Abstract
Recent compelling epidemiological studies indicate a strong association of obesity with thyroid cancer. Obesity has been shown to promote thyroid cancer progression and exacerbate poor outcome in thyroid cancer patients. However, the molecular mechanisms by which obesity increases thyroid cancer risk and facilitates cancer progression are not completely understood. Obesity induces complex pathological changes including hyperglycemia, hyperinsulinemia, hyperlipidemia, oxidative stress, adipokines, and inflammatory responses. These changes can affect the development and progression of cancer through highly complex interactions in vivo. The deleterious effect of obesity may differ according to the different cancer types. In view of the increased incidence of thyroid cancer in parallel with the widespread occurrence of obesity in the past decades, it is imperative to clarify how obesity affects thyroid carcinogenesis. This review focuses on molecular mechanisms by which obesity aggravates thyroid carcinogenesis as elucidated by mouse models of thyroid cancer.
Keywords: Thyroid Carcinogenesis, Signal Transducer And Activator Of Transcription (STAT3), Anaplastic Thyroid Cancer (ATC), STAT3 Signaling, Adenosine Monophosphate-activated Kinase (AMPK)
Introduction
Thyroid cancer is the most common endocrine malignancy, and its incidence has risen rapidly in recent decades all over the world [1–5]. The increased use of ultrasonography for the detection of small papillary thyroid carcinoma (PTC) cannot fully explain this increase in thyroid cancer incidence [1]. Previous epidemiological studies suggested that only about half of this increase results from screening using ultrasonography for early detection while the rest could be attributable to environmental factors such as obesity and cigarette smoking [1, 3, 6–8].
The prevalence of obesity has been increasing, not only in adults but also in children and adolescents, in parallel with the increases in thyroid cancer incidence in the USA and elsewhere [9–11]. Several epidemiological studies provide compelling evidence that obesity is associated with an increased risk of thyroid cancer [9–14]. A recent meta-analysis found that the risk of thyroid cancer was 25% greater in overweight and 55% greater in obese individuals than their normal-weight peers [13]. A pooled analysis of 22 prospective studies showed that each 5-unit increase of body mass index (BMI), young-adult BMI, adulthood BMI gain, and waist circumference (per 5 cm) were associated with 6, 13, 7, and 3% greater risks of thyroid cancer, respectively [14]. These associations were consistently observed in all major pathological subtypes of thyroid cancer except medullary thyroid cancer [13, 14]. Several studies also suggested that overweight and obesity are significantly associated with more aggressive clinicopathological features in patients with thyroid cancer [15–17]. These findings underscore the need to study molecular mechanisms underlying the effects of obesity on thyroid carcinogenesis.
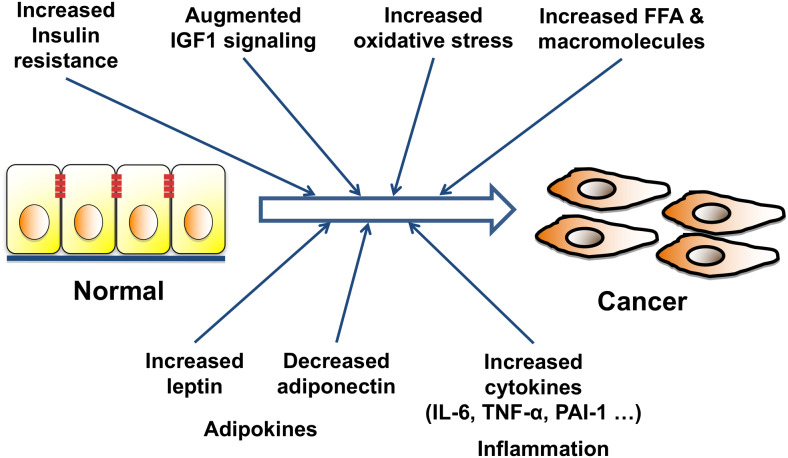
Obesity is known to cause an array of complex and diverse metabolic changes through accumulation of adipocytes. Adipose tissue is considered an endocrine organ that secretes various adipokines involved in metabolic regulation and inflammatory processes [18]. Dysregulation of the endocrine function of adipose tissue leads to hyperglycemia, hyperlipidemia, and insulin resistance. As illustrated in Fig. 1, obesity, due to excessive accumulation of adipose tissues, leads to the increased secretion of adipokines, increased inflammatory responses, and other metabolic changes that could impact the development and progression of many cancers. In this review, we highlight the molecular mechanisms by which obesity aggravates thyroid carcinogenesis which has been uncoveed in studies using mouse models of thyroid cancer.
Fig. 1.
Various pathways may explain the mechanism by which obesity induces carcinogenesis and exacerbates cancer progression. Obesity increases adipocytes, and the extent of systemic inflammation, and oxidative stress. Insulin resistance induced by obesity results in increased serum insulin levels, augmented IGF-1 signaling, increases serum leptin levels and suppresses serum adiponectin levels. Increases in insulin resistance, IGF-1 signaling, oxidative stress, free fatty acid (FFA), inflammatory signaling, and changes in adiopkines and other macromolecules, may contribute to cancer progression, and metastasis
Obesity in Mouse Models of Thyroid Cancer
A diet-induced obesity model has been widely used to evaluate the effects of obesity on human diseases. The effect of obesity on thyroid carcinogenesis was studied using mouse models of follicular thyroid cancer (FTC). The mouse, which harbors a knock-in dominant negative mutation (denoted as PV) in the Thrb gene, spontaneously develops metastatic FTC [19]. The ThrbPV/PV mice showed pathological progression of capsular and vascular invasion, lung metastases, and anaplastic changes similar to human thyroid cancer [20]. Additionally, deletion of one allele of the Pten gene (phosphatase and tensin homolog deleted from chromosome 10) into ThrbPV/PV mice reduced the time required for spontaneous development and progression of FTC in ThrbPV/PVPten+/− mice [21]. The loss of one allele of the tumor suppressor, the Pten gene, induces further overactivation of phosphatidylinositol 3-kinase (PI3K) and protein kinase B (AKT) signaling pathway in thyroid cancer of ThrbPV/PVPten+/− mice with more aggressive thyroid cancer and shorter survival than in ThrbPV/PV mice [21].
