates in at least two independent experiments.
Statistical Analysis
Normal distribution of data was analyzed using the Shapiro-Wilks test. Student’s t test was performed between two conditions, and for comparisons between more than two groups, one-way ANOVA was used, followed by Dunnet or Tukey correction for multiple testing. Differences were considered significant when p < 0.05, and results are presented as mean ± SEM. All the statistical analyses and the graphs presented were done using GraphPad Prism software (Windows version 6.01, GraphPad Software, La Jolla, CA, USA. Website:
Results
20-HETE Increases Viability of Androgen-Sensitive Prostate Cancer Cells
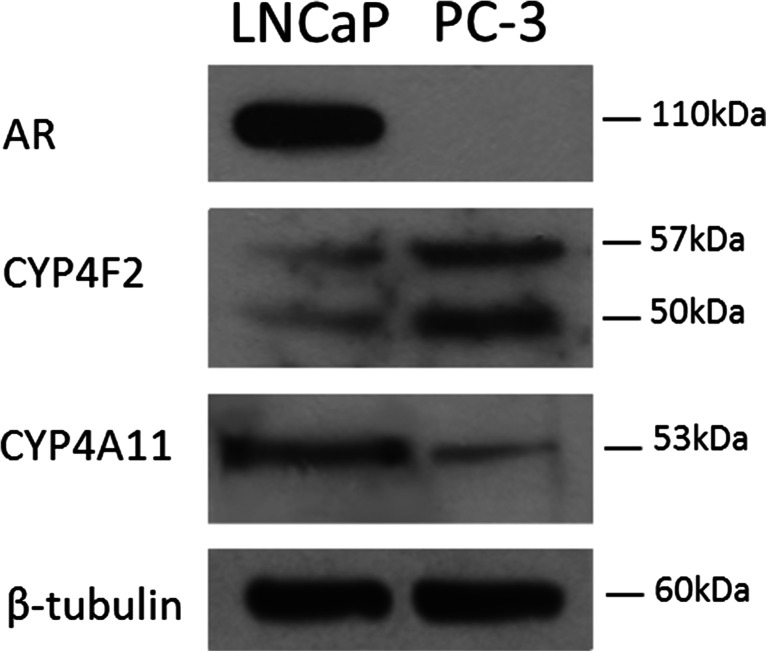
The role of 20-HETE on the viability of prostate cancer cells was first addressed by studying the effect of HET0016 (an inhibitor of 20-HETE synthesis). For HET0016 to have any effect on cell survival through the decrease in 20-HETE availability, LNCaP (androgen-sensitive) and PC-3 (androgen-insensitive) cells should produce 20-HETE. As previously reported, the presence of the androgen receptor protein and the two main human ω-hydroxylases, CYP4F2 and CYP4A11, were observed in LNCaP cells [27]. Additionally, our results also show the expression of both CYP isoforms in PC-3 cells (Fig. 1). Standard concentrations of HET0016 (1 and 10 μM) proved to be effective for reducing the intracellular levels of 20-HETE after 6 h incubation in complete media (Table 2).
Fig. 1.
Characterization of the expressions of the two main ω-hydroxylase isoforms in LNCaP and PC-3 cells. Cells were grown in complete media; expressions of the androgen receptor (AR), CYP4F2, and CYP4A11 were analyzed in cell homogenates by Western blot. β-Tubulin served as loading control
Table 2.
Intracellular levels of 20-HETE in prostate cancer cell lines
| Cell line | 20-HETE (ng/mg protein) | ||
|---|---|---|---|
| Control | HET0016 | ||
| 1 μM | 10 μM | ||
| LNCaP | 312.8 ± 62.0 | 139.0 ± 28.9* | 22.97 ± 0.4** |
| PC-3 | 207.3 ± 9.0 | 55.1 ± 1.0* | N.D. |
Effect of HET0016. Cells grown in complete media were incubated for 6 h with vehicle (control) or the indicated concentrations of HET0016. Cell homogenates were analyzed for 20-HETE by ELISA. LNCaP cells n = 2 in duplicates, ANOVA p = 0.0002 followed by Dunnet. PC-3 cells n = 2 in duplicates, t test p < 0.0001. Values are mean ± SEM
N.D. not determined
*p < 0.01; **p < 0.01 vs. control
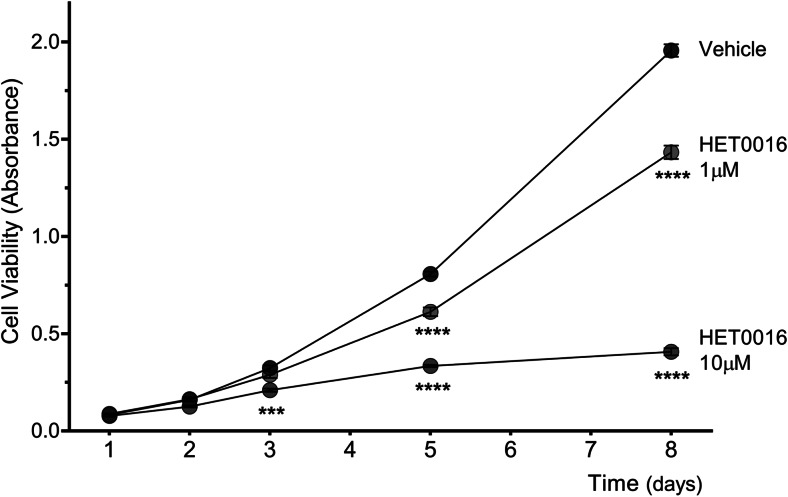
Time-course studies showed a significant drop in the viability of LNCaP cells grown in the presence of 10 μM HET0016 since 72 h incubation (p < 0.001 vs. time-related control) which persisted and was strikingly evident after 8 days (p < 0.0001). Also, the effect of 1 μM HET0016 on cell viability became significant since day 5 (p < 0.0001) and persisted until the end of the experiment (p < 0.0001) (Fig. 2). On the contrary, treatment with HET0016 did not affect the viability of PC-3 cells at any concentration tested (data not shown).
Fig. 2.
