Abstract
Adrenal Cortical Carcinoma (ACC) is a rare malignancy with an incidence of 1.0 per million per year in the Netherlands. Median survival varies according to the European Network for the Study of Adrenal Tumours (ENS@T) tumour stage. It is unknown whether time until development of metastases is of influence on prognosis. To asses this, data were retrospectively obtained from centres of the Dutch Adrenal Network. Patients who presented with ACC between January 1, 2004 and October 31, 2013 were included. Date of detection of metastases, number of metastases and affected organs were registered. One hundred sixty patients were included in the analysis. Synchronous metastases were defined as diagnosis of metastasis ≤6 months after the initial diagnosis of ACC. Overall survival rate was calculated from the date of diagnosis of metastasis until death from any cause. At first presentation, 50 patients (31 %) had ACC with metastases (ENS@T stage IV). Another 67 (42 %) developed metastases during follow-up. Amongst the 117 patients with metastases, 84 (72 %) patients had synchronous metastases and 33 (28 %) developed metachronous metastases. Diagnosis of synchronous metastases (p = 0.046), more than one affected organ (p < 0.001) and four or more metastases (p < 0.001) were found to be associated with reduced overall survival. Limitations included retrospective design and limited details regarding pathological data. We conclude that synchronous metastases of ACC are associated with a poorer prognosis compared to metachronous metastases of ACC. The clinical characteristics associated with prognosis in this study support the view to refine the prognostic classification for patients with stage IV ACC.
Keywords: Charlson Comorbidity Index, Adrenal Tumour, Adrenocortical Carcinoma, Mitotane, Synchronous Metastasis
Introduction
Adrenal Cortical Carcinoma (ACC) is a rare malignancy with an incidence of 1.0 per million in the Netherlands [1]. Median survival varies according to tumour stage, from 159 to 5 months for patients with ENS@T stage I–II and ENS@T stage IV, respectively [1]. ACC usually presents with symptoms caused by hormone production or local symptoms due to tumour growth. Radical surgical resection of locoregional tumour lesions is a mainstay of the curative treatment for ACC. Nevertheless, 75–85 % of patients who underwent radical resection showed recurrence of local disease often with concurrent distant metastases [2, 3]. Options for palliative treatment are limited.
Approximately, 30 % of patients already present with metastases at the time of diagnosis [4]. The most common sites for metastases are the lungs (∼40 %), liver (∼40 %), skeleton (∼30 %) and lymph nodes (∼25 %) [5–7].
For various malignancies, it has been described that the distinction between synchronous and metachronous metastases has a prognostic value [8, 9]. It is currently unknown, however, whether these different time patterns of metastases are associated with the outcome of patients with ACC. In addition, the influence of the number of metastases and affected organs on prognosis in patients with ACC is not well known. The number of involved organs in patients with stage IV ACC has been suggested to be useful for the prediction of outcome [10].
The primary objective of the present retrospective study was to investigate if there was a difference in survival of ACC between patients with synchronous metastases and patients with metachronous metastases. In addition, we aimed to study the effect of the number of metastases and affected organs on survival.
Methods
Data were retrospectively obtained from the nine centres of the Dutch Adrenal Network (DAN). The DAN includes eight Dutch university hospitals (Erasmus Medical Centre Rotterdam, Leiden University Medical Centre, VU Medical Centre Amsterdam, Radboud University Medical Centre Nijmegen, University Medical Centre Groningen, Academic Medical Centre Amsterdam, University Medical Centre Utrecht and Maastricht University Medical Centre) and Máxima Medical Centre (MMC). It has initiated the registration of clinical data on ACC in a national database, e.g. for research purposes and the improvement of patient care [11, 12].
Patients who presented with ACC between January 1, 2004 and October 31, 2013 were included. Start and end of the observation period coincided with the founding year of the DAN and the latest update of the DAN database, respectively.
In order to make the inclusion as complete as possible, hospital pathology departments, whom all participate in a nationwide network system (PALGA), were asked to compose a lists of all patients diagnosed with any adrenal tumour. Furthermore, the Dutch hospital diagnosis registry system, the Diagnosis Treatment Combination (DTC) system, was screened for patients labelled with an adrenal disease DTC code.
Inclusion criteria were age ≥18 years and confirmed diagnosis of ACC, either histological (Weiss, Van Slooten, Ki67) or by a combination of clinical analysis, hormonal analysis (e.g. urinary steroid profiling, dexamethasone suppression test) and imaging data e.g. computed tomography (CT). Patient records were assessed to obtain retrospective information on patient characteristics, metastases, radiology imaging (CT, Magnetic Resonance Imaging and positron emission tomography and treatment). Comorbidity was classified according to the Charlson Comorbidity Index (CCI) [13, 14].
