Abstract
A partial response (PR) has been proposed as a surrogate for overall survival in advanced adrenocortical carcinoma (ACC). The primary endpoint of the study was to characterize the time until a PR in patients with metastatic ACC treated with a standard therapy is achieved. Long-term survivors were selected to allow evaluation of delayed tumor response to mitotane. Records from patients with metastatic ACC that survived for > 24 months were retrieved. Tumor response was analyzed according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria. Time until a tumor response, after treatment initiation or therapeutic plasma mitotane level, was analyzed. Sixty-eight patients were analyzed. The first-line systemic therapy was mitotane as a monotherapy (M) (n = 57) or cytotoxic polychemotherapy plus/minus mitotane (PC ± M) (n = 11). The second-line therapy was M (n = 2) or PC ± M (n = 41). Thirty-two PRs occurred in 30/68 patients (44.1%): this was obtained for 13 (40.6%) during M and during PC ± M for 19/32 responders (59.4%). PRs were observed within 6 months of starting M or PC ± M in 76.9 and 94.7% of responses, respectively, within 6 months of therapeutic plasma mitotane being first observed in 88.9% of responses with M and in 53.3% of responses with PC ± M. All PRs (but one) occurred within 1 year after initiating treatment. To conclude, Most patients with metastatic ACC and long survival times had PRs within the first 6 months of standard systemic therapy, and almost all within the first year. The absence of response after that period could be considered as a treatment failure. Maintenance of mitotane therapy in non-responders after 1 year should be questioned in future randomized trials.
Keywords: Plasma Mitotane, Mitotane Therapy, Polychemotherapy (PC), Response Evaluation Criteria In Solid Tumors (RECIST), Advanced Adrenocortical Carcinoma (ACC)
Introduction
The prognosis of patients with metastatic adrenocortical carcinoma (ACC) is usually poor with a survival time of less than 1.5 years [1, 2]; however, survival rates are heterogeneous [3–5]. Mitotane plus local therapeutic options are recommended for patients that have favorable prognostic parameters, whereas polychemotherapy plus mitotane is recommended for those with unfavorable prognostic parameters [4]. No new therapeutic options have emerged over the last decades. In the absence of randomized trials, the recommendations are based on expert opinions that take into account several parameters, like the prognostic heterogeneity of ACC, the antitumor activity of mitotane and/or cisplatin-based chemotherapy, and the delayed action of mitotane. Indeed, in palliative care, partial responses to mitotane, when given as a monochemotherapy, have been reported in 13–33% of cases with response durations of 2–190 months [5–7]. Partial response rates at the time of systemic polychemotherapy, combined or not with mitotane, have been reported in 11–30% of cases, with median durations of progression-free survival between 2 and 5 months.
However, the characteristics and kinetics of tumor response remain unclear after initiating standard therapy in patients with advanced ACC. Previous studies, mainly retrospective, have shown a relationship between the level of plasma mitotane and overall survival. Based on these results, plasma mitotane is considered a standard of care, and a targeted level of ≥ 14 mg/L is recommended when tolerance is acceptable [8, 9]. In addition, a few studies have suggested that maintaining this level of therapeutic plasma mitotane is associated with prolonged antitumor efficacy [8, 10]. In addition, achieving a partial response constitutes a critical objective of treatment as it has been associated with prolonged overall survival in several retrospective studies [11–13]. However, no precise recommendation has been made regarding how long clinicians should wait before considering mitotane therapy has become inefficient within a given patient.
It is a consensual statement that a prolonged period of evaluation is warranted as mitotane-antitumor activity is delayed, as reflected by the time to achieve a therapeutic level of plasma mitotane. Thus, mitotane therapy, combined with chemotherapy, is frequently maintained for months or years in ACC patients, when a first progression is evident, or in case of poor prognostic factors. The precise time at which mitotane therapy should be stopped is unclear, exposing patients to undue toxicity and to adverse drug interactions.
The objective of this retrospective study from the French COMETE-Cancer network was to characterize the time until a partial response after starting the standard systemic therapy in patients with metastatic ACC. In order to consider the delayed antitumor action of mitotane, we selected a subpopulation of patients with metastatic ACC that had prolonged survival rates, thus allowing a prolonged follow-up and evaluation of the potential delay until the antitumor action of mitotane was activated. We only included patients that had survived for ≥ 24 months as this is approximately the double of expected survival time for patients with metastatic ACC. We also characterized the prognostic factors and the rates of response of the standard therapy in this selected subgroup of ACC patients.
