Abstract
The clinical features of thymic carcinoid (TC) and bronchial carcinoid (BC) tumors as part of multiple endocrine neoplasia type 1 (MEN1) have been rarely described and their importance in clinical practice is debated. The objective of this study was to describe the clinical presentation and outcome of this uncommon manifestation of MEN1 in a tertiary care center setting. We present the clinical features of patients with MEN1 and either TC or BC evaluated at the Mayo Clinic from 1977 to 2013. A total of 348 patients with MEN1 were evaluated and the prevalence of TC was 2.0 % (n = 7) and of BC 4.9 % (n = 17). The majority of the patients with BC were men (61 %) diagnosed on routine screening (77 %) and BC was not the confirmed cause of death in any patient. In contrast, TC patients were all men and during follow-up 43 % died due to TC complications. We conclude that TC and BC tumors are uncommon, but important components of MEN1. BC were most commonly diagnosed during routine screening and associated with an indolent course. TC were predominantly seen in men and associated with a more aggressive behavior.
Keywords: Pituitary Adenoma, Mayo Clinic, Carcinoid Tumor, Primary Hyperparathyroidism, Routine Screening
Introduction
Multiple endocrine neoplasia type 1 (MEN1) is an autosomal dominant tumor syndrome arising from an inactivating mutation on a tumor suppressor gene located on chromosome 11. Diagnosis of MEN1 can be established if an individual presents with two or more primary MEN1-associated endocrine tumors (parathyroid adenoma/hyperplasia, gastroenteropancreatic tumor, and pituitary adenoma). In addition to these most common findings, adrenal, bronchial carcinoid (BC), thymic carcinoid (TC), and skin tumors may occur [1].
BC and TC tumors occur in 3.6–8.4 % of the patients with MEN1 [2, 3]. BC in patients with MEN1 occur more frequently in women and have not been associated with increased mortality [4, 5]. In reports from Europe and North America, TC occurs almost exclusively in men; although also male-predominant in a Japanese report, the gender association was less exclusive [6–9, 1]. TC has also been associated with an aggressive clinical course and increased risk of death [6–9].
Due to its rare occurrence, the literature is lacking on descriptions of prevalence and clinical impact of BC and TC tumors in patients with MEN1. Pending prospective and randomized trials, important clinical questions regarding the management of these patients (e.g., the need for tumor detection testing) can be indirectly answered in retrospective series from different geographical areas and centers. To this end, our goal was to describe the clinical presentation of patients with MEN1 who also had TC or BC and who were treated at the Mayo Clinic during a 36-year period.
Materials and Methods
This study was approved by the Mayo Clinic Institutional Review Board with all patients consenting to participation in research. We performed an electronic search of the medical records to identify patients with MEN1 who were seen at Mayo Clinic in Rochester, Minnesota from 1977 to 2013. This initial group of records was reviewed and a diagnosis of MEN1 was confirmed based on the clinical presentation including two or more of the tumors associated with MEN1, patients with one tumor and documented MEN1 germline mutation, and those patients with a positive family history and documented MEN1 germline mutation [1]. Within this group, patients with BC and TC were identified based on histopathological findings. All the demographic and clinicopathologic information was extracted from these records. This was a retrospective study based on 36 years of clinical experience. The evaluation and treatment of patients with MEN1 was determined by the treating physician. There was no specific protocol used to determine the need, methods, or timing of screening for BC or TC. In addition, during this time frame, the availability of imaging technologies also changed.
Continuous variables (e.g., age) are summarized as mean ± standard deviation. Categorical variables are described as percentages.
Results
A total of 348 patients fulfilled the diagnostic criteria for MEN1. Mean age at diagnosis of MEN1 was 37.2 years (range, 11 months–78 years). The majority of the patients were female (56.0 %). Mean duration of follow-up from the time of diagnosis was 7.6 ± 8.3 years. BC were the found in 17 cases (4.9 %) and TC in seven (2.0 %) in this cohort of 348 MEN1 patients.
Bronchial Carcinoid Tumors
We identified 17 cases of BC in our population of patients with MEN1. Four patients were excluded from our descriptive analysis since they had their initial surgery at a different institution and both their pathology slides and surgical reports were not available for our review. Detailed clinical and surgical characteristics of the 13 cases included are shown in Table 1.
Table 1.
