Abstract
Brain tumors are associated with increased mortality and morbidity and are the most common cancer type in children and young adults. The present review focuses on the interplay between leptin, the most extensively studied adipokine, and the onset, development, and treatment of primary brain and intracranial tumors. The two main mechanisms for increased leptin levels in intracranial tumor survivors, leptin resistance caused by hypothalamic damage, or secondary to obesity, are discussed. The contradicting mechanistic observations on leptin being able to both promote tumorinogenesis (e.g., in gliomas) as well as inhibit it (e.g., in adenomas) are also reported. Additionally, the relevant current and future clinical applications, including most notably the proposed use of serum leptin measurements for non-invasive brain tumor diagnostics, are also reported.
Keywords: Increased Leptin Levels, Leptin Resistance, Leptin System, Lateral Hypothalamic Area, Glioma Stem-like Cells
Introduction
White adipose tissue (WAT) was traditionally thought to only serve as a long-term fuel reserve, which during food deprivation is mobilized by releasing fatty acids for oxidation in other organs. According to this classical view, adipose tissue increases in size during periods of positive energy balance and decreases when the energy expenditure is greater than the food intake. In recent years, however, ongoing research, due to the alarming increase in the prevalence of obesity in modern societies, has changed our perspective on WAT, which is now recognized as a major endocrine and secretory organ involved in body metabolism and homeostasis [1–6].
The main event introducing this new concept of WAT was the discovery of the satiety factor leptin (derived from the Greek word “leptos,” which means “thin”) in 1994 [7]. Leptin is primarily produced in adipose tissue, and its circulating levels are positively associated with the amount of body fat mass [8, 9]. Following the discovery of leptin, more than six hundred hormones, termed adipokines (or adipocytokines), which are either exclusively or partly secreted by WAT, have been identified [10]. Adipokines act in various autocrine, paracrine, and endocrine roles.
Adipokines influence appetite and satiety, regulate energy expenditure, and control insulin secretion, in addition to glucose and fat metabolism. They are also involved in neuroendocrine axis regulation, endothelial function, atherogenesis and cardiovascular function, fertility, immune function, and fetal growth [11–19]. Accumulating evidence has suggested that their altered secretion from adipose tissue plays a key role in mechanisms related to cancer [20].
Primary brain tumors are relatively rare in middle-aged individuals compared to metastatic ones, but they are increasingly prevalent in children and young adults [21]. Central nervous system (CNS) tumors are associated with increased mortality and morbidity, being responsible for around 30 and 20% of total cancer-related deaths, respectively [22]. Among children survivors of primary CNS tumors, long-term consequences are common; hence, research on inherited, environmental, and developmental risk factors is ongoing [21]. Approximately three out of four primary brain tumors in children and young adults are gliomas, including pilocytic astrocytoma, anaplastic astrocytoma, glioblastoma, oligodendroglioma, anaplastic oligodendroglioma, ependymomas, anaplastic ependymoma, and mixed gliomas.
Globally, primary brain tumors are more common in males than females, with a ratio ranging up to 3.5 [23]. Nevertheless, studies on groups of both pre-pubertal and post-pubertal patients indicate that the different prevalence of brain tumors between males and females cannot be attributed to the sexual hormone dimorphism. On the other hand, pituitary adenomas have been found to be much more prevalent in females than males, with a ratio of up to 7 [24]. This ratio varies along the decades of life, with a peak around the age of 35, indicating a dependence on the sexual hormone dimorphism.
The aim of the present review is twofold: firstly, to investigate to what extent the levels of leptin can be used as a prognostic factor of primary brain tumors and pituitary adenomas in children, before and after treatment, and secondly, to report on current findings related to the mechanistic involvement of leptin in intracranial tumor pathogenesis.
Expression and Production of Leptin
The leptin gene (Lep (ob)) well conserved among species, encodes an 18-kDa protein containing a signal sequence that is cleaved to produce the mature 16-kDa hormone in humans. [7]. A 96% homology between the rat and mouse ob gene exists, while the nucleotide and protein are 84 and 82% homologous to the human ob gene, respectively [25]. Although WAT still remains the major site of leptin synthesis and the major contributor to leptin circulating levels [9], leptin has also been detected in several additional sites, including brown adipose tissue (BAT), the stomach, placenta, mammary gland, ovarian follicles, the heart and skeletal muscles, the pituitary gland, and the brain [26].
In humans, serum leptin levels are significantly higher in females compared to males [27] and exhibit a significant 24-h pattern with a nocturnal maximum [28]. Leptin levels increase in overweight and obese individuals who develop leptin resistance, a condition characterized by the inability of leptin to control appetite or energy balance [9].
Nutritional status regulates leptin synthesis in WAT. Fasting induces a rapid reduction both in ob gene expression and leptin circulating levels, which are reversed upon refeeding, as found in rodents [29, 30]. Low leptin levels in mice suppress the feeling of satiety and increase the drive to feed, activate pathways to decrease energy use, and suppress the growth and reproductive endocrine axes [13]. Various hormones, including catecholamines (adrenaline and noradrenaline) in mice [29], as well as insulin [31], glucocorticoids, estrogens [32], and testosterone [33] in humans, regulate leptin expression in WAT. Furthermore, based on rodent models, leptin levels were found to increase in response to inflammatory cytokines such as tumor necrosis factor (TNF)-α, sepsis, and acute infection [34–36]. As found in mice, the sympathetic nervous system is the main regulator of leptin production that provides a negative feedback loop to adipose tissue in the production of the hormone [29].
Functions of Leptin
Apart from its interaction with central neuroendocrine systems leading to the inhibition of food intake [19, 37], leptin signaling regulates several additional pathways implicated in epithelial cell proliferation, adhesion, inflammation, and angiogenesis. Leptin increases β3 integrin [38], leukemia inhibitory factor (LIF), leukemia inhibitory factor receptor (LIF-R), interleukin (IL)-1, IL-1 receptor (IL-1R), IL-1 receptor antagonist (IL-1ra), vascular endothelial growth factor (VEGF), and VEGF receptor [39]. Thus, excessive and uncontrolled leptin signaling can lead to cell proliferation in malignancies and/or increased angiogenesis and this way contributes to tumor growth and metastasis [40–44].
Leptin exerts its biological effects via its receptor OB-R, a member of the cytokine I superfamily [45]. Ligand-dependent activation of OB-R plays a key role in proliferation, survival, and migration of epithelial cells [46]. Thus, leptin/OB-R signaling has been linked to the progression of breast, endometrial, pancreatic, bladder, colon, kidney, esophageal, lung, liver, prostate, ovarian, skin, thyroid, and brain cancers [47–49]. In the brain, OB-R exists as six isoforms: an OB-R long form (OB-Rb), four short forms (OB-Ra, -Rc, -Rd, and -Rf), and a soluble isoform (OB-Re) [50]. All isoforms have identical extracellular ligand-binding domains, but differ in the length of the cytoplasmic tail [51]. OB-Rb is highly, although not exclusively, expressed in the brain, in particular the hypothalamus [52] and is the only isoform exhibiting signaling capability. It consists of an extracellular, a trans-membrane, and an intracellular domain.
OB-Rb is essential for leptin’s weight-reducing effects and is highly expressed in hypothalamic neurons, T cells and vascular endothelial cells. In the CNS, OB-Rb is mainly expressed in the hypothalamic arcuate nucleus (ARC), dorsomedial nucleus (DMH), paraventricular nucleus (PVN), ventromedial nucleus (VMH), and the lateral hypothalamic area (LHA) [53, 54]. These nuclei express neuropeptides and neurotransmitters that regulate food intake and body weight, including the orexigenic neuropeptide Y (NPY), which responds through its receptors to absent, or low levels of leptin and the melanocyte-stimulating hormone (MSH), which acts through its melanocortin-4 receptor in response to an increased plasma leptin concentration. NPY mRNA levels are increased in ob/ob mice and decreased after leptin treatment [55].