Time-course studies of the effect of HET0016 on LNCaP cancer cell viability. LNCaP cells were grown in complete media for the indicated period in the presence of vehicle or HET0016 (1–10 μM). Results are expressed as absorbance values (n = 2 in triplicates) (ANOVA p < 0.0001; ***p < 0.001; ****p < 0.0001 vs. time-paired controls). Values are presented as the mean ± SEM
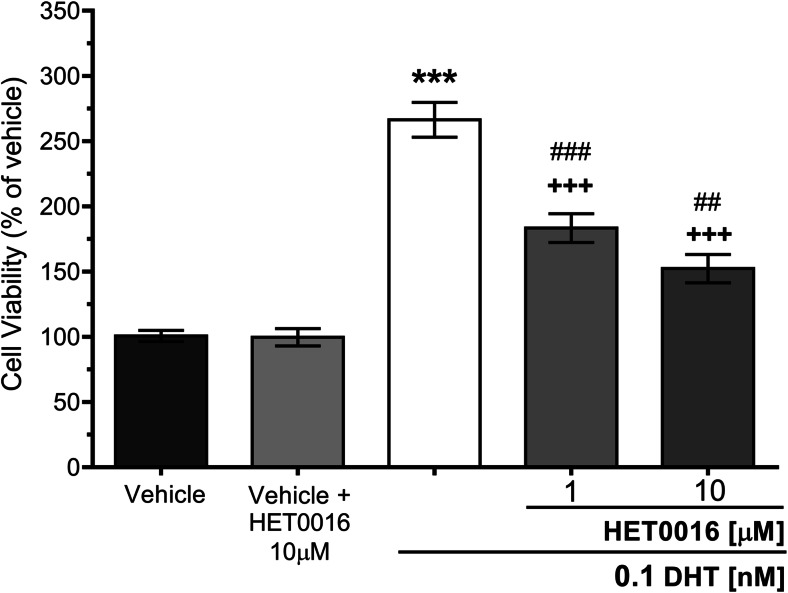
Since prostate cancer tumor growth is androgen-dependent, the following experiments were aimed at assessing whether 20-HETE production is necessary for androgen-promoted viability in LNCaP cells. The optimal concentration of DHT was determined in preliminary experiments. LNCaP cells were incubated for 5 days in steroid-deprived media with 0.1 nM DHT alone or in the presence of 1 or 10 μM HET0016. DHT increased by 166% the viability of LNCaP cells (p < 0.001 vs. vehicle), and this effect was reduced between 30 and 42% by HET0016 (vs. DHT alone, p < 0.001, for 1 or 10 μM). Interestingly, in contrast to the effect observed on cells grown in complete media, HET0016 alone had no impact on the viability of cells grown in steroid-deprived media when compared to vehicle (Fig. 3).
Fig. 3.
Effect of HET0016 on DHT-induced viability in LNCaP cells. Cells were grown in steroid-deprived media for 48 h before the addition of DHT and/or HET0016, and cultures were continued in the specified conditions for another 5 days. Cell viability was assessed by the MTT assay, and results are expressed as percentage of control cells (vehicle-treated cells or vehicle + HET0016-treated cells) (n = 3–4, in sextuplicates) (ANOVA p < 0.0001); (***p < 0.001 vs. vehicle; ##p < 0.01 vs. vehicle + HET0016, 10 μM; ###p < 0.001; +++p < 0.001 vs. DHT 0.1 nM). Values are presented as the mean ± SEM
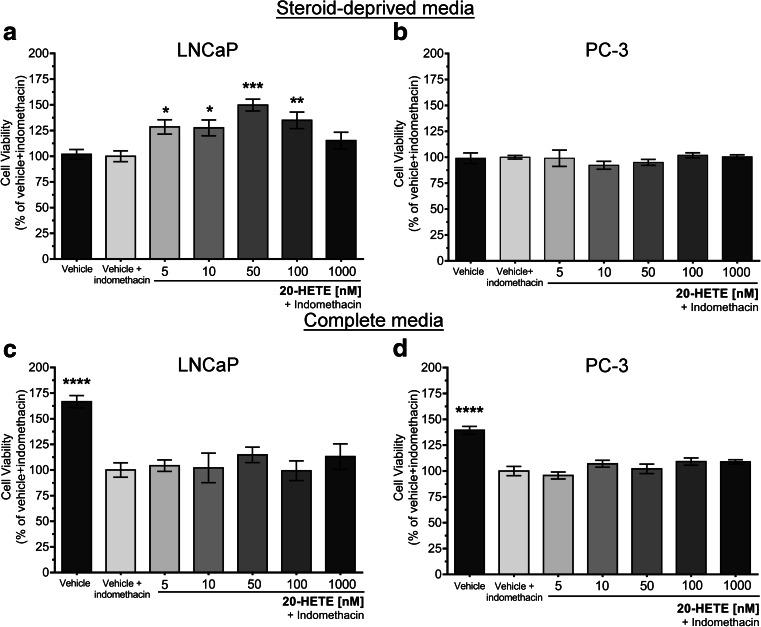
To confirm the effect of 20-HETE on cell viability, LNCaP and PC-3 cells were grown in steroid-deprived media for 5 days in the presence of 20-HETE at concentrations varying from 5 to 1000 nM. The addition of indomethacin (to prevent COX-dependent 20-HETE degradation) had no effect per se on cell growth. A significant increase in cell viability in response to 20-HETE treatment was observed only in LNCaP cells starting from 5 nM up to 100 nM, with 50 nM being the concentration with the highest effect (50% increase in viability) (p < 0.001 vs. vehicle + indomethacin) (Fig. 4a). On the opposite, and in line with the observations described above, no change in viability was observed in PC-3 cells grown in the presence of a wide range of 20-HETE concentrations (Fig. 4b). Also, in complete media, 20-HETE did not affect LNCaP and PC-3 cell viability when compared to control condition (indomethacin-incubated cells) (Fig. 4c, d); in this condition, indomethacin reduced viability by 65 and 40% compared to vehicle in LNCaP and PC-3 cells, respectively.
Fig. 4.
Effect of 20-HETE on the viability of prostate cancer cells. Cells were grown in steroid-deprived (a, b) or complete media (c, d) for 48 h before the addition of the indicated concentrations of 20-HETE plus indomethacin (10 μM), and cultures were continued for another 5 days. Cell viability was assessed by the MTT assay. Results are expressed as percentage of control cells (vehicle plus indomethacin-treated cells). a n = 3, in sextuplicates (ANOVA p < 0.0001; *p < 0.05; **p < 0.01; ***p < 0.001 vs. control). b n = 3–4, in sextuplicates (ANOVA p = 0.3543). c (n = 2, in sextuplicates) (ANOVA p < 0.0001; ****p < 0.0001 vs. control). d n = 2, in sextuplicates (ANOVA p < 0.0001; ****p < 0.0001 vs. control). Values are presented as the mean ± SEM
Inhibition of the Synthesis of 20-HETE Reduces Proliferation (Ki-67 Staining) of Androgen-Sensitive Prostate Cancer Cells
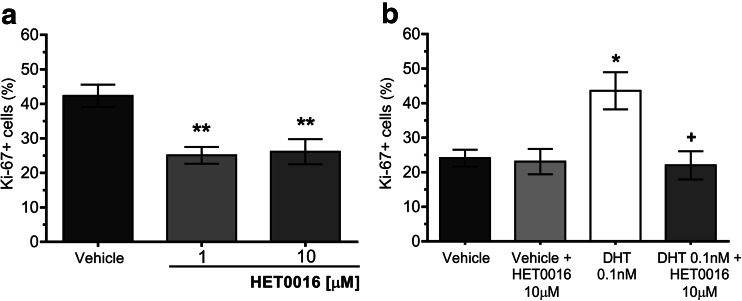
In control conditions, 43.6 ± 3.5% of cells cultured in complete media were positive for Ki-67 staining (Ki-67+). Incubation with HET0016 (1 or 10 μM) for 48 h reduced the number of Ki-67+ cells to 23.4 ± 2.7 and 27.7 ± 3.3%, which represents a decrease of approximately 40% related to the control (p < 0.01 vs. vehicle for both) (Fig. 5a). The effect of 20-HETE withdrawal on cell proliferation was further analyzed on LNCaP cells grown in steroid-deprived media supplemented with DHT. Under basal conditions, 24.1 ± 2.4% cells were Ki-67+, and the addition of DHT, 0.1 nM (48 h), increased this value to 43.6 ± 5.4% (p < 0.05 vs. vehicle), whereas the co-incubation with HET0016, 10 μM, completely abolished the effect of DHT rendering only 22.0 ± 4.1% cells labeled with Ki-67 (p < 0.05 vs. DHT). Similar to what was observed on cell viability, HET0016 alone had no impact on proliferation in cells grown in steroid-deprived media (Fig. 5b).
