Abstract
Oncogene-induced senescence (OIS) explains the phenomenon of cellular senescence triggered by the action of oncogenes. It is a mechanism adopted by a cell to inhibit progression of benign tumors into malignancy, occurs in premalignant lesions, and is almost never present in malignant lesions. BRAF mutations occur in about 40–45% of all papillary thyroid carcinomas (PTCs) and of which 99.7% is the BRAFV600E mutation. A unique phenotype of the BRAFV600E mutation is the upregulation of the thyroid-stimulating hormone receptor (TSHR) on thyrocyte membranes. Despite the overexpression of the receptor, BRAFV600E cells undergo cell cycle arrest leading to OIS via a negative feedback signaling mechanism. A simultaneous increase in serum thyroid-stimulating hormone (TSH) in response to hypothyroidism (common in autoimmune diseases such as Hashimoto’s thyroiditis) would cause senescent tumor cells to overcome OIS and proceed towards malignancy, hence showing the importance of TSH/TSHR signaling in the development of PTCs. Increase in TSH/TSHR signaling triggers an increase in levels of downstream enzymes such as manganese superoxide dismutase (MnSOD) and dual-specific phosphatase 6 (DUSP6) which eventually results in the production of oncogenic proteins such as c-Myc. Therefore, the detection of these genetic alterations as effective biomarkers for premalignant lesions of PTC is important in clinical settings and techniques such as polymerase chain reaction-mediated restriction fragment length polymorphism (PCR-RFLP) and real-time PCR can be used to detect the BRAFV600E point mutation and overexpression of TSHR, MnSOD, and DUSP6, respectively.
Keywords: Papillary Thyroid Carcinomas (PTC), BRAFV600E Mutation, Manganese Superoxide Dismutase (MnSOD), Thyroid-stimulating Hormone Receptor (TSHR), Dual Specificity Phosphatase (DUSP6)
Introduction
Oncogene-induced senescence (OIS) and apoptosis play an important role in suppressing tumorigenesis by inhibiting proliferation of cells at risk of malignant transformation [1]. As its name implies, OIS is a type of cellular senescence which is not triggered in normal cells, but in cells that possess a proto-oncogene transformed into an oncogene.
Serrano and coworkers (1997) set the first indication of the mechanism of OIS through in vitro studies which demonstrated that oncogenic RAS had the capability of triggering permanent G1 arrest in primary human or rodent cells. This growth arrest was accompanied by the accumulation of p53 and p16 and the inactivation of either p53 or p16 resulted in the overcome of RAS-induced growth arrest in rodent cells [2].
OIS is phenotypically indistinguishable from normal cellular senescence. Senescence was originally defined for primary fibroblasts which had a limited proliferative capacity and determined genetics. At the twilight of their lifespan, these cells undergo morphological and physiological changes together with alterations in gene expression resulting in permanent cease of proliferation [2]. These in vitro findings soon led to the speculation that in vivo mechanisms too must exist as a phenomenon of proliferative arrest in benign tumors [3–6].
OIS As a Prognostic Indicator of Malignancy
OIS is the mechanism of a cell to inhibit malignant transformation of benign tumors and is a method of natural tumor suppression [7]. It occurs early in tumorigenesis, hence senescent tumor cells are abundant in premalignant lesions but almost absent in malignant tumors [8]. The fact that OIS occurs in early stages of malignancy suggests that it restricts growth of oncogenically stressed cells, thus limiting the tumor to a non-aggressive, premalignant state. Hence, the detection of proteins/genes involved in OIS would serve as a useful biomarker to detect premalignant lesions enabling cancer screening. A single oncogene is insufficient to trigger senescence and also insufficient to generate malignant tumors (Fig. 1). Therefore, oncogenic stress increases progressively during tumorigenesis and at one point, when benign tumors have already been initiated but not yet reached their full malignant phenotype, senescence is triggered [8]. According to Michaloglou and coworkers (2005), the overexpression of BRAFV600E in cultured human melanocytes causes growth arrest [9].
Fig. 1.
Mechanism of how cells/thyrocytes overcome OIS. a Oncogene activation in normal cells can lead to OIS. In order to overcome OIS and initiate tumorigenesis, further oncogene activation or tumor suppressor gene inactivation is required. b In thyrocytes, the BRAFV600E mutation results in OIS. But the mutation causes overexpression of TSHR on thyrocyte membranes. Hence, OIS can be overcome by increased TSH/TSHR signaling leading to tumorigenesis
BRAFV600E Mutation in Papillary Thyroid Carcinoma
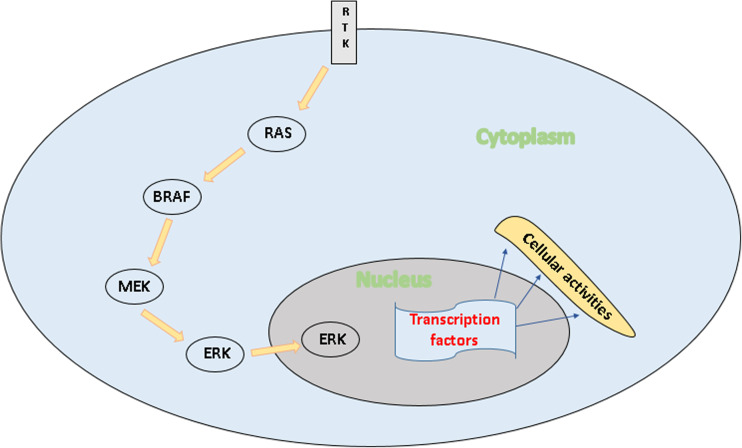
The BRAFV600E mutation is prevalent in the kinase domain (exon 15) of the protein which the BRAF gene encodes. According to the current consensus, the frequency of occurrence of BRAF mutations in papillary thyroid carcinoma (PTC) is about 40–45% of all PTC patients and the occurrence of the BRAFV600E mutation is about 99.7% among BRAF-mutated PTCs [10]. The BRAF gene belongs to a class of genes know as oncogenes, which when mutated, have the potential to transform normal cells into malignant cells. This proto-oncogene encodes a serine/threonine kinase BRAF which belongs to the family of RAF proteins which are intracellular effectors of the mitogen-activated protein kinase (MAPK) signaling cascade and helps to transmit signals from the outside of the cell to the cell nucleus. This signal transduction pathway called MAPK pathway or mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathway is important for growth and cell proliferation, differentiation, cell movement, and apoptosis. RAS binding and protein recruitment to the cell membrane triggers the activation of these kinases which then phosphorylate and activate MAPK/ERK kinase (MEK) which in turn activates ERK and downstream effectors of the MAPK signaling cascade (Fig. 2) [11].
