Abstract
Obesity is associated with increased risk of breast cancer in postmenopausal but not in premenopausal women. Many factors may be responsible for this difference. The aim of this study was to determine the mechanisms by which the genes related to the AMPK pathway, inflammation, and estrogen actions are affected by adiposity in breast tissue with the objective of identifying differences that may explain the different breast cancer risk in premenopausal and postmenopausal women. Random fine needle aspirates (rFNAs) of breast tissue were collected from 57 premenopausal and 55 postmenopausal women and were classified as normal weight, overweight, or obese. Expression levels of 21 target genes were determined using a TaqMan Low Density Array procedure. Breast tissue estradiol levels were measured by a liquid chromatography-tandem mass spectrometry procedure, and serum estradiol and follicle-stimulating hormone (FSH) were measured by a radioimmunoassay and an enzyme-linked immunosorbent assay, respectively. We found that in postmenopausal women, serum and tissue estradiol levels were increased in those who were overweight, and serum FSH levels were decreased in obese status. Interestingly, RPS6KB1, an AMPK downstream-responsive gene for protein synthesis and cell growth, and estrogen receptor α (encoded by the ESR1 gene) and its target gene GATA3 were significantly decreased in rFNA of premenopausal, obese women. In postmenopausal women, RPS6KB1, ESR1, and GATA3 expression remained unchanged in relation to adiposity. However, prostaglandin-endoperoxide synthase 2 (PTGS2), cyclin D1 (CCND1), and another ESR1 target gene, TFF1, were elevated in rFNA of obese postmenopausal women. Thus, as bodyweight increases, gene expression is indicative of increased proliferation in postmenopausal women but decreased proliferation in premenopausal women. Overall, our data reveal a novel process by which obesity promotes the risk of breast cancer in postmenopausal but not premenopausal women.
Keywords: Trefoil Factor Family (TFF1), AMPK Pathway, AMP-dependent Protein Kinase (AMPK), CREB-regulated Transcription Coactivator (CRTC2), acetyl-CoA Carboxylase Alpha (ACACA)
Introduction
Approximately 1.5 billion adults in the world are overweight (BMI ≥ 25 kg/m2), and more than 500 million are obese (BMI ≥ 30 kg/m2). In the USA, the majority (> 60%) of adults are overweight, and obesity rates are continuously increasing [1]. Obesity is associated with increased breast cancer risk in postmenopausal women but decreased cancer risk in premenopausal women [2–5]. However, the underlying mechanisms linking obesity to alterations of breast cancer risk in women remain unclear.
Estrogen, a unique product of the aromatase enzyme, is an important risk factor for the development of estrogen receptor α (ERα, encoded by the ESR1 gene)-positive breast cancer [2, 6]. In premenopausal women, the biosynthesis of estradiol (E2), a biologically active estrogen, occurs primarily in the ovaries but aromatase expression and estrogen formation [mainly estrone (E1)] also occur in the preadipocytes (fibroblasts) of breast adipose tissue and subcutaneous fat in other areas of the body [3, 7]. When the ovaries cease to produce E2 in postmenopausal women, local aromatase expression in the breast preadipocytes provides the main source of estrogens (E1 and E2) that drive ERα-positive breast cancer development [8]. The activation of the master energy regulator AMP-dependent protein kinase (AMPK) inhibits aromatase expression and estrogen formation in breast adipose fibroblasts via transcriptional factors cAMP-responsive element-binding protein (CREB) and CREB-regulated transcription coactivator 2 (CRTC2) [9]. Rapid cell growth requires the active synthesis of proteins, ribosomal RNA, and lipids, all of which are suppressed by AMPK activation. Conversely, a chronic inflammatory condition associated with obesity results in type 1 cytokines [e.g., tumor necrosis factor β (TNFβ)] and prostaglandin E2 (PGE2) which further promote the transcription of aromatase and increase local estrogen formation via activation of transcription factors of CCAAT-enhancer-binding protein-β (C/EBPB) and JUNB [10–12]. However, the differential expression of PGE2 and AMPK in premenopausal or postmenopausal breast tissue is unknown.
The Ser/Thr protein kinase AMPK is recognized as a master sensor of cellular energy availability and a connection for the convergence of endocrine signals including estrogens, androgens, inflammatory factors, leptin, and adiponectin [13, 14]. AMPK is a heterotrimeric enzyme consisting of a catalytic (α) and two regulatory subunits [15]. Two isoforms of AMPKα (AMPKα1 and α2) are encoded by PRKAA1 and PRKAA2, respectively. One of the major upstream kinases that activate AMPK is liver kinase B1 (LKB1), also known as serine/threonine kinase 11 (STK11) [16]. Activation occurs through phosphorylation of AMPKα on Thr-172. Activated AMPK inhibits mTOR and subsequently downregulates ribosomal protein S6 kinase (RPS6KB1) which leads to reduced protein synthesis, reduced cellular growth, and decreased proliferation [17]. AMPK activation also turns off fatty acid synthesis via phosphorylation and inactivation of acetyl-CoA carboxylase alpha (ACACA) [18] and increases glycolysis via activation of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 2 (PFKFB2).
Phosphorylation of LKB1, AMPK, and their downstream kinases is generally considered as a common mechanism for activation of the catabolic pathways. However, the levels of gene expression may play an important role as well. For example, the tumor suppressor p53 can increase the expression of AMPKβ and TSC2, a downstream mediator of AMPK signaling. Increased gene expression of either AMPKβ or TSC2 can negatively regulate the IGF-I/AKT/mTOR pathway after stress [19, 20]. Moreover, fatty acid synthesis is particularly active in proliferating cells [21], and AMPK turns this off by a dual mechanism: (1) phosphorylation and inactivation of ACACA [22] and (2) downregulation of ACACA as well as fatty acid synthase and other lipogenic genes [23, 24] .
Prostaglandin-endoperoxide synthase (PTGS) is the rate-limiting enzyme for prostaglandin production. There are three isozymes of PTGS: a constitutively expressed PTGS1, an inducible PTGS2, and a PTGS1 splice variant named as PTGS3, which differ in their regulation of expression and tissue distribution [25]. PTGS1 is distributed almost ubiquitously. However, PTGS2, also known as cyclooxygenase-2 or COX-2, is constitutively expressed in some tissues in physiological conditions (such as the endothelium, kidney, and brain) and in pathological conditions (such as cancer) [26]. In cancer cells, PTGS2 is responsible for the production of inflammatory PGE2, which plays important roles in breast tumor development, growth, vascularization, and resistance to apoptosis [27]. Elevated levels of PTGS2 and PGE2 indicate poor prognosis of breast cancer [28] .
