Abstract
Mycobacterium tuberculosis can persist for many years within host lung tissue without causing clinical disease. Little is known about the state in which the bacilli survive, although it is frequently referred to as dormancy. Some evidence suggests that cells survive in nutrient-deprived stationary phase. Therefore, we are studying stationary-phase survival of Mycobacterium smegmatis as a model for mycobacterial persistence. M. smegmatis cultures could survive 650 days of either carbon, nitrogen, or phosphorus starvation. In carbon-limited medium, cells entered stationary phase before the carbon source (glycerol) had been completely depleted and glycerol uptake from the medium continued during the early stages of stationary phase. These results suggest that the cells are able to sense when the glycerol is approaching limiting concentrations and initiate a shutdown into stationary phase, which involves the uptake of the remaining glycerol from the medium. During early stationary phase, cells underwent reductive cell division and became more resistant to osmotic and acid stress and pool mRNA stabilized. Stationary-phase cells were also more resistant to oxidative stress, but this resistance was induced during late exponential phase in a cell-density-dependent manner. Upon recovery in fresh medium, stationary-phase cultures showed an immediate increase in protein synthesis irrespective of culture age. Colony morphology variants accumulated in stationary-phase cultures. A flat colony variant was seen in 75% of all long-term-stationary-phase cultures and frequently took over the whole population. Cryo scanning electron microscopy showed that the colony organization was different in flat colony strains, flat colonies appearing less well organized than wild-type colonies. Competition experiments with an exponential-phase-adapted wild-type strain showed that the flat strain had a competitive advantage in stationary phase, as well a providing evidence that growth and cell division occur in stationary-phase cultures of M. smegmatis. These results argue against stationary-phase M. smegmatis cultures entering a quiescent state akin to dormancy but support the idea that they are a dynamic population of cells.
Mycobacterium tuberculosis causes more than 25% of avoidable adult deaths in the developing world (51). Far from being under control, tuberculosis is on the increase in both developing and industrialized countries (51). In addition, there is the widespread emergence of drug-resistant strains, making some cases of tuberculosis effectively untreatable (53). Although one-third of the world’s population is estimated to be infected with M. tuberculosis, not every person will develop the disease (60). M. tuberculosis enters and survives in macrophages, and if the infection is controlled successfully, either by the immune system or with the help of antibiotics, lesions are formed and then walled off from the immune system (for a review, see reference 73). These lesions consist of dead macrophages and viable bacteria that are not killed by antibiotics normally used in tuberculosis treatment (36–38). Within these lesions, M. tuberculosis can survive for months or years, only to reinitiate the disease when the immune system of the host becomes compromised (45). Indeed, a large number of tuberculosis cases in industrialized countries are thought to be due to reactivation of these latent infections (67).
The physiological state in which M. tuberculosis survives in the lesions is not known. In the literature, this stage of the disease has been referred to as latency or dormancy, and dormancy has also been used to describe the physiological state in which the bacteria exist (9, 16, 67, 74). In bacterial physiology, the term dormancy is used to define “ a reversible state of low metabolic activity, in which cells can persist for extended periods without division” (26). Clear examples are Bacillus subtilis endospores (14) and Micrococcus luteus nutrient-starved cells (25), both of which require specific signals to be able to resuscitate. Although the term dormancy is now regularly used to describe the state in which M. tuberculosis survives within the lung, there is no direct experimental evidence to suggest that persisting M. tuberculosis cells survive in such a state within lesions (4). However, there is evidence that the bacteria do survive in a nongrowing state. Wallace (63) showed that in vitro-grown stationary-phase M. tuberculosis cells were more resistant to 53°C than exponentially growing cells. M. tuberculosis isolated from mice with latent tuberculosis were resistant to as high a temperature as in vitro-grown stationary-phase cells. In contrast, M. tuberculosis isolated from mice with acute infections, although less sensitive to killing at 53°C than exponentially growing cells, were significantly more sensitive than cells from mice with latent tuberculosis (63). Increased stress resistance is a well-documented property of stationary-phase bacteria (30, 35, 48, 58).
Entry of a bacterial population into stationary phase can be caused in several ways, including accumulation of toxic by-products and environmental stresses such as low temperatures, acidity, and high osmolarity. An important reason for nongrowth in many bacteria is the absence of sufficient nutrients in the environment to sustain growth (28). Bacteria have evolved different ways of adapting to limitation of nutrients. Some bacterial species produce specialized structures, such as highly resistant dormant spores (e.g., Bacillus spp.) and fruiting bodies, as has been observed in Myxococcus species (for a review, see references 11 and 27). However, many bacteria do not form specialized structures upon nutrient starvation. The morphological and physiological changes that do occur in these so-called nondifferentiating bacteria have been well documented for the gram-negative species Escherichia coli, Vibrio sp., and Salmonella typhimurium and include a decrease in cell size, increased stress resistance, increases in RNA stability, and major changes in protein synthesis (29, 30, 35, 43, 48, 58).
There is some evidence suggesting that persisting mycobacteria in lung lesions are nutritionally starved. Nyka showed that M. tuberculosis cells in lung lesions differ in their morphology and staining properties from those growing in vitro (41, 42). They are small spherical cells rather than rods and are chromophobic (they are not stained with conventional strains and are not acid fast). This type of cell could be obtained in vitro by starving M. tuberculosis, Mycobacterium kansasii, or Mycobacterium phlei cultures in sterile distilled water or in agar blocks made up of 3% agar in distilled water. When the in vitro-starved cells were added to a nutrient-rich liquid medium, even after 2 years of starvation, they regained their acid fastness and started growing (42). So not only do starved cells become chromophobic, like cells isolated from lung lesions, but they can also survive for at least 2 years without the presence of nutrients and then recover rapidly when fresh nutrients are encountered. These observations led us to use nutrient starvation as a model for the state in which persisting M. tuberculosis survives. It is not known what limiting nutrient keeps M. tuberculosis in stationary phase during the latent phase of the disease. Wayne has proposed that within calcified lung lesions oxygen is the growth-limiting factor and has developed an O2-limited model for mycobacterial persistence (67–69). Although mycobacteria are obligate aerobes, cultures can survive stationary phase induced by anaerobiosis (70).
In this paper, we report the stationary-phase response of mycobacteria. Throughout this work, nonpathogenic, fast-growing Mycobacterium smegmatis was used as our model organism. We report on the survival of M. smegmatis during starvation for carbon, nitrogen, or phosphorus and focus on carbon starvation to study the physiological changes that occur when M. smegmatis enters stationary phase.
MATERIALS AND METHODS
Growth and starvation of bacteria.
The M. smegmatis strain used for all starvation experiments was mc2155 (59). For competition experiments (see below), the following strains were derived from mc2155 (Table 1): (i) MS1, an exponential-phase-adapted stock of mc2155, created by growing mc2155 exponentially for 40 doublings before freezing the cells as a glycerol stock culture; (ii) MS1-1, a spontaneous rifampin-resistant mutant of MS1, isolated by plating MS1 onto Lab-lemco medium (see below) with 90 μg of rifampin ml−1; (iii) MSf4 and MSf7, flat-colony-morphology strains isolated from cultures of mc2155 that had been starved for carbon for 17 and 10 months, respectively; (iv) MSf7-2, a spontaneous streptomycin-resistant mutant of MSf7, isolated by plating MSf7 onto Lab-lemco with 20 μg of streptomycin ml−1 and (v) MSn2 and MSn3, normal-colony-morphology strains isolated from cultures of mc2155 that had been starved for carbon for 17 and 14 months, respectively.
TABLE 1.
Properties of strains used to examine the stable phenotypic changes that occur in stationary-phase cultures of M. smegmatis
| Strain | Relevant phenotype | Doubling time in minimal medium (h) |
|---|---|---|
| mc2155 | Wild type | 3 |
| MS1 | Exponentially adapted mc2155 | 3 |
| MS1-1 | Spontaneous rifampin-resistant strain of MS1 | 3.5 |
| MSf4 | Flat strain from 17-month-old culturea | 5.5 |
| MSf7 | Flat strain from 10-month-old culturea | 4.5 |
| MSf7-2 | Spontaneous streptomycin-resistant strain of MSf7 | 3.5 |
| MSn2 | Normal strain from 17-month-old culturea | 4 |
| MSn3 | Normal strain from 14-month-old culturea | 3 |
All old strains were isolated from carbon-limited stationary-phase cultures
Cultures were grown in either Hartmans-de Bont minimal medium (19) or Lab-lemco medium. Lab-lemco medium was used as the plating medium for viable plate counts, for some recovery experiments, and for competition experiments and contained (per liter) peptone (10 g), NaCl (5 g), Lab-lemco (Oxoid) (5 g), and Tween 80 (0.05%, vol/vol) to reduce the natural tendency of the cells to stick together in large clumps. For solid medium, agar was added at 15 g liter−1 and Tween 80 was left out. Hartmans-de Bont minimal medium was used for starvation experiments. To prevent precipitation of salts during preparation of this medium, components were added in the listed order. Ten milliliters of a 100-fold-concentrated stock of trace minerals was diluted in 965 ml of H2O (final composition per liter, EDTA [0.01 g], MgCl2 · 6H2O [0.1 g], CaCl2 · 2H2O [1 mg], NaMoO4 · 2H2O [0.2 mg], CoCl2 · 6H2O [0.4 mg], MnCl2 · 2H2O [1 mg], ZnSO4 · 7H2O [2 mg], FeSO4 · 7H2O [5 mg], CuSO4 · 5H2O [0.2 mg]). Then, 2.0 g of the nitrogen source, (NH4)2SO4, was dissolved (final concentration 15 mM), and Tween 80 was added to 0.05%, vol/vol. The carbon source, glycerol, was added to 27.4 mM (0.2%, vol/vol). The solution was made up to 990 ml with H2O and autoclaved. Finally, 10 ml of a 100-fold-concentrated, autoclaved stock of the phosphates was added to yield final concentrations of 8.9 mM K2HPO4 (1.55 g liter−1) and 7.08 mM NaH2PO4 (0.85 g liter−1). The final pH of the medium was 7.0. This medium could be easily manipulated to vary the amount of the carbon, nitrogen, or phosphorus source. For carbon starvation, the amount of glycerol was reduced to 11 mM (0.08%, vol/vol). For nitrogen starvation, (NH2)2SO4 was reduced 100-fold to 0.15 mM. For phosphorus starvation, both K2HPO4 and NaH2PO4 were reduced 100-fold to 0.16 mM, 3-(N-morpholino)propanesulfonic acid (MOPS) was added at 50 mM to replace lost buffering capacity, and the pH was adjusted to pH 7. Nutrient levels were known to be limiting as cultures entered stationary phase at significantly lower optical densities (ODs) than if normal nutrient concentrations were used. In normal Hartmans-de Bont medium, cultures entered stationary phase at an OD at 600 nm (OD600) of 2.5. This OD600 was reduced to 1.8 (2 × 108 CFU ml−1) in the carbon starvation medium, to 0.8 (2 × 107 CFU ml−1) in the nitrogen starvation medium, and to 2.0 (8 × 107 CFU ml−1) in the phosphorus starvation medium. Although the density at which an exponential culture enters stationary phase may influence its subsequent survival, we did not find this for M. smegmatis (unpublished results).
