Abstract
The study aims to compare serial changes in prostate-specific antigen (PSA), testosterone, dehydroepiandrosterone (DHEA), and androstenedione in patients treated with either of the antiandrogen agents, bicalutamide or flutamide, using a randomized controlled study. Patients had to meet the following inclusion criteria: (1) presence of histopathologically confirmed prostate cancer, (2) prostate cancer treatment naive, (3) no current treatment with luteinizing hormone-releasing hormone (LH-RH) agonist for sexual interest and physical capacity, (4) clinical stage T1–cT3N0M0, (5) Gleason score ≤7, and (6) Cooperative Oncology Group performance status 0–1. Patients were randomly allocated to two groups: flutamide and bicalutamide monotherapy group 1:1. PSA levels were significantly decreased in both groups at 4 weeks. PSA levels were significantly lower in the bicalutamide group compared with the flutamide group at 4 and 8 weeks. Testosterone levels in the bicalutamide group were significantly higher than the baseline levels between 4 and 24 weeks of treatment. Testosterone levels in the flutamide group were significantly increased at 4 and 12 weeks and returned to baseline levels at 16 and 24 weeks. DHEA levels in the bicalutamide group were unchanged from baseline at 4 and 24 weeks. However, DHEA levels in the flutamide group were decreased at 24 weeks. Androstenedione levels increased slightly in both groups, but the increase did not reach statistical significance. PSA, testosterone, and DHEA levels significantly differed between bicalutamide and flutamide monotherapy.
Keywords: Prostate Cancer, Testosterone Level, Flutamide, Bicalutamide, Abiraterone
Introduction
Prostate cancer is the second most frequently diagnosed cancer and the sixth common cause of death in men, worldwide. More than 250,000 men worldwide die annually because of prostate cancer [1]. The incidence of prostate cancer was the fourth highest [2] among cancers in Japan in 2006, and was the ninth commonest cause of death in Japan in 2013 [2].
Many treatment modalities are available for localized prostate cancer, including active surveillance, radical prostatectomy, radiation therapy, hormone therapy, and combination therapy [3–5]. Treatment options for patients older than 70 years with localized prostate cancer remain controversial. As many elderly men are ineligible or unsuitable candidates for definitive radiation therapy or radical prostatectomy or are unwilling to undergo observation protocols, hormone therapy is increasingly being considered. A number of reports have described the feasibility of antiandrogen monotherapy [6–9]. Monotherapy offers improved quality of life compared to castration in terms of sexual interest and physical capacity [10].
Bicalutamide and flutamide are generally used for prostate cancer as non-steroidal antiandrogen drugs. However, there have been no studies examining differences between the use of bicalutamide and flutamide as antiandrogen monotherapy [11, 12]. We therefore conducted a randomized control trial to compare serial changes in prostate-specific antigen (PSA), testosterone, dehydroepiandrosterone (DHEA), and androstenedione levels in patients treated with either of the antiandrogen agents, bicalutamide or flutamide.
Materials and Methods
The protocol of this clinical study was approved by the Institutional Review Board of Nara Medical University Hospital. Written informed consent was obtained from every enrolled patient prior to administration of antiandrogen therapy in accordance with good clinical practice [13].
Patient Selection
Patients were registered from January 2007 to December 2010. Patients were required to meet the following inclusion criteria: (1) presence of histopathologically confirmed prostate cancer, (2) prostate cancer treatment naive, (3) no current treatment with luteinizing hormone-releasing hormone (LH-RH) agonist for sexual interest and physical capacity, (4) clinical stage T1–cT3N0M0, (5) Gleason score ≤7, and (6) Cooperative Oncology Group performance status 0–1.
Patients were also required to meet the following criteria at entry: white blood cell count of 3300 to 8000/mm3, platelet count of ≥7.5 × 104/mm3, hemoglobin of ≥10 g/dl, normal levels of asparatate aminotransferase and alanine aminotransferase, serum creatinine of ≤1.5 mg/dL, and PSA of ≤50 ng/mL.
We suggested antiandrogen monotherapy in the present study to all patients meeting the criteria who were ineligible or unsuitable candidates for definitive radiation therapy or radical prostatectomy because of high age or are unwilling to undergo observation protocols.