In the present study, we investigated the genes regulating aromatase expression and those responding to estrogen action in normal weight, overweight, and obese premenopausal and postmenopausal women, especially genes associated with the AMPK pathway and inflammation. The differential expression of these genes may help to explain the difference in breast cancer risk associated with increasing adiposity in premenopausal and postmenopausal women [29, 30].
Materials and Methods
Subjects
The procedures have been described in detail in a previous publication [31]. In brief, healthy women aged 35 to 60 and willing to undergo a random fine-needle aspiration (rFNA) of the breast within 14 weeks of having a normal mammogram were recruited to the study. If the time elapsed was greater than 14 weeks, a new mammogram was obtained. Fifty-seven women were premenopausal, having regular menstrual cycles of between 25 and 32 days, and 55 women were postmenopausal based on not having had a menstrual period for 12 months and having serum E2 < 110 pmol/l and follicle-stimulating hormone (FSH) of > 30 mIU/ml. Excluded were women with a personal history of breast cancer, recent biopsy, or surgical excision for a breast abnormality, and those with any other cancer within the previous 5 years (with the exception of non-melanoma skin cancer or cervical carcinoma in situ). Women were required to have intact and healthy bilateral breasts, without implants or history of radiation for any indication. Prior and current medications were restricted as follows. Medication for breast cancer prevention (e.g., tamoxifen, raloxifene, or aromatase inhibitor) for up to 12 months was permitted if the last dose was taken 2 years or more prior to participation. Oral contraceptive, hormone replacement therapy, or vaginal/topical hormonal preparations were not permitted within 3 months of study enrollment. Concurrent use of daily aspirin, other non-steroidal anti-inflammatory drugs (NSAIDs), fish oil supplements (omega-3 fatty acids), multivitamins, or vitamins C and E were not allowed within 2 weeks of the rFNA procedure. Women were excluded if they had a history of pregnancy or lactation within the prior 2 years, if they had any condition that in the opinion of the investigator it may not make it safe to take part, or if they were unable to give voluntary consent. Recruitment was focused to enroll similar numbers of premenopausal and postmenopausal women. Study patient characteristics are shown in Table 1.
Table 1.
Patient demographic information
| Group | Premenopausal n = 57 | Postmenopausal n = 55 | |
|---|---|---|---|
| Normal | No. of subjects | n = 18 | n = 20 |
| BMI (kg/m2) | 19.9–24.9 | 18.7–24.9 | |
| Median age (range) | 46 (35–52) | 57 (47–60) | |
| Caucasian (%) | 95 | 95 | |
| Overweight | No. of subjects | 20 | 16 |
| BMI (kg/m2) | 25.1–29.6 | 25.1–29.8 | |
| Median age (range) | 43 (37–50) | 56 (41–59) | |
| Caucasian (%) | 85 | 75 | |
| Obese | No. of subjects | 19 | 19 |
| BMI (kg/m2) | 31.3–51.3 | 30.2–50.8 | |
| Median age (range) | 43 (37–51) | 53 (45–60) | |
| Caucasian (%) | 85 | 80 | |
Eligible participants were scheduled for an in-person clinic visit where study procedures, in most cases, occurred in a single visit. Participants completed personal and medical history questionnaires and vital signs. Of those approached for the study, 88.3% agreed to participate and 11.7% were ineligible based on the exclusion criteria. Height and weight were measured and BMI was calculated. Subjects with BMI of 18.5 to 24.9 kg/m2 were classified as normal body weight; those between 25 and 29.9 kg/m2 were classified as overweight; and those ≥ 30 kg/m2 were classified as obese. Blood for hormone levels and rFNA cells were collected following a breast exam and mammogram.
The rFNA procedure involved inserting a 1.5-in. 21-gauge needle around the periphery of the areola and drawing out tissue. The tissue within the syringe was rinsed into 15 ml of phosphate buffered saline, pH 7.4. The procedure was repeated 10 times encircling the areola. This provided a representative sample of the breast, including parenchymal tissue, connective tissue, fat, and vasculature. The lipid was separated by centrifugation at 4000g for 10 min at 4 °C for steroid analyses. Coarse connective tissue was then removed from the pellet by filtration through a 20-μm nylon mesh, and the filtrate was used for the mRNA analyses. The study was approved by the Institutional Review Boards of Northwestern University and Johns Hopkins University.
Total RNA Extraction and Preamplification of cDNA
Total RNA was extracted from rFNA samples using TRIzol reagent (Invitrogen) and purified using RNeasy Plus Micro Kit (Qiagen). RNA was then treated with DNase and checked for integrity using Agilent 2100 Bioanalyzer. Total RNA (100 ng) was reverse-transcribed using High Capacity RNA-to-cDNA Master Mix (Applied Biosystems). The cDNA was further amplified by preamplification PCR (14 cycles) using TaqMan® PreAmp Master Mix and Pooled Assay Mix. The preamplification cDNA was diluted 1:20 with 1× TE buffer before applying quantitative RT-PCR.
Quantitative RT-PCR Using TaqMan Low-Density Assay (TLDA) Microfluidic Cards
Primers for the 21 target genes and three housekeeping genes (GAPDH, HPRT1, and 18S for normalization) were preloaded in 384-well TLDA Microfluidic Cards from Life Technologies for gene expression assays. Assays were designed with small amplicons (< 100 bp) to enhance detection sensitivity. Mixtures of the preamplification cDNAs and TaqMan Real-Time PCR Master Mix were loaded to the TLDA Microfluidic Cards. Three ERα target genes [GATA3, progesterone receptor (PGR), and trefoil family factor 1 (TFF1)], which were not included in 384-well TLDA Microfluidic Cards, were determined by regular real-time PCR assay. Real-time PCR reactions were then carried out in an Applied Biosystems 7900HT machine. For each target gene, the expression level was normalized against the average expression of three housekeeping genes.