Patients and Methods
Patients and Data Collection
Between 1 January 2002 and 31 December 2014, patients with stage IV ACC followed up in centers within the French COMETE-Cancer network and registered in the database of the European Network for the Study of Adrenal Tumors (ENSAT) were identified. Inclusion criteria were a confirmed diagnosis of ACC by the network pathologists [14], survival for > 24 months after metastasis had been diagnosed, an evaluable RECIST metastatic disease, and available imaging during the follow-up period.
The following relevant prognostic parameters were collected at the time that metastasis was diagnosed: age (< 50/≥ 50 years), gender, presence of hormone- or tumor-related symptoms (yes/no), Weiss score (≤ 6/> 6), Ki67 percentage (< 20%/≥ 20%), mitotic count (< 20/≥ 20), R status of the primary surgery (complete resection, R0; microscopic residual disease, R1; macroscopic residual disease, R2; resection not known, Rx), and the number of organs affected by the tumor. Stage IV patients were categorized into subgroups according to the number of involved organs (IVa, 2; IVb, 3; IVc, > 3). Patients with metastasis at the time that ACC was first diagnosed were categorized as synchronous; patients with delayed occurrence of metastasis were classified as metachronous. The medical records were reviewed by an on-site investigator (DV).
According to recent data, the pejorative prognostic factors including grade, resection status, age, and symptoms (GRAS) were characterized, i.e., a Weiss score > 6, Ki67 analyses ≥ 20%, a mitotic count ≥ 20, R1 or R2 status of adrenal surgery, age ≥ 50 years, presence of tumor-related or hormone-related symptoms, and more than two organs affected by the tumor [15].
All patients signed a written informed consent. The present study upheld the revised (2001) 1975 Helsinki Declaration.
Treatment Options
Standard antitumor treatments were recorded and classified into two main subgroups: (i) mitotane as a monotherapy (M), given to obtain a 14–30 mg/L therapeutic level [8], plus or minus locoregional therapy that included surgery of the primary tumor and/or liver trans-arterial chemoembolization and/or radiofrequency and/or radiotherapy, and (ii) systemic polychemotherapy (PC) combined or not with mitotane (PC ± M). As the number of patients that received PC + M was greater than the number that received systemic polychemotherapy alone, we decided not to separate these two groups for the analyses.
The first-line systemic therapy was defined as the first therapy (mitotane and/or systemic polychemotherapy) performed at the time when metastasis was diagnosed and before the first tumor progression was evidenced according to a local RECIST 1.1 evaluation. The second-line systemic therapy was defined as the second therapy performed before the second tumor progression was evidenced. The third-line therapy was defined as the third therapy performed before the third tumor progression was evidenced.
Response Criteria
All the patients’ files were reviewed by a single on-site investigator (DV) according to the local RECIST 1.1 criteria evaluated by thorax and abdomen computed tomography images performed at 2–4-month intervals [16]. In brief, a complete response was defined as the disappearance of all target lesions; a partial response was defined as at least a 30% decrease in the largest diameters of the targeted lesions; progressive disease was defined as at least a 20% increase in the largest diameters of the targeted lesions; otherwise, the tumor was classified as a stable disease. Disease control rate (DCR) was defined by the sum of cases that had a complete response, a partial response, or a stable disease: this was first assessed during M or PC ± M therapy and also when first therapeutic plasma mitotane level was obtained.
Analyses of responses were performed per patient and per type of treatment. The time to obtain a partial response was calculated from treatment initiation (M or PC ± M) and, then for patients treated with mitotane, from the plasma mitotane level first increased to > 14 mg/L.
Statistical Analyses
The primary endpoint of the study was to characterize the time until a tumor response after initiating treatment (M or PC ± M) in patients with metastatic ACC, according to the RECIST 1.1 criteria as determined by per patient and per type of treatment. In cases of combined locoregional therapies, targets outside the field targeted were selected to avoid any interference with the results from the locoregional therapies. The secondary endpoints evaluated disease control rate, the duration of response, median progression-free survival after each therapeutic option was initiated, and after plasma mitotane level first became above 14 mg/L. Factors associated with a partial response or DCR of more than 6 months were looked for, using the chi-square or Fisher’s exact test on the mENSAT-GRAS parameters, as defined above.
Quantitative data was expressed as their means and ranges or their medians and interquartile ranges (IRs), as appropriate. Comparisons between quantitative variables were made using the Kruskal-Wallis test.
The results were considered to be statistically significant if p < 0.05.