Definitions
The primary endpoint was overall survival (OS). OS was calculated from the date of first diagnosis of metastases to death from any cause. One-year survival and 2-year survival were also calculated from the date of diagnosis of metastases. Conditional survival derived from the concept of conditional probability was calculated using the traditional Kaplan-Meier. The mathematical definition used can be expressed as follows: [15–18].
Metastases were defined as synchronous when found <6 months after the initial diagnosis of ACC [8, 9, 19]. Consequently, ‘metachronous metastases’ was defined as metastases diagnosed ≥6 months after the initial diagnosis of ACC.
The number of metastases was classified as ‘limited’ if there were one to three metastases and as ‘extended’ if there were four or more metastases [20]. A distinction was made between metastases found in only one organ/lymph node or in more than one organ/lymph node localization (discriminated organs: liver, lung, bone, lymph nodes under diaphragm, lymph nodes above diaphragm and elsewhere). Multiple metastases found in one organ were registered as one affected organ in the analysis of number of affected organs.
Diagnostic delay was calculated from the first hospital visit for ACC-related symptoms or signs until the moment the diagnosis of ACC were confirmed.
The effect of resection of the primary tumour on overall survival was assessed amongst patients with synchronous metastases. In addition, to further investigate the impact of ENS@T stage at primary diagnosis, its impact on survival was analysed within the group of synchronous metastases. The IBM SPSS Statistics 22 was used for analysis. T tests and Pearson chi-square tests were used to analyse patient characteristics. Univariate Cox regression was used to analyse the effect of synchronous metastases vs. metachronous metastases, one or more involved organs, number of metastases and different involved organs. Those factors found to be significant were analysed in a multivariate Cox-regression analysis. Cox regression was used to evaluate the effect of different treatments on 1-year survival and 2-year survival as well as overall survival. Survival was calculated using the Kaplan-Meier method and applied log-rank test to assess differences in survival rates.
Results
One hundred ninety-two patients were identified with adrenal tumour disease upon screening of provided PALGA and DTC patient lists. Adrenal metastases of other primary malignant origin, adenomas and pheochromocytomas were excluded at first screening. Finally, a total of 160 patients from the DAN centres fulfilled the inclusion criteria. Thirty-two patients were excluded: 30 patients were not diagnosed in the time period set for this study, 1 patient had an ectopic ACC and 1 patient’s file was incomplete and data not retrievable.
Patient characteristics are summarised in Table 1. The ratio female to male patients was 1.6:1. Most patients in this study had stage II disease at diagnosis (36 %) followed by stage IV disease (31 %). In 153 patients, ACC was confirmed based on histology of biopsy or tumour resection material. There were 7 patients in whom the diagnosis was solely based on the combination of clinical presentation, hormonal and imaging analysis and therefore treated as ACC (patients were not operated nor was there a biopsy performed). Median duration of follow-up was 17 months (range 0–118 months). Three patients in remission were lost to follow-up because they were referred back to their treating physician in a non-DAN hospital, and follow-up data were not available. During follow-up, 87 patients died as a direct result of ACC, 4 as a result of treatment toxicity and 2 patients died due to other causes (1 patient died of a pulmonary infection and the other patient died as a result of melanoma). At the end of follow-up, 23 patients were alive with disease and 44 patients were alive without evidence of disease.
Table 1.
Clinical characteristics of study population
| Sex | N (% of total 160) | |
|---|---|---|
| Male | 61 (38 %) | |
| Female | 99 (62 %) | |
| Age (years) | ||
| Median | 55 (Range: 19–89) | |
| Follow-up (months) | ||
| Median | 17 (Range: 0–118) | |
| Survival (months) | ||
| Median | 31 (Range: 0–118) | |
| ENS@T Stage at diagnosis | Survival median | |
| Unknown | 2 (1 %) | |
| I | 9 (6 %) | * |
| II | 58 (36 %) | 62 Months |
| III | 41 (26 %) | 32 Months |
| IV | 50 (31 %) | 7 Months |
| Metastases | ||
| None | 43 (27 %) | |
| Synchronous metastases | 84 (53 %) | |
| Metachronous metastases | 33 (21 %) | |
| At the time of first metastases | Any time during follow-up | |
| Liver | 43 (27 %) | 65 (41 %) |
| Lung | 58 (36 %) | 79 (49 %) |
| Bone | 8 (5 %) | 21 (13 %) |
| Lymph nodes under diaphragm | 32 (20 %) | 52 (33 %) |
| Lymph nodes above diaphragm | 11 (7 %) | 27 (17 %) |
| Other location | 16 (10 %) | 47 (29 %) |
*Could not be calculated
Survival
Of the 160 included patients, 117 (73 %) were diagnosed with metastases during the follow-up period of this study: 84 patients had synchronous metastases and 33 developed metachronous metastases. Figure 1 shows the Kaplan-Meier curve of the time interval between initial diagnosis and detection of the first metastasis.