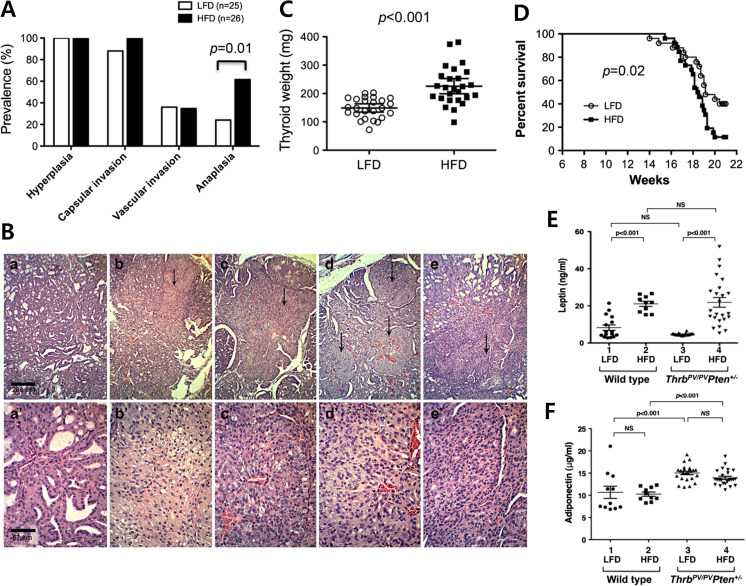
This double mutant mouse model has provided an opportunity to evaluate the effects of diet-induced obesity on the carcinogenesis of the thyroid [22] . Feeding ThrbPV/PVPten+/− mice with high fat diet (HFD) for 15 weeks successfully induced the phenotype of obesity in this mouse model with body weight gain and increases of adiposity [22]. Virtually all ThrbPV/PVPten+/− mice exhibited early-phase thyroid cancer developments such as thyroid hyperplasia and capsular invasion (Fig. 2a), which were similarly detected in the double mutant mice treated with HFD. Remarkably, anaplastic transformation of thyroid cancer was promoted by HFD-induced obese ThrbPV/PVPten+/− mice (Fig. 2b) [22]. About 62% of thyroid cancers in the HFD group underwent anaplastic transformation versus only 24% in the low fat diet (LFD) group (Fig. 2a). Microscopic examination of H&E-stained tumor sections of representative HFD group samples indicated a loss of glandular differentiation and spindle cell anaplasia (Fig. 2b). The tumor weights of the HFD group (n = 26) were significantly higher than those of the LFD group (n = 25, p < 0.001), as shown in Fig. 2c. The survival of the HFD group was significantly shorter than the LFD group (p = 0.02, Fig. 2d) [22].
Fig. 2.
HFD-induced obesity induces aggressive phenotype in ThrbPV/PVPten+/− mice. a Pathological changes in thyroid cancer progression in high-fat diet (HFD) group and low-fat diet (LFD) group. There was significant increase in anaplastic transformation after HFD-induced obesity. b Anaplastic transformations in HFD-induced obese ThrbPV/PVPten+/− mice. H&E-stained thyroid tumor sections of LFD-treated ThrbPV/PVPten+/− mice are shown in panels a and a′at low magnification and high magnification, respectively. H&E-stained thyroid tumor sections of HFD-treated ThrbPV/PVPten+/− mice are shown in panels b, c, d, and e and in b′, c′, d′, and e′ at low and high magnification, respectively. Representative samples of anaplastic loci indicated by arrows are apparent at low magnification in panels b, c, d, and e (×20). The foci are selected shown at high magnification in the corresponding panels b′, c′, d′, and e′ (×80). c The weights of thyroid tumors in HFD group and LFD group, presented as mean ± SE. The difference was analyzed by Student’s t test. d The survival of ThrbPV/PVPten+/− mice was shorter in HFD group than LFD group. The Kaplan-Meier curves were compared by the log-rank test. e, f The serum leptin and adiponectin concentrations in wild-type and ThrbPV/PVPten+/− mice treated with LFD or HFD. [24]
Currently, it was not entirely clear the underlying molecular mechanisms by which obesity led to anaplasia. Anaplastic transformation occurs in the advanced stage of thyroid carcinogenesis, suggesting that additional “hits” could be acquired during cancer progression. Indeed, findings from next generation sequencing supported the notion that anaplastic thyroid cancer was derived from well differentiated thyroid carcinoma through accumulation of genetic abnormalities [23, 24]. In HFD-ThrbPV/PVPten+/− mice, serum leptin was elevated, resulting in the activation of JAK-STAT3 signaling [22]. Several downstream effectors in JAK-STAT3 signaling, such as c-MYC, were found to be also elevated [22]. It is known that c-MYC induces cell de-differentiation. In human anaplastic thyroid cancer, c-MYC was shown over-expressed [25]. One mechanism by which over-expression of c-MYC was found via activation of super-enhancers through chromatin remodeling [26]. However, the over-expression of c-MYC could also be due to gene amplification and/or other genetic aberration events. Such changes could be broadly considered as additional “hits” to affect anaplastic transformation, which is linked closely with the up-stream events initiated from obesity.
Obesity affects the secretion of adipokines, the hormones secreted by adipocytes, with increased leptin and decreased adiponectin levels being well known examples [27]. It is known that leptin binds to its receptor, leading to the activation of intracellular Janus kinase 2 (JAK2)-signal transducer and activator of transcription 3 (STAT3) and mitogen-activated protein kinase (MAPK) signaling system, thereby promoting the growth of cancer cells [28, 29]. On the other hand, adiponectin inhibits the PI3K-AKT-mechanistic target of rapamycin (mTOR) signaling through activation of adenosine monophosphate-activated kinase (AMPK), which plays an important role in intracellular energy metabolism and inhibits the growth of cancer cells [28, 30]. Thus, the decrease of adiponectin caused by obesity may play an important role in the development and progression of cancer. These findings suggest that the change in adipokine plays an important role in thyroid cancer. A previous study revealed that leptin and leptin receptors were over-expressed in human PTCs and were associated with a more aggressive cancer phenotype [31]. Studies also suggested that the expression of both leptin and its receptor was regulated by insulin epigenetically [32]. An in vitro study using thyroid cancer cells demonstrated that leptin induced cancer cell proliferation and migration and inhibited apoptosis [33, 34]. In ThrbPV/PVPten+/− mice, serum leptin levels were significantly increased by HFD-induced obesity (Fig. 2e), whereas there was no signification difference in serum adiponectin levels (Fig. 2f) [22]. Increased serum leptin levels were detected in obese ThrbPV/PVPten+/− mice [22]. Moreover, over-activation of the JAK2-STAT3 signaling pathway and increased expression of STAT3 target genes were found in obese ThrbPV/PVPten+/− mice [22]. These findings indicate that increased serum leptin levels are involved in promoting the progression of thyroid cancer by activating the JAK2- STAT3 signaling.