Fig. 2.
Schematic diagram of the involvement of BRAF in the MAPK/ERK signaling cascade. RTK receptor tyrosine kinase. RTK receives mitogenic stimulus which activates RAS. RAS binds and phosphorylates BRAF which in turn binds and phosphorylates MEK. MEK activates ERK which translocates to the nucleus to initiate transcription of growth-regulatory genes by binding to transcription factors
Almost all the point mutations of the BRAF gene involve nucleotide 1799 which results in a single-base change from thymine to adenine at the 1799th position of the coding sequence of the BRAF gene, abbreviated as T1799A which corresponds to a subsequent change in amino acid at the 600th codon in the translated protein, replacing valine with glutamic acid, hence V600E.
The mutation results in the constitutive activation of the mutant BRAF protein which exhibits increased kinase activity and also results in the constitutive activation of downstream signaling pathways leading to increased cell proliferation [12]. In the dephosphorylated state, the wild-type BRAF protein possesses hydrophobic interactions between the interaction loop and the ATP-binding site, which maintains the protein in its inactive conformation. The V600E substitution disrupts these hydrophobic interactions and enables the formation of new interactions which keep the protein in a catalytically active conformation [11].
Origin of PTC
The presence of the BRAFV600E mutation in thyrocytes causes increased expression of the thyroid-stimulating hormone receptor (TSHR) on the thyrocyte membrane and also causes cells to enter senescence. In conditions which cause hypothyroidism such as Hashimoto’s thyroiditis that are known to increase serum thyroid-stimulating hormone (TSH) levels, these BRAF-mutated thyrocytes overcome OIS via TSH/TSHR signaling resulting in full-blown PTC.
The thyroid gland contains two main types of cells, thyrocytes (follicular cells) and parafollicular cells (C cells). Follicular cells synthesize thyroid hormone, and excess production of it results in a condition called hyperthyroidism. In contrast, hypothyroidism arises due to the reduced production of thyroid hormone. Hypothyroidism occurs in Hashimoto’s thyroiditis due to the destruction of follicular epithelial cells. The excessive or reduced production of thyroid hormone is regulated by TSH secreted by the pituitary gland. Parafollicular cells produce the hormone calcitonin that regulates calcium metabolism in the body [13].
Thyroid enlargement is medically referred to as a goiter. Both tumors and non-tumoral conditions result in enlargement of the thyroid gland including Hashimoto’s thyroiditis. Tumors in the thyroid gland can be categorized as benign (non-cancerous) or malignant (cancerous). Malignant tumors arise from follicular and parafollicular cells having undergone changes that enable them to divide and proliferate abnormally (malignant transformation). These cancers metastasize compared to their benign counterparts. The main types of thyroid carcinomas arising from malignant tumors are (i) differentiated thyroid carcinoma, which includes PTC, follicular thyroid carcinoma (FTC), and Hurthle cell carcinoma (HCC), (ii) medullary thyroid carcinoma (MTC), and (iii) anaplastic thyroid carcinoma (ATC) (aggressive undifferentiated tumor) [13]. PTC, FTC, HCC, and ATC arise from follicular cells (thyrocytes) whereas MTC arises from parafollicular cells. The differences in these cancers have been subjected to extensive studies as they affect the seriousness of the cancer and the type of treatment required.
Although PTC is generally a slow-growing tumor type with a good prognosis, aggressive forms with local invasion or distant metastasis which are rare may occur [14].
MAPK Signaling Pathway: the Cell’s Fundamental Signaling Cascade
MAPK pathways are evolutionarily conserved kinase modules that link extracellular signals to the machinery that controls fundamental cellular processes such as growth, proliferation, differentiation, migration, and apoptosis. These pathways comprise of a three-tier kinase module (MAP3K-MAP2K-MAPK) in which eventually, a MAPK is activated upon phosphorylation by a mitogen-activated protein kinase kinase (MAPKK) [15].
According to the current consensus, tumorigenesis requires deregulation of at least six cellular processes [16] and cancer cells should acquire the following capabilities: independence of proliferation signals, evasion of apoptosis, insensitivity to anti-growth signals, unlimited replicative potential, ability to invade and metastasize, ability to attract and sustain angiogenesis for nutrient supply, acquisition of drug resistance, and avoidance of OIS. Abnormalities in MAPK signaling pathways affect some, if not all these processes and play a critical role in the development and progression of cancer [17]. The BRAF protein plays a key role in the MAPK pathway and thus mutant BRAF protein could deregulate the MAPK pathway to increase cell proliferation and initiate tumorigenesis. However, in thyrocytes, the BRAFV600E mutation triggers BRAFV600E-induced senescence rather than overgrowth. These cells do not undergo malignant transformation under normal TSH levels.
BRAFV600E-Induced Senescence
BRAFV600E mutation promotes dedifferentiation of thyrocytes in PTC by downregulation of genes involved in thyroid hormone synthesis [18]. The BRAF oncogene is known to induce senescence-like growth inhibitory responses when ectopically expressed in normal cells such as thyrocytes, human dermal fibroblasts, and melanocytes. This phenomenon is known as OIS and is triggered by the tumor suppressor gene p53 in the face of abnormal/aberrant RAF/MAPK/ERK activation. In order to undergo malignant transformation, this OIS must be overcome by additional stimuli such as tumor suppressor gene inactivation, additional oncogene activation, and inactivation of aberrant RAF/MAPK/ERK signaling [19]. Increased expression of the p53 tumor suppressor gene was demonstrated in tumor transplants undergoing OIS while the repression of the p53 gene through inactivation has been shown to bring about reversion of OIS, enabling tumor transplants to grow in mice resulting in ATC [18].
A study conducted by Cisowski and coworkers (2015) demonstrated the mutual exclusivity of BRAFV600E and KRASG12D mutations in tumorigenesis. Their results suggested that the expression of physiological levels of both oncogenes BRAF and KRAS hyperactivates AKT and ERK signaling pathways but reduces proliferation by activating certain cell cycle inhibitory proteins. Therefore, a benign tumor cell with the BRAFV600E mutation which later acquires the KRASG12D mutation would likely senesce and would be outcompeted by mutant cells with a single mutation [20].
Other rare mutations which involve BRAF activation in PTC include K601E point mutation, small in-frame insertions or deletions surrounding codon 600, and AKAP9-BRAF rearrangement which is found to be more common in PTCs associated with radiation exposure [11].
