Abstract
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor best known for its ability to mediate the effects of environmental toxins such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD or dioxin), polycyclic aromatic hydrocarbons (PAHs), benzene, and polychlorinated biphenyls (PCBs) through the initiation of transcription of a number of metabolically active enzymes. Therefore, the AHR has been studied mostly in the context of xenobiotic signaling. However, several studies have shown that the AHR is constitutively active and plays an important role in general cell physiology, independently of its activity as a xenobiotic receptor and in the absence of exogenous ligands. Within the pituitary, activation of the AHR by environmental toxins has been implicated in disruption of gonadal development and fertility. Studies carried out predominantly in mouse models have revealed the detrimental influence of several environmental toxins on specific cell lineages of the pituitary tissue mediated by activation of AHR and its downstream effectors. Activation of AHR during fetal development adversely affected pituitary development while adult models exposed to AHR ligands demonstrated varying degrees of pituitary dysfunction. Such dysfunction may arise as a result of direct effects on pituitary cells or indirect effects on the hypothalamic-pituitary-gonadal axis. This review offers in-depth analysis of all aspects of AHR biology, with a particular focus on its role and activity within the adenohypophysis and specifically in pituitary tumorigenesis. A novel mechanism by which the AHR may play a direct role in pituitary cell proliferation and tumor formation is postulated. This review therefore attempts to cover all aspects of the AHR’s role in the pituitary tissue, from fetal development to adult physiology and the pathophysiology underlying endocrine disruption and pituitary tumorigenesis.
Keywords: Aryl Hydrocarbon Receptor (AHR), Xenobiotic Signaling, Pituitary Cells, Aryl Hydrocarbon Receptor Nuclear Translocator (ARNT), AhR Repressor (AHRR)
The AHR: Structure, Function, and Xenobiotic Signaling
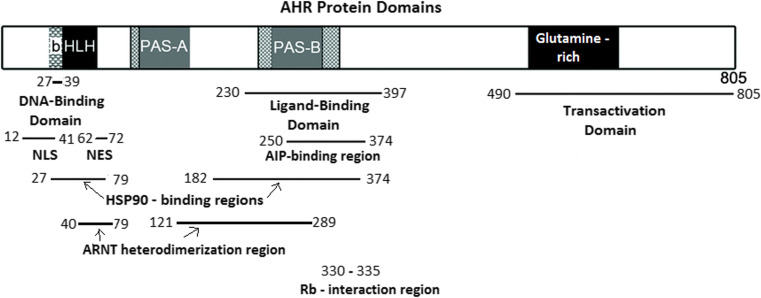
Aryl hydrocarbon receptor (AHR) is best characterized with regards to its xenobiotic function as a mediator of environmental toxin processing through the transcriptional regulation of genes that degrade, metabolize, and excrete toxins including dioxins, polycyclic aromatic hydrocarbons (PAHs), benzene, and polychlorinated biphenyls (PCBs) [1]. The AHR is a cytosolic ligand-activated transcription factor belonging to the basic helix-loop-helix (bHLH)/Period (Per)-aryl hydrocarbon receptor nuclear translocator (ARNT)-single minded (SIM) (PAS) family of transcription factors. The structure of the AHR gene and protein is similar to other members of the bHLH/PAS family members, although among this family of transcription factors, the AHR is the only one that requires ligand activation [2]. The AHR gene located on chromosome 7p21 in the human karyotype is highly conserved in animals and expressed in almost all tissues, indicating a ubiquitous role in general cell physiology [3]. The AHR protein is composed of several structural domains, an N-terminal bHLH domain responsible for DNA binding, a glutamine-rich C-terminal transcriptional activation domain (TAD), and a median PAS domain with two degenerate repeats, PAS-A and PAS-B, that serve as ligand binding domains and mediate interactions with a number of other proteins including chaperones (Fig. 1). The region containing the two PAS domains and part of the bHLH domain is responsible for the formation of heterodimers between AHR and other PAS domain containing proteins such as ARNT which is an essential partner for the xenobiotic activity of AHR [4].
Fig. 1.
AHR protein domains and the relevant amino acid sequences responsible for interactions with other proteins. bHLH basic helix-loop-helix, PAS PerARNT-Sim homology domain, NLS nuclear localization sequence, NES nuclear export sequence
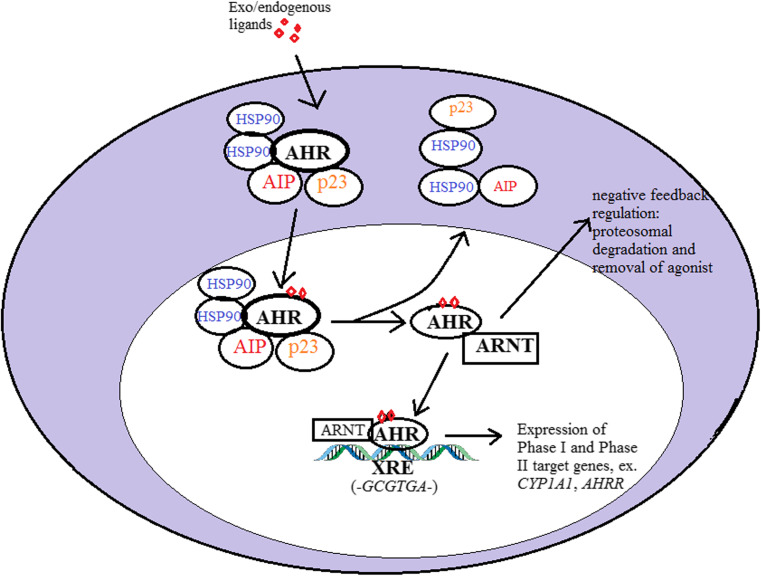
In the absence of exogenous or endogenous ligands, the inactive AHR is held in the cytosol in a complex made up of the heat shock protein 90 (HSP90), p23, and the AHR-interacting protein (AIP) co-chaperone proteins [5]. In the presence of an agonist, ligand binding to the AHR causes a conformational change that exposes the nuclear localization sequence (NLS) and results in its nuclear translocation. Upon entering the nucleus, AHR, with the help of its transcriptionally active partner ARNT, dissociates from its chaperone complex and heterodimerizes with ARNT, forming a transcriptionally active complex which is required for DNA binding [6, 7]. The AHR/ARNT heterodimer directs transcription of target genes that have the consensus xenobiotic response elements (XRE)—5′T/GnGCGTGA/CG/CA-3′—in their promoter regions [8, 9]. Once AHR has completed its function, it is exported outside the nucleus where it is degraded by the proteasome-ubiquitin system [10]. Figure 2 below highlights the principal stages of canonical xenobiotic signaling in response to AHR agonists.
Fig. 2.
Classical AHR-directed xenobiotic signaling in response to an AHR exogenous or endogenous agonist. Upon binding the ligand, AHR translocates to the nucleus where it dissociates from its cytoplasmic complex and heterodimerizes with ARNT. The AHR/ARNT complex binds to target xenobiotic response elements (XRE) containing the consensus binding sequence and recruits transcriptional machinery to facilitate the transcription of AHR target genes. This is followed by a series of negative feedback mechanisms that restrict AHR activity and minimize the cytotoxic impact of the agonist on target cells
Ligand-activated AHR mediates the transcription of xenobiotic metabolizing phase I and phase II enzymes, most notably, the cytochrome P450 (CYP) family of enzymes. Phase I enzymes most commonly transcribed in response to AHR activation include CYP1A1, CYP1B1, and CYP1A2 while well-known phase II responsive genes include glutathione S-transferase (GST), NADPH, and UDP-glucuronosyltransferase. These enzymes can metabolize and degrade AHR agonists into harmless substances that can be cleared by the body [11]. Another very important AHR target gene is the AHR repressor (AHRR); the function of which is to directly inhibit AHR transactivation by competing with ARNT for the activating site on the receptor and hence, upon binding AHR, renders it inactive. Being a transcriptional target of AHR, AHRR exerts a direct negative feedback on the xenobiotic response of AHR by limiting its activation [12]. Similarly, phase I enzymes such as the CYP proteins act as negative feedback controls by degrading the xenobiotic toxins that activate the AHR. In fact, the half-life of inactive AHR protein is 28 h while the half-life of agonist-activated AHR is around 3 h, demonstrating the importance of this negative feedback loop in restricting AHR activation [13].
Besides being tightly regulated, AHR responses are also ligand- and cell context-specific. Similar agonists can cause different responses in different cell types, and similarly, different agonists may cause activation of different response pathways in the same cell type. Studies aimed at the identification of endogenous activating ligands of the AHR which are likely to account for its constitutive activity in the absence of known exogenous environmental ligands have been reported in the literature [14]. More recently, the expression of a novel target of AHR, stanniocalcin 2 (STC2), was noted to be increased by the novel endogenous ligand cinnabarinic acid but not by classical exogenous agonists TCDD and benzo α-pyrene (BαP) in primary hepatocytes, while in regulatory T cells, cinnabarinic acid, but not TCDD, stimulates the production of interleukin-22 through AHR activation and transcriptional regulation [15, 16]. Novel endogenous agonists of AHR have been identified from metabolic by-products, such as kynurenine, which is a relatively weak AHR agonist produced from the metabolic breakdown of tryptophan and capable of activating AHR sufficiently to elicit a noticeable response in many cell contexts [17].
Murine models have established that environmental toxins, TCDD in particular, require the presence of a functional AHR protein to impact the biology of living cells. Using AHR knock out mice, three studies have shown that TCDD toxicity is abolished in the absence of a functional AHR. However, the observation of other seemingly unrelated phenotypes in these mice including reduced reproductive ability, lower body weight, decreased liver size with fibrotic bodies, impaired immune response, and hypertrophy or hyperplasia of a number of organs with age [18–21] prompted a search for a role for AHR that went beyond that of a xenobiotic biosensor.
Non-xenobiotic Functions of the AHR
The AHR is highly conserved in several invertebrates and vertebrates but it is only in the higher vertebrates that it is able to bind and detoxify harmful substances indicating that this function is a late development in the evolutionary biology of this receptor. However, the AHR is unable to fully metabolize all its substrates. TCDD, which is a potent activator of AHR, is itself very slowly and incompletely degraded by AHR-mediated enzyme activity [22]. The arrival of novel high-throughput technologies further highlighted the complex and variable nature of the AHR biology, providing increasing evidence for a role of the AHR beyond that of a xenobiotic receptor.
RNA expression profiles comparing AHR wild-type mice to AHR-null mice in the absence of exogenous ligands identified differential expression of around 400 genes in the liver, showcasing the constitutive role of AHR in hepatic biology [23]. Similarly, complementary chromatin immunoprecipitation DNA microarray of AHR binding sites revealed that around 50% of AHR protein was bound to DNA regions lacking an XRE core sequence after TCDD treatment, thereby increasing the spectrum of putative AHR target genes beyond that encompassing classical xenobiotic response genes [24]. There is now an increasing body of evidence supporting a role/s of AHR in cellular homeostasis, including cell cycle regulation, epithelial barrier function, cell migration, and alteration of immunological responses.
