Abstract
Nuclear localization of insulin-like growth factor receptor type 1 (IGF-1R) has been described as adverse prognostic factor in some cancers. We studied the expression and localization of IGF-1R in paediatric patients with gliomas, as well as its association with World Health Organization (WHO) grading and survival. We conducted a single cohort, prospective study of paediatric patients with gliomas. Samples were taken at the time of the initial surgery; IGF-1R expression and localization were characterized by immunohistochemistry (IHC), subcellular fractionation and western blotting. Tumours (47/53) showed positive staining for IGF-1R by IHC. IGF-1R nuclear labelling was observed in 10/47 cases. IGF-1R staining was mostly non-nuclear in low-grade tumours, while IGF-1R nuclear labelling was predominant in high-grade gliomas (p = 0.0001). Survival was significantly longer in patients with gliomas having non-nuclear IGF-1R localization than in patients with nuclear IGF-1R tumours (p = 0.016). In gliomas, IGF-1R nuclear localization was significantly associated with both high-grade tumours and increased risk of death. Based on a prospective design, we provide evidence of a potential usefulness of intracellular localization of IGF-1R as prognostic factor in paediatric patients with gliomas.
Electronic supplementary material
The online version of this article (10.1007/s12672-018-0328-7) contains supplementary material, which is available to authorized users.
Keywords: Pediatric Gliomas, Insulin-like Growth Factor Type-2 Receptor, High-grade Gliomas, KAPA SYBR FAST qPCR Master, Fractionation Buffer
Introduction
Gliomas are the most common solid tumours in children, accounting for approximately half of all paediatric central nervous system (CNS) tumours [1]. The World Health Organization (WHO) classification of CNS tumours organizes gliomas according to their histological characteristics and divides them into four grades of malignancy (I–IV) [2]. Paediatric gliomas are very different from their adult counterparts, most of them are low grade (I and II) and do not develop into high-grade (III and IV) tumours [3]. In 2016, the WHO classification of CNS tumours was updated in order to include molecular findings as diagnostic biomarkers and prognostic factors [4]. For instance, in adult gliomas, the presence of IDH mutations defines tumour classification and patient management [5]. Conversely, in paediatric gliomas, molecular biomarkers with prognostic value are rarely found, and the histologic and grading WHO classification remains the “gold standard” for the diagnosis and patient management [4].
In 1997, Glick et al. provided the first evidence of the presence of insulin and insulin-like growth factor (IGF) receptors in human CNS tumours [6]. The IGF system of ligands and receptors plays important physiological roles to ensure cellular survival and proliferation of many tissues. Several components of the IGF system are known to be present in tumours from both adults and paediatric patients. In particular, the presence of type 1 receptor for IGF (IGF-1R) has been described as a prerequisite for the acquisition of a neoplastic phenotype [7–10].
IGF-1R is a membrane receptor, belonging to the tyrosine kinase family of receptors, and signalling through tyrosine kinase that targets the phosphorylation of molecules such as IRS proteins, phosphatidylinositol 3-kinase and others. However, its nuclear localization has also been described in cells of melanoma [11], renal cancer [12] and sarcomas [13]. Intact IGF-1R was detected in the nuclei of human malignant and non-malignant cells, showing an association between higher levels of nuclear IGF-1R with poor prognosis in patients with clear cell renal cancer [12]. Nuclear IGF-1R expression has been shown to be an adverse prognostic factor in both embryonal rhabdomyosarcoma [13] and synovial sarcomas [14]. Although the function of the IGF-1R in the nucleus is still unknown, these results suggest that nuclear IGF-1R localization may contribute to the development of an aggressive phenotype.
In spite of the growing evidence showing the presence of IGF-1R in the nucleus of different human cancer cells [15–19], no reports have focused on paediatric gliomas. The present study was designed to explore the IGF-IR expression and intracellular localization in paediatric gliomas and to assess whether IGF-1R intracellular localization is associated with WHO histological grading and patient survival.
Materials and Methods
Study Design and Patients
We performed an observational, single cohort, prospective study in the tertiary paediatric hospital “Dr. Ricardo Gutiérrez” in Buenos Aires (Argentina). All patients under 19 years of age undergoing surgery for glioma between June 2012 and December 2016 were included. Patients with previous radiotherapy were excluded. Only histological samples obtained at the first surgery were studied. The diagnosis of glioma was ascertained following the morphological criteria according to the WHO classification system [2]. The available clinical data for each patient included age, gender, date of surgery, extent of surgical resection, adjuvant treatment and follow-up.
The protocol was approved by the Institutional Review Board of Hospital “Dr. Ricardo Gutiérrez” and conducted according to Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association Declaration of Helsinki. Patients and/or parents or legal guardians gave informed consent or assent, according to national and state regulations.
Exposure and Outcome Measures
Tumours were classified following the “4th edition of WHO classification of CNS tumours” in four grades of malignancy and grouped as low grade (gliomas classified as grades I and II) and high grade (gliomas classified as grades III and IV) for their analysis [2]. Although a new WHO classification of tumours of CNS has been recently published based on additional molecular parameters [4], gliomas are still classified into low (I and II) and high (III and IV) grades. All gliomas included in this study remained classified as previously, in low and high grades, regardless of the molecular profiling.
IGF-1R immunohistochemical labelling was qualitatively scored as negative when no staining was observed or positive when staining was observed in at least one area of the specimen. Positive gliomas were further classified as specimens with nuclear labelling when IGF-1R staining was observed in at least 10 positive nuclei per field at a × 40 magnification. All patients were treated by the same team of neurosurgeons and paediatric oncologists, according to a standardized institutional treatment guidance, adapted from international consensus [20, 21]. Follow-up started at surgery and ended in January 2017. At the end of the study, patients were classified as dead or alive, free of disease or with tumour persistence. Progression of the disease was defined as any changes during follow-up that required adjuvant treatment. Recurrence was defined as the reappearance of disease in any localization, following complete tumour eradication. Overall survival was calculated from the time of surgery to death or to the end of study. Patients were classified as dropouts at the end of the study when the time from the last visit was longer than 12 months.
The primary outcome was the proportion of patients harbouring gliomas with IGF-1R nuclear staining in high-grade vs. low-grade gliomas and the secondary outcome the overall survival in patients with tumours showing non-nuclear vs. nuclear IGF-1R staining with at least 6 months of follow-up.
Tumour Samples
Tumour samples were fixed using 4% paraformaldehyde buffer for histology and immunohistochemistry or snap frozen and kept at − 80 °C for protein and RNA analysis when available.