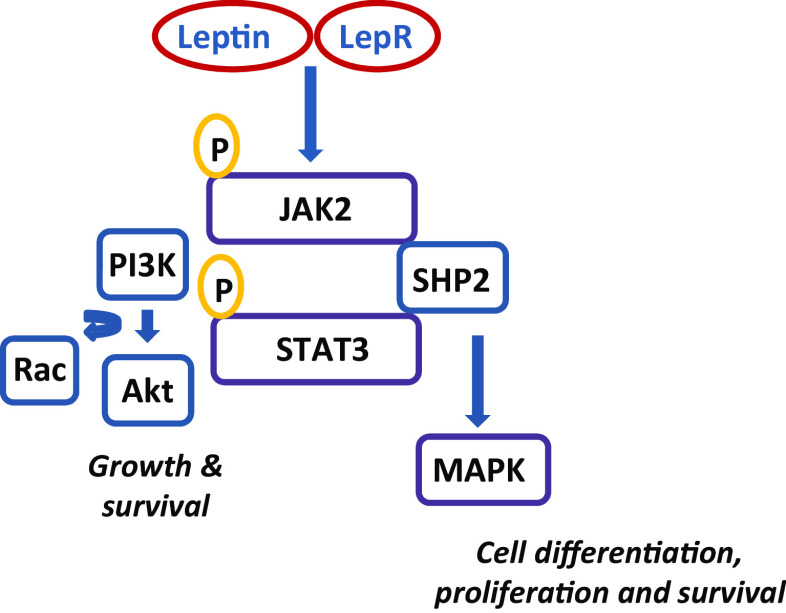
The leptin interaction with OB-R (Fig. 1), activates multiple intracellular pathways including the Janus kinase 2-signal transducer and activator of transcription 3 (JAK2/STAT3) signaling pathway, the most important regulator of energy homeostasis [56, 57]. With the exception of OB-Re, all OB-R isoforms contain a highly conserved, proline-rich sequence in their cytoplasmic tail that is required for JAK2 activation. Additional pathways activated include the mitogen-activated protein kinase extracellular signal-activated kinase 1/2 (MAPK/ERK1/2) and phosphatidylinositol-3 kinase-protein kinase B (PI3K/PKB, the latter also known as AKT, a serine/threonine kinase) [58]. Leptin stimulation of murine OB-Rb involves phosphorylation of two specific tyrosine residues (Tyr985 and Tyr1138) in the C-terminal domain. The main leptin signaling pathway in the hypothalamus is activated upon STAT3 to Tyr1138 [59]. OB-R activity is regulated by additional mechanisms including dimerization and translocation of STAT3 to the nucleus which then promotes gene expression and increases the levels of Src homology 2 domain containing the protein, suppressor of cytokine signaling-3 (SOCS-3). SOCS-3 protein binds to Tyr985 of OB-R and mediates the negative regulation of the STAT pathway [59]. The protein tyrosine phosphatase SHP2 (Src homology region 2-containing protein tyrosine phosphatase 2) also acts as a regulator of OB-R/STAT3 [60, 61] and OB-R/ERK activities [62].
Fig. 1.
The effects of leptin are mediated through activation of multiple intracellular pathways upon binding of leptin with OB-R. These include JAK2/STAT3, MAPK, and the PI3/Akt signaling pathways. SHP2 acts as a regulator of OB-R/STAT3 and OB-R/MAPK activities
Physiological Effects of Leptin in the Brain
Summarizing, the leptin receptor OB-Rb, as previously reported, is primarily expressed in hypothalamic nuclei (most prominently in ARC and VMN), as well as other neuronal populations in the midbrain and brainstem [63]. Leptin has been shown to exert a neurotrophic effect on hypothalamic, hippocampal, and cortical neurons, mainly those related to the feeding circuits and the behaviors that are important for energy homeostasis in general [64]. For a recent review discussing the role of leptin in the neurobiology of appetite and eating, as part of a complex neuroendocrine system, see [65]. Even more interestingly, based on studies in mice, leptin has been shown to play a role in neuronal and glial development and growth, as well as the modulation of synaptic plasticity [66–68].
Leptin and Intracranial Tumor Prognosis
Given the fact that leptin exerts most of its biological action via the brain, studies of the leptin regulatory system after treatment of intracranial tumors are of particular interest. Both surgical resection as well as radiotherapy are known to influence adjacent and neighboring to the tumor structures, so given the high expression of leptin receptors in the hypothalamus, most relevant studies focus on the effect of the treatment of hypothalamic and pituitary tumors on leptin levels and signaling (Table 1).
Table 1.
Leptin/leptin receptors and brain tumor prognosis
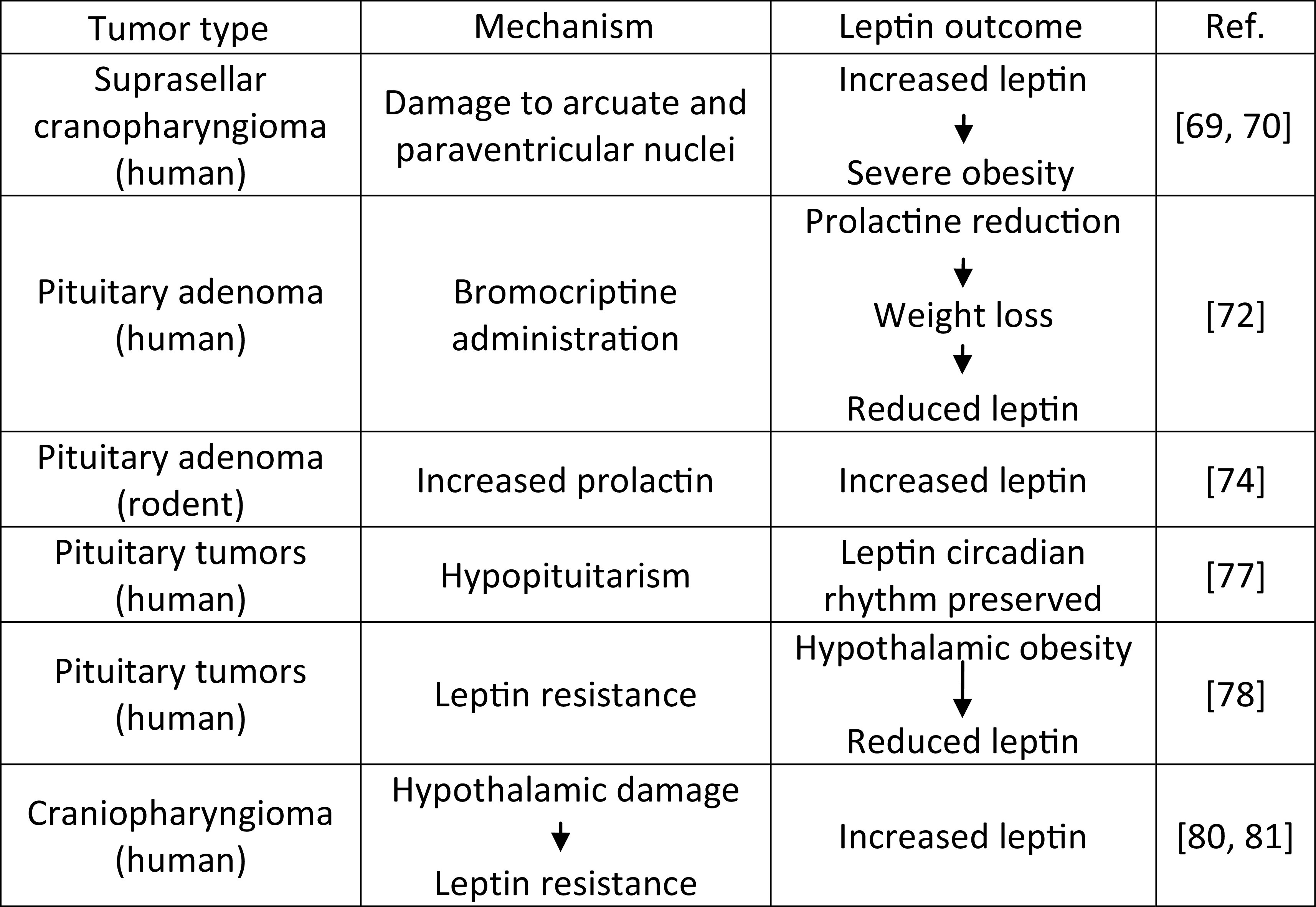
Leptin serum levels in obese supracellular craniopharyngioma-operated patients were referred elevated compared to obese controls [69]. The observed severe obesity in such patients was attributed to a disturbed feedback in the leptin mechanism. The end result of this process seems analogous to that of the mechanism that leads to high leptin levels in obese patients where leptin resistance has already been developed. Additionally, it was noted that patients with an intrasellar tumor presented higher serum leptin levels comparable to controls [69]. These findings were explained by the hypothesis that it is the suprasellar tumor localization only that can lead to damage to the ARC and the PVN, either by the tumor itself or the treatment interventions. These nuclei are known to play a pivotal role in leptin signaling, thus being spared by an intrasellar tumor or its treatment process can explain the normal leptin levels on these patients. Further supporting the above hypothesis of “hypothalamic obesity” on brain tumor patients, Ferretti et al. have consequently reported two cases of familial giant pituitary adenomas characterized by progressing obesity and high serum leptin levels [70]. Based on the finding that the weight gain was increased after tumor radiotherapy, as well as the fact that post-operative episellar hemorrhage was observed in one of the two patients, the authors have concluded that hypothalamic damage and subsequent dysregulation of leptin signaling, with the development of a “leptin resistance” syndrome, is the most plausible explanation for the obesity described in these patients [70].