Involvement in Tissue Development and Differentiation
In mouse models, AHR protein is detectable from day 10 post fertilization. The observed severe developmental problems in AHR-null mice support a role in normal development [25]. Additionally, female mice treated with dioxin during pregnancy have significantly smaller pups and smaller litter sizes, and lose more pups during the pregnancy. The pups also suffer growth retardation and delayed or absent sexual development, possibly through a direct or indirect effect on the hormone physiology responsible for sexual maturity [26–30]. In humans, children whose mothers were suspected to have been exposed to dioxin during pregnancy also have disorders of sexual differentiation and maturation [31, 32]. The AHR has been shown to be an important regulator of hematopoietic stem cell (HSC) differentiation, with the AHR acting as a repressor of HSC proliferation and differentiation by maintaining these cells in a quiescent state. According to the authors, AHR is required to maintain a population of HSCs to ensure the development of new hematopoietic cells [33]. TCDD-treated mice undergo thymic atrophy leading to a decreased number of T and B cells which fail to mature [34, 35]. Owing to its role in regulating B and T cell populations, mice lacking functional AHR develop lymphomas due to the lack of AHR-induced repression of B and T cell proliferation [36]. In hematopoietic progenitor cells, AHR mediates cell fate, with chronic AHR activation causing megakaryocyte differentiation in mice [37]. AHR was also shown to regulate the metabolic ability of regulatory type 1T (Tr1) cells through its interaction with hypoxia-induced factor 1α (HIF1α), promoting differentiation of Tr1 cells in normal conditions, while in hypoxic environments, HIF1α inactivates AHR, inhibiting Tr1 differentiation [38]. AHR also interacts directly with SOX2, a master regulator of “stemness” that determines cell fate in multiple cell types, through which AHR is able to determine the development of stem cells in breast cancer [39]. The influence of AHR particularly on cell lineages of immune cells proposes a putative role for the AHR in regulating immune system responses, an avenue that has lately garnered significant interest in the field of cancer immunotherapy [40, 41].
Involvement in Inflammation and Immunity
AHR has been shown to alter both the innate and adaptive immune response systems in mouse models. AHR-null mice challenged with liposaccharide (LPS) led to much lower cytokine expression when compared to wild-type, with putative XREs found on the promoters of genes for the interleukins IL-1β, IL-6, Il-8, and IL-21 in different cell settings and with different mechanisms of action [42–44]. As a result, several AHR agonists have been shown to elicit inflammation, particularly by direct topical exposure on skin, leading to tumor formation in mouse models [45, 46]. Murine skin tissue exposed to AHR agonists has increased expression on IL-1β, Tnf, Il-6, Tgfβ, and Cyp1b1 genes in additional to chronic inflammation [47]. Similar effects on cytokine and chemokine levels have been observed in various other tissues, such as gliomas and breast and hematopoietic cells, and have been shown to be a result of the interaction between AHR and members of the nuclear factor NF-κβ, RelA (p65), and RelB [48–50]. AHR was also able to potentiate the expression of the prostaglandin COX2 in response to BαP agonist in cells of epithelial origin through an XRE located on the COX2 promoter [36, 51]. The AHR has also been shown to regulate B and T cell function and may be involved in immune tolerance. TCDD significantly inhibited the ability of CH12.LX B cells from becoming activated, and expressed immunoglobulins in response to LPS presence [52]. TCDD also induced expression of the suppressor of cytokine signaling (Soc2) gene in B cells further immunosuppressing the response to microbial presence [53]. In fact, the potential immunosuppressive role of AHR agonists has been proposed as a target for immunotherapy in cancer. Tryptophan metabolites such as kynurenine act as endogenous agonists of the AHR and play a significant role in immune tolerance of tumor cells resulting in dendritic cells with defective antigen presentation and may also repress T cell function [54, 55].
Involvement in Cell Cycle and Cancer
Several lines of evidence also indicate a role for the AHR in regulation of the cell cycle and hence cell growth and proliferation and an emerging; albeit unclear, role in cancer biology is gradually being revealed. Studies carried out on several cell lines have shown that AHR activity, both in the presence and absence of applied exogenous ligand, is able to alter cell cycle progression. Rat 5L hepatoma cells treated with TCDD showed a marked reduction in cell cycle progression, with knock down of AHR resulting in loss of this behavior [56–58]. Addition of different AHR agonists was able to significantly reduce cell proliferation in a number of studies [59–61]. Interestingly, removal of AHR in some cell types also resulted in cell cycle arrest in specific cell lines of liver, epithelial, and breast origin [58, 62, 63]. Although several studies reported the cell cycle-regulating ability of AHR requires an AHR-activating agonist, other studies on varying cell types reported a non-agonist-driven mechanism of AHR action. Transient over-expression of AHR in murine embryonic fibroblast 3T3-L1 cells repressed cell cycle progression in the absence of any exogenous activating ligand [64]. Similarly, AHR protein lacking a ligand transactivation domain was still able to lower cell proliferation rates in MCF-7 cells [61]. Bone osteosarcoma SAOS-2 cells over-expressing AHR undergo a reduction in S phase transition both in ligand-stimulated and unstimulated conditions, indicating that ligand activation of AHR is not required for the cell cycle repression to occur in these cells [65]. An analysis of AHR expression in 967 established cancer cell lines found relatively high expression in chondrosarcomas, esophageal, pancreatic and liver cell types while many leukemic cell types had low expression of AHR [66]. However, since most of this work was carried out in immortalized cell lines with genetically modified cell cycles, these results must be interpreted with caution.
However, murine models also replicate some findings obtained from cell cultures. AHR-null mice display hyperproliferative growth of stomach tissue [18, 25] while mice with defective AHR display higher indices of proliferation, polyploidy, and inflammation, thereby showing that the role of AHR on cell cycle and proliferation is highly cell-specific [67, 68]. AHR was greatly reduced in mouse liver tumors resulting from retinoblastoma (Rb) gene ablation [69], and AHR-null transgenic adenocarcinoma of mouse prostate (TRAMP) mice develop prostate tumors with much greater frequency than that of wild-type mice [67, 70]. Similarly, diethyl-nitrosamine-treated AHR-null mice developed hepatic tumors in much greater frequency than AHR wild-type mice [68]. Some human tumors also display altered AHR signaling. Primary leukemia cell cultures from patients with acute lymphoblastic leukemia had reduced expression of the AHR gene [71] while cancerous tissue in breast, lung, gastric, pancreatic, and prostate tissues had higher AHR expression [72–78]. Therefore, although several lines of evidence exist to validate a role for AHR in the regulation of cell cycle and cell proliferation, the evidence does not point to a clear indication of whether AHR acts as an oncogene or tumor suppressor, and this is likely due to cell-type and agonist specificity that alter its behavior in relation to the cell cycle, as is the case for its xenobiotic function.
Studies regarding the mechanism(s) by which AHR interacts with the cell cycle demonstrated a number of interesting avenues. Ge and Elfrink [79] and Puga and colleagues [65] revealed a direct interaction between AHR, the hypophosphorylated retinoblastoma (Rb) protein, and the transcriptional cofactor p300 in 5L hepatoma and MCF-7 breast carcinoma cells. The Rb protein in its hypophosphorylated or active state inhibits the G1/S transition by binding to and repressing E2F transcription factors which are responsible for progression into the S phase and DNA replication [80]. AHR binds to active Rb and maintains it in its hypophosphorylated state, thereby prolonging the inhibition of E2F factors and causing cell cycle arrest [65]. Another mechanism that has been proposed involves the increased transcriptional expression of cell cycle regulator genes, in particular CDKN1B, coding for p27/Kip1, and CDKN1A, coding for p21/Cip1 proteins which are important inhibitors of cyclin-dependent kinases (CDKs) and hence inhibitors of the cell cycle [81–83]. AHR has been shown to induce expression of the p27 gene in murine cell lines following TCDD addition while in vivo murine models have been shown to respond to TCDD by induction of p21 gene expression [83, 84]. Mouse liver cells undergoing regeneration after partial hepatectomy revealed that p21 expression was induced by TCDD during regeneration suppression and that transactivation of p21 gene expression was occurring directly through AHR binding to the Cdkn1a promoter containing a 5′-GGGA-3′ tetranucleotide repeat motif which the authors termed the non-consensus xenobiotic binding site (NC-XRE). Therefore, the AHR may be able to modulate cell cycle progression through both XRE and NC-XRE pathways which are likely to act synergistically or antagonistically depending on cell type and presence of agonist [84]. Additional mechanisms by which AHR might be involved in tumorigenesis include the transcriptional targeting of genes involved in cancer formation, such as fibroblast growth factor 9 (FGF9) and vascular endothelial growth factor A (VEGFa), both potent mediators of cell growth and angiogenesis which possess XREs in their promoters and have been shown to increase in expression in response to AHR agonists in head and neck squamous cell carcinoma cell lines [85]. Additionally, the ability of AHR to influence inflammatory cytokines described earlier may play a role in mediating local cell growth through pro-inflammatory responses. A summary of these mechanisms is given below (Fig. 3).
Fig. 3.
Summary of some of the possible mechanisms through which AHR alters the cell cycle and proliferation of cells. 1 AHR has been shown to interact with the retinoblastoma (Rb) protein both in the presence and absence of ligand and maintain Rb in its active hypophosphorylated state which is able to repress E2F transcription factors, thereby blocking cell cycle progression. 2 AHR is able to transactivate the expression of mitogenic proteins (FGF4, VEGFA, and platelet-derived growth factor (PDGF)) which have active XREs and might increase local cell proliferation. 3 AHR was also shown to be able to modulate cytokine expression and hence influence local inflammation which can lead to cell proliferation. 4 AHR was also reported to activate the transcription of the important cyclin-dependent kinase inhibitors (CDKNs) p21 and p27 through both XRE and non-consensus (NC-XRE)-driven mechanisms. CDKNs block phosphorylation of Rb and inhibit interactions between CDKs and cyclins, thereby halting cell cycle progression from the G1 to S phase
The AHR has also been implicated in other aspects related to carcinogenesis, most notably apoptosis and cell migration; both of which are hallmarks of cancer progression and metastasis. TCDD inhibited apoptosis in a several lymphoma cell lines through altered COX-2 and Bcl-2 expression [36]. A comparison of 1c1c7 cells having functional AHR to others with reduced AHR expression found a difference in processing of caspases leading to different responses to pro-apoptotic signals [86]. Some studies have indicated the presence of an activated AHR during cell contact loss involving Jun N-terminal kinase (JNK) activation, and several cell lines have increased cell migration with an activated AHR [76, 87–89]. AHR was also shown to regulate the expression of several key genes involved in cell adhesion, such as E-cadherin, proteases, cytokines, and cytokines that can influence cell adhesion and migration [76, 90, 91]. However, the influence of AHR on cell–cell contact and migration might once again be cell context-specific. In triple-negative breast cancer (TNBC), increased tryptophan metabolism resulted in increased kynurenine, an endogenous AHR ligand. The subsequent activation of AHR resulted in resistance to anoikis and increased metastatic potential in TNBC through increases migration behavior, a result which was confirmed using classical AHR agonists TCDD and BαP [92, 93]. Inversely, in lung cancer models, increased expression of AHR was able to inhibit migration through an increase in autophagy [94]. Similarly, 4T1.2 rodent mammary tumor cells treated with TCDD showed a 50% reduction in metastasis to the lungs and mammary glands [95]. Therefore, once again, the elusive nature of AHR biology on cell carcinogenesis is greatly dependent on context although a clear functional impact is repeatedly reported.