IGF-1R IHC
Tumour samples were fixed in 10% neutral formaldehyde and paraffin embedded; 5-μm-thick sections were stained with hematoxylin and eosin. Immunostaining was performed on paraffin sections using an indirect biotin-avidin method on tumour slices mounted on charged glass slides as previously described [22]. Briefly, sections were dewaxed in xylene and rehydrated. Antigen retrieval was performed using 10 mM EDTA buffer (pH 8.0) in a microwave oven for 10 min, and endogenous peroxidase activity was blocked using 3% H2O2 for 30 min. Non-specific binding was blocked with normal horse serum (ABC Vectastain) for other 30 min. Sections were incubated with anti IGF-1Rβ-subunit antibody (Cat no. 3027, CST, Boston MA, USA, 1:750 dilution) at − 4 °C overnight. Antibody reactivity was detected using the Vectastain Universal ABC detection system (Vector Laboratories, CA 94010, USA). Sections of human liver tissue served as negative control for immunostaining. Samples of human kidney tissue were used as positive controls for both nuclear and non-nuclear labelling and for control for non-specific binding of DAB and secondary antibody (absence of primary antibody). Specificity validation was carried out using a specific blocking peptide (IGF-I Receptor β Blocking Peptide,CST no.1525, supplementary methods). The cellular distribution of IGF-1R detailed under “exposure and outcome measures” was assessed using an optic microscope.
Subcellular Fractionation and Western Blotting
To confirm IGF-1R intracellular localization, fresh available tumour samples were processed for subcellular fractionation and western blotting, following standard protocols. Tumour homogenates were prepared using fractionation buffer (250 mM Sucrose, 20 mM HEPES, 10 mM KCL, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA) containing protease inhibitors. Lysates were passed through a 25-Ga needle, incubated on ice for 20 min and centrifuged at low speed. Pellets were washed with fractionation buffer and suspended in nuclear fractionation buffer (also containing 10% glycerol and 0.1% sodium dodecyl sulfate) followed by sonication to obtain nuclear fraction. Supernatants were centrifuged again and supernatant fraction were kept as cytosolic and membrane (non-nuclear) fraction. Available fresh or frozen tumour samples were also processed for whole lysate preparation [23]. Protein concentration was determined using Bradford reagent. Protein extracts from both subcellular fractionation and total lysates were resolved by SDS-PAGE and transferred to polyvinylidene fluoride membranes. Blots were blocked and probed with antibodies against IGF-1R β-subunit (Cat no. 3027, CST, Boston, MA, USA), cyclin D1 (SC no. 718, Santa Cruz Inc., Dallas, TX, USA), β-actin (CST no. 4970, CST, Boston, MA, USA), as loading control for non-nuclear fraction and whole lysates, and Lamin B (SC no. 6217, Santa Cruz Inc., Dallas, TX, USA) as a marker for nuclear fraction [24].
RT-PCR
RNA from gliomas was obtained using Direct-Zol RNA Kit (Zymo Research, Irvine, CA) following manufacturer’s protocol. RT-PCR was performed using 500 ng of RNA of each sample with random hexamers to prime the reverse transcription catalysed by Super Script II (Invitrogen, Carlsbad, CA). Resulting cDNA was diluted by 1:30, and 3 μl from each dilution was subjected to RT-PCR in triplicates using Kapa Sybr Fast qPCR master mix (Kapa Biosystems, Boston, MA) in Step One Plus Real-Time PCR Systems (Life Technologies, Carlsbad, CA). Human IGF-1R was studied using the following primers (Fw: GCAACCACGAGGCTGAGAAG Rv: GTCACTGGCCCAGGAATGTC) and normalized to TATA binding protein (TBP) as internal control. mRNA values were calculated using relative quantitation method [25] and are presented as fold change compared to low-grade gliomas or non-nuclear IGF-1R localization.
Statistical Analysis
For categorical variables, cross-tabulations were analysed using the Fisher’s exact test. Kaplan–Meier survival plots were constructed to determine whether nuclear IGF-1R localization influenced patient’s survival. Log rank (Mantel–Cox) test was applied to compare survival functions. Cox regression analysis was used to adjust for confounders and validated by the maximum likelihood test. All p values were two sided, and probabilities less than 0.05 were considered significant. SPSS 18.0 software (SPSS, Chicago, IL, USA) was used for all statistical analysis.
Results
The cohort included 53 patients (24 females), with a median age of 8.5 ± 5.1 years (range 0.87–18.32 years). Glioma localization was supratentorial in 25 patients and infratentorial in 28, 6 of them were brainstem tumours. One patient had tuberous sclerosis. Low-grade gliomas were the most frequent tumours (43 out of 53) and 66% of the total cohort were pilocytic astrocytomas (Table 1).
Table 1.