Activated oncogenes, when expressed in primary cells, block cell proliferation by inducing senescence or apoptosis. Both these mechanisms play an important role in suppressing tumorigenesis in vivo by preventing the risk of malignant transformation via cellular proliferation [21].
TSH Signaling Overcomes OIS
Another interesting phenomenon which has been highlighted in a study conducted by Kim and coworkers (2014) that provokes the initiation of PTC is the upregulation of TSHR on thyrocyte membranes [19]. Overexpression of TSHR is a unique phenotype of BRAFV600E PTC which was previously demonstrated by a study which showed elevated TSHR expression in PTCs and poorly differentiated carcinomas by immunohistochemical analysis as well as real-time PCR when compared to undifferentiated carcinomas [22]. Animal studies have suggested the importance of TSH signaling to overcome BRAFV600E-induced senescence and promote thyroid tumorigenesis [19].
As mentioned previously, the BRAFV600E mutation is said to be responsible for OIS, which inhibits malignant transformation of cells. However, studies on transgenic mice have shown that OIS cannot be overcome if TSH levels are within normal range [19]. In mice with normal serum TSH levels or blocked TSH signaling, it was found that tumorigenesis may occur but is significantly delayed, resulting in small and localized benign tumors [18].
Hence, TSH signaling can overcome BRAFV600E-induced senescence and initiate tumorigenesis. However, this could only occur if there is an upregulation of TSHR in thyrocytes, which is stimulated by the BRAFV600E mutation. For example, autoimmune diseases such as Hashimoto’s thyroiditis result in hypothyroidism, where TSH levels increase in order to compensate for the reduced function of the thyroid gland. Hence, if such an individual possesses the BRAFV600E mutation, the coupled effect of increased TSH and overexpression of TSHR on thyrocyte membranes results in inactivation of BRAFV600E-induced senescence leading to full-blown PTC.
It is a proven fact that well-differentiated thyroid carcinomas express TSHR suggesting that TSH can act as a cancer stimulus. Several previous studies have demonstrated that serum TSH concentration at presentation, even when within normal range, can act as an independent predictor of the prevalence of thyroid malignancy (Table 1). Incidence of PTC in patients with autoimmune disease of the thyroid such as Hashimoto’s thyroiditis and Graves’ disease is supportive of the role of TSHR in cancer progression. Some experts suggest that TSHR stimulation is associated with increased cancer risk and aggressiveness despite the negative data suggesting a reciprocal relationship between TSHR mRNA and cancer aggressiveness. Because Hashimoto’s thyroiditis is associated with hypothyroidism which results in elevated TSH and Graves’ disease is associated with TSHR stimulation, the link between autoimmune thyroid diseases and thyroid carcinoma may be the TSH receptor [27].
Table 1.
Variation of percentage prevalence of thyroid malignancy with serum TSH concentration at presentation
| Serum TSH concentration (mIU/l)a | Prevalence of malignancy (%) | Reference |
|---|---|---|
| < 0.4 | 2.8 | Boelaert et al. [23] |
| 0.4–0.9 | 3.7 | |
| 1.0–1.7 | 8.3 | |
| 1.8–5.5 | 12.3 | |
| > 5.5 | 29.6 | |
| < 0.06 | 16 | Haymart et al. [24] |
| 0.4–1.39 | 25 | |
| 1.4–4.99 | 35 | |
| ≥ 5.00 | 52 | |
| < 0.4 | 8.0 | Polyzos et al. [25] |
| 0.4–0.8 | 5.0 | |
| 0.9–1.4 | 7.9 | |
| 1.5–4.0 | 18.2 | |
| > 4.0 | 5.3 | |
| ≤ 0.3 | 5.8 | Shi et al. [26] |
| 0.3–1.0 | 10.0 | |
| 1.0–1.9 | 15.3 | |
| 1.9–4.8 | 21.6 | |
| > 4.8 | 36.1 |
aMilli-international units/l
Furthermore, the study conducted on the thyroid tissue of rats by Nishida and coworkers (1997) showed an elevated level of manganese superoxide dismutase (MnSOD) in thyroid tissues administered with TSH and in cultured thyrocytes in medium supplemented with TSH [28]. Similarly, Kim and coworkers (2014) reported that an increased level of TSH is associated with an increase in expression of genes that encode for scavenger proteins of reactive oxygen species (ROS), such as MnSOD in thyrocytes possessing the BRAFV600E mutation (due to increased TSH/TSHR signaling) and hence enhancing the RAS/AKT signaling pathway that leads to increased cell proliferation. It was found that OIS was due to a compensatory mechanism of the p53 gene against BRAFV600E-driven tumor progression and increased TSH signaling can overcome BRAFV600E-induced senescence by downregulating the p53 gene resulting in upregulation of the AKT pathway, leading to tumor progression [19].
BRAFV600E Influences Expression of Key Genes in Thyrocytes
In a study conducted by Franco and coworkers (2011), BRAFV600E knock-in mice were found to be profoundly hypothyroid due to the downregulation of key genes required for iodine transport and thyroid hormone synthesis. Thus, mice with PTC harboring the BRAF mutation had lower expression of thyroglobulin (Tg), thyroid peroxidase (TPO), and Na-I symporter (NIS) compared to mice with PTC that did not harbor the mutation [29]. Therefore, this could also be a reason for BRAFV600E to contribute to growth cycle arrest and senescence.
Rosignolo and coworkers (2015) analyzed the effect of the BRAFV600E mutation on the expression of the thyroid hormone-β receptor (TRβ) using malignant tumor tissue and contralateral non-tumor (normal) tissue collected from 36 patients immediately after thyroidectomy by dividing them into two groups based on the presence or absence of the BRAFV600E mutation. The analysis of the level of gene expression of TRβ in these malignant tumor tissues with respect to their non-tumor counterparts using real-time PCR indicated that the levels of TRβ were downregulated in all PTCs irrespective of the presence or absence of the BRAFV600E mutation demonstrating the downregulation of TRβ as a common feature of PTCs and is not associated with the aggressiveness of PTCs [30]. The functional role of TRβ in thyroid tumorigenesis was further supported by the work carried out by Kim and coworkers (2013) demonstrating the reactivation of silenced TRβ gene expression in thyroid cancer cells when treated with demethylating agents that lead to delayed thyroid tumor progression [31].