Interestingly, it is the lack of leptin that has a much more pronounced effect than its increase, even considerably, over the physiological levels, i.e., leptin-deficient individuals are going to be obese, whereas significant doses of leptin administered to patients with common obesity will most probably not lead to considerable weight decrease [71]. Following that line of thought, it is highly improbable that increased leptin action, either via increased leptin concentration, or via increased leptin receptor expression would be the cause of anorexia or cachexia. This notion was supported by experimental animal models [72]. It was shown in a rat brain tumor model that the OB-R mRNA was not increased in the hypothalamus of rats with C6 glioma, even though they exhibited anorexia. At the time, this seemed to further support the hypothesis that the leptin system dysregulation is a primary suspect in cases of obesity following brain tumors, but not in cases of anorexia/cachexia, which were thus thought to be mediated by other leptin-independent pathways [72].
Caution should be taken when comparing the results of animal models to those of patient studies. For instance, a much more recent study on children with brain tumors, some of them surgically treated, but all of them treated with chemotherapy and radiotherapy, reported a tendency for increased serum leptin levels in cachectic children with malignant brain tumors when compared to non-cachectic ones, during the phase of maintenance treatment [73]. Such findings can potentially explain the root of severe undernourishment noted in some patients during maintenance treatment. Additionally, these findings are highly indicative of the existing differences in the leptin signaling system between rodents and humans. A similar study showed that in contrast to the maintenance phase, no statistically significant correlation was found between leptin levels and nutritional status, as expressed by body mass index (BMI), during or after the treatment itself. Moreover, during the same initial period, no difference in leptin concentrations was noted between children with tumors and normal controls, even though during the course of treatment, the BMI of the children with malignancies was reduced in a statistically significant manner. The small number of patients enrolled could negatively affect the validity of the results, but the outcome is highly indicative of the complexity of the leptin system. Even though it initially seems to be functioning properly, either the tumor itself or its treatment trigger is a delayed, or secondary, leptin dysregulation. Taking this into consideration will not only allow for better designed longitudinal studies on this matter but it is also essential for clinicians to keep this in mind for the long-term patient management.
Another characteristic example of why leptin studies on rodent models sometimes fail to be representative of the situation in humans is due to prolactin (PRL). In a study of pituitary adenoma survivors treated with the dopamine agonist bromocriptine (BC) for persistent hyperprolactinemia, prolactin and leptin serum levels were not found to be directly correlated [74]. Although BC led to PRL levels reduction and weight loss, it was only in a later phase that leptin was reduced, which is indicative of an indirect leptin reduction via the BMI decrease, rather than a direct one via interaction with a prolactin pathway. These observed findings on humans seemed to contradict the results of previous, analogous studies in rodents, where increased prolactin levels have led to a statistically significant leptin increase, compared to controls. This is another difference in the leptin system between rodents and humans.
Returning to the analogy between obesity after craniopharyngioma surgery and common obesity as far as leptin concentration is concerned, one should consider the supporting results reported by Srinivasan et al. [75]. Motivated by the fact that around 50% of patients treated at childhood age for craniopharyngioma are obese at follow-up, the authors of this study have followed 15 patients, all of them treated by means of surgical tumor resection and only one receiving adjuvant radiotherapy. They have concluded that patients after craniopharyngioma removal have increased abdominal fat and a less favorable lipid profile compared to obese controls, which are features of the metabolic syndrome. Nevertheless, compared to controls with common obesity, craniopharyngioma survivors exhibited neither decreased insulin sensitivity nor statistically significant different serum leptin levels. Although the authors mainly focused on the unmet (due to the limited sample size) question of whether or not GH treatment can have a significant effect on the abdominal adiposity and dyslipidemic profile, an interesting question with respect to leptin remains: given that leptin concentration was elevated in both obese groups in the study (craniopharyngioma survivors and controls), was this phenomenon an indication of a “leptin resistance syndrome” being caused by the tumor or its treatment? Or is the obesity in craniopharyngioma survivors mediated mainly by another mechanism and increased leptin levels occur in the same way as for the case of leptin resistance being developed in common obesity? At this point, it should be noted that a similar trend of serum leptin concentrations not differing significantly from BMI-matched controls was observed in a study including 14 adult-onset GH deficiency patients, following pituitary tumor treatment, by means of both surgery and radiotherapy for the majority of the patients [76].
Kousta et al. have performed a study on 14 hypopituitary patients, as a result of surgical pituitary tumor excision and radiotherapy for all patients but one that received only radiotherapy, with or without some remaining pituitary function [77]. The study did not focus on the leptin serum levels of these patients with respect to controls, but tried to identify changes in the circadian rhythm and sex dimorphism that are specific for this group. It was observed that leptin levels followed the same circadian rhythm, with women having higher leptin serum levels than men, as seen in the literature for healthy controls. Based on that, the authors have concluded that the diurnal leptin levels are independent of an intact hypothalamic-pituitary axis function. This finding indicates the complex mechanism of leptin regulation and action and the care that must be taken when evaluating the effects of a tumor or its treatment on leptin regulation: some aspects might be affected and some not.
To better answer the question of whether post-intracranial tumor obesity is linked to the development of leptin resistance due to hypothalamic damage on the hypothalamic nuclei that are rich in OB-Rs, the notion of free leptin index (FLI) is also used. FLI is defined as the ratio of total serum leptin to the soluble leptin receptor OB-Re and indicates the amount of circulating leptin that is available for its signaling pathway. In one study, higher plasma leptin levels corrected for BMI compared to BMI-matched controls were found in patients who developed hypothalamic obesity post-pituitary tumor treatment along with a tendency for a higher FLI for the hypothalamic obesity group of patients [78]. It should be mentioned however that similar OB-Re levels were found in all groups. This study has thus not only highly indicated the contribution of leptin resistance in the pathogenesis of the well-described hypothalamic obesity in this patient group but also hinted at the fact that such leptin resistance is most probably more severe than what is encountered in cases of common obesity.
Nevertheless, the hypothesis of “leptin resistance syndrome” is not the only one for explaining hypothalamic obesity. Growth hormone (GH) deficiency that can occur as a result of intracranial tumor or its treatment (especially cranial irradiation) is known to cause obesity with predominant abdominal adiposity [79]. Following the onset of obesity, a secondary leptin increase is to be expected. GH therapy in pituitary tumor survivors should reverse or avoid this effect, but to what extent, this might be difficult to assess. Under the conventional hormone replacement by GH administration in 42 craniopharyngioma survivors, serum leptin levels normalized over the fat mass and remained significantly higher compared to controls’ levels, even after correcting for BMI. The authors speculated that this was proof that owing to direct hypothalamic damage, a lack of sensitivity to endogenous leptin is developed, disrupting the brain circuits related to feeding behavior and energy homeostasis [80]. The same group, two years later, reported the differential effect of craniopharyngioma and its treatment, between males and females focusing on the effect of GH replacement therapy on bone mineral density (BMD) [81]. Leptin resistance, as indicated by the ratio of leptin/kg of fat mass, was found to be more increased in female than male patients, with the female group only displaying a statistically significant difference compared to controls. Leptin resistance was also considered to be one of the causes of BMD reduction as opposed to the expected beneficial effects of increased BMI on BMD that are observed in cases of common obesity.