Study Design
Following registration, the central registration center randomly allocated an equal number of subjects to two groups: the flutamide and bicalutamide groups. Subjects in the flutamide group received 375 mg flutamide daily, and those in the bicalutamide group received 80 mg bicalutamide daily, for 24 weeks. Serum PSA and testosterone levels were measured every 4 weeks. Serum DHEA and androstenedione levels were measured at 4 and 24 weeks following initiation of antiandrogen therapy. Baseline levels of each parameter were also measured before treatment initiation (Fig. 1).
Fig. 1.
Study design
Statistical Analyses
All statistical analyses were performed using SPSS for Windows (version 20.0; IBM, Armonk, NY, USA). The Mann–Whitney U test was used for the comparison of continuous variables. The chi-squared test was used for the comparison of categorical variables. Differences were considered statistically significant if P values were <0.05.
Results
Twenty-two patients who met the inclusion criteria and consented to this study were enrolled in the present study. Eleven patients were recruited into the flutamide group and 11 other into the bicalutamide group. All patients enrolled in this study completed the planned treatment. Patient characteristics are shown in Table 1. There were no significant differences in age, PSA levels, testosterone levels, DHEA levels, androstenedione levels, Gleason score (GS), or clinical T category between the two groups at baseline.
Table 1.
Characteristics of patients
| Flutamide group (n = 11) | Bicalutamide group (n = 11) | P | |
|---|---|---|---|
| Age (median, years) | 79 (71–86) | 79 (67–82) | 0.84† |
| PSA (median, ng/mL) | 12.4 (4.7–17.6) | 10.4 (3.8–38.6) | 0.69† |
| Testosterone (median, ng/mL) | 4.7 (2.0–9.4) | 4.5 (2.8–7.7) | 0.90† |
| DHEA (median, ng/mL) | 59 (18–167) | 73 (36–230) | 0.09† |
| Angiostenegione (median, ng/mL) | 1.2 (0.6–1.9) | 1.5 (0.9–3.0) | 0.11† |
| Gleason score | |||
| 6 | 2 | 3 | 0.91‡ |
| 7 | 9 | 8 | |
| 8–10 | 0 | 0 | |
| Clinical T stage | |||
| T1 | 5 | 6 | 0.62‡ |
| T2 | 5 | 5 | |
| T3 | 1 | 0 | |
| T4 | 0 | 0 | |
† Mann–Whitney U test; ‡ chi-square test
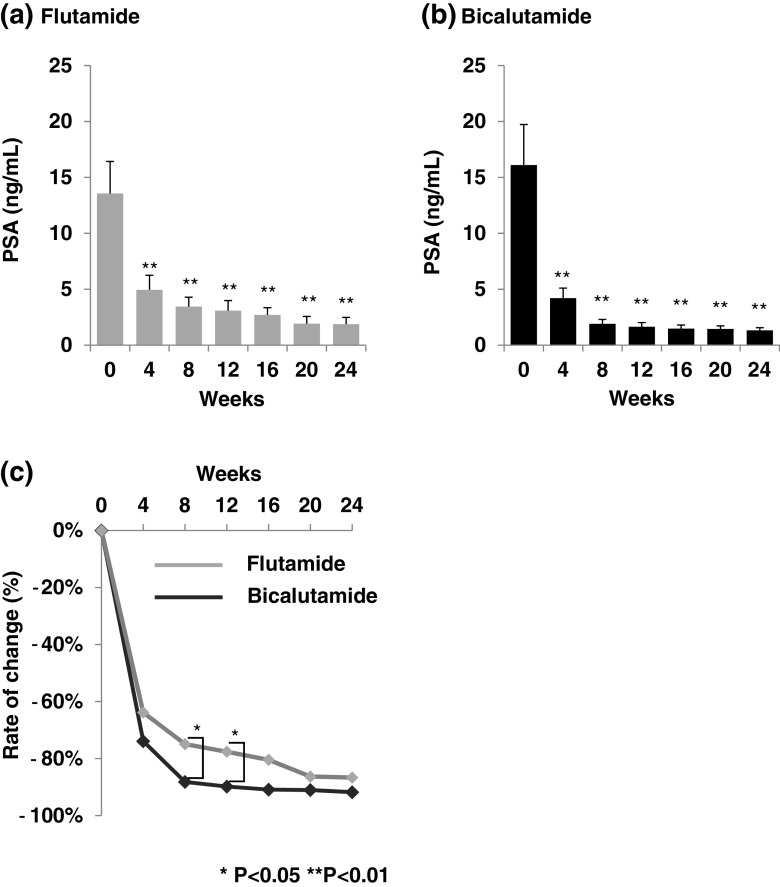
PSA levels in both the flutamide and bicalutamide groups were significantly decreased at 4 weeks. PSA levels were significantly lower in the bicalutamide group compared to the flutamide group at 8 and 12 weeks (Fig. 2). Testosterone levels in the flutamide group were significantly increased at 4, 8, and 12 weeks but returned to baseline levels at 16, 20, and 24 weeks. On the other hand, testosterone levels in the bicalutamide group were significantly increased at 4 weeks and remained at an increased level. Testosterone levels were significantly higher in the bicalutamide group compared to the flutamide group at 24 weeks (Fig. 3). DHEA levels in the flutamide group were significantly decreased at 24 weeks. However, DHEA levels in the bicalutamide group were not significantly changed from baseline at 4 and 24 weeks. DHEA levels were significantly lower in the flutamide group compared to the bicalutamide group at 24 weeks (Fig. 4). Androstenedione levels in the flutamide and bicalutamide groups increased slightly, but increases were not statistically significant. No significant difference in androstenedione levels was observed between the two groups (Fig. 5).