Serum and Breast Tissue Hormones
Blood samples were obtained from all women at the time rFNA was obtained. E2 was measured by a radioimmunoassay from Beckman-Coulter (Brea, CA, DSL-4800). FSH was measured by an enzyme-linked immunosorbent assay (ELISA) with a kit from ALPCO (Salem, NH, 11-FSHHU-E01). E1 and E2 in rFNA of breast tissue were determined using liquid chromatography-tandem mass spectrometry (LC-MS2) at the IIT Research Institute, as described previously [30, 31].
Statistical Analysis
Results are expressed as mean ± s.e.m., unless otherwise indicated. Statistically significant differences at P < 0.05 were determined using a two-tailed Student’s t test or ANOVA with Fisher’s LSD or Tukey’s multiple comparison tests. The relationship between BMI and the gene transcript was determined by linear regression analysis. All data were transformed to natural log values for analysis and were adjusted for age, except those in Fig. 2. Statistical analysis was performed using the SYSTAT 13 software and the GraphPad Prism software.
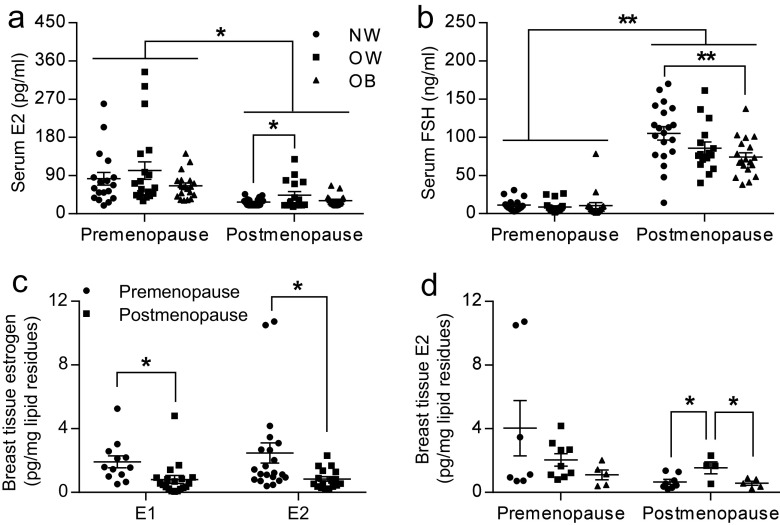
Fig. 2.
Serum E2 (a) and FSH (b) were measured in premenopausal and postmenopausal women who are normal-weight (n = 18 for pre and n = 20 for post), overweight (n = 20 for pre and n = 16 for post), and obese (n = 19 for both pre and post). c rFNA breast tissue estrogen (E1 and E2) was measured by LC-MS2 assay in premenopausal and postmenopausal women. For E1, n = 12 for premenopausal and n = 21 for postmenopausal women. For E2, n = 19 for premenopausal and n = 17 for postmenopausal women. d E2 in rFNA breast tissue were measured in normal-weight (n = 7 for pre and n = 8 for post), overweight (n = 9 for pre and n = 4 for post), and obese women (n = 5 for both pre and post) of both premenopausal and postmenopausal status. *P < 0.05; **P < 0.01
Results
Serum and Tissue E2 Levels Are Higher in Postmenopausal Overweight Women
The patient demographic information is shown in Table 1. Among the 112 women, 57 were premenopausal (35–52 years of age) and 55 were postmenopausal (41–60 years of age). The median age of the premenopausal women was 43 years and that of the postmenopausal women was 55 years. The age and race distribution were balanced among normal weight, overweight, and obese groups. Genes selected for expression analysis were based on their known and hypothesized actions in regulating aromatase activity and estrogen formation in breast tissue, such as the genes associated with the AMPK pathway and inflammation (Table 2). In the rFNAs, we found that aromatase (CYP19A1) mRNA levels were significantly and positively correlated with BMI without regard to menopausal status (P = 0.0463; Fig. 1a). Previous studies have shown that undifferentiated preadipocytes express higher levels of aromatase, which is responsible for increased production of estrogen. Estrogens produced in preadipocytes in turn exert an intracrine effect to enhance antiadipogenic properties of tumor necrosis factor α (TNFα) by selectively increasing the expression of the TNF receptor superfamily member 1A (TNFRSF1A), which mediates inhibition of adipocyte differentiation [32]. Consistent with this previous finding, we found that TNFRSF1A mRNA levels were also significantly correlated with BMI (P = 0.0065; Fig. 1b). Perilipin 2 (PLIN2) is an adipose differentiation-related protein and a lipid droplet-specific marker for metabolically active cells [33]. We found that PLIN2 mRNA levels in breast tissue were significantly correlated with BMI, as expected (P = 0.0150; Fig. 1c).
Table 2.
Gene expression differences between normal-weight, overweight, and obese women in premenopausal and postmenopausal states. *P < 0.05 stands for significance of ANOVA prior to post hoc testing
| Gene symbol | Gene name | P values from ANOVA | |
|---|---|---|---|
| Premenopause n = 57 | Postmenopause n = 55 | ||
| Estrogen synthase, receptor and responsive genes | |||
| CYP19A1 | Cytochrome P450, family 19, subfamily A, polypeptide 1 | 0.511 | 0.585 |
| ESR1 | Estrogen receptor 1 | 0.071 | 0.357 |
| CCND1 | Cyclin D1 | 0.879 | 0.001* |
| SUSD3 | Sushi domain containing 3 | 0.331 | 0.335 |
| PLIN2 | Perilipin 2 | 0.324 | 0.077 |
| Genes associated with the AMPK pathway | |||
| STK11 | Serine/threonine kinase 11 | 0.556 | 0.793 |
| PRKAA1 | Protein kinase, AMP-activated, alpha 1 catalytic subunit | 0.194 | 0.845 |
| PRKAA2 | Protein kinase, AMP-activated, alpha 2 catalytic subunit | 0.274 | 0.646 |
| MTOR | Mechanistic target of rapamycin (serine/threonine kinase) | 0.482 | 0.127 |
| RPS6KB1 | Ribosomal protein S6 kinase, 70 kDa, polypeptide 1 | 0.037* | 0.773 |
| EEF2 | Eukaryotic translation elongation factor 2 | 0.293 | 0.031* |
| ACACA | Acetyl-CoA carboxylase alpha | 0.896 | 0.585 |
| PFKFB2 | 6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 2 | 0.944 | 0.985 |
| CRTC2 | CREB-regulated transcription coactivator 2 | 0.292 | 0.247 |
| CREB1 | cAMP-responsive element-binding protein 1 | 0.967 | 0.863 |
| Genes associated with the inflammation | |||
| TNF | Tumor necrosis factor | 0.590 | 0.668 |
| TNFRSF1A | Tumor necrosis factor receptor superfamily, member 1A | 0.390 | 0.845 |
| TNFRSF1B | Tumor necrosis factor receptor superfamily, member 1B | 0.567 | 0.949 |
| PTGS2 | Cytochrome c oxidase subunit II | 0.565 | 0.041* |
| JUNB | Jun B proto-oncogene | 0.770 | 0.641 |
| CEBPB | CCAAT/enhancer-binding protein (C/EBP), beta | 0.419 | 0.588 |
*P < 0.05 for comparison between normal-weight, overweight, and obese women
Fig. 1.