Starter cultures were prepared by inoculating M. smegmatis from plates or from glycerol stocks into 5 ml of carbon-limited Hartmans-de Bont minimal medium in a 30-ml screw-cap tube. These cultures were grown at 37°C in a shaking incubator (200 rpm) until they reached stationary phase. Three hundred-microliter volumes were used to inoculate 50 ml of medium in 250-ml conical flasks, to give an OD600 of about 0.002. Starter cultures had never been in stationary phase for more than 6 days when the were used for inoculation.
Growth and survival were measured as OD600 with a Shimadzu MPS-2000 spectrophotometer, by dry-weight (biomass) measurements, and by cell counting. Dry weights were determined by filtering a known volume (usually 25 to 50 ml) of a culture onto a 0.2-μm-pore-size filter of known weight and drying the filter at 80°C to a constant weight. Total cell numbers were counted microscopically with a hemocytometer slide to determine the number of cells in a known volume of medium. Viable counts were done by plating appropriate dilutions (in room temperature phosphate-buffered saline [PBS] with 0.05% Tween 80) of a 100-μl culture sample onto solidified room temperature lab-lemco medium. M. smegmatis cells tended to stick together in clumps that increased in size and became more compact towards the end of growth and during stationary phase. Microscopic examination indicated that not all clumps were broken up into single cells during viable plate counting. For total cell counts, one clump was counted as one cell, except when the cells in the clumps were loosely attached so individual cells were easily distinguished (this was only the case during exponential growth).
Microscopy.
The lengths of bacteria were measured microscopically by using a calibrated eye piece graticule in Nomarski interference contrast microscopy. Changes in cell morphology were also monitored by scanning electron microscopy (SEM). For SEM, 1-ml culture samples were first fixed in an equal volume of primary fix (2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M sodium cacodylate buffer [pH 7.2]) and incubated overnight at 4°C to fix the bacterial protein. The bacteria were collected by filtration onto a 0.2-μm-pore-size Nuclepore polycarbonate filter (Agar Scientific). One milliliter of secondary fix (1% osmium tetroxide in 0.1 M sodium cacodylate buffer [pH 7.2]) was added to the filter unit with a syringe and left for 1 to 2 h to fix the bacterial lipids. The filters were then flushed with water and removed from the filter unit. They were immediately frozen in liquid nitrogen and freeze-dried overnight at −60°C (ultimate vacuum, 7 × 10−2 torr). Dried filters were mounted on microscope stubs and sputter coated for 3 min at 30 mA to obtain a 600-Å layer of gold (Poleron E5000 sputter coater). Cells were examined with a Philips 50 (model B) SEM.
Whole colonies on agar were studied by cryo SEM. The colony was cut out of the agar as an agar block and mounted on electroconductive aqueous colloidal graphite (DAG; Agar Scientific) on a mounting stub, quickly submersed in liquid N2 (−196°C), and frozen under vacuum. While under vacuum, the sample was transferred to the microscope stage, where the temperature was increased to −66°C to sublime off water that had settled on top of the specimen by condensation. When all the surface water had been removed (inspected by electron microscopy), the temperature was reduced to between −100 and −150°C. The sample was then sputter coated for 3 min as described above and transferred back to the microscope. Photographs were taken before and after sputter coating, but no differences were observed. Sections through colonies were obtained by freeze fracture: colonies frozen in liquid nitrogen were split with a razor blade and a small hammer, after which the samples were treated like the whole colonies described above.
Determination of glycerol concentrations.
Glycerol concentrations of cultures going into carbon-limited stationary phase were measured by the method described by Burton (7). The method relies on the conversion of glycerol into formaldehyde, which can be detected spectrophotometrically at 570 nm in the presence of chromotropic acid reagent. A standard curve was prepared with glycerol concentrations between 0.001 and 1 mM.
Stress challenge experiments.
Two M. smegmatis cultures were inoculated into carbon-limited medium to an initial OD600 of approximately 0.001. One culture was grown to mid-exponential phase (OD600, approximately 0.5), and the other was grown to approximately 8 h into stationary phase. A stress condition was then applied to both cultures, and survival was monitored over a period of 3 to 9 h by viable plate counting. Oxidative stress was applied by adding H2O2 to a final concentration of 18 or 36 mM. Osmotic stress was applied by the addition of NaCl to a final concentration of 5 M. NaCl (2.92 g) was quickly dissolved in 10 ml of culture by vortexing for 30 s. Acid stress was applied by adding 1 M HCl to lower the pH to 2. The pHs of the cultures were checked at the start and end of the experiment with pH indicator strips.
Recovery experiments.
Recovery experiments were done with carbon-limited Hartmans-de Bont minimal medium (10 mM glycerol) or with Lab-lemco medium. M. smegmatis cultures were grown to stationary phase in carbon-limited Hartmans-de Bont minimal medium. To determine the recovery potential of starved cultures, 100-μl samples were removed at different time points in stationary phase and diluted into 50 ml of fresh Hartmans-de Bont minimal medium or Lab-lemco. Growth of this culture was monitored and culture viability was determined immediately after inoculation (time [t] = 0) and at a time point (t = x) during visible exponential growth (usually when the culture density was between OD600s 0.2 and 0.7). The number of doublings that had occurred between t = 0 and t = x was calculated, and this in combination with the known 3-h doubling time yielded the time the culture had spent growing in exponential phase. The lag time was then calculated by subtracting the time spent growing from the total time (t = x). The doubling time of recovering cultures was checked by monitoring growth (OD600) throughout the experiment in several recovering cultures. Due to the long and variable lag phases of recovering cultures, a more accurate direct determination of lag phases was impractical.
Determination of protein synthesis.
M. smegmatis cultures were grown in carbon-limited minimal medium. At different times during stationary phase, 100-μl samples were removed and diluted into fresh minimal medium as described above. Immediately after dilution, and at intervals during the lag phase, 0.5-ml samples were removed and 1 μCi of [35S]methionine (Amersham) was added. These samples were incubated at room temperature for 15 min and then quenched with 1 ml of cold 7.5% trichloroacetic acid (TCA) containing 1 mM unlabelled methionine for 60 min on ice to stop labelling and to precipitate the proteins. The samples were then heated at 90°C for 30 min, cooled, and collected on 25-mm-diameter glass fiber filter discs (pore size, 1 μm; Gelman Science). Filters were flushed with 10 ml of 7.5% cold TCA. The filters were removed from the filtering unit and added to a scintillation vial with 2 ml of Cocktail T Scintran (BDH Chemicals Ltd.), and radioactivity was counted in a model 1214 Rackbeta liquid scintillation counter (LKB Wallace). Each recovery experiment was done in duplicate, and determination of the protein synthesis rate was based on results from three samples per time point.
Determination of pool mRNA stability.
This was determined as described previously (1, 62). mRNA stability was estimated as the loss of potential to incorporate [35S]methionine after inhibition of transcription by rifampin. Control experiments were run to ensure that the concentration of rifampin used inhibited the rate of RNA synthesis rapidly and with the same kinetics at each sample time. This was done by measuring the residual RNA synthesis after the addition of rifampin. Samples (0.5 ml) were pulse-labelled for 20 s with 1 μCi of [3H]uridine at time points 0 to 300 s after the addition of 10 μl of 500 μg of fresh rifampin ml−1. After the pulse, samples were quenched with 4 ml of ice-cold 7.5% TCA with 12.5 μg of herring sperm DNA ml−1 as a carrier. Samples were kept on ice for at least 15 min before collection of cells on 25-mm-diameter glass fiber filter discs (pore size, 1 μm; Gelman Science). Filters were washed with 5 ml of 7.5% TCA, and the levels of radioactivity on the filters were determined as described above. This protocol was repeated for carbon-starved and exponentially growing cultures to ensure that the kinetics of inhibition were similar. In order to measure the half-life of the mRNA pool, 0.5-ml culture samples were pulse-labelled for 2 min with 1 μCi of [35S]methionine at time points between 0 and 60 min after the addition of 10 μl of 500 μg of rifampin ml−1. Samples were then quenched with 1.0 ml of ice-cold 7.5% TCA with 1 mM methionine and left for at least 60 min. Samples were then heated at 90°C for 30 min, cooled, collected on glass fiber discs, and then washed with 10 ml of 7.5% TCA. The radioactivity was then counted.
Competition experiments.
Competition experiments were adapted from the experiments described for E. coli by Zambrano et al. (76). Although colony morphology variants were isolated from cultures grown in Hartmans-de Bont minimal medium, competition experiments were done with cultures grown in Lab-lemco medium. This was done because the effects of competition experiments with E. coli strains in minimal medium are observed only after several months while effects in rich medium are observed within 2 weeks (15). To be able to distinguish between the two populations in a competition experiment, spontaneous antibiotic-resistant strains were selected from MS1 and MSf7. Although we tried to obtain mutants resistant to kanamycin, tetracycline, streptomycin, or rifampin, we successfully selected only streptomycin- and rifampin-resistant strains of MS1 and streptomycin-resistant strains of MSf7. Surprisingly, the streptomycin-resistant mutant of MSf7, MSf7-2, grew faster in minimal medium than the parental strain.
The two strains to be competed were first inoculated into lab-lemco medium directly from glycerol stocks. At 1 day into stationary phase, these cultures were used to inoculate fresh lab-lemco medium and grown to 3 days into stationary phase. The two strains were then mixed in a 1:1,000 ratio (5 μl of minority culture in 5 ml of majority culture) and incubated at 37°C. At intervals, 100-μl samples were diluted in PBS plus 0.5%, vol/vol, Tween 80 and plated onto Lab-lemco with or without the appropriate antibiotics to distinguish the minority culture from the majority culture. Competitions were carried out in duplicate. Controls consisted of (i) a culture for a reverse competition (majority culture becomes the minority culture) to test for possible effects of the medium and (ii) 5-ml cultures of the strains without competitors present to test for reversion and stationary-phase survival.