Recent studies compared the effects of leptin and orally available leptin-derived peptide, OB3 in thyroid cancer cells [35]. OB3 has shown to be effective appetite suppression similarly as metformin in preclinical studies [36, 37]. Leptin stimulated thyroid cancer cell invasion through activation of STAT3 and MAPK pathway. However OB3 did not affect cancer cell proliferation and invasion [35]. Besides, OB3 reported to suppress leptin-induced signaling and progression of ovarian cancer cells [38]. These findings suggested that potential reduction of serum leptin levels by metformin or OB3 could be useful to suppress leptin-induced stimulation in cancer progression.
JAK-STAT3 Signaling in Obese Mouse Models of Thyroid Cancer
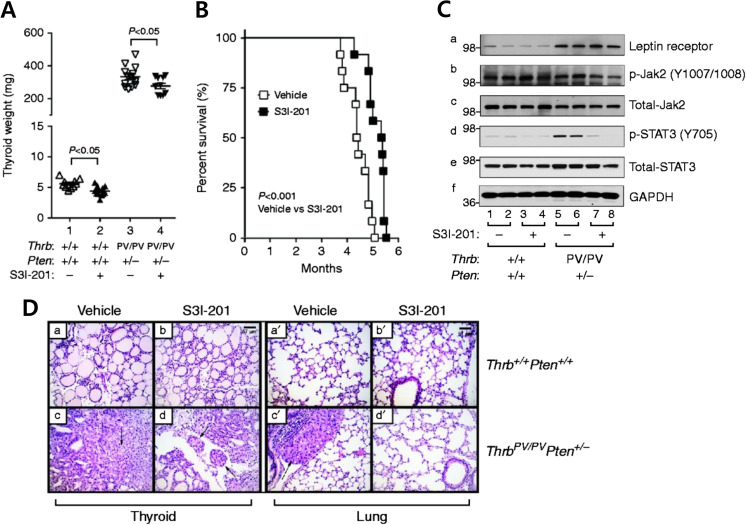
Activation of the JAK-STAT3 signaling pathway has been shown to be associated with increased invasion and metastasis in various types of cancer [39]. In normal cells STAT3 signaling is tightly controlled by a negative feedback mechanism involving the suppressors of cytokine signaling (SOCS) and tyrosine phosphatases, whereas in cancer cells constitutive activation of STAT3 is common [39]. Overexpression of STAT3 signaling is also associated with lymphatic metastasis of thyroid cancer [40, 41]. The role of JAK-STAT3 signaling in the anaplastic transformation of FTC was confirmed by treating ThrbPV/PVPten+/− mice with the STAT3 selective inhibitor, S3I-201 [42]. This inhibitor, which was identified through structure-based virtual screening of the National Cancer Institute libraries, has shown its effectiveness in the inhibition of STAT3 activity in both in vitro and in vivo studies [43]. S3I-201 treatment significantly reduced the thyroid tumor weight of obese ThrbPV/PVPten+/− mice (thyroid cancer incidence Fig. 3a). The median survival of obese ThrbPV/PVPten+/− mice treated with S3I-201 was significantly longer (1 month) than vehicle-treated mice (Fig. 3b). Moreover, this inhibitor suppressed the phosphorylation of tyrosine 705 (Y705), critical for STAT3 activation (Fig. 3c). Inhibition of STAT3 activity led to the suppression of downstream target genes such as Ccnd1, Myc, Bcl2, Mcl1, and Socs3, thus contributing to the delayed tumor growth and survival [42]. The reduced expression of Ccnd1, Myc, and Bcl2 can inhibit cancer cell proliferation and promote cancer cell survival [44]. Detailed molecular analysis also revealed that inhibition of STAT3 reduces the expression of cell cycle regulators to inhibit thyroid tumor cell proliferation without evidence of any change in thyroid function [42]. Inhibition of the STAT3 signaling pathway also delayed thyroid cancer progression. As shown in Fig. 3d, the anaplasia phenotype in vehicle-treated thyroid tumors reverted to a less progressed vascular invasion (panel d). While we saw metastases in the lung of vehicle-treated obese ThrbPV/PVPten+/− mice (panel c′), none were observed in the lung of inhibitor-treated mice ((panel d′), Fig. 3d). These findings further strengthen the notion that elevated leptin could promote carcinogenesis via JAK-STAT3 signaling in obese ThrbPV/PVPten+/− mice.
Table 1.
Summary of phenotypical changes in a mouse model of thyroid cancer
| Animal model | Intervention | Phenotype | Reference |
|---|---|---|---|
| Thrb PV/PV Pten +/− | HFD-induced obesity | Large tumor size, shorter survival, more anaplastic change | 22 |
| Thrb PV/PV Pten +/− | STAT3 inhibitor, S3I-201 | Delay tumor growth and survival, inhibit lung metastasis | 42 |
| Thrb PV/PV Pten +/− | Metformin | Reduce capsular invasion, vascular invasion, and anaplasia | 61 |
Fig. 3.