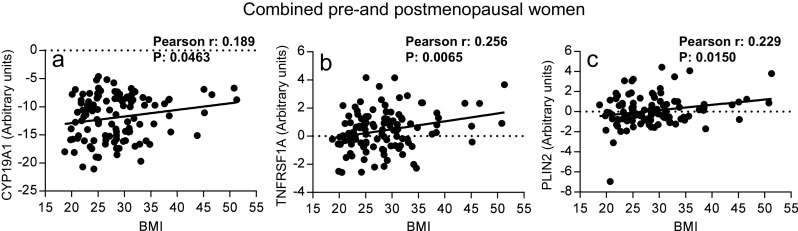
Expression of CYP19A1 (a), TNFRSF1A (b), and PLIN2 (c) was significantly correlated with BMI in all rFNA breast tissue samples. n = 112
To determine whether increased breast tissue aromatase expression in overweight/obese women is accompanied by a change in tissue or serum estrogen levels, we compared peripheral serum concentrations of E2 and FSH in premenopausal and postmenopausal women (Fig. 2a, b). We also measured tissue concentrations of E2 in premenopausal and postmenopausal women with various degrees of adiposity (Fig. 2c, d). It is known that increased serum E2 levels are associated with decreased serum FSH levels via negative feedback in the hypothalamus and pituitary glands. As expected, serum E2 levels were significantly lower (P < 0.05) and serum FSH levels were significant higher (P < 0.01) in postmenopausal women than in premenopausal women. In premenopausal women, serum E2 and FSH levels did not differ between normal weight, overweight, and obese groups. Serum E2 levels in postmenopausal women, however, were higher in overweight women compared to normal-weight women (P < 0.05). FSH levels were lower in overweight/obese postmenopausal women but only reached significance in obese women compared to normal women (P < 0.01)(Fig. 2b). Generally, breast tissue E1 and E2 levels were lower in postmenopausal women than in premenopausal women (P < 0.05, Fig. 2c). Furthermore, breast tissue E2 levels were higher in overweight women compared to normal-weight women in postmenopausal women (P < 0.05), but did not differ among all three groups of premenopausal women. These results show that both serum and local breast tissue E2 levels were higher in overweight as compared to normal-weight postmenopausal women.
Effect of Obesity on the AMPK Pathway and Estrogen Receptor α and Its Target Genes in Premenopausal Women
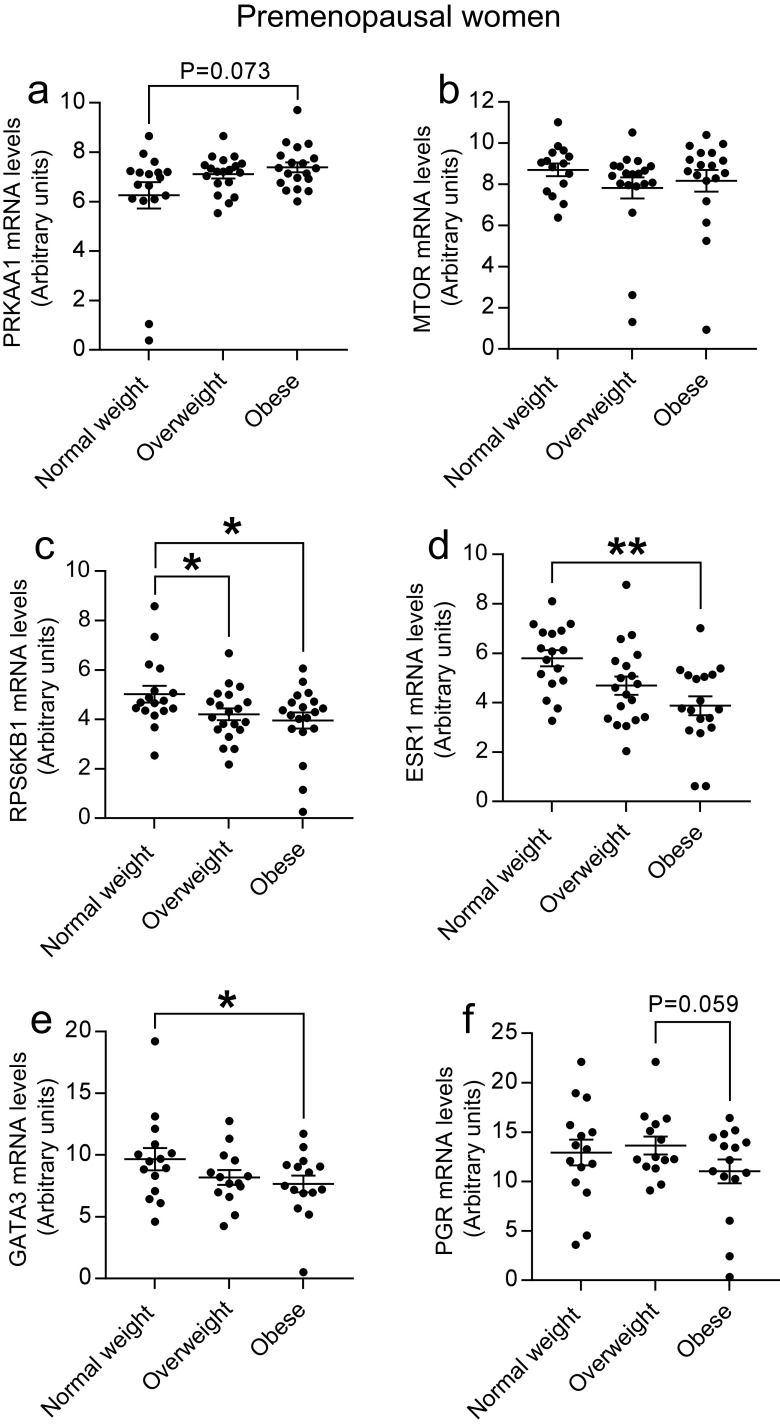
To determine the AMPK signaling pathway in the human breast tissue and its relationship with increased adiposity (obesity) and menopausal status, we analyzed genes involved in the AMPK pathway and its downstream responders (Table 2 and Fig. 3a–c). In premenopausal samples, we first used one-way ANOVA to determine whether there are any overall statistically significant differences of each gene expression between normal-weight, overweight, and obese groups (Table 2). To define the detailed differences between those groups, post hoc multiple comparison analyses were used to further characterize effects of adiposity in those statistically significant genes identified by ANOVA (e.g., RPS6KB1 and ESR1). The genes either regulated RPS6KB1 (i.e., PRKKA1 and MTOR) or regulated by ESR1 (GATA3, PGR, and TFF1) were also compared using post hoc multiple comparison analyses. We found that the mRNA levels of PRKAA1 tended to be higher (P = 0.073) and mRNA levels of MTOR tended to be lower (P = 0.171) in obese women as compared to normal-weight women, although significance was not reached. Most importantly, RPS6KB1, an AMPK downstream target gene and a ribosomal protein kinase responsible for protein biosynthesis and cell growth, was significantly lower in both obese (P = 0.020) and overweight women (P = 0.030) than in normal-weight women (Fig. 3a–c). However, ACACA, a rate-limiting enzyme in fatty acid synthesis, did not differ in three groups of premenopausal women (Table 2). No significant changes occurred in expression of several other AMPK pathway-related genes (STK11, PRKAA2, CRTC2, CREB1, PFKFB2, and EEF2) (Table 2). In addition, ERα (encoded by ESR1) expression was decreased in obese women than in normal-weight women (P = 0.003, Fig. 3d). Next, we determined the expression levels of classical ERα target genes (GATA3, PGR, and TFF1) in premenopausal women. Consistent with a decrease in ESR1 in premenopausal obese women, GATA3 mRNA levels were significantly decreased in obese women compared with normal weight women (P = 0.046, Fig. 3e) and PGR mRNA levels were also decreased in obese women, marginally significant as compared to overweight women (P = 0.059, Fig. 3f). TFF1 mRNA levels did not differ between three groups (data not shown). Decreased expression of RPS6KB1, ESR1, and GATA3 may be responsible for reduced breast cancer risk in premenopausal obese women. In contrast, the mRNA levels of RPS6KB1, ESR1, GATA3, and PGR in breast tissue remained unchanged among normal-weight, overweight, and obese postmenopausal women (Table 2 and data not shown).