Fig. 2.
Mean serum PSA levels. a Serum PSA levels in the flutamide group. b Serum PSA levels in the bicalutamide group. c Difference in serum PSA levels from baseline at each time-point
Fig. 3.
Mean serum testosterone levels. a Serum testosterone levels in the flutamide group. b Serum testosterone levels in the bicalutamide group. c Difference in serum testosterone levels from baseline at each time-point
Fig. 4.
Mean serum DHEA levels. a Serum DHEA levels in the flutamide group. b Serum DHEA levels in the bicalutamide group. c Difference in serum DHEA levels from baseline at each time-point
Fig. 5.
Mean serum androstenedione levels. a Serum androstenedione levels in the flutamide group. b Serum androstenedione levels in the bicalutamide group. c Difference in serum androstenedione levels from baseline at each time-point
Discussion
PSA levels were significantly lower in the bicalutamide group compared to the flutamide group at 8 and 12 weeks in the present study. Non-steroidal antiandrogens exert their effects through competitive inhibition of testosterone binding. The difference between the two groups may therefore be due to the different affinities of flutamide and bicalutamide. Bicalutamide has a four times higher affinity than 2-hydroxyl-flutamide and the active metabolite of flutamide [14, 15]. This difference in affinity may have influenced PSA levels. Furthermore, new antiandrogen drug (enzalutamide) has higher affinity than bicalutamide [16]. Tombal et al. reported the good efficacy and safety of enzalutamide monotherapy [17]. So, enzaltamide may lower PSA significantly compared to bicalutamide and flutamide on the use of antiandrogen drugs as monotherapy.
Testosterone levels in the flutamide group returned to baseline levels; however, testosterone levels in the bicalutamide group remained increased after 4 weeks. This finding has been previously reported. Lund et al. reported that testosterone levels increased until 12 weeks following initiation of flutamide and returned to baseline levels after 12 weeks [18]. On the other hand, testosterone levels in patients treated with bicalutamide as monotherapy remained increased after 24 weeks [19]. Our results corroborate these previous reports.
In our study, DHEA levels in the flutamide group were significantly decreased at 24 weeks. On the other hand, no significant change in DHEA levels was observed in the bicalutamide group. These findings indicate flutamide decreases DHEA levels while bicalutamide does not. A number of reports have demonstrated suppression of DHEA is associated with suppression of prostate cancer. Narimoto et al. [20] reported that good responders treated with flutamide as a second line agent demonstrated a significant decrease in DHEA levels compared with poor responders. Ketoconazole and abiraterone, inhibitors of cytochrome P (CYP) 17, suppress castration-resistant prostate cancer [21, 22]. CYP17 is a key enzyme of androgen biosynthesis and involved in the production of DHEA from cholesterol. These results indicate suppression of DHEA is an important mechanism in the treatment of prostate cancer.
Androstenedione levels did not change in either the bicalutamide or the flutamide group in the present study. Balzano et al. reported no change in androstenedione levels with flutamide therapy, though the study population was very small [23]. Androstenedione levels have been shown to increase with bicalutamide therapy, though the study involved patients with benign prostatic hyperplasia [19]. Our results corroborate these previous studies.