Clinical features of patients with bronchial carcinoid (BC) tumors in patients with multiple endocrine neoplasia type 1 (MEN1)
| Case | Age at diagnosis of MEN1 (years) | Age at diagnosis of BC tumor (years) | Sex | Diagnosis | Therapy | Lymph nodes and surgical margins report and number positive for metastatic BC tumor | Recurrence | Follow-up (years) | Death during follow-up (cause) | Tumor size, location, and pathology findings |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39 | 39 | F | Reflux symptoms, followed by chest CT scan | Mediastinoscopy and wedge resection | 1/5 positive peribronchial lymph node | No | 10 | No | Right lower lobe, size not known, grade 1 neuroendocrine carcinoma |
| 2 | 38 | 40 | F | Screening chest x-ray | Mediastinoscopy and wedge resection | 0/14 lymph nodes | No | 13 | No | Left lower lobe 1 cm, BC tumor IA |
| 3 | 53 | 69 | M | Screening chest CT scan | Wedge resection | Lymph nodes not evaluated | No | 11 | Yes (unrelated cause) | Left upper lobe, size not known, BC tumor IA. |
| 4 | 25 | 34 | M | Screening chest x-ray | Lobectomy with mediastinal lymphadenectomy | 0/13 lymph nodes | No | 12 | No | Right middle lobe, 2.1 × 1.5 × 1.5 cm BC tumor |
| 5 | 24 | 42 | M | Screening chest CT scan | Wedge resection | Negative margins, no lymph nodes examined | No | 7 | No | Two right BC tumors, right lower lobe 1.5 × 1.4 cm and right middle lobe 0.6 × 0.5 cm |
| 6 | 25 | 37 | F | Screening chest CT scan | Mediastinoscopy and wedge resection | Negative margins, 0/2 lymph nodes | Residual symptomatic pulmonary nodules; required second lobectomy | 2 | No | First surgery typical BC tumor left lower lobe 1.1 × 1.4 cm; second surgery atypical BC right middle lobe 0.8 × 0.5 × 0.5 cm and right upper lobe 1.5 × 1.4 × 1.4 cm |
| 7 | 41 | 42 | M | Screening with chest x-ray | Mediastinoscopy and lobectomy | Negative margins; 0/14 lymph nodes | No | 9 | Yes (unknown) | Left lower lobe 2.7 × 2.5 × 2.5 cm BC tumor |
| 8 | 54 | 56 | F | Screening with chest x-ray | Lobectomy with mediastinal lymphadenectomy | Negative margins, 0/17 lymph nodes | Yes, 10 years postoperatively, subcentimeter nodule, no symptoms | 12 | Yes (unknown) | Left upper lobe 2.5 × 1.5 cm BC tumor |
| 9 | 50 | 40 | M | Screening with chest x-ray | Wedge resection | Negative margins | Yes, 2 years postoperatively, new nodule same area, required resection | 18 | No | Initial surgery right lower lobe 2 cm BC tumor; second surgery right lower lobe 1.5 cm BC tumor |
| 10 | 42 | 42 | F | Cough followed by chest CT scan | Wedge resection with mediastinal lymphadenectomy | Negative margins, 0/2 lymph nodes | Yes, asymptomatic subcentimeter nodules | 10 | No | Right lower lobe 1.6 × 1.5 × 2.5 cm BC tumor |
| 11 | 40 | 40 | M | Shortness of breath followed by chest x-ray | Lobectomy | Negative margins | Yes, 1 year postoperatively, asymptomatic subcentimeter nodules | 5 | No | Two BC tumors, right middle lobe 1.2 cm and right lower lobe 2.8 × 2.5 × 1.4 cm |
| 12 | 53 | 53 | M | Screening with chest CT scan | Mediastinoscopy | Negative margins, 0/9 lymph nodes | No | 1 | No | Right upper lobe 1.3 × 0.7 × 0.5 cm atypical BC tumor |
| 13 | 52 | 53 | M | Screening with chest CT scan | Mediastinoscopy and segmentectomy | Negative margins, 1/8 lymph nodes | No | 0.5 | No | Left lower lobe 0.9 × 0.8 × 0.7 cm grade 1 BC tumor |
F female, M male
In patients with MEN1 and BC, the mean age at diagnosis of MEN1 was 41.2 ± 11.0 years and for BC 45.1 ± 9.8 years. For BC, the majority of the patients were men (61.5 %, n = 8). BC was the presenting tumor of MEN1 in four patients. The mean duration of follow-up was 8.6 ± 5.3 years. A history of smoking was present in six patients (46.1 %).