Fig. 5.
Effect of HET0016 on the proliferation of LNCaP cells. Cells synchronized by serum deprivation for 16 h were further grown for another 48 h in a complete media in the presence of vehicle (control) or HET0016 or b steroid-deprived media in the presence of vehicle (control) or the indicated drugs. The expression of the nuclear Ki-67 antigen was analyzed by immunocytochemistry, and quantitation of cell proliferation is expressed as percentage of Ki-67+ cells. a n = 2, in triplicates (ANOVA p = 0.0045; **p < 0.01 vs. vehicle). b n = 3, in triplicates (ANOVA p = 0.0078; *p < 0.05 vs. vehicle; +p < 0.05 vs. DHT 0.1 nM). Values are presented as the mean ± SEM
Unexpectedly, incubation with 20-HETE (48 h) in steroid-deprived media had no effect on cell proliferation. The percentage of Ki-67+ cells grown in control conditions (vehicle, 24.1 ± 2.4%) was modified neither by indomethacin alone (26.8 ± 0.8%) nor by 20-HETE, 50 nM, added in the presence of indomethacin (26.3 ± 1.9%) (data not shown).
20-HETE Prevents Apoptosis in Androgen-Dependent Prostate Cancer Cells
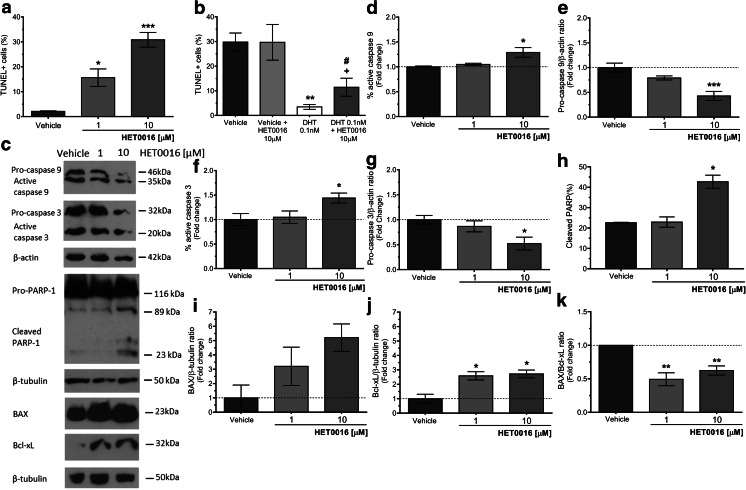
TUNEL assay was performed on LNCaP cells grown in complete media with HET0016 for 5 days. In control conditions, only 2.1 ± 0.2% of cells were positive for TUNEL staining (TUNEL+), and incubation with HET0016, 1 and 10 μM, significantly increased this value to 15.6 ± 3.7 and 30.8 ± 2.9% (p < 0.05 and p < 0.001 vs. vehicle, respectively) (Fig. 6a). For LNCaP cells grown in steroid-deprived media, the proportion of TUNEL+ cells was 29.8 ± 3.7%, and was not changed by HET0016. Supplementation with DHT, 0.1 nM, reduced TUNEL+ cells to 3.5 ± 1.0% (p < 0.01 vs. vehicle), and the addition of HET0016, 10 μM, to cells supplemented with DHT increased TUNEL+ cells to 11.5 ± 3.7% (p < 0.05 vs. DHT) (Fig. 6b).
Fig. 6.
Effect of HET0016 on the apoptosis of LNCaP cells. Cells were grown for 5 days in complete media (a), in steroid-deprived media (b), or for 48 h in complete media (c–k) in the presence of vehicle (control) or the indicated drugs. Nuclear TUNEL staining was analyzed by immunofluorescence, and the quantitation is expressed as percentage of TUNEL+ cells (a, b). Also, cells were lysed and the expressions of the proteins involved in apoptotic pathways were detected by Western blot (c). Densitometric analyses are presented as follows: Caspase 9 measured as percentage of active Caspase 9 (d) and pro-Caspase 9/β-actin ratio (e), Caspase 3 measured as percentage of active Caspase 3 (f) and pro-Caspase 3/β-actin ratio (g), PARP-1 measured as percentage of cleaved PARP-1 (h), BAX/β-tubulin ratio (i), Bcl-xL/β-tubulin ratio (j), and BAX/Bcl-xL ratio (k). Results are expressed as fold change of the control cells (vehicle-treated cells). a n = 2–3, in duplicates (ANOVA p = 0.0010; *p < 0.05; ***p < 0.001 vs. vehicle). b n = 4, in duplicates (ANOVA p = 0.0034; **p < 0.01 vs. vehicle; #p < 0.05 vs. vehicle + HET0016; +p < 0.05 vs. DHT 0.1 nM). d n = 3 (ANOVA p = 0.0139; *p < 0.05 vs. vehicle). e n = 3 (ANOVA p = 0.0006; ***p < 0.001 vs. vehicle). f n = 3 (ANOVA p = 0.0410; *p < 0.05 vs. vehicle). g n = 3 (ANOVA p = 0.0212; *p < 0.05 vs. vehicle). h n = 2 (ANOVA p = 0.0145; *p < 0.05 vs. vehicle). i n = 3 (ANOVA p = 0.1394). j n = 3 (ANOVA p = 0.0102; *p < 0.05 vs. vehicle). k n = 3 (ANOVA p = 0.0043; **p < 0.01 vs. vehicle). Values are presented as the mean ± SEM
Cell lysates were collected after 48 h of HET0016 treatment to assess proteins involved in apoptotic pathways. As shown in Fig. 6c–k, HET0016, 10 μM, increased the cleavage of Caspase-9 and Caspase-3 by 20 and 43%, respectively (p < 0.05 vs. vehicle for both), reflecting a direct increment in the active form of the enzymes (Fig. 6d, f). Also, a 100% increase in PARP-1 cleavage was observed (p < 0.05 vs. vehicle) accounting for its degradation (Fig. 6h). As for the expression of the pro-apoptotic protein BAX, an increase was also found in the presence of HET0016, although statistically not significant. However, as a result of the concomitant increase in the expression of the anti-apoptotic protein Bcl-xL, the BAX/Bcl-xL expression ratio decreased by 40% (p < 0.05 vs. vehicle) following incubation with HET0016 (Fig. 6k).