A last note shall be made with respect to the leptin system and GH dysregulation: after resection of suprasellar brain tumors, no difference in serum leptin levels was found between patients with and without GWGH (growth without GH) [82]. Moreover, even when patients were divided into an obese/overweight and a normal BMI group, no difference in serum leptin levels between those two groups was noted. Such findings indicate that for GWGH patients, a primary leptin system dysregulation plays a minor role in the onset of obesity. These findings also hint at a decoupling of the GH axis and the leptin system, indicating that primary leptin resistance due to hypothalamic damage (and not secondary due to developing obesity) must indeed be an important mechanism in the onset of obesity in intracranial tumor survivors.
Another possible explanation of leptin increase and obesity that has been proposed reported that a lesion of the VMH by either the tumor itself or its treatment leads to a dysregulation of the vagally mediated insulin release from the β-pancreatic cells [83]. The increase in insulin secretion can in turn cause weight gain and obesity. An interesting follow-up question occurs: given the known role of the VMH in leptin signaling pathways, to what extent the observed hyperleptinemia is a primary effect of the VMH lesion, or a secondary effect of the obesity onset? Based on a follow-up study of 27 children with craniopharyngioma, further evidence exists to support the latter proposition [84]. It is suggested that the degree of hypothalamic involvement before the surgical resection of the craniopharyngioma determines the evolution of the patient BMI, as the greater the hypothalamic involvement, the more increased the insulin secretion post-tumor resection and the bigger the subsequent BMI increase. Then, leptin plasma concentrations increase at a second phase, based on the established BMI [84].
Leptin and Intracranial Tumor Pathophysiology
From early on after its discovery, leptin has been shown to play an important role in the pathogenesis of primary brain and pituitary tumors, a relationship supported both by in vivo and in vitro data.
In one of the first relevant studies, Shimon et al. have observed that the “functioning” version, i.e., the long isoform, OB-Rb, is only expressed in human fetal and adult pituitary tumor tissues, but not in normal adult pituitaries [85]. In order to further investigate the relationship between the leptin and the GH system, they used cultures of the harvested human fetal and tumor pituitary cells. The in vitro leptin administration resulted in a specific stimulation of GH release (although had no effect on PRL, LH, FHS, or ACTH), for cultures of fetal cells with a gestation age of up to 3 months. Interestingly enough, GH- or PRL-secreting adenoma cells presented different response to leptin administration. Nevertheless, caution is required when evaluating the results, due to the limited sample size. Studying a larger sample group, including the human pituitary cell line HP75, as well as cultures of harvested human normal pituitary cells and ACTH, GH, PRL, gonadotroph, and null cell adenomas, Jin et al. have found that OB-Rb and OB-Ra are expressed by most human anterior pituitary cell types, while leptin expression is reduced in pituitary adenomas [86]. This study revealed a major difference in results with respect to the experiment of Shimon et al.: both the long and short OBs were found to be expressed by normal and neoplastic anterior pituitary cells alike, contrarily to the prior observation of no OB-R expression in normal pituitary cells. Differences such as the specimen origin and the fact that the latter study used normal pituitary tissue stemmed from brain tumor surgery, whereas the normal pituitary tissue used by the former study was obtained by autopsy, may resulted in increased sample degradation prior to fixation. In any case, the expression of both leptin and its receptors by normal adult and tumor pituitary tissue alike led to a formation of an autocrine loop hypothesis in the pituitary, where the leptin system plays a significant role in normal conditions and its dysregulation is a major candidate for tumor onset and development. However, in vitro leptin administration had a diverse effect in different cell lines’ growth: it inhibited all growth in the human HP75 and in the rat pituitary GH3 lines, but not on the OTB-1 and LβT2 ones, hence showing a complex regulatory role in tumor dynamics that needed to be further investigated [86].
At this point, an important question arises: while measuring the leptin content in different cell types by means of antibody-based detection with light microscopy gives us an image of leptin content and most probably leptin RNA expression levels, is it enough to understand leptin dynamics? To answer this question, apart from measuring leptin content in different cell types, sub-cellular localization of leptin with increased spatial resolution would be the next logical step. To that end, the experimental setup of Vidal et al. [87], using electron instead of light microscopy, was employed. Studying both normal and tumorous pituitary tissue extracted during surgery, the authors observed similarities and differences compared with the experiment of Jin et al. In detail, leptin was detected in most hormone-producing glandular cells in human pituitaries, with the exception of lactotroph, and stellate cells as opposed to Vidal et al. possibly because of the different sensitivity of immunohistochemical methods applied, between light and electron microscopy. Moving further on to adenomas, leptin was only identified in corticotroph adenomas and even then, the immunoreactivity was approximately half than that of normal corticotroph cells. These findings are clearly in tandem with the previously observed inhibition of cell growth in some immortalized cell lines by leptin, hinting at the fact that lack of endogenous leptin can indeed be one of the first steps that leads to unchecked cell multiplication and growth. It would also indicate exogenous leptin administration as a possible solution for pituitary tumor treatment, a possibility that needed to be explored in subsequent studies.
The use of electron microscopy in the aforementioned study of Vidal et al. [87] not only increased the accuracy of leptin detection in different cell types but also verified leptin localization in secretory granules in the hormone-producing glandular cells, both in healthy and diseased pituitaries. This information suggested a novel secretory pathway for leptin in the pituitary, different to that for adipose tissue and as one expects, it can further increase complexity in the leptin system, as leptin can be stored and secreted as a response to specific stimuli, together with pituitary hormones.
The autocrine loop hypothesis may be strongly supported by experimental data for the case of normal pituitaries, but it does not seem to necessarily apply to brain tumors, as shown by two relevant studies in 2001. Working on several tumor samples from different brain tumor types, Knerr et al. observed that leptin mRNA was not consistently expressed in all specimens and even when it was, its expression levels were very low [88]. In contrast to that, OB-Rb gene expression was high with respect to leptin in all of the tumor types, although a note needs to be made on the fact that no comparison with normal controls was performed. Nevertheless, based on these findings, it was concluded that the impact of autocrine leptin is minimal in brain tumors, whereas there is still a significant chance that exogenous leptin administration can play an important, most possible therapeutic role. In another study performed the same year, Korbonits et al. have focused on pituitary adenomas and converged on the same hypothesis [89]. In detail, leptin long receptor (OB-Rb) mRNA was again found non-consistently expressed at varying levels and leptin mRNA was also observed randomly and at low levels in human adenomas, consolidating the hypothesis that for these tumors, correlation of leptin and OB-Rb levels was not found. Additionally, this study addressed the still open at the time question of possible differences in tissue obtained from either autopsies or biopsies, as discussed before for the works of Shimon et al. and Jin et al. [85, 86]. Leptin mRNA was not detected in normal tissue from autopsies, but was detected in normal tissue biopsies, validating the explanation of Shimon et al. for superior quality of biopsied specimens. Finally, it was observed that adenoma cells in vitro randomly secreted leptin, with an additional interesting finding of a significant difference between the considerable release of leptin and the low leptin mRNA transcription rates [80]. Could this be correlated to the storage of leptin in secretory granules that could allow for temporally decoupling the leptin mRNA transcription and the release of leptin into the cell environment?
And while studies based directly on measurements performed on human tumor samples have a particular significance, in vitro studies of cultured cells from these specimens are of increasing importance towards a more mechanistic approach of the leptin system function and regulation. For example, in such an in vitro study, the leptin priming of cultured GH-secreting human pituitary adenoma cells was found to have a double effect: a slight, dose-dependent reduction of the spontaneous GH discharge, as well as an increase of the GHRH-induced GH discharge into the medium [90]. Although the first part of this leptin effect follows the low GH levels observed in obesity, the second part contradicts what happens in obese patients, where the GHRH-induced GH is reduced too. However, no speculation was made for this finding. In what they seemed to agree with previous knowledge though, was once again the non-consistent expression of leptin long receptor by the cultured adenoma cells.
