Abstract
Interferon-alpha (IFN-alpha) is recommended in neuroendocrine tumors (NET). Malignant pheochromocytoma and paragangliomas (MPPGLs) constitute a rare subgroup of NET with few treatment options. IFN-alpha efficacy in patients with MPPGLs was evaluated in a single-center retrospective study. Progression-free survival (PFS) was the primary endpoint according to RECIST 1.1 and/or PERCIST 1.0, and response rate, safety, and symptomatic efficacy were secondary endpoints. Fourteen patients received peginterferon alfa-2a (90 to 180 μg/week) or interferon alfa-2b (1.5 to 3 million units × 3/week) at our institution between December 2005 and February 2014 as the first (n = 7), second (n = 3), or subsequent line (n = 4) of treatment. Most of the patients had a slowly progressive disease before IFN-alpha initiation. Eight patients were men (57%); the median age was 44. At the beginning of treatment, 12 patients had progressive disease demonstrated by FDG-PET (n = 9), MIBG (n = 1), or CT scan (n = 2). Most of the patients treated (64%) had metastatic disease limited to or predominantly located in the bones. During IFN-alpha therapy, bone-directed loco-regional treatments were performed in 9 patients (range 1–4). Median PFS was 17.2 months (95% CI [12.1–58.3]). We observed 3 partial metabolic responses, 9 stable diseases, and 2 progressive diseases. No partial response according to RECIST 1.1 was observed. Symptomatic relief of pain, headaches, diarrhea, or sweating occurred in 6 out of 10 symptomatic pts. Most frequent all grade IFN-α-related toxicities were asthenia (n = 10), lymphopenia (n = 7), thrombopenia (n = 6), and anemia (n = 5). Median overall survival was 7.5 years (95% CI [4–NR]). This study suggests symptomatic response and tumor control effect with interferon-alpha in progressive MPPGLs.
Keywords: Partial Metabolic Response, Locoregional Treatment, Overall Survival (OS), Single-center Retrospective Study, Paraganglioma
Introduction
Pheochromocytomas and paragangliomas (PP) are neuroendocrine tumors (NET) which originate from the adrenal medulla and from the parasympathetic or sympathetic neural ganglia, respectively [1]. Malignancy is defined by the presence of pheochromocytoma or paraganglioma tumor cells in sites other than the primary tumor [2, 3]. In patients with metastatic disease, the bone is the most frequent site. In the French nationwide database of 90 patients, 56% of the metastatic patients had bone metastases (BM), of whom 17% had bone-only metastases (BOM) [4]. In the MD Anderson database, 71% of the 137 patients had BM, with 20% of the patients having BOM and 72% experiencing skeletal-related events (SRE) [5]. While the frequency of bone metastasis is one of the hallmarks of malignant pheochromocytoma/paraganglioma (MPPGLS), the disease is also characterized by a heterogeneous prognosis with 46% of MPPGLs remaining progression-free at 1 year without any therapeutic intervention [4]. In the MD Anderson retrospective series, median overall survival was 12 years for patients with BOM, 7.5 years for patients with non-osseous metastases, and 5 years for patients with both bone metastases and non-osseous metastases [5].
In line with the abovementioned prolonged overall survival but also in the absence of curative option, MPPGL patient management requires carefully weighted risk-benefit strategy. A watchful wait-and-see strategy, combined or not with imaging-guided locoregional options, is recommended for MPPGL patients with no symptoms, slow progression, and a low tumor burden [2, 3, 6]. In addition, it is of major importance to expanding the therapeutic arsenal not only for patients with slowly progressing tumors but also for those with more aggressive disease with therapeutic modalities such as anti-angiogenics or chemotherapy. In the context of the extreme scarcity of MPP, lessons from other NET primaries and retrospective analyses performed in expert centers may provide the first signal of efficacy of new or available therapeutic options.
Interferon-alpha (IFN-alpha) has been studied for the treatment of neuroendocrine tumors and has been shown to provide symptomatic and biochemical response in up to 70% of the patients with carcinoid syndrome [7] and 30 to 75% of the patients with disease stabilization [8, 9]. To date, interferon-alpha remains a recommended option for the management of patients with digestive and pulmonary neuroendocrine tumors [10, 11], especially in subgroups of patients with slowly progressive tumors whose characteristics could mirror subgroups of MPPGL patients. In neuroendocrine tumor of other origin, little is known about IFN-alpha activity. IFN-alpha has been studied in a rat pheochromocytoma cell line and was suggested to have an impact on cell proliferation [12]; moreover, we recently reported a tumor response to pegylated interferon in a patient with metastatic MPPGL [13]. Here, we report our retrospective experience with the use of interferon-alpha in MPPGL patients.