Crosstalk with Other Signaling Pathways in Cancer
Adding to the complexity of AHR’s biological role, it has now been clearly established that AHR signaling crosstalks with a number of other pathways which can affect each other in turn and hence complicate further the degree of variability that is the function of AHR. Of particular interest are the interactions between AHR and hormonally regulated nuclear hormone receptors and their role in carcinogenesis. AHR has been shown to interact functionally with the estrogen receptor α (ERα) and repress its transcriptional activity by sequestering it to the XRE on AHR target genes. TCDD was able to repress ER target gene expression in the presence of estradiol in MCF-7 cells, an ER-positive breast cancer cell line. Additionally, AHR enhanced the proteosomal degradation of ERα [96, 97]. AHR also interacts with two members of the NF-κβ family, RelA (p65) and RelB [50, 98]. Interaction with these proteins potentiated the expression of the oncogene Myc, which contains six XRE sites on its promoter, in particular breast cancer cell lines [49] while another study found that AHR represses Myc expression in another breast cancer cell line, Hs578T, since removal of the six XREs from the Myc promoter resulted in a fivefold increase in MYC-reporter activity [99]. AHR has also been shown to interact functionally with the cAMP signaling pathway, an important secondary messenger cascade pathway regulating a number of functions in almost all cell types, particularly in endocrine cells where cAMP regulates hormone secretion [100]. cAMP stimulation through forskolin treatment of Hepa1 cells resulted in localization of AHR to the nucleus without the requirement of its DNA-binding partner ARNT. The authors hypothesize that activation through cAMP causing the AHR to change conformation allowing novel protein-protein interactions and to become active in a ligand-independent manner. cAMP did not activate XRE-driven transcription but instead inhibited TCDD-mediated response on xenobiotic activity [101]. The significance of the AHR-cAMP interaction is not yet fully understood, and hence, the role of this pathway in regulating cell biology is yet to be elucidated, but given the ubiquitous role of cAMP signaling particularly in hormone regulation, such an interaction may yet prove highly influential.
Once again, most of the evidence for the role of AHR in such diverse molecular and physiological pathways was carried out in either cell cultures or mouse/rat models. The mouse and human AHR protein share 85% of sequence homology with quite significant difference in the C-terminal end containing the transactivation domain where they share much less homology (58%). In fact, mouse and human AHRs mediate quite different transcriptional activity in hepatocytes [102–104], and TCDD has a tenfold higher affinity for mouse AHR than the human protein [105]. Therefore, studies carried out in mice might be under- or over-estimating the effects when superimposed to the human biology. However, the wealth of information available regarding the multitude of functions regulated by the AHR in such diverse systems and with the intricacies of cell-type and agonist (or lack of) specificity add another level of complexity to the role that AHR plays in human cell physiology.
AHR in the Pituitary Gland
Given the body of evidence implicating ligand activation and non-ligand activation of AHR in endocrine disruption and tumorigenesis in various cell types, very little information regarding the role of AHR in the pituitary gland, the master regulator of endocrine function, is available. AHR appears to be expressed in all cell lineages of the pituitary gland as demonstrated by immunohistochemical or mRNA analyses of both mouse and human pituitary tissues [106–108]. Studies have proven the responsiveness of the pituitary gland to AHR agonists via the classical xenobiotic signaling pathway. In a commonly used pituitary cell line, GH3, a rat sommatotroph/lactotroph cell line, the AHR agonist β-naphthoflavone induced expression of Cyp1a1 gene and suppressed AHR expression [109]. Similarly, in rat primary pituitary cells, TCDD was able to activate AHR and expression of its target genes Cyp1a1, Cyp1b1, and Cyp1a2 [108]. In C57Bl/6 mice, TCDD and β-naphthoflavone, a strong and weak AHR agonist, respectively, both increased expression of AHR target genes Cyp1a1 and Ahrr in pituitary cells [110].
Disruption of Endocrine Pituitary Function by AHR Activation
Most work relating to the action/s of AHR to the pituitary has focused on its endocrine disruptive ability. AHR agonists have long been shown to affect the hypothalamic-pituitary-gonadal (HPG) axis in human beings. As described earlier, both pregnant human women exposed to dioxin and lab female mice treated with TCDD during pregnancy display altered reproductive and growth potential in their young, showing that dioxin can alter the HPG axis even in utero [27, 111, 112]. Similarly, TCDD treatment of mice during early gestation reduced pituitary expression of both gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), leading to impaired sexual maturity and reproduction, together with impaired growth hormone (GH) production in fetal and neonatal rats resulting in smaller pups with reduced IGF-1 [30, 113–116]. Takeda and colleagues [29] used two strains of mice with different AHR affinities for TCDD and showed that the effect of TCDD on hormone expression was AHR-dependent. The C57Bl/6L mice having higher sensitivity to dioxin showed a much higher effect of the toxin on the repression of gonadotropin expression, while the DBA/2J mice with low affinity for TCDD showed little to no effect on LH and FSH expression. The C57BL/6L mice also had a significant reduction in the gonadotropin-releasing hormone receptor (GnRHR), and therefore, identifying a mechanism by which the reduction in gonadotropins was achieved. The same group also showed that by impairing the HPG axis at a critical period during development, these impairments could be imprinted into the mouse models and carried forward into other generations [29, 114]. The authors also proposed a mechanism by which the reduction in LH and FSH is achieved via agonist-activated AHR, through the induction of expression of histone deacetylase (HDAC) enzymes which possess functional XREs in their promoter region. They hypothesized that an increase in HDAC expression would cause epigenetic silencing of the gonadotropin and GH genes although no specific evidence is provided for this mechanism [29].
Different studies have shown different sensibilities of hormone expression to TCDD toxicity. Most studies agree that the gonadotropins, LH and FSH, are the most affected, while evidence for the effect on GH and prolactin is more inconsistent, and the effect on the thyroid-stimulating hormone (TSH) and adrenocorticotropic hormone (ACTH) production appears to be minimal [29, 117–119]. The mouse and rat GH genes have one and two XRE core motifs, respectively, in their promoters in which authors suggest that they could bind AHR and reduce expression of GH by competing for enhancer sites, thereby inhibiting other transcription factors from binding and transcriptionally activating the GH expression [120, 121]. In rat pituitary cells, TCDD was able to increase estrogen receptor (Esr1) mRNA expression and hindered estradiol-induced prolactin (Prl1) gene expression by sequestering the ER to XRE DAN sequences together with the AHR [108].
Studies using a less potent AHR agonist, β-naphthoflavone, have yielded similar results. In GH3 cells, activation of AHR by β-naphthoflavone caused reduction in prolactin expression while GH was unaffected [109]. However, in the rainbow trout pituitary, β-naphthoflavone decreased expression of Gh and pro-opiomelanocortin (Pomc) genes. Pomc is a precursor protein to the hormone ACTH in the anterior pituitary and α-melanocyte stimulating hormone (MSH) from the intermediate pituitary which regulates the production of melanin [122]. Both β-naphthoflavone and TCDD treatment in mouse pituitary primary cells and AtT-20 adrenocorticotropinoma mouse cells caused a threefold increase in Pomc gene expression and protein expression of its products ACTH and beta-endorphin. The authors suggest that this is a direct result of AHR transcriptional regulation since several XRE sequences were found upstream of the murine Pomc gene [123]. While in the cynomolgus monkey, TCDD treatment caused an increase in corticotrophin-releasing hormone from the paraventricular nucleus of the hypothalamus but no effect on Pomc expression levels [124]. In the rainbow trout, treatment with xenoestrogens, weak activators of AHR, caused a significant increase in GH and prolactin expression in pituitary tissue. TCDD treatment also yielded the same outcome on prolactin and GH expression and pre-treatment with an AHR antagonist, and attenuated the effect on prolactin but not on GH expression although the effects where not consistent since a biphasic behavior was observed, with stimulation occurring at low concentrations of TCDD and inhibition at high concentrations [125]. Therefore, as can be observed the role of AHR in regulation the endocrine function of the pituitary appears complex, and different species, cell contexts, and agonists can all lead to different effects observed on pituitary hormonogenesis and secretion.
Involvement in Pituitary Development (Proliferation), Growth, and Tumorigenesis
AHR agonists and activity are not only significantly linked to hormonal deregulation of the pituitary but significant evidence is now being put forward for an equally significant function in regulation pituitary cell growth, proliferation, and apoptosis. Again, most evidence for such a role is available from either cell lines of rat or mouse origin, or from animal models, and points towards such a function.
In rat primary cells, AHR agonists, benzene and 2-ethyl-phthalate, altered cell energy and viability at different time points but not dose-dependently. This study also reported an increase in Ahr and Aip, and the oncogenes Pttg and Cyclin D1 gene expression as a result of agonist treatment at 24- and 96-h post treatment which the authors suggest account for the increase in cell proliferation. However, they do not provide evidence as to whether these two agonists also induced expression of classical AHR targets or whether the effect on gene expression was direct or indirect [126]. In GH3 cells, β-naphthoflavone reduced the expression levels of the anti-proliferative cytokine TGFβ1 but cell proliferation was not affected [109]. Long-term benzene exposure of GH3 cells increased GH expression and production and was associated with an increase in AHR and somatostatin type 2 receptor (Sstr2) as well as a decrease in Aip and Zac1 expression, both important tumor suppressors [127].
Another study using dioxin and non-dioxin-like polychlorinated biphenyls (PCBs) on primary mouse pituitary cells revealed different effects of these AHR agonists on pituitary cell apoptosis and proliferation. A mixture of PCBs induced apoptosis through a caspase 3 activation pathway while two non-dioxin-like aromatic compounds that do not activate AHR also increased apoptosis, although the authors do not conclude whether this effect is due to direct toxicity or mediated by intracellular responses to the compounds. Interestingly, one dioxin-like PCB, PCB153, which acts as an agonist to AHR, caused an anti-apoptotic effect and increased pituitary cell proliferation. However, attenuation of AHR with an antagonist failed to hinder the anti-apoptotic effect of PCB153, showing that this PCB may be acting independently of AHR in its anti-apoptotic effect. TCDD increased apoptosis of mouse pituitary cells, and AHR antagonist was able to hinder this response, showing a direct AHR-mediated effect of TCDD on apoptosis in these cells [128]. Other endocrine-disrupting chemicals such as perfluorinated compounds have been shown to affect pituitary cell growth and interfere with both AHR and thyroid hormone receptor functions in GH3 cells [129].