As seen above, the main challenge for investigators on the first years after leptin discovery was the detection and the precise localization of leptin on different normal or transformed cell types, either originating directly from tumor samples or from cells being cultured in the laboratory. It was nearly a decade after leptin discovery that the interest started to considerably shift not only towards the mere presence or not of leptin in tumors but also towards its correlation with specific tumor phenotypes and especially with abilities such as proliferation and invasiveness. Being one of the first to comprehensively address this matter, Isono et al. have investigated extracted adenoma tissue from nearly 100 patients, investigating the link between leptin expression in these tumors, in relation with cell proliferation and invasiveness [91]. Leptin expression was significantly higher in non-invasive, functioning adenomas, compared to invasive ones, a relationship that persisted regardless of tumor size. In addition, leptin expression levels were positively correlated to ACTH- and then GH-expressing functioning adenomas, hinting at a significance of the functioning adenoma type characterization as a possible prognostic factor, based on its invasiveness. Furthermore, the study moved on to identify that among non-invasive adenomas, microadenomas tended to express increased leptin levels compared to larger adenomas, presenting a negative correlation between leptin expression levels and tumor cell proliferation, which again could be exploited towards a better patient management. Interestingly, no such relation with respect to leptin and proliferation or invasiveness was found in non-functioning adenomas. A connection seems to exist between the above observations and the proposed mechanism of leptin storage and secretion together with pituitary hormones that has already been discussed previously [87]. Table 2 summarizes the above observations on the presence of different leptin system elements in healthy and tumorous tissue.
Table 2.
Leptin system elements and their presence in normal/tumorous tissue
| Leptin system element | Mechanism | Leptin outcome | Ref. |
|---|---|---|---|
| OB-Rb | Human fetal adult pituitary tumor tissue | Expressed | [85] |
| Normal adult pituitary | Not expressed | ||
| Leptin | Normal pituitary cells | Detected | [86] |
| Pituitary adenomas | Reduced | ||
| OB-Rb | Normal anterior pituitary cells | Expressed | [86] |
| Neoplastic anterior pituitary cells | Expressed | ||
| Leptin mRNA | Normal tissue from autopsies | Not expressed | [89] |
| Normal tissue from biopsies | Expressed | ||
| Leptin | Non-invasive functioning adenomas | High levels detected | [91] |
| Invasive adenomas | Low levels detected | ||
| Leptin | ACTH- and GH-expressing functioning adenomas | High levels detected | [91] |
| Other functioning adenomas | Low levels detected | ||
| Leptin | Non-invasive microadenomas | High levels detected | [91] |
| Non-invasive macroadenomas | Low levels detected |
The prospect of leptin as a therapeutic target has not only been investigated for the case of adenomas. Important breakthroughs have been performed on gliomas, though in comparison to adenomas, for these tumor types, leptin seems to lead to a less favorable tumor phenotype and behavior. For instance, LN18 and LN229 human glioblastoma multiforme (GBM) cells were found in vitro to be able to produce biologically active leptin, together with the necessary OB-Rb necessary for its action [92]. This autocrine loop seemed to exert a clear pro-angiogenic effect in endothelial cells, in what the authors considered to be both a VEGF-dependent (having proven STAT3 activation by leptin) as well as non-dependent pathway. Such effects were blocked by the OB-R competitive antagonist AcA1. The authors have thus hypothesized and proven a synergistic effect of the OB-R antagonist AcA1 and the anti-VEGF factor SU1498, whose combination was able to significantly reduce tumor cell proliferation. As the authors themselves pointed out, their study was designed on the basis of several preceding ones that have correlated leptin expression with angiogenesis induction and a malignant, chemotherapy-resistant phenotype in gliomas [92].
Proving the above synergistic effect though has not only deepened our mechanistic knowledge of glioma development but has also offered important insight for future therapeutic effort directions. In a 2013 study by Han et al. [93], the previously mentioned STAT3 pathway activation by leptin that was already observed was further investigated. In detail, the authors focused on the implication of this pathway activation on the glioblastoma chemotherapeutic resistance, both in vitro, using the U87 human glioma cell line, and in vivo. It was observed that the STAT3-mediated SOX2/OCT signaling axis maintained the stem/progenitor cell properties (i.e., invasiveness and proliferation) of glioblastoma OB-R positive cells, leading to temozolomide (TMZ) resistance. The percentage of OB-R positive cells was also increased in recurring tumors after resection, presenting a clear link between the leptin—OB-R axis and the tumor malignancy and self-renewal. For nude mice injected with such tumor cells, the tumor volume was also significantly increased after leptin treatment, hinting at a molecular link between increased leptin levels and glioblastoma occurrence, aggressiveness, and recurrence. Another recent study by the same group supporting this theory relied on the same U87 human glioma cell line and described the enhancing of glioma stem-like cells’ (CD133+) invasive ability that was induced by leptin [94]. It was shown that this effect was mediated by the JAK/STAT3 signaling pathway activation by leptin, after which, mRNA expression of known invasive factors such as matrix metalloproteinase (MMP)-9, β-integrin, N-cadherin, and vimentin is increased too. The aforementioned observations are presented summarily in Table 3. Towards an updated therapeutic approach based on regulation of the leptin signaling pathway, it is also important to remember that increased leptin levels at the tumor can either occur locally due to an autocrine loop or can be a result of increased systemic leptin levels, with the most prominent example being obesity.
Table 3.
Leptin and brain tumors: mechanistic observations
| Tumor/Cell type | Investigated factor | Mechanism | Outcome | Ref. |
|---|---|---|---|---|
| Cultured GH-secreting human pituitary adenoma cells | Leptin priming | Spontaneous GH discharge reduced | Low GH levels in obesity | [90] |
| GHRH-induced discharge increased | Low GHRH-induced GH in obesity | |||
| Glioblastoma cells | OB-Rb expression | STAT3, SOX2/OCT signaling axis |
Invasiveness and proliferation ↓ temozolide resistance |
[93] |
| Glioblastoma tumors | Resection and recurrence | Percentage of OB-Rb+ cells increased | Increased malignancy and self-renewal | [93] |
| OB-Rb+ tumors in nude mice | Leptin treatment | (Not specified) | Increased tumor volume | [93] |
| Glioma stem-like cells (CD133+) | Leptin treatment |
JAK/STAT3 signaling pathway ↓ Increased MMP9, β-integrin, N-cadherin, and vimentin expression |
Increased invasive ability | [94] |
Leptin as Tumor Biomarker
Based on the increasing evidence of the correlation between leptin signaling and glioblastomas, one could propose that leptin serum levels could be used for diagnostic purposes, starting for these types of tumors. This seems to be the case, since leptin was successfully identified as one of several biomarkers that can be used in a model of early glioblastoma diagnosis that relies on a simple serum analysis [95]. In total, 15 different cytokine/angiogenesis factors were investigated, with 7 of them having a statistically significant difference in concentration between glioblastoma patients and controls. Out of them, leptin was the least sensitive (40%), yet the second most specific (74%) marker investigated. Obviously, increased leptin levels due to obesity can pose an important challenge, but even though this study did not explicitly discuss or address the BMI factor, the diagnostic method proposed was reported to have up to 87.5 and 100% sensitivity and specificity, respectively, so the proposed line of thought seems at least promising.
Conclusions
Dysregulations of the leptin system can be both a consequence as well as a cause for primary brain and pituitary tumors. In the first case, there seems to be two main mechanisms for increased leptin levels in intracranial tumor survivors, caused by the tumor itself or its treatment, surgical or radiological: (a) primary leptin resistance due to hypothalamic damage and (b) secondary leptin resistance caused by developing obesity. Differentiating between these two cases could provide the clinician with useful information, although it cannot be ruled out that both mechanisms occur to a certain extent at the same time.
In the second case, locally produced leptin (as part of an autocrine loop in brain regions, e.g., the pituitary), systemically increased leptin (e.g., due to obesity), and exogenous leptin can all influence cancer pathogenesis. Data is still conflicting though, indicating a differential effect of leptin on specific tumor types. For instance, in functioning adenomas, leptin levels are negatively correlated with tumor invasiveness, while in gliomas, leptin expression leads to angiogenesis, invasiveness, increased proliferation, resistance to chemotherapy, and improved self-renewal.