Subjects and Methods
Population
Patients with MPPGLs who were treated with IFN-alpha at Gustave Roussy between December 2005 and February 2014 were included in this retrospective study. IFN-alpha treatment was initiated in patients with a good performance status (WHO PS 0–2), a reviewed pathological diagnosis of MPPGL (AAG), and a slowly progressive disease as defined by computed tomography (CT) scan/magnetic resonance imaging (MRI) and/or best scintigraphic evaluation (either [18F]-fluorodeoxyglucose-positron emission tomography (FDG-PET) or Iodine-123-metaiodobenzylguanidine (123I-MIBG)). Patients with rapidly progressive disease were treated by either chemotherapy [14] and/or tyrosine kinase inhibitor [15] as part of the FIRSMAPP trial (NCT01371201) when feasible. Patients were not treated if they had uncontrolled cardiovascular conditions such as uncontrolled hypertension, congestive heart failure, or unstable coronary disease; persistent laboratory abnormalities such as hematopoietic abnormalities (absolute neutrophil count <1500 cells/mm3 or thrombopenia <100,000 cells/mm3); hepatic impairment (bilirubin >2 × upper limit of normal (ULN) or alanine aminotransferase/aspartate aminotransferase activity >5 × ULN); and severe renal insufficiency (glomerular filtration rate <30 ml/min). Genetic testing to screen for germline mutations in PP predisposing genes (SDHB, SDHD, SDHC, SDHA, VHL, RET, TMEM127, and MAX) was carried out on genomic DNA at the Laboratory of Genetics of Hôpital Européen Georges Pompidou as previously described [16]. The following clinical characteristics were recorded: time since diagnosis (first evidence of disease); time since first surgery; time since first metastasis; age; sex; WHO performance status; weight; hormone- or tumor-related symptoms; chromogranin A serum concentrations [17, 18]; urinary concentrations of metanephrines including metanephrine, normetanephrine, and methoxytyramine [19]; 123I-MIBG scintigraphy, somatostatin receptor scintigraphy, or FDG-PET status [20]; primary location; and metastatic locations based on lung and abdomen-pelvic CT and/or liver and bone MRI [21, 22]. Patients with hormone-related symptoms were defined as functioning MPPGL patients. MPPGLs with metastases only or mainly in the bone and no more than five extra-bone metastatic sites were defined as bone-predominant disease.
Treatment
Interferon-alpha was initially given as interferon alfa-2b subcutaneous injections at an initial dose of 1.5 million units (MU) three times a week increased to 3 MU × 3/week if well tolerated. According to its 1-week administration schedule, peginterferon alfa-2a at 90 μg per week for 1 month, increased to 180 μg/week if well tolerated, was the schedule of choice since it has been shown to have a better tolerability in NET patients [23]. Treatment was continued every 28 days-cycle according to toxicity assessment. IFN-alpha was administered until disease progression or unacceptable toxicity. The IFN-α dose was reduced in case of grade 3 or long-lasting uncontrolled grade 2 toxicity.
Loco-regional treatments to the bone were allowed and recorded, including radiation therapy, interventional radiology procedures (radiofrequency ablation, cryotherapy, and kyphoplasty) as previously described [24, 25], and surgery.
This study was approved by the Gustave Roussy institutional review board; every patient medical case was discussed in the setting of our regional multidisciplinary adrenal tumor board of the COMETE-Cancer network in order to confirm IFN-alpha therapy, and informed consent was obtained from all the patients.
Efficacy and Safety Assessment
Safety was assessed throughout the study by monitoring and recording adverse events, vital signs, clinical chemistry, and hematology. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTC, v.4).
Evaluation of tumor response was performed at the time of this retrospective study according to local review using RECIST 1.1 criteria (CC) on computed tomography (CT) scans and/or magnetic resonance imaging (MRI) performed at the time of initial screening and every 12 weeks when available. In non-RECIST 1.1-evaluable patients, determination of objective response was performed according to PERCIST 1.0 criteria (MT). FDG-PET was performed at the time of initial screening and every 12 weeks.
Design and Statistical Analysis
This is a single-center retrospective study. The primary objective was to determine progression-free survival (PFS) according to RECIST 1.1 or PERCIST 1.0 criteria performed every 3 months. Secondary objectives were objective response evaluation by RECIST 1.1 or PERCIST 1.0, the duration of response, safety, and overall survival.
Descriptive quantitative data were expressed as mean ± standard deviation, and qualitative data were expressed as a percentage. Median follow-up, overall survival (OS), and PFS were estimated by the Kaplan-Meier method. PFS was defined by the time elapsed between the first dose of IFN-α and either progression or death by any cause, and OS was defined by the time elapsed between the first dose of IFN-α and death by any cause. Data were censored at last follow-up for patients without progression or death. Data analysis and graphs were done with GraphPad Prism 6.0 (GraphPad Software, Inc).