AHR in Pituitary Tumors
In general, few studies have looked into the effects of AHR signaling on pituitary cell growth and proliferation, and equally even fewer studies have taken an in-depth look at AHR signaling and its role in human pituitary adenoma (PA) formation or progression. One early study focused on the incidence of PA in the town of Seveso, Italy, which was exposed to high dioxin levels due to a chemical plant accident in 1976. Using a reference zone with a population of 38,624 persons against an area of dioxin exposure composed of around 6500 persons, the study identified 3 cases and 42 cases of PA in the exposed and reference areas, respectively, leading to the conclusion that dioxin exposure did not lead to a significantly different incidence of PA in a 20-year study period [130]. However, in another study in the Sicilian city of Messina in which industrial pollution raised concerns of environmental pollution, an epidemiological study found a higher incidence of acromegaly resulting from GH-secreting tumors in areas of higher exposure as compared to that in areas with low to medium exposure to pollutants [131].
In order to further elucidate the effects of AHR signaling and environmental contaminants on PA incidence, the same group carried out genetic screening of AHR polymorphisms in inhabitants occupying highly polluted regions. The polymorphisms analyzed, namely the rs2066853 and rs4986826 variants, were associated with variable induction of AHR target genes CYP1A1 and CYP1A2 and were found to alter the stability of the AHR ligand binding domain [132, 133]. The rs2066853 variant was found to correlate with higher IGF-1 levels, more invasive PAs, and higher risk of developing a secondary adenoma in patients with acromegaly. Both polymorphisms also appear to increase the risk of developing PA in polluted areas as compared to other areas with lower dioxin levels [134, 135]. Therefore, epidemiological evidence does support a role for AHR in PA tumorigenesis.
Most research on PA genetic predisposition has focused on a partner of the AHR, the AIP; several germ-line genetic mutations of which have been consistently linked to familial incidence of PA or FIPA. Genetic studies on familial cases of PA have found that about 40% of patients, particularly those with GH and prolactinomas, carry a germ-line mutation in the AIP gene which has been shown to function as a tumor suppressor and loss of heterozygosity (LOH) of this gene is a frequent occurrence even, thought to a lesser extent, in sporadic cases of PA. Patients with AIP mutations tend to be younger and have more aggressive tumors which respond less to medical treatment with somatostatin or dopamine analogues [136, 137]. AIP is a co-chaperone protein that interacts with many other proteins besides the AHR, and the precise role of AIP in predisposition to PA has remained a subject of controversy. Most mutations within the AIP are thought to disrupt the protein-protein interactions between AIP and its partners, and therefore, the search is underway to find the relevant pathways through which AIP deregulation might increase susceptibility to PA incidence. Work from our group and others have proposed that AIP, acting through interactions with several members of the cAMP signaling pathway, maintains a low cAMP threshold and loss of function of AIP causes an increase in cAMP levels that predisposes normal pituitary tissue to increased hormonal release which induces local cell growth and proliferation [138–140].
The effect of AIP on AHR signaling and activity is not completely understood. Some studies reported that AIP increases the transcriptional activity of AHR. Other studies showed that AIP reduction results in a reduction in AHR expression and activity while AIP over-expression reduces AHR nuclear translocation, thereby reducing its transactivation potential [141–149]. A consistent observation was that AIP protects AHR protein from ubiquitin-dependent degradation, hence suggesting that AIP may be required for proper AHR function [149, 150]. However, these functional studies of the AIP interaction with AHR were not carried out in the context of the pituitary. Recent work by Lecoq and colleagues [151] reported the effect of AIP mutants in patients with PA on AHR signaling in patient fibroblasts and GH3 cells. AIP protein expression in patient fibroblasts carrying mutations was significantly reduced, but the expression of AHR protein was unaffected. Unstimulated and agonist-stimulated AHRs in these fibroblasts carrying mutant AIP resulted in a reduction in expression of AHR target genes AHRR and CYP1B1. Furthermore, knock down of Aip in GH3 cells caused a reduction in agonist-stimulated AHR expression of target genes. These results would suggest that deregulated AIP would result in a reduction in AHR activity within the pituitary. The presence of AIP mutations also caused a decrease in GH secretion by GH3 cells when stimulated with kynurenine agonist [151]. The presence of AIP mutations in PA was found to correlate with a reduced expression of ARNT, the trans-activating partner of AHR, thereby providing a mechanism by which a reduction in AIP function may lead to reduction in AHR activity [152].
More specific analyses of the involvement of AHR in PA has centered around its expression and localization in PAs from patient samples. A study by Jaffrain-Rae and colleagues [106] found AHR expression to be lower in PAs compared to AIP expression using immunohistochemical techniques and quantitative PCR. Additionally, AHR was also found to be reduced in invasive tumors and in tumors with AIP mutations, suggesting a possible protective role in tumorigenesis. Another study by the same group again reported a decrease in AHR expression in the more aggressive tumors, with AHR expression correlating negatively with tumor size and suprasellar extension. Additionally, AIP and cytoplasmic AHR expression correlated significantly showing the role of AIP for stabilizing AHR in the cytoplasm. The authors also report that GH-secreting tumors with higher nuclear AHR staining were smaller than those with low nuclear AHR, suggesting a tumor-suppressive role for nuclear AHR [107]. The AHR signaling pathway also figured prominently in a protein data mining experiment carried out by Zhan and Desiderio [153] using the ingenuity pathway analysis (IPA) on a number of proteomic expression screens. In their analyses, the AHR signaling pathway was found to be consistently altered in all tumor types and featured in the top ten significantly altered pathways in all analyses. However, the authors failed to indicate whether the AHR pathway identified there activated or repressed in the PAs studied.
A recent study carried out by our laboratory on local pituitary adenomas also identified the AHR signaling pathway as a significantly altered pathway in all tumor types analyzed. We carried out mRNA expression analysis using microarrays on PAs resected locally and compared their expression profiles to a pooled control RNA and confirmed our findings using a total of 31 PAs of various sub-types (19 non-secreting PA, 8 GH-secreting PA, 2 PRL-secreting PA, 2 ACTH-secreting PA). IPA carried out on these samples revealed the AHR signaling pathway to be constantly down-regulated in all tumor types versus control RNA. Hence, our findings also support a protective role for AHR in PA tumorigenesis. However, we wanted to verify such a role and also identify possible mechanisms by which the AHR might be acting as a putative tumor suppressor in PA. Functional analyses using the GH3 cell line revealed that over-expression of AHR, both in the absence and presence of exogenous ligand BαP, was able to reduce GH3 cell proliferation, an effect which was reversed upon knock down of endogenous AHR. Further analyses revealed that over-expression of AHR was enough to reduce the cell cycle progression of GH3 cells. In order to see how this inhibition of cell cycle progression was achieved, we decided to look at the pathways already uncovered connecting the AHR to the cell cycle. Using immunoprecipitation, we verified the interaction between AHR and Rb1 in GH3 cells and using reporter assays, confirmed the inhibition of E2F-mediated transcriptional activity upon AHR over-expression. Endogenous AHR silencing using siRNA reversed this effect while the exogenous AHR agonist had little effect on this behavior. Additionally, AHR over-expression was able to increase the expression of Cdkn1b gene (p27) in GH3 cells, offering another pathway through which blockade of cell cycle progression could be achieved. We have therefore identified a putative mechanism by AHR may be protective in pituitary tumorigenesis, although other avenues of action cannot be excluded, such as the interaction between AIP and AHR or numerous other interactions that may be at play (in preparation).
In conclusion, this review attempts to cover all the aspects of AHR biology in order to highlight the complexity surrounding the role of this nuclear receptor in its many diverse settings and contexts. Within the pituitary, AHR once again appears to play a highly complex role/s, from regulating endocrine signaling to regulating physiological cell growth and division as well as mediating the disruptive effect of environmental toxins. A significant role in pituitary tumorigenesis has also been suggested, and evidence is increasing for a role of the AHR in tumor biology in all tissue types, with the pituitary being no exception.
Acknowledgments
The authors would like to acknowledge their funding bodies for allowing the work that has led to this review. R Formosa is funded by the REACH HIGH Scholars Programme – Post-Doctoral Grant. The grant is part-financed by the European Union, Operational Programme II—Cohesion Policy 2014–2020 Investing in human capital to create more Opportunities and promote the wellbeing of society—European Social Fund. J Vassallo is funded by the University of Malta Research Fund Committee Allocation (PHBRP07-02) and Faculty of Medicine and Surgery Funds (MDSIN08-22).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interests.
Contributor Information
Robert Formosa, Email: robert.formosa@um.edu.mt.
Josanne Vassallo, Phone: 00356 2340 1881, Email: josanne.vassallo@um.edu.mt.