Demographic data and IGF-1R IHC of paediatric patients harbouring gliomas
| ID | WHO grade | Age (years) | Sex | Time to death/last follow-up (months) | Pathology classification | Loc | SR | Clinical status | IHQ IGF-1R | Treatment new surgery/RT/CT/ | Mutations |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | I | 1.1 | M | 51.95 | Ganglioglioma | S/H | C | A | C | No | ND |
| 2 | I | 18.2 | M | 51.68 | Angiocentric glioma | S/H | C | A | Neg | No | ND |
| 3 | I | 13.6 | M | 49.15 | Pilocytic astrocytoma | I/C | P | DO | C | No | ND |
| 4 | I | 4.8 | F | 48.46 | Pilocytic astrocytoma | I/C | C | A | Neg | No | ND |
| 5 | I | 7.4 | M | 43.63 | Giant cell astrocytoma | S/M | C | A | C | No | ND |
| 6 | I | 7.1 | M | 40.57 | Pilocytic astrocytoma | I/C | C | A | C | No | ND |
| 7 | I | 3.7 | F | 39.29 | Pilocytic astrocytoma | I/C | C | A | C | No | ND |
| 8 | I | 10.6 | M | 36.72 | Pilocytic astrocytoma | S/OP | P | DO | C | No | ND |
| 9 | I | 13.3 | F | 29.98 | Ganglioglioma | I/C | P | A;T | C | No | ND |
| 10 | I | 7.8 | M | 28.92 | Pilocytic astrocytoma | I/C | C | A;T | Neg | Surgery (R) | BRAF V600E neg./H3K27M neg./ATRX neg. |
| 11 | I | 3.9 | F | 27.36 | Pilocytic astrocytoma | I/C | C | A | C | No | ND |
| 12 | I | 18.3 | M | 26.88 | Pilocytic astrocytoma | I/C | C | A | Neg | No | ND |
| 13 | I | 8.8 | M | 25.48 | Pilocytic astrocytoma | S/OP | P | A;T | C | CT/VB | ND |
| 14 | I | 7.9 | M | 24.56 | Ganglioglioma | S/H | C | A | C | Surgery (R)/CT (Carb, V) | ND |
| 15 | I | 13.0 | F | 23.64 | Pilocytic astrocytoma | I/B | C | A | C | No | ND |
| 16 | I | 16.0 | M | 23.38 | Pilocytic astrocytoma | S/T | P | A;T | C | Surgery (P) | ND |
| 17 | I | 17.8 | F | 20.42 | Pilocytic astrocytoma | S/M | P | A;T | C | No | BRAF V600E neg/ATRX neg. |
| 18 | I | 2.5 | F | 18.51 | Pilocytic astrocytoma | I/C | C | A | C | No | BRAF V600E neg/ATRX neg./IDH neg. |
| 19 | I | 10.7 | M | 16.04 | Pilocytic astrocytoma | I/B | P | A | C | No | ND |
| 20 | I | 7.3 | M | 15.55 | Pilocytic astrocytoma | I/C | C | A | C | No | ND |
| 21 | I | 1.0 | F | 13.91 | Pilocytic astrocytoma | I/B | B | A;T | C | No | BRAF V600E neg |
| 22 | I | 11.3 | F | 4.41 | Pilocytic astrocytoma | I/C | C | A | C | No | BRAF V600E neg |
| 23 | I | 9.9 | M | 3.16 | Pilocytic astrocytoma | I/C | P | A | Neg | No | BRAF V600E neg |
| 24 | I | 17.0 | M | 2.24 | Pilocytic astrocytoma | I/C | C | A | C | No | ND |
| 25 | I | 7.4 | F | 1.78 | Pilocytic astrocytoma | I/C | C | A | C | No | BRAF V600E neg |
| 26 | I | 1.0 | F | 54.72 | Pilomyxoid astrocytoma | I/C | P | A;T | Neg | No | ND |
| 27 | I | 1.5 | F | 52.32 | Pilomyxoid astrocytoma | S/OP | B | A;T | N | CT (VB–Carb–V–TMZ–vemurafenib) | BRAF V600E pos |
| 28 | I | 3.6 | F | 48.46 | Pilomyxoid astrocytoma | S/M | P | A;T | N | No | ND |
| 29 | I | 10.9 | M | 47.77 | Pilomyxoid astrocytoma | S/M | B | A;T | C | No | ND |
| 30 | I | 7.9 | F | 45.96 | Pilomyxoid astrocytoma | S/M | C | A | C | No | ND |
| 31 | I | 0.9 | M | 37.91 | Pilomyxoid astrocytoma | I/B | C | A | C | No | ND |
| 32 | I | 3.0 | F | 36.53 | Pilomyxoid astrocytoma | S/OP | P | A;T | C | Surgery (P)/CT (VB) | ND |
| 33 | I | 7.4 | F | 34.65 | Pilomyxoid astrocytoma | I/B | P | A | C | Surgery (P)/CT (VB–V–C) | ND |
| 34 | I | 1.5 | F | 25.71 | Pilomyxoid astrocytoma | S/M | P | A;T | C | CT (VB–V–Carb) | ND |
| 35 | I | 6.4 | M | 24.07 | Pilomyxoid astrocytoma | I/B | P | A;T | C | Surgery (P)/CT (VB) | ND |
| 36 | I | 12.1 | F | 23.74 | Pilomyxoid astrocytoma | I/C | C | A | C | No | ND |
| 37 | I | 3.8 | F | 20.28 | Pilomyxoid astrocytoma | I/C | C | A | C | No | ND |
| 38 | I | 11.0 | M | 14.43 | Pilomyxoid astrocytoma | I/C | C | A | N | No | BRAF V600E neg |
| 39 | I | 11.6 | M | 12.13 | Pilomyxoid astrocytoma | I/C | P | A;T | C | No | ND |
| 40 | I | 2.0 | M | 2.10 | Pilomyxoid astrocytoma | I/C | C | A | C | No | ND |
| 41 | II | 12.5 | M | 46.88 | Pleom. xanthoastrocytoma | S/H | C | A | C | No | ND |
| 42 | II | 11.0 | M | 42.02 | Pleom. xanthoastrocytoma | S/H | C | A | C | No | ND |
| 43 | II | 1.7 | F | 22.72 | Diffuse astrocytoma NOS | S/OP | P | A;T | C | CT (VB) | BRAF V600E neg/ATRX neg. |
| 44 | III | 7.2 | M | 16.56 | Anaplastic astrocytoma NOS | S/T | P | D | N | RT/CT (TMZ) | ND |
| 45 | III | 12.2 | M | 4.68 | Anaplastic astrocytoma NOS | I/C | P | D | N | RT | ND |
| 46 | III | 11.8 | F | 47.40 | Anaplastic astrocytoma NOS | S/H | P | A | C | RT/CT (TMZ) | ND |