The effects of STAT3 inhibitor, S3I-201, in HFD-treated obese ThrbPV/PVPten+/− mice. a Survival curves for HFD-ThrbPV/PVPten+/− mice treated with S3I-201 or vehicle. Intraperitoneal injection three times a week from 8 weeks of age until they had to be euthanized because of sickness. Data are presented by Kaplan-Meier methods and analyzed by log-rank test. The p values are indicated. b Thyroid weights of vehicle-treated or S3I-201-treated wild-type or ThrbPV/PVPten+/−. c Western blot analysis of protein abundance of leptin receptor, phosphorylated -JAK2 (Y1007/1008), total JAK-2, phosphorylated STAT3 (Y705), total-STAT3, and GAPDH as a loading control after treatment with vehicle or S3I-201 in wild-type and ThrbPV/PVPten+/− mice. d Representative examples of hematoxylin and eosin (H&E)-stained thyroid sections from wild-type mice treated with vehicle (panel a) and S3I-201 (panel b) and lung sections treated with vehicle (panel a′) and S3I-201 (panel b′) and thyroid tumor sections from ThrbPV/PVPten+/− treated with vehicle (panel c) and S3I-201 (panel d) and lung sections treated with vehicle (panel c′) and S3I-201 (panel d′). The arrows indicate vascular invasion (panel d) and anaplasia (panel c). [36]
Therapeutic Potential of Metformin in Obesity Associated with Thyroid Cancer
With well-established efficacy and safety profiles, metformin is the most widely used anti-diabetes drug for treatment of patients with type II diabetes. Metformin has shown anti-cancer activities in both in vitro and in vivo studies for many cancers [45]. The molecular basis by which metformin acts beneficially for patients remains inconclusive. Both direct and indirect effects of metformin have been proposed for anti-cancer properties, such as a direct inhibition of the AMPK/mTOR signaling pathway and/or an indirect lowering of glucose, insulin, and anti-inflammatory effects [46, 47]. Clinical studies have shown that metformin has anti-proliferative effects in early breast cancer and suppresses precancerous lesions in colorectal cancer [48–50]. Results of meta-analyses suggest a possible role for metformin in the primary prevention of cancer, as well as survival benefits of metformin’s use in cancer patients [51–53]. A recent meta-analysis also supports the use of metformin as an adjuvant therapeutic agent, especially in patients with colorectal and prostatic cancer who have undergone radical radiotherapy [54].
In two studies, the use of metformin was associated with better prognostic factors and treatment outcomes in diabetic patients with thyroid cancer [55, 56]. One of these studies showed that primary tumor size is smaller in patients treated with metformin, suggesting it inhibits tumor growth [55]. The other cohort study reported significantly lower recurrence of differentiated thyroid cancer in diabetic patients treated with metformin [56]. Several in vitro studies revealed that metformin inhibits cell proliferation in thyroid cancer cells including PTC, medullary, and anaplastic thyroid cancer (ATC) cells [55, 57–60]. Metformin inhibits cancer cell growth by activation of AMPK and downregulation of the mTOR signaling pathway in PTC and FTC cell lines [55]. In ATC cell lines, metformin showed anti-mitogenic effects by inhibition of cell cycle progression and induction of apoptosis [57]. Metformin also potentiated the effects of chemotherapeutic agents such as doxorubicin and cisplatin in ATC cells [57]. These observations suggest the possibility of clinical use of metformin as an adjuvant treatment for patients with ATC.
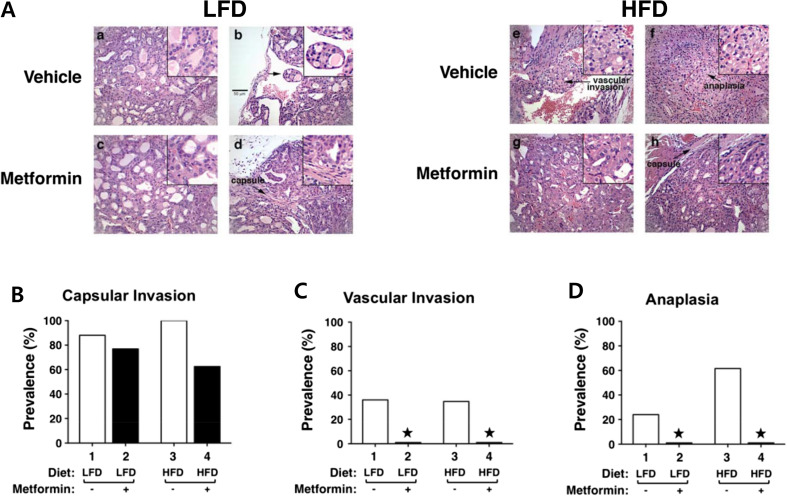
The beneficial effects of metformin were also demonstrated in vivo in HFD-induced obese ThrbPV/PVPten+/− mice. HFD-induced obesity promoted the anaplastic transformation of thyroid cancer in ThrbPV/PVPten+/− mice [22]. HFD promoted tumor progression from extensive hyperplasia (Fig. 4a, panel a) and early vascular invasion (panel b) in the thyroid of LFD-ThrbPV/PVPten+/− mice to the more advanced stages of vascular invasion (panel e) and anaplasia (panel f) [61]. Treatment with metformin from the age of 6 to 21 weeks reduced tumor progression from vascular invasion (Fig. 4a, panel b) to hyperplasia (panel d) in LFD-ThrbPV/PVPten+/− mice [61]. More impressively, metformin markedly reduced the tumor phenotypes from vascular invasion (Fig. 4a, panel e) and anaplasia (panel f) to capsular invasion (panel h) in HFD-ThrbPV/PVPten+/− mice [61]. The beneficial effects of metformin on the pathological changes are clearly evident in reduced capsular invasion (Fig. 4b) and blocked occurrence of vascular invasion (Fig. 4c) and anaplastic transformation (Fig. 4d) in HFD-ThrbPV/PVPten+/− mice [61] . These beneficial effects of metformin on thyroid cancer progression support the use of metformin as an adjuvant therapy for patients with refractory thyroid cancer or ATC.
Fig. 4.