References
- 1.Xu C, Li CY, Kong AN. Induction of phase I, II and II drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005;28(3):249–268. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- 2.Bersten DC, Sullivan AE, Peet DJ, Whitelaw ML. bHLH-PAS proteins in cancer. Nat Rev Canc. 2013;13:827–841. doi: 10.1038/nrc3621. [DOI] [PubMed] [Google Scholar]
- 3.Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 2009;30(9):447–454. doi: 10.1016/j.it.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Beischlag TV, Morales JL, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18(3):207–250. doi: 10.1615/CritRevEukarGeneExpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer BK, Perdew GH. Characterization of the AhR-hsp90-XAP2 core complex and the role of the immunophilin-related protein XAP2 in the Ahr stabilization. Biochemistry. 1999;38(28):8907–8917. doi: 10.1021/bi982223w. [DOI] [PubMed] [Google Scholar]
- 6.Probst MR, Reisz-Porszasz S, Agbunag RV, Ong MS, Hankinson O. Role of the aryl hydrocarbon receptor nuclear translocator protein in aryl hydrocarbon (dioxin) receptor action. Mol Pharmacol. 1993;44:511–518. [PubMed] [Google Scholar]
- 7.Whitelaw M, Pongratz I, Wilhelmsson A, Gustafsson JA, Poellinger L. Ligand-dependent recruitment of the Arnt co-regulator determines DNA recognition by the dioxin receptor. Mol Cell Biol. 1993;13:2504–2514. doi: 10.1128/MCB.13.4.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denison MS, Heath-Pagliuso S. The Ah receptor: a regulator of the biochemical and toxicological actions of structurally diverse chemicals. Bull Environ Contam Toxicol. 1998;61:557–568. doi: 10.1007/PL00002973. [DOI] [PubMed] [Google Scholar]
- 9.Lusska A, Shen E, Whitlock JP. Protein-DNA interactions at a dioxin-responsive enhancer. J Biol Chem. 1993;268:6575–6580. [PubMed] [Google Scholar]
- 10.Davarinos NA, Pollenz RS (1999) Aryl hydrocarbon receptor imported into the nucleus following ligand binding is rapidly degraded via cytoplasmic proteasome following nuclear export. 274(40):28708–28715 [DOI] [PubMed]
- 11.Ma Q. Induction of CYP1A1. The AhR/DRE paradigm: transcription, receptor regulation and expanding biological roles. Curr Drug Metab. 2001;2(2):149–164. doi: 10.2174/1389200013338603. [DOI] [PubMed] [Google Scholar]
- 12.Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev. 1999;13(1):20–25. doi: 10.1101/gad.13.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss C, Kolluri SK, Keifer F, Gottlicher M. Complementation of the Ah receptor deficiency in hepatoma cells: negative feedback regulation and cell cycle control by the Ah receptor. Exp Cell Res. 1996;226(1):154–163. doi: 10.1006/excr.1996.0214. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21(1):102–116. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harper TA, Joshi AD, Elferink CJ. Identification of stanniocalcin 2 as a novel aryl hydrocarbon receptor target gene. J Pharmacol Exp Ther. 2013;344(3):579–588. doi: 10.1124/jpet.112.201111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowe MM, Mold JE, Kanwar B, Huang Y, Louie A, Pollastri MP, et al. Identification of cinnabaric acid as a novel endogenous aryl hydrocarbon receptor ligand that drives IL-22 production. PLoS One. 2014;9(2):e87877. doi: 10.1371/journal.pone.0087877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. Aninteraction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185(6):3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterisation of a murine Ahr null allele: involvement of the Ar receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93(13):6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, Nakao K, Ema M, Sogawa K, Yasuda M, Katsuki M, Fujii-Kuriyama Y. Loss of the teratogenis response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ahr (dioxin) receptor. Genes Cells. 1997;2(10):645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzales FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-biding Ah receptor. Science. 1995;268(5211):722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzales FJ. Lesions of aryl hydrocarbon receptor-deficient mice. Vet Pathol. 1997;34(6):605–614. doi: 10.1177/030098589703400609. [DOI] [PubMed] [Google Scholar]
- 22.Bohonowych JE, Denison MS. Persistent binding of ligands to the aryl hydrocarbon receptor. Toxicol Sci. 2007;98:99–109. doi: 10.1093/toxsci/kfm085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tijet N, Boutros PC, Moffat ID, Okey AB, Tuomisto J, Pohjanvirta R. Aryl hydrocarbon receptor regulates distinct dioxin-dependant and dioxin-independent gene batteries. Mol Pharmacol. 2006;69(1):140–153. doi: 10.1124/mol.105.018705. [DOI] [PubMed] [Google Scholar]
- 24.Dere E, Lo R, Celius T, Matthews J, Zacharowski TR. Integration of genome-wide computation DRE search, AhR ChIP-chip and gene expression analyses of TCDD-elicited responses in the mouse liver. BMC Genomics. 2011;12:365. doi: 10.1186/1471-2164-12-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzales FJ, Fernandez-Salguero P. The aryl hydrocarbon receptor: studies using AhR-null mice. Drug Metab Dispos. 1998;26(12):1194–1198. [PubMed] [Google Scholar]
- 26.Bjerke DL, Sommer RJ, Moore RW, Peterson RE. Effects of in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on responsiveness of the male rat reproductive system to testosterone stimulation in adulthood. Toxicol Appl Pharmacol. 1994;127(2):250–257. doi: 10.1006/taap.1994.1159. [DOI] [PubMed] [Google Scholar]
- 27.Le G, Wolf C, Mann P, Ostby JS. In utero exposure to low doses of 2,3,7,8-tetrachlorodibenzo-p-dioxin alters reproductive development of female Long Evans hooded rat offspring. Toxicol Appl Pharmacol. 1997;146(2):237–244. doi: 10.1006/taap.1997.8222. [DOI] [PubMed] [Google Scholar]
- 28.Huukonen H, Unkila M, Pohjanvirta R, Tuomisto J. Developmental toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in the most TCDD-resistant and susceptible rat strains. Toxicol Appl Pharmacol. 1994;124(2):174–180. doi: 10.1006/taap.1994.1021. [DOI] [PubMed] [Google Scholar]
- 29.Takeda T, Taura J, Hattori Y, Ishii Y, Yamada H. Dioxin-induced retardation of development through a reduction in the expression on pituitary hormones and possible involvement of an aryl hydrocarbon receptor in this defect: a comparative study using two strains of mice with different sensitivities to dioxin. Toxicol Appl Pharmacol. 2014;278(3):220–229. doi: 10.1016/j.taap.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 30.Takeda T, Fujii M, Hattori Y, Yamamoto M, Shimazoe T, Ishii Y, Himeno M, Yamada H. Maternal exposure to dioxin imprints sexual immaturity of the pups through fixing the status of the reduced expression of hypothalamic gonadotropin-releasing hormone. Mol Pharmacol. 2014;85:74–82. doi: 10.1124/mol.113.088575. [DOI] [PubMed] [Google Scholar]
- 31.Ho HM, Oshima K, Watanabe G, Taya K, Strawn EY, Hutz RJ. TCDD increases inhibin A production by human luteinized granulosa cells in vitro. J Reprod Dev. 2006;52(4):523–528. doi: 10.1262/jrd.18006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsukimori K, Morokuma S, Hori T, Takahashi K, Hirata T, Otera Y, Fukushima K, Kawamoto T, Wake N. Characterisation of placental transfer of polychlorinated dibenzo-p-dioxins, dibenzofurans and polychlorinated biphenyls in normal pregnancy. J Obstet Gynaecol Res. 2013;39(1):83–90. doi: 10.1111/j.1447-0756.2012.01906.x. [DOI] [PubMed] [Google Scholar]
- 33.Singh KP, Casado FL, Opanashuk LA, Gasiewicz TA. The aryl hydrocarbon receptor has a normal function in the regulation of hematopoietic and other stem/progenitor cell populations. Biochem Pharmacol. 2009;77(4):577–587. doi: 10.1016/j.bcp.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thurmond TS, Gasiewicz TA. A single dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin produces a time- and dose-dependent alteration in the murine bone marrow B-lymphocyte maturation profile. Toxicol Sci. 2000;58(1):88–95. doi: 10.1093/toxsci/58.1.88. [DOI] [PubMed] [Google Scholar]
- 35.Wyman A, Lavin AL, Wilding GE, Gasiewicz TA. 2,3,7,8-Tetrachlorodibenzo-p-dioxin does not directly alter the phenotype of maturing B cells in a murine co-culture system. Toxicol Appl Pharmacol. 2002;180(3):164–177. doi: 10.1006/taap.2002.9396. [DOI] [PubMed] [Google Scholar]
- 36.Vogel CF, Li W, Sciullo E, Newman J, Hammock B, Reader JR, Tuscano J, Matsumura F. Pathogenesis of aryl hydrocarbon receptor-mediated development of lymphoma is associated with increased cyclooxygenase-2 expression. Am J Pathol. 2007;171(5):1538–1548. doi: 10.2353/ajpath.2007.070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith BW, Rozelle SS, Leung A, Ubellacker J, Parks A, Nah SK, et al. The aryl hydrocarbon receptor directs hematopoetic progenitor cell expansion and differentiation. Blood. 2013;122(3):376–385. doi: 10.1182/blood-2012-11-466722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mascanfroni D, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Mat Med. 2015;21(6):638–646. doi: 10.1038/nm.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanford EA, Wang Z, Novikov O, Mulas F, Landesman-Bollag MS, Smith BW, Seldin DC, Murphy GJ, Sherr DH. The role of the aryl hydrocarbon receptor in the development of cells with the molecular and functional characteristics of cancer stem-like cells. BMC Biol. 2016;14:20. doi: 10.1186/s12915-016-0240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Platten M, von Knebel DN, Oezen I, Wick W, Ochs K. Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Front Immunol. 2014;5:673. doi: 10.3389/fimmu.2014.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adams JL, Smothers J, Srinivasan R, Hoos A. Big opportunities for small molecules in immune-oncology. Nature Rev Drug Discov. 2015;14(9):603–622. doi: 10.1038/nrd4596. [DOI] [PubMed] [Google Scholar]