Results
Patient Characteristics
Between December 2005 and February 2014, 14 patients were treated with IFN-alpha at our institution; there were 8 males (57%) with a median age of 44. Patient characteristics are summarized in Table 1. Ten patients (71%) had a paraganglioma and 4 patients had a pheochromocytoma. Most of the patients treated (65%) had metastatic disease limited to or predominantly located on the bones. All but one patient had a surgical resection of the primary, and all patients had bone metastases, with 5 patients (36%) having bone-only disease and 4 patients having bone-predominant disease (29%); 10 patients had metachronous metastases. Eight patients had hypertension and six patients had bone pain. Half of the patients received IFN-alpha as first-line therapy; 21 and 29% received it as second or third or more line, respectively.
Table 1.
Patient characteristics
| Characteristics | N = 14 (%) |
|---|---|
| Median age (years) | 44 |
| Male | 8 (57%) |
| Genetic status | |
| No mutation | 5 (36%) |
| SDHB mutation | 9 (64%) |
| Primary site | |
| Adrenal | 4 (29%) |
| Abdomen | 7 (50%) |
| Neck | 3 (21%) |
| Hypertension | 8 (57%) |
| Metanephrin secretion | 9 (64%) |
| Chromogranin A secretion | 11 (79%) |
| Surgery of primary site | 13 (93%) |
| Median time from diagnosis to metastatic disease |
12.3 months (range 0–338 months) |
| Metastatic at diagnosis | 4 (29%) |
| Bone metastases only | 5 (36%) |
| Median time to progression before IFN-alpha initiation | 9.4 months (range 3–33) |
| Number of previous systemic therapies | |
| 0 | 7 (50%) |
| 1 | 3 (21%) |
| ≥2 | 4 (29%) |
Interferon-alpha was administered within a median time from diagnosis of malignancy of 13.7 months (range 2.4–62.6 months), as interferon alfa-2b at an initial dose of 1.5 million units (MU) three times a week increased to 3 MUI × 3/week in 3 patients and as peginterferon alfa-2a at 90 μg per week for 1 month increased to 180 μg/week if well tolerated in the remaining 11 patients. Most of the patients had progressive disease within 1 year before IFN-alpha treatment, documented by appearance of a new lesion on best scintigraphy (FDG-PET or MIBG) except two patients for whom progression was demonstrated within 18 and 33 months, respectively (Table 3). Median time to progression before initiation of IFN-alpha-α was 9.4 months (range 3–33 months).
Table 3.
Patient treatment summary
| Patient | Mutation | Reason of IFN treatment | Imaging exam (baseline and follow-up) | New lesion on scintigraphy (FDG or MIBG) at baseline | Response to IFN PERCIST 1.0/RECIST 1.1 | Loco-regional treatment associated | Duration of treatment with IFN | SRE (n) |
|---|---|---|---|---|---|---|---|---|
| #1 | SDHB | Progression | MRI and FDG-PET | NA | NA/NA | None | 4 months | 0 |
| #2 | SDHB | Progression within 7 months | MRI and FDG-PET | Yes, epidural compression on MRI | SD/NE | Kiphoplasty L1 and L4 | 54 months | 2 |
| #3 | No | Progression within 18 months | FDG-PET and CT scan | Yes | SD/SD | None | 6 months, discontinuation for fever and arthralgiaa | 0 |
| #4 | SDHB | Progression within 10 months | CT scan, FDG-PET, and octreo scan | Yes | PMR/NA | Radiofrequency ablation and kiphoplasty T4, left iliac and left femur | 25 months | 0 |
| #5 | SDHB | Progression within 9 months | CT scan and octreo scan | Yes | SD/PD | None | 11 months | 0 |
| #6 | SDHB | Progression within 6 months | FDG-PET | Yes | PMR/NE | C1 to C5 radiation | 19 months | 1 |
| #7 | SDHB | Progression within 6 months | FDG-PET and CT scan | Yes | PMR/SD | C1 radiation | 14 months | 1 |
| #8 | SDHB | Progression within 7 months | FDG-PET and MRI | Yes | SD/NA | T2T3 surgery and radiation therapy | 37 months, treatment discontinuation for asthenia | 1 |
| #9 | No | Progression within 6 months | MIBG | Yes | SD/NE | Sacro-iliac joint and right femur radiation therapy | 17 months | 1 |
| #10 | SDHB | Progression within 8 months | FDG-PET | Yes | PMR/NE | Kiphoplasty L4, radiofrequency ablation T4, left rib and manubrium | 40 months | 1 |
| #11 | No | Progression within 33 months | MIBG and CT scan | Yes | SD/SD | None | 3 months, treatment until MIBG therapy | 0 |
| #12 | SDHB | Progression within 4 months | FDG-PET and scan | Yes | SD/SD | L2 radiofrequency ablation | 7 months until progressive disease with new bone lesion | 0 |