| 47 | III | 11.3 | M | 44.88 | Anaplastic astrocytoma NOS | S/M | P | A;T | N | RD/CT (TMZ–procarbazine–V-Lomustina) | 1p19q codeletion neg. |
| 48 | III | 5.0 | M | 37.08 | Anaplastic astrocytoma NOS | S/M | P | A;T | N | RT/CT (TMZ–bevacizumab) | ND |
| 49 | III | 1.5 | F | 19.08 | Anaplastic astrocytoma NOS | S/H | P | A | N | CT (V–Carb–TMZ) | ATRX neg. |
| 50 | III | 11.3 | F | 10.80 | Anaplastic astrocytoma NOS | S/H | P | D | C | RT/CT (TMZ–bevacizumab) | ND |
| 51 | IV | 14.0 | M | 13.20 | Glioblastoma NOS | I/C | C | D | N | RT/CT (TMZ–nimotuzumab) | ND |
| 52 | IV | 14.6 | F | 6.60 | Diffuse midline glioma, H3 K27M-mutant | S/H | P | A;T | N | CT (TMZ/RT) | BRAF V600E neg./H3K27M pos./IDH neg. |
| 53 | IV | 13.6 | M | 6.00 | Glioblastoma NOS | S/H | P | D | C | No | BRAF V600E neg/ATRX pos. |
References: Sex: M male, F female. Pathology classification (according to “2016 World Health Organization Classification of Tumors of the Central Nervous System”): NOS not otherwise specified. Loc localization: S supratentorial, I infratentorial, C cerebellar, H hemispheric, OP optic pathway, M midline, B brainstem, T thalamic, CPA cerebellopontine angle. SR surgical resection: C complete resection, P partial resection, B biopsy. Clinical status: A alive, A;T alive with tumour, D dead, DO dropout. IHQ IGF-1R: C citoplasmatic, N nuclear, Neg negative. Treatment: RT radiotherapy, CT chemotherapy, VB vinblastine, TMZ temozolomide, Carb carboplatin, V vincristine, R recurrence, P progression. Mutations: ND not determined, Neg negative, Pos positive
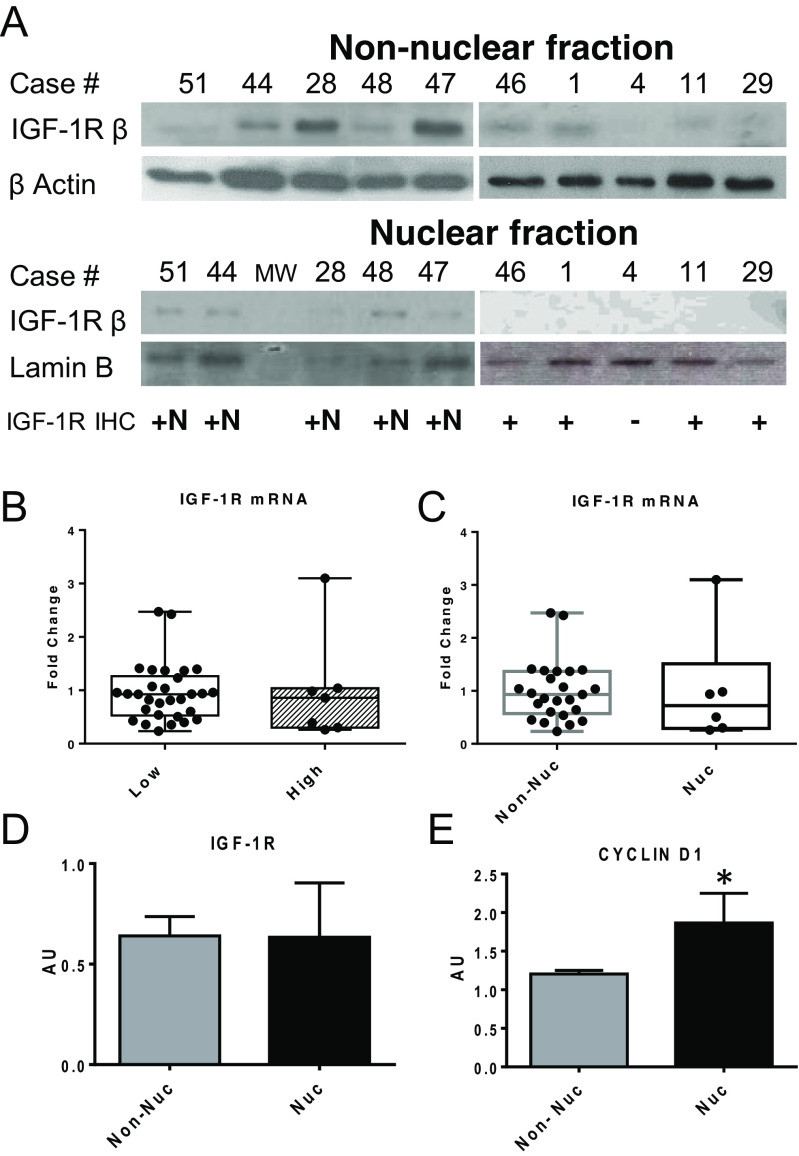
Immunostaining for IGF-1R images are shown in Fig. 1. Most gliomas (47 out of 53 specimens) showed positive staining for IGF-1R by IHC (Table 1). All six cases with negative staining were low-grade gliomas. Positive staining for IGF-1R was exclusively localized in the cytoplasm in 37 cases and present in both cytoplasm and nucleus in 10 tumours (Table 2). We did not find specimens with exclusive nuclear labelling for IGF-1R. The specificity of nuclear and non-nuclear IGF-1R labelling was validated using a specific blocking peptide for IGF-1R antibody (Suppl Fig. 1). The distribution of positive nuclear and non-nuclear staining according to tumour grading is summarized in Table 3. IGF-1R staining was mostly non-nuclear in low-grade tumours, while IGF-1R nuclear staining was predominant in high-grade gliomas (Pearson χ2 21.05; p = 0.0001). Nuclear IGF-1R localization was confirmed by subcellular fractionation followed by western blotting of non-nuclear and nuclear fractions. IGF-1R was detected in both fractions of all available samples that had been scored as nuclear by IHC (Fig. 2a), and it was detected and replicated in non-nuclear fraction in 14/14 scored as non-nuclear by IHC (data not shown). To assess if IGF-1R nuclear localization was related to its level of expression, we performed RT-PCR for IGF-1R. We found no differences in IGF-1R expression levels when comparing low- vs. high-grade gliomas as well as when comparing tumours with non-nuclear vs. nuclear IGF-1R localization (Fig. 2b, c). These results were confirmed by western blot using whole protein extracts (Fig. 2d). Since IGF-1R nuclear localization was previously associated with an increase in cyclin D1 expression, we studied cyclin D1 levels in our samples. We found that cyclin D1 protein levels were increased in gliomas with nuclear compared to non-nuclear IGF-1R staining (p < 0.05, Mann–Whitney test) (Fig. 2e).
Fig. 1.
IGF-1R immunohistochemistry (DAB, counterstaining with H). a Liver tissue, negative control (× 20), bar 50 μm. b Kidney tissue, positive control (× 20), bar 50 μm. c Kidney tissue, positive control, nuclear labelling (× 100), bar 10 μm, nuclear and non-nuclear staining (asterisk). d Grade I glioma, negative (× 100), bar 10 μm. e Grade III glioma, positive non-nuclear (× 100), bar 10 μm, non-nuclear staining (asterisk). f Grade III glioma, positive non-nuclear and nuclear (× 100), bar 10 μm, nuclear and non-nuclear staining (asterisk)
Table 2.
IGF-1R expression and intracellular localization in paediatric gliomas per WHO grading
| WHO grades | Negative | Positive non-nuclear | Positive nuclear | Total |
|---|---|---|---|---|
| I | 6 | 31 | 3 | 40 |
| II | 0 | 3 | 0 | 3 |
| III | 0 | 2 | 5 | 7 |
| IV | 0 | 1 | 2 | 3 |
| Total | 6 | 37 | 10 | 53 |
Table 3.