- 42.Di Natale BC, Murray IA, Schroder JC, FLaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ, Perdew GH. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signalling. Toxicol Sci. 2010;115(1):89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Apoteh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Wiener HL, Kuchroo VK. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11(9):854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacob A, Tomkiewicz-Raulet C, Jamet C, Bendayan R, Massicot F, Coumoul X, Decleves X. Aryl hydrocarbon receptor upregulates IL-1β expression in hCMEC/D3 human cerebral microvascular endothelial cells after TCDD exposure. Toxicol in Vitro. 2017;4:200–204. doi: 10.1016/j.tiv.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto O, Tokura Y. Photocontact dermatitis and chloracne: two major occupational and environmental skin diseases induced by different actions of halogenated chemicals. J Dermatol Sci. 2003;31(2):85–94. doi: 10.1016/S0923-1811(03)00097-5. [DOI] [PubMed] [Google Scholar]
- 46.Rundhaug JE, Fischer SM. Molecular mechanisms of mouse skin tumor promotion. Cancers (Basel) 2010;2(2):436–482. doi: 10.3390/cancers2020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Souza VR, Cabrera WK, Galvan A, Ribeiro OG, De Franco M, Vorraro F, Starobinas N, Massa S, Dragani TA, Ibanez OM. Aryl hydrocarbon receptor polymorphism modulates DMBA-induced inflammation and carcinogenesis in phenotypically selected mice. Int J Cancer. 2009;12(6):1478–1482. doi: 10.1002/ijc.24066. [DOI] [PubMed] [Google Scholar]
- 48.Gramatzki D, Pantazis G, Schittenhelm J, Tabatabai G, Kohle C, Wick W, Schwarz M, Weller M, Tritschler I. Aryl hydrocarbon receptor inhibition downregulates the TGF-beta/Smad pathway in human glioblastoma cells. Oncogene. 2009;28(28):2593–2605. doi: 10.1038/onc.2009.104. [DOI] [PubMed] [Google Scholar]
- 49.Kim DW, Gazourian L, Quadri SA, Romieu-Mourez R, Sherr DH, Sonenshein GE. The RelA NF-kappaB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene. 2000;19(48):5498–5506. doi: 10.1038/sj.onc.1203945. [DOI] [PubMed] [Google Scholar]
- 50.Vogel CF, Matsumura F. A new cross-talk between the aryl hydrocarbon receptor and RelB, a member of the NF-kappaB family. Biochem Pharmacol. 2009;77(4):734–745. doi: 10.1016/j.bcp.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller ME, Holloway AC, Foster WG. Benzo-[a]-pyrene increases invasion in MDA-MB-231 breast cancer cells via increased COX-II expression and prostaglandin E2 (PGE2) output. Clin Exp Metastasis. 2005;22:149–156. doi: 10.1007/s10585-005-6536-x. [DOI] [PubMed] [Google Scholar]
- 52.Suh J, Jeon YJ, Kim HM, Kang JS, Kaminski NE, Yang KH. Aryl hydrocarbon receptor-dependent inhibition of AP-1 activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin in activated B cells. Toxicol Appl Pharmacol. 2002;181(2):116–123. doi: 10.1006/taap.2002.9403. [DOI] [PubMed] [Google Scholar]
- 53.Boverhof DR, Tam E, Harbey AS, Crawford RB, Kaminski NE, Zacharewski TR. 2,3,7,8-Tetrachlorodibenzo-p-dioxin induces suppressor of cytokine signalling 2 in murine B cells. Mol Pharmacol. 2004;66(6):1662–1670. doi: 10.1124/mol.104.002915. [DOI] [PubMed] [Google Scholar]
- 54.Belladonna M, Orabona C, Grohmann U, Puccetti P. TGF-B and kynurenines as the key to infectious tolerance. Trends Mol Med. 2009;15:41–49. doi: 10.1016/j.molmed.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 55.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 56.Wiebel F, Klose U, Kiefer F. Toxicity of 2,3,7,8-tetracholordibenzo-p-dioxin in vitro: H4IIIEC3-derived 5l hepatoma cells as a model system. Toxicol Lett. 1991;55(2):161–169. doi: 10.1016/0378-4274(91)90130-X. [DOI] [PubMed] [Google Scholar]
- 57.Göttlicher M, Wiebel FJ. 2,3,7,8-Tetrachlorodibenzo-p-dioxin causes unbalanced growth in 5L rat hepatoma cells. Toxicol Appl Pharmacol. 1991;111:496–503. doi: 10.1016/0041-008X(91)90253-B. [DOI] [PubMed] [Google Scholar]
- 58.Ma Q, Whitlock JP. The aromatic hydrocarbon receptor modulates the Hepa 1c1c7 cell cycle and differentiated state independently of dioxin. Mol Cell Biol. 1996;16(5):2144–2150. doi: 10.1128/MCB.16.5.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bauman JW, Goldsworthy TL, Dunn CS, Fox TR. Inhibitory effects of 2,3,7,8-tetracholorodibenzo-p-dioxin on rat hepatocyte proliferation induced by 2/3 partial hepatectomy. Cell Prolif. 1995;28:437–451. doi: 10.1111/j.1365-2184.1995.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 60.Levine-Fridman A, Chen L, Elferink CJ. Cytochrome P4401A1 promotes G1 phase cell cycle progression by controlling aryl hydrocarbon receptor activity. Mol Pharmacol. 2004;65:461–469. doi: 10.1124/mol.65.2.461. [DOI] [PubMed] [Google Scholar]
- 61.Marlowe JL, Knudsen ES, Schwemberger S, Puga A. The aryl hydrocarbon receptor displaces p300 from E2F-dependent promoters and represses S phase-specific gene expression. J Biol Chem. 2004;279(28):29013–29022. doi: 10.1074/jbc.M404315200. [DOI] [PubMed] [Google Scholar]
- 62.Shimba S, Komiyama K, Moro I, Tezuka M. Overexpression of the aryl hydrocarbon receptor (AhR) accelerates the cell proliferation of A549 cells. J Biochem. 2002;132(5):795–802. doi: 10.1093/oxfordjournals.jbchem.a003289. [DOI] [PubMed] [Google Scholar]
- 63.Barhoover MA, Hall JM, Greenlee WF, Thomas RS. Aryl hydrocarbon receptor regulates cell cycle progression in human breast cancer cells via a functional interaction with cyclin-dependent kinase 4. Mol Pharmacol. 2010;77(2):195–201. doi: 10.1124/mol.109.059675. [DOI] [PubMed] [Google Scholar]
- 64.Shimba S, Wada T, Tezuka M. Aryl hydrocarbon receptor (Ahr) in involved in negative regulation of adipose differentiation in 3T3-L1 cells: Ahr inhibits differentiation independently of dioxin. J Cell Sci. 2001;114(15):2809–2817. doi: 10.1242/jcs.114.15.2809. [DOI] [PubMed] [Google Scholar]
- 65.Puga A, Barnes SJ, Dalton TP, Chang CY, Knudsen E, Maier MA. Aromatic hydrocarbon receptor interaction with the retinoblastoma protein potentiates repression of E2F-dependent transcription and cell cycle arrest. J Biol Chem. 2000;275(4):2943–2950. doi: 10.1074/jbc.275.4.2943. [DOI] [PubMed] [Google Scholar]
- 66.O’Donnell EF, Kopparapu PR, Koch DC, Jang HS, Phillips JL, Tanguay RL, Kerkvliet N, Kolluri SK. The aryl hydrocarbon receptor mediates leflunomide-induced growth inhibition of melanoma cells. PLoS One. 2012;7(7):e40926. doi: 10.1371/journal.pone.0040926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fritz WA, Lin TM, Safe S, Moore RW, Peterson RE. The selective aryl hydrocarbon receptor modulator 6-methyl-1,3,8-trichlorodibenzofuran inhibits prostate tumor metastasis in TRAMP mice. Biochem Pharmacol. 2009;77(7):1151–1160. doi: 10.1016/j.bcp.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fan Y, Boivin GP, Knudsen ES, Nebert DW, Xia Y, Puga A. The aryl hydrocarbon receptor functions as a tumor suppressor of liver carcinogenesis. Cancer Res. 2010;70(1):212–220. doi: 10.1158/0008-5472.CAN-09-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peng L, Mayhew CN, Schnekenburger M, Knudsen ES, Puga A. Repression of Ahr receptor and induction of transforming growth factor-beta genes in DEN-induced mouse liver tumors. Toxicology. 2008;246(2–3):242–247. doi: 10.1016/j.tox.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fritz WA, Lin TM, Cardiff RD, Peterson RE. The aryl hydrocarbon receptor inhibits prostate carcinogenesis in TRAMP mice. Carcinogenesis. 2007;28(2):497–505. doi: 10.1093/carcin/bgl179. [DOI] [PubMed] [Google Scholar]
- 71.Mulero-Navarro S, Carvajal-Gonzalez JM, Herranz M, Ballestar E, Fraga MF, Ropero S, Esteller M, Fernandez-Salguero PM. The dioxin receptor is silenced by promoter hypermethylation in human acute lymphoblastic leukemia through inhibition of Sp1 binding. Carcinogenesis. 2006;27(5):1099–1104. doi: 10.1093/carcin/bgi344. [DOI] [PubMed] [Google Scholar]
- 72.Koliopanos A, Kleeff J, Xiao Y, Safe S, Zimmermann A, Buchler MW, Freiss H. Increased aryl hydrocarbon receptor expression offers a potential target for pancreatic cancer. Oncogene. 2002;21(39):6059–6070. doi: 10.1038/sj.onc.1205633. [DOI] [PubMed] [Google Scholar]
- 73.Feng S, Cao Z, Wang X. Role of aryl hydrocarbon receptor in cancer. Biochim Biophys Acta. 2013;1836(2):197–210. doi: 10.1016/j.bbcan.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 74.Lin P, Chang H, Tsai WT, Wu MH, Liao YS, Chen JT, Su JM. Overexpression of aryl hydrocarbon receptor in human lung carcinomas. Toxicol Pathol. 2003;31(1):22–30. doi: 10.1080/01926230309746. [DOI] [PubMed] [Google Scholar]
- 75.Ishida M, Mikami S, Kikuchi E, Kosaka T, Miyajima A, Nakagawa K, Mukai M, Okada Y, Oya M. Activation of the aryl hydrocarbon receptor pathway enhances cancer cell invasion by upregulating the MMP expression and is associated with poor prognosis in upper urinary tract urothelial cancer. Carcinogenesis. 2010;31(2):287–295. doi: 10.1093/carcin/bgp222. [DOI] [PubMed] [Google Scholar]
- 76.Peng TL, Chen J, Mao W, Song X, Chen MH. Aryl hydrocarbon receptor pathway activation enhances gastric cancer cell invasiveness likely through a c-Jun-dependent induction of matrix metalloproteinase-9. BMC Cell Biol. 2009;16:10–27. doi: 10.1186/1471-2121-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gluschneider U, Hidas G, Cojocaru G, Yutkin V, Ben-Neriah Y, Pikarsky E. Beta-TrCP inhibition reduces prostate cancer cell growth via upregulation od the aryl hydrocarbon receptor. PLoS one. 2010;5(2):e9060. doi: 10.1371/journal.pone.0009060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang S, Kim K, Jin UH, Pfent C, Cao H, Amendt B, Liu X, Wilson-Robies H, Safe S. Aryl hydrocarbon receptor agonists induce microRNA-335 expression and inhibit lung metastasis of estrogen receptor negative breast cancer cells. Mol Cancer Ther. 2012;11(1):109–118. doi: 10.1158/1535-7163.MCT-11-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ge NL, Elferink CJ. A direct interaction between the aryl hydrocarbon receptor and retinoblastoma protein. Linking dioxin signaling to the cell cycle. J Biol Chem. 1998;273:22708–22713. doi: 10.1074/jbc.273.35.22708. [DOI] [PubMed] [Google Scholar]