Regarding other manifestations of MEN1, primary hyperparathyroidism was diagnosed in all the patients with BC. Eleven patients had gastroenteropancreatic neuroendocrine tumors (eight nonfunctional, two gastrinoma, and one insulinoma). Six patients had a pituitary adenoma [three prolactinomas, two nonfunctional, and one plurihormonal (prolactin and growth hormone)]. Five adrenal adenomas were present in five patients, and one patient had parathyroid carcinoma.
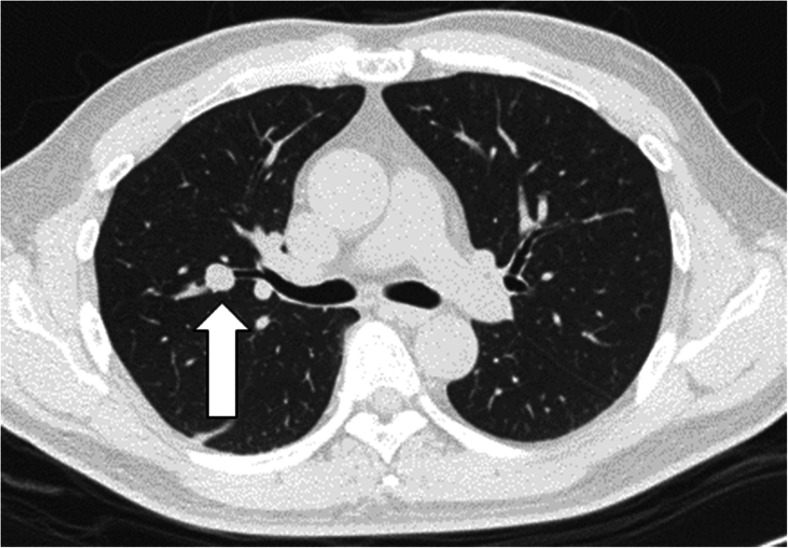
The majority of the patients were diagnosed on routine screening (n = 10, 77 %) with only three patients having symptoms at the time of diagnosis. When present, BC symptoms included cough, shortness of breath, or gastroesophageal reflux. The initial imaging modality for diagnosis was a chest roentgenogram (chest x-ray) in six patients and chest computed tomography (CT) in the remainder. A typical chest CT is shown in Fig. 1.
Fig. 1.
Smooth and spherical 1.1 cm endobronchial/partially endobronchial nodule (arrow) within a subsegmental bronchus branching off the anterior segmental bronchus of the right upper lobe, which proved to be a bronchial carcinoid tumor
All BC patients underwent surgical resection which varied from wedge resection to lobectomy with mediastinal lymphadenectomy.
Three patients died during the follow-up (23 %). One died from a cause unrelated to his BC, and in the other two patients, the cause of death was unknown.
None of the patients had distant metastasis at diagnosis nor developed them during follow-up. One of the patients had residual disease following the initial BC surgery and developed cough. Four patients had clinically suspected local recurrence of the disease with subcentimeter lung nodules. Three of them were asymptomatic and one required an additional surgical intervention (the exact cause for why intervention was offered is not clear).
Thymic Carcinoid Tumors
Seven patients were diagnosed with a TC. One of the patients had his initial surgery in an outside institution, but all of his records and pathology slides were retrieved so he was included in the descriptive analysis.
All TC patients were men and three were smokers (43 %). The mean age at diagnosis of MEN1 was 38 ± 9.9 years and the mean age at diagnosis of TC was 43 ± 12.4 years. One patient had thymic carcinoid as his first presenting tumor of MEN1. The clinical features are described in Table 2.
Table 2.