Fig. 1.
Kaplan-Meier curve of the time in days between diagnosis of adrenocortical carcinoma and the diagnosis of the first metastases (n = 160). The cutoff (6 months) between synchronous and metachronous metastases is marked by the dotted line
At 2-year survival, 28 (33 %) of the patients with synchronous metastases and 19 (58 %) of the patients with metachronous metastases were still alive (p = 0.036).
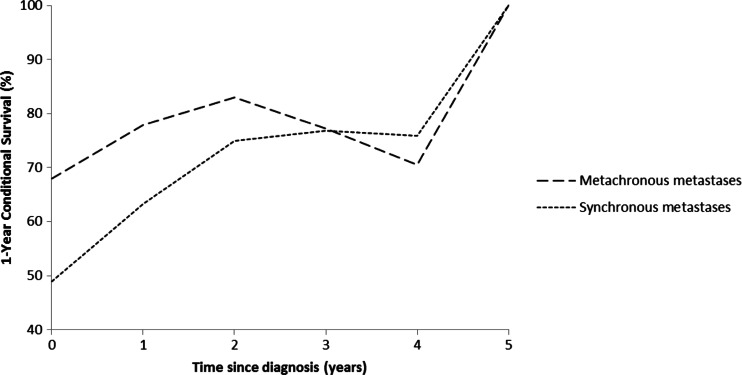
At the end of follow-up of this study, 16 patients (19 %) with synchronous and 13 patients (39 %) with metachronous metastases were still alive. Median overall survival after diagnosis of the first metastases for patients with synchronous or metachronous metastases was 12 and 29 months, respectively (p = 0.046; Fig. 2). The 1-year conditional survival for ACC patients with synchronous and metachronous metastases is presented in Fig. 3.
Fig. 2.

Overall survival in months for a synchronous (n = 84) vs. metachronous (n = 33) metastases; b 1–3 metastases (n = 40) vs. ≥4 metastases (n = 77); c 1 affected organ (n = 75) vs. ≥2 (n = 42) affected organs
Fig. 3.
One-year conditional survival CS (1|s) in ACC patients with synchronous (N = 84) and metachronous (N = 33) metastases
Upon first diagnosis of metastatic disease, 40 patients (34 %) had one to three metastases. The other 77 patients (66 %) had four or more metastases. Median survival was 44 months for patients with 1–3 metastases and 7 months for patients with ≥4 metastases (p < 0.001). Overall survival for these two groups was significantly different with 50 and 12 %, respectively (p < 0.001). Also, the number of affected organs was of influence on median survival. In 75 patients with only one affected organ, median survival was 24 months; in 42 patients with two or more involved organs, median survival was 4 months. Overall survival percentages were 33 and 10 %, respectively (p < 0.001) (Fig. 2). When analysing overall survival in a selected group of patients with ≥2 affected organs (n = 42) by the factor synchronous vs. metachronous metastases, a log-rank p value of 0.006 was found.
The results of synchronous disease, ≥2 affected organs and ≥4 metastases on 1-, 2-year and overall survival in univariate and multivariate analysis are shown in Table 2.
Table 2.
Univariate and multivariate analyses of 1-, 2-year and overall survival for synchronous metastases, ≥2 affected organs and ≥4 metastases
| 1-Year survival | 2-Year survival | Overall survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95 % CI | p | HR | 95 % CI | p | HR | 95 % CI | p | |
| Univariate analysis | |||||||||
| Synchronous metastases | .94 | .87–1.01 | 0.096 | 1.86 | 1.03–3.34 | 0.039 | 1.66 | 1.02–2.75 | 0.048 |
| ≥2 Affected organs | 3.52 | 2.025–6.134 | <0.001 | 2.95 | 1.83–4.74 | <0.001 | 2.82 | 1.82–4.35 | <0.001 |
| ≥4 Metastases | 3.30 | 1.61–6.78 | 0.001 | 3.38 | 1.85–6.2 | <0.001 | 3.26 | 1.95–5.45 | <0.001 |
| Multivariate analysis | |||||||||
| Synchronous metastases | .94 | .87–1.01 | 0.101 | 1.85 | 1.02–2.36 | 0.044 | 1.66 | .99–2.8 | 0.055 |
| ≥2 Affected organs | 2.80 | 1.46–5.35 | 0.002 | 2.19 | 1.27–3.77 | 0.005 | 2.07 | 1.2–3.42 | 0.005 |
| ≥4 Metastases | 1.80 | .76–4.09 | 0.189 | 2.15 | 1.08–4.27 | 0.029 | 2.19 | 1.22–3.94 | 0.009 |
Affected Organs and Therapy
Overall survival between subgroups with only one affected organ system (i.e., metastasis only in the lung and liver of lymph nodes) was not different.