On the other hand, the work carried out by Romitti and coworkers (2012) on mRNA and protein expression, and activity of the thyroid hormone-inactivating type 3 deiodinase gene (DIO3) in PTCs demonstrated that DIO3 expression is induced in PTC and this induction is likely to be driven by the constitutive activation of the MAPK signaling pathway. Furthermore, in the same study, it was shown that PTC cells possessing BRAFV600E (which exhibit elevated MAPK pathway) had the highest levels of DIO3 mRNA and activity suggesting that this may be involved in aggressive PTC tumors [32] and tumorigenesis is affected by the alterations in levels of DIO3 by changing intracellular concentrations of thyroid hormone to interfere with cell proliferation and differentiation [33].
Moreover, the work carried out by Cantara and coworkers (2012) on the effect of an E3 ubiquitin protein ligase praja2 (product of a novel cancer-related gene) in the progression of thyroid carcinoma found praja2 to be markedly overexpressed in differentiated PTCs, reduced in less differentiated variants, and almost undetectable in ATCs. Generally, the expression of praja2 is regulated by cAMP/PKA signaling and the overexpression of praja2 is associated with increased cAMP/PKA signaling and hyperactivated MAPK pathway which is a characteristic of BRAF-mutated and RET fusion PTCs. As expected, praja2 expression was increased in differentiated histotypes but showed a progressive decline in less differentiated histotypes such as ATCs due to the lack of association of ATC with BRAF and RET mutations [34]. Despite the increased and decreased expressions of praja2 associated with differentiated and less differentiated histotypes, the role of praja2 in BRAFV600E-induced senescence is yet to be clearly understood.
Furthermore, in another study conducted by Zou and coworkers (2015) on mice harboring either the KRASG12D or BRAFV600E mutation, the expression of Sprouty1 (SPRY1) was analyzed to determine its role in thyroid carcinogenesis in addition to the effect of long-term TSH stimulation in KRASG12D knock-in mice. The Sprouty (SPRY) family of proteins is generally involved in negative feedback regulation of the MAPK pathway and in this study, it was observed that KRASG12D mice stimulated with TSH developed FTC through activation of the PI3K/AKT signaling pathway and mice with BRAFV600E developed PTC through activation of both PI3K/AKT and MAPK/ERK signaling pathways. In the same study, expression analysis based on real-time PCR showed that the SPRY1 expression was significantly higher in mutant compared to that of wild-type mice for KRAS in contrast to that of the mutant and wild-type mice for BRAF that showed significantly lower level of SPRY1 expression for the former compared to the latter. Using the outcomes of this study, it was suggested that (i) the initiation of FTC is associated with activation of the PI3K/AKT pathway through the activation of p-AKT and reduced expression of p-ERK by the increased expression of SPRY1 in KRASG12D cells when treated with TSH, (ii) the initiation of PTC (instead of FTC) is associated with the activation of both MAPK/ERK and PI3K/AKT pathways through the increased expression of both p-ERK and p-AKT by the reduced expression of SPRY1 in BRAFV600E cells, and (iii) the SPRY1 acts as a molecular switch to control and regulate the transformation of thyroid follicular cells into malignant state (FTC or PTC) [35].
TSHR: the Mediator of TSH Signaling
TSHR is a surface glycoprotein belonging to the G-protein-coupled receptor family, described as the master switch in regulating thyroid growth. TSHR is expressed in both benign and malignant thyrocytes and is a target for therapeutic drugs to inhibit TSHR-mediated malignancy [36]. Once its ligand TSH binds to TSHR, it triggers downstream effects such as increasing thyroid-specific gene transcription, controlling iodine uptake, Tg and TPO synthesis, and thyroid hormone production [37–39].
The study conducted by Kim and coworkers (2014) demonstrated that PTCs that express BRAFV600E have a higher level of expression of TSHR, indicating this elevation of expression as a unique phenotype of the BRAFV600E mutation [19]. On the contrary, according to Xing and coworkers (2013), the hyperactivation of TSHR signaling as those acquired through activating mutations in TSHR is well known to cause benign tumors that almost never turn malignant, suggesting that TSHR signaling may be protective against transformation to malignancy. However, low-serum TSH levels caused by genetic variants may predispose to an increased risk of PTC suggesting the dichotomous role of TSH/TSHR signaling in the development of thyroid cancer, i.e., (i) TSH/TSHR signaling may suppress malignant transformation of thyrocytes and therefore suppress the occurrence of thyroid cancer but (ii) may promote progression of thyroid cancer once initiated by oncogenic alterations [40].
Further, Xing and coworkers (2013) demonstrated the fundamental role of TSH/TSHR signaling in thyroid cell proliferation using a mouse model having thyroid-specific knock-in of BRAFV600E (LSL-BRAFV600E TPO-Cre). These mice developed aggressive PTC, but when crossed with TSHR −/− mice, the offspring failed to develop PTC [40].
Contrary to the observations of Kim and coworkers (2014) which stated that increase in TSHR expression is a unique phenotype of BRAFV600E, studies which demonstrate methylation of the promoter region of the TSHR gene indicate that TSHR expression is suppressed in BRAFV600E-mutated cells. Upon administration of a DNA methylation inhibitor, TSHR mRNA expression was increased in both normal and PTC cell lines [19]. Similarly, Khan and Pandith (2014) found a higher frequency of TSHR gene methylation in malignant thyroid tumors resulting in epigenetic silencing of the gene, which suggests the role of TSHR hypermethylation in the progression and aggressiveness of thyroid tumors [41].
Another study conducted by Lu and coworkers (2010) using a mouse model of FTC on the influence of TSH/TSHR signaling on tumorigenesis stated that TSH is the major stimulator of thyrocyte proliferation. In this study, mice harboring a dominantly negative mutation (PV) in TRβ, TRβ PV/PV, were crossed with TSHR gene knock-out mice, TSHR −/− in order to eliminate the TSH growth stimulating effect. The TRβ PV/PV TSHR −/− mice exhibited impaired thyroid growth with virtually no occurrence of FTC further strengthening the fact that TSH/TSHR signaling plays a major role in thyroid carcinogenesis [42].
RAS/AKT Signaling Is Reactivated by Increased TSH and DUSP6
The serine/threonine kinase AKT regulates several biological processes including cell survival, proliferation, growth, and glycogen metabolism. Studies have shown that BRAFV600E induces ERK1/2 phosphorylation (Fig. 3) which in turn inhibits RAS activity. Inhibition of RAS activity by p-ERK1/2 is a well-characterized feedback signaling mechanism. The presence of BRAFV600E inhibits RAS-dependent AKT activation by way of phosphorylated ERK1/2 and thus induces OIS. The AKT signaling pathway activates several oncogenic proteins including c-Myc. It was observed that RAS/AKT signaling pathway is reactivated by TSH in BRAFV600E cells by the overexpression of MnSOD, which scavenges ROS and inhibits its levels. This releases the inhibition on DUSP6 levels (Fig. 3), causing the phosphorylation of ERK1/2, thus inhibiting OIS and promoting tumorigenesis by upregulation of c-Myc [19].