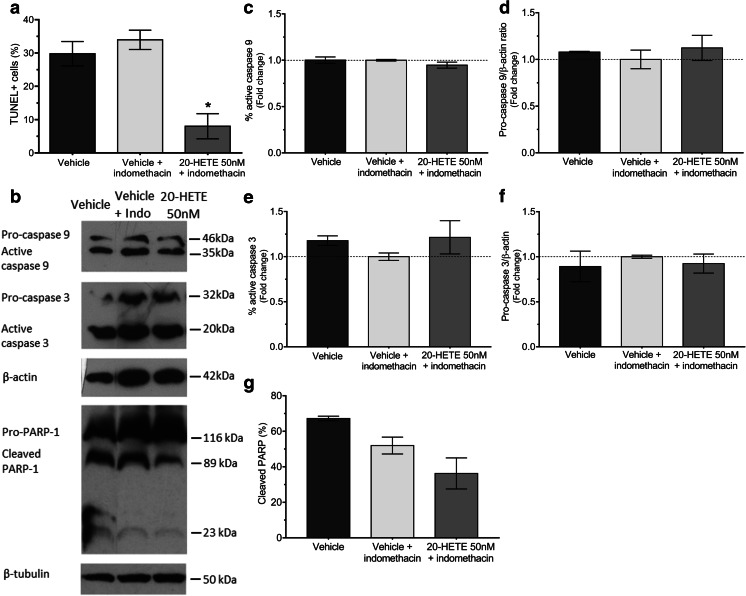
Furthermore, the anti-apoptotic effect of 20-HETE in LNCaP cells was confirmed by TUNEL assay and PARP-1 cleavage. For cells grown in steroid-deprived media, the proportion of TUNEL+ cells was 29.8 ± 3.7% and was not changed by indomethacin. 20-HETE (50 nM) reduced TUNEL+ cells to 8.0 ± 3.8% (p < 0.05 vs. vehicle + indomethacin) (Fig. 7a). Moreover, Western blot analysis showed a decrease in the cleavage of PARP-1 in 20-HETE- incubated cells even though it did not reach statistical significance. No changes were observed in the expression of active Caspases (Fig. 7b–g).
Fig. 7.
Effect of 20-HETE on the apoptosis of LNCaP cells. Cells were grown for 5 days (a) or for 48 h (b–g) in steroid-deprived media in the presence of vehicle (control), 20-HETE (50 nM), and/or indomethacin (10 μM). Nuclear TUNEL staining was analyzed by immunocytochemistry, and quantitation is expressed as percentage of TUNEL+ cells (a). Also, cells were lysed and the expressions of the proteins involved in apoptotic pathways were detected by Western blot (b). Densitometric analyses are presented as follows: Caspase 9 measured as percentage of active Caspase 9 (c) and pro-Caspase 9/β-tubulin ratio (d), Caspase 3 measured as percentage of active Caspase 3 (e) and pro-Caspase 3/β-tubulin ratio (f), and PARP-1 measured as percentage of cleaved PARP-1 (g). Results are expressed as fold change of the control cells (vehicle + indomethacin-treated cells). a n = 3–4, in duplicates (ANOVA p = 0.0085; *p < 0.05 vs. control and control + indomethacin). c n = 2 (ANOVA p = 0.3513). d n = 3 (ANOVA p = 0.7257). e n = 3 (ANOVA p = 0.3204). f n = 3 (ANOVA p = 0.8337). g n = 2 (ANOVA p = 0.0723). Values are presented as the mean ± SEM. Indo indomethacin
Dihydrotestosterone Increases Endogenous CYP4F2 Expression in LNCaP Cells
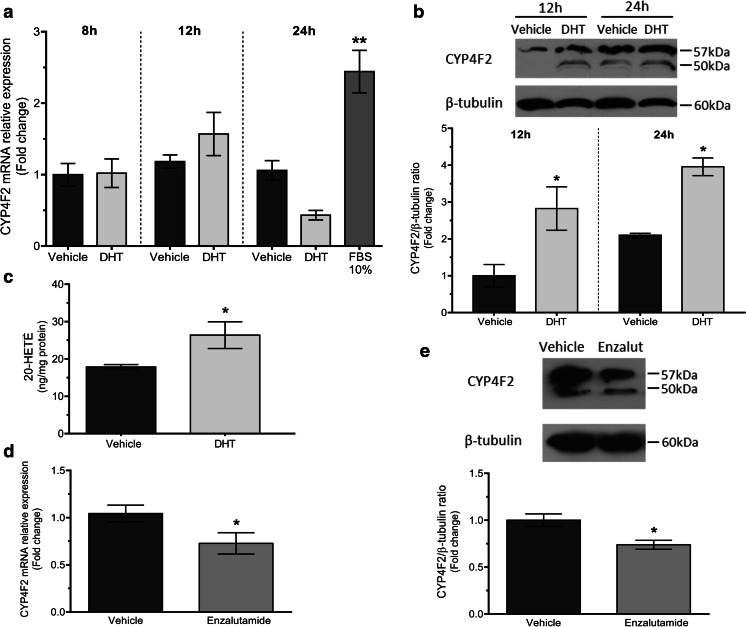
LNCaP cells were grown in steroid-deprived media for 48 h before the addition of DHT (10 nM) and further incubated for 8, 12, and 24 h. Real-time PCR results indicate that CYP4F2 mRNA levels in LNCaP cells grown in control conditions (normalized to HPRT-1 mRNA levels) remained stable during the experimental period. However, a slight (although not significant) increase was observed in cells that had been exposed to DHT for 12 h compared to their time-related control. Also, the mRNA expression in cells grown in complete media for 24 h showed a 1.38-fold increase vs. its time-related control grown in steroid-deprived media (p < 0.01) (Fig. 8a).
Fig. 8.
Androgen regulation of CYP4F2 expression in LNCaP cells. a, b LNCaP cells were grown in steroid-deprived media for 8, 12, or 24 h in the presence of vehicle (control); in DHT, 10 nM; or in complete media (FBS 10%). Then, they were collected and subjected to RNA and protein extraction for the quantitation of CYP4F2 mRNA normalized to HPRT-1 (qPCR) (a) and the analysis of CYP4F2 protein expression normalized to β-tubulin (Western blot) (b). The expression levels were calculated as the values relative to that of a calibrator sample (control, 8 or 12 h for qPCR and WB, respectively). c Cells were grown in steroid-deprived media for 24 h, in the presence of vehicle or DHT, 10 nM, for the measurement of intracellular 20-HETE levels by ELISA. d, e LNCaP cells were grown in complete media for 24 h in the presence of vehicle (control) or enzalutamide, 10 μM, and subjected to the analysis of CYP4F2 mRNA expression normalized to HPRT-1 (d) and protein expression normalized to β-tubulin (e). a n = 2–3, in duplicates (ANOVA p = 0.0005; **p < 0.01 vs. all conditions except DHT, 12 h). b n = 3 (ANOVA p = 0.0023; *p < 0.05 vs. time-related control). c n = 2, in duplicates (t test p = 0.0297). d n = 2, in duplicates (t test p = 0.045). e n = 2 (t test p = 0.0193). Values are presented as the mean ± SEM
The expression of CYP4F2 protein in cells treated with DHT for 12 and 24 h was 2.8-and 1.9-fold higher than the signal obtained from their respective time-related controls (p < 0.05 for both) (Fig. 8b). The functional relevance of this latter observation was confirmed by the increase in the intracellular 20-HETE levels measured after 24 h cell incubation with DHT (p < 0.05 vs. control) (Fig. 8c).