The vast majority of current information on the role of leptin originates from association studies, where showing a clear causal relationship is challenging at best. The fact that the studies available also rely on different number of subjects, different treatment between subjects, measurements of leptin in both tissue or blood, and measured outcomes at varying times after the therapeutic intervention should also be taken into consideration when analyzing the current literature knowledge. Especially for the case of human studies, future data will be more valuable when obtained for larger numbers of patients undergoing the same treatment, for tumors with as many common clinical, anatomical, and pathological characteristics as possible. Additionally, a better mechanistic understanding of the leptin effect in different brain tumor subtypes is necessary before attempting its directed targeting for therapeutic reasons, because a wrongful intervention in the leptin system could lead to the exactly opposite outcome. A far more attractive option that has already yielded positive results is the diagnostic exploitation of leptin levels as a biomarker in a multi-factor model of early brain tumor detection based on a simple blood serum examination.
References
- 1.Sakurai T, Ogasawara J, Shirato K, Izawa T, Oh-Ishi S, Ishibashi Y, Radak Z, Ohno H, Kizaki T. Exercise training attenuates the dysregulated expression of adipokines and oxidative stress in white adipose tissue. Oxidative Med Cell Longev. 2017;2017:9410954. doi: 10.1155/2017/9410954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopes HF, Correa-Giannella ML, Consolim-Colombo FM, Egan BM. Visceral adiposity syndrome. Diabetol Metab Syndr. 2016;8:40. doi: 10.1186/s13098-016-0156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parimisetty A, Dorsemans A-C, Awada R, Ravanan P, Diotel N, Lefebvre d’Hellencourt C. Secret talk between adipose tissue and central nervous system via secreted factors-an emerging frontier in the neurodegenerative research. J Neuroinflammation. 2016;13:67. doi: 10.1186/s12974-016-0530-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Pan R, Pfeifer A. Fat tissues, the brite and the dark sides. Pflugers Arch. 2016;468:1803–1807. doi: 10.1007/s00424-016-1884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaszkiewicz M, Townsend KL. Adipose tissue and energy expenditure: central and peripheral neural activation pathways. Curr Obes Rep. 2016;5:241–250. doi: 10.1007/s13679-016-0216-9. [DOI] [PubMed] [Google Scholar]
- 6.DiSpirito JR, Mathis D. Immunological contributions to adipose tissue homeostasis. Semin Immunol. 2015;27:315–321. doi: 10.1016/j.smim.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 8.Frederich RC, Lollmann B, Hamann A, Napolitano-Rosen A, Kahn BB, Lowell BB, Flier JS. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest. 1995;96:1658–1663. doi: 10.1172/JCI118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 10.Lehr S, Hartwig S, Sell H. Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteomics Clin Appl. 2012;6:91–101. doi: 10.1002/prca.201100052. [DOI] [PubMed] [Google Scholar]
- 11.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 12.Matarese G, La Rocca C, Moon HS, Huh JY, Brinkoetter MT, Chou S, Perna F, Greco D, Kilim HP, Gao C, Arampatzi K, Wang Z, Mantzoros CS. Selective capacity of metreleptin administration to reconstitute CD4+ T-cell number in females with acquired hypoleptinemia. Proc Natl Acad Sci U S A. 2013;110:E818–E827. doi: 10.1073/pnas.1214554110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 14.Carbone F, La Rocca C, Matarese G. Immunological functions of leptin and adiponectin. Biochimie. 2012;94:2082–2088. doi: 10.1016/j.biochi.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 15.De Rosa V, Procaccini C, Cali G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Mantzoros CS, Moschos S, Avramopoulos I, Kaklamani V, Liolios A, Doulgerakis DE, Griveas I, Katsilambros N, Flier JS. Leptin concentrations in relation to body mass index and the tumor necrosis factor-alpha system in humans. J Clin Endocrinol Metab. 1997;82:3408–3413. doi: 10.1210/jcem.82.10.4323. [DOI] [PubMed] [Google Scholar]
- 17.Fasshauer M, Bluher M, Stumvoll M. Adipokines in gestational diabetes. Lancet Diabetes Endocrinol. 2014;2:488–499. doi: 10.1016/S2213-8587(13)70176-1. [DOI] [PubMed] [Google Scholar]
- 18.Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, Hamnvik OP, Koniaris A. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301:E567–E584. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 20.Howard JM, Beddy P, Ennis D, Keogan M, Pidgeon GP, Reynolds JV. Associations between leptin and adiponectin receptor upregulation, visceral obesity and tumour stage in oesophageal and junctional adenocarcinoma. Br J Surg. 2010;97:1020–1027. doi: 10.1002/bjs.7072. [DOI] [PubMed] [Google Scholar]
- 21.Walsh KM, Ohgaki H, Wrensch MR. Epidemiology. Handb Clin Neurol. 2016;134:3–18. doi: 10.1016/B978-0-12-802997-8.00001-3. [DOI] [PubMed] [Google Scholar]
- 22.McNeill KA. Epidemiology of brain tumors. Neurol Clin. 2016;34:981–998. doi: 10.1016/j.ncl.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Sun T, Warrington NM, Rubin JB. Why does Jack, and not Jill, break his crown? Sex disparity in brain tumors. Biol Sex Differ. 2012;3:3. doi: 10.1186/2042-6410-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mindermann T, Wilson CB. Age-related and gender-related occurrence of pituitary adenomas. Clin Endocrinol. 1994;41:359–364. doi: 10.1111/j.1365-2265.1994.tb02557.x. [DOI] [PubMed] [Google Scholar]
- 25.Clement K, Garner C, Hager J, Philippi A, LeDuc C, Carey A, Harris TJR, Jury C, Cardon LR, Basdevant A, Demenais F, Guy-Grand B, North M, Froguel P. Indication for linkage of the human OB gene region with extreme obesity. Diabetes. 1996;45:687–690. doi: 10.2337/diab.45.5.687. [DOI] [PubMed] [Google Scholar]
- 26.Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001;60:329–339. doi: 10.1079/PNS200194. [DOI] [PubMed] [Google Scholar]
- 27.Havel PJ, Kasim-Karakas S, Dubuc GR, Mueller W, Phinney SD. Gender differences in plasma leptin concentrations. Nat Med. 1996;2:949–950. doi: 10.1038/nm0996-949b. [DOI] [PubMed] [Google Scholar]
- 28.Heptulla R, Smitten A, Teague B, Tamborlane WV, Ma Y-Z, Caprio S. Temporal patterns of circulating leptin levels in lean and obese adolescents: relationships to insulin, growth hormone, and free fatty acids rhythmicity. J Clin Endocrinol Metab. 2001;86:90–96. doi: 10.1210/jcem.86.1.7136. [DOI] [PubMed] [Google Scholar]
- 29.Trayhurn P, Thomas ME, Duncan JS, Rayner DV. Effects of fasting and refeeding on ob gene expression in white adipose tissue of lean and obese (ob/ob) mice. FEBS Lett. 1995;368:488–490. doi: 10.1016/0014-5793(95)00719-P. [DOI] [PubMed] [Google Scholar]
- 30.Hardie LJ, Rayner DV, Holmes S, Trayhurn P. Circulating leptin levels are modulated by fasting, cold exposure and insulin administration in lean but not Zucker (fa/fa) rats as measured by ELISA. Biochem Biophys Res Commun. 1996;223:660–665. doi: 10.1006/bbrc.1996.0951. [DOI] [PubMed] [Google Scholar]
- 31.Havel PJ. Peripheral signals conveying metabolic information to the brain: short-term and long-term regulation of food intake and energy homeostasis. Exp Biol Med. 2001;226:963–977. doi: 10.1177/153537020122601102. [DOI] [PubMed] [Google Scholar]
- 32.Castracane VD, Kraemer RR, Franken MA, Kraemer GR, Gimpel T. Serum leptin concentration in women: effect of age, obesity, and estrogen administration. Fertil Steril. 1998;70:472–477. doi: 10.1016/S0015-0282(98)00187-3. [DOI] [PubMed] [Google Scholar]