Fig. 3.
TSH signaling inhibits BRAFV600E-induced senescence through phosphorylation of ERK1/2. TSH thyroid-stimulating hormone; TSHR thyroid-stimulating hormone receptor; MnSOD manganese superoxide dismutase; ROS reactive oxygen species; DUSP6 dual-specific phosphatase 6. (a) Mitogenic stimulus activates RAS which in turn activates mutant BRAF protein and triggers the MAPK/ERK signaling cascade which results in the phosphorylation of ERK1/2. Phosphorylated ERK1/2 (p-ERK1/2) inhibits RAS activity and hence inhibits RAS-dependent AKT signaling, which if activated, produces oncogenic proteins such as c-Myc. This feedback inhibition induces OIS in BRAFV600E PTC thyrocytes. (b) Once serum TSH increases, the coupled effect of increased number of TSHR (unique phenotype of BRAFV600E) on the thyrocyte membrane and increased TSH triggers increased TSH/TSHR signaling which results in increased expression of MnSOD. MnSOD normally inhibits the levels of ROS and ROS (scavenged by MnSOD) in turn inhibits the levels of DUSP6. The increase in MnSOD relieves the inhibition on DUSP6 by ROS resulting in increased levels of DUSP6. High levels of DUSP6 cause the dephosphorylation of p-ERK1/2 (the result of MAPK/ERK signaling), hence lifting the feedback inhibition on RAS. The reactivation of RAS activates the RAS-dependent AKT signaling pathway resulting in the production of oncogenic proteins such as c-Myc leading progression into malignancy
A study conducted by Innocenti and coworkers (2013) showed high levels of DUSP6 (dual-specific phosphatase 6) mRNA and proteins in all analyzed PTC cell lines which were associated with high ERK1/2 activation in the analyzed thyroid carcinomas. Furthermore, functional experiments of DUSP6 silencing in four PTC cell lines showed decrease in neoplastic properties suggesting that DUSP6 may have a pro-tumorigenic role in thyroid tumorigenesis [43].
Negative Feedback Signaling Causes OIS
OIS limits cell proliferation. It was found that mutations in the RAF gene induce a global negative feedback response which inhibits RAS-dependent signaling pathways and its effectors such as the AKT pathway, thus triggering senescence [44]. Oncogenic BRAF-induced senescence occurs though an autocrine/paracrine pathway which establishes a negative feedback loop. Previous studies have demonstrated that melanocytes harboring the BRAFV600E mutation initially exhibit a proliferative burst resulting in clonal expansion followed by growth arrest. Subsequently, Wajapeyee and coworkers (2007) explained this biphasic response. The first phase causes initial expression of BRAFV600E leading to increased MEK/ERK signaling resulting in a transient proliferative signal. The second phase of BRAFV600E expression results in the synthesis and secretion of IGFBP7 which inhibits the MEK/ERK signaling pathway via an autocrine/paracrine pathway thus activating senescence [1].
Detection of Point Mutations of BRAF
Currently, there are several techniques used for the detection of known mutations and single nucleotide polymorphisms (SNPs) with their respective degrees of accuracy. In the analysis of the BRAFV600E mutation, the exon 15 of the kinase domain of the BRAF gene is initially amplified by PCR and base substitutions are detected by restriction digest, allele-specific hybridization, by ligation, or non-ligation of adjacent probes.
Allelic variations or point mutations alter the restriction recognition sites in DNA and this principle is used to cleave the amplified fragment specifically using restriction endonucleases. The specific restriction endonuclease will cleave the DNA only when the perfect recognition sequence is present [45]. Polymerase chain reaction-mediated restriction fragment length polymorphism (PCR-RFLP) is a popular technique for detection of point mutations but RFLP results must be confirmed by DNA sequencing which is gold standard in the detection of mutations. PCR-RFLP exploits the fact that most SNPs and point mutations either create or abolish a restriction recognition site. Despite the widespread use of PCR-RFLP in the detection of point mutations, one major drawback of this technique is that not all mutations contain restriction sites for enzymes and hence limiting its usage in such analyses.
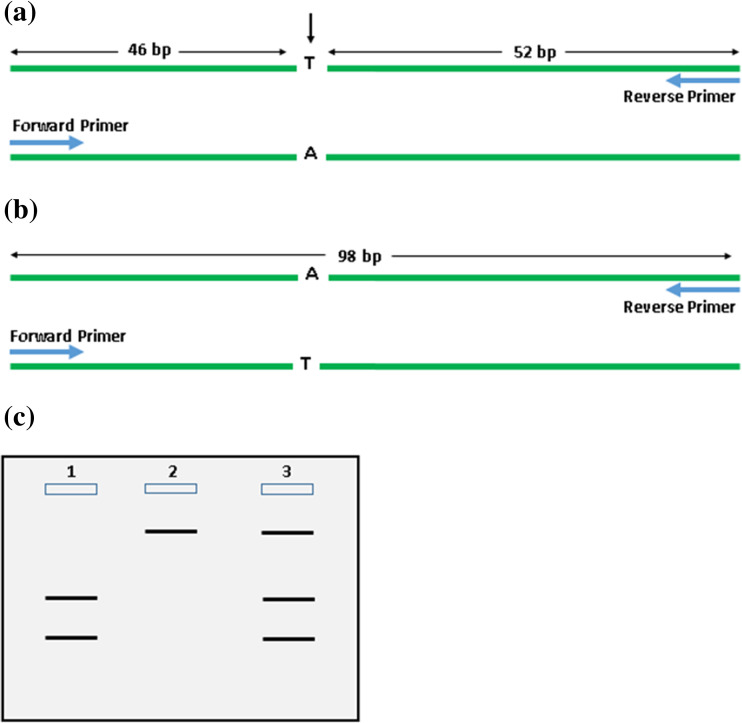
Ranjbari and coworkers (2013) extracted DNA from 63 formalin-fixed paraffin-embedded (FFPE) PTC tumor tissues and amplified the exon 15 of the BRAF gene by PCR: 5′-TCA TGA AGA CCT CAC AGT AAA AAT-3′ (forward) and 5′-TGG ATC CAG ACA ACT GT T CAA-3′ (reverse). The PCR product was subsequently digested by the restriction enzyme TspR1 which cleaves at the site of base substitution. Polyacrylamide gel (8%) was used to distinguish the fragments of different sizes where in the presence of the wild-type genotype, two distinct fragments of lengths 46 and 52 bp were produced and in the case of the mutant genotype, an intact 98-bp fragment was produced (Fig. 4). Since most malignant tumors contain both normal and mutant thyrocytes, a mixed banding pattern would most frequently be observed (Fig. 4c) [46].