RESULTS
Effect of nutrient starvation on survival and cell size.
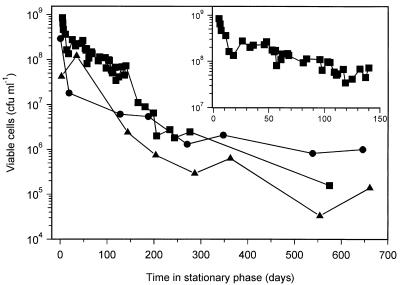
The long-term survival of a total of 34 M. smegmatis cultures following starvation by nutrient exhaustion for carbon, nitrogen, or phosphorus was monitored for up to 650 days. Figure 1 shows the survival of a representative culture from each starvation condition. In 24 carbon-starved cultures, the viability never dropped below 104 CFU ml−1, and in most cultures, it did not decrease below 105 CFU ml−1. During the first 10 to 20 days there was a relatively rapid 10-fold loss in viability, followed by a period of very gradual decline between 20 and 150 days (Fig. 1, inset). There was a further period of more rapid loss in viability between 150 and 200 days (Fig. 1). In five nitrogen-starved cultures, the levels of viability after 650 days had not decreased below 106 CFU ml−1, and in five phosphorus-starved cultures, levels of viability after 650 days of stationary phase varied between 103 and 106 CFU ml−1. During long-term starvation for either carbon, nitrogen, or phosphorus, we observed following plating populations with different colony morphologies that were not seen in exponential-phase cultures. We started detecting these variants usually after the cultures had been at least 1 month in stationary phase, but because the detection limit was only ever 1% of the total number of viable cells, it is possible that they arose earlier. The predominant colony variant had a flat morphology rather than the normal dome-shaped morphology of colonies on Lab-lemco medium. Some of the properties of cells from colonies with this flat morphology will be described below.
FIG. 1.
Long-term survival of M. smegmatis following nutrient starvation by exhaustion for either carbon (■), nitrogen (•), or phosphorus (▴). Survival was determined by plate counting of viable cells in samples taken from cultures throughout the experiment. Survival during the first 150 days of carbon-limited stationary phase is shown in the inset.
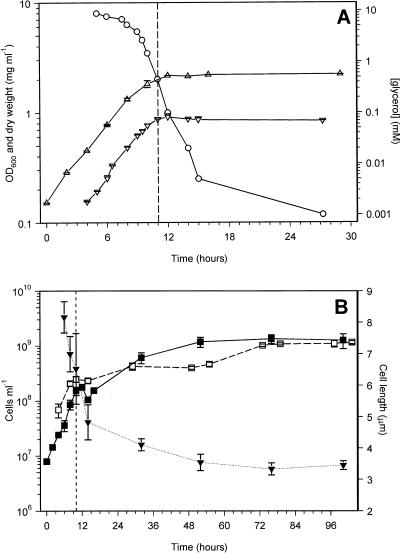
The kinetics of entry into carbon-limited stationary phase were investigated in more detail by monitoring viable and total cell counts, biomass (dry weight), and OD600 (Fig. 2). The point of entry into stationary phase was taken as the point at which the biomass (dry weight) stopped increasing, and this corresponded with the plateau in the OD600 measurements (Fig. 2A). Therefore, OD600 was used to determine the point of stationary-phase entry in all subsequent experiments. Upon entry into stationary phase, the viable and total cell counts stopped increasing for at least 3 h, but this was followed by an approximately 10-fold increase in cell numbers over the next 48 h (Fig. 2B). During this period the cell length decreased from 6.52 ± 1.12 μm (mean ± standard error [SE]) at the start of stationary phase to 3.54 ± 0.26 μm, with an earlier decrease from 8.2 ± 0.5 μm at late exponential phase, indicating that reductive cell division occurred in these cultures (Fig. 2B). In contrast, in both nitrogen- and phosphorus-starved cultures there was a long deceleration period into stationary phase, where biomass and cell numbers kept increasing at a reduced rate. This was accompanied by a reduction in cell length from approximately 6 μm at the start of stationary phase to 4.57 ± 0.84 and 4.81 ± 0.90 μm at 8 days of stationary phase in nitrogen- and phosphorus-starved cultures, respectively (data not shown).
FIG. 2.
Growth of M. smegmatis and entry into carbon-limited stationary phase. (A) Changes in optical density (▵), biomass (dry weight, ▿), and glycerol concentration (○). (B) Changes in viable (■) and total (□) cell numbers and in cell length (▾). The glycerol concentration experiment was performed four times with similar results. OD600, biomass, and viable count results are means ± SE of results of duplicate experiments. Mean cell lengths ± SE are shown, with numbers of cells varying between 12 and 30. Total cell counts are means ± SE of determinations from between 8 and 10 hemocytometer fields of 2.5 × 10−4 mm3. The vertical dashed lines indicate the point of entry into stationary phase.
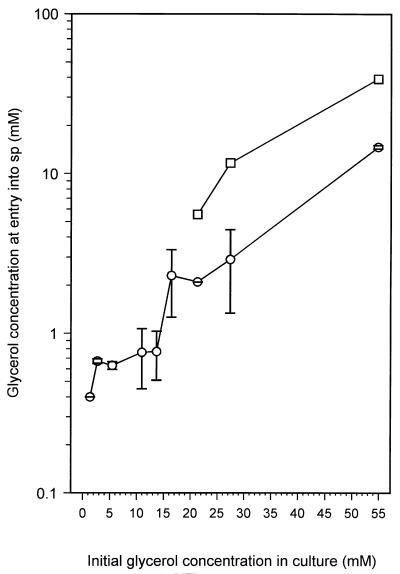
Figure 2A shows the depletion of the carbon source, glycerol, during exponential growth and entry into carbon-limited stationary phase. During growth, glycerol was depleted at an increasing rate, until at the onset of stationary phase it has decreased from 11 mM to approximately 0.5 mM. However, during the first 4 h in stationary phase, the concentration of glycerol continued to fall rapidly to approximately 0.005 mM and then more slowly over the next 12 h. After 2 days in stationary phase, it had dropped to below detectable levels (<0.001 mM [data not shown]). Therefore, while these cultures entered stationary phase due to carbon (glycerol) limitation, glycerol had not been exhausted from the medium at the onset of stationary phase. Glycerol continued to be used during the early stages of stationary phase, coinciding with the period of reductive cell division. When M. smegmatis was grown with other limiting amounts of glycerol (1.37 up to 16.4 mM), the glycerol depletion curves were similar to that shown in Fig. 2A. In addition, all these cultures, with the exception of the 16.4 mM glycerol culture, entered stationary phase at glycerol concentrations of approximately 0.5 to 0.7 mM (Fig. 3). This result supports the idea that cells are able to sense when the glycerol is approaching limiting concentrations and start a shutdown into stationary phase which involves the uptake of remaining glycerol from the medium, perhaps allowing its use as a carbon and energy source during the adaptation process. At present we cannot explain why the culture with 16.4 mM glycerol enters stationary phase with a higher level of glycerol still present. In this culture, and in those that were not carbon limited (concentrations of glycerol of 21.3 mM and above), the glycerol level at entry into stationary phase was 2 mM or higher. The lack of correspondence between the actual and expected levels of glycerol left in the medium at the onset of stationary phase in non-carbon-limited cultures (Fig. 3) can be explained by cells assimilating excess glycerol, perhaps for conversion into storage compounds. In support of this, the glycerol concentration after entry of cultures into non-carbon-limited stationary phase decreased rapidly during the first 5 to 6 h after entry, followed by a slower decline to levels below the detection limit (data not shown).
FIG. 3.
Effect of changing the initial glycerol concentration in the culture on the glycerol concentration at the point of entry into stationary phase (○). Stationary-phase entry was the point at which the OD600 stabilized (Fig. 2A). Complete experiments were done at least three times for each initial glycerol concentration except for the experiment with 21.3 mM glycerol, which was done only once. At least two glycerol determinations were done for each data point, and values shown are means ± SE. It was experimentally determined that 15.7 mM glycerol was used during exponential growth of the culture, with an initial glycerol concentration of 16.4 mM. For cultures where the amount of glycerol was not limiting (≥16.4 mM), the concentration of glycerol that was expected to be left in the medium upon entry into stationary phase was calculated by subtracting 15.7 from the initial concentration (□).
M. smegmatis cells loosely stick together in small clumps during growth in minimal medium. These clumps become larger as exponential phase progresses, and they become more compact after entry into stationary phase. During prolonged starvation, the number of clumps relative to the number of single cells increased (data not shown). Microscopic examination indicated that not all clumps were broken up into single cells during dilution prior to plating, and so the viabilities shown in Fig. 1 and 2 are likely to be underestimates of the true viability.
In the experiments described in Fig. 1 and 2, nutrient starvation was induced by exhaustion of a limiting nutrient from the medium. An alternative method of achieving nutrient starvation is to wash and resuspend an exponential-phase culture in a buffer or medium lacking one or more nutrients (6, 56). When exponentially growing cultures (OD600, 0.2 to 1.3) and early-stationary-phase M. smegmatis cells (OD600, 1.8) were starved by resuspension in Hartmans-de Bont minimal medium without glycerol or in PBS with 0.05% Tween 80, their survival rates were not appreciably different from cultures starved by nutrient exhaustion for at least the 70 days that viability was monitored (data not shown). Also, all resuspended cultures underwent reductive cell division, the number of viable cells increasing between 10- and 50-fold within 2 days of resuspension. This increase suggests that exponentially growing M. smegmatis cells can adapt quickly to sudden nutrient depletion and is in contrast to the finding that glycerol depletion following entry into stationary phase may be important in stationary-phase adaptation. However, it has not been ruled out that the Tween 80 in the resuspension medium, which M. smegmatis can hydrolyze (50), provides the exponentially growing cells with enough energy to prepare for stationary phase.
Changes in pool mRNA stability in stationary-phase M. smegmatis.