Results
Population Characteristics
Sixty-eight patients (45 women) with metastatic ACC that had survived for > 24 months after metastasis was diagnosed were included in this study. They were followed in eight French centers over a median period of 49.8 months (IR 30–75 months; range 24–1320). The main clinical features are summarized in Table 1. Twenty-eight patients (41.2%) had metachronous metastases, i.e., after ACC was diagnosed, with a median disease duration of 24 months before metastasis was diagnosed (range 2–348 months). Before reaching stage IV, 13 patients received mitotane as an adjuvant therapy. Sixty-five patients (95.6%) had at least one pejorative mENSAT-GRAS prognostic factor. Forty-seven patients (69.1%) had hormone-related symptoms. Thirty-six patients (57%) had a Weiss score > 6, 21 patients (46%) had a Ki67 index of ≥ 20%, and 15 patients (27%) had a mitotic count of ≥ 20. At diagnosis of metastasis, a median of two organs was affected (including primary and lymph nodes). Adrenal R0 surgery was performed in 65 patients (96%), including 28 patients before metastasis.
Table 1.
Characteristics of the patients
| Parameters | Number of evaluable patients | Number of patients (%) |
|---|---|---|
| Age (years) at diagnosis of stage IV | 68 | |
| ≥ 50 | 23 (33.8) | |
| < 50 | 45 (66.2%) | |
| Gender | 68 | |
| Female | 45 (66.2) | |
| Male | 23 (33.8) | |
| Hormone-related symptoms | 68 | 47 (69.1%) |
| Glucocorticoids | 41 | |
| Androgens | 25 | |
| Estrogens | 3 | |
| Mineralocorticoid | 1 | |
| Multi-hormonal secretion | 23 | |
| Metastasis | 68 | |
| Synchronous | 40 | |
| Metachronous | 28 | |
| Resection of the primary tumor | 68 | |
| R0* | 65 (95.6%) | |
| R1–R2* | 3 (4.4) | |
| Pathology | 68 | |
| Weiss score > 6 | 63 | 36 (57) |
| Ki67 ≥ 20 | 46 | 21 (46) |
| Mitotic count ≥ 20 | 56 | 15 (27) |
| mENSAT stage at diagnosis | 68 | |
| Stages I–II | 19 | |
| Stage III | 9 | |
| Stage IV | 40 | |
| Number of metastatic organs | 68 | |
| n = 1 | 28 (41.2) | |
| n = 2 | 28 (41.2) | |
| n = 3 | 10 (14.6) | |
| n = 4 | 1 (1.5) | |
| n = 5 | 1 (1.5) | |
| Localization of metastases | 68 | |
| Primary or local recurrence | 37 (54.4) | |
| Lung | 34 (50) | |
| Liver | 22 (32.3) | |
| Bone | 7 (10.3) | |
| Peritoneum | 12 (17.7) | |
| Other | 11 (16.2) | |
| Disease-free interval before metastasis ≥ 12 months | 28 | 20 (29.4) |
| Number of pejorative prognostic mENSAT-GRAS factors** | 68 | |
| n = 0 | 3 (4.4) | |
| n = 1 | 15 (22.0) | |
| n = 2 | 22 (32.3) | |
| n = 3 | 15 (22.1) | |
| n = 4 | 13 (19.2) | |
| Therapy before metastasis diagnosis | 68 | |
| Adrenal surgery | 28 (41.2) | |
| Adrenal radiotherapy | 2 (2.9) | |
| Adjuvant mitotane therapy | 13 (19.1) |
*R0, complete resection; R1, microscopic residual disease; R2, macroscopic residual disease
**Pejorative prognostic mENSAT-GRAS factors: age ≥ 50 years, presence of tumor-related symptoms, Weiss score > 6, Ki67 ≥ 20, mitotic count ≥ 20, > 2 tumor-involved organs, and R1 or R2 status of adrenal surgery
Systemic Therapies
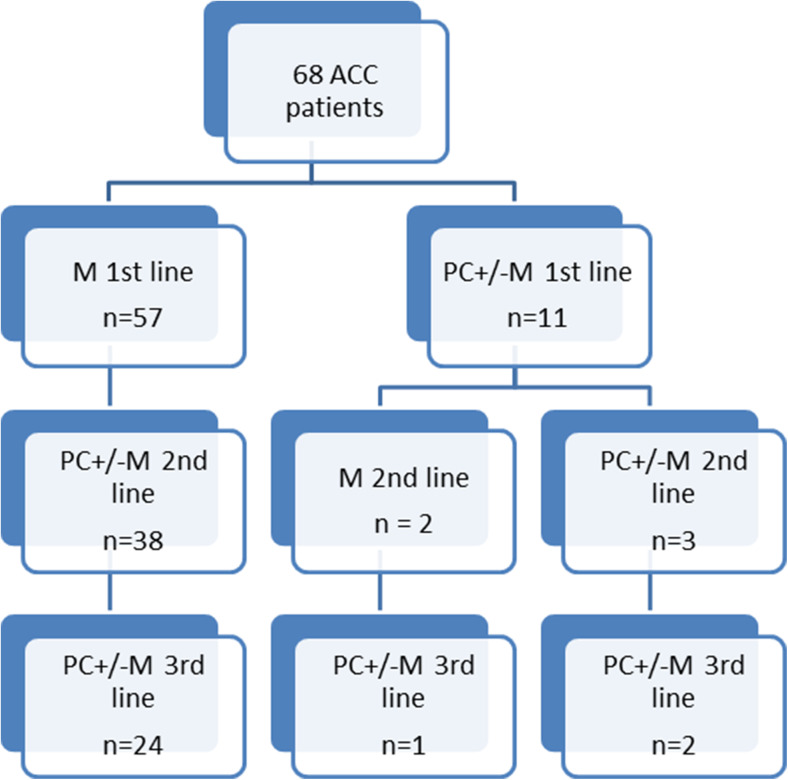
Figure 1 provides details of the systemic therapies. All patients underwent first-line systemic therapy. Of the 68 patients, 57 received mitotane as a first-line monotherapy (84%), which was combined with locoregional guided therapy in 36 patients (53%). Forty-three patients remained on mitotane until the end of the third-line systemic therapy and/or until death.