Distribution of nuclear and non-nuclear staining per tumour grading
| IGF-1R IHC | Non-nuclear | Nuclear | Total |
|---|---|---|---|
| Low-grade gliomas | 40 | 3 | 43 |
| High-grade gliomas | 3 | 7** | 10 |
| Total | 43 | 10 | 53 |
**Pearson χ2 21.05, p = 0.0001
Fig. 2.
Nuclear localization and level of expression of IGF-1R in paediatric gliomas. a IGF-1R presence in the nuclei of tumour homogenates of gliomas scored as nuclear by IHC was verified by subcellular fractionation followed by Western Blot of non-nuclear (46, 1, 4, 11, 29 cases) and nuclear fractions in cases 51,44, 28, 48 and 47. β-Actin and Lamin B were used as markers of non-nuclear and nuclear fractions respectively. b, c IGF-1R expression levels assessed by RT-PCR, expressed as fold change, showed no statistical differences when comparing low-grade (n = 36) vs. high-grade (n = 8) gliomas or non-nuclear (n = 36) vs. nuclear (n = 8) IGF-1R localization. d, e IGF-1R and cyclin D1 protein levels assessed by western blot: IGF-1R protein levels were similar between tumours with non-nuclear vs. nuclear IGF-1R localization, while cyclin D1 protein levels were significantly higher in tumours with nuclear IGF-1R localization. *p < 0.05, Mann–Whitney test. MW molecular weight marker
Mean follow-up was 28.9 ± 15.6 months (range 1.78–54.7 months). There were two dropouts, both corresponding to grade I gliomas, none of them with IGF-1R nuclear labelling by IHC. Ten patients (27%) with low-grade gliomas and positive staining for IGF-1R required adjuvant treatment (chemotherapy and/or radiotherapy) due to tumour progression (n = 8) or recurrence (n = 2).
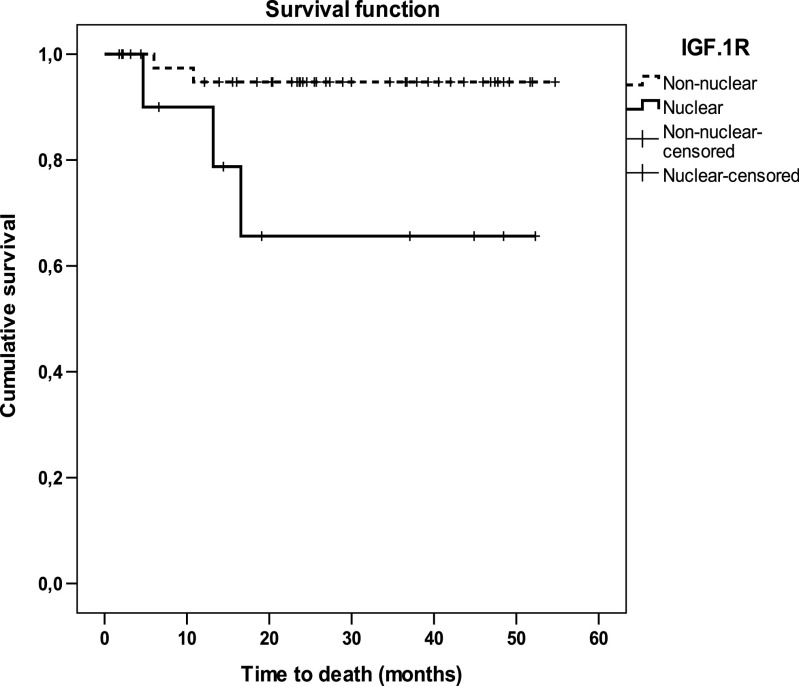
Survival was analysed according to IGF-1R localization of all patients with at least 6 months of follow-up. Mean follow-up was 32 months (range 52.3–6.6) in patients with gliomas having nuclear IGF-1R localization and 31.3 months (range 52.0–12.1) in patients with non-nuclear IGF-1R gliomas. Kaplan–Meier plots showed that survival time was significantly longer in patients with non-nuclear IGF-1R gliomas (mean 45.0 months, 95% CI 42.7–47.4 months) than in patients with nuclear IGF-1R gliomas (30.6 months, 95% CI 18.9–42.3 months, Mantel–Cox Log rank test 5.76, p = 0.016; Fig. 3). Indeed, the risk of death was significantly increased in patients bearing gliomas with nuclear IGF-1R labelling (hazard ratio 6.7, 95% CI 1.11–40.04, p = 0.038).
Fig. 3.
Kaplan–Meier Survival analysis in paediatric patients with gliomas according to IGF-1R cellular localization in their primary tumours. Log rank test 5.75, **p = 0.016. Censored: a “Censored” case is a patient that did not have the event (death) until the last recorded time of follow-up (indicated with a vertical dash)
Discussion
This study describes the expression and subcellular localization of IGF-1R in a large cohort of paediatric patients with gliomas. We found that IGF-1R nuclear localization was associated with high-grade tumours and increased risk of death. Several studies have described the IGFs system in CNS tumours in both adult and paediatric patients, including the IGF-1R [6, 26, 27]. However, as far as we know, this is the first study on IGF-1R intracellular localization in CNS tumour cells and its association with clinical outcome.
The first report of IGF-1R presence in the nucleus was published by Chen and Roy in 1996 in kidney cells of Syrian hamsters [28]. Interestingly, in these cells, the treatment with the oncogenic drug stilbene oestrogen doubled nuclear levels of IGF-1R. Although nuclear translocation of other tyrosine kinase receptors and their functions as transcription factors were subsequently reported [29], it was not until 2010 that nuclear IGF-1R localization was further characterized. Aleksic et al. showed that nuclear IGF-1R is detectable not only in human cancer cells (primary renal, breast and prostate cancer cells) but also in the nuclei of an important number of normal cells [12]. However, no CNS tissues were included in this study. In contrast to other proteins transported to the nucleus, IGF-1R does not contain the canonical nuclear localization sequence (NLS). The mechanism of its nuclear translocation begins to be unveiled [19, 30].
In agreement with reports in gliomas of the adult [27, 31–33], our results demonstrated that most paediatric gliomas showed positive staining for IGF-1R by IHC. Furthermore, we also showed that IGF-1R immunoreactivity was almost exclusively localized in the non-nuclear compartment (89.7%). Only 10 cases showed IGF-1R in both non-nuclear and nuclear localization, while we did not find any tumours with exclusive IGF-1R nuclear staining, a feature that has been observed in other tumours [16]. Nuclear IGF-1R localization in our study was significantly associated with high-grade gliomas. Moreover, the few tumours that did not show any IGF-1R staining corresponded to low-grade tumours.