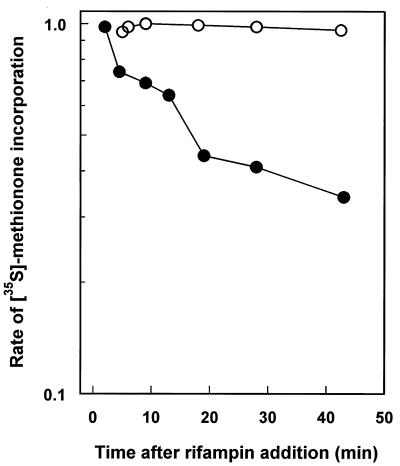
There are a number examples of the stabilization of the mRNA pool in response to nutrient starvation (1, 62). We investigated whether there was a similar change in the pool mRNA stability of M. smegmatis. The pool mRNA stability was determined as the rate of the decay of the potential to synthesize proteins, determined by the rate of incorporation of [35S]methione, after complete inhibition of transcriptional initiation by rifampin. In Fig. 4 the rates of [35S]methionine incorporation are shown for exponential and 20-h-stationary-phase cultures of M. smegmatis after rifampin addition. Exponentially growing cells had a decay half-life of 18 min. Clearly, the carbon-starved, stationary-phase cells had a markedly lower rate of decay of methionine incorporation following rifampin addition. The most likely explanation for this lower rate of decay is increased pool mRNA stability in carbon-starved cultures. By extrapolation of the data in Fig. 4, the decay half-time for the carbon-starved culture was approximately 550 min, representing a 30-fold stabilization of pool mRNA over that of exponential cultures. However, as we have used an indirect method to measure pool mRNA stability changes, the possibility that the loss of methionine incorporation is entirely or in part due to changes in translational efficiency cannot be ruled out (31).
FIG. 4.
Residual protein synthesis, after inhibition of mRNA synthesis with rifampin, in exponentially growing and stationary-phase cultures. At time zero rifampin (0.1 μg ml−1) was added to the culture, and at various times the protein synthesis was determined by determining the rate of incorporation of [35S]methionine. Rates are given relative to the rate at time zero. ○, exponential-phase culture; •, 20-h-carbon-starved, stationary-phase culture.
Development of a stress-resistant state in stationary-phase M. smegmatis.
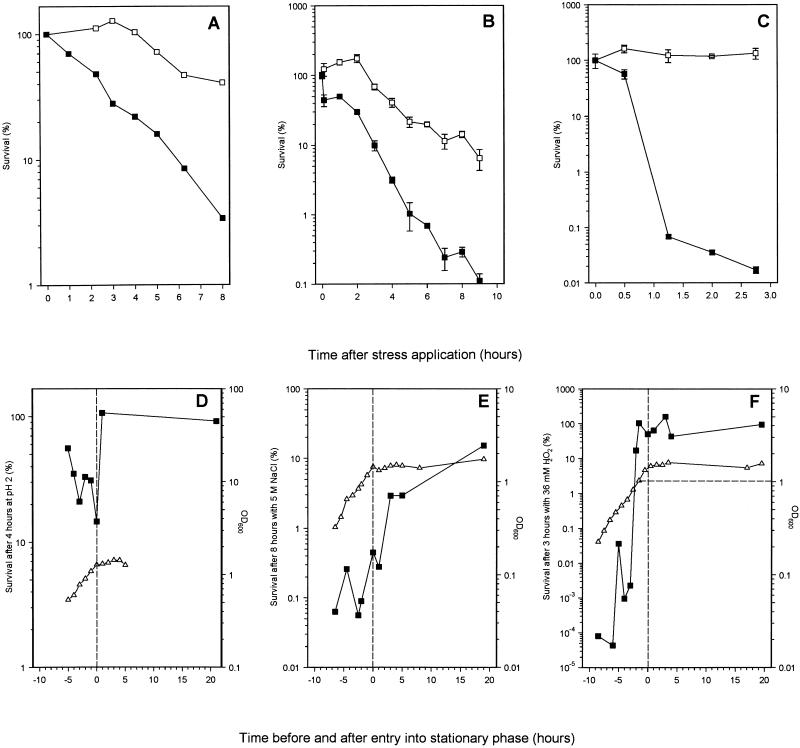
Gram-negative bacteria are known to become resistant to a variety of environmental stresses after they have entered stationary phase (17, 22, 35, 58, 62). We determined whether this was the case for M. smegmatis by subjecting mid-exponential-phase and 8-h-stationary-phase cultures to acid, osmotic, and oxidative stress (Fig. 5). Stationary-phase cultures were significantly more resistant to all three stresses than exponentially growing cultures. At pH 4 and above, both growing and starved M. smegmatis cells survived equally well (data not shown), while at pHs 3 and 2, stationary-phase cells survived better than growing cells. Below pH 2, both stationary-phase and exponential-phase cells died rapidly. The survival kinetics at pH 2 are shown in Fig. 5A. Survival of the growing culture declined steadily to 3.4% after 8 h of incubation compared with the 41% survival of the stationary-phase culture (Fig. 5A). Similarly, after 9 h of exposure to 5 M NaCl, the survival of the exponentially growing culture was 0.1% compared with 7% for the stationary-phase culture (Fig. 5B). The difference in oxidative-stress resistance between growing and starved cultures was most pronounced. Cultures were exposed to 36 mM H2O2, and after 3 h, carbon-starved cells had lost no viability while the exponential-phase cells had viability reduced to 0.02% (Fig. 5C). To rule out the possibility that increased stationary-phase resistance to H2O2 was simply due to a larger population and not to increased resistance of individual cells, stationary-phase cultures were diluted to densities similar to those of the exponential-phase cultures (5 × 107 CFU ml−1). The levels of H2O2 resistance in diluted and undiluted stationary-phase cultures were very similar (data not shown).
FIG. 5.
(A to C) Stress resistance of exponentially growing (■) and 8-h-carbon-starved stationary-phase (□) cultures of M. smegmatis. (A) Acid stress by addition of 1 M HCl to lower the pH to 2; (B) osmotic stress by addition of 5 M NaCl; (C) oxidative stress by addition of 36 mM H2O2. Samples were taken at various times after the application of the specific stress and plated to determine viability. (D to E) Induction of acid, osmotic-, and oxidative-stress resistance (■) during the growth of M. smegmatis measured as OD600 (▵). Samples were taken throughout growth, and viability was determined by plate counting before and after applying a stress condition for fixed periods (indicated on the y axes). Acid (D), osmotic (E), and oxidative (F) stresses were applied as described for panels A to C, respectively. The vertical dashed lines indicate the point of entry into stationary phase. The horizontal dashed line in panel F indicates the OD at which maximal resistance was reached. Experiments were performed at least three times, and the results of a representative experiment are shown. Where values have errors, these are means ± standard deviations of determinations from three plates.
The induction kinetics of resistance to each stress during growth and entry into stationary phase were determined. For acid and osmotic stress, it was clear that the largest increases in resistance occurred after the cells had entered stationary phase (Fig. 5D and E), suggesting that these were stationary-phase-induced responses. However, although there was variation in the degrees of survival around 4 to 5 h before stationary-phase entry, resistance to oxidative stress began to increase dramatically 3 h before entry into stationary phase, when the culture reached an OD600 of 0.8. Maximum resistance was reached 2 h before the culture entered stationary phase (Fig. 5F). This result indicated that resistance to oxidative stress was not a stationary-phase-induced response, and we investigated if it was cell density dependent. Resistance to H2O2 was monitored in cultures that entered stationary phase at different cell densities (which was achieved by varying the starting concentrations of glycerol) (Fig. 6A). Cultures with glycerol concentrations of 27.4 and 11 mM showed maximum induction of resistance to oxidative stress when the OD600 reached 0.8, at 5 and 2.5 h before entry into stationary phase, respectively. In the culture with 5.5 mM glycerol, the time of entry into stationary phase, the reaching of an OD600 of 0.8, and maximal H2O2 resistance were almost coincident. However, a culture with 1.4 mM glycerol, which did not reach an OD600 of 0.8, did not induce comparable levels of H2O2 resistance (Fig. 6). These data are consistent with a cell-density-dependent, rather than a stationary-phase-dependent, induction of H2O2 resistance in M. smegmatis.
FIG. 6.
Cell-density-dependent induction of oxidative stress resistance in M. smegmatis. Cultures were grown in Hartmans-de Bont minimal medium with different, limiting amounts of glycerol so that they entered stationary phase at different densities. (A) Growth curves of four cultures of M. smegmatis grown in Hartmans-de Bont minimal medium with 1.4 mM (□), 5.5 mM (▾), 11 mM (•), or 27.4 mM glycerol (⋆). (B) Induction of oxidative-stress resistance in the cultures whose growth curves are shown in panel A. Samples were taken throughout growth, and viability was determined before and after exposing the samples to 36 mM H2O2 for 3 h. The horizontal dashed line in panel A indicates the threshold OD600, after which resistance is induced. The vertical dashed line in both graphs indicates the start of stationary phase.
Recovery from carbon starvation.
The recovery potentials of carbon-starved cultures were determined at different time points during stationary phase by diluting 100 μl of a starved culture into 50 ml of fresh carbon-limited minimal medium or lab-lemco medium and determining the lag period before cells entered exponential growth. During the first 2 days in stationary phase, the recovery lag phases were less than 4 h in both minimal medium and Lab-lemco medium (Table 2). Between 2 and 7 days of stationary phase, lag phases upon recovery increased to around 10 to 15 h in minimal medium (5 to 10 h in Lab-lemco medium) and stayed at this level until cells were 20 to 30 days into stationary phase, when the lag phases increased to 20 to 25 h in minimal medium (17 h in Lab-lemco). Recovery in minimal medium was monitored for cultures at up to 250 days of stationary phase, and the lag phases remained stable around 20 to 25 h between day 30 and day 250 (Table 2). This stability is unlike what occurs with Vibrio sp. strain Ant-300, in which the length of the lag phase during recovery was directly proportional to the time spent in stationary phase, for at least the first 2 months (3). Measurements of protein synthesis by [35S]methionine incorporation of cultures recovering from either 2, 10, or 60 to 75 days stationary phase showed that, irrespective of how long the cells had spent in stationary phase, there was an immediate response to new nutrients with a sudden increase in [35S]methionine incorporation from 10−4 cpm CFU−1 to 0.033 ± 0.015 (mean ± standard deviation; n = 6) cpm CFU−1. The level of protein synthesis then remained stable at this increased level until close to the start of exponential phase. The main difference between recovering cultures of different ages was the length of this stable period of elevated protein synthesis: 4 h for cells recovering from 2 days at stationary phase, up to 12 h for cells recovery from 10 days at stationary phase, and up to 20 h for cells starved for 75 days (data not shown).