Fig. 3.
Expression of PRKAA1 (a), MTOR (b), RPS6KB1 (c), ESR1 (d), GATA3 (e), and PGR (f) in rFNA breast tissue samples of premenopausal women. n = 18 for normal weight, n = 20 for overweight, and n = 19 for obese. *P < 0.05; **P < 0.01
PTGS2, CCND1, and TFF1 Are Highly Expressed in Breast Tissue of Postmenopausal Obese Women
To determine the inflammatory effects on the human breast tissue and its relationship with increased adiposity and menopausal status, we analyzed genes related to inflammation and estrogen responsiveness using one-way ANOVA (Table 2) followed by post hoc multiple comparison analyses (Fig. 4a, b). We found that in postmenopausal women, PTGS2 expression was significantly higher in obese women (P = 0.017), and tended to be higher in overweight (P = 0.064) compared to normal-weight women (Fig. 4a). Cyclin D1 (encoded by the CCND1 gene) is an estrogen target gene that regulates the cell cycle during G1/S transition in breast epithelial cells. In postmenopausal women, we found that breast tissue CCND1 mRNA levels were significantly higher in obese women than in normal-weight and overweight women (P = 0.013 and P = 0.0004, respectively, Fig. 4b). Moreover, the mRNA levels of ERα target gene TFF1 were significantly increased in obese postmenopausal women (P = 0.034, Fig. 4c). In contrast, mRNA levels of PTGS2, CCND1, and TFF1 were not significantly affected by adiposity in premenopausal breast samples. It is clear that cellular proliferative pathways were upregulated with increased adiposity in postmenopausal women but not in premenopausal women. No significant changes were found in expression of several other inflammatory genes with adiposity (TNF, TNFRSF1A, TNFRSF1B, JUNB, and CEBPB) in premenopausal or postmenopausal women. The mRNA levels of EEF2 were not consistent between the pairs of contrasts. It was decreased in overweight women as compared to normal-weight women (P = 0.030) but restored to normal levels in obese women (Table 2 and data not shown).
Fig. 4.
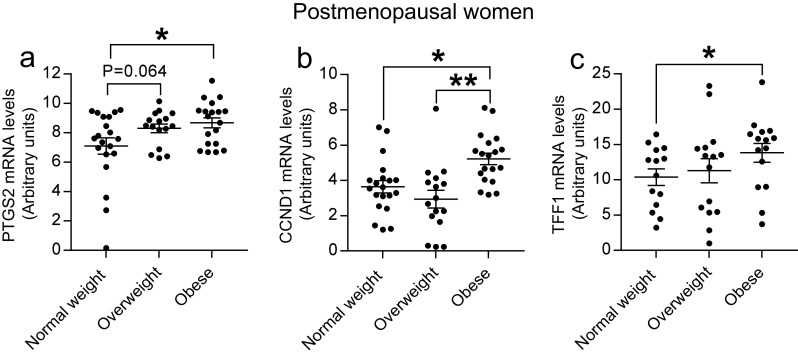
Expression of PTGS2 (a), CCND1 (b), and TFF1 (c) in rFNA breast tissue samples of postmenopausal women. n = 20 for normal weight, n = 16 for overweight, and n = 19 for obese. *P < 0.05; **P < 0.01
Discussion
Obesity increases breast cancer risk by 1.5- to 3-fold in postmenopausal women but is protective in premenopausal women [34, 35]. In premenopausal women, we found that the mRNA levels of RPS6KB1, an important target of PRKAA1, were significantly lower in obese breast tissue, indicating decreased protein synthesis and cell growth. The observed decrease in ESR1 and its target gene GATA3 with increasing adiposity also results in a lower proliferative potential in premenopausal women. Decreased expression of RPS6KB1, ESR1, and GATA3 is likely responsible for reduced breast cancer risk in premenopausal women. Such results are consistent with the observation that overweight and obese premenopausal women are at lower risk of breast cancer [30, 34, 36], even though the prognosis of established breast cancer is worse in obese women for both premenopausal and postmenopausal women [37]. In postmenopausal women, mRNA levels of PTGS2, CCND1, and TFF1 were significantly higher in overweight/obese breast tissue, suggesting increased cell proliferation and increased breast cancer risks associated with increasing adiposity.