- 33.Blum WF, Englaro P, Hanitsch S, Juul A, Hertel NT, Muller J, Skakkebaek NE, Heiman ML, Birkett M, Attanasio AM, Kiess W, Rascher W. Plasma leptin levels in healthy children and adolescents: dependence on body mass index, body fat mass, gender, pubertal stage, and testosterone. J Clin Endocrinol Metab. 1997;82:2904–2910. doi: 10.1210/jcem.82.9.4251. [DOI] [PubMed] [Google Scholar]
- 34.Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet DJ, 3rd, Flier JS, Lowell BB, Fraker DL, Alexander HR. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171–175. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1 beta mediates leptin induction during inflammation. Am J Physiol. 1998;274:R204–R208. doi: 10.1152/ajpregu.1998.274.1.R204. [DOI] [PubMed] [Google Scholar]
- 36.Gualillo O, Eiras S, Lago F, Dieguez C, Casanueva FF. Elevated serum leptin concentrations induced by experimental acute inflammation. Life Sci. 2000;67:2433–2441. doi: 10.1016/S0024-3205(00)00827-4. [DOI] [PubMed] [Google Scholar]
- 37.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez RR, Leavis P. Leptin upregulates beta3-integrin expression and interleukin-1beta, upregulates leptin and leptin receptor expression in human endometrial epithelial cell cultures. Endocrine. 2001;16:21–28. doi: 10.1385/ENDO:16:1:21. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez RR, Devoto L, Campana A, Bischof P. Effects of leptin, interleukin-1alpha, interleukin-6, and transforming growth factor-beta on markers of trophoblast invasive phenotype: integrins and metalloproteinases. Endocrine. 2001;15:157–164. doi: 10.1385/ENDO:15:2:157. [DOI] [PubMed] [Google Scholar]
- 40.Ria R, Vacca A, Ribatti D, Di Raimondo F, Merchionne F, Dammacco F. Alpha(v)beta(3) integrin engagement enhances cell invasiveness in human multiple myeloma. Haematologica. 2002;87:836–845. [PubMed] [Google Scholar]
- 41.Gulluoglu S, Sahin M, Tuysuz EC, Yaltirik CK, Kuskucu A, Ozkan F, Sahin F, Ture U, Bayrak OF (2017) Leukemia inhibitory factor promotes aggressiveness of chordoma. Oncol Res 25:1177–1188 [DOI] [PMC free article] [PubMed]
- 42.Voronov E, Apte RN (2017) Targeting the tumor microenvironment by intervention in Interleukin-1 biology. Curr Pharm Des 23:4893–4905 [DOI] [PubMed]
- 43.Plate KH, Breier G, Millauer B, Ullrich A, Risau W. Up-regulation of vascular endothelial growth factor and its cognate receptors in a rat glioma model of tumor angiogenesis. Cancer Res. 1993;53:5822–5827. [PubMed] [Google Scholar]
- 44.Salm F, Dimitrova V, von Bueren AO, Ćwiek P, Rehrauer H, Djonov V, Anderle P, Arcaro A (2015) The phosphoinositide 3-kinase p110α isoform regulates leukemia inhibitory factor receptor expression via c-Myc and miR-125b to promote cell proliferation in medulloblastoma. PLoS One 10:e0123958 [DOI] [PMC free article] [PubMed]
- 45.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Arthur Campfield L, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 46.Cleary MP, Juneja SC, Phillips FC, Hu X, Grande JP, Maihle NJ. Leptin receptor-deficient MMTV-TGF-alpha/Lepr(db)Lepr(db) female mice do not develop oncogene-induced mammary tumors. Exp Biol Med. 2004;229:182–193. doi: 10.1177/153537020422900207. [DOI] [PubMed] [Google Scholar]
- 47.Lipsey CC, Harbuzariu A, Daley-Brown D, Gonzalez-Perez RR. Oncogenic role of leptin and notch interleukin-1 leptin crosstalk outcome in cancer. World J Methodol. 2016;6:43–55. doi: 10.5662/wjm.v6.i1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mullen M, Gonzalez-Perez RR (2016) Leptin-induced JAK/STAT signaling and cancer growth. Vaccines 4(3):26 [DOI] [PMC free article] [PubMed]
- 49.Wauman J, Zabeau L, Tavernier J. The leptin receptor complex: heavier than expected? Front Endocrinol. 2017;8:30. doi: 10.3389/fendo.2017.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee G-H, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 51.Koerner A, Kratzsch J, Kiess W. Adipocytokines: leptin—the classical, resistin—the controversical, adiponectin—the promising, and more to come. Best Pract Res Clin Endocrinol Metab. 2005;19:525–546. doi: 10.1016/j.beem.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, Friedman JM. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci U S A. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21:263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- 54.Elmquist JK. Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S78–S82. doi: 10.1038/sj.ijo.0801918. [DOI] [PubMed] [Google Scholar]
- 55.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 56.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 57.Chan JL, Moschos SJ, Bullen J, Heist K, Li X, Kim Y-B, Kahn BB, Mantzoros CS. Recombinant methionyl human leptin administration activates signal transducer and activator of transcription 3 signaling in peripheral blood mononuclear cells in vivo and regulates soluble tumor necrosis factor-alpha receptor levels in humans with relative leptin deficiency. J Clin Endocrinol Metab. 2005;90:1625–1631. doi: 10.1210/jc.2004-1823. [DOI] [PubMed] [Google Scholar]
- 58.Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Nat Acad Sci U S A. 1996;93:6231–6235. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carpenter LR, Farruggella TJ, Symes A, Karow ML, Yancopoulos GD, Stahl N. Enhancing leptin response by preventing SH2-containing phosphatase 2 interaction with Ob receptor. Proc Natl Acad Sci U S A. 1998;95:6061–6066. doi: 10.1073/pnas.95.11.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.David M, Zhou G, Pine R, Dixon JE, Larner AC. The SH2 domain-containing tyrosine phosphatase PTP1D is required for interferon alpha/beta-induced gene expression. J Biol Chem. 1996;271:15862–15865. doi: 10.1074/jbc.271.27.15862. [DOI] [PubMed] [Google Scholar]
- 61.You M, Yu D-H, Feng G-S. Shp-2 tyrosine phosphatase functions as a negative regulator of the interferon-stimulated Jak/STAT pathway. Mol Cell Biol. 1999;19:2416–2424. doi: 10.1128/MCB.19.3.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Haihua G, Neel BG, Myers MG, Jr, Flier JS. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276:4747–4755. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 63.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 65.Subramaniapillai M, McIntyre RS. A review of the neurobiology of obesity and the available pharmacotherapies. CNS Spectr. 2017;22:29–38. doi: 10.1017/S1092852917000839. [DOI] [PubMed] [Google Scholar]
- 66.Ahima RS, Bjorbaek C, Osei S, Flier JS. Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology. 1999;140:2755–2762. doi: 10.1210/endo.140.6.6774. [DOI] [PubMed] [Google Scholar]
- 67.Udagawa J, Nimura M, Otani H. Leptin affects oligodendroglial development in the mouse embryonic cerebral cortex. Neuro Endocrinol Lett. 2006;27:177–182. [PubMed] [Google Scholar]
- 68.Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci Off J Soc Neurosci. 2001;21:Rc186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roth C, Wilken B, Hanefeld F, Schroter W, Leonhardt U. Hyperphagia in children with craniopharyngioma is associated with hyperleptinaemia and a failure in the downregulation of appetite. Eur J Endocrinol / Eur Fed Endocr Soc. 1998;138:89–91. doi: 10.1530/eje.0.1380089. [DOI] [PubMed] [Google Scholar]
- 70.Ferretti E, Jaffrain Rea ML, Asteria C, Di Stefano D, Esposito V, Ferrante L, Daniele P, Tiberti C, Gallucci M, Bosman C, Alesse E, Gulino A, Beck-Peccoz P, Tamburrano G. Two familial giant pituitary adenomas associated with overweight: clinical, morphological and genetic features. Eur J Endocrinol / Eur Fed Endocr Soc. 2001;144:227–235. doi: 10.1530/eje.0.1440227. [DOI] [PubMed] [Google Scholar]