| #13 | No | Progression within 3 months | FDG-PET and MRI | Yes | NA/NA | L4 and sacrum cryotherapy | 37 months, pause | 0 |
| #14 | No | Progression within 3 months | FDG-PET | Yes | SD/NE | None | 12.6 months, discontinued for skin toxicity, stabilization remains at last follow-up | 0 |
NA not available, NE not evaluable, SD stable disease, PD progressive disease, PMR partial metabolic response
aPatient #3 was admitted to ICU 1 month after discontinuation of IFN-α for thoracic pain + hypertension + pulmonary edema + renal insufficiency which led to cardiac arrest
Safety
Interferon-alpha was well tolerated with very few grade 3–4 toxicities (asthenia n = 2, neutropenia n = 2, and thrombopenia n = 1); most of the side effects were grade 1–2, with the most frequent being asthenia (71%), lymphopenia (50%), and neutropenia (42%) with no infectious complication. Toxicity for the whole population is summarized in Table 2. The IFN-alpha dose was reduced in four patients: because of asthenia in three patients (one grade 2 and two grade 3) and because of grade 2 weight loss in one patient. One patient was diagnosed with Graves’ disease during IFN-alpha treatment, which was treated with carbimazole. One patient was admitted to the intensive care unit 1 month after IFN-alpha discontinuation because of fever, lymphopenia, and anemia with no evidence of infectious disease and normal blood pressure. She complained of thoracic pain, hypertension, pulmonary edema, and renal insufficiency; her condition worsened and she died of cardiac arrest. Disease progression was hypothesized to be the cause of death.
Table 2.
Toxicity of interferon-α treatment
| Toxicity | Grade 1 | Grade 2 | Grade 3–4 | All grades |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Asthenia | 5 (36%) | 3 (21%) | 2 (14%) | 10 (71%) |
| Myalgia | 3 (21%) | None | None | 3 (21%) |
| Diarrhea | 1 (7%) | 2 (14%) | None | 3 (21%) |
| Anemia | 4 (29%) | 1 (7%) | None | 5 (36%) |
| Neutropenia | 2 (14%) | 2 (14%) | 2 (14%) | 6 (42%) |
| Lymphopenia | 5 (36%) | 1 (7%) | 1 (7%) | 7 (50%) |
| Thrombopenia | 5 (36%) | None | None | 5 (36%) |
| Thyroid dysfunction | None | 1 (7%) | None | 1 (7%) |
| AST/ALT elevation | 3 (21%) | 1 (7%) | None | 4 (28%) |
| Other toxicity (n=) | Alopecia G1(1) G2(1), hypertension G1(1) G3(1), weight loss G2 (2), anorexia G2 (1), hypersomnia G2 (1), depression G1 (1), skin toxicity G1 and G21) | 10 (71%) | ||
G grade
Efficacy
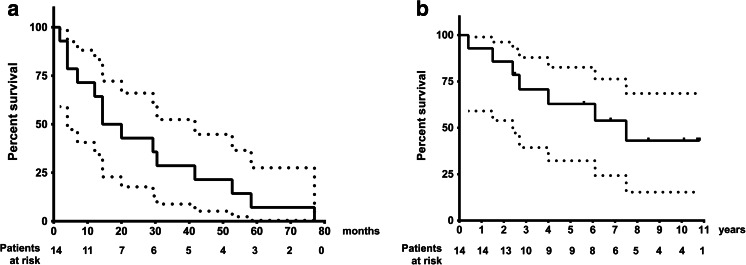
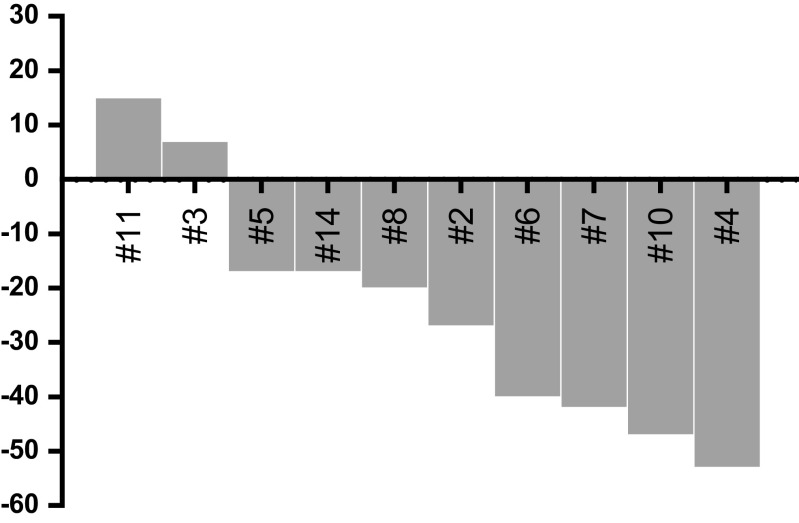
Twelve out of 14 patients were deemed evaluable for tumor response according to RECIST 1.1 and/or PERCIST 1.0. The two remaining patients, for whom no imaging picture was available for review at the time of the retrospective analysis, were considered to have stable and progressive disease, respectively, by their treating physician based on clinical and metanephrine results. After a median follow-up of 8.5 years (95% CI [6.8–NR]), median PFS was 17.2 months (95% CI [12.1–58.3]) (Fig. 1a) and median overall survival was 7.5 years (95% CI [4–NR]) (Fig. 1b). There was no difference in terms of PFS nor OS in SDHB- mutated as compared to non-mutated patients (logrank test, p = 0.453 and 0.0901, respectively). Owing to bone-only disease in five patients and discrepancies in the different imaging modalities used during follow-up in four patients, only five patients were evaluable according to RECIST 1.1: four stable diseases and one progressive disease were observed as best response. Twelve patients were evaluated by scintigraphy (FDG-PET, n = 10, and 131I-MIBG, n = 2): four partial metabolic responses, six stable diseases according to FDG-PET PERCIST 1.0 (Fig. 2), and two stable diseases on MIBG were observed as best response. Five patients were evaluated according to both RECIST 1.1 and PERCIST 1.0 with some discrepancies between these evaluation procedures; three patients were classified as having stable disease by both imaging procedures, one patient who had a metabolic response was considered to have RECIST 1.1 stable disease, and another patient had PERCIST 1.0 stable metabolic disease and RECIST 1.1 progressive disease (Table 3). Altogether, we observed three partial metabolic responses, nine stable diseases, and two progressive diseases (Fig. 2 and Table 3) which led to a 79 and 64% disease control rate at 6 and 12 months, respectively. Symptomatic relief of pain, headaches, paradoxical diarrhea, or sweating occurred in 60% of the 10 symptomatic patients. During IFN-alpha treatment, 8 kyphoplasties, 3 radiofrequency ablations, 5 radiation therapies, and 1 surgery were performed in 9 out of the 14 patients of this study in order to prevent bone fracture and/or pain.