Scher et al. demonstrated the efficacy of second-line hormonal therapy with flutamide for refractory prostate cancer previously treated with bicalutamide [24]. Switching of antiandrogen drugs in patients who relapse following initial hormonal therapy is now considered a second-line treatment. However, the efficacy of switching antiandrogen drugs as a second-line hormonal therapy remains to be demonstrated. Hara et al. demonstrated that androgen receptor mutations were important in antiandrogen withdrawal syndrome and that flutamide may be effective as a second-line therapy for refractory prostate cancer previously treated with bicalutamide [25]. Narimoto et al. demonstrated that flutamide suppresses DHEA and androstenedione levels and that androstenedione contributes to the progression of prostate cancer [20]. Suppression of androstenedione levels is thought to suppress castration-resistant prostate cancer [20–22]. Ketoconazole and abiraterone, inhibitors of cytochrome P (CYP) 17, can suppress prostate cancer resistant to bicalutamide or flutamide by suppressing androstenedione. In our study, DHEA levels decreased in the flutamide group though levels did not change in the bicalutamide group. Androstenedione levels did not change in either the flutamide or bicalutamide group. These results indicate DHEA may play a major role in suppressing prostate cancer in patients who relapse following initial hormonal therapy with bicalutamide and then treated with flutamide as the second hormonal therapy without inhibiting CYP17.
The small study population was a limitation of our study. We report preliminary results of flutamide and bicalutamide monotherapy for prostate cancer in this study. This study was the first randomized control trial comparing serial changes in PSA, testosterone, DHEA, and androstengion levels between patients receiving either of the antiandrogen monotherapy agents, bicalutamide, or flutamide.
Conclusion
The present study demonstrated differences in serial PSA and androgenic hormone levels between patients receiving the antiandrogen monotherapy agents, flutamide, and bicalutamide.
Acknowledgments
We are very grateful to Yoshihiko Hirao (Osaka Gyomeikan Hospital) for his valuable cooperation in our study.
Conflict of Interest
The authors have no conflicts of interest.
Footnotes
Highlight
We demonstrated the randomized study of antiandrogen monotherapy.
Patients were allocated to two groups: flutamide and bicalutamid group.
We compared changes in PSA, testosterone, DHEA, and androstenedione
PSA, testosterone, and DHEA levels significantly differed between both groups.
References
- 1.Jemal A. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Center for Cancer Control and Information Services, National Cancer Center, Japan 2013. http://ganjoho.jp/en/professional/statistics/table_download.html
- 3.Ischia J, Gleave M. Radical prostatectomy in high-risk prostate cancer. Int J Urol. 2013;20:290–300. doi: 10.1111/iju.12069. [DOI] [PubMed] [Google Scholar]
- 4.Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11:1066–1073. doi: 10.1016/S1470-2045(10)70223-0. [DOI] [PubMed] [Google Scholar]
- 5.Warde P, Mason M, Ding K, Kirkbride P, Brundage M, Cowan R, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378:2104–2111. doi: 10.1016/S0140-6736(11)61095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wirth MP, See WA, McLeod DG, Iversen P, Morris T, Carroll K, et al. Bicalutamide 150 mg in addition to standard care in patients with localized or locally advanced prostate cancer: results from the second analysis of the early prostate cancer program at median followup of 5.4 years. J Urol. 2004;172:1865–1870. doi: 10.1097/01.ju.0000140159.94703.80. [DOI] [PubMed] [Google Scholar]
- 7.Kang YJ, Kim KH, Lee KS. Efficacy of bicalutamide 150-mg monotherapy compared with combined androgen blockade in patients with locally advanced prostate cancer. Korean J Urol. 2014;55:315–320. doi: 10.4111/kju.2014.55.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banez LL, Blake GW, Mcleod DG, et al. Combined low-dose flutamide plus finasteride vs low-dose flutamide monotherapy for recurrent prostate cancer: a comparative analysis of two phase II trials with a long-term follow up. BJU Int. 2009;104:301–304. doi: 10.1111/j.1464-410X.2009.08400.x. [DOI] [PubMed] [Google Scholar]