Effects of metformin on thyroid cancer progression of LFD- or HFD-ThrbPV/PVPten+/− mice. a Representative examples of hematoxylin and eosin (H&E)-stained thyroid sections from the vehicle-treated LFD (panels a and b), metformin-treated LFD (panels c and d), vehicle-treated HFD (panels e and f), metformin-treated HFD (panels g and h) groups of ThrbPV/PVPten+/− mice. The magnification is ×166. Arrows indicate pathological features of vascular invasion (panel e), capsular invasion (panels d and h) and anaplasia (panel f). The detailed pathological features are enlarged in a higher magnification of ×332. b–d Pathologic analysis of the vehicle-treated LFD (n = 25), metformin-treated LFD (n = 13), vehicle-treated HFD (n = 26), and metformin-treated HFD (n = 18) groups of ThrbPV/PVPten+/− mice. The prevalence of each pathologic feature in mice treated with vehicle or metformin is shown as percentage of occurrence for capsular invasion (b), vascular invasion (c), and anaplasia (d). *Represents no occurrence. [55]
Metformin acts through three different mechanisms to delay thyroid carcinogenesis of HFD-ThrbPV/PVPten+/− mice. The first is that metformin inhibits the activation of the STAT3 signaling pathway in this mouse model. One study found that the activation of leptin-JAK2-STAT3 signaling accounts for the HFD-induced promotion of thyroid cancer progression in ThrbPV/PVPten+/− mice [22]. Another showed that metformin treatment reduces the HFD-induced activation of STAT3 signaling by decreasing the extent of phosphorylation of STAT3 at tyrosine 705, which is critical for STAT3 activation, in these mouse thyroid tumor tissues [61]. The second mechanism is that metformin inhibits the extracellular signal-regulated kinase (ERK) signaling pathway. Leptin mediates its effects via not only STAT3 signaling, but also ERK signaling [62]. That metformin treatment attenuates the HFD-induced activation of ERK signaling is made evident by the reduced phosphorylation of ERK proteins in thyroid tumors [61]. The third mechanism by which metformin delays thyroid carcinogenesis is through its effect on cytoskeletal structure, cancer cell motility, and migration via inhibition of the epithelial-mesenchymal-transition (EMT) and fibronectin (FN)-integrin signaling pathway in HFD-ThrbPV/PVPten+/− mice [61]. Metformin treatment significantly decreases the expression of vimentin, a type III intermediate filament protein and a major cytoskeletal component in mesenchymal cells [61]. FN and its receptors such as integrins α6, β1, and β3 were abundant in thyroid tumors from HFD-ThrbPV/PVPten+/− mice, and metformin treatment markedly reduced the protein levels of FN and integrins [61]. Currently, the detailed molecular basis underlying the metabolic effects of metformin are not completely understood. However, these findings suggest that metformin could act could act in vivo in ways other than inhibiting the AMPK and mTOR pathways. These newly discovered pathways mediated by metformin could play a critical role in thyroid cancer.
Perspectives and Future Directions
The in vivo molecular evidence presented in this review clearly demonstrates that obesity could impact thyroid carcinogenesis in a mouse model of thyroid cancer, ThrbPV/PVPten+/− mice. HFD-induced obesity accelerates the growth and progression of thyroid cancer, notably shortening survival and promoting anaplastic transformation. These changes were elucidated via the HFD-induced elevated leptin to act through the JAK2-STAT3 signaling pathway. Preclinical targeting of the STAT3 by using a STAT3-specific inhibitor, S3I-201, further validated the relevance and the critical role of the leptin downstream effector in obesity-activated thyroid carcinogenesis. That STAT3 could be a potential target raised the possibility that the findings from the mouse model could be translated to clinical practice. Oral STAT3 inhibitors and anti-sense STAT3 (AZD9150) are emerging as potential anti-cancer agents and are being tested in various phases of clinical trials for solid tumors, refractory hematological malignancies, lung, and head and neck cancers [63–69]. Currently, there are no ongoing trials for STAT3 inhibitors in obesity-activated thyroid cancer. However, the successful trials of STAT3 inhibitors in the cancers just mentioned could pave the way for future testing of STAT3 inhibitors on thyroid cancer exacerbated by obesity.
The finding that metformin could delay obesity-activated thyroid cancer progression provides an extraordinary opportunity for a novel treatment modality for thyroid cancer. At present, five active phase III clinical trials are using metformin as a single or combination treatment strategy in breast, endometrial, prostatic cancer, colorectal, and hepatocellular carcinoma (https://clinicaltrials.gov). Two clinical trials in colorectal (NCT02614339) and hepatocellular carcinoma (NCT03184493) are in the clinical setting of adjuvant therapy after the initial cancer treatment. The other clinical trial is for the combination treatment with standard chemotherapy in advanced or recurrent endometrial cancer (NCT02065687). Interestingly, two active phase III clinical trials using metformin are for preventing progression of low-risk prostatic cancer (NCT01864096) and progression of breast cancer in patients with atypical hyperplasia or in situ breast cancer (NCT 01905046). However, no ongoing clinical trial is evaluating therapeutic effects of metformin on thyroid cancer. Two active clinical trials are for the purpose of mitigating the side effects of radioactive iodine treatment by the use of metformin in patients with differentiated thyroid cancer (NCT0309847) and evaluating the effects of metformin use on volume change of benign thyroid nodules (NCT03183752). Taken together, the current trials testing metformin as a therapeutic for other cancers and the preclinical positive findings thus far from the mouse model suggest that metformin could be beneficial for obesity-activated thyroid cancer. Future studies are needed to bring metformin to the forefront of treatment modalities for thyroid cancer.
The present review highlights the contribution of the leptin-JAK2-STAT3 signaling pathway to the development and progression of obesity-activated thyroid cancer. This, however, is only one aberrant pathway that is affected by obesity. Obesity causes a barrage of changes in the human body associated with cancer development, including alterations in insulin/IGF-1 signaling, fatty acids/lipid signaling, oxidative stress, adipokines, and inflammation. It would be useful to dissect the contributions of these different signaling pathways in obesity-activated thyroid carcinogenesis. By doing so, a better picture of how obesity impacts thyroid carcinogenesis would emerge. Importantly, these molecular mechanisms thus identified could be used as the basis for discovering and applying novel therapeutic targets for cancer treatment associated with obesity.