Fig. 1.
Survival curves, dotted lines: 95% confidence interval. a Progression-free survival. Median PFS, 17.2 months (95% CI [12.1–58.3]). b Overall survival. Median OS, 7.5 years (95% CI [4–NR])
Fig. 2.
Waterfall plot of FDG-PET PERCIST objective responses
Discussion
The therapeutic strategy for metastatic pheochromocytoma/paraganglioma remains palliative and aims to control hormonal secretion and tumor burden. With respect to antitumor control, treatment strategies include a wait-and-see policy, locoregional therapies, systemic chemotherapy, and radiopharmaceutical agents, depending on the presence of tumor-related symptoms, tumor burden, and progression of the disease (Table 3) [2, 3, 6]. In NET patients, RECIST, which estimates the change in tumor burden, together with the appearance of new lesions, remains the mainstay of therapeutic result evaluation. In MPP patients, the high frequency of bone metastases makes the search for alternative modality of evaluation critical and currently ongoing in the running FIRSTMAPP trial. Based on the insufficient sensitivity of hormone markers in MPP patients for diagnosis [4, 26] and the lack of data regarding their role for the follow-up of metastatic patients, we used PERCIST 1.0 or RECIST 1.1 criteria for assessing tumor response in the present study. However, the definition of progressive disease according to RECIST (increase of the sum of lesions of more than 20%) has been determined for clinical trials and does not always reflect the heterogeneity of the natural histories of MPPGLs. Indeed, slowly progressive diseases, which could be defined as “infra-RECIST” progressions, are often seen in clinical practice. In these cases, one or several metastatic sites may be harmful and therefore require loco-regional treatment and/or systemic treatment before a proper RECSIT progression occurs. Interferon-alpha could be part of the therapeutic options in these cases.
Interferon-alpha has shown a disease-stabilization effect in NET of the digestive tract and efficacy against carcinoid syndrome [8, 9, 23, 27–29]. Here, we report a 17.2-month median PFS and a 64% disease control rate for more than 12 months in MPPGL patients who had, for most of them, a progression of the disease within the year before starting treatment. Median overall survival was 7.5 years in our study which is longer than the 3.5 years of the Huang et al. series [30] and the 4.6 years of the Asai et al. series, while it is in the range of what was seen in the MD Anderson series (median OS 6.4 years) [31] and in the Fishbein et al. recent series (69% 5-year OS) [32]. These discrepancies between overall survivals in the different retrospective studies of MPPGL treatment modalities probably reflect a selection bias which may have led to the inclusion of patients with more indolent disease for IFN-alpha treatment. Most of the responses we observed were stable diseases, explaining the frequent combination with loco-regional treatment in order to prevent bone-related symptoms and/or decrease the tumor burden [5]. Metabolic responses according to PERCIST were observed in four patients (Fig. 1). Most of the patients included in this study had a time to progression of more than 6 months before starting IFN-alpha with a median time to progression before IFN-alpha initiation of 9.4 months which compares favorably with the 17.2-month median PFS under treatment. This therapeutic option may, therefore, be considered for controlling tumor burden in combination with loco-regional treatments of the most at-risk metastatic sites. Whether interferon has a role in hormone secretion control in MPPGL patients remains to be elucidated. However, we observed hypertension control in two out of two patients who had uncontrolled hypertension before starting interferon and we did not observe any hormone secretion-related side effect.