Of the patients with only lung metastases (n = 27), 44 % received chemotherapy (50 % EDP, 42 % Streptozotocin, 8 % Cisplatin), 17 % had a metastasectomy and 89 % received mitotane. For the patients with only liver metastases (n = 20), 45 % received chemotherapy (78 % EDP, 11 % Streptozotocin, 11 % Cisplatin), 45 % had a metastasectomy, 30 % received radiofrequency ablation (RFA) and 75 % received mitotane. In the group of patients with only lymph nodes under the diaphragm (n = 14), 36 % received chemotherapy (80 % EDP, 20 % Streptozotocin), 57 % had a metastasectomy and 79 % were treated with mitotane.
One-year, 2-year and overall survival was not different between patients who received chemotherapy and those who did not in patients with only liver metastases (n = 20), only lung metastases (n = 27) or only metastases in the lymph nodes under the diaphragm (n = 14). The small number of patients treated with radiotherapy precludes separate analysis of its potential benefit.
Mitotane had a significant impact on one-year (p = 0.017), two-year (p = 0.007) and overall survival (p = 0.007) in the group of patients with only metastases in the lymph nodes under the diaphragm in comparison to those who did not receive mitotane in this group. In other subgroup analysis, mitotane showed no significant impact on survival in our population.
RFA was performed in 6 patients with only liver metastases, and none of these patients died within 2 years (p = 0.046). Overall survival, however, of this subgroup was not different from the whole group of patients with liver metastases only (p = 0.158).
Metastasectomy appeared to have a significant impact for patients with lung metastases only on overall survival (p = 0.012) in comparison to patients with lung metastases only who did not undergo a metastasectomy.
Patients with Synchronous Metastases
Amongst the patients with synchronous metastases of ACC (n = 84), 50 patients (60 %) were ENS@T stage IV ACC at primary diagnosis, 22 stage III (26 %) and 12 stage II (14 %). Median time interval until diagnosis of metastasis was 0, mean time 17 days (range 0–119 days). Age and CCI did not differ significantly between patients who presented with stage IV at initial diagnosis or stage II–III. Patients with stage IV disease at initial diagnosis had significantly more affected organs compared to patients who developed metastases within 6 months after primary diagnosis (p = 0.006). Characteristics of these two subgroups are summarised in Table 3. Diagnostic delay (time in days until diagnosis of ACC was confirmed) did not differ significantly between these subgroups (p = 0.060).
Table 3.
Patient characteristics of patients with synchronous metastases: stage IV at diagnosis vs. metastases ≤6 months but not at diagnosis
| Stage IV at diagnosis (n = 50) | Metastases ≤6 months but not at diagnosis (n = 34) | ||||
|---|---|---|---|---|---|
| Sex (ns) | |||||
| Male | 16 | 32 % | Stage II | Stage III | 50 % |
| 3 | 14 | ||||
| Female | 34 | 68 % | 9 | 8 | 40 % |
| Age (ns) | |||||
| Mean | 53 | (Range: 24–77) | 51 | (Range: 21–81) | |
| Charlson Comorbidity Index (ns) | |||||
| Mean | 1.8 | 1.4 | |||
| Survival (months, p = 0.003) | |||||
| Median | 7 | (Range: 0–114) | 23 | (Range: 0–103) | |
| Number of metastatic organs (p = 0.006) | |||||
| 1 | 25 | 50 % | 27 | 79 % | |
| ≥2 | 25 | 50 % | 7 | 21 % | |
| Number of metastases (ns) | |||||
| 1–3 Metastases | 14 | 28 % | 11 | 33 % | |
| 4+ Metastases | 36 | 72 % | 23 | 67 % | |
| Adrenalectomy (p = 0.001) | |||||
| Yes | 34 | 68 % | 33 | 97 % | |
| No | 16 | 32 % | 1 | 3 % | |
| Diagnostic delay (months, ns) | |||||
| Median | 1.3 | (Range: 0–11) | 2.1 | (Range: 0–33) | |
Significant differences between the two groups are indicated with a p value
ns not significant
The 1-year, 2-year and overall survival rates of patients who were stage IV at first presentation (n = 50) and of those who developed stage IV within 6 months after the initial primary diagnosis (n = 34) were significantly different (Table 3). Median survival was 7 and 23 months, respectively (p = 0.003).