Fig. 1.
Flowchart of patients and treatments
Of the 11 patients that underwent first-line polychemotherapy (platinum based: n = 8, 73%), mitotane was combined in 9 (81.8%) patients. One patient was enrolled in a trial as a first-line therapy.
Forty-three patients (63.2%) underwent second-line systemic therapy. Two patients (4.6%) received mitotane as a monotherapy. Forty-one patients (95.4%) received cytotoxic polychemotherapy (platinum based, n = 31, 75.6%). Of these, mitotane was combined in 34 patients (82.9%) or locoregional therapy in 5 patients (12.2%). Two patients were enrolled in a trial on second-line therapy.
Twenty-seven patients (39.7%) underwent third-line systemic therapy. They all underwent third-line polychemotherapy (platinum based, n = 6, 22.2%). Mitotane was combined in 22 patients (81.5%). Three patients received targeted therapy as a third-line polychemotherapy (sunitinib, n = 1; phase 1, n = 2).
Per-Patient Analysis
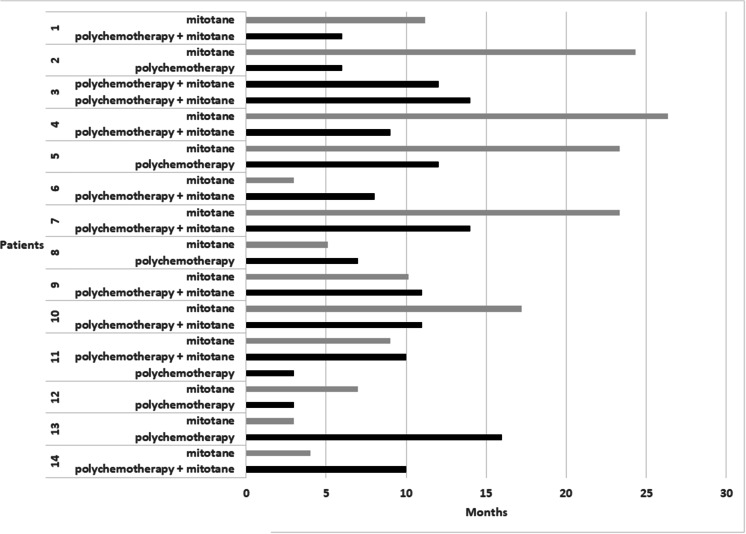
A median of two systemic therapies was administered per patient. Considering all the lines of treatment, the best response was a partial response in 30 patients (44.1%) and tumor stabilization in 33 patients (48.6%). Two patients experienced two partial responses. Twelve patients experienced two stabilizations, including one patient that experienced three stabilizations (Fig. 2). No progression prior to a partial response was evidenced within one given line of therapy.
Fig. 2.
Duration of progression-free survival with mitotane as a monotherapy or with polychemotherapy ± mitotane in patients with multiple responses or stabilizations. Gray lines refer to mitotane as a monotherapy and black lines to polychemotherapy ± mitotane. Fourteen patients experienced multiple partial responses (patients 1 and 2) or multiple stabilizations (patients 3 to 14)
Per-Type Treatment Analysis
At the time of M, a partial response was observed in 13 patients (22.0%; 95% confidence interval 11.46–32.61), stabilization in 33 patients (55.9%), and tumor progression in 13 (22.0%) of the 59 patients. A partial response was obtained during first- or second-line systemic therapy in 12 (92.3%) and 1 (7.7%) of the patients, respectively. After withdrawal of the 13 patients that received adjuvant M prior to a diagnosis of metastasis, a partial response was observed in 11 (20%), stabilization in 31 (56.4%), and tumor progression in 13 (23.6%) of the 55 patients. Median progression-free survival after initiating first-line mitotane was 17 months (IR 10–25 months; range 3–160). DCRs at 6 and 12 months were 67.8% (40/59 patients) and 54.2% (32/59 patients), respectively.