This is a single-center retrospective study which has several biases. First, the little number of included patients, which is related to the extreme rarity of the disease [2], prevents the drawing of a definitive conclusion. Second, this is not a prospective, placebo-controlled study and it is not, therefore, possible to definitely conclude on the real clinical impact of IFN-alpha. However, by treating mostly patients with progressive disease, we have done our best to ensure that disease stabilization observed during treatment could be related to IFN-alpha. Third, we could not conclude on the hormonal effect of IFN-alpha because of our lack of data due to the retrospective setting.
We believe that interferon-alpha may have a role in the management of MPPGL patients, especially for those patients with slowly progressive, bone-only or bone-predominant disease, by its action of disease stabilization in association with loco-regional treatment like for bone metastases, for example, in order to shrink the tumor burden and prevent SREs [2, 5]. Several systemic treatment options are available for MPPGL patients with progressive and/or significant tumor burden. In this setting, MIBG therapy and CVD are the most frequently reported in the literature. First, in studies of MIBG therapy, which can be offered to 50% of MPP (based on MIBG uptake on diagnostic imaging studies), a tumor response rate of 22 to 48% and a median PFS of 24 to 36 months were observed [33–37]. Second, systemic chemotherapy with CVD has been reported by several groups with response rates of 25 to 55% according to different criteria (WHO, RECIST 1.1, clinical and biochemical) and median PFS of 20 to 40 months in responders and 3.3 to 5 months in non-responders [30–32, 38, 39]. However, it is difficult to draw conclusions from these different studies because response criteria used were very heterogeneous and the progressive status of the tumor before therapy was not specified in most of the studies. Indeed, it is of major importance to carefully describe the progression rate of the tumor before treatment because the median PFS of untreated MPPGL was 13.5 months in the French retrospective study [4], with half of the MPPGL patients not having experienced any tumor progression at 1 year. These reflect the heterogeneity of the natural course of the disease and argue for prioritization and selection of treatment options according to the tumor burden, the progression rate, availability of treatments, comorbidities, and preference of the patients. Therefore, we believe that interferon-alpha may participate in a “strategic management” of patients with MPPGLs by postponing the initiation of systemic therapies such as anti-angiogenics or chemotherapy and may be an alternative to MIBG therapy, especially in patients with MIBG-negative tumors and slowly progressive disease.
To conclude, our retrospective study suggests that interferon-alpha provides partial metabolic responses and prolonged disease stabilizations with acceptable toxicity in patients with slowly progressive metastatic pheochromocytoma/paraganglioma. Further prospective studies are needed in order to confirm these retrospective results.
Acknowledgements
None.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1.DeLellis RA, Lloyd RV, Heitz PU, Eng C (2004) Pathology and genetics of tumours of endocrine organs. IARC WHO Classification of Tumours
- 2.Baudin E, Habra MA, Deschamps F, et al. Therapy of endocrine disease: treatment of malignant pheochromocytoma and paraganglioma. Eur J Endocrinol. 2014;171:R111–R122. doi: 10.1530/EJE-14-0113. [DOI] [PubMed] [Google Scholar]
- 3.Jimenez C, Rohren E, Habra MA, et al. Current and future treatments for malignant pheochromocytoma and sympathetic paraganglioma. Curr Oncol Rep. 2013;15:356–371. doi: 10.1007/s11912-013-0320-x. [DOI] [PubMed] [Google Scholar]
- 4.Hescot S, Leboulleux S, Amar L et al (2013) One-year progression-free survival of therapy-naive patients with malignant pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. doi:10.1210/jc.2013-1907 [DOI] [PubMed]
- 5.Ayala-Ramirez M, Palmer JL, Hofmann M-C, et al. Bone metastases and skeletal-related events in patients with malignant pheochromocytoma and sympathetic paraganglioma. J Clin Endocrinol Metab. 2013;98:1492–1497. doi: 10.1210/jc.2012-4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berruti A, Baudin E, Gelderblom H, et al. Adrenal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii131–vii138. doi: 10.1093/annonc/mds231. [DOI] [PubMed] [Google Scholar]