- 9.Monk JP, Halabi S, Picus J, et al. Efficacy of peripheral androgen blockade in prostate cancer patients with biochemical failure after definitive local therapy. Cancer. 2012;118:4139–4147. doi: 10.1002/cncr.26732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iversen P, Tyrrell CJ, Kaisary AV, Anderson JB, Baert L, Tammela T, et al. Casodex (bicalutamide) 150 mg monotherapy compared with castration in patients with previously untreated nonmetastatic prostate cancer: results from two multicenter randomized trials at a median follow-up of 4 years. Urology. 1998;51:389–396. doi: 10.1016/S0090-4295(98)00004-1. [DOI] [PubMed] [Google Scholar]
- 11.Carlström K, Pousette A, Stege R. Flutamide has no effect on adrenal androgen response to acute ACTH stimulation in patients with prostatic cancer. Prostate. 1990;17:219–225. doi: 10.1002/pros.2990170305. [DOI] [PubMed] [Google Scholar]
- 12.Ayub M, Levell MJ. Suppression of plasma androgens by the antiandrogen flutamide in prostatic cancer patients treated with Zoladex, a GnRH analogue. Clin Endcrinol. 1990;32:329–339. doi: 10.1111/j.1365-2265.1990.tb00874.x. [DOI] [PubMed] [Google Scholar]
- 13.Ministerial Ordinance on Good Clinical Practice for Drugs. Ordinance of the Ministry of Health and Welfare No. 28 of March 27,1997. http://www.pmda.go.jp/english/service/pdf/ministerial/20110307No_28.pdf
- 14.Teutsch G, Goubet F, Battmann T, Bonfils A, Bouchoux F, Cerede E, et al. Non-steroidal antiandrogens: synthesis and biological prole of high-affinity ligands for the androgen receptor. J Steroid Biochem Mol Bio. 1994;48:111–119. doi: 10.1016/0960-0760(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 15.Furr BJ, Valcaccia B, Curry B, Woodburn JR, Chesterson G, Tucker H. ICI 176,334: a novel non-steroidal, peripherally selective antiandrogen. J Endocrinol. 1987;113:R7–R9. doi: 10.1677/joe.0.113R007. [DOI] [PubMed] [Google Scholar]
- 16.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;329:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tombal B, Borre M, Rathenborg P, et al. Enzalutamide monotherapy in hormone-naive prostate cancer: primary analysis of an open-label, single-arm, phase 2 study. Lancet Oncol. 2014;15:592–600. doi: 10.1016/S1470-2045(14)70129-9. [DOI] [PubMed] [Google Scholar]
- 18.Lund F, Rasmussen F. Flutamide versus stilboestrol in the management of advanced prostatic cancer. A controlled prospective study. Br J Urol. 1988;61:140–142. doi: 10.1111/j.1464-410X.1988.tb05062.x. [DOI] [PubMed] [Google Scholar]
- 19.Eri LM, Haug E, Tveter KJ. Effects on the endocrine system of long-term treatment with the non-steroidal antiandrogen Casodex in patients with benign prostatic hyperplasia. Br J Urol. 1995;75:335–340. doi: 10.1111/j.1464-410X.1995.tb07345.x. [DOI] [PubMed] [Google Scholar]
- 20.Narimoto K, Mizokami A, Izumi K, Mihara S, Sawada K, Sugata T, et al. Adrenal androgen levels as predictors of outcome in castration-resistant prostate cancer patients treated with combined androgen blockade using flutamide as a second-line antiandrogen. Int J Urol. 2010;17:337–345. doi: 10.1111/j.1442-2042.2010.02473.x. [DOI] [PubMed] [Google Scholar]
- 21.Ryan CJ, Halabi S, Ou SS, Vogelzang NJ, Kantoff P, Small EJ. Adrenal androgen levels as predictors of outcome in prostate cancer patients treated with ketoconazole plus antiandrogen withdrawal: results from a cancer and leukemia group B study. Clin Cancer Res. 2007;13:2030–2037. doi: 10.1158/1078-0432.CCR-06-2344. [DOI] [PubMed] [Google Scholar]
- 22.Attard G, Reid AH, A’Hern R, Parker C, Oommen NB, Folkerd E, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–3748. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balzano S, Cappa M, Migliari R, Scarpa RM, Danielli E, Campus G, et al. The effect of flutamide on basal and ACTH-stimulated plasma levels of adrenal androgens in patients with advanced prostate cancer. J Endocrinol Invest. 1988;11:693–696. doi: 10.1007/BF03350920. [DOI] [PubMed] [Google Scholar]
- 24.Scher HI, Liebertz C, Kelly WK, Mazumdar M, Brett C, Schwartz L, et al (1997) Bicalutamide for advanced prostate cancer: the natural versus treated history of disease. J Clin Oncol 15:2928–2938 [DOI] [PubMed]
- 25.Hara T, Miyazaki J, Araki H, Yamaoka M, Kanzaki N, Kusaka M, et al. Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Res. 2003;63:149–153. [PubMed] [Google Scholar]