At the time of PC ± M, a partial response was evidenced in 19 patients (38%; 95% CI 24.6–51.5) and stabilization in 18 (36%) out of the 50 patients. After withdrawal of the 13 patients that received adjuvant mitotane, a partial response was observed in 15 (36.6%), stabilization in 14 (34.1%), and tumor progression in 12 (29.3%) of the 41 patients. Partial responses (PRs) were observed at the time of platinum-based polychemotherapy or another type (gemcitabine, n = 1; Vepesid + doxorubicin, n = 1; streptozotocin, n = 2) of chemotherapy in 15 and in 4 of the 19 patients, respectively. PRs were observed at the time of PC + M in all 19 patients (first-line: n = 5, second-line: n = 13, third-line: n = 1). Median progression-free survival after initiating PC ± M was 10 months (IR 5–23 months; range 3–24). DCRs at 6 and 12 months were 62% (31/50 patients) and 24% (12/50 patients), respectively.
No correlation regarding the mENSAT-GRAS parameters (age ≥ 50 years, presence of tumor-related symptoms, Weiss score > 6, Ki67 ≥ 20, mitotic count ≥ 20, more than two organs affected, R1 or R2 status of adrenal surgery) with a partial response or with a longer DCR was found.
Analyses of Tumor Response as a Function of Time
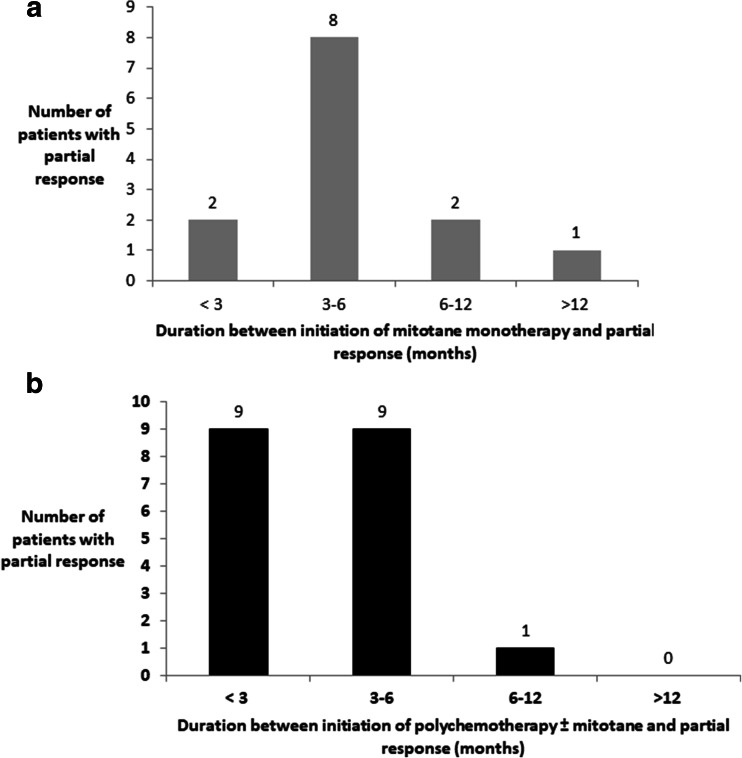
After initiating mitotane, a partial response was obtained after a median of 4 months (IR 3–5 months). A partial response was observed within 6 months after initiating mitotane monotherapy in 76.9% of responses (10/13), in < 3 months in two responses (15% of responders), between 3 and 6 months in eight responses (62%), between 6 and 12 months in two responses (15%), and in > 12 months in one response (8%) (Fig. 3).
Fig. 3.
Partial response per type of systemic therapy as a function of time from treatment initiation. a From initiation of mitotane as a monotherapy. b From initiation of polychemotherapy ± mitotane
After initiating PC ± M, a partial response was obtained after a median of 3 months (IR 2–4 months). A PR was observed within 6 months after initiating PC ± M in 94.7% of responses (18/19), in < 3 months in nine responses (47.4%), between 3 and 6 months in nine responses (47.4%), and between 6 and 12 months in one response (5.2%) (Fig. 3). Partial responses occurred more rapidly after initiating PC ± M than after initiating mitotane as a monotherapy (p = 0.012).