- 7.Öberg K. Biotherapies for GEP-NETs. Best Pract Res Clin Gastroenterol. 2012;26:833–841. doi: 10.1016/j.bpg.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Bajetta E, Zilembo N, Di Bartolomeo M, et al. Treatment of metastatic carcinoids and other neuroendocrine tumors with recombinant interferon-alpha-2a: a study by the Italian trials in Medical Oncology Group. Cancer. 1993;72(10):3099–3105. doi: 10.1002/1097-0142(19931115)72:10<3099::AID-CNCR2820721035>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Dahan L, Bonnetain F, Rougier P, et al. Phase III trial of chemotherapy using 5-fluorouracil and streptozotocin compared with interferon alpha for advanced carcinoid tumors: FNCLCC-FFCD 9710. Endocr Relat Cancer. 2009;16:1351–1361. doi: 10.1677/ERC-09-0104. [DOI] [PubMed] [Google Scholar]
- 10.Caplin ME, Baudin E, Ferolla P, et al. Pulmonary neuroendocrine carcinoid tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26:1604–1620. doi: 10.1093/annonc/mdv041. [DOI] [PubMed] [Google Scholar]
- 11.Pavel M, O’Toole D, Costa F, et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. 2016;103:172–185. doi: 10.1159/000443167. [DOI] [PubMed] [Google Scholar]
- 12.Motylewska E, Lawnicka H, Kowalewicz-Kulbat M, et al. Interferon alpha and rapamycin inhibit the growth of pheochromocytoma PC12 line in vitro. Endokrynol Pol. 2013;64:368–374. doi: 10.5603/EP.2013.0020. [DOI] [PubMed] [Google Scholar]
- 13.Bahougne T, Imperiale A, Averous G, et al. Successful response to pegylated interferon alpha in a patient with recurrent paraganglioma. Endocr Relat Cancer. 2017;24:L7–L11. doi: 10.1530/ERC-16-0431. [DOI] [PubMed] [Google Scholar]
- 14.Hadoux J, Favier J, Scoazec J-Y, et al. SDHB mutations are associated with response to temozolomide in patients with metastatic pheochromocytoma or paraganglioma. Int J Cancer. 2014;135:2711–2720. doi: 10.1002/ijc.28913. [DOI] [PubMed] [Google Scholar]
- 15.Ayala-Ramirez M, Chougnet CN, Habra MA, et al. Treatment with sunitinib for patients with progressive metastatic pheochromocytomas and sympathetic paragangliomas. J Clin Endocrinol Metab. 2012;97:4040–4050. doi: 10.1210/jc.2012-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buffet A, Venisse A, Nau V, et al. A decade (2001-2010) of genetic testing for pheochromocytoma and paraganglioma. Horm Metab Res. 2012;44:359–366. doi: 10.1055/s-0032-1304594. [DOI] [PubMed] [Google Scholar]
- 17.Baudin E, Bidart JM, Bachelot A, et al. Impact of chromogranin A measurement in the work-up of neuroendocrine tumors. Ann Oncol. 2001;12(Suppl 2):S79–S82. doi: 10.1093/annonc/12.suppl_2.S79. [DOI] [PubMed] [Google Scholar]
- 18.Baudin E, Gigliotti A, Ducreux M, et al. Neuron-specific enolase and chromogranin A as markers of neuroendocrine tumours. Br J Cancer. 1998;78(8):1102–1107. doi: 10.1038/bjc.1998.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amar L, Peyrard S, Rossignol P, et al. Changes in urinary total metanephrine excretion in recurrent and malignant pheochromocytomas and secreting paragangliomas. Ann N Y Acad Sci. 2006;1073:383–391. doi: 10.1196/annals.1353.042. [DOI] [PubMed] [Google Scholar]
- 20.Abgral R, Leboulleux S, Déandreis D, et al. Performance of (18) fluorodeoxyglucose-positron emission tomography and somatostatin receptor scintigraphy for high Ki67 (≥10%) well-differentiated endocrine carcinoma staging. J Clin Endocrinol Metab. 2011;96:665–671. doi: 10.1210/jc.2010-2022. [DOI] [PubMed] [Google Scholar]
- 21.Dromain C, de Baere T, Lumbroso J, et al. Detection of liver metastases from endocrine tumors: a prospective comparison of somatostatin receptor scintigraphy, computed tomography, and magnetic resonance imaging. J Clin Oncol. 2005;23:70–78. doi: 10.1200/JCO.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Leboulleux S, Dromain C, Vataire AL, et al. Prediction and diagnosis of bone metastases in well-differentiated gastro-entero-pancreatic endocrine cancer: a prospective comparison of whole body magnetic resonance imaging and somatostatin receptor scintigraphy. J Clin Endocrinol Metab. 2008;93:3021–3028. doi: 10.1210/jc.2008-0459. [DOI] [PubMed] [Google Scholar]