Clinical features of patients with thymic carcinoid (TC) tumors in patients with multiple endocrine neoplasia type 1 (MEN1)
| Case | Age at diagnosis of MEN1 (years) | Age at diagnosis of TC tumor (years) | MEN1 features | Symptoms | Tumor size (cm) |
|---|---|---|---|---|---|
| 1 | 53 | 55 | PHP/AA | Chest pain/dyspnea | 10 × 10 × 3 |
| 2 | 36 | 36 | PHP/gastrinoma | Thymic resection at the time of parathyroidectomy | 9 × 1.5 × 1 |
| 3 | 47 | 39 | PHP/NF NET/AA | Screening (chest x-ray) | 5.5 × 1.5 × 1 |
| 4 | 31 | 32 | PHP/insulinoma | Screening (chest-x-ray) | 11 × 6 × 7 |
| 5 | 27 | 41 | PHP | Chest pain | 7 × 6 × 4 |
| 6 | 43 | 65 | PHP/VIPoma/ZE/AA | Chest pain | 8 × 7.5 × 5 |
| 7 | 29 | 33 | PHP/insulinoma/AA/PRL | Chest pain | 5.5 |
PHP primary hyperparathyroidism, AA adrenal adenoma, NF NET nonfunctional neuroendocrine tumor, ZES Zollinger Ellison syndrome, PRL prolactinoma
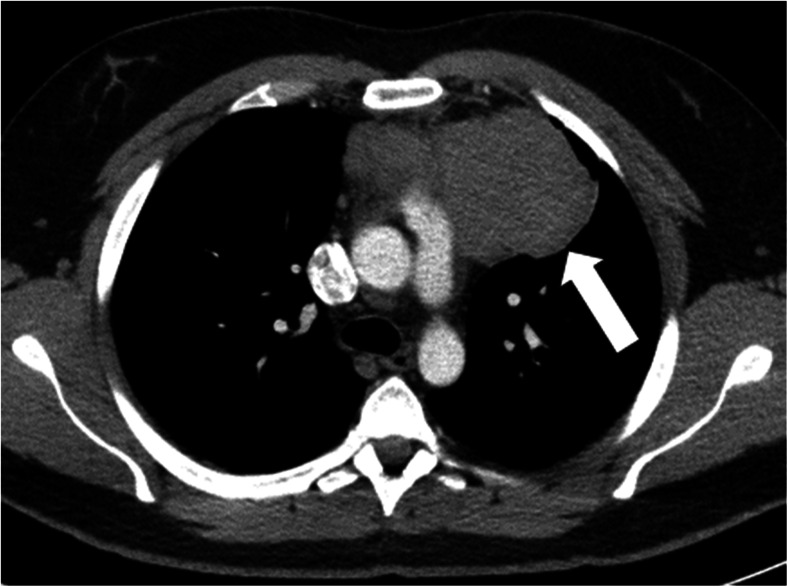
Of note, all patients had primary hyperparathyroidism as a manifestation of MEN1. Four patients were diagnosed based on symptoms (57.1 %) that led to further evaluation. The initial imaging used for diagnosis was a chest x-ray, which showed a mediastinal mass in six patients. The remaining patient was diagnosed during parathyroid surgery. A typical CT scan showing a TC is shown in Fig. 2.
Fig. 2.
Left 6.8 × 6.4 cm anterior mediastinal mass (arrow), which proved to be a thymic carcinoid tumor
Mortality was reported in four patients (57 %) during follow-up. Three of these patients died from known complications related to their thymic carcinoid, and in one case the cause was not known. In one patient, there was local progression complicated with pleural effusions and superior vena cava syndrome. Metastatic disease was present in the other two fatalities. In addition, recurrence and advanced disease were common. The treatment and outcomes for each of the patients are described in Table 3.
Table 3.
Treatment and outcomes of patients with thymic carcinoid (TC) tumors
| Case | Extent of disease at diagnosis | Therapy | Recurrence | Follow-up (years) | Death |
|---|---|---|---|---|---|
| 1 | Local extension with tumor in entire anterior mediastinum and pleural cavities | SR + RT | Residual tumor with local progression | 3 | Yes, due to TC |
| 2 | No local extension or distal metastasis | SR at diagnosis; at time of recurrence 5-FU + RT + somatostatin analog | At 6 years postoperatively, anterior mediastinal mass with pericardial effusion; at 8 years, metastatic disease to sacrum and ribs | 10 | Yes, unknown cause |
| 3 | No local extension or distant metastasisa | SR; brain resection of metastatic lesion + WBRT 4200 | At 4 years postoperatively with bone and brain metastases | 4 | Yes, due to TC |
| 4 | No local extension or distant metastasis | SR | Local recurrence at 11 years postoperatively | 11 | No |
| 5 | Local extension with positive surgical margins, vascular and lymph node invasion | SR | At 6 years postoperatively brain metastases and at 7 years lung metastases | 8 | Yes, due to TC |
| 6 | No local extension or distant metastasis | SR | No | 5 | No |
| 7 | Local extension with positive surgical margins | Cisplatin + etoposide followed by SR and RT; at time of recurrence started on tyrosine kinase inhibitor | At 3.5 years postoperatively, mediastinal and lung nodules | 3 | No |
SR surgical resection, RT radiation therapy, 5FU 5 fluorouracil, WBRT whole brain radiation therapy
aScreening chest roentgenogram showed a mediastinal mass that was resected and reported as a benign thymic tumor. Four years later, the patient presented with neurologic symptoms and metastatic disease to the brain was diagnosed
A comparison between the clinical features of TC and BC tumors is shown in Table 4.