- 80.Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12(1):2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- 81.Kolluri SK, Weiss C, Koff A, Gottlicher M. p27(Kip1) induction and inhibition of proliferation by the intracellular Ah receptor in developing thymus and hepatoma cells. Genes Dev. 1999;13(13):1742–1753. doi: 10.1101/gad.13.13.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Elferink CJ, Ge NL, Levine A. Maximal aryl hydrocarbon receptor activity depends on an interaction with the retinoblastoma protein. Mol Pharmacol. 2001;59(4):664–673. doi: 10.1124/mol.59.4.664. [DOI] [PubMed] [Google Scholar]
- 83.Faust D, Kletting S, Ueberham E, Dietrich C. Aryl hydrocarbon receptor-dependent cell cycle arrest in isolated mouse oval cells. Toxicol Lett. 2013;223(1):73–80. doi: 10.1016/j.toxlet.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 84.Jackson DP, Li H, Mitchell KA, Joshi AD, Elferink CJ. Ah receptor-mediated suppression of liver regeneration through NC-XRE-driven p21Cip1 expression. Mol Pharmacol. 2014;85(4):533–541. doi: 10.1124/mol.113.089730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.John K, Lahoti TS, Wagner K, Hughes JM, Perdew GH. The Ah receptor regulates growth factor expression in head and neck squamous cell carcinoma cell lines. Mol Carcinogen. 2014;53:765–776. doi: 10.1002/mc.22032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caruso JA, Mathieu PA, Joiakim A, Zhang H, Reiners JJ., Jr Aryl hydrocarbon receptor modulation of tumour necrosis factor-alpha-induced apoptosis and lysosomal disruption in a hepatoma model that is caspase-8-independent. J Biol Chem. 2006;281(16):10954–10967. doi: 10.1074/jbc.M508383200. [DOI] [PubMed] [Google Scholar]
- 87.Cho YC, Zheng W, Jefcoate CR. Disruption of cell-cell contact maximally but transiently activated AhR-mediated transcription in 10T1/2 fibroblasts. Toxicol Appl Pharmacol. 2004;199:220–238. doi: 10.1016/j.taap.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 88.Ikuta T, Kobayashi Y, Kawahiri K. Cell density regulates intracellular localization of aryl hydrocarbon receptor. J Biol Chem. 2004;279:19209–19216. doi: 10.1074/jbc.M310492200. [DOI] [PubMed] [Google Scholar]
- 89.Diry M, Tomkiewicz C, Koehle C, Coumoul X, Walter Bock K, Barouki R, Transy C. Activation of the dioxin/aryl hydrocarbon receptor (AhR) modulates cell plasticity through a JNK-dependent mechanism. Oncogene. 2006;25:5570–5574. doi: 10.1038/sj.onc.1209553. [DOI] [PubMed] [Google Scholar]
- 90.Niermann T, Schmutz S, Erne P, Resink T. Aryl hydrocarbon receptor ligands repress T-cadherin expression in vascular smooth muscle cells. Biochem Biophys Res Commun. 2003;300:943–949. doi: 10.1016/S0006-291X(02)02970-4. [DOI] [PubMed] [Google Scholar]
- 91.DiNatale BC, Perdew GH. Ah receptor antagonism inhibits constitutive and cytokine inducible IL6 production in the head and neck tumor cell lines. Mol Carcinog. 2011;50:173–183. doi: 10.1002/mc.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Novikov O, Wang Z, Stanford EA, Parks AJ, Ramirez-Cardenas A, Landesman E, et al. An aryl hydrocarbon receptor-mediated amplification loop that enforces cell migration in ER−/PR−/Her2-Human breast cancer cells. Mol Pharmacol. 2016;90(5):674–688. doi: 10.1124/mol.116.105361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.D’Amato NC, Rogers TJ, Gordon MA, Greene LI, Cochrane DR, Spoelstra NS, et al. A TDO2-AhR signalling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res. 2015;75(21):4651–4664. doi: 10.1158/0008-5472.CAN-15-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsai CH, Li CH, Cheng YW, Lee CC, Liao PL, Lin CH, Huang SH, Kang JJ. The inhibition of lung cancer cell migration by AhR-regulated autophagy. Sci Rep. 2017;7:41927. doi: 10.1038/srep41927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang T, Wyrick KL, Meadows GG, Wills TB, Vorderstrasse BA. Activation of the aryl hydrocarbon receptor by TCDD inhibits mammary tumor metastasis in a syngeneic mouse model of breast cancer. Toxicol Sci. 2011;124(2):291–298. doi: 10.1093/toxsci/kfr247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Spink DC, Katz BH, Hussain MM, Pentecost BT, Cao Z, Spink BC. Estrogen regulates Ah responsiveness in MCF-7 breast cancer cells. Carcinogenesis. 2003;24(12):1941–1950. doi: 10.1093/carcin/bgg162. [DOI] [PubMed] [Google Scholar]
- 97.Wormke M, Stoner M, Saville B, Walker K, Abdelrahim M, Burghardt R, Safe S. The aryl hydrocarbon receptor mediates degradation of estrogen receptor α through activation of proteasomes. Mol Cell Biol. 2003;23:1843–1855. doi: 10.1128/MCB.23.6.1843-1855.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tian Y, Ke S, Denison MS, Rabson AB, Galo MA. Ah receptor and NF-kappa B interactions, a potential mechanism for dioxin toxicity. J Biol Chem. 1999;274(1):510–515. doi: 10.1074/jbc.274.1.510. [DOI] [PubMed] [Google Scholar]
- 99.Yang X, Liu D, Murray TJ, Mitchell GC, Hesterman EV, Karchner SI, Merson RR, Hahn ME, Sherr DH. The aryl hydrocarbon receptor constitutively represses c-myc transcription in human mammary tumor cells. Oncogene. 2005;24(53):7869–7881. doi: 10.1038/sj.onc.1208938. [DOI] [PubMed] [Google Scholar]
- 100.Mergl Z, Acs Z, Makara GB. Growth hormone secretion and activation of cyclic AMP by growth hormone releasing hormone and gamma-aminobutyric acid in the neonatal rat pituitary. Life Sci. 1995;56(8):579–585. doi: 10.1016/0024-3205(94)00490-J. [DOI] [PubMed] [Google Scholar]
- 101.Oesch-Bartlomowicz B, Huelster A, Wiss O, Antoniou-Lipfert P, Dietrich C, Arand M, Weiss C, Bockamp E, Oesch F. Aryl hydrocarbon receptor activation by cAMP vs dioxin: divergent signalling pathways. PNAS USA. 2005;102(26):9218–9223. doi: 10.1073/pnas.0503488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Flaveny C, Reen RK, Kusnadi A, Perdew GH (2008) The mouse and human Ah receptor differ in recognition of LXXLL motifs. Arch Biochem Biophys 471(215–223) [DOI] [PMC free article] [PubMed]
- 103.Flaveny CA, Murray IA, Perdew GH. Differential gene regulation by the human and mouse Aryl hydrocarbon receptor. Toxicol Sci. 2010;114(2):217–225. doi: 10.1093/toxsci/kfp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Black MB, Budinsky RA, Dombkowski A, Cukovic D, LeCluyse EL, Ferguson SS, Thomas RS, Rowlands JC. Cross-species comparisons of transcriptomic alterations in human and rat primary hepatocytes exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2012;127:199–215. doi: 10.1093/toxsci/kfs069. [DOI] [PubMed] [Google Scholar]
- 105.Ramadoss P, Perdew GH. Use of 2-axido-3-[125I]-iodo-7,8-dibromodibenzo-p-dioxin as a probe to determine the relative ligand affinity of human and mouse aryl hydrocarbon receptor in cultured cells. Mol Pharmacol. 2004;66:129–136. doi: 10.1124/mol.66.1.129. [DOI] [PubMed] [Google Scholar]
- 106.Jaffrain-Rae ML, Angelini M, Gargano D, Tichomirowa MA, Daly AF, Vanbellinghen JF, D'Innocenzo E, Barlier A, Giangaspero F, Esposito V, et al. Expression of aryl hydrocarbon receptor (AHR) and AHR-interacting protein in pituitary adenomas: pathological and clinical implications. Endocr Relat Cancer. 2009;16:1029–1043. doi: 10.1677/ERC-09-0094. [DOI] [PubMed] [Google Scholar]
- 107.Jaffrain-Rae M, Rotondi S, Turchi A, Occhi G, Barlier A, Peverelli E, Rostomyan L, Defilles C, Angelini M, Oliva MA, et al. Somatostatin analogues increase AIP expression in somatotropinomas, irrespective of Gsp mutations. Endocr Relat Cancer. 2013;20(5):753–766. doi: 10.1530/ERC-12-0322. [DOI] [PubMed] [Google Scholar]
- 108.Cao J, Patisaul HB, Peterssen SL. Aryl hydrocarbon receptor activation in lactotropes and gonadotropes interferes with estradiol-dependent and -independent preprolactin, glycoprotein alpha and luteinizing hormone beta gene expression. Mol Cell Endocrinol. 2011;333(2):151–159. doi: 10.1016/j.mce.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moran TB, Brannick KE, Raetzman LT. Aryl hydrocarbon receptor activity modulates prolactin expression in the pituitary. Toxicol Appl Pharmacol. 2012;265(1):139–145. doi: 10.1016/j.taap.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huang P, Ceccatelli S, Hoegberg P, Sten Shi TJ, Hakansson H, Rannug A. TCDD-induced expression of Ah receptor responsive gene in the pituitary and brain of cellular retinol-binding protein (CRBP-1) knockout mice. Toxicol Appl Pharmacol. 2003;192(3):262–274. doi: 10.1016/S0041-008X(03)00296-5. [DOI] [PubMed] [Google Scholar]
- 111.Mably TA, Bjerke DL, Moore RW, Gendron-Fitzpatrick A, Peterson RE. In utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin. 3. Effects on spermatogenesis and reproductive capability. Toxicol Appl Pharmacol. 1992;114:118–126. doi: 10.1016/0041-008X(92)90103-Y. [DOI] [PubMed] [Google Scholar]
- 112.Guo YL, Hsu PC, Hsu CC, Lambert GH. Semen quality after prenatal exposure to polychlorinated biphenyls and dibenzofurans. Lancet. 2000;356:1240–1212. doi: 10.1016/S0140-6736(00)02792-6. [DOI] [PubMed] [Google Scholar]
- 113.Mutoh J, Taketoh J, Okamura K, Kagawa T, Ishida T, Ishii Y, Yamada H. Fetal pituitary gonadotropin as an initial target of dioxin in its impairment of cholesterol transportation and steroidogenesis in rats. Endocrinology. 2006;147(2):927–936. doi: 10.1210/en.2005-1125. [DOI] [PubMed] [Google Scholar]
- 114.Takeda T, Matsumoto Y, Koga T, Mutoh J, Mishimura Y, Shimazoe T, Ishii Y, Ishida T, Yamada H. Maternal exposure to dioxin disrupts gonadotropin production in fetal rats and imprints defects in sexual behaviour. J Pharmacol Exp Ther. 2009;329(3):1091–1099. doi: 10.1124/jpet.109.151282. [DOI] [PubMed] [Google Scholar]
- 115.Taketoh J, Mutoh J, Takeda T, Ogishima T, Takeda S, Ishii Y, Ishida T, Yamada H. Suppression of fetal testicular cytochrome P450 17 by maternal exposure to 2,3,7,8- tetrachlorodibenzo-p-dioxin: a mechanism involving an initial effect on gonadotropin synthesis in the pituitary. Life Sci. 2007;80(14):1259–1267. doi: 10.1016/j.lfs.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 116.Hattori Y, Takeda T, Fujii M, Taura J, Ishii Y, Yamada H. Dioxin-induced fetal growth retardation: the role of a preceding attenuation in the circulating level of glucocorticoid. Endocrine. 2014;47:572–580. doi: 10.1007/s12020-014-0257-3. [DOI] [PubMed] [Google Scholar]
- 117.Kohn MC. Effects of TCDD on thyroid hormone homeostasis in the rat. Drug Chem Toxicol. 2000;23(1):259–277. doi: 10.1081/DCT-100100114. [DOI] [PubMed] [Google Scholar]