TABLE 2.
Effect of the time spent in carbon-limited stationary phase on the lengths of lag phases of recovering cultures of M. smegmatis.
| Time spent in stationary phase (days) | Length of lag phase upon recovery (h)a in:
|
|
|---|---|---|
| Minimal medium | Lab-lemco medium | |
| 0–2 | ≤4 | ≤4 |
| 2–7 | Increase to 10–15 | Increase to 5–10 |
| 7–20 | 10–15 | 5–10 |
| 20–250 | 20–25 | approx 17 |
Recovery in minimal medium was examined for 10 different cultures; recovery in Lab-lemco medium was examined for 6 cultures. Lag phases showed trends that could be roughly grouped in four time periods.
Stable phenotypic changes occur in nutrient-starved cultures of M. smegmatis.
During the experiments to determine long-term-stationary-phase survival, strains with several different colony morphology phenotypes were observed. By far the most obvious and abundant were those with a flat, dry, and granular morphology rather than the domed, moist, and smooth morphology of wild-type M. smegmatis colonies when plated on Lab-lemco medium (Fig. 7A and B). Of the 34 cultures monitored for up to 650 days, flat colony variants were seen in 75% (18 of 24) of carbon-starved, 100% (5 of 5) of nitrogen-starved, and 60% (3 of 5) of phosphorus-starved cultures. Flat colonies were usually larger than wild-type colonies, and when grown in liquid culture, the flat colony variants formed large clumps that adhered to the walls of the flasks, suggesting that their surfaces were more hydrophobic than those of wild-type cells.
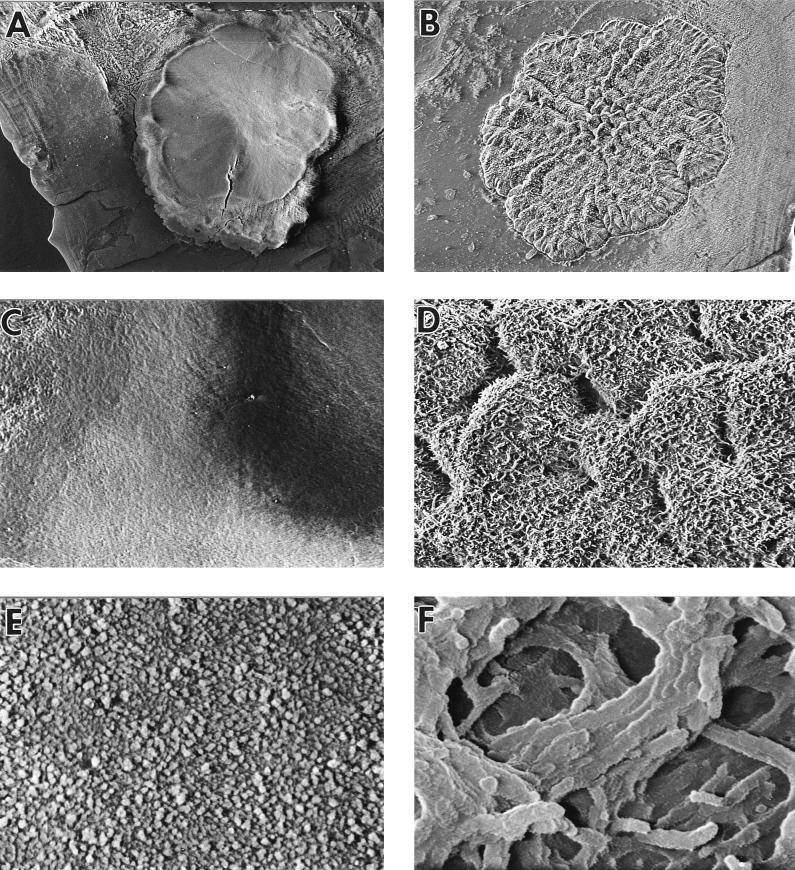
FIG. 7.
Cryo scanning electron micrographs of a wild-type colony (A, C, and E) and a flat colony variant (B, D, and F). (A and B) Colonies viewed from above at a magnification of ×15; (C and D) surfaces of colonies at a magnification of ×120; (E and F) surfaces of colonies at a magnification of ×3,750.
Flat strains were purified and subcultured in either liquid or solid medium at least five times without loss of the phenotype. Only after repeated subculturing were some colonies with normal morphology again observed. This indicated that the flat phenotype is a highly stable, heritable property but that it can revert. The flat colony phenotype may arise from mutation or from a genetic switch, such as phase variation, or be a physiological adaptation. These possibilities cannot be distinguished from the present data. Two-dimensional gel electrophoresis of lysates from cells that had been pulse-labelled with [35S]methionine showed that although protein synthesis profiles of stationary-phase flat strains were virtually identical to that of stationary-phase, wild-type M. smegmatis, there were at least seven proteins with different synthesis rates (data not shown). In most stationary-phase cultures, once flat strains had arisen, they increased in number until the proportion of flat cells plateaued. However, in some cultures, the proportion of flat cells increased until they had virtually replaced all the cells with wild-type colony morphology, while in other cultures, the proportion of flat cells decreased again after an initial increase. The appearance of flat strains in stationary-phase cultures and their increase in number over time suggested that cells in stationary-phase cultures of M. smegmatis are metabolically active and that they may be growing and dividing.
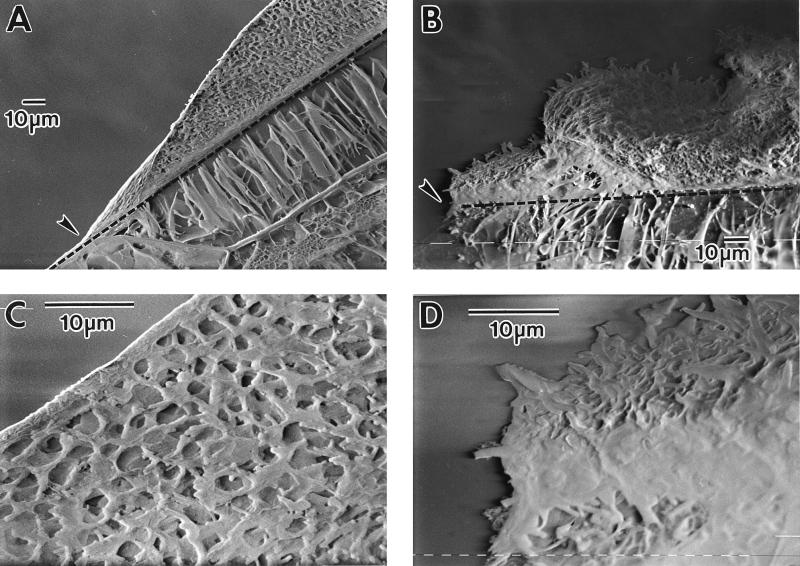
Microscopic examination by cryo SEM showed that wild-type colonies were covered by a layer of extracellular material, which obscured any detail of individual cells. This was absent in flat colonies, in which individual cells were clearly seen at higher magnifications (Fig. 7C to F). This layer consisted partly of water, as when the colony was subsequently freeze-dried to remove water, the surface layer changed to strands rather than a continuous sheet (data not shown). When colonies were sectioned, the layer covering normal colonies was distinguished clearly (Fig. 8A and C). Also, the smooth and irregular surfaces of the domed and flat colonies, respectively, were clearly visible from these side views, and the flat colony appeared a less organized structure throughout than the normal colony (Fig. 8). There was no obvious difference in cell morphology between single cells from wild-type and flat colonies examined by SEM following suspension of colonies in PBS (data not shown).
FIG. 8.
Cross-section through a wild-type (A and C) and flat (B and D) colony, viewed by cryo SEM. (A and B) Magnification, ×480. The edge of the colonies is indicated by arrows, and the dashed line shows the division between the agar and the colony. (C and D) Parts of panels A and B at a magnification of ×1,875.
Flat-colony variants have reduced exponential growth rates.
The growth rates of two flat strains, MSf4 and MSf7, isolated from separate cultures that had been in stationary phase for 17 and 10 months, respectively, were compared with the growth rates of MS1, an exponential-phase-adapted strain of M. smegmatis mc2155, and with the growth rates of two normal-colony-morphology strains, MSn3 and MSn2, isolated from 14- and 17-month old stationary-phase cultures, respectively (Table 1). The strains mc2155 and MS1 had doubling times of 3 h in both Lab-lemco and Hartmans-de Bont minimal medium, while both flat strains had lower rates of growth, with doubling times between 4 and 5.5 h. The “old normal” strain Msn2 also had a lower rate of growth (td [doubling time], approximately 4 h), while the doubling time of MSn3 was not increased compared to that of MS1. These results suggested that stationary-phase-adapted cells of M. smegmatis may have acquired mutations in stationary phase that made them less fit for rapid exponential growth, but there is no direct evidence to link this phenotype specifically with acquisition of flat colony morphology.
Stationary-phase-adapted strains have a competitive growth advantage in stationary phase.
Clearly, variants with new phenotypes arise and accumulate in stationary-phase M. smegmatis cultures. We next examined whether these strains had acquired a competitive advantage in stationary phase over exponential-phase-adapted cells, similar to the GASP (growth-advantage-in-stationary-phase) mutations described for E. coli (75, 76). To test this, competition experiments were set up between old (stationary-phase-adapted) and young (exponential-phase-adapted) strains. In order to successfully distinguish between the two populations in a competition, spontaneous antibiotic-resistant mutants of MS1 and the flat-colony variant MSf7 were isolated (Table 1).