Given the stimulation of the expression of CYP4F2 by DHT, the role of the androgen receptor was explored by using the antagonist enzalutamide [28]. CYP4F2 mRNA and protein contents were analyzed in homogenates from LNCaP cells grown in complete media in the presence or absence of enzalutamide (10 μM, 24 h). A 30% decrease in the expression of CYP4F2 mRNA (p < 0.05 vs. control), along with a 25% decrease in protein levels (p < 0.02 vs. control), was observed in enzalutamide-treated cells (Fig. 8d, e).
Inhibition of ω-Hydroxylases Decreases the Expression and Activity of the Androgen Receptor
Next experiments were aimed to evaluate whether 20-HETE availability may affect the expression or function of the androgen receptor.
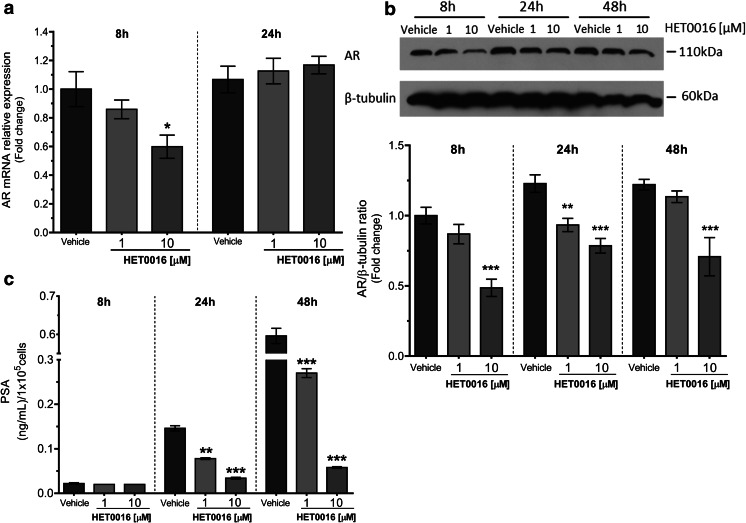
LNCaP cells were grown in complete media in the absence or presence of 1 or 10 μM HET0016 for 8, 24, and 48 h and were tested for the expression of the androgen receptor and the production of PSA. There was a 40% decrease in the mRNA levels of the androgen receptor after 8 h incubation with 10 μM HET0016 (p < 0.05 vs. vehicle), which normalized after 24 h (Fig. 9a). This was confirmed by Western blot analysis, which also showed a decrease in androgen receptor protein levels at all the time points studied (p < 0.01–0.001 vs. time-related control) (Fig. 9b).
Fig. 9.
Role of 20-HETE in the regulation of the expression of the androgen receptor in LNCaP cells. LNCaP cells were grown in complete media for 8, 24, or 48 h in the presence of vehicle (control) and HET0016, 1 or 10 μM. Cells were collected and subjected to RNA and protein extractions for the quantitation of androgen receptor (AR) mRNA normalized to HPRT-1 (qPCR) (a) and the analysis of AR protein expression normalized to β-tubulin (b). The expression levels were calculated as the values relative to that of a calibrator sample (control, 8 h). Culture supernatants were collected for the measurement of secreted prostate-specific antigen (PSA) (c); results are presented as nanograms of PSA every 1 × 105 cells. a n = 2–3, in duplicates (8 h: ANOVA p = 0.0183; *p < 0.05 vs. vehicle; 24 h: ANOVA p = 0.5870). b n = 2 (8 h: ANOVA p < 0.0001; ***p < 0.001 vs. vehicle; 24 h: ANOVA p < 0.0001; **p < 0.01; ***p < 0.001 vs. vehicle; 48 h: ANOVA p = 0.0008; ***p < 0.001 vs. vehicle). c n = 2, in duplicates (8 h: ANOVA p = 0.4648; 24 h: ANOVA p = 0.0006; **p < 0.01; ***p < 0.001 vs. vehicle; 48 h: ANOVA p = 0.0002; ***p < 0.001 vs. vehicle). Values are presented as the mean ± SEM
In line with the reduction in androgen receptor protein levels in HET0016-treated cells, incubation with 1 or 10 μM HET0016 resulted in a decrease in PSA protein secretion since 24 h (p < 0.01 and 0.001 vs. time-related control for 1 and 10 μM, respectively), which persisted for up to 48 h (p < 0.001 vs. time-related control for both) (Fig. 9c).
Discussion
Previous in vivo and in vitro studies have shown that 20-HETE promotes cancer cell proliferation, migration, angiogenesis, survival, and invasion in several types of tumors such as gliomas, gliosarcomas, renal, lung, and breast cancers [12–14, 29, 30]. However, neither the characteristics of the expression and regulation of the enzymes responsible for its synthesis nor its role in prostate cancer has been cleared so far.
Results from the present study support an important role of 20-HETE in prostate cancer biology. This is based on the following findings: (1) Measurable levels of 20-HETE and the expression of the enzymes responsible for its synthesis were found in androgen-sensitive and androgen-insensitive prostate cancer cell lines; (2) the availability of 20-HETE was necessary to sustain androgen-induced viability as the result of an increase in cell proliferation and the inhibition of apoptosis; and (3) the expression and catalytic activity of CYP4F2 were enhanced by DHT, and the expression and function of the androgen receptor were dependent on the availability of 20-HETE.
Our results confirm the expression of the two main human 20-hydroxylases CYP4F2 and CYP4A11 in LNCaP cells found by other authors [27] and describe their presence in PC-3 cells. The inhibition of the synthesis of 20-HETE by HET0016 proved to be effective in reducing androgen-induced cell viability solely in androgen-sensitive cells but not in PC-3 cells. This difference may be related to the dissimilar need of androgens or other steroids and supports previous reports regarding the difference in the intracellular pathways involved in the preservation of cell viability between both cell lines [31, 32].
The concentrations of HET0016 used in the present study (1–10 μM) are in the range of the ones previously used by other authors [12, 29]. The IC50 for the inhibition of the arachidonic acid ω-hydroxylase activity in renal microsomes is 8.9 nM, whereas the IC50 for the inhibition of its metabolism through epoxygenases or cyclooxygenases is 2.8 and 2.3 μM, respectively [33]. Therefore, to rule out the possibility that the effects of HET0016 here described might be attributed to some inhibitory effect on the synthesis of PGs or EETs, the response to the addition of 20-HETE on cell viability was also tested.
The addition of 20-HETE in a range of concentrations similar to the ones reported by other authors to be effective on enhancing lung endothelial cell survival (10–1000 nM) [34] increased by up to 50% the viability of LNCaP cells grown in steroid-deprived media without affecting LNCaP cell growth in complete media. The plethora of other growth factors available in complete media may explain the lack of effect of 20-HETE in the latter condition. In line with the lack of effect of HET0016 in PC-3 cells, the addition of 20-HETE did not modify PC-3 cell viability in complete or in steroid-deprived media.
One of the mechanisms suggested to be involved in 20-HETE-induced cell survival in endothelial [34], glioma [12], and gliosarcoma [29] cells is to support cell proliferation. However, in steroid-deprived media, 20-HETE did not induce LNCaP cell proliferation. This might be attributed to the deficiency of other pro-proliferative factors in these conditions that may synergize with 20-HETE to promote cell proliferation. Nevertheless, HET0016 reduced cell proliferation promoted by androgens whether supplied by FBS in complete media or by exogenously added DHT. The fact that, similar to the effect on cell viability, the effect of HET0016 on cell proliferation was dependent on the availability of androgens in the culture media suggests that the actions of 20-HETE rely on the presence and function of the androgen receptor.