Fig. 4.
Schematic diagram showing PCR-RFLP analysis of the BRAFV600E mutation using the restriction enzyme TspR1. a BRAF exon 15 containing wild-type genotype is amplified by PCR primers and cleaved with TspR1 which gives two bands of lengths 46 and 52 bp. The arrow indicates cleavage by restriction enzyme. b BRAF exon 15 containing mutant genotype is amplified by PCR primers and cleaved with TspR1 which gives an intact band of length 98 bp. c Banding pattern visualized via polyacrylamide gel electrophoresis; lane 1 shows the banding pattern of a wild-type genotype; lane 2 shows the banding pattern of a mutant genotype; lane 3 shows the banding pattern of a heterogeneous malignant tumor consisting of both wild-type and mutant genotypes
Detection of Expression Levels of TSHR, MnSOD, and DUSP6 Using Real-Time PCR
Real-time PCR monitors the amplification of a targeted DNA molecule (RNA molecule reverse-transcribed into cDNA) during the course of PCR, in real-time and not at its end as in conventional PCR. The two most commonly used methods to analyze data from real-time quantitative PCR experiments are absolute quantification and relative quantification. Absolute quantification is performed by the determination of the input copy number of the transcript of interest by relating the PCR signal to a standard curve [47]. On the contrary, relative quantification describes the change in target gene expression relative to a constitutively expressed reference gene. It is easier to perform than absolute quantification and in theory, is adequate to investigate physiological changes in level of gene expression [48]. The reference gene is often a housekeeping gene and can be co-amplified in the same tube in a multiplex assay using a sequence-specific DNA probe or can be amplified in a separate tube either using a sequence-specific DNA probe or non-sequence-specific fluorescent dye such as SYBR Green I [49].
In a study that analyzed the expression of TSHR, MnSOD, and DUSP6 in normal and BRAFV600E PTC cells by real-time PCR using β-actin as the reference gene, Kim and coworkers (2014) extracted total cellular RNA from normal and PTC tissues followed by first-strand cDNA synthesis using oligo-dT primers. DUSP6 to β-actin and MnSOD to β-actin ratios were analyzed in normal and BRAFV600E-expressing cells treated with or without TSH and showed the activation of DUSP6 by TSH and the induction of expression of MnSOD in PTC tissues in addition to a moderate increase in expression of TSHR in BRAFV600E PTC cells with respect to normal thyrocytes [19].
Diagnosis of BRAFV600E and Therapeutic Studies
A molecular diagnostic assay that detects the prevalence of the BRAFV600E mutation in thyroid cell aspirates would help to screen patients with hypothyroid goiters and to predict the risk of developing TSH-triggered PTC. Patients positive for the BRAF mutation would be further screened for increased expression levels of TSHR, MnSOD, and DUSP6. Such assays would increase prognosis of the disease by seeking early medical treatment for the BRAF mutation in patients with only the mutation who have not yet developed PTC. For patients with the BRAFV600E mutation and PTC, early chemotherapeutic treatment can be sought, or if necessary, complete excision of the thyroid gland can be performed.
The effect of therapy on mutation/alteration of gene expression was studied by Cheng and coworkers (2017) and they tested the effects of BRAF/MEK inhibitor dabrafenib/selumetinib alone and in combination with HER inhibitor lapatinib to sensitize BRAFV600E-mutated thyroid cancer cells to redifferentiation therapy and their effect on expression and function of iodine-handling genes [50]. Malignant tumors of the thyroid consist of thyrocytes which have undergone dedifferentiation accompanied by the progressive loss of thyroid-specific functions causing these cells to be refractory to treatments such as radioiodine therapy. For example, the BRAFV600E mutation causes downregulation of genes required for iodine transport and thyroid hormone synthesis. Thus, BRAF/MEK inhibitors facilitate treatment with radioiodine in redifferentiation therapy. The results of this study showed that dabrafenib/selumetinib, when administered alone, increased iodine uptake and toxicity and suppressed glucose metabolism in BRAFV600E PTC cells. However, when combined with lapatinib, increase in iodine-handling gene expression, cell membrane localization of NIS, radioiodine uptake and toxicity were observed. Cheng and coworkers concluded that lapatinib prevented the MAPK pathway and sensitized BRAFV600E PTC cells to BRAF/MEK inhibitors facilitating redifferentiation therapy [50].
Another study conducted by Anelli and coworkers (2017) on zebrafish with the BRAFV600E mutation identified TWIST2 as a key downstream effector of BRAF. By genetically inactivating a TWIST2 orthologue using CRISPR/Cas9, the effects of BRAFV600E in the thyrocytes of zebrafish with thyroid carcinoma were suppressed, restoring thyroid morphology and hormone synthesis [51].
Conclusion
PTC containing the BRAFV600E mutation induces a characteristic OIS in which the thyrocytes begin to senesce under normal TSH levels. The mutation also results in the upregulation of TSHR on the membranes of thyrocytes. In autoimmune conditions such as Hashimoto’s thyroiditis which cause hypothyroidism, the level of serum TSH is elevated. The combined effect of increased serum TSH and increased TSHR causes increased TSH/TSHR signaling which inhibits OIS and results in thyrocytes progressing into malignancy. Studies have shown that the prevalence of malignancy increases with elevated serum TSH levels. Thus, it could be concluded that TSH/TSHR signaling plays an important role in thyroid gland tumorigenesis in BRAFV600E-mutated PTC thyrocytes. Hence, techniques such as PCR-RFLP and real-time PCR would be useful in the detection and screening of premalignant lesions of PTC.