A report of adult patients with brain tumours including gliomas found a weak correlation between IGF-1R mRNA expression levels and WHO grading [34]. Another study showed that nuclear accumulation of IGF-1R in tumour cells was dependent on the levels of IGF-1R expression and that the ratio nuclear/non-nuclear IGF-1R localization was almost 20 times higher in tumour cell lines compared to their normal counterparts [15]. We sought whether the levels of IGF-1R expression had clinical relevance but found no differences in mRNA levels of IGF-1R when we compared low- vs. high-grade gliomas or tumours with nuclear vs. non-nuclear IGF-1R staining. Our results suggest that the amount of IGF-1R may not impact its cellular localization in paediatric gliomas and that nuclear IGF-1R localization seems to be more relevant than its level of expression. Studying nuclear IGF-1R functions, Warsito et al. showed that into the nucleus, the receptor binds to enhancer regions and works as a transcriptional cofactor, stimulating promoter activity of LEF1 on downstream target genes cyclin D1 and axin2, increasing their protein levels [35]. In agreement, we found an increase in cyclin D1 protein levels in paediatric gliomas with nuclear IGF-1R staining, as compared to those with non-nuclear labelling. Although we did not perform specific experiments to test the hypothesis, these findings may suggest a possible stimulatory role for nuclear IGF-1R in cell cycle progression. Because aberrant activation of the mitogen-activated protein kinase (MAPK) pathway, due to activating mutations of BRAF or fusions genes involving BRAF or RAF, is a common event in the tumourigenesis of paediatric low-grade astrocytomas [36] [37], and mutations of genes involved in cell cycle regulation are involved in high-grade gliomas [5], we cannot rule out the possibility that the genetic status of the tumours, included in our study, might also contribute to an increment in cyclin D1. Nonetheless, our data suggest that nuclear IGF-1R localization may contribute to an aggressive behaviour of gliomas from paediatric patients.
With an average follow-up of more than 2 years, we found that patients bearing gliomas with nuclear IGF-1R labelling had a significantly shorter survival time than those with non-nuclear IGF-1R gliomas. In an ongoing prospective study [38], we have analysed 64 paediatric non-glioma CNS tumours, 33 of them high grade. Interestingly enough, only one of them showed IGF-1R nuclear localization and it was an anaplastic ependymoma, a high-grade tumour that some authors consider gliomas. We would like to underscore that after studying 117 paediatric CNS tumours, we have only found IGF-1R nuclear localization in gliomas. This suggests that nuclear translocation is restricted to this cell line in CNS paediatric tumours. We found IGF-1R nuclear localization in only 3 of 43 low-grade gliomas. Remarkably, one of them is a progressive tumour that has failed to respond to the first three therapeutic lines. In most patients with partial resection of low-grade gliomas, adjuvant therapy is prescribed only when tumours progress and re-resection is not feasible. The availability of prognostic biomarkers to identify which tumours are likely to progress can determine the need of adjuvant therapy. An earlier intervention might help to minimize severe adverse effects, especially in very young children.
In conclusion, we report the intracellular localization of IGF-1R in CNS gliomas from paediatric patients, showing that nuclear IGF-1R localization is associated to high grade in these tumours. Based on a prospective design, we provide evidence of a potential usefulness of intracellular localization of IGF-1R as prognostic factor in paediatric patients with gliomas. Larger studies will be necessary to assess if nuclear IGF-1R expression is an independent prognostic factor in gliomas, especially in low-grade tumours.
Electronic supplementary material
(GIF 269 kb)
(DOCX 13 kb)
Acknowledgements
We are grateful to Dr. Rodolfo Rey and Dr. Saul Malozowski for critical comments and helpful discussions on the manuscript.
Funding Information
This study was supported by Grant #0100214, 2013–2015 Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), awarded to PAP, and Grant 2013–2015 from the Instituto Nacional del Cáncer, Ministerio de Salud de la Nación, República Argentina, awarded to PAP. FC is a recipient of a research fellowship from the Consejo de Investigación en Salud, Gobierno de la Ciudad Autónoma de Buenos Aires and from the Instituto Nacional del Cáncer, Ministerio de Salud de la Nación, República Argentina.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The protocol was approved by the Institutional Review Board of Hospital “Dr. Ricardo Gutiérrez,” and all procedures performed in this study were conducted according to Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association Declaration of Helsinki of 1964 and its later amendments.
Informed Consent
All participants included in this study (patients and/or parents or legal guardians) gave informed consent or assent, according to national and state regulations.
Footnotes
Florencia Clément and Ayelen Martin contributed equally to this work.
References
- 1.Bauchet L, Rigau V, Mathieu-Daudé H, Fabbro-Peray P, Palenzuela G, Figarella-Branger D, Moritz J, Puget S, Bauchet F, Pallusseau L, Duffau H, Coubes P, Trétarre B, Labrousse F, Dhellemmes P. Clinical epidemiology for childhood primary central nervous system tumours. J Neuro-Oncol. 2009;92:87–98. doi: 10.1007/s11060-008-9740-0. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu D, Poon W. Pilocytic astrocytomas do not show most of the genetic changes commonly seen in diffuse astrocytomas. Histopathology. 2000;37:437–444. doi: 10.1046/j.1365-2559.2000.01005.x. [DOI] [PubMed] [Google Scholar]