The regulation of the androgen receptor expression may be undertaken at different steps: transcriptional level, modulation of mRNA stability, protein synthesis, and post-translational modifications [35, 36]. The incubation of LNCaP cells with HET0016 decreased the androgen receptor mRNA, which suggests that the availability of 20-HETE is necessary for the normal androgen receptor transcription. However, our results do not preclude further actions of 20-HETE on the levels of androgen receptor protein and the release of PSA or for maintaining androgen receptor mRNA stability. It is possible to speculate that the lower expression of the androgen receptor in conditions of 20-HETE shortage is related, among other possibilities, to a deficient epidermal growth factor (EGF)-dependent phosphorylation at Mitogen-activated protein kinase (MAPK) or Protein kinase C (PKC) consensus sites, a step that regulates androgen receptor nuclear-cytoplasmic shuttling in CWR-R1 prostate cancer cells [37]. Thus, the lower levels of androgen receptor after HET0016 treatment may account for the decrease in LNCaP cell viability and proliferation, as well as for the increase in cell apoptosis. Proliferation of LNCaP cells is tightly regulated by androgen receptors, through the control of the expression of proteins involved in cell cycle progression such as cyclins E and A [38] and the MAPK/ERK cascade [39]. Activated ERK-1/2 translocates to the nucleus and directly phosphorylates transcription factors [40] that coordinately regulate the expression of several genes involved in cell proliferation [40]. It has been recently described that 20-HETE signals through G protein-coupled receptor GPR75 (Gq), whose stimulation activates the epidermal growth factor receptor (EGFR)-MAPK-IKK-NFkB signaling pathway [41]. Indeed, in renal proximal tubule cells, 20-HETE activates c-Src leading to the phosphorylation of EGFR and resulting in epithelial renal cell proliferation [42], whereas in glioma or gliosarcoma cells, HET0016 reduced phosphorylation of p42/p44 MAPK and of EGFR [12, 29]. Also, a number of studies have shown that 20-HETE activates PKC-dependent phosphorylation in renal epithelial cells [43, 44]. Thus, the similarity of these latter signals with the ones activated by androgens, described above [40, 45], may support the speculation that some of them might act downstream 20-HETE in the signaling cascade of AR.
It is known that steroid depletion induces cell cycle arrest and an increase in apoptotic cells [46–48]. This has been also proved in prostate biopsy samples from patients under androgen deprivation therapy and has been regarded as one of the mechanisms leading to tumor size reduction [49]. As expected, the number of TUNEL+ cells was higher when grown in steroid-deprived media than in those grown in complete media; also, HET0016 increased the number of TUNEL+ LNCaP cells grown in complete media in a dose-dependent manner.
This increase in the apoptotic cell fraction was further confirmed by the increase in PARP-1 cleavage as well as in the active fraction of Caspases 3 and 9. Although, in line with previous reports [50], these events point to a Caspase-dependent cell death involving the intrinsic pathway, the BAX/Bcl-xL ratio suggests a prevalence of the anti-apoptotic state. At least two points should be considered regarding this last observation: (1) the raise in other pro-apoptotic proteins such as BAD or BAK may have overcome this putative anti-apoptotic state, or a lower expression of other anti-apoptotic proteins, such as Bcl-2, may have inclined the balance towards a final pro-apoptotic state [51], and (2) the increase in the ratio BAX/Bcl-xL may constitute late compensatory anti-apoptotic mechanisms that at this point are insufficient to prevent apoptosis.
In contrast, the addition of 20-HETE to LNCaP cells grown in androgen-deprived media decreased cell apoptosis. However, the fact that neither HET0016 could abolish the anti-apoptotic effect of DHT, nor was the anti-apoptotic effect of 20-HETE per se of the same magnitude as the one elicited by DHT, suggests that the intracellular pathway, which involves 20-HETE, is not the only one triggered in DHT-induced protection from apoptosis.
Although 20-HETE has pro-apoptotic actions in cardiomyocytes, podocytes, and renal cells [52, 53], there is extensive evidence demonstrating anti-apoptotic action in lung tissue and pulmonary artery epithelial cells [34, 54, 55]. Thus, the interaction of pro- and anti-survival signaling pathways triggered by 20-HETE in different cell types is still not clear.
Most of the results discussed so far point to 20-HETE as a second messenger whose availability is necessary for the normal function of the androgen receptor. These findings along with the reduction of androgen receptor expression by HET0016 suggest an interplay between the expression and activity of 20-HETE-producing enzymes and the androgen receptor.
The lower expression of CYP4F2 in the presence of enzalutamide strongly points to an androgen receptor-mediated tonic regulation of CYP4F2 expression. However, although in our experimental conditions incubation with DHT failed to significantly increase CYP4F2 mRNA, an androgen-mediated CYP4F2 regulation at the transcriptional level cannot be ruled out. The increase in CYP4F2 protein, and in 20-HETE levels, by DHT suggests post-traductional CYP4F2 modifications by androgens, as previously described for other proteins [56]. In line, other authors have described that the expression of 20-hydroxylases in mouse and rat normal renal and prostatic tissues is increased by androgens [16, 57, 58]. Although analysis of the promoter region of CYP4F2 has not revealed a hormone response element, it has been reported that in human primary hepatocytes, as well as in HEPG2 cells, CYP4F2 transcription is mainly regulated by sterol regulatory element-binding proteins (SREBPs) [59].
Ongoing studies will unravel the complex mechanisms that could be implicated in the interactions between 20-hydroxylases and the androgen receptor.
Clinically, understanding of these androgen receptor signaling pathways is important, as they may represent potential mechanisms of resistance to the androgen deprivation therapy. Androgen receptor antagonists including casodex and flutamide have no effect on non-genomic androgen receptor signaling, as evidenced by ERK phosphorylation in the presence of these drugs [60]. The mechanism by which most of prostate tumors acquire castration resistance after androgen deprivation therapy is still not completely understood, but recent evidence that even low levels of testosterone can activate the androgen receptor and its signaling cascade has led to the search for novel therapeutic strategies that specifically target members of the androgen receptor pathway [26, 61]. In this study, we revealed that 20-hydroxyeicosatetraenoic acid (20-HETE), a 20-hydroxylated arachidonic acid metabolite, is involved in androgen-mediated prostate tumor cell survival and may be potentially targetable in the development of novel agents to inhibit all forms of the androgen receptor signaling in prostate cancer.