Significant differences were found in serum E2 levels of postmenopausal women among the normal-weight, overweight, and obese groups, but the actual differences were probably greater. Because serum E2 levels are very low in postmenopausal women, the currently available assays may not differentiate their exact levels when below the limit of detection (< 20 pg/ml) in this study. Undetectable levels were found in 8 out of 20 normal-weight, 6 out of 16 overweight, but only 1 out of 19 obese postmenopausal subjects. Once E2 levels below the limit of detection, we designated E2 levels as 20 pg/ml. Therefore, the actual E2 levels in the normal-weight group could be much lower. Lower serum levels of FSH in overweight/obese women further confirmed this speculation.
Although the CYP19A1 gene expression is correlated with BMI across the full age range of BMI, both serum and tissue E2 levels were found to be lower in obese than in overweight postmenopausal women. This observation requires further study, but we speculate that the number of estrogen secreting preadipocytes may be lower in obese women. In this regard, Isakson et al. have shown that the number of preadipocytes able to differentiate into adipose cells is negatively correlated with both BMI and adipocyte cell size [38]. However, whether this affects the ability of the preadipocytes to produce estrogen is unknown. Because tissue estrogen levels were calculated per unit of lipid fluid, the lower estrogen concentration in adipose tissue of obese women may simply be due to the dilution effect of the large quantity of lipid in breasts of obese compared with overweight women. The total amount of E2 levels produced by adipose tissue is probably not lower in obese women. The lower serum E2 levels may reflect a lower rate of diffusion from the lower concentration of estrogen in the fatty tissue of obese women. Paradoxically, serum FSH levels were much lower in obese women than in overweight women. Based on the negative feedback relationship of estrogen on FSH secretion, this would indicate that serum estrogen is higher in obese women. The higher expression of estrogen-responsive gene TFF1 further supports the higher levels of serum E2 in obese postmenopausal women as do previous publications [39, 40]. These inconsistent results remain unexplained.
Parenchymal differences between tissue samples from premenopausal and postmenopausal women may occur as a result of age-related breast involution. However, we limited the age of the postmenopausal women to 60 years, and thus the median age difference between premenopausal (43 years) and postmenopausal women (55 years) was only 12 years to minimize differences in the proportion of cellular components. Also, we removed the lipids and the coarse connective tissue from all samples prior to analysis of the transcripts. Those procedures minimized the age effect of breast tissue on the gene expression in this study.
This study investigated a number of gene expressions involved in the AMPK pathway in human breast tissues in premenopausal and postmenopausal women including an AMPK kinase (STK11), AMPKs (PRKAA1 and PRKAA2), and a number of downstream genes, such as MTOR, RPS6KB1, and EEF2 for protein synthesis and cell growth, ACACA for fatty acid synthesis, PFKFB2 for controlling glycolysis, and CRTC2 and CREB1 for regulation of the aromatase expression. With increasing adiposity in premenopausal women, PRKAA1 and MTOR tended to be more highly expressed, and most importantly, RPS6KB1 was significantly suppressed only in premenopausal women. EEF2 in the breast is importantly involved with synthesis of milk proteins and proteins for cell proliferation [41, 42]. Although it was significant by ANOVA in postmenopausal women (Table 2), the inconsistent change of EEF2 with adiposity makes these changes difficult to evaluate. The kinase gene expression for fatty acid synthesis, glycolysis, and the CRTC2/CREB-mediated aromatase expression remained unchanged in premenopausal and postmenopausal women with differences in adiposity. The kinases in the AMPK pathway are largely regulated by phosphorylation and/or activity. We recognize that regulation of a number of the enzymes by phosphorylation may be more important than by changes in the gene expression. However, the level of the gene expression is also an important component of the regulatory process [22–24]. Thus, the upregulated PRKAA1 gene expression followed by the downregulated RPS6KB1 gene expression in the AMPK pathway in premenopausal woman breast with increasing adiposity may explain, in part, the decreased risk of breast cancer in premenopausal obese women. The present study is limited to changes in the expression of genes associated with energy utilization, estrogen activity, and inflammation. Future studies will expand to explore the kinase phosphorylation of the AMPK pathway in normal-weight, overweight, and obese premenopausal and postmenopausal women. We have no simple explanation for the apparent association of PRKAA1 with transcripts of genes associated protein synthesis in premenopausal but not postmenopausal women. However, there are many factors that can influence the activity of PRKAA1. These include LKB1, sirtuin 1, free fatty acids, estrogens, androgens, and adipokines [43], so there may be differences in the input to PRKAA1 in premenopausal and postmenopausal women.
The association of inflammatory factors with obesity is well documented [44–46]. PTGS2 is also overexpressed in malignant breast epithelial cells, and the resultant PGE2 increases aromatase expression and estrogen production in breast adipose fibroblasts via paracrine pattern [47]. It has been shown that the positive association of PTGS2 with greater risk of breast cancer is limited to postmenopausal women [48]. A remarkable finding in this study was the further demonstration that PTGS2 expression increased with increasing adiposity in postmenopausal women, but not in premenopausal women. Both non-activation of the protective AMPK pathway and increased inflammatory responses may be responsible for the increased risk of breast cancer in postmenopausal obese women, as evidenced by epidemiological studies [49, 50]. As a result, the AMPK activator (e.g., biguanides) and anti-inflammatory drugs may be therapeutic options for breast cancer prevention and treatment in postmenopausal obese women.