Table 4.
Clinical features of thymic (TC) and bronchial carcinoid (BC) tumors in multiple endocrine neoplasia type1 (MEN1)
| Feature | BC (n = 13) | TC (n = 7) |
|---|---|---|
| Mean age at diagnosis, years | 45.1 | 43.0 |
| Men, % | 61 % | 100 % |
| BC or TC as initial feature of MEN1 | 31 % | 14.3 % |
| Symptoms at diagnosis | 23.1 % | 57.1 % |
| Death a | 0 % | 57.1 % |
| Distant metastasis | 0 % | 57.1 % |
aIn the BC tumor group, there were two deaths of unknown cause. In the TC group, the cause of death in one patient was unknown
Discussion
In the present study, we analyzed the clinical features of thymic and bronchial carcinoids identified in a cohort of 348 patients with MEN1. The prevalence of BC was 4.9 % (3.7 % if only cases with histology verified at our institution are included) and 2.0 % for TC based on histology.
Bronchial Carcinoid Tumors
A previous study that reported the presence of BC in members of the Tasman MEN1 kindred found a prevalence of 5 % in a total of 129 patients, of which only 28 % had chest CT. In their series, 83 % of the cases occurred in women [5]. A study from the Dutch MEN1 database found a prevalence of BC tumors of 13 % when using both imaging findings and pathology as the basis for diagnosis. When the analysis was restricted to diagnosis based on pathology, 16 cases were found out of 323 MEN1 patients (5 %) [4]. The prevalence of BC in our cohort is similar to the Tasman kindred and the Dutch series (based on pathology); we also found a male predominance with 67 % of our cases occurring in men. Our findings are consistent with those in the Tasman kindred regarding the clinical presentation with the majority of our patients diagnosed by routine screening and no patient developing metastatic disease during follow-up [5]. In addition, in the Dutch cohort, no patient died from a complication of bronchial carcinoid and the 10-year survival was noted at 71 % [4]. In our cohort, three of the patients died during follow-up, but the cause of death was not known in two cases and not related to a carcinoid tumor in the other. We did find patients with local recurrence in our series, but only one of them required further surgical intervention which eventually resolved his symptoms.
Thymic Carcinoid Tumors
The prevalence of TC in patients with MEN1 has been reported to be between 2.6 and 8 % [4, 6–9]. In a prospective study performed at the National Institute of Health (NIH), seven patients (8 %) with TC were identified from a group of 85 patients who were followed prospectively [7]. On the other hand, in a study that looked at a European registry, seven cases (2.6 %) of TC were identified in a group of 761 patients with MEN1 [8]. This variation in prevalence is likely explained by the prospective nature of the NIH study in which patients were systematically screened in comparison to the retrospective studies.
TC has been reported as having an almost exclusive male predominance in most of the case series with the exception of a Japanese series in which 36 % of the patients were women [9]. In our series, all the patients were men which is consistent with other Western series; the cause for this sex distribution is not clearly understood [6–8, 10, 11].
The majority of the patients were diagnosed based on initial symptoms (57 %) followed by an abnormal chest x-ray. This is similar to previous retrospective series in which the majority of the patients were symptomatic in comparison with the NIH prospective series in which 71 % of the patients were asymptomatic at the time of diagnosis [6–8]. This discrepancy with the NIH study can again be explained by the prospective study design.
TC were associated with increased morbidity and mortality. Only one of the patients was free of recurrence after 5 years of follow-up. From the six remaining patients, three had locally advanced disease and three had recurrence. Four patients died during follow-up, and in three of them (43 %), the cause of death was related to TC. The morbidity associated with TC is consistent with the current literature in which local invasion, distant metastasis, and recurrence during follow-up are common [4, 6, 7, 10]. On the other hand, we again see significant mortality in the retrospective studies but not in the NIH prospective study [4, 6–8, 10].