- 4.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):1–18. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network, Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, Rheinbay E, Miller CR, Vitucci M, Morozova O, Robertson AG, Noushmehr H, Laird PW, Cherniack AD, Akbani R, Huse JT, Ciriello G, Poisson LM, Barnholtz-Sloan JS, Berger MS, Brennan C, Colen RR, Colman H, Flanders AE, Giannini C, Grifford M, Iavarone A, Jain R, Joseph I, Kim J, Kasaian K, Mikkelsen T, Murray BA, O’Neill BP, Pachter L, Parsons DW, Sougnez C, Sulman EP, Vandenberg SR, Van Meir EG, von Deimling A, Zhang H, Crain D, Lau K, Mallery D, Morris S, Paulauskis J, Penny R, Shelton T, Sherman M, Yena P, Black A, Bowen J, Dicostanzo K, Gastier-Foster J, Leraas KM, Lichtenberg TM, Pierson CR, Ramirez NC, Taylor C, Weaver S, Wise L, Zmuda E, Davidsen T, Demchok JA, Eley G, Ferguson ML, Hutter CM, Mills Shaw KR, Ozenberger BA, Sheth M, Sofia HJ, Tarnuzzer R, Wang Z, Yang L, Zenklusen JC, Ayala B, Baboud J, Chudamani S, Jensen MA, Liu J, Pihl T, Raman R, Wan Y, Wu Y, Ally A, Auman JT, Balasundaram M, Balu S, Baylin SB, Beroukhim R, Bootwalla MS, Bowlby R, Bristow CA, Brooks D, Butterfield Y, Carlsen R, Carter S, Chin L, Chu A, Chuah E, Cibulskis K, Clarke A, Coetzee SG, Dhalla N, Fennell T, Fisher S, Gabriel S, Getz G, Gibbs R, Guin R, Hadjipanayis A, Hayes DN, Hinoue T, Hoadley K, Holt RA, Hoyle AP, Jefferys SR, Jones S, Jones CD, Kucherlapati R, Lai PH, Lander E, Lee S, Lichtenstein L, Ma Y, Maglinte DT, Mahadeshwar HS, Marra MA, Mayo M, Meng S, Meyerson ML, Mieczkowski PA, Moore RA, Mose LE, Mungall AJ, Pantazi A, Parfenov M, Park PJ, Parker JS, Perou CM, Protopopov A, Ren X, Roach J, Sabedot TS, Schein J, Schumacher SE, Seidman JG, Seth S, Shen H, Simons JV, Sipahimalani P, Soloway MG, Song X, Sun H, Tabak B, Tam A, Tan D, Tang J, Thiessen N, Triche T Jr, Van Den Berg DJ, Veluvolu U, Waring S, Weisenberger DJ, Wilkerson MD, Wong T, Wu J, Xi L, Xu AW, Yang L, Zack TI, Zhang J, Aksoy BA, Arachchi H, Benz C, Bernard B, Carlin D, Cho J, DiCara D, Frazer S, Fuller GN, Gao J, Gehlenborg N, Haussler D, Heiman DI, Iype L, Jacobsen A, Ju Z, Katzman S, Kim H, Knijnenburg T, Kreisberg RB, Lawrence MS, Lee W, Leinonen K, Lin P, Ling S, Liu W, Liu Y, Liu Y, Lu Y, Mills G, Ng S, Noble MS, Paull E, Rao A, Reynolds S, Saksena G, Sanborn Z, Sander C, Schultz N, Senbabaoglu Y, Shen R, Shmulevich I, Sinha R, Stuart J, Sumer SO, Sun Y, Tasman N, Taylor BS, Voet D, Weinhold N, Weinstein JN, Yang D, Yoshihara K, Zheng S, Zhang W, Zou L, Abel T, Sadeghi S, Cohen ML, Eschbacher J, Hattab EM, Raghunathan A, Schniederjan MJ, Aziz D, Barnett G, Barrett W, Bigner DD, Boice L, Brewer C, Calatozzolo C, Campos B, Carlotti CG Jr, Chan TA, Cuppini L, Curley E, Cuzzubbo S, Devine K, DiMeco F, Duell R, Elder JB, Fehrenbach A, Finocchiaro G, Friedman W, Fulop J, Gardner J, Hermes B, Herold-Mende C, Jungk C, Kendler A, Lehman NL, Lipp E, Liu O, Mandt R, McGraw M, Mclendon R, McPherson C, Neder L, Nguyen P, Noss A, Nunziata R, Ostrom QT, Palmer C, Perin A, Pollo B, Potapov A, Potapova O, Rathmell WK, Rotin D, Scarpace L, Schilero C, Senecal K, Shimmel K, Shurkhay V, Sifri S, Singh R, Sloan AE, Smolenski K, Staugaitis SM, Steele R, Thorne L, Tirapelli DP, Unterberg A, Vallurupalli M, Wang Y, Warnick R, Williams F, Wolinsky Y, Bell S, Rosenberg M, Stewart C, Huang F, Grimsby JL, Radenbaugh AJ, Zhang J. (2015) Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372(26):2481–2498 [DOI] [PMC free article] [PubMed]
- 6.Glick RP, Lichtor T, Unterman TG. Insulin-like growth factors in central nervous system tumors. J Neuro-Oncol. 1997;35(3):315–325. doi: 10.1023/A:1005876819455. [DOI] [PubMed] [Google Scholar]
- 7.Lodhia KA, Tienchaiananda P, Haluska P. Understanding the key to targeting the IGF Axis in cancer: a biomarker assessment. Front Oncol. 2015;5:142. doi: 10.3389/fonc.2015.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weroha SJ, Haluska P. IGF system in cancer. Endocrinology Metabolism Clinics North America. 2012;41(2):335–350. doi: 10.1016/j.ecl.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4(7):505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 10.Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107(6):873–877. doi: 10.1002/ijc.11487. [DOI] [PubMed] [Google Scholar]
- 11.B. Sehat, A. Tofigh, Y. Lin, E. Trocmé, U. Liljedahl, J. Lagergren and O. Larsson. 2010. SUMOylation mediates the nuclear translocation and signaling of the IGF-1 receptor. Science Signaling 3 (108): p. ra10. [DOI] [PubMed]
- 12.Aleksic T, Chitnis MM, Perestenko OV, Gao S, Thomas PH, Turner GD, Protheroe AS, Howarth M, Macaulay VM. Type 1 insulin-like growth factor receptor translocates to the nucleus of human tumor cells. Cancer Res. 2010;70:6412–6419. doi: 10.1158/0008-5472.CAN-10-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Gaal JC, Roeffen MH, Flucke UE, van der Laak JA, van der Heijden G, de Bont ES, Suurmeijer AJ, Versleijen-Jonkers YM, van der Graaf WT. Simultaneous targeting of insulin-like growth factor-1 receptor and anaplastic lymphoma kinase in embryonal and alveolar rhabdomyosarcoma: a rational choice. Eur J Cancer. 2013;49(16):3462–3470. doi: 10.1016/j.ejca.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 14.Palmerini E, Benassi MS, Quattrini I, Pazzaglia L, Donati D, Benini S, Gamberi G, Gambarotti M, Picci P, Ferrari S. Prognostic and predictive role of CXCR4, IGF-1R and Ezrin expression in localized synovial sarcoma: is chemotaxis important to tumor response? Orphanet J Rare Diseases. 2015;10:6. doi: 10.1186/s13023-014-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng H, Lin Y, Badin M, Vasilcanu D, Strömberg T, Jernberg-Wiklund H, Sehat B, Larsson O. Over-accumulation of nuclear IGF-1 receptor in tumor cells requires elevated expression of the receptor and the SUMO-conjugating enzyme Ubc9. Biochem Biophys Res Commun. 2011;404(2):667–671. doi: 10.1016/j.bbrc.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 16.Asmane I, Watkin E, Alberti L, Duc A, Marec-Berard P, Ray-Coquard I, Cassier P, Decouvelaere A-V, Ranchère D, Kurtz J-E, Bergerat J-P, Blay J-Y. Insulin-like growth factor type 1 receptor (IGF-1R) exclusive nuclear staining: a predictive biomarker for IGF-1R monoclonal antibody (Ab) therapy in sarcomas. Eur J Cancer. 2012;48(16):3027–3035. doi: 10.1016/j.ejca.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Bodzin AS, Wei Z, Hurtt R, Gu T, Doria C. Gefitinib resistance in HCC mahlavu cells: upregulation of CD133 expression, activation of IGF-1R signaling pathway, and enhancement of IGF-1R nuclear translocation. J Cell Physiol. 2012;227(7):2947–2952. doi: 10.1002/jcp.23041. [DOI] [PubMed] [Google Scholar]