Tumor Response Rate as a Function of Plasma Mitotane Level
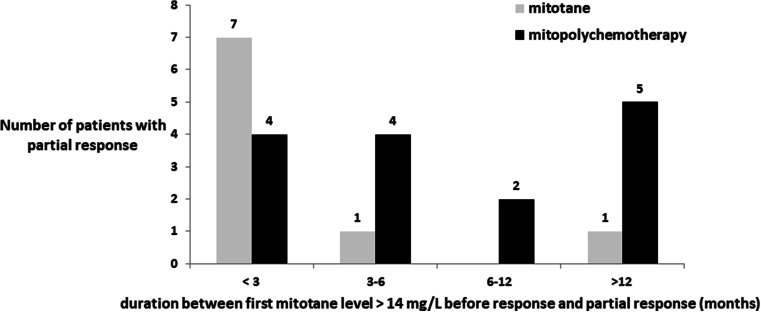
In the subgroup of patients that experienced a partial response, levels of plasma mitotane before a response were available for 31/32 of responses (96.9%), with a mean of five plasma mitotane levels per patient (range 1–13). Of these 31 responses, plasma mitotane levels above 14 mg/L were not reached before a partial response in seven cases. Of these seven cases, maximal mitotane levels before a partial response were 7.8, 9, and 11 mg/L in the three patients under M and were 3.6, 5.4, 6, and 6 mg/L in the four patients under PC + M at the time of a partial response.
Of the 24 cases with therapeutic plasma mitotane levels before a response, a partial response was observed in 9 cases receiving M and 15 cases receiving PC ± M. These partial responses were observed before 3 months, at 3–6 months, at 6–12 months, or after 12 months in 11 (45.9%), 5 (20.8%), 2 (8.3%), and 6 (25%) patients, respectively, after plasma mitotane level first became above 14 mg/L (Fig. 4). These partial responses were observed within 6 months of first therapeutic plasma mitotane in 88.9% (8/9) of partial responses with mitotane monotherapy. In contrast, partial responses were observed within 6 months of first therapeutic plasma mitotane in 53.3% (8/15) of partial responses with PC + M.
Fig. 4.
Partial response per type of systemic therapy as a function of time from when mitotane level was first observed above 14 mg/L. “As a function of time from when mitotane level was first observed above 14 mg/L” corresponds to the time when therapeutic plasma level was first observed above 14 mg/L in each patient, and not the initiation of mitotane as a monotherapy or with polychemotherapy. Data not available for a patient that received polychemotherapy + mitotane
Discussion
Today, management of patients with metastatic ACC is based on expert opinions rather than results from phase III randomized trials. Indeed, no placebo-controlled trial has been performed and therapeutic standards have not been compared. Critical questions, such as the optimal duration of mitotane therapy for patients with advanced ACC, remain unsolved. To provide our first insights into this critical question, the time until a partial response was analyzed in patients with advanced ACC and long-term survival rates, selected to take into account the delayed antitumor mitotane activity.
Our retrospective study provides several new clinical results that could trigger future prospective randomized trials. Firstly, in patients with metastatic ACC and long-term survival, most partial responses occurred during the first 6 months following initiation of therapy with either mitotane monotherapy or polychemotherapy, and almost all partial responses occurred within the first year. Secondly, partial responses tended to be more often observed with polychemotherapy than with mitotane therapy alone. Thirdly, partial responses also occurred more rapidly with polychemotherapy than with mitotane therapy alone. In addition, obtaining a therapeutic plasma mitotane level shortened the time until a partial response occurred in patients that received mitotane alone compared to those that received polychemotherapy. Finally, twofold partial responses were rare and no progression prior to a partial response was observed within one line of treatment. To the best of our knowledge, these results are new and have not been published elsewhere.
Based on these results, several points could be challenged.
-
i.
Mitotane withdrawal in the absence of a partial tumor response within 12 months of initiating mitotane and/or polychemotherapy could be performed. In patients with metastatic ACC and receiving standard therapeutic options, this could mean that the withdrawal of mitotane should be discussed for non-secreting ACC patients whereas it could be reduced to an antisecretory dose in secreting ACC patients. A decision to withdraw or reduce mitotane therapy may become even more obvious if there is progression under both M and PC ± M.
-
ii.
The upfront first-line use of polychemotherapy in all cases of advanced ACC, whatever the prognostic factors, could also be proposed, especially when tumor reduction is expected. Indeed, a partial response was more frequently observed at the time of polychemotherapy, and the mENSAT-GRAS parameters did not affect these results. This result was confirmed after exclusion of patients that received mitotane as an adjuvant therapy. As a consequence, the early use of polychemotherapy as a trigger for a partial response could be reevaluated in all cases of advanced ACC, whatever their prognostic parameters. In this new proposal, mitotane would be used as a maintenance therapy rather than as a first-line monotherapy, as recommended for patients with a good prognosis, to potentially prolong the best response. Our proposal is further reinforced by the shorter time until a partial response occurred in patients that received polychemotherapy compared to mitotane alone.