- 23.Pavel ME, Baum U, Hahn EG, et al. Efficacy and tolerability of pegylated IFN-α in patients with neuroendocrine gastroenteropancreatic carcinomas. J Interf Cytokine Res. 2006;26:8–13. doi: 10.1089/jir.2006.26.8. [DOI] [PubMed] [Google Scholar]
- 24.Deschamps F, Farouil G, de Baere T. Percutaneous ablation of bone tumors. Diagn Interv Imaging. 2014;95:659–663. doi: 10.1016/j.diii.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Deschamps F, Farouil G, Ternes N, et al. Thermal ablation techniques: a curative treatment of bone metastases in selected patients? Eur Radiol. 2014;24:1971–1980. doi: 10.1007/s00330-014-3202-1. [DOI] [PubMed] [Google Scholar]
- 26.Gimenez-Roqueplo A-P, Caumont-Prim A, Houzard C, et al. Imaging work-up for screening of paraganglioma and pheochromocytoma in SDHx mutation carriers: a multicenter prospective study from the PGL.EVA Investigators. J Clin Endocrinol Metab. 2013;98:E162–E173. doi: 10.1210/jc.2012-2975. [DOI] [PubMed] [Google Scholar]
- 27.Faiss S, Pape U-F, Böhmig M, et al. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors—the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21:2689–2696. doi: 10.1200/JCO.2003.12.142. [DOI] [PubMed] [Google Scholar]
- 28.Kölby L, Persson G, Franzén S, Ahrén B. Randomized clinical trial of the effect of interferon alpha on survival in patients with disseminated midgut carcinoid tumours. Br J Surg. 2003;90:687–693. doi: 10.1002/bjs.4149. [DOI] [PubMed] [Google Scholar]
- 29.Mirvis E, Mandair D, Garcia-Hernandez J, et al. Role of interferon-alpha in patients with neuroendocrine tumors: a retrospective study. Anticancer Res. 2014;34:6601–6607. [PubMed] [Google Scholar]
- 30.Huang H, Abraham J, Hung E, et al. Treatment of malignant pheochromocytoma/paraganglioma with cyclophosphamide, vincristine, and dacarbazine: recommendation from a 22-year follow-up of 18 patients. Cancer. 2008;113:2020–2028. doi: 10.1002/cncr.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayala-Ramirez M, Feng L, Habra MA, et al. Clinical benefits of systemic chemotherapy for patients with metastatic pheochromocytomas or sympathetic extra-adrenal paragangliomas: insights from the largest single-institutional experience. Cancer. 2012;118:2804–2812. doi: 10.1002/cncr.26577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fishbein L, Ben-Maimon S, Keefe S et al (2017) SDHB mutation carriers with malignant pheochromocytoma respond better to CVD. Endocr Relat Cancer. doi:10.1530/ERC-17-0086 [DOI] [PubMed]
- 33.Gonias S, Goldsby R, Matthay KK, et al. Phase II study of high-dose 131Imetaiodobenzylguanidine therapy for patients with metastatic pheochromocytoma and paraganglioma. J Clin Oncol. 2009;27:4162–4168. doi: 10.1200/JCO.2008.21.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carrasquillo JA, Pandit-Taskar N, Chen CC. I-131 Metaiodobenzylguanidine therapy of pheochromocytoma and paraganglioma. Semin Nucl Med. 2016;46:203–214. doi: 10.1053/j.semnuclmed.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Krempf M, Lumbroso J, Mornex R, et al. Treatment of malignant pheochromocytoma with 131metaiodobenzylguanidine: a French multicenter study. J Nucl Biol Med. 1991;35(4):284–287. [PubMed] [Google Scholar]
- 36.Safford SD, Coleman RE, Gockerman JP, et al. Iodine −131 Imetaiodobenzylguanidine is an effective treatment for malignant pheochromocytoma and paraganglioma. Surgery. 2003;134:956–962. doi: 10.1016/S0039-6060(03)00426-4. [DOI] [PubMed] [Google Scholar]
- 37.Wakabayashi H, Taki J, Inaki A, et al. Prognostic values of initial responses to low-dose 131I-MIBG therapy in patients with malignant pheochromocytoma and paraganglioma. Ann Nucl Med. 2013;27:839–846. doi: 10.1007/s12149-013-0755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asai N, Iwashita T, Matsuyama M, Takahashi M. Mechanism of activation of the ret proto-oncogene by multiple endocrine neoplasia 2A mutations. Mol Cell Biol. 1995;15(3):1613–1619. doi: 10.1128/MCB.15.3.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanabe A, Naruse M, Nomura K, et al. Combination chemotherapy with cyclophosphamide, vincristine, and dacarbazine in patients with malignant pheochromocytoma and paraganglioma. Horm Cancer. 2013;4:103–110. doi: 10.1007/s12672-013-0133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]