Acknowledgements
This work was supported by grant 1006/2016 from the National Cancer Institute (INC-Argentina). The authors are grateful to Drs. Elba Vázquez (University of Buenos Aires, Buenos Aires, Argentina), Adriana de Siervi (IBYME-CONICET, Buenos Aires, Argentina), and Richard Roman (University of Mississippi Medical Center, USA) for the supply of PC-3 cells, androgen receptor primers, and HET0016, respectively, and to Dr. Marcela Venara (CEDIE-CONICET, Buenos Aires, Argentina) for assistance with immunocytochemical studies.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Rose DP, Connolly JM. Effects of fatty acids and eicosanoid synthesis inhibitors on the growth of two human prostate cancer cell lines. Prostate. 1991;18:243–254. doi: 10.1002/pros.2990180306. [DOI] [PubMed] [Google Scholar]
- 2.Pandalai PK, Pilat MJ, Yamazaki K, et al. The effects of omega-3 and omega-6 fatty acids on in vitro prostate cancer growth. Anticancer Res. 1996;16:815–820. [PubMed] [Google Scholar]
- 3.Berquin IM, Min Y, Wu R, et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J Clin Invest. 2007;117:1866–1875. doi: 10.1172/JCI31494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bidoli E, Talamini R, Bosetti C, et al. Macronutrients, fatty acids, cholesterol and prostate cancer risk. Ann Oncol Off J Eur Soc Med Oncol. 2005;16:152–157. doi: 10.1093/annonc/mdi010. [DOI] [PubMed] [Google Scholar]
- 5.Graff JR, Deddens JA, Konicek BW, et al. Integrin-linked kinase expression increases with prostate tumor grade. Clin Cancer Res. 2001;7:1987–1991. [PubMed] [Google Scholar]
- 6.Oleksowicz L, Liu Y, Bracken RB, et al. Secretory phospholipase A2-IIa is a target gene of the HER/HER2-elicited pathway and a potential plasma biomarker for poor prognosis of prostate cancer. Prostate. 2012;72:1140–1149. doi: 10.1002/pros.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sved P, Scott KF, McLeod D, et al. Oncogenic action of secreted phospholipase A2 in prostate cancer. Cancer Res. 2004;64:6934–6940. doi: 10.1158/0008-5472.CAN-03-3018. [DOI] [PubMed] [Google Scholar]
- 8.Schneider C, Pozzi A. Cyclooxygenases and lipoxygenases in cancer. Cancer Metastasis Rev. 2011;30:277–294. doi: 10.1007/s10555-011-9310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroetz DL, Xu F. Regulation and inhibition of arachidonic acid Ω-hydroxylases and 20-hete formation. Annu Rev Pharmacol Toxicol. 2005;45:413–438. doi: 10.1146/annurev.pharmtox.45.120403.100045. [DOI] [PubMed] [Google Scholar]
- 10.Lasker JM, Chen WB, Wolf I, et al. Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of Cyp4F2 and Cyp4A11. J Biol Chem. 2000;275:4118–4126. doi: 10.1074/jbc.275.6.4118. [DOI] [PubMed] [Google Scholar]
- 11.Powell PK, Wolf I, Jin R, Lasker JM. Metabolism of arachidonic acid to 20-hydroxy-5,8,11, 14-eicosatetraenoic acid by P450 enzymes in human liver: involvement of CYP4F2 and CYP4A11. J Pharmacol Exp Ther. 1998;285:1327–1336. [PubMed] [Google Scholar]
- 12.Guo M, Roman RJ, Falck JR, et al. Human U251 glioma cell proliferation is suppressed by HET0016 [N-hydroxy-N’-(4-butyl-2-methylphenyl)formamidine], a selective inhibitor of CYP4A. J Pharmacol Exp Ther. 2005;315:526–533. doi: 10.1124/jpet.105.088567. [DOI] [PubMed] [Google Scholar]
- 13.Alexanian A, Rufanova VA, Miller B, et al. Down-regulation of 20-HETE synthesis and signaling inhibits renal adenocarcinoma cell proliferation and tumor growth. Anticancer Res. 2009;29:3819–3824. [PMC free article] [PubMed] [Google Scholar]
- 14.Borin TF, Zuccari DAPC, Jardim-Perassi BV, et al. HET0016, a selective inhibitor of 20-HETE synthesis, decreases pro-angiogenic factors and inhibits growth of triple negative breast cancer in mice. PLoS One. 2014;9:1–20. doi: 10.1371/journal.pone.0116247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexanian A, Miller B, Roman RJ, Sorokin A. 20-HETE-producing enzymes are up-regulated in human cancers. Cancer Genomics Proteomics. 2012;9:163–169. [PMC free article] [PubMed] [Google Scholar]
- 16.Strömstedt M, Hayashi S, Zaphiropoulos PG, Gustafsson JA. Cloning and characterization of a novel member of the cytochrome P450 subfamily IVA in rat prostate. DNA Cell Biol. 1990;9:569–577. doi: 10.1089/dna.1990.9.569. [DOI] [PubMed] [Google Scholar]
- 17.Wu C-C, Mei S, Cheng J, et al. Androgen-sensitive hypertension associates with upregulated vascular CYP4A12–20-HETE synthase. J Am Soc Nephrol. 2013;24:1288–1296. doi: 10.1681/ASN.2012070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasudevan H, Yuen VG, McNeill JH. Testosterone-dependent increase in blood pressure is mediated by elevated Cyp4A expression in fructose-fed rats. Mol Cell Biochem. 2012;359:409–418. doi: 10.1007/s11010-011-1035-7. [DOI] [PubMed] [Google Scholar]
- 19.Singh H, Cheng J, Deng H, et al. Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension. 2007;50:123–129. doi: 10.1161/HYPERTENSIONAHA.107.089599. [DOI] [PubMed] [Google Scholar]
- 20.Huggins C, Hodges CV. Studies of prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatase in metastatic carcinoma of the prostate. Arch Surg. 1941;43:209. doi: 10.1001/archsurg.1941.01210140043004. [DOI] [PubMed] [Google Scholar]
- 21.Perner S, Cronauer MVMV, Schrader AJAJ, et al. Adaptive responses of androgen receptor signaling in castration-resistant prostate cancer. Oncotarget. 2015;6:35542–35555. doi: 10.18632/oncotarget.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nithipatikom K, Isbell MA, See WA, Campbell WB. Elevated 12- and 20-hydroxyeicosatetraenoic acid in urine of patients with prostatic diseases. Cancer Lett. 2006;233:219–225. doi: 10.1016/j.canlet.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 23.Rieger MA, Ebner R, Bell DR, et al. Identification of a novel mammary-restricted cytochrome P450, CYP4Z1, with overexpression in breast carcinoma identification of a novel mammary-restricted cytochrome P450, CYP4Z1, with overexpression in breast carcinoma. Cancer Res. 2004;64:2357–2364. doi: 10.1158/0008-5472.CAN-03-0849. [DOI] [PubMed] [Google Scholar]
- 24.Tradonsky A, Rubin T, Beck R, et al. A search for reliable molecular markers of prognosis in prostate cancer. Am J Clin Pathol. 2012;137:918–930. doi: 10.1309/AJCPF3QWIG8FWXIH. [DOI] [PubMed] [Google Scholar]
- 25.Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013;32:5501–5511. doi: 10.1038/onc.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanella L, Di Giacomo C, Acquaviva R, et al. Effects of ellagic acid on angiogenic factors in prostate cancer cells. Cancers (Basel) 2013;5:726–738. doi: 10.3390/cancers5020726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo M, Roman RJ, Fenstermacher JD, et al. 9L gliosarcoma cell proliferation and tumor growth in rats are suppressed by N-hydroxy-N’-(4-butyl-2-methylphenol) formamidine (HET0016), a selective inhibitor of CYP4A. J Pharmacol Exp Ther. 2006;317:97–108. doi: 10.1124/jpet.105.097782. [DOI] [PubMed] [Google Scholar]