Acknowledgments
We regret any reference omissions due to length limitation. We wish to thank all colleagues and collaborators who have contributed to the work described in this review. The research described in this review by the authors and their colleagues at the National Cancer Institute was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
References
- 1.Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12(11):646–653. doi: 10.1038/nrendo.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295(18):2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 3.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988-2005. Cancer. 2009;115(16):3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 4.Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”—screening and overdiagnosis. N Engl J Med. 2014;371(19):1765–1767. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 5.Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, Rothman N, Zhang Y. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control. 2009;20(5):525–531. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Udelsman R, Zhang Y. The epidemic of thyroid cancer in the United States: the role of endocrinologists and ultrasounds. Thyroid. 2014;24(3):472–479. doi: 10.1089/thy.2013.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, Devesa SS. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol Biomark Prev. 2009;18(3):784–791. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brito JP, Gionfriddo M, Morris JC, Montori VM. Overdiagnosis of thyroid cancer and graves’ disease. Thyroid. 2014;24(2):402–403. doi: 10.1089/thy.2013.0425. [DOI] [PubMed] [Google Scholar]
- 9.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 10.Kitahara CM, Platz EA, Freeman LE, Hsing AW, Linet MS, Park Y, Schairer C, Schatzkin A, Shikany JM, Berrington de Gonzalez A. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomark Prev. 2011;20(3):464–472. doi: 10.1158/1055-9965.EPI-10-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han JM, Kim TY, Jeon MJ, Yim JH, Kim WG, Song DE, Hong SJ, Bae SJ, Kim HK, Shin MH, Shong YK, Kim WB. Obesity is a risk factor for thyroid cancer in a large, ultrasonographically screened population. Eur J Endocrinol. 2013;168(6):879–886. doi: 10.1530/EJE-13-0065. [DOI] [PubMed] [Google Scholar]
- 12.Ma J, Huang M, Wang L, Ye W, Tong Y, Wang H (2015) Obesity and risk of thyroid cancer: evidence from a meta-analysis of 21 observational studies. Med Sci Monit:21283–21291 [DOI] [PMC free article] [PubMed]
- 13.Schmid D, Ricci C, Behrens G, Leitzmann MF. Adiposity and risk of thyroid cancer: a systematic review and meta-analysis. Obes Rev. 2015;16(12):1042–1054. doi: 10.1111/obr.12321. [DOI] [PubMed] [Google Scholar]
- 14.Kitahara CM, McCullough ML, Franceschi S, Rinaldi S, Wolk A, Neta G, Olov Adami H, Anderson K, Andreotti G, Beane Freeman LE, Bernstein L, Buring JE, Clavel-Chapelon F, De Roo LA, Gao YT, Gaziano JM, Giles GG, Hakansson N, Horn-Ross PL, Kirsh VA, Linet MS, MacInnis RJ, Orsini N, Park Y, Patel AV, Purdue MP, Riboli E, Robien K, Rohan T, Sandler DP, Schairer C, Schneider AB, Sesso HD, Shu XO, Singh PN, van den Brandt PA, Ward E, Weiderpass E, White E, Xiang YB, Zeleniuch-Jacquotte A, Zheng W, Hartge P, de Gonzalez AB. Anthropometric factors and thyroid cancer risk by histological subtype: pooled analysis of 22 prospective studies. Thyroid. 2016;26(2):306–318. doi: 10.1089/thy.2015.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HJ, Kim NK, Choi JH, Sohn SY, Kim SW, Jin SM, Jang HW, Suh S, Min YK, Chung JH, Kim SW. Associations between body mass index and clinico-pathological characteristics of papillary thyroid cancer. Clin Endocrinol. 2013;78(1):134–140. doi: 10.1111/j.1365-2265.2012.04506.x. [DOI] [PubMed] [Google Scholar]
- 16.Tresallet C, Seman M, Tissier F, Buffet C, Lupinacci RM, Vuarnesson H, Leenhardt L, Menegaux F. The incidence of papillary thyroid carcinoma and outcomes in operative patients according to their body mass indices. Surgery. 2014;156(5):1145–1152. doi: 10.1016/j.surg.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Choi JS, Kim EK, Moon HJ, Kwak JY. Higher body mass index may be a predictor of extrathyroidal extension in patients with papillary thyroid microcarcinoma. Endocrine. 2015;48(1):264–271. doi: 10.1007/s12020-014-0293-z. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83(2):461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 19.Kaneshige M, Kaneshige K, Zhu X, Dace A, Garrett L, Carter TA, Kazlauskaite R, Pankratz DG, Wynshaw-Boris A, Refetoff S, Weintraub B, Willingham MC, Barlow C, Cheng S. Mice with a targeted mutation in the thyroid hormone beta receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc Natl Acad Sci U S A. 2000;97(24):13209–13214. doi: 10.1073/pnas.230285997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki H, Willingham MC, Cheng SY. Mice with a mutation in the thyroid hormone receptor beta gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid. 2002;12(11):963–969. doi: 10.1089/105072502320908295. [DOI] [PubMed] [Google Scholar]
- 21.Guigon CJ, Zhao L, Willingham MC, Cheng SY. PTEN deficiency accelerates tumour progression in a mouse model of thyroid cancer. Oncogene. 2009;28(4):509–517. doi: 10.1038/onc.2008.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim WG, Park JW, Willingham MC, Cheng SY. Diet-induced obesity increases tumor growth and promotes anaplastic change in thyroid cancer in a mouse model. Endocrinology. 2013;154(8):2936–2947. doi: 10.1210/en.2013-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126(3):1052–1066. doi: 10.1172/JCI85271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeon MJ, Chun SM, Kim D, Kwon H, Jang EK, Kim TY, Kim WB, Shong YK, Jang SJ, Song DE, Kim WG. Genomic alterations of anaplastic thyroid carcinoma detected by targeted massive parallel sequencing in a BRAF(V600E) mutation-prevalent area. Thyroid. 2016;26(5):683–690. doi: 10.1089/thy.2015.0506. [DOI] [PubMed] [Google Scholar]