Limitations
The present study has several limitations related primarily to its retrospective nature. Although we studied a large number of patients with MEN1, not all of the patients were screened for thymic and bronchial carcinoids, which can result in decreased detection of early cases and a perceived aggressive behavior due to a late diagnosis. We do not have information regarding the number of patients in which screening was performed and no abnormalities were found. In addition, the imaging technology and the frequency of screening have changed during the last 36 years. Due to the retrospective nature of our study, measurement bias is present since the decision to screen for these tumors as well as the decision to treat (in case of pulmonary nodules) was based on the physician and patient’s preferences and not in a predefined protocol. In addition, we also present a single tertiary center experience that might be prone to referral bias and identification of a more severe spectrum of disease, which might impact the generalizability of our results. It is interesting to note that even with these caveats we were able to find different behaviors between the bronchial and thymic carcinoid tumors in this population.
Implications for Practice and Research
The clinical presentation of TC and BC is overall poorly understood with a limited number of studies available. A summary of identified studies are listed in Tables 5 and 6. Our findings support the current available literature, with TC and BC tumors occurring in a small proportion of patients with MEN1 with a prevalence of 4.9 and 2.0 %, respectively. BC were most commonly diagnosed during routine screening and were not associated with distant metastasis and rarely with local recurrence. On the other hand, TC tumors were predominantly seen in men and were symptomatic at the time of diagnosis. TC were associated with locally advanced disease, recurrence, and mortality.
Table 5.
Summary of selected MEN1 series with thymic carcinoid (TC) tumors
| Author | Type of study | Location | Number of MEN1 cases | TC tumors | Prevalence (%) | Male (%) | Outcome |
|---|---|---|---|---|---|---|---|
| de Laat et al. 2014 | Observational study using a national database | Netherlands | 323 | 11 | 3.4 | 91 | 7 deceased by the end of follow-up (10-year survival of 25 %) |
| Ferolla et al. 2005 | Observational study using a national registry | Italy | 221 | 7 | 3.2 | 100 | 3 died during follow-up (range, 23–120 months) |
| Gibril et al. 2003 | Prospective observational, single referral center | US | 85 | 7 | 8.2 | 100 | 1 died during follow-up (range, 0.13–15.3 years) |
| Goudet et al. 2009 | Observational study using national registry | France | 761 | 21 | 2.8 | 95.2 | 10 died during follow-up (10-year probability of survival 36.1 %) |
| Sakurai et al. 2013 | Observational study using a registry | Japan | 560 | 28 | 5.0 | 64.3 | 8 died during follow-up (median follow-up of 6.7 years; 10-year probability of survival of 30.3 %) |
| Teh et al. 1997 | Observational, different referral centers | Australia/Malaysia | 204 | 10 | 4.9 % | 100 | 9 patients died during follow-up (mean follow-up of 4.5 years) |
| Teh et al. 1998 | Observational, different referral centers | Australia/Malaysia | Not clear | 10 | Not clear | 100 | 1 patient died during follow-up (range, 2–162 months) |
| Present study | Observational, single referral center | US | 348 | 7 | 2.0 | 100 | 4 patients died during follow-up (range, 3–11 years) |
Table 6.
Summary of selected MEN1 series with bronchial carcinoid (BC) tumors
| Author | Type of study | Location | Number of MEN1 cases | Number of BC tumors | Prevalence (%) | Male (%) | Diagnosis | Outcome |
|---|---|---|---|---|---|---|---|---|
| de Laat et al. 2014 | Observational study using a national database | Netherlands | 323 | 42 | 13.3 | 48 | Histology and CT imaging | 5 died during follow-up but not related to BC (10-year survival of 71.1 %) |
| Sachithanandan et al. 2005 | Observational using a surveillance program | Tasmania | 129 | 6 | 4.7 | 17 | Histology | 2 died during follow-up |
| Present study | Observational, single center | US | 348 | 17 | 4.8 | 61 | Histology | 3 deaths during follow-up (2 unknown cause and 1 unrelated to BC). Range of follow-up 0.5–18 years |
Further studies are needed to explore the indications for surgical management of bronchial carcinoid tumors since the prevalence of these tumors based on imaging can be up to 13 % of patients with MEN1 but mortality is not significantly increased [4, 5]. In addition, the imaging modality and frequency of screening for these tumors should be investigated with prospective multicenter studies that will be less prone to bias. However, until future studies provide the needed guidance, the current recommendation [1] to periodically screen patients with MEN1 for both TC and BC (every 1–2 years using CT or MRI) is reasonable because these neoplasms can be associated with morbidity and mortality.