- 71.Rosenbaum M, Leibel RL. Role of leptin in energy homeostasis in humans. J Endocrinol. 2014;223:T83–T96. doi: 10.1530/JOE-14-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ilyin SE, Gayle D, Gonzalez-Gomez I, Miele ME, Plata-Salaman CR. Brain tumor development in rats is associated with changes in central nervous system cytokine and neuropeptide systems. Brain Res Bull. 1999;48:363–373. doi: 10.1016/S0361-9230(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 73.Musiol K, Sobol G, Mizia-Malarz A, Wos H. Leptin concentration and nutritional status in the course of treatment in children with brain tumours—preliminary report. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2014;30:131–136. doi: 10.1007/s00381-013-2183-8. [DOI] [PubMed] [Google Scholar]
- 74.Doknic M, Pekic S, Zarkovic M, Medic-Stojanoska M, Dieguez C, Casanueva F, Popovic V. Dopaminergic tone and obesity: an insight from prolactinomas treated with bromocriptine. Eur J Endocrinol / Eur Fed Endocr Soc. 2002;147:77–84. doi: 10.1530/eje.0.1470077. [DOI] [PubMed] [Google Scholar]
- 75.Srinivasan S, Ogle GD, Garnett SP, Briody JN, Lee JW, Cowell CT. Features of the metabolic syndrome after childhood craniopharyngioma. J Clin Endocrinol Metab. 2004;89:81–86. doi: 10.1210/jc.2003-030442. [DOI] [PubMed] [Google Scholar]
- 76.Malik IA, English PJ, Ghatei MA, Bloom SR, MacFarlane IA, Wilding JP. The relationship of ghrelin to biochemical and anthropometric markers of adult growth hormone deficiency. Clin Endocrinol. 2004;60:137–141. doi: 10.1111/j.1365-2265.2004.01929.x. [DOI] [PubMed] [Google Scholar]
- 77.Kousta E, Chrisoulidou A, Lawrence NJ, al-Shoumer KA, Parker KH, McCarthy MI, Johnston DG. The circadian rhythm of leptin is preserved in growth hormone deficient hypopituitary adults. Clin Endocrinol. 1998;48:685–690. doi: 10.1046/j.1365-2265.1998.00498.x. [DOI] [PubMed] [Google Scholar]
- 78.Guran T, Turan S, Bereket A, Akcay T, Unluguzel G, Bas F, Gunoz H, Saka N, Bundak R, Darendeliler F, Isguven P, Yildiz M, Adal E, Sarikaya S, Baygin LA, Memioglu N, Onal H, Ercan O, Haklar G. The role of leptin, soluble leptin receptor, resistin, and insulin secretory dynamics in the pathogenesis of hypothalamic obesity in children. Eur J Pediatr. 2009;168:1043–1048. doi: 10.1007/s00431-008-0876-x. [DOI] [PubMed] [Google Scholar]
- 79.Rasmussen MH. Obesity, growth hormone and weight loss. Mol Cell Endocrinol. 2010;316:147–153. doi: 10.1016/j.mce.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 80.Holmer H, Ekman B, Bjork J, Nordstom CH, Popovic V, Siversson A, Erfurth EM. Hypothalamic involvement predicts cardiovascular risk in adults with childhood onset craniopharyngioma on long-term GH therapy. Eur J Endocrinol / Eur Fed Endocr Soc. 2009;161:671–679. doi: 10.1530/EJE-09-0449. [DOI] [PubMed] [Google Scholar]
- 81.Holmer H, Popovic V, Ekman B, Follin C, Siversson AB, Erfurth EM. Hypothalamic involvement and insufficient sex steroid supplementation are associated with low bone mineral density in women with childhood onset craniopharyngioma. Eur J Endocrinol / Eur Fed Endocr Soc. 2011;165:25–31. doi: 10.1530/EJE-11-0229. [DOI] [PubMed] [Google Scholar]
- 82.Iwayama H, Kamijo T, Ueda N. Hyperinsulinemia may promote growth without GH in children after resection of suprasellar brain tumors. Endocrine. 2011;40:130–133. doi: 10.1007/s12020-011-9493-y. [DOI] [PubMed] [Google Scholar]
- 83.Siviero-Miachon AA, Spinola-Castro AM, Guerra-Junior G. Adiposity in childhood cancer survivors: insights into obesity physiopathology. Arq Bras Endocrinol Metabol. 2009;53:190–200. doi: 10.1590/S0004-27302009000200011. [DOI] [PubMed] [Google Scholar]
- 84.Trivin C, Busiah K, Mahlaoui N, Recasens C, Souberbielle JC, Zerah M, Sainte-Rose C, Brauner R. Childhood craniopharyngioma: greater hypothalamic involvement before surgery is associated with higher homeostasis model insulin resistance index. BMC Pediatr. 2009;9:24. doi: 10.1186/1471-2431-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimon I, Yan X, Magoffin DA, Friedman TC, Melmed S. Intact leptin receptor is selectively expressed in human fetal pituitary and pituitary adenomas and signals human fetal pituitary growth hormone secretion. J Clin Endocrinol Metab. 1998;83:4059–4064. doi: 10.1210/jcem.83.11.5273. [DOI] [PubMed] [Google Scholar]
- 86.Jin L, Burguera BG, Couce ME, Scheithauer BW, Lamsan J, Eberhardt NL, Kulig E, Lloyd RV. Leptin and leptin receptor expression in normal and neoplastic human pituitary: evidence of a regulatory role for leptin on pituitary cell proliferation. J Clin Endocrinol Metab. 1999;84:2903–2911. doi: 10.1210/jcem.84.8.5908. [DOI] [PubMed] [Google Scholar]
- 87.Vidal S, Cohen SM, Horvath E, Kovacs K, Scheithauer BW, Burguera BG, Lloyd RV. Subcellular localization of leptin in non-tumorous and adenomatous human pituitaries: an immuno-ultrastructural study. J Histochem Cytochem Off J Histochem Soc. 2000;48:1147–1152. doi: 10.1177/002215540004800811. [DOI] [PubMed] [Google Scholar]
- 88.Knerr I, Schuster S, Nomikos P, Buchfelder M, Dotsch J, Schoof E, Fahlbusch R, Rascher W. Gene expression of adrenomedullin, leptin, their receptors and neuropeptide Y in hormone-secreting and non-functioning pituitary adenomas, meningiomas and malignant intracranial tumours in humans. Neuropathol Appl Neurobiol. 2001;27:215–222. doi: 10.1046/j.0305-1846.2001.00324.x. [DOI] [PubMed] [Google Scholar]
- 89.Korbonits M, Chitnis MM, Gueorguiev M, Norman D, Rosenfelder N, Suliman M, Jones TH, Noonan K, Fabbri A, Besser GM, Burrin JM, Grossman AB. The release of leptin and its effect on hormone release from human pituitary adenomas. Clin Endocrinol. 2001;54:781–789. doi: 10.1046/j.1365-2265.2001.01279.x. [DOI] [PubMed] [Google Scholar]
- 90.Giusti M, Bocca L, Florio T, Corsaro A, Spaziante R, Schettini G, Minuto F. In vitro effect of human recombinant leptin and expression of leptin receptors on growth hormone-secreting human pituitary adenomas. Clin Endocrinol. 2002;57:449–455. doi: 10.1046/j.1365-2265.2002.01612.x. [DOI] [PubMed] [Google Scholar]
- 91.Isono M, Inoue R, Kamida T, Kobayashi H, Matsuyama J. Significance of leptin expression in invasive potential of pituitary adenomas. Clin Neurol Neurosurg. 2003;105:111–116. doi: 10.1016/S0303-8467(02)00129-4. [DOI] [PubMed] [Google Scholar]
- 92.Ferla R, Bonomi M, Otvos L, Jr, Surmacz E. Glioblastoma-derived leptin induces tube formation and growth of endothelial cells: comparison with VEGF effects. BMC Cancer. 2011;11:303. doi: 10.1186/1471-2407-11-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Han G, Wang L, Zhao W, Yue Z, Zhao R, Li Y, Zhou X, Hu X, Liu J. High expression of leptin receptor leads to temozolomide resistance with exhibiting stem/progenitor cell features in gliobalastoma. Cell Cycle. 2013;12:3833–3840. doi: 10.4161/cc.26809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han G, Zhao W, Wang L, Yue Z, Zhao R, Li Y, Zhou X, Hu X, Liu J. Leptin enhances the invasive ability of glioma stem-like cells depending on leptin receptor expression. Brain Res. 2014;1543:1–8. doi: 10.1016/j.brainres.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 95.Hands JR, Abel P, Ashton K, Dawson T, Davis C, Lea RW, McIntosh AJ, Baker MJ. Investigating the rapid diagnosis of gliomas from serum samples using infrared spectroscopy and cytokine and angiogenesis factors. Anal Bioanal Chem. 2013;405:7347–7355. doi: 10.1007/s00216-013-7163-z. [DOI] [PubMed] [Google Scholar]