Sixty-seven of the synchronous metastasized patients did undergo an adrenalectomy for their primary tumour, whereas in 17 patients the primary tumour was not resected. All but 1 of the patients who did not undergo an adrenalectomy (n = 17) had stage IV disease at the moment of diagnosis of ACC. Patients with only one synchronous affected organ were more frequently submitted to a surgical resection of the primary adrenal tumour (p = 0.023). Of the 67 patients undergoing an adrenalectomy, 22 patients also had a metastasectomy. In contrast, of the 17 patients with synchronous metastases not undergoing an adrenalectomy, only 2 patients had a diagnostic metastasectomy to confirm ACC and determine the therapeutic strategy.
Median survival of patients with synchronous metastases who did or did not undergo adrenalectomy was 16.6 (range 0–113.8 months) and 1.8 months (range 0–16.5 months), respectively (p < 0.001) (Fig. 4). This result did not change after correction for number of affected organs and CCI.
Fig. 4.
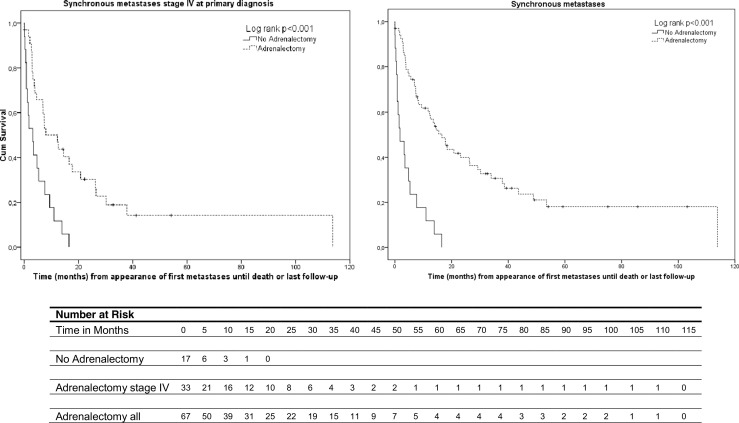
a Overall survival in ACC patients with synchronous metastases and stage IV at primary diagnosis treated with adrenalectomy (n = 50; 34 underwent an adrenalectomy, 16 did not) p < 0.001. b Overall survival in all ACC patients with synchronous metastases (n = 84; 67 underwent an adrenalectomy, 17 did not) p < 0.001
Discussion
In different types of cancer, the moment metastases occur has been shown to have a significant impact on survival. Synchronous metastases, defined as metastases originating within 6 months after diagnosis of the primary tumour, are associated with a significant negative effect on prognosis in the colon [21] and lung cancer [22]. A trend towards better survival in ACC patients with metachronous metastases compared to synchronous metastases has previously been described [10]. To our knowledge, this is the first study to describe that timing of metastases is associated with overall survival in ACC. Late synchronous metastases, i.e. development of stage IV disease within 6 months after stage II–III at primary diagnosis, were associated with improved survival compared to patients that present with stage IV disease.
The number of affected organs has been considered a prognostic factor for survival in metastatic ACC [10, 23]. Our results confirm that the number of affected organs is indeed a prognostic factor.
Earlier studies evaluating the relationship between the number of metastatic lesions and survival have been inconclusive: In a cohort of 124 patients with ACC, Asssie et al. identified the presence of more than 5 metastatic lesions or the involvement of more than two organs as predictive factors for survival [10]. In the study by den Winkel et al., number of metastases, synchronous vs. metachronous metastatic disease and lymph node involvement did not significantly influence survival in a small cohort of 24 patients [24]. Our study shows a significant negative effect of having ≥4 metastases on survival, suggesting that an increasing number of metastases is prognostically unfavourable. Moreover, our data suggest that adrenal cancer spread to the lymph nodes affects survival to the same extent as hematogenic metastases. These results support a modified ENS@T classification observed heterogeneity in survival of patients with stage IV ACC [25].
Although it has been suggested that oligometastatic disease is associated with more favourable behaviour than extended metastatic disease, this has not resulted in a tailored treatment approach in ACC [26]. The low efficacy of current treatment options for ACC [27] raises the question whether patients with limited metastatic disease might benefit from a different approach. Recent research shows that a radical adrenalectomy and metastasectomy in patients with synchronous, limited metastatic disease, resulted in a more favourable prognosis [28]. We found that a metastasectomy for ACC patients with lung metastases was associated with significant better overall survival. It has been suggested that patients with locally recurrent ACC also benefit from surgical treatment, but we did not examine the impact of local adrenal recurrence on prognosis [29]. Previous studies demonstrated a better prognosis in patients with a time to local recurrence longer than 1 year, and those in whom a complete resection of the local recurrence was performed [30].