In postmenopausal women, without the AMPK protection effects, breast tissue is vulnerable to the detrimental factors associated with breast cancer proliferation. We found that CCND1, an indicator of proliferative changes in the breast [48], was increased in obese postmenopausal women. As an estrogen target gene, the increased expression of CCND1 may be induced by higher serum E2 levels in overweight women compared to normal-weight women. Moreover, the high PTGS2 associated with increased PGE2 can induce CCND1 expression via PKA/PKC-mediated aromatase expression and estrogen production as well as other signaling pathways such as PI3K/Akt and MAPKs [51, 52]. The increased expression of CCND1 is possibly associated with other inflammatory factors such as TNF and IL-11 as a consequence of the inflammatory processes in adipose tissue of obese individuals [32].
In summary, distinct differences in association of gene expression patterns with obesity were found in rFNA of breast tissue of premenopausal and postmenopausal women. In premenopausal women, expression of genes that are involved in estrogen action such as ESR1 and GATA3 and genes involved in cell proliferation such as RPS6KB1 was lower in breast tissue with increasing adiposity. In postmenopausal women, the response to increasing adiposity was related to increased inflammatory molecule PTGS2, increased ESR1 target gene TFF1, and a significant elevation of cell cycle G1/S checkpoint gene CCND1. The differences between the expression patterns in premenopausal and postmenopausal obese women are consistent with the observed differences in breast cancer risks associated with these age groups.
Acknowledgements
This work was supported by the Avon Breast Cancer Foundation. We thank the technical expertise and help of Dr. Jun Bai.
Compliance with Ethical Standards
The study was approved by the Institutional Review Boards of Northwestern University and Johns Hopkins University and was performed in accordance with the ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 2.Brown KA, Simpson ER. Obesity and breast cancer: progress to understanding the relationship. Cancer Res. 2010;70(1):4–7. doi: 10.1158/0008-5472.CAN-09-2257. [DOI] [PubMed] [Google Scholar]
- 3.Bulun SE, Chen D, Moy I, Brooks DC, Zhao H. Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol Metab. 2012;23(2):83–89. doi: 10.1016/j.tem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cleary MP, Grossmann ME. Mini review: obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150(6):2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3(9):565–574. doi: 10.1016/S1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 6.Bulun SE, Lin Z, Zhao H, Lu M, Amin S, Reierstad S, Chen D. Regulation of aromatase expression in breast cancer tissue. Ann N Y Acad Sci. 2009;1155:121–131. doi: 10.1111/j.1749-6632.2009.03705.x. [DOI] [PubMed] [Google Scholar]
- 7.Bulun SE, Sharda G, Rink J, Sharma S, Simpson ER. Distribution of aromatase P450 transcripts and adipose fibroblasts in the human breast. J Clin Endocrinol Metab. 1996;81(3):1273–1277. doi: 10.1210/jcem.81.3.8772611. [DOI] [PubMed] [Google Scholar]
- 8.Chetrite GS, Cortes-Prieto J, Philippe JC, Wright F, Pasqualini JR. Comparison of estrogen concentrations, estrone sulfatase and aromatase activities in normal, and in cancerous, human breast tissues. J Steroid Biochem Mol Biol. 2000;72(1–2):23–27. doi: 10.1016/S0960-0760(00)00040-6. [DOI] [PubMed] [Google Scholar]
- 9.Brown KA, McInnes KJ, Hunger NI, Oakhill JS, Steinberg GR, Simpson ER. Subcellular localization of cyclic AMP-responsive element binding protein-regulated transcription coactivator 2 provides a link between obesity and breast cancer in postmenopausal women. Cancer Res. 2009;69(13):5392–5399. doi: 10.1158/0008-5472.CAN-09-0108. [DOI] [PubMed] [Google Scholar]
- 10.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, du B, Brogi E, Crawford CB, Kopelovich L, Subbaramaiah K, Dannenberg AJ. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4(7):1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, et al. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2(4):356–365. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Sun X, Casbas-Hernandez P, Bigelow C, Makowski L, Joseph Jerry D, Smith Schneider S, Troester MA. Normal breast tissue of obese women is enriched for macrophage markers and macrophage-associated gene expression. Breast Cancer Res Treat. 2012;131(3):1003–1012. doi: 10.1007/s10549-011-1789-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown KA, Samarajeewa NU, Simpson ER. Endocrine-related cancers and the role of AMPK. Mol Cell Endocrinol. 2013;366(2):170–179. doi: 10.1016/j.mce.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 14.Thompson AM. Molecular pathways: preclinical models and clinical trials with metformin in breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20(10):2508–2515. doi: 10.1158/1078-0432.CCR-13-0354. [DOI] [PubMed] [Google Scholar]
- 15.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9(8):563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310(5754):1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441(7092):424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 18.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25(18):1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoh J, Jin S, Parrado T, Edington J, Levine AJ, Ott J. The p53MH algorithm and its application in detecting p53-responsive genes. Proc Natl Acad Sci U S A. 2002;99(13):8467–8472. doi: 10.1073/pnas.132268899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Z, Hu W, de Stanchina E, Teresky AK, Jin S, Lowe S, Levine AJ. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007;67(7):3043–3053. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 21.Metallo CM, Walther JL, Stephanopoulos G. Evaluation of 13C isotopic tracers for metabolic flux analysis in mammalian cells. J Biotechnol. 2009;144(3):167–174. doi: 10.1016/j.jbiotec.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies SP, Carling D, Munday MR, Hardie DG. Diurnal rhythm of phosphorylation of rat liver acetyl-CoA carboxylase by the AMP-activated protein kinase, demonstrated using freeze-clamping. Effects of high fat diets. Eur J Biochem. 1992;203(3):615–623. doi: 10.1111/j.1432-1033.1992.tb16591.x. [DOI] [PubMed] [Google Scholar]