-
iii.
Monitoring plasma mitotane should remain standard: this is because of the more frequent partial response and faster time until a partial response was observed in patients that received mitotane once a therapeutic plasma level had been reached. Monitoring plasma mitotane may help refine the decision to start combined polychemotherapy. A tumoral partial response was more frequently associated with plasma mitotane levels that were above 14 mg/L. Thus, it could be considered whether mitotane therapy should be stopped or reduced in patients that have lower plasma mitotane level. Interestingly, the influence of therapeutic plasma mitotane level was mainly observed in patients treated with mitotane alone, thus reinforcing the independent antitumor role of polychemotherapy.
Randomized trials that compare early versus late introduction of polychemotherapy, as well as mitotane withdrawal or maintenance after 12 months with mitotane alone or polychemotherapy initiation, could answer these critical questions. Stratification for prognostic factors, hormone-secreting status, and plasma mitotane levels would help refine the results. Such studies would also answer the question regarding the prognostic value of tumor stabilization in patients with advanced ACC. Whether tumor stabilization alone, under M or PC ± M, should be considered a benefit or a failure of standard therapeutic options is currently unknown.
The limitations of our study include its retrospective nature, the choice of a highly selected population of patients with advanced ACC based on their prolonged survival rate, and the absence of a morphological central review. Among these factors, the choice of long-term survival was mandatory due to mitotane’s kinetics. However, this strategy may have increased the rate of response to the standard therapy. Indeed, despite at least one pejorative mENSAT-GRAS parameter being present in 95.6% of patients, we observed 44.1% partial responses that could be classified as good, which were more than those in the general population of ACC patients and may have participated in the longer survival times. However, it is unlikely that our primary objective, the time until a response, was affected by selecting this subgroup of patients with advanced ACC. In addition, the higher rate of partial responses in patients receiving PC ± M may have been biased by selecting a subpopulation of patients resistant to mitotane, as 13 patients progressed during adjuvant therapy. However, the rate of PRs in M or PC + M after removing the data for these 13 patients confirmed the higher rate of PR in the PC + M subgroup. Finally, the higher number of partial responses in those receiving PC ± M may also have been biased by higher plasma mitotane levels at the time of PC + M therapy.
In conclusion, our study shows that most of the partial responses of patients with ACC and treated with mitotane and/or polychemotherapy occurred within 6 months, and almost all occurred within 12 months. This challenges the use of long-term maintenance mitotane in all ACC patients after 12 months. A shorter time until a partial response was observed after a therapeutic plasma mitotane level was obtained and also at the time of polychemotherapy, thus urging evaluation of the best sequence regimen for these advanced patients. Future prospective trials should answer both questions.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Compliance with Ethical Standards
The present study upheld the revised (2001) 1975 Helsinki Declaration.
Conflict of Interest
E. Baudin has received honoraria from HRA. The other coauthors have nothing to declare.
References
- 1.Bilimoria KY, Shen WT, Elaraj D, Bentrem DJ, Winchester DJ, Kebebew E, Sturgeon C. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113:3130–3136. doi: 10.1002/cncr.23886. [DOI] [PubMed] [Google Scholar]
- 2.Lughezzani G, Sun M, Perrotte P, Jeldres C, Alasker A, Isbarn H, Budaus L, Shariat SF, Guazzoni G, Montorsi F, Karakiewicz PI. The European Network for the Study of Adrenal Tumors staging system is prognostically superior to the international union against cancer-staging system: a North American validation. Eur J Cancer. 2010;46:713–719. doi: 10.1016/j.ejca.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Baudin E. Adrenocortical carcinoma. Endocrinol Metab Clin N Am. 2015;44:411–434. doi: 10.1016/j.ecl.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Berruti A, Baudin E, Gelderblom H, Haak HR, Porpiglia F, Fassnacht M, Pentheroudakis G. Adrenal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii131–vii138. doi: 10.1093/annonc/mds231. [DOI] [PubMed] [Google Scholar]