- 18.Sarfstein R, Pasmanik-Chor M, Yeheskel A, Edry L, Shomron N, Warman N, Wertheimer E, Maor S, Shochat L, Werner H. Insulin-like growth factor-I receptor (IGF-IR) translocates to nucleus and autoregulates IGF-IR gene expression in breast cancer cells. J Biol Chem. 2012;287(4):2766–2776. doi: 10.1074/jbc.M111.281782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Packham S, Warsito D, Lin Y, Sadi S, Karlsson R, Sehat B, Larsson O. Nuclear translocation of IGF-1R via p150(Glued) and an importin-β/RanBP2-dependent pathway in cancer cells. Oncogene. 2015;34(17):2227–2238. doi: 10.1038/onc.2014.165. [DOI] [PubMed] [Google Scholar]
- 20.Gnekow AK. Recommendations of the brain tumor subcommittee for the reporting of trials. SIOP Brain Tumor Subcommittee. International Society of Pediatric Oncology. Med Pediatr Oncol. 1995;24(2):104–108. doi: 10.1002/mpo.2950240209. [DOI] [PubMed] [Google Scholar]
- 21.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2009;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez MC, Martin A, Venara M, Calcagno ML, Sanso G, Quintana S, Chemes HE, Barontini M, Pennisi PA. Overexpression of the insulin-like growth factor 1 receptor (IGF-1R) is associated with malignancy in familial pheochromocytomas and paragangliomas. Clin Endocrinol. 2013;79(5):623–630. doi: 10.1111/cen.12205. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez MC, Venara M, Nowicki S, Chemes HE, Barontini M, Pennisi PA. Igf-I regulates pheochromocytoma cell proliferation and survival in vitro and in vivo. Endocrinology. 2012;153:3724–3734. doi: 10.1210/en.2012-1107. [DOI] [PubMed] [Google Scholar]
- 24.Wakeman JA, Sarkar D, Shields B, Davies ML. BRACHYURY confers cancer stem cell characteristics on colorectal cancer cells. Int J Cancer. 2012;130(2):328–337. doi: 10.1002/ijc.26029. [DOI] [PubMed] [Google Scholar]
- 25.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zumkeller W, Westphal M. The IGF/IGFBP system in CNS malignancy. Mol Pathol. 2001;54:227–229. doi: 10.1136/mp.54.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maris C, D’Haene N, Trépant AL, Le Mercier M, Sauvage S, Allard J, Rorive S, Demetter P, Decaestecker C, Salmon I. IGF-IR: a new prognostic biomarker for human glioblastoma. Br J Cancer. 2015;113(5):729–737. doi: 10.1038/bjc.2015.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chena C, Oberleyb TD, Roy D. Inhibition of stilbene estrogen-induced cell proliferation of renal epithelial cells through the modulation of insulin-like growth factor-I receptor expression. Cancer Lett. 1996;105:51–59. doi: 10.1016/0304-3835(96)04263-2. [DOI] [PubMed] [Google Scholar]
- 29.Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3(9):802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 30.Sarfstein R, Werner H. Minireview: nuclear insulin and insulin-like growth factor-1 receptors: a novel paradigm in signal transduction. Endocrinology. 2013;154(5):1672–1679. doi: 10.1210/en.2012-2165. [DOI] [PubMed] [Google Scholar]
- 31.Merrill MJ, Edwards NA. Insulin-like growth factor-I receptors in human glial tumors. J Clin Endocrinol Metab. 1990;71(1):199–209. doi: 10.1210/jcem-71-1-199. [DOI] [PubMed] [Google Scholar]
- 32.Galanopoulos T, Neville-golden J, Maxwell M. Expression of insulin-like growth factors I and II and their receptor mRNAs in primary human astrocytomas and meningiomas: in vivo studies using in situ hybridization and immunocytochemistry. Int J Cancer. 1992;50:215–222. doi: 10.1002/ijc.2910500210. [DOI] [PubMed] [Google Scholar]
- 33.Gammeltoft S, Ballotti R, Kowalski A, Westermark B, Van Obberghen E. Expression of two types of receptor for insulin-like growth factors in human malignant glioma. Cancer Res. 1988;48(5):1233–1237. [PubMed] [Google Scholar]
- 34.Hirano H, Lopes MB, Laws ER, Asakura T, Goto M, Carpenter JE, Karns LR, VandenBerg SR. Insulin-like growth factor-1 content and pattern of expression correlates with histopathologic grade in diffusely infiltrating astrocytomas. Neuro-Oncology. 1999;1(2):109–119. doi: 10.1093/neuonc/1.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warsito D, Sjöström S, Andersson S, Larsson O, Sehat B. Nuclear IGF1R is a transcriptional co-activator of LEF1/TCF. EMBO Rep. 2012;13(3):244–250. doi: 10.1038/embor.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y-H, Gutmann DH. The molecular and cell biology of pediatric low-grade gliomas. Oncogene. 2014;33(16):2019–2026. doi: 10.1038/onc.2013.148. [DOI] [PubMed] [Google Scholar]
- 37.Dias-Santagata D, Lam Q, Vernovsky K, Vena N, Lennerz JK, Borger DR, Batchelor TT, Ligon KL, Iafrate AJ, Ligon AH, Louis DN, Santagata S. Braf V600E mutations are common in pleomorphic xanthoastrocytoma: Diagnostic and therapeutic implications. PLoS One. 2011;6(3):e17948. doi: 10.1371/journal.pone.0017948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.F. Clément, M. Venara, S. Maglio, A. Martin, C. Matho, C. Petre, M. García Lombardi, I. Bergadá and Pennisi. 2015. Characterization of IGF-1 receptor expression and localization in paediatric tumours of the central nervous system upon diagnosis according to WHO 2007 grading. Endocrine Reviews (36) 2, Suppl
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(GIF 269 kb)
(DOCX 13 kb)