In this study, adrenalectomy had a strong influence on survival, even in the presence of metastases. Patients with only one synchronous affected organ more frequently underwent an adrenalectomy than patients with two or more affected organs. Of notice, we were unable to find any other difference in clinical characteristics between patients who did undergo an adrenalectomy and those who did not. We had expected that patients with a bad performance status at disease presentation would have been less likely to have undergone surgical resection. The improved survival that is associated with surgical intervention [31, 32] might overrule decision-making based on performance status and stage of disease in this group to a certain extent.
We found that patients who presented with stage IV disease at diagnosis had a much shorter survival than patients with stage II–III disease at diagnosis who developed late synchronous metastases. Thus, even within the group of synchronous metastases, a difference in survival was observed between early and late metastases. Future analysis should focus on identifying this specific subgroup of patients with ACC which exhibits less aggressive behaviour, who may benefit from adrenalectomy [33].
Our study is limited because of its retrospective nature. There were missing data, especially pathological data. The present study, however, was performed in a relatively large and well-defined cohort of adrenocortical cancer patients that reliably reflects the Dutch ACC population as a whole [1].
Current treatment options in ACC are limited, and new therapeutic options are anticipated [34]. Metachronous metastases are associated with better overall survival. Local therapy directed at metastasis should be considered in patients with limited metachronous disease. Otherwise, mitotane in combination with chemotherapy according to the FIRM-ACT protocol is indicated in case of more extended metachronous or synchronous metastases [34], although the latter patient group often has a bad prognosis despite systemic therapy [27]. The role of other local therapies already implemented in other malignancies has not been thoroughly investigated in ACC. Stereotactic radiotherapy for lung metastases could be a promising alternative to surgical intervention. Radiofrequency ablation (RFA) for liver metastases, as a local-regional treatment with relatively little morbidity, should be further investigated as a therapeutic option in the treatment of selected cases of liver metastases in ACC.
In conclusion, synchronous metastases of ACC are associated with a worse prognosis compared to metachronous metastases of ACC and patients with late synchronous metastases have a better prognosis than those with metastases at initial diagnosis. Further refinement of ENSAT stage IV disease should be considered, as these prognostic differences could be taken into account when determining the optimal treatment for a patient with ACC. Number of affected organs, number of metastases and timing of development of metastases influence survival and could potentially be considered in therapeutic decision making.
Acknowledgments
We would like to express our gratitude to all the members of the Dutch Adrenal Network for their contribution to the database. Special thanks goes to TMA Kerkhofs and A Pastoors for their significant assistance with the data collection.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
On behalf of the Dutch Adrenal Network
References
- 1.Kerkhofs TMA, Verhoeven RH, Van der Zwan JM, Dieleman J, Kerstens MN, Links TP, Van de Poll-Franse LV, Haak HR. Adrenocortical carcinoma: a population-based study on incidence and survival in the netherlands since 1993. Eur J Cancer. 2013;49(11):2579–2586. doi: 10.1016/j.ejca.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 2.Pommier RF, Brennan MF. An eleven-year experience with adrenocortical carcinoma. Surgery. 1992;112:963–963. [PubMed] [Google Scholar]
- 3.Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, Rossetto R, Buci L, Sperone P, Grossrubatscher E, Reimondo G, Bollito E, Papotti M, Saeger W, Hahner S, Koschker AC, Arvat E, Ambrosi B, Loli P, Lombardi G, Mannelli M, Bruzzi P, Mantero F, Allolio B, Dogliotti L, Berruti A. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356(23):2372–2380. doi: 10.1056/NEJMoa063360. [DOI] [PubMed] [Google Scholar]