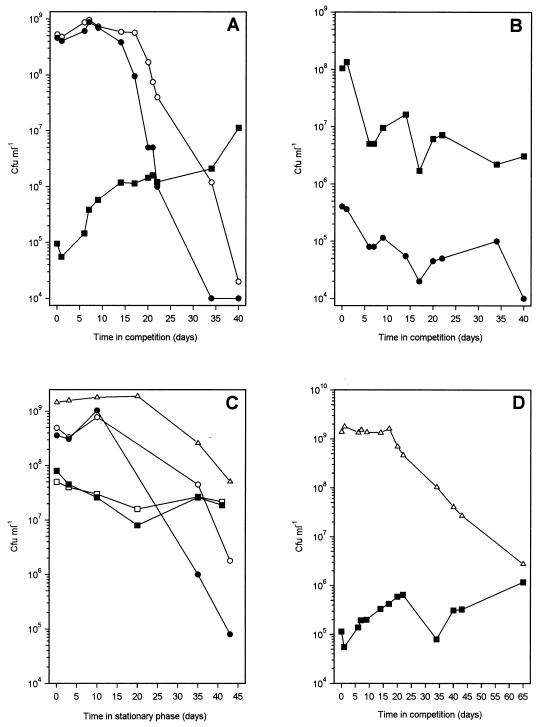
Competition experiments were set up between the old flat strain MSf7-2 (Strr) and the young exponential-phase-adapted wild-type strain MS1-1 (Rifr). Five microliters from a 3-day-stationary-phase culture of MSf7-2 was added to 5 ml of a 3-day-stationary-phase culture of MS1-1, and the viability of each population was monitored by plating the strain onto lab-lemco with the appropriate antibiotics (Fig. 9A). Control experiments consisted of (i) a reverse competition, i.e., a majority of MSf7-2 cells with a minority of MS1-1 cells (Fig. 9B), and (ii) monitoring the stationary-phase survival of pure cultures of MS1-1 and MSf7-2 (Fig. 9C). First, while noting that the survival curves for MS1 and MS1-1 were markedly different from those in Fig. 1 because the experiments were performed with different media, it is clear that the flat strain MSf7-2 survives much better in stationary phase than MS1-1 or MS1 (Fig. 9C). Second, during the 40-day competition whose results are shown in Fig. 9A, the number of viable MS1-1 cells declined approximately 105-fold, 100-fold more than the number of viable cells in the pure control culture in Fig. 9C, while there was a gradual increase in the number of MSf7-2 cells from 105 to 107 CFU ml−1. In contrast, the viability of the MSf7-2 control culture had decreased 10-fold during the same period (Fig. 9C). This indicated that the stationary-phase-adapted MSf7-2 strain had a competitive advantage over the exponential-phase-adapted MS1-1 strain in stationary phase. When the competition was reversed (Fig. 9B), the MS1-1 minority could not take over the population. Instead, there was an approximately 50-fold decline in viability, similar to the reduction in viability of the pure control culture of MSf1-1 (Fig. 9C).
FIG. 9.
Competition experiments. (A) Competition between a minority strain, MSf7-2 (Strr, stationary-phase-adapted flat strain), identified by plating with streptomycin (■), and a majority strain, MS1-1 (Rifr, exponential-phase-adapted, normal-colony-morphology strain), plated with (•) and without (○) rifampin. Five microliters of a 3-day-stationary-phase culture of MSf7-2 was mixed with 5 ml of a 3-day-stationary-phase culture of MS1-1. After 30 days, the number of rifampin-resistant MS1-1 cells dropped to ≤1 × 104. (B) Reverse competition between the MSf7-2 majority strain (■, plated with streptomycin) and the MS1-1 minority strain (•, plated with rifampin). (C) Shown are an MS1-1 control (no MSf7-2 added), plated with (•) and without (○) rifampin; an MSf7-2 control (no MS1-1 added), plated with (■) and without (□) streptomycin; and an MS1 control to the strain used in panel D (no MSf7-2 added), plated without antibiotics (▵). (D) Competition between a minority strain, MSf7-2 (■), and a majority strain, MS1 (▵). All competitions were performed three times with similar results. Plating errors were within 10% of the viable counts. Note that although the proportion of the majority cells to minority cells inoculated was always 1,000:1 (vol/vol), there appeared to be 10,000-fold more MS1-1 cells than MSf7-2 cells in Fig. 8A, compared with only 500-fold more MSf7-2 cells in Fig. 8B. This was due to the fact that flat cultures clumped more, which reduced the viable counts of flat strains approximately 10-fold compared with counts for the wild type under similar conditions.
The rifampin-resistant mutation was unstable in stationary-phase MS1-1 cells. From 12 days into the competition (Fig. 9A) as well as in pure culture (Fig. 9C), a larger number of cells lost rifampin resistance. It is possible that the mutation is deleterious to survival in stationary phase. To check whether this loss of resistance influenced the competition results of Fig. 9A, a similar competition was set up with MS1 as the majority strain (Fig. 9D). Because this strain did not have an antibiotic-resistant marker, it was not possible to accurately perform the reverse competition with MS1 in the minority. Results of a control experiment in which survival of pure cultures of MSf7-2 and MS1 was monitored are shown in Fig. 9C. In this competition, the number of flat strain MSf7-2 cells showed an overall increase, while MS1 showed a small but significantly greater loss in viability than the control culture. These results indicate that MSf7-2 has a competitive advantage in stationary phase compared with MS1 and MS1-1, suggesting, but no proving, that stationary-phase cultures of M. smegmatis accumulate mutations that confer a GASP phenotype similar to those described for E. coli (75, 76) and Pseudomonas putida (12). In addition, the competition experiments provide strong evidence for the ability of M. smegmatis to grow and divide in stationary-phase cultures. It seems highly probable that the nutrients released from dying MS1-1 and MS1 cells allow the regrowth of MSf7-2. Together with the ability of flat variants to appear and take over stationary-phase cultures of M. smegmatis, our results provide a very strong indication that nutrient-limited stationary-phase cultures of M. smegmatis do not uniformly enter a dormant state but are a dynamic population.
DISCUSSION
In this work, we have reported the stationary-phase-survival response of M. smegmatis. M. smegmatis cultures were able to survive carbon, nitrogen, or phosphorus starvation for at least 650 days. The high level of survival found was consistent with that reported for M. tuberculosis, M. kansasii, M. phlei (42), and Mycobacterium fortuitum (33), studies in which cells were starved by resuspension rather than nutrient exhaustion. To compare the survival of M. smegmatis with that of other bacterial species, several problems arise. First, it is difficult to compare the results of starvation studies if different media or starvation methods were used, since the type of medium used for growth can affect stationary-phase survival. We found that M. smegmatis starved in minimal medium lost only 10-fold viability after 100 days carbon starvation (Fig. 1) but that cultures grown in lab-lemco medium lost up to 100-fold viability over the same period (Fig. 9). Second, the method of starvation can also affect starvation survival; for example, in Rhizobium leguminosarum, cells starved by nutrient exhaustion survive much better than exponentially growing cells starved by resuspension (62). Third, survival can depend on culture cell density at the onset of nutrient starvation (18, 49, 61), although preliminary experiments suggest that this is also not the case for M. smegmatis (unpublished results). Fourth, although stationary-phase-survival studies have now been reported for many species, there are few reports in which survival has been recorded for periods longer than 1 or 2 months. One example, however, is the study in which Pseudomonas syringae strains were starved for 24 years in distilled water (24). These cultures showed only a relatively small drop in viability from 108 to around 105 to 106 CFU ml−1.
We have described some of the changes that occur when M. smegmatis enters carbon-limited stationary phase. Cells underwent reductive cell division, resulting in a 10-fold increase in cell numbers, which meant that there was no net loss of viability during the first 65 days of starvation. Reductive cell division has been well described for gram-negative bacteria (23, 28, 30, 64). In marine Vibrio, spp., carbon-starved (but not nitrogen- or phosphorus-starved) cells undergo extreme reductive cell division, forming ultramicrocells (23, 48). Although nitrogen- and phosphorus-starved M. smegmatis cells were smaller than exponentially growing cells, they were still much larger than carbon-starved cells.
Starved cells do not have the energy and resources required to adapt quickly to a changing environment, and a general increase in stress resistance following entry into stationary phase is likely to enhance future survival prospects. Indeed, the development of a generalized stress-resistant state in stationary phase is a widespread characteristic of many bacteria (17, 20, 30, 44, 66) and was shown here to apply to M. smegmatis as well. Upon entry into stationary phase, M. smegmatis became more resistant to acid and osmotic stress. However, resistance to oxidative stress was induced before cells entered stationary phase in a density-dependent manner; cell density has previously been shown to regulate the oxidative-stress defenses in R. leguminosarum (8).
M. smegmatis showed clumping during both growth phase and stationary phase. During prolonged starvation, the proportion of clumps to single cells increased (data not shown). An interesting possibility is that clumping promotes stationary-phase survival, as cells stuck to other cells will be able to use cellular contents released by dying neighbors in the clump before they can diffuse out to the environment. Nyka (42) starved mycobacteria in agar blocks and monitored their loss of acid fastness as a measure of entry into a so-called dormant state. Cells in clumps were the last to become chromophobic, and even after 2 years of starvation some cells in clumps were still acid fast. The increase in clumping in stationary phase may be the result of an increase in cell wall hydrophobicity in stationary-phase cells, as has been reported for other bacteria (54, 58). A lot of work has been done on cell surface-exposed lipids and polysaccharides of exponentially growing M. smegmatis, M. tuberculosis, and other mycobacteria (32, 46, 47). However, there have not been many reports on changes in cell wall components when cells enter stationary phase. It has recently been reported that cell wall thickening occurs in static, O2-starved M. tuberculosis and BCG cells, but not in M. smegmatis cells (9).
The energy required to successfully adapt to stationary-phase conditions is generally thought to come from utilization of storage compounds in addition to degradation of unnecessary proteins and mRNA in the cell (43, 48, 58). In M. smegmatis cultures, additional energy was available, because cultures entered carbon-starvation-induced stationary phase when there was still 0.5 to 0.7 mM glycerol in the medium. In comparison, when Klebsiella pneumoniae is grown in synthetic medium with glucose as the growth-limiting substrate, exhaustion of glucose in the medium is coincident with entry into stationary phase (34). E. coli, grown in continuous culture in a chemostat, induces its stationary-phase response when the glucose concentration reaches 0.0001 mM (40). The glycerol depletion curve observed here for M. smegmatis showed an interesting parallel to the O2 depletion curve of an O2-limited culture of M. tuberculosis, in which stationary phase was entered when the O2 level had decreased from 100 to around 10% saturation, after which the concentration dropped readily to 0.1 and more slowly to 0.01% O2 saturation (69). Perhaps mycobacteria are able to sense low levels of a range of nutrients and start adapting to stationary phase well before they have run out, using the energy derived from the remaining nutrients in the adaptation process. Both carbon limitation and oxygen limitation are expected to lead to energy deprivation in this obligate aerobe.