Acknowledgements
The authors would like to recognize and thank Dr. N.V. Chandrasekharan of the Faculty of Science, University of Colombo, for providing motivation to write this review article.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interests.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132(3):363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serrano M, Lin AW, Mccurrach ME, Beach D, Lowe SW. Oncogenic Ras provokes premature cell senescence associated with accumulation of p53 and p16 INK4a. Cell. 1997;88(5):593–602. doi: 10.1016/S0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 3.Bennett DC. Human melanocyte senescence and melanoma susceptibility genes. Oncogene. 2003;22(20):3063–3069. doi: 10.1038/sj.onc.1206446. [DOI] [PubMed] [Google Scholar]
- 4.Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11(11):527–531. doi: 10.1016/S0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- 5.Bringold F, Serrano M. Tumor suppressors and oncogenes in cellular senescence. Exp Gerontol. 2000;35(3):317–329. doi: 10.1016/S0531-5565(00)00083-8. [DOI] [PubMed] [Google Scholar]
- 6.Lowe SW, Sherr CJ. Tumor suppression by Ink4a-Arf : progress and puzzles. Curr Opin Genet Dev. 2003;13(1):77–83. doi: 10.1016/S0959-437X(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 7.Courtois-Cox S, Jones SL, Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene. 2008;27(20):2801–2809. doi: 10.1038/sj.onc.1210950. [DOI] [PubMed] [Google Scholar]
- 8.Collado M, Serrano M. The power and the promise of oncogene-induced senescence markers. Nat Rev Cancer. 2006;6(6):472–476. doi: 10.1038/nrc1884. [DOI] [PubMed] [Google Scholar]
- 9.Michaloglou C, Vredeveld LCW, Soengas MS, Denoyelle C, Majoor M, Shay JW, Mooi WJ, Kuilman T, Van Der Horst CMAM, Peeper DS. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436(7051):720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 10.Espinosa AJ, Gilbert J, Fagin (2015) BRAF c.1799T>A (V600E) mutation in thyroid cancer. My cancer genome. https://mycancergenome.org/content/disease/thyroid-cancer/braf/54. Accessed 01 Aug 2017
- 11.Nikiforov YE. Thyroid Carcinoma: molecular pathways and therapeutic targets. Mod Pathol. 2008;21:S37–S43. doi: 10.1038/modpathol.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X. BRAF mutations: prognostic and therapeutic markers in human cancers. Austin J Clin Pathol. 2014;1:1–2. [Google Scholar]
- 13.American Cancer Society. The thyroid gland. https://www.cancer.org/cancer/thyroid-cancer.html. Accessed 02 Aug 2017
- 14.Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LDR, Barletta JA, Wenig BM, al Ghuzlan A, Kakudo K, Giordano TJ, Alves VA, Khanafshar E, Asa SL, el-Naggar AK, Gooding WE, Hodak SP, Lloyd RV, Maytal G, Mete O, Nikiforova MN, Nosé V, Papotti M, Poller DN, Sadow PM, Tischler AS, Tuttle RM, Wall KB, LiVolsi VA, Randolph GW, Ghossein RA. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2(8):1023–1029. doi: 10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26(22):3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 16.Johnson R, Spiegelman B, Hanahan D, Wisdom R. Cellular transformation and malignancy induced by ras require c-jun. Mol Cell Biol. 1996;16(8):4504–4511. doi: 10.1128/MCB.16.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 18.Zou M, Baite EY, Al-rijjal RA, Parhar RS, Al-mohanna FA, et al. TSH overcomes Braf V600E-induced senescence to promote tumor progression via downregulation of p53 expression in papillary thyroid cancer. Oncogene. 2015;35(15):1909–1918. doi: 10.1038/onc.2015.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YH, Choi YW. TSH signaling overcomes B-RafV600E–induced senescence in papillary thyroid carcinogenesis. Neoplasia. 2014;16(12):1107–1120. doi: 10.1016/j.neo.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cisowski J, Sayin VI, Liu M, Karlsson C, Bergo MO. Oncogene-induced senescence underlies the mutual exclusive nature of oncogenic KRAS and BRAF. Oncogene. 2015;35(10):1328–1333. doi: 10.1038/onc.2015.186. [DOI] [PubMed] [Google Scholar]
- 21.Vizioli MG, Possik PA, Tarantino E, Meissl K, Borrello MG, Miranda C, Anania MC, Pagliardini S, Seregni E, Pierotti MA, Pilotti S, Peeper DS, Greco A. Evidence of oncogene-induced senescence in thyroid carcinogenesis. Endocr Relat Cancer. 2011;18(6):743–757. doi: 10.1530/ERC-11-0240. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto H, Sakamoto A, Fujiwara M. Decreased expression of the thyroid-stimulating hormone receptor in poorly-differentiated carcinoma of the thyroid. Oncol Rep. 2008;19(6):1405–1411. [PubMed] [Google Scholar]
- 23.Boelaert K, Horacek J, Holder RL, Watkinson JC, Sheppard MC, Franklyn JA. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab. 2015;91(11):4295–4301. doi: 10.1210/jc.2006-0527. [DOI] [PubMed] [Google Scholar]
- 24.Haymart MR, Glinberg SL, Liu J, Sippel RS, Jaume JC, Chen H. Higher serum TSH in thyroid cancer patients occurs independent of age and correlates with extrathyroidal extension. Clin Endocrinol. 2009;71(3):434–4399. doi: 10.1111/j.1365-2265.2008.03489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polyzos SA, Efstathiadou Z, Poulakos P, Slavakis A, So D, et al. Serum thyrotropin concentration as a biochemical predictor of thyroid malignancy in patients presenting with thyroid nodules. J Cancer Res Clin Oncol. 2008;134(9):953–960. doi: 10.1007/s00432-008-0373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi L, Li Y, Guan H, Li C, Shi L, Shan Z, Teng W. Usefulness of serum thyrotropin for risk prediction of differentiated thyroid cancers does not apply to microcarcinomas: results of 1,870 Chinese patients with thyroid nodules. Endocr J. 2012;59(11):973–980. doi: 10.1507/endocrj.EJ12-0154. [DOI] [PubMed] [Google Scholar]
- 27.Boelaert K. The association between serum TSH concentration and thyroid cancer. Endocr Relat Cancer. 2009;16(4):1065–1072. doi: 10.1677/ERC-09-0150. [DOI] [PubMed] [Google Scholar]