- 5.Haak HR, Hermans J, van de Velde CJ, Lentjes EG, Goslings BM, Fleuren GJ, Krans HM. Optimal treatment of adrenocortical carcinoma with mitotane: results in a consecutive series of 96 patients. Br J Cancer. 1994;69:947–951. doi: 10.1038/bjc.1994.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luton JP, Cerdas S, Billaud L, Thomas G, Guilhaume B, Bertagna X, Laudat MH, Louvel A, Chapuis Y, Blondeau P, et al. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med. 1990;322:1195–1201. doi: 10.1056/NEJM199004263221705. [DOI] [PubMed] [Google Scholar]
- 7.Barzon L, Fallo F, Sonino N, Daniele O, Boscaro M. Adrenocortical carcinoma: experience in 45 patients. Oncology. 1997;54:490–496. doi: 10.1159/000227608. [DOI] [PubMed] [Google Scholar]
- 8.Hermsen, I. G., M. Fassnacht, M. Terzolo, S. Houterman, J. den Hartigh, S. Leboulleux, F. Daffara, A. Berruti, R. Chadarevian, M. Schlumberger, B. Allolio, H. R. Haak and E. Baudin. 2011. Plasma concentrations of o,p′DDD, o,p′DDA, and o,p′DDE as predictors of tumor response to mitotane in adrenocortical carcinoma: results of a retrospective ENS@T multicenter study. J Clin Endocrinol Metab 96: 1844–1851 [DOI] [PubMed]
- 9.Kerkhofs TM, Baudin E, Terzolo M, Allolio B, Chadarevian R, Mueller HH, Skogseid B, Leboulleux S, Mantero F, Haak HR, Fassnacht M. Comparison of two mitotane starting dose regimens in patients with advanced adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98:4759–4767. doi: 10.1210/jc.2013-2281. [DOI] [PubMed] [Google Scholar]
- 10.Terzolo M, Baudin AE, Ardito A, Kroiss M, Leboulleux S, Daffara F, Perotti P, Feelders RA, deVries JH, Zaggia B, De Francia S, Volante M, Haak HR, Allolio B, Al Ghuzlan A, Fassnacht M, Berruti A. Mitotane levels predict the outcome of patients with adrenocortical carcinoma treated adjuvantly following radical resection. Eur J Endocrinol. 2013;169:263–270. doi: 10.1530/EJE-13-0242. [DOI] [PubMed] [Google Scholar]
- 11.Bukowski RM, Wolfe M, Levine HS, Crawford DE, Stephens RL, Gaynor E, Harker WG. Phase II trial of mitotane and cisplatin in patients with adrenal carcinoma: a Southwest Oncology Group study. J Clin Oncol. 1993;11:161–165. doi: 10.1200/JCO.1993.11.1.161. [DOI] [PubMed] [Google Scholar]
- 12.Malandrino, P., A. Al Ghuzlan, M. Castaing, J. Young, B. Caillou, J. P. Travagli, D. Elias, T. de Baere, C. Dromain, A. Paci, P. Chanson, M. Schlumberger, S. Leboulleux and E. Baudin. 2010. Prognostic markers of survival after combined mitotane- and platinum-based chemotherapy in metastatic adrenocortical carcinoma. Endocr Relat Cancer 17: 797–807 [DOI] [PubMed]
- 13.Berruti A, Terzolo M, Sperone P, Pia A, Della Casa S, Gross DJ, Carnaghi C, Casali P, Porpiglia F, Mantero F, Reimondo G, Angeli A, Dogliotti L. Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: a large prospective phase II trial. Endocr Relat Cancer. 2005;12:657–666. doi: 10.1677/erc.1.01025. [DOI] [PubMed] [Google Scholar]
- 14.Tissier F, Aubert S, Leteurtre E, Al Ghuzlan A, Patey M, Decaussin M, Doucet L, Gobet F, Hoang C, Mazerolles C, Monges G, Renaudin K, Sturm N, Trouette H, Vacher-Lavenu MC, Viallon V, Baudin E, Bertagna X, Coste J, Libe R. Adrenocortical tumors: improving the practice of the Weiss system through virtual microscopy: a National Program of the French Network INCa-COMETE. Am J Surg Pathol. 2012;36:1194–1201. doi: 10.1097/PAS.0b013e31825a6308. [DOI] [PubMed] [Google Scholar]
- 15.Libe R, Borget I, Ronchi CL, Zaggia B, Kroiss M, Kerkhofs T, Bertherat J, Volante M, Quinkler M, Chabre O, Bala M, Tabarin A, Beuschlein F, Vezzosi D, Deutschbein T, Borson-Chazot F, Hermsen I, Stell A, Fottner C, Leboulleux S, Hahner S, Mannelli M, Berruti A, Haak H, Terzolo M, Fassnacht M, Baudin E. Prognostic factors in stage III-IV adrenocortical carcinomas (ACC): an European Network for the Study of Adrenal Tumor (ENSAT) study. Ann Oncol. 2015;26:2119–2125. doi: 10.1093/annonc/mdv329. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]