Our data does not support the idea that M. smegmatis cultures enter a dormant state upon entry into stationary phase. Indeed, several observations argue against stationary-phase cultures of M. smegmatis becoming dormant. First, protein synthesis levels increased almost immediately upon addition of fresh nutrients irrespective of the age of the cells. This result is similar to what has been observed during recovery of stationary-phase cultures of Vibrio sp. strain S14 (2) and R. leguminosarum (62) and indicates that stationary-phase M. smegmatis maintain sufficient metabolic activity to be able to respond immediately to fresh nutrients. In similar experiments, recovering E. coli cells were found to start protein synthesis 3 min after nutrient addition (57). Second and most importantly, we have shown evidence for continuous cell growth and division in stationary-phase cultures, both from the observation that strains with altered colony morphology appear and are able to take over stationary-phase cultures and from the results of competition experiments between stationary-phase-adapted and exponential-phase-adapted strains of M. smegmatis. We showed that the flat strain MSf7-2 had acquired a growth advantage in stationary phase, outcompeting exponential-phase-adapted strains in competition experiments. Whether the competitive advantage of MSf7-2 is analogous to the accumulation of GASP mutations, demonstrated with stationary-phase cultures of E. coli and P. putida, and whether its advantage is linked to the flat phenotype remain to be determined (76). In conclusion, our demonstration that flat strains could arise and replace wild-type cells in pure cultures and in mixed-culture competition experiments indicates that stationary-phase cultures of M. smegmatis are not static populations of dormant cells but are metabolically active, dynamic populations that are able to grow and divide. Whether stationary-phase cultures of M. tuberculosis are similarly dynamic remains to be seen. Work reported on O2-starved stationary-phase M. tuberculosis (9, 68–70) certainly does not prove entry into a dormant state as defined in the introduction. It is important that the experiments described here for M. smegmatis be repeated with species of the M. tuberculosis complex to gain an understanding of the physiological status of nongrowing cultures of these pathogens.
Our experiments do not rule out the possibility that a fraction of the population enters a dormant state, resulting in a loss of the ability to plate, but clearly this survival route is not followed by the whole culture. Cultures of mycobacteria are heterogeneous due to cell clumping, and it is possible that both dormancy and growth take place in the same culture. The dynamism of stationary-phase populations have previously been demonstrated with E. coli and P. putida (12, 75, 76). With P. putida Eberl et al. (12) demonstrated the appearance of small colony mutants in cultures approximately 3 weeks into phosphate starvation, which eventually replaced the wild type. In E. coli, the first GASP mutation to develop in stationary-phase populations is in the rpoS gene and leads to attenuation of the stationary-phase sigma factor ςS (76).
Varying colony morphology types have been described for M. kansasii (5), Mycobacterium intracellulare (39), and Mycobacterium avium, which has three predominant colony forms that differ in their virulence levels (52). In a growing list of bacterial species, changes in colony morphology are due to the phase-variable expression of outer membrane (10, 21, 65, 71, 72) or cell wall components (55). It is possible that a phase variation mechanism operates in M. smegmatis. Rough and smooth colonies of mycobacteria may be formed due to differences in the types of lipids and polysaccharides that are exposed on the surface (5, 13, 52). However, Lemassu et al. (32) found no qualitative or quantitative differences in extracellular material from M. tuberculosis and M. kansasii rough and smooth colonies (mainly polysaccharide that was similar in composition to the surface-exposed polysaccharides). They did not rule out the possibility that the difference in colony morphology was caused by differences in protein composition. Considering that in M. smegmatis 75% of surface-exposed material was reported to consist of protein (32), it would be useful to look in more detail at differences in protein profiles of cell wall fractions from normal domed and flat M. smegmatis colonies.
ACKNOWLEDGMENTS
This work was funded by the Wellcome Trust, via a Wellcome Prize Studentship to M.J.S. and grants to H.D.W.
We are very grateful to Douglas Young for his support at the start of this project, to Ian Morris for his enthusiastic help with the electron microscope, work, to Melinda Pitt for help with the mRNA stability experiments, and to Mike Barer and Paul Wheeler for useful discussions.
REFERENCES
- 1.Albertson N H, Nystrom T, Kjelleberg S. Functional mRNA half-lives in the marine Vibrio sp. S14 during starvation and recovery. J Gen Microbiol. 1990;136:2195–2199. [Google Scholar]
- 2.Albertson N H, Nystrom T, Kjelleberg S. Macromolecular synthesis during recovery of the marine Vibrio sp. S14 from starvation. J Gen Microbiol. 1990;136:2201–2207. [Google Scholar]
- 3.Amy P S, Pauling C, Morita R Y. Recovery from nutrient starvation by a marine Vibrio sp. Appl Environ Microbiol. 1983;45:1685–1690. doi: 10.1128/aem.45.5.1685-1690.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barer M R. Viable but non-culturable and dormant bacteria: time to resolve an oxymoron and a misnomer? J Med Microbiol. 1997;46:629–631. doi: 10.1099/00222615-46-8-629. [DOI] [PubMed] [Google Scholar]
- 5.Belisle J T, Brennan P J. Chemical basis of rough and smooth variation in mycobacteria. J Bacteriol. 1989;171:3465–3470. doi: 10.1128/jb.171.6.3465-3470.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belisle J T, McNeil M R, Chatterjee D, Inamine J M, Brennan P J. Expression of the core lipopeptide of the glycopeptidolipid surface antigens in rough mutants of Mycobacterium avium. J Biol Chem. 1993;268:10510–10516. [PubMed] [Google Scholar]
- 7.Burton R M. The determination of glycerol and dihydroxyacetone. Methods Enzymol. 1957;3:246–248. [Google Scholar]
- 8.Crockford A J, Davis G A, Williams H D. Evidence for cell-density-dependent regulation of catalase activity in Rhizobium leguminosarum bv. phaseoli. Microbiology. 1995;141:843–851. [Google Scholar]
- 9.Cunningham A F, Spreadbury C L. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton alpha-crystallin homolog. J Bacteriol. 1998;180:801–808. doi: 10.1128/jb.180.4.801-808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diderichsen B. flu, a metastable gene controlling surface properties of Escherichia coli. J Bacteriol. 1980;141:858–867. doi: 10.1128/jb.141.2.858-867.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dworkin M. Recent advances in the social and developmental biology of the myxobacteria. Microbiol Rev. 1996;60:70–102. doi: 10.1128/mr.60.1.70-102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eberl L, Givskov M, Sternberg C, Moller S, Christiansen G, Molin S. Physiological responses of Pseudomonas putida KT2442 to phosphate starvation. Microbiology. 1996;142:155–163. doi: 10.1099/13500872-142-1-155. [DOI] [PubMed] [Google Scholar]
- 13.Ensign J C. Long-term starvation of rod and spherical cells of Arthrobacter crystallopoietes. J Bacteriol. 1970;103:569–577. doi: 10.1128/jb.103.3.569-577.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993;57:1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkel S H, Zinser E, Gupta S, Kolter R. Life and death in stationary phase. Mol Microbiol NATO ASI Series H. 1998;103:3–16. [Google Scholar]
- 16.Gangadharam P R J. Mycobacterial dormancy. Tubercle Lung Dis. 1995;76:477–479. doi: 10.1016/0962-8479(95)90521-9. [DOI] [PubMed] [Google Scholar]
- 17.Givskov M, Eberl L, Molin S. Response to nutrient starvation in Pseudomonas putida Kt2442: two-dimensional electrophoretic analysis of starvation- and stress-induced proteins. J Bacteriol. 1994;176:4816–4824. doi: 10.1128/jb.176.16.4816-4824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison A P. The response of Bacterium lactis aerogenes when held at growth temperature in the absence of nutriment: an analysis of survival curves. Proc R Soc Lond B. 1960;152:418–428. [Google Scholar]
- 19.Hartmans S, De Bont J A M. The genus Mycobacterium—nonmedical. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes, a handbook on the biology of bacteria: ecophysiology, isolation, identifcation, application. 2nd ed. II. New York, N.Y: Springer-Verlag New York Inc.; 1992. pp. 1215–1237. [Google Scholar]
- 20.Hecker M, Schumann W, Volker U. Heat-shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 21.Henderson I R, Meehan M, Owen P. Antigen 43, a phase-variable bipartite outer membrane protein, determines colony morphology and autoaggregation in Escherichia coli K-12. FEMS Microbiol Lett. 1997;149:115–120. doi: 10.1111/j.1574-6968.1997.tb10317.x. [DOI] [PubMed] [Google Scholar]
- 22.Hengge-Aronis R. The role of rpoS in early stationary phase gene regulation in Escherichia coli K12. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. [Google Scholar]
- 23.Holmquist L, Kjelleberg S. Changes in viability, respiratory activity and morphology of the marine Vibrio sp. strain S14 during starvation of individual nutrients and subsequent recovery. FEMS Microbiol Ecol. 1993;12:215–224. [Google Scholar]
- 24.Iacobellis N S, DeVay J E. Long-term storage of plant-pathogenic bacteria in sterile distilled water. Appl Environ Microbiol. 1986;52:388–389. doi: 10.1128/aem.52.2.388-389.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaprelyants A S, Kell D B. Dormancy in stationary-phase cultures of Micrococcus luteus: flow cytometric analysis of starvation and resuscitation. Appl Environ Microb. 1993;59:3187–3196. doi: 10.1128/aem.59.10.3187-3196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kell D B, Kaprelyants A S, Grafen A. Pheromones, social behavior and the functions of secondary metabolism in bacteria. Trends Ecol Evol. 1995;10:126–129. doi: 10.1016/s0169-5347(00)89013-8. [DOI] [PubMed] [Google Scholar]
- 27.Kim S K, Kaiser D, Kuspa A. Control of cell density and pattern by intercellular signalling in Myxococcus development. Annu Rev Microbiol. 1992;46:117–139. doi: 10.1146/annurev.mi.46.100192.001001. [DOI] [PubMed] [Google Scholar]
- 28.Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. [Google Scholar]