- 118.Bestervelt LL, Cai Y, Piper DW, Nolan CJ, Pitt JA, Piper WN. TCDD alters pituitary-adrenal functional I: adrenal responsiveness to exogenous ACTH. Neurotoxicol Teratol. 1993;15:365–367. doi: 10.1016/0892-0362(93)90052-P. [DOI] [PubMed] [Google Scholar]
- 119.Bestervelt LL, Pitt JA, Nolan CJ, Cai Y, Piper DW, Dybowski JA, Dayharsh GA, Piper WN. In vitro 2,3,7,8-tetrachlorodibenzo-p-dioxin interference with the anterior pituitary hormone adrenocorticotropin. Toxicol Sci. 1998;44:107–115. doi: 10.1006/toxs.1998.2480. [DOI] [PubMed] [Google Scholar]
- 120.Savage JJ, Yaden BC, Kiratipranon P, Rhodes SJ. Transcriptional control during mammalian anterior pituitary development. Gene. 2003;319:1–19. doi: 10.1016/S0378-1119(03)00804-7. [DOI] [PubMed] [Google Scholar]
- 121.Lipkin SM, Naar AM, Kalla KA, Sack RA, Rosenfeld MG. Identification of a novel zinc finger protein binding a conserved element critical for Pit-1-depeneent growth hormone gene expression. Genes Dev. 1993;7(9):1674–1687. doi: 10.1101/gad.7.9.1674. [DOI] [PubMed] [Google Scholar]
- 122.Aluru N, Vijavan MM. Brain transcriptomics in response to beta-naphthoflavone treatment in rainbow trout: the role of aryl hydrocarbon receptor signalling. Aquat Toxicol. 2008;87(1):1–12. doi: 10.1016/j.aquatox.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 123.Huang P, Ceccatelli S, Hakansson H, Grandison L, Rannug A. Constitutive and TCDD-induced expression of Ah receptor-responsive genes in the pituitary. Neurotoxicology. 2002;23(6):783–793. doi: 10.1016/S0161-813X(02)00040-2. [DOI] [PubMed] [Google Scholar]
- 124.Shridhar S, Farley A, Reid RL, Foster WG, Van Vugt DA. The effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on corticotrophin-releasing hormone, arginine vasopressin, and pro-opiomelanocortin mRNA levels in the hypothalamus of the cynomolgus monkey. Toxicol Sci. 2001;63(2):181–188. doi: 10.1093/toxsci/63.2.181. [DOI] [PubMed] [Google Scholar]
- 125.Elango A, Sphephard B, Chen TT. Effects of endocrine disruptors on the expression of growth hormone and prolactin mRNA in the rainbow trout pituitary. Gen Comp Endocrinol. 2006;145(2):116–127. doi: 10.1016/j.ygcen.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 126.Tapella L, Sesta A, Cassarino MF, Zunino V, Catalano MG, Pecori Gialdi F (2016) Benzene and 2-ethyl-phthalate induce proliferation in normal rat pituitary cells. Pituitary 1–12 [DOI] [PMC free article] [PubMed]
- 127.Zunino V, Catalano MG, Guaraldi F, D’angelo V, Arvat E, Fortunati N (2014) Exposure to benzene modifies SSTR2-ZAC1 signalling pathway in GH3 cells pituitary adenoma cells. 38th National Meeting of the Italian Society of Endocrinology p117, PD17
- 128.Raggi F, Russo D, Urbani C, Sardella C, Manetti L, Cappellani C, Lupi I, Tomisti L, Martino E, Marcocci C, Bogazzi F. Divergent effects of dioxin- or non-dioxin-like polychlorinated biphenyls on the apoptosis of primary cell culture from the mouse pituitary gland. PLoS One. 2016;11(1):e0146729. doi: 10.1371/journal.pone.0146729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Long M, Ghisari M, Bonefeld-Jorgensen EC. Effects of perfluoroalkyl acids on the function of the thyroid hormone and the aryl hydrocarbon receptor. Environ Sci Pollut Res Int. 2013;20(11):8045–8056. doi: 10.1007/s11356-013-1628-7. [DOI] [PubMed] [Google Scholar]
- 130.Pesatori AC, Baccarelli A, Consonni D, Lania A, Beck-Peccoz P, Bertazzi PA, Spada A. Aryl hydrocarbon receptor-interacting protein and pituitary adenomas: a population-based study on subjects exposed to dioxin after the Seveso, Italy, accident. Eur J Endocrinol. 2008;159(6):699–703. doi: 10.1530/EJE-08-0593. [DOI] [PubMed] [Google Scholar]
- 131.Cannavo S, Ferrau F, Ragonese M, Curto L, Torre ML, Magistri M, Marchese A, Alibrandi A, Trimarchi F. Increased prevalence of acromegaly in a highly polluted area. Eur J Endocrinol. 2010;163(4):509–513. doi: 10.1530/EJE-10-0465. [DOI] [PubMed] [Google Scholar]
- 132.Harper PA, Wong JM, Lam MS, Okey AB. Polymorphisms in the human AH receptor. Chem Biol Interact. 2002;141(1–2):161–187. doi: 10.1016/S0009-2797(02)00071-6. [DOI] [PubMed] [Google Scholar]
- 133.Aftabi Y, Colagar AH, Mehrnejad F. An in silico approach to investigate the source of the controversial interpretations about the phenotypic results of the human AhR-gene G1661A polymorphism. J Theor Biol. 2016;393:1–15. doi: 10.1016/j.jtbi.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 134.Cannavo S, Ferrau F, Ragonese M, Romeo PD, Torre ML, Puglisi S, De Menis E, Arnaldi G, Salpietro C, Cotta OR, Albani A, Ruggeri RM, Trimarchi F. Increased frequency of the rs2066853 variant of the aryl hydrocarbon receptor gene in patients with acromegaly. Clin Endocrinol. 2014;81(2):249–253. doi: 10.1111/cen.12424. [DOI] [PubMed] [Google Scholar]
- 135.Cannavo S, Ragonese M, Puglisi S, Romeo PD, Torre ML, Alibrandi A, Scaroni C, Occhi G, Ceccaro F, Regazzo D, De Menis E, Sartorato P, Arnaldi G, Trementino L, Trimarchi F, Ferrau F. Acromegaly is more severe in patients with AHR or AIP gene variants living in highly polluted areas. J Clin Endocrinol Metab. 2016;101(4):1872–1879. doi: 10.1210/jc.2015-4191. [DOI] [PubMed] [Google Scholar]
- 136.Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, et al. Pituiary adenoma predisposition caused by germline mutations in the AIP gene. Science. 2006;312(5777):1228–1230. doi: 10.1126/science.1126100. [DOI] [PubMed] [Google Scholar]
- 137.Beckers A, Aaltonen LA, Daly AF, Karhu A. Familial isolated pituitary adenomas (FIPA) and the pituitary adenoma predisposition due to mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocr Rev. 2013;34(2):239–277. doi: 10.1210/er.2012-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Formosa R, Xuereb-Anastasi A, Vassallo J. Aip regulates cAMP signalling and GH secretion in GH3 cells. Endocr Relat Cancer. 2013;20(4):495–505. doi: 10.1530/ERC-13-0043. [DOI] [PubMed] [Google Scholar]
- 139.Tuominen I, Heliovaara E, Raitila A, Rautiainen MR, Mehine M, Katainen R, et al. AIP inactivation leads to pituitary tumorigenesis through defective Gαi-cAMP signalling. Oncogene. 2015;34:1174–1184. doi: 10.1038/onc.2014.50. [DOI] [PubMed] [Google Scholar]
- 140.Schernthaner-Reiter MH, Trivellin G, Starost MF, Stratakis CA (2016) Interaction between AIP and the protein kinase A in a pituitary tumor formation. Endocrine Abstracts from the Endocrine Society conference 2016, SUN532
- 141.Pollenz RS, Dougherty EJ. Redifining the role of the endogenous XAP2 and C-terminal hsp70-interacting protein on the endogenous Ah receptors expressed in mouse and rat cell lines. J Biol Chem. 2005;280(39):33346–33356. doi: 10.1074/jbc.M506619200. [DOI] [PubMed] [Google Scholar]
- 142.Carver LA, Bradfield CA. Ligand-dependent interaction of the aryl hydrocarbon receptor with a novel immunophilin homolog in vivo. J Biol Chem. 1997;272(17):11452–11456. doi: 10.1074/jbc.272.17.11452. [DOI] [PubMed] [Google Scholar]
- 143.Ma Q, Whitlock JP. A novel cytoplasmic protein that interacts with the Ah receptor, contains tetratricopeptide repeat motifs, and augments the transcriptional response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Chem. 1997;272(14):8878–8884. doi: 10.1074/jbc.272.14.8878. [DOI] [PubMed] [Google Scholar]
- 144.Meyer BK, Pray-Grant MG, Vanden Heuvel JP, Perdew GH. Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Moll Cell Biol. 1998;18:978–988. doi: 10.1128/MCB.18.2.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bell DR, Poland A. Binding of the aryl hydrocarbon receptor (AhR) to the AhR-interacting protein: the role of HSP90. J Biol Chem. 2000;275:36407–36414. doi: 10.1074/jbc.M004236200. [DOI] [PubMed] [Google Scholar]
- 146.Nukuya M, Lin BC, Glover E, Moran SM, Kennedy GD, Bradfield CA. The aryl hydrocarbon receptor-interacting protein (AIP) is required for dioxin-induced hepatotoxicity but not for the induction of the Cyp1a1 and Cyp1a1 genes. J Biol Chem. 2010;285(46):35599–35605. doi: 10.1074/jbc.M110.132043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ramadoss P, Petrulis JR, Hollingshead BD, Kusnadi A, Perdew GH. Divergent roles of hepatitis B virus X-associated protein 2 (XAP2) in human versus mouse AH receptor complexes. Biochemistry. 2004;43(3):700–709. doi: 10.1021/bi035827v. [DOI] [PubMed] [Google Scholar]
- 148.Hollingshead BD, Petrulis JR, Perdew GH. The aryl hydrocarbon (Ah) receptor transcriptional regulator hepatitis B virus-associated protein 2 antagonizes p23 biding to Ah receptor-Hsp90 complexes and is dispensible for receptor function. J Biol Chem. 2004;279(44):45652–45661. doi: 10.1074/jbc.M407840200. [DOI] [PubMed] [Google Scholar]
- 149.Kazlauskas A, Poellinger L, Pongratz I. The immunophilin-like protein XAP2 regulates ubiquitination and subcellular localization of the dioxin receptor. J Biol Chem. 2000;275(52):41317–41324. doi: 10.1074/jbc.M007765200. [DOI] [PubMed] [Google Scholar]
- 150.Morales JL, Perdew GH. Carboxyl terminus of hsc7-interacting protein (CHIP) can remodel mature aryl hydrocarbon receptor (AhR) complexes and mediate ubiquitination of both the AhR and the 90 kDa heat shock protein (hsp90) in vitro. Biochemistry. 2007;46(2):610–621. doi: 10.1021/bi062165b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lecoq AL, Viengchareun S, Hage M, Bouligand J, Young J, Boutron A, Zizzari P, Lombes M, Chanson P, Kamenicky P. AIP mutations impair AhR signalling in pituitary adenoma patients fibroblasts and in GH3 cells. Endocr Relat Cancer. 2016;23(5):433–443. doi: 10.1530/ERC-16-0041. [DOI] [PubMed] [Google Scholar]
- 152.Heliovaara E, Raitila A, Launnonen V, Paetau A, Arola J, Lehtonen H, et al. The expression of AIP-related molecules in elucidation of cellular pathways in pituitary adenomas. Am J Pathol. 2009;175(6):2501–2507. doi: 10.2353/ajpath.2009.081131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhan X, Desiderio DM. Signaling pathways networks mined from human pituitary adenoma proteomics data. BMC Bio Genomics. 2010;3:13. doi: 10.1186/1755-8794-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]