- 4.Koschker AC, Fassnacht M, Hahner S, Weismann D, Allolio B. Adrenocortical carcinoma—improving patient care by establishing new structures. Exp Clin Endocrinol Diabetes. 2006;2:45–51. doi: 10.1055/s-2006-923808. [DOI] [PubMed] [Google Scholar]
- 5.Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab. 2009;23(2):273–289. doi: 10.1016/j.beem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Lafemina J, Brennan MF. Adrenocortical carcinoma: past, present, and future. J Surg Oncol. 2012;106(5):586–594. doi: 10.1002/jso.23112. [DOI] [PubMed] [Google Scholar]
- 7.Glover AR, Ip JC, Zhao JT, Soon PS, Robinson BG, Sidhu SB. Current management options for recurrent adrenocortical carcinoma. Oncol Targets Ther. 2013;6:635–643. doi: 10.2147/OTT.S34956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mekenkamp LJ, Koopman M, Teerenstra S, van Krieken JH, Mol L, Nagtegaal ID, Punt CJ. Clinicopathological features and outcome in advanced colorectal cancer patients with synchronous vs metachronous metastases. Br J Cancer. 2010;103(2):159–164. doi: 10.1038/sj.bjc.6605737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howell GM, Carty SE, Armstrong MJ, Stang MT, McCoy KL, Bartlett DL, Yip L. Outcome and prognostic factors after adrenalectomy for patients with distant adrenal metastasis. Ann Surg Oncol. 2013;20(11):3491–3496. doi: 10.1245/s10434-013-3050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assie G, Antoni G, Tissier F, Caillou B, Abiven G, Gicquel C, Leboulleux S, Travagli JP, Dromain C, Bertagna X, Bertherat J, Schlumberger M, Baudin E. Prognostic parameters of metastatic adrenocortical carcinoma. J Clin Endocrinol Metab. 2007;92(1):148–154. doi: 10.1210/jc.2006-0706. [DOI] [PubMed] [Google Scholar]
- 11.Hermsen IG, Kerkhofs TMA, Butter G, Kievit J, van Eijck CH, Nieveen van Dijkum EJ, Haak HR, Dutch Adrenal Network Surgery in adrenocortical carcinoma: importance of national cooperation and centralized surgery. Surgery. 2012;152(1):50–56. doi: 10.1016/j.surg.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Kerkhofs TMA, Verhoeven RH, Bonjer HJ, van Dijkum EJ, Vriens MR, De Vries J, Van Eijck CH, Bonsing BA, Van de Poll-Franse LV, Haak HR, Network DA. Surgery for adrenocortical carcinoma in the netherlands: analysis of the national cancer registry data. Eur J Endocrinol. 2013;169(1):83–89. doi: 10.1530/EJE-13-0142. [DOI] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 15.Hieke S, Kleber M, Konig C, Engelhardt M, Schumacher M. Conditional survival: a useful concept to provide information on how prognosis evolves over time. Clin Cancer Res. 2015;21(7):1530–1536. doi: 10.1158/1078-0432.CCR-14-2154. [DOI] [PubMed] [Google Scholar]
- 16.Zamboni BA, Yothers G, Choi M, Fuller CD, Dignam JJ, Raich PC, Thomas CR, Jr, O’Connell MJ, Wolmark N, Wang SJ. Conditional survival and the choice of conditioning set for patients with colon cancer: an analysis of NSABP trials C-03 through C-07. J Clin Oncol. 2010;28(15):2544–2548. doi: 10.1200/JCO.2009.23.0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zabor EC, Gonen M, Chapman PB, Panageas KS. Dynamic prognostication using conditional survival estimates. Cancer. 2013;119(20):3589–3592. doi: 10.1002/cncr.28273. [DOI] [PubMed] [Google Scholar]
- 18.van Houwelingen H, Putter H (2011) Dynamic prediction in clinical survival analysis. CRC Press
- 19.Turanli S. Importance of the development time of isolated bone metastasis in breast cancer. Langenbecks Arch Surg. 2012;397(6):967–972. doi: 10.1007/s00423-009-0561-1. [DOI] [PubMed] [Google Scholar]
- 20.Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8(6):378–382. doi: 10.1038/nrclinonc.2011.44. [DOI] [PubMed] [Google Scholar]