- 25.Enomoto K, Zhu X, Park S, Zhao L, Zhu YJ, Willingham MC, Qi J, Copland JA, Meltzer P, Cheng SY. Targeting MYC as a therapeutic intervention for anaplastic thyroid cancer. J Clin Endocrinol Metab. 2017;102(7):2268–2280. doi: 10.1210/jc.2016-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu X, Enomoto K, Zhao L, Zhu YJ, Willingham MC, Meltzer P, Qi J, Cheng SY. Bromodomain and extraterminal protein inhibitor JQ1 suppresses thyroid tumor growth in a mouse model. Clin Cancer Res. 2017;23(2):430–440. doi: 10.1158/1078-0432.CCR-16-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014;220(2):T47–T59. doi: 10.1530/JOE-13-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11(12):886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 29.Vansaun MN. Molecular pathways: adiponectin and leptin signaling in cancer. Clin Cancer Res. 2013;19(8):1926–1932. doi: 10.1158/1078-0432.CCR-12-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hebbard L, Ranscht B. Multifaceted roles of adiponectin in cancer. Best Pract Res Clin Endocrinol Metab. 2014;28(1):59–69. doi: 10.1016/j.beem.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng SP, Chi CW, Tzen CY, Yang TL, Lee JJ, Liu TP, Liu CL. Clinicopathologic significance of leptin and leptin receptor expressions in papillary thyroid carcinoma. Surgery. 2010;147(6):847–853. doi: 10.1016/j.surg.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Cheng SP, Liu CL, Hsu YC, Chang YC, Huang SY, Lee JJ. Regulation of leptin receptor expression in human papillary thyroid cancer cells. Biomed Pharmacother. 2012;66(6):469–473. doi: 10.1016/j.biopha.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Uddin S, Bavi P, Siraj AK, Ahmed M, Al-Rasheed M, Hussain AR, Ahmed M, Amin T, Alzahrani A, Al-Dayel F, Abubaker J, Bu R, Al-Kuraya KS. Leptin-R and its association with PI3K/AKT signaling pathway in papillary thyroid carcinoma. Endocr Relat Cancer. 2010;17(1):191–202. doi: 10.1677/ERC-09-0153. [DOI] [PubMed] [Google Scholar]
- 34.Cheng SP, Yin PH, Hsu YC, Chang YC, Huang SY, Lee JJ, Chi CW. Leptin enhances migration of human papillary thyroid cancer cells through the PI3K/AKT and MEK/ERK signaling pathways. Oncol Rep. 2011;26(5):1265–1271. doi: 10.3892/or.2011.1388. [DOI] [PubMed] [Google Scholar]
- 35.Yang YC, Chin YT, Hsieh MT, Lai HY, Ke CC, Crawford DR, Lee OK, Fu E, Mousa SA, Grasso P, Liu LF, Chang HY, Tang HY, Lin HY, Davis PJ. Novel leptin OB3 peptide-induced signaling and progression in thyroid cancers: comparison with leptin. Oncotarget. 2016;7(19):27641–27654. doi: 10.18632/oncotarget.8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee DW, Leinung MC, Grasso P. Oral delivery of mouse [D-Leu-4]-OB3, a synthetic peptide amide with leptin-like activity, in male Swiss Webster mice: a study comparing the pharmacokinetics of oral delivery to intraperitoneal, subcutaneous, intramuscular, and intranasal administration. Regul Pept. 2010;160(1–3):129–132. doi: 10.1016/j.regpep.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Novakovic ZM, Leinung MC, Grasso P (2013) [D-Leu-4]-OB3, an orally bioavailable leptin-related synthetic peptide insulin sensitizer: a study comparing the efficacies of [D-Leu-4]-OB3 and metformin on energy balance and glycemic regulation in insulin-deficient male Swiss Webster mice. Peptides:43167–43173 [DOI] [PubMed]
- 38.Chin YT, Wang LM, Hsieh MT, Shih YJ, Nana AW, Changou CA, Yang YSH, Chiu HC, Fu E, Davis PJ, Tang HY, Lin HY. Leptin OB3 peptide suppresses leptin-induced signaling and progression in ovarian cancer cells. J Biomed Sci. 2017;24(1):51. doi: 10.1186/s12929-017-0356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu H, Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev Cancer. 2004;4(2):97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Gill A, Atmore B, Johns A, Delbridge L, Lai R, McMullen T. Upregulation of the signal transducers and activators of transcription 3 (STAT3) pathway in lymphatic metastases of papillary thyroid cancer. Int J Clin Exp Pathol. 2011;4(4):356–362. [PMC free article] [PubMed] [Google Scholar]
- 41.Dong W, Cui J, Tian X, He L, Wang Z, Zhang P, Zhang H. Aberrant sonic hedgehog signaling pathway and STAT3 activation in papillary thyroid cancer. Int J Clin Exp Med. 2014;7(7):1786–1793. [PMC free article] [PubMed] [Google Scholar]
- 42.Park JW, Han CR, Zhao L, Willingham MC, Cheng SY. Inhibition of STAT3 activity delays obesity-induced thyroid carcinogenesis in a mouse model. Endocr Relat Cancer. 2016;23(1):53–63. doi: 10.1530/ERC-15-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence NJ, Sebti SM, Turkson J. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci U S A. 2007;104(18):7391–7396. doi: 10.1073/pnas.0609757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Yue P, Page BD, Li T, Zhao W, Namanja AT, Paladino D, Zhao J, Chen Y, Gunning PT, Turkson J. Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts. Proc Natl Acad Sci U S A. 2012;109(24):9623–9628. doi: 10.1073/pnas.1121606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dowling RJ, Niraula S, Stambolic V, Goodwin PJ. Metformin in cancer: translational challenges. J Mol Endocrinol. 2012;48(3):R31–R43. doi: 10.1530/JME-12-0007. [DOI] [PubMed] [Google Scholar]
- 46.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67(22):10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 47.Dowling RJ, Goodwin PJ, Stambolic V (2011) Understanding the benefit of metformin use in cancer treatment. BMC Med 933 [DOI] [PMC free article] [PubMed]
- 48.Hadad S, Iwamoto T, Jordan L, Purdie C, Bray S, Baker L, Jellema G, Deharo S, Hardie DG, Pusztai L, Moulder-Thompson S, Dewar JA, Thompson AM. Evidence for biological effects of metformin in operable breast cancer: a pre-operative, window-of-opportunity, randomized trial. Breast Cancer Res Treat. 2011;128(3):783–794. doi: 10.1007/s10549-011-1612-1. [DOI] [PubMed] [Google Scholar]
- 49.Niraula S, Dowling RJ, Ennis M, Chang MC, Done SJ, Hood N, Escallon J, Leong WL, McCready DR, Reedijk M, Stambolic V, Goodwin PJ. Metformin in early breast cancer: a prospective window of opportunity neoadjuvant study. Breast Cancer Res Treat. 2012;135(3):821–830. doi: 10.1007/s10549-012-2223-1. [DOI] [PubMed] [Google Scholar]