- 30.Yu W, Chen L, Yang Y-Q, et al. Cytochrome P450 ω-hydroxylase promotes angiogenesis and metastasis by upregulation of VEGF and MMP-9 in non-small cell lung cancer. Cancer Chemother Pharmacol. 2011;68:619–629. doi: 10.1007/s00280-010-1521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horoszewicz JS, Leong SS, Kawinski E, et al. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- 32.Kaighn ME, Narayan KS, Ohnuki Y, et al. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Investig Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- 33.Miyata N, Taniguchi K, Seki T, et al. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol. 2001;133:325–329. doi: 10.1038/sj.bjp.0704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhanasekaran A, Bodiga S, Gruenloh S, et al. 20-HETE increases survival and decreases apoptosis in pulmonary arteries and pulmonary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2009;296:H777–H786. doi: 10.1152/ajpheart.01087.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kashiwagi E, Shiota M, Yokomizo A, et al. EP2 signaling mediates suppressive effects of celecoxib on androgen receptor expression and cell proliferation in prostate cancer. Prostate Cancer Prostatic Dis. 2014;17:10–17. doi: 10.1038/pcan.2013.53. [DOI] [PubMed] [Google Scholar]
- 36.Burnstein KL. Regulation of androgen receptor levels: implications for prostate cancer progression and therapy. J Cell Biochem. 2005;95:657–669. doi: 10.1002/jcb.20460. [DOI] [PubMed] [Google Scholar]
- 37.Ponguta LA, Gregory CW, French FS, Wilson EM. Site-specific androgen receptor serine phosphorylation linked to epidermal growth factor-dependent growth of castration-recurrent prostate cancer. J Biol Chem. 2008;283:20989–21001. doi: 10.1074/jbc.M802392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Y, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66:7783–7792. doi: 10.1158/0008-5472.CAN-05-4472. [DOI] [PubMed] [Google Scholar]
- 39.Migliaccio A, Castoria G, Di Domenico M, et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gille H, Kortenjann M, Thomae O, et al. ERK phosphorylation potentiates elk-1-mediated ternary complex formation and transactivation. EMBO J. 1995;14:951–962. doi: 10.1002/j.1460-2075.1995.tb07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia V, Gilani A, Shkolnik B et al (2017) 20-HETE signals through G protein-coupled receptor GPR75 (Gq) to affect vascular function and trigger hypertension. Circ Res. doi:10.1161/CIRCRESAHA.116.310525 [DOI] [PMC free article] [PubMed]
- 42.Akbulut T, Regner KR, Roman RJ, et al. 20-HETE activates the Raf/MEK/ERK pathway in renal epithelial cells through an EGFR- and c-Src-dependent mechanism. Am J Physiol Renal Physiol. 2009;297:F662–F670. doi: 10.1152/ajprenal.00146.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nowicki S, Chen SL, Aizman O, et al. 20-Hydroxyeicosa-tetraenoic acid (20 HETE) activates protein kinase C. Role in regulation of rat renal Na+,K+−ATPase. J Clin Invest. 1997;99:1224–1230. doi: 10.1172/JCI119279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng Q, Han Y, Bao Y, et al. 20-HETE increases NADPH oxidase-derived ROS production and stimulates the L-type Ca2+ channel via a PKC-dependent mechanism in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2010;299:H1109–H1117. doi: 10.1152/ajpheart.00067.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16:2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- 46.Knudsen KE, Arden KC, Cavenee WK. Multiple G 1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J Biol Chem. 1998;273:20213–20222. doi: 10.1074/jbc.273.32.20213. [DOI] [PubMed] [Google Scholar]
- 47.Eto M, Bennouna J, Hunter OC, et al. C16 ceramide accumulates following androgen ablation in LNCaP prostate cancer cells. Prostate. 2003;57:66–79. doi: 10.1002/pros.10275. [DOI] [PubMed] [Google Scholar]
- 48.Bhardwaj A, Singh S, Srivastava SK, et al. Modulation of protein phosphatase 2A activity alters androgen-independent growth of prostate cancer cells: therapeutic implications. Mol Cancer Ther. 2011;10:720–731. doi: 10.1158/1535-7163.MCT-10-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohlson N, Wikström P, Stattin P, Bergh A. Cell proliferation and apoptosis in prostate tumors and adjacent non-malignant prostate tissue in patients at different time-points after castration treatment. Prostate. 2005;62:307–315. doi: 10.1002/pros.20139. [DOI] [PubMed] [Google Scholar]
- 50.Wang Z, Tang X, Li Y, et al. 20-Hydroxyeicosatetraenoic acid inhibits the apoptotic responses in pulmonary artery smooth muscle cells. Eur J Pharmacol. 2008;588:9–17. doi: 10.1016/j.ejphar.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 51.Portt L, Norman G, Clapp C, et al. Anti-apoptosis and cell survival: a review. Biochim Biophys Acta - Mol Cell Res. 2011;1813:238–259. doi: 10.1016/j.bbamcr.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 52.Bao Y, Wang X, Li W, et al. 20-Hydroxyeicosatetraenoic acid induces apoptosis in neonatal rat cardiomyocytes through mitochondrial-dependent pathways. J Cardiovasc Pharmacol. 2011;57:294–301. doi: 10.1097/FJC.0b013e3182073c78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eid AA, Gorin Y, Fagg BM, et al. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes. 2009;58:1201–1211. doi: 10.2337/db08-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacobs ER, Bodiga S, Ali I, et al. Tissue protection and endothelial cell signaling by 20-HETE analogs in intact ex vivo lung slices. Exp Cell Res. 2012;318:2143–2152. doi: 10.1016/j.yexcr.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bodiga S, Gruenloh SK, Gao Y, et al. 20-HETE-induced nitric oxide production in pulmonary artery endothelial cells is mediated by NADPH oxidase, H2O2, and PI3-kinase/Akt. Am J Physiol Lung Cell Mol Physiol. 2010;298:L564–L574. doi: 10.1152/ajplung.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Svensson C, Ceder J, Iglesias-Gato D, et al. REST mediates androgen receptor actions on gene repression and predicts early recurrence of prostate cancer. Nucleic Acids Res. 2014;42:999–1015. doi: 10.1093/nar/gkt921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holla VR, Adas F, Imig JD, et al. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci U S A. 2001;98:5211–5216. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh H, Schwartzman ML. Renal vascular cytochrome P450-derived eicosanoids in androgen-induced hypertension. Pharmacol Reports. 2008;60:29–37. [PubMed] [Google Scholar]
- 59.Hsu M-H, Savas U, Griffin KJ, Johnson EF. Regulation of human cytochrome P450 4F2 expression by sterol regulatory element-binding protein and lovastatin. J Biol Chem. 2007;282:5225–5236. doi: 10.1074/jbc.M608176200. [DOI] [PubMed] [Google Scholar]
- 60.Peterziel H, Mink S, Schonert A, et al. Rapid signalling by androgen receptor in prostate cancer cells. Oncogene. 1999;18:6322–6329. doi: 10.1038/sj.onc.1203032. [DOI] [PubMed] [Google Scholar]
- 61.Bishr M, Saad F. Overview of the latest treatments for castration-resistant prostate cancer. Nat Rev Urol. 2013;10:522. doi: 10.1038/nrurol.2013.137. [DOI] [PubMed] [Google Scholar]