- 28.Nishida S, Nakano T, Kimoto S, Kusunoki T, Suzuki K, Taniguchi N, Murata K, Tomura TT. Induction of manganese superoxide dismutase by thyroid stimulating hormone in rat thyroid cells. FEBS Lett. 1997;416(1):69–71. doi: 10.1016/S0014-5793(97)01171-X. [DOI] [PubMed] [Google Scholar]
- 29.Franco AT, Malaguarnera R, Refetoff S, Liao X, Kimura S, et al. Receptor thyrotrophin signaling dependence in mice tumor initiation thyroid. Proc Natl Acad Sci U S A. 2011;108(4):1615–1620. doi: 10.1073/pnas.1015557108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosignolo F, Maggisano V, Sponziello M, Celano M, Di Gioia CRT, D’Agostino M, Giacomelli L, Verrienti A, Dima M, Pecce V, Durante C. Reduced expression of THRβ in papillary thyroid carcinomas: relationship with BRAF mutation, aggressiveness and miR expression. J Endocrinol Investig. 2015;38(12):1283–1289. doi: 10.1007/s40618-015-0309-4. [DOI] [PubMed] [Google Scholar]
- 31.Kim WG, Zhu X, Kim DW, Zhang L, Kebebew E, Cheng SY. Reactivation of the silenced thyroid hormone receptor β gene expression delays thyroid tumor progression. Endocrinology. 2013;154(1):25–35. doi: 10.1210/en.2012-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romitti M, Wajner SM, Zennig N, Goemann IM, Bueno AL, Meyer EL, Maia AL. Increased type 3 deiodinase expression in papillary thyroid carcinoma. Thyroid. 2012;22(9):897–904. doi: 10.1089/thy.2012.0031. [DOI] [PubMed] [Google Scholar]
- 33.Romitti M, Wajner SM, Ceolin L, Ferreira CV, Ribeiro RV, et al. MAPK and SHH pathways modulate type 3 deiodinase expression in papillary thyroid carcinoma. Endocr Relat Cancer. 2016;23(3):135–146. doi: 10.1530/ERC-15-0162. [DOI] [PubMed] [Google Scholar]
- 34.Cantara S, D’Angeli F, Toti P, Lignitto L, Castagna MG, Capuano S, Prabhakar BS, Feliciello A, Pacini F. Expression of the ring ligase PRAJA2 in thyroid cancer. J Clin Endocrinol Metab. 2012;97(11):4253–4259. doi: 10.1210/jc.2012-2360. [DOI] [PubMed] [Google Scholar]
- 35.Zou M, Baitei EY, Al-Rijjal RA, Parhar RS, Al-Mohanna FA, Kimura S, Pritchard C, BinEssa H, Alanazi AA, Alzahrani AS, Akhtar M, Assiri AM, Meyer BF, Shi Y. KRASG12D-mediated oncogenic transformation of thyroid follicular cells requires long-term TSH stimulation and is regulated by SPRY1. Lab Investig. 2015;95(11):1269–1277. doi: 10.1038/labinvest.2015.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowe CW, Paul JW, Gedye C, Tolosa JM, Bendinelli C, McGrath S, Smith R. Targeting the TSH receptor in thyroid cancer. Endocr Relat Cancer. 2016;24(6):R191–R202. doi: 10.1530/ERC-17-0010. [DOI] [PubMed] [Google Scholar]
- 37.Roger P, Taton M, Sande JVAN, Dumont JE. Mitogenic effects of thyrotropin and adenosine 3′,5′-monophosphate in differentiated normal human thyroid cells in vitro. J Clin Endocrinol Metab. 1988;66(6):1158–1165. doi: 10.1210/jcem-66-6-1158. [DOI] [PubMed] [Google Scholar]
- 38.Vassart G, Dumont JE. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev. 1992;13(3):596–611. doi: 10.1210/edrv-13-3-596. [DOI] [PubMed] [Google Scholar]
- 39.Bruno R, Ferretti E, Tosi E, Arturi F, Giannasio P, Mattei T, Scipioni A, Presta I, Morisi R, Gulino A, Filetti S, Russo D. Modulation of thyroid-specific gene expression in normal and nodular human thyroid tissues from adults: an in vivo effect of thyrotropin. J Clin Endocrinol Metab. 2005;90(10):5692–5697. doi: 10.1210/jc.2005-0800. [DOI] [PubMed] [Google Scholar]
- 40.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13(3):184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan MS, Pandith AA. Epigenetic silencing of TSHR gene in thyroid cancer patients in relation to their BRAF V600E mutation status. Endocrine. 2014;47(2):449–455. doi: 10.1007/s12020-014-0319-6. [DOI] [PubMed] [Google Scholar]
- 42.Lu C, Zhao L, Ying H, Willingham MC, Cheng S-y. Growth activation alone is not sufficient to cause metastatic thyroid cancer in a mouse model of follicular thyroid carcinoma. Endocrinology. 2010;151(4):1929–1939. doi: 10.1210/en.2009-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Innocenti DD, Romeo P, Tarantino E, Sensi M, Cassinelli G, et al. DUSP6 / MKP3 is overexpressed in papillary and poorly differentiated thyroid carcinoma and contributes to neoplastic properties of thyroid cancer cells. Endocr Relat Cancer. 2013;20(1):23–37. doi: 10.1530/ERC-12-0078. [DOI] [PubMed] [Google Scholar]
- 44.Williams SMG, Reczek EE, Johnson BW, Mcgillicuddy LT, Johannessen CM, et al. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10(6):459–472. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nollau P, Wagener C. Methods for detection of point mutations: performance and quality assessment. Clin Chem. 1997;43(7):1114–1128. [PubMed] [Google Scholar]
- 46.Ranjbari N, Almasi S, Mohammadi-Asl J, Rahim F. BRAF mutations in Iranian patients with papillary thyroid carcinoma. Asian Pac J Cancer Prev. 2013;14(4):2521–2523. doi: 10.7314/APJCP.2013.14.4.2521. [DOI] [PubMed] [Google Scholar]
- 47.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 48.Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29(9):e45–445. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agretti P, Chiovato L, De Marco G, Marcocci C, Mazzi B. Real-time PCR provides evidence for thyrotropin receptor mRNA expression in orbital as well as in extraorbital tissues. Eur J Endocrinol. 2002;147(6):733–739. doi: 10.1530/eje.0.1470733. [DOI] [PubMed] [Google Scholar]
- 50.Cheng L, Jin Y, Liu M, Ruan M, Chen L. HER inhibitor promotes BRAF/MEK inhibitor-induced redifferentiation in papillary thyroid cancer harboring BRAFV600E. Oncotarget. 2017;8(12):19843–19854. doi: 10.18632/oncotarget.15773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anelli V, Villefranc JA, Chhangawala S, Martinez-McFaline R, Riva E, Nguyen A, Verma A, Bareja R, Chen Z, Scognamiglio T, Elemento O, Houvras Y. Oncogenic BRAF disrupts thyroid morphogenesis and function via twist expression. elife. 2017;6:e20728. doi: 10.7554/eLife.20728. [DOI] [PMC free article] [PubMed] [Google Scholar]