- 50.Hosono K, Endo H, Takahashi H, Sugiyama M, Sakai E, Uchiyama T, Suzuki K, Iida H, Sakamoto Y, Yoneda K, Koide T, Tokoro C, Abe Y, Inamori M, Nakagama H, Nakajima A. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev Res (Phila) 2010;3(9):1077–1083. doi: 10.1158/1940-6207.CAPR-10-0186. [DOI] [PubMed] [Google Scholar]
- 51.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, Gandini S. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3(11):1451–1461. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 52.Zhang P, Li H, Tan X, Chen L, Wang S. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol. 2013;37(3):207–218. doi: 10.1016/j.canep.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 53.Zhang ZJ, Li S. The prognostic value of metformin for cancer patients with concurrent diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16(8):707–710. doi: 10.1111/dom.12267. [DOI] [PubMed] [Google Scholar]
- 54.Coyle C, Cafferty FH, Vale C, Langley RE. Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Ann Oncol. 2016;27(12):2184–2195. doi: 10.1093/annonc/mdw410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klubo-Gwiezdzinska J, Costello J, Jr., Patel A, Bauer A, Jensen K, Mete M, Burman KD, Wartofsky L, Vasko V (2013) Treatment with metformin is associated with higher remission rate in diabetic patients with thyroid cancer. J Clin Endocrinol Metab 98(8):3269–3279, DOI: 10.1210/jc.2012-3799 [DOI] [PubMed]
- 56.Jang EK, Kim WG, Kwon H, Choi YM, Jeon MJ, Kim TY, Shong YK, Kim WB, Kim EY. Metformin is associated with a favorable outcome in diabetic patients with cervical lymph node metastasis of differentiated thyroid cancer. Eur Thyroid J. 2015;4(3):181–188. doi: 10.1159/000437365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen G, Xu S, Renko K, Derwahl M. Metformin inhibits growth of thyroid carcinoma cells, suppresses self-renewal of derived cancer stem cells, and potentiates the effect of chemotherapeutic agents. J Clin Endocrinol Metab. 2012;97(4):E510–E520. doi: 10.1210/jc.2011-1754. [DOI] [PubMed] [Google Scholar]
- 58.Klubo-Gwiezdzinska J, Jensen K, Costello J, Patel A, Hoperia V, Bauer A, Burman KD, Wartofsky L, Vasko V. Metformin inhibits growth and decreases resistance to anoikis in medullary thyroid cancer cells. Endocr Relat Cancer. 2012;19(3):447–456. doi: 10.1530/ERC-12-0046. [DOI] [PubMed] [Google Scholar]
- 59.Shen CT, Wei WJ, Qiu ZL, Song HJ, Zhang XY, Sun ZK, Luo QY. Metformin reduces glycometabolism of papillary thyroid carcinoma in vitro and in vivo. J Mol Endocrinol. 2017;58(1):15–23. doi: 10.1530/JME-16-0134. [DOI] [PubMed] [Google Scholar]
- 60.Han B, Cui H, Kang L, Zhang X, Jin Z, Lu L, Fan Z. Metformin inhibits thyroid cancer cell growth, migration, and EMT through the mTOR pathway. Tumour Biol. 2015;36(8):6295–6304. doi: 10.1007/s13277-015-3315-4. [DOI] [PubMed] [Google Scholar]
- 61.Park J, Kim WG, Zhao L, Enomoto K, Willingham M, Cheng SY. Metformin blocks progression of obesity-activated thyroid cancer in a mouse model. Oncotarget. 2016;7(23):34832–34844. doi: 10.18632/oncotarget.8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Z, Shen J, WK W, Yu X, Liang J, Qiu G, Liu J. Leptin induces cyclin D1 expression and proliferation of human nucleus pulposus cells via JAK/STAT, PI3K/Akt and MEK/ERK pathways. PLoS One. 2012;7(12):e53176. doi: 10.1371/journal.pone.0053176. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Bendell JC, Hong DS, Burris HA, 3rd, Naing A, Jones SF, Falchook G, Bricmont P, Elekes A, Rock EP, Kurzrock R. Phase 1, open-label, dose-escalation, and pharmacokinetic study of STAT3 inhibitor OPB-31121 in subjects with advanced solid tumors. Cancer Chemother Pharmacol. 2014;74(1):125–130. doi: 10.1007/s00280-014-2480-2. [DOI] [PubMed] [Google Scholar]
- 64.DY O, Lee SH, Han SW, Kim MJ, Kim TM, Kim TY, Heo DS, Yuasa M, Yanagihara Y, Bang YJ. Phase I Study of OPB-31121, an oral STAT3 inhibitor, in patients with advanced solid tumors. Cancer Res Treat. 2015;47(4):607–615. doi: 10.4143/crt.2014.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okusaka T, Ueno H, Ikeda M, Mitsunaga S, Ozaka M, Ishii H, Yokosuka O, Ooka Y, Yoshimoto R, Yanagihara Y, Okita K. Phase 1 and pharmacological trial of OPB-31121, a signal transducer and activator of transcription-3 inhibitor, in patients with advanced hepatocellular carcinoma. Hepatol Res. 2015;45(13):1283–1291. doi: 10.1111/hepr.12504. [DOI] [PubMed] [Google Scholar]
- 66.Wong AL, Soo RA, Tan DS, Lee SC, Lim JS, Marban PC, Kong LR, Lee YJ, Wang LZ, Thuya WL, Soong R, Yee MQ, Chin TM, Cordero MT, Asuncion BR, Pang B, Pervaiz S, Hirpara JL, Sinha A, WW X, Yuasa M, Tsunoda T, Motoyama M, Yamauchi T, Goh BC. Phase I and biomarker study of OPB-51602, a novel signal transducer and activator of transcription (STAT) 3 inhibitor, in patients with refractory solid malignancies. Ann Oncol. 2015;26(5):998–1005. doi: 10.1093/annonc/mdv026. [DOI] [PubMed] [Google Scholar]
- 67.Ogura M, Uchida T, Terui Y, Hayakawa F, Kobayashi Y, Taniwaki M, Takamatsu Y, Naoe T, Tobinai K, Munakata W, Yamauchi T, Kageyama A, Yuasa M, Motoyama M, Tsunoda T, Hatake K. Phase I study of OPB-51602, an oral inhibitor of signal transducer and activator of transcription 3, in patients with relapsed/refractory hematological malignancies. Cancer Sci. 2015;106(7):896–901. doi: 10.1111/cas.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sen M, Thomas SM, Kim S, Yeh JI, Ferris RL, Johnson JT, Duvvuri U, Lee J, Sahu N, Joyce S, Freilino ML, Shi H, Li C, Ly D, Rapireddy S, Etter JP, Li PK, Wang L, Chiosea S, Seethala RR, Gooding WE, Chen X, Kaminski N, Pandit K, Johnson DE, Grandis JR. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: implications for cancer therapy. Cancer Discov. 2012;2(8):694–705. doi: 10.1158/2159-8290.CD-12-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hong D, Kurzrock R, Kim Y, Woessner R, Younes A, Nemunaitis J, Fowler N, Zhou T, Schmidt J, Jo M, Lee SJ, Yamashita M, Hughes SG, Fayad L, Piha-Paul S, Nadella MV, Mohseni M, Lawson D, Reimer C, Blakey DC, Xiao X, Hsu J, Revenko A, Monia BP, MacLeod AR. AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer. Sci Transl Med. 2015;7(314):314ra185. doi: 10.1126/scitranslmed.aac5272. [DOI] [PMC free article] [PubMed] [Google Scholar]