- 21.Kumar R, Price TJ, Beeke C, Jain K, Patel G, Padbury R, Young GP, Roder D, Townsend A, Bishnoi S, Karapetis CS. Colorectal cancer survival: an analysis of patients with metastatic disease synchronous and metachronous with the primary tumor. Clin Colorectal Cancer. 2014;13(2):87–93. doi: 10.1016/j.clcc.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Tanvetyanon T, Robinson LA, Schell MJ, Strong VE, Kapoor R, Coit DG, Bepler G. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small-cell lung cancer: a systematic review and pooled analysis. J Clin Oncol. 2008;26(7):1142–1147. doi: 10.1200/JCO.2007.14.2091. [DOI] [PubMed] [Google Scholar]
- 23.Malandrino P, Al Ghuzlan A, Castaing M, Young J, Caillou B, Travagli JP, Elias D, de Baere T, Dromain C, Paci A, Chanson P, Schlumberger M, Leboulleux S, Baudin E. Prognostic markers of survival after combined mitotane- and platinum-based chemotherapy in metastatic adrenocortical carcinoma. Endocr Relat Cancer. 2010;17(3):797–807. doi: 10.1677/ERC-09-0341. [DOI] [PubMed] [Google Scholar]
- 24.op den Winkel J, Pfannschmidt J, Muley T, Grunewald C, Dienemann H, Fassnacht M, Allolio B. Metastatic adrenocortical carcinoma: results of 56 pulmonary metastasectomies in 24 patients. Ann Thorac Surg. 2011;92(6):1965–1970. doi: 10.1016/j.athoracsur.2011.07.088. [DOI] [PubMed] [Google Scholar]
- 25.Libe R, Borget I, Ronchi CL, Zaggia B, Kroiss M, Kerkhofs T, Bertherat J, Volante M, Quinkler M, Chabre O, Bala M, Tabarin A, Beuschlein F, Vezzosi D, Deutschbein T, Borson-Chazot F, Hermsen I, Stell A, Fottner C, Leboulleux S, Hahner S, Mannelli M, Berruti A, Haak H, Terzolo M, Fassnacht M, Baudin E, ENSAT network Prognostic factors in stage III-IV adrenocortical carcinomas (ACC): an european network for the study of adrenal tumor (ENSAT) study. Ann Oncol. 2015;26(10):2119–2125. doi: 10.1093/annonc/mdv329. [DOI] [PubMed] [Google Scholar]
- 26.Huang F, Wu G, Yang K. Oligometastasis and oligo-recurrence: more than a mirage. Radiat Oncol. 2014;9:230-014–0230-6. doi: 10.1186/s13014-014-0230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S, Schade-Brittinger C, Lacroix A, Jarzab B, Sorbye H, Torpy DJ, Stepan V, Schteingart DE, Arlt W, Kroiss M, Leboulleux S, Sperone P, Sundin A, Hermsen I, Hahner S, Willenberg HS, Tabarin A, Quinkler M, de lF, Schlumberger M, Mantero F, Weismann D, Beuschlein F, Gelderblom H, Wilmink H, Sender M, Edgerly M, Kenn W, Fojo T, Müller H, Skogseid B (2012) Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med
- 28.Dy BM, Strajina V, Cayo AK, Richards ML, Farley DR, Grant CS, Harmsen WS, Evans DB, Grubbs EG, Bible KC, Young WF, Perrier ND, Que FG, Nagorney DM, Lee JE, Thompson GB (2014) Surgical resection of synchronously metastatic adrenocortical cancer. Ann Surg Oncol [DOI] [PubMed]
- 29.Dy BM, Wise KB, Richards ML, Young WF, Jr, Grant CS, Bible KC, Rosedahl J, Harmsen WS, Farley DR, Thompson GB. Operative intervention for recurrent adrenocortical cancer. Surgery. 2013;154(6):1292–9. doi: 10.1016/j.surg.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 30.Erdogan I, Deutschbein T, Jurowich C, Kroiss M, Ronchi C, Quinkler M, Waldmann J, Willenberg HS, Beuschlein F, Fottner C, Klose S, Heidemeier A, Brix D, Fenske W, Hahner S, Reibetanz J, Allolio B, Fassnacht M, German Adrenocortical Carcinoma Study Group The role of surgery in the management of recurrent adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98(1):181–191. doi: 10.1210/jc.2012-2559. [DOI] [PubMed] [Google Scholar]
- 31.Livhits M, Li N, Yeh MW, Harari A. Surgery is associated with improved survival for adrenocortical cancer, even in metastatic disease. Surgery. 2014;156(6):1531–40. doi: 10.1016/j.surg.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dy BM, Strajina V, Cayo AK, Richards ML, Farley DR, Grant CS, Harmsen WS, Evans DB, Grubbs EG, Bible KC, Young WF, Perrier ND, Que FG, Nagorney DM, Lee JE, Thompson GB. Surgical resection of synchronously metastatic adrenocortical cancer. Ann Surg Oncol. 2015;22(1):146–151. doi: 10.1245/s10434-014-3944-7. [DOI] [PubMed] [Google Scholar]
- 33.Hermsen IG, Gelderblom H, Kievit J, Romijn JA, Haak HR. Extremely long survival in six patients despite recurrent and metastatic adrenal carcinoma. Eur J Endocrinol. 2008;158(6):911–919. doi: 10.1530/EJE-07-0723. [DOI] [PubMed] [Google Scholar]
- 34.Kerkhofs TMA, Ettaieb MH, Hermsen IG, Haak HR (2015) Developing treatment for adrenocortical carcinoma. Endocr Relat Cancer [DOI] [PubMed]