- 23.Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120(7):2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leclerc I, Kahn A, Doiron B. The 5'-AMP-activated protein kinase inhibits the transcriptional stimulation by glucose in liver cells, acting through the glucose response complex. FEBS Lett. 1998;431(2):180–184. doi: 10.1016/S0014-5793(98)00745-5. [DOI] [PubMed] [Google Scholar]
- 25.Rouzer CA, Marnett LJ. Cyclooxygenases: structural and functional insights. J Lipid Res. 2009;50:Suppl:S29–Suppl:S34. doi: 10.1194/jlr.R800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flower RJ. The development of COX2 inhibitors. Nat Rev Drug Discov. 2003;2(3):179–191. doi: 10.1038/nrd1034. [DOI] [PubMed] [Google Scholar]
- 27.Howe LR. Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res. 2007;9(4):210. doi: 10.1186/bcr1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kochel TJ, Goloubeva OG, Fulton AM. Upregulation of cyclooxygenase-2/prostaglandin E2 (COX-2/PGE2) pathway member multiple drug resistance-associated protein 4 (MRP4) and downregulation of prostaglandin transporter (PGT) and 15-prostaglandin dehydrogenase (15-PGDH) in triple-negative breast cancer. Breast Cancer (Auckl) 2016;10:61–70. doi: 10.4137/BCBCR.S38529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomark Prev. 2009;18(10):2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 30.van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, Fraser G, Goldbohm RA, Graham S, Kushi L, Marshall JR, Miller AB, Rohan T, Smith-Warner SA, Speizer FE, Willett WC, Wolk A, Hunter DJ. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152(6):514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 31.Stearns V, Fackler MJ, Hafeez S, Lopez Bujanda Z, Chatterton RT, Jacobs LK et al (2016) Gene methylation and cytological atypia in random fine needle aspirates for assessment of breast cancer risk. Cancer Prev Res (Phila). 10.1158/1940-6207.CAPR-15-0377 [DOI] [PMC free article] [PubMed]
- 32.Deb S, Amin S, Imir AG, Yilmaz MB, Suzuki T, Sasano H, Bulun SE. Estrogen regulates expression of tumor necrosis factor receptors in breast adipose fibroblasts. J Clin Endocrinol Metab. 2004;89(8):4018–4024. doi: 10.1210/jc.2004-0127. [DOI] [PubMed] [Google Scholar]
- 33.Sletten A, Seline A, Rudd A, Logsdon M, Listenberger LL (2014) Surface features of the lipid droplet mediate perilipin 2 localization. Biochem Biophys Res Commun. 10.1016/j.bbrc.2014.08.097 [DOI] [PMC free article] [PubMed]
- 34.John EM, Sangaramoorthy M, Hines LM, Stern MC, Baumgartner KB, Giuliano AR, Wolff RK, Slattery ML (2014) Overall and abdominal adiposity and premenopausal breast cancer risk among Hispanic women: the breast cancer health disparities study. Cancer Epidemiol Biomark Prev. 10.1158/1055-9965.EPI-13-1007-T [DOI] [PMC free article] [PubMed]
- 35.Carmichael AR, Bates T. Obesity and breast cancer: a review of the literature. Breast. 2004;13(2):85–92. doi: 10.1016/j.breast.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Bandera EV, Chandran U, Hong CC, Troester MA, Bethea TN, Adams-Campbell LL, Haiman CA, Park SY, Olshan AF, Ambrosone CB, Palmer JR, Rosenberg L. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res Treat. 2015;150(3):655–666. doi: 10.1007/s10549-015-3353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150(6):2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 2009;58(7):1550–1557. doi: 10.2337/db08-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, Stanczyk FZ, Stephenson HE, Jr, Falk RT, Miller R, Schatzkin A, Allen DS, Fentiman IS, Key TJ, Wang DY, Dowsett M, Thomas HV, Hankinson SE, Toniolo P, Akhmedkhanov A, Koenig K, Shore RE, Zeleniuch-Jacquotte A, Berrino F, Muti P, Micheli A, Krogh V, Sieri S, Pala V, Venturelli E, Secreto G, Barrett-Connor E, Laughlin GA, Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE, Cauley JA, Kuller LH, Cummings SR, Helzlsouer KJ, Alberg AJ, Bush TL, Comstock GW, Gordon GB, Miller SR, Longcope C, Endogenous Hormones Breast Cancer Collaborative Group Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95(16):1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 40.Judd HL, Lucas WE, Yen SS. Serum 17 beta-estradiol and estrone levels in postmenopausal women with and without endometrial cancer. J Clin Endocrinol Metab. 1976;43(2):272–278. doi: 10.1210/jcem-43-2-272. [DOI] [PubMed] [Google Scholar]
- 41.Kaul G, Pattan G, Rafeequi T. Eukaryotic elongation factor-2 (eEF2): its regulation and peptide chain elongation. Cell Biochem Funct. 2011;29(3):227–234. doi: 10.1002/cbf.1740. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi AA, Nones K, Roy NC, McNabb WC, Mackenzie DS, Pacheco D, et al. Initiation and elongation steps of mRNA translation are involved in the increase in milk protein yield caused by growth hormone administration during lactation. J Dairy Sci. 2009;92(5):1889–1899. doi: 10.3168/jds.2008-1334. [DOI] [PubMed] [Google Scholar]
- 43.Zhao H, Orhan YC, Zha X, Esencan E, Chatterton RT, Bulun SE. AMP-activated protein kinase and energy balance in breast cancer. Am J Transl Res. 2017;9(2):197–213. [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrante AW., Jr Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262(4):408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 45.Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance—a mini-review. Gerontology. 2009;55(4):379–386. doi: 10.1159/000212758. [DOI] [PubMed] [Google Scholar]
- 46.Hsieh PS, Lu KC, Chiang CF, Chen CH. Suppressive effect of COX2 inhibitor on the progression of adipose inflammation in high-fat-induced obese rats. Eur J Clin Investig. 2010;40(2):164–171. doi: 10.1111/j.1365-2362.2009.02239.x. [DOI] [PubMed] [Google Scholar]
- 47.Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57(3):359–383. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- 48.Ristimaki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62(3):632–635. [PubMed] [Google Scholar]
- 49.Bulun SE, Chen D, Moy I, Brooks DC, Zhao H. Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol Metab. 2012;23(2):83–89. doi: 10.1016/j.tem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown KA, Simpson ER. Obesity and breast cancer: progress to understanding the relationship. Cancer Res. 2010;70(1):4–7. doi: 10.1158/0008-5472.CAN-09-2257. [DOI] [PubMed] [Google Scholar]
- 51.Hull MA, Ko SC, Hawcroft G. Prostaglandin EP receptors: targets for treatment and prevention of colorectal cancer? Mol Cancer Ther. 2004;3(8):1031–1039. [PubMed] [Google Scholar]
- 52.Chen D, Reierstad S, Lu M, Lin Z, Ishikawa H, Bulun SE. Regulation of breast cancer-associated aromatase promoters. Cancer Lett. 2009;273(1):15–27. doi: 10.1016/j.canlet.2008.05.038. [DOI] [PubMed] [Google Scholar]