- 29.Kjelleberg S, Albertson N, Flardh K, Holmquist L, Jouperjaan A, Marouga R, Ostling J, Svenblad B, Weichart D. How do non-differentiating bacteria adapt to starvation? Antonie Leeuwenhoek Int J Gen Mol Microbiol. 1993;63:333–341. doi: 10.1007/BF00871228. [DOI] [PubMed] [Google Scholar]
- 30.Kolter R, Siegele D A, Tormo A. The stationary phase of the bacterial life cycle. Annu Rev Microbiol. 1993;47:855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- 31.Kurland C G, Mikkola R. The impact of nutritional state on the microevolution of ribosomes. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 225–237. [Google Scholar]
- 32.Lemassu A, Ortalomagne A, Bardou F, Silve G, Laneelle M A, Daffe M. Extracellular and surface-exposed polysaccharides of non-tuberculous mycobacteria. Microbiology. 1996;142:1513–1520. doi: 10.1099/13500872-142-6-1513. [DOI] [PubMed] [Google Scholar]
- 33.Majtan V, Drobnica L. Effect of glycerol on viability and other properties of starved Mycobacterium fortuitum. Can J Microbiol. 1979;25:600–604. doi: 10.1139/m79-086. [DOI] [PubMed] [Google Scholar]
- 34.Mason A C, Egli T. Dynamics of microbial growth in the decelerating and stationary phase of batch culture. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 81–102. [Google Scholar]
- 35.Matin A. Molecular analysis of the starvation stress in Escherichia coli. FEMS Microbiol Ecol. 1990;74:185–195. [Google Scholar]
- 36.McCune R M, Feldmann F M, Lambert H P, McDermont W. Microbial persistence. I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J Exp Med. 1966;123:445–468. doi: 10.1084/jem.123.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCune R M, Tompsett R. The fate of Mycobacterium tuberculosis in mouse tissues as determined using the microbial enumeration technique. I. The persistence of drug-susceptible tubercle bacilli in the tissues despite prolonged antimicrobial therapy. J Exp Med. 1956;104:737–762. doi: 10.1084/jem.104.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCune R M, Tompsett R, McDermott W. The fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. II. The conversion of tuberculous infection to the latent state by the administration of pyrazinamide and a companion drug. J Exp Med. 1956;104:763–802. doi: 10.1084/jem.104.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizuguchi Y, Fukunaga M, Taniguchi H. Plasmid deoxyribonucleic acid and translucent-to-opaque variation in Mycobacterium intracellulare 103. J Bacteriol. 1981;146:656–659. doi: 10.1128/jb.146.2.656-659.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Notley L, Ferenci T. Induction of Rpos-dependent functions in glucose-limited continuous culture: what level of nutrient limitation induces the stationary phase of Escherichia coli? J Bacteriol. 1996;178:1465–1468. doi: 10.1128/jb.178.5.1465-1468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nyka W. Method for staining both acid-fast and chromophobic tubercle bacilli with carbolfuchsin. J Bacteriol. 1967;93:1458–1460. doi: 10.1128/jb.93.4.1458-1460.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nyka W. Studies on the effect of starvation on mycobacteria. Infect Immun. 1974;9:843–850. doi: 10.1128/iai.9.5.843-850.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nystrom T, Albertson N H, Flardh K, Kjelleberg S. Physiological and molecular adaptation to starvation and recovery from starvation by the marine Vibrio sp. S14. FEMS Microbiol Ecol. 1990;74:129–140. [Google Scholar]
- 44.Nystrom T, Olsson R M, Kjelleberg S. Survival, stress resistance, and alterations in protein expression in the marine Vibrio sp. strain S14 during starvation for different individual nutrients. Appl Environ Microbiol. 1992;58:55–65. doi: 10.1128/aem.58.1.55-65.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orme I M. A mouse model of the recrudescence of latent tuberculosis in the elderly. Am Rev Respir Dis. 1988;137:716–718. doi: 10.1164/ajrccm/137.3.716. [DOI] [PubMed] [Google Scholar]
- 46.Ortalomagne A, Dupont M A, Lemassu A, Anderson A B, Gounon P, Daffe M. Molecular composition of the outermost capsular material of the tubercle bacillus. Microbiology. 1995;141:1609–1620. doi: 10.1099/13500872-141-7-1609. [DOI] [PubMed] [Google Scholar]
- 47.Ortalomagne A, Lemassu A, Laneelle M A, Bardou F, Silve G, Gounon P, Marchal G, Daffe M. Identification of the surface-exposed lipids on the cell envelopes of Mycobacterium tuberculosis and other mycobacteiral species. J Bacteriol. 1996;178:456–461. doi: 10.1128/jb.178.2.456-461.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostling J, Holmquist L, Flardh K, Svenblad B, Jouper-Jaan A, Kjelleberg S. Starvation and recovery of Vibrio. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 103–127. [Google Scholar]
- 49.Postgate J R, Hunter J R. The survival of starved bacteria. J Gen Microbiol. 1962;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- 50.Ratledge C. Nutrition, growth and metabolism. In: Ratledge S, Stanford J L, editors. Biology of the mycobacteria. Vol. 1. London, United Kingdom: Academic Press Inc., Ltd.; 1982. pp. 186–212. [Google Scholar]
- 51.Raviglione M C, Snider D, Kochi A. Global epidemiology of tuberculosis. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 52.Reddy V M, Lunaherrera J, Gangadharam P R J. Pathobiological significance of colony morphology in Mycobacterium avium complex. Microb Pathog. 1996;21:97–109. doi: 10.1006/mpat.1996.0046. [DOI] [PubMed] [Google Scholar]
- 53.Riley L W. Drug-resistant tuberculosis. Clin Infect Dis. 1993;17:S442–S446. doi: 10.1093/clinids/17.supplement_2.s442. [DOI] [PubMed] [Google Scholar]
- 54.Rosenberg M, Gutnick D, Rosenberg E. Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett. 1980;9:29–33. [Google Scholar]
- 55.Saluja S K, Weiser J N. The genetic basis of colony opacity in Streptococcus pneumoniae: evidence for the effect of box elements on the frequency of phenotypic variation. Mol Microbiol. 1995;16:215–227. doi: 10.1111/j.1365-2958.1995.tb02294.x. [DOI] [PubMed] [Google Scholar]
- 56.Siegele D A, Almiron M, Kolter R. Approaches to the study of survival and death in stationary phase Escherichia coli. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 151–169. [Google Scholar]
- 57.Siegele D A, Guynn L J. Escherichia coli proteins synthesized during recovery from starvation. J Bacteriol. 1996;178:6352–6356. doi: 10.1128/jb.178.21.6352-6356.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siegele D A, Kolter R. Life after log. J Bacteriol. 1992;174:345–348. doi: 10.1128/jb.174.2.345-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Snapper S B, Melton R E, Mustafa S, Kieser T, Jacobs W R., Jr Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol. 1990;4:1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 60.Sudre P, ten Dam G, Kochi A. Tuberculosis: a global review of the situation today. Bull W H O. 1992;70:149–159. [PMC free article] [PubMed] [Google Scholar]
- 61.Thorne S H. Stationary phase survival of Rhizobium leguminosarum. Ph.D. thesis. London, United Kingdom: University of London; 1997. [Google Scholar]
- 62.Thorne S H, Williams H D. Adaptation to nutrient starvation in Rhizobium leguminosarum bv. phaseoli: analysis of survival, stress resistance, and changes in macromolecular synthesis during entry to and exit from stationary phase. J Bacteriol. 1997;179:6894–6901. doi: 10.1128/jb.179.22.6894-6901.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wallace J G. The heat resistance of tubercle bacilli in the lungs of infected mice. Am Rev Respir Dis. 1961;83:866. doi: 10.1164/arrd.1961.83.6.866. [DOI] [PubMed] [Google Scholar]
- 64.Wanner U, Egli T. Dynamics of microbial growth and cell composition in batch culture. FEMS Microbiol Rev. 1990;75:19–44. doi: 10.1111/j.1574-6968.1990.tb04084.x. [DOI] [PubMed] [Google Scholar]
- 65.Warne S R, Varley J M, Boulnois G J, Norton M G. Identification and characterization of a gene that controls colony morphology and auto-aggregation in Escherichia coli K12. J Gen Microbiol. 1990;136:455–462. doi: 10.1099/00221287-136-3-455. [DOI] [PubMed] [Google Scholar]
- 66.Watson S P, Clements M O, Foster S J. Characterization of the starvation-survival response of Staphylococcus aureus. J Bacteriol. 1998;180:1750–1758. doi: 10.1128/jb.180.7.1750-1758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wayne L G. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur J Clin Microbiol Infect Dis. 1994;13:908–914. doi: 10.1007/BF02111491. [DOI] [PubMed] [Google Scholar]
- 68.Wayne L G. Dynamics of submerged growth of Mycobacterium tuberculosis under aerobic and microaerophilic conditions. Am Rev Respir Dis. 1976;114:807–811. doi: 10.1164/arrd.1976.114.4.807. [DOI] [PubMed] [Google Scholar]
- 69.Wayne L G, Hayes L G. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun. 1996;64:2062–2069. doi: 10.1128/iai.64.6.2062-2069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wayne L G, Lin K-Y. Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infect Immun. 1982;37:1042–1049. doi: 10.1128/iai.37.3.1042-1049.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weiser J N, Chong S T H, Greenberg D, Fong W. Identification and characterization of a cell envelope protein of Haemophilus influenzae contributing to phase variation in colony opacity and nasopharyngeal colonization. Mol Microbiol. 1995;17:555–564. doi: 10.1111/j.1365-2958.1995.mmi_17030555.x. [DOI] [PubMed] [Google Scholar]
- 72.Weisser J N, Love J M, Moxon E R. The molecular mechanism of phase variation of H. influenzae lipopolysaccharide. Cell. 1989;59:657–665. doi: 10.1016/0092-8674(89)90011-1. [DOI] [PubMed] [Google Scholar]
- 73.Young D B, Duncan K. Prospects for new interventions in the treatment and prevention of mycobacterial disease. Annu Rev Microbiol. 1995;49:641–673. doi: 10.1146/annurev.mi.49.100195.003233. [DOI] [PubMed] [Google Scholar]
- 74.Yuan Y, Crane D D, Barry C E., III Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial alpha-crystallin homolog. J Bacteriol. 1995;178:4484–4492. doi: 10.1128/jb.178.15.4484-4492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zambrano M M, Kolter R. Gasping for life in stationary phase. Cell. 1996;86:181–184. doi: 10.1016/s0092-8674(00)80089-6. [DOI] [PubMed] [Google Scholar]
- 76.Zambrano M M, Siegele D A, Almiron M, Tormo A, Kolter R. Microbial competition: Escherichia coli mutants that take over stationary phase cultures. Science. 1993;259:1757–1760. doi: 10.1126/science.7681219. [DOI] [PubMed] [Google Scholar]