Acknowledgments
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, Melmed S, Sakurai A, Tonelli F, Brandi ML. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1) J Clin Endocrinol Metab. 2012;97(9):2990–3011. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 2.Sakurai A, Suzuki S, Kosugi S, Okamoto T, Uchino S, Miya A, Imai T, et al. Multiple endocrine neoplasia type 1 in Japan: establishment and analysis of a multicentre database. Clin Endocrinol (Oxf) 2012;76(4):533–539. doi: 10.1111/j.1365-2265.2011.04227.x. [DOI] [PubMed] [Google Scholar]
- 3.Trump D, Farren B, Wooding C, Pang JT, Besser GM, Buchanan KD, Edwards CR, et al. Clinical studies of multiple endocrine neoplasia type 1 (MEN1) QJM. 1996;89(9):653–669. doi: 10.1093/qjmed/89.9.653. [DOI] [PubMed] [Google Scholar]
- 4.de Laat JM, Pieterman CR, van den Broek MF, Twisk JW, Hermus AR, Dekkers OM, de Herder WW, et al. Natural course and survival of neuroendocrine tumors of thymus and lung in MEN1 patients. J Clin Endocrinol Metab. 2014;99(9):3325–3333. doi: 10.1210/jc.2014-1560. [DOI] [PubMed] [Google Scholar]
- 5.Sachithanandan N, Harle RA, Burgess JR. Bronchopulmonary carcinoid in multiple endocrine neoplasia type 1. Cancer. 2005;103(3):509–515. doi: 10.1002/cncr.20825. [DOI] [PubMed] [Google Scholar]
- 6.Ferolla P, Falchetti A, Filosso P, Tomassetti P, Tamburrano G, Avenia N, Daddi G, et al. Thymic neuroendocrine carcinoma (carcinoid) in multiple endocrine neoplasia type 1 syndrome: the Italian series. J Clin Endocrinol Metab. 2005;90(5):2603–2609. doi: 10.1210/jc.2004-1155. [DOI] [PubMed] [Google Scholar]
- 7.Gibril F, Chen YJ, Schrump DS, Vortmeyer A, Zhuang Z, Lubensky IA, Reynolds JC, et al. Prospective study of thymic carcinoids in patients with multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 2003;88(3):1066–1081. doi: 10.1210/jc.2002-021314. [DOI] [PubMed] [Google Scholar]
- 8.Goudet P, Murat A, Cardot-Bauters C, Emy P, Baudin E, du Boullay Choplin H, Chapuis Y, et al. Thymic neuroendocrine tumors in multiple endocrine neoplasia type 1: a comparative study on 21 cases among a series of 761 MEN1 from the GTE (Groupe des Tumeurs Endocrines) World J Surg. 2009;33(6):1197–1207. doi: 10.1007/s00268-009-9980-y. [DOI] [PubMed] [Google Scholar]
- 9.Sakurai A, Imai T, Kikumori T, Horiuchi K, Okamoto T, Uchino S, Kosugi S, et al. Thymic neuroendocrine tumour in multiple endocrine neoplasia type 1: female patients are not rare exceptions. Clin Endocrinol (Oxf) 2013;78(2):248–254. doi: 10.1111/j.1365-2265.2012.04467.x. [DOI] [PubMed] [Google Scholar]
- 10.Teh BT, McArdle J, Chan SP, Menon J, Hartley L, Pullan P, Ho J, et al. Clinicopathologic studies of thymic carcinoids in multiple endocrine neoplasia type 1. Medicine. 1997;76(1):21–29. doi: 10.1097/00005792-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Teh BT, Zedenius J, Kytola S, Skogseid B, Trotter J, Choplin H, Twigg S, et al. Thymic carcinoids in multiple endocrine neoplasia type 1. Ann Surg. 1998;228(1):99–105. doi: 10.1097/00000658-199807000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]