Abstract
Two mutations (C228T and C250T) in the promoter region of the telomerase reverse transcriptase (TERT) have recently been described in different types of cancer including follicular cell-derived thyroid cancer (TC). In this paper, we reviewed the rates of these mutations in different types and subtypes of TC, their association with a number of clinical and histopathological features and outcome of TC, and their potential diagnostic and prognostic roles in TC. The overall rate of these mutations in TC is about 14 % with least prevalence in the well-differentiated subtypes of papillary thyroid cancer (10–13 %). Their rates increase significantly with increasing aggressiveness of TC reaching about 40 % in the undifferentiated and anaplastic thyroid cancers. There is also clear association with increasing age of patients at the time of diagnosis of TC. The evidence is compelling but with some conflicting results for associations between TERT promoter mutations and tumor size, extrathyroidal invasion, distant metastases, high tumor TNM stage, BRAF V600E mutation, recurrence, and mortality. A couple of studies reported a potential diagnostic role for TERT promoter mutations in thyroid nodules with indeterminate cytology of fine needle aspiration biopsy. These studies showed 100 % specificity but very low sensitivity of 7–10 %. The sensitivity increases significantly when TERT promoter mutation testing is combined with other gene mutations, particularly BRAF V600E and RAS mutations. Although TERT promoter mutations seem to play significant roles in the pathogenesis of TC, the mechanisms by which they contribute to carcinogenesis remain elusive and future work is needed to fully assess the roles, interactions, and impact of these mutations on the pathogenesis, diagnosis, prognosis, and therapeutics of TC.
Keywords: Thyroid Cancer, Thyroid Nodule, Papillary Thyroid Cancer, BRAF V600E Mutation, Anaplastic Thyroid Cancer
Introduction
Somatic cells are not immortal and normally undergo a limited number of divisions before they enter senescence [1]. This phenomenon was described by Leonard Hayflick in 1961 [2]. Hayflick observed that human fetal cells divide in culture about 40–60 times on average before they stop cell division and become senescent [2]. The reason for lack of cell immortality was not clear until the seminal work of Elizabeth Blackburn and Carolyn Greider in the late 1980 demonstrated that cells lose part of the telomeres with each division until they are unable to divide [3, 4]. Telomeres are nucleoprotein complexes that function as molecular cups to protect the ends of the chromosomes from excessive shortening with each cell division [5]. They are composed of several DNA repeats of the sequence TTAGGG [5]. With each cell division, telomeres become shorter and telomeric DNA is lost [6, 7]. When this shortening of telomere length reaches a critical point, cells cannot divide anymore and enter senescence [6, 7]. For cells to overcome the Hayflick limit and become immortal, they have to develop mechanisms that maintain the telomere length [8, 9]. This is usually achieved by activation of a telomerase enzyme, a ribonucleoprotein polymerase which adds hexatelomeric DNA fragments TTAGGG using a short segment of RNA as a template [10]. Although TERT is the most common mechanism by which telomers are maintained, alternative mechanisms independent of telomerase are sometimes utilized for telomeric lengthening, so called ALT [9, 11].
Cancer cells are characterized by rapid and durable ability to divide. This entails the need to maintain telomeric length. In more than 90 % of cases, this is achieved by reactivation of telomerase [10]. Telomerase is overexpressed in a significant majority of cancers [8]. Normal cells and benign adenomas rarely have an increased expression of telomerase, but self-renewing cells such as stem and fetal cells frequently have increased amounts of telomerase [5, 12]. Telomerase itself is a nucleoprotein complex with a number of proteins and RNA [13, 14]. The most important components are the telomerase reverse transcriptase subunit (TERT), the telomerase RNA component (TERC), and dyskerin (DKC1 gene) [15–17]. The TERC serves as a template against which the TERT synthesizes the telomeric fragments TTAGGG. TERT gene is a 35 kb gene located on chromosome 5. It contains 16 exons and a promotor region of 330 base pairs [18]. Mutations in the coding regions of the gene are rare [19]. Uniquely, mutations in the promotor region have recently been described in a large number of cancers [1, 20]. These mutations cluster mainly in two hotspots, 1 295 228 C > T and 1 295 250 (commonly called C228T and C250T, respectively). These are transition mutations corresponding to nucleotides −124 and −146 upstream of the initiation codon ATG, respectively [1, 20]. Since its original description in thyroid cancer (TC) in 2013 [21], a significant interest in C228T and C250T mutations has accumulated and several publications have been generated over the last 2.5 years. Therefore, a review of this subject is timely in order to examine and summarize these data on TERT promoter mutations in TC. This is the aim of this review.
Methods
We searched the PubMed and Scopus up to 1 October 2015 using search terms “TERT,” “TERT promoter mutations,” “Telomerase,” “Telomers,” “Thyroid,” “Thyroid Neoplasms,” “Thyroid Cancer,” “Thyroid Tumor.” We included only studies in which TERT promoter mutations were screened in a non-selected sample of thyroid tumors and the objectives of the study were to find out the prevalence of these mutations in TC and /or their association with histopathological features and outcome of TC. We identified 15 studies that met these selection criteria. We expressed categorical values in rates and ratios as appropriate and the continuous variables as median and range or mean ± SD. A meta-analysis was performed for studies with available data on PTC, the most common type of TC, using comprehensive meta-analysis version 3 software to test for associations between different clinicopathological features and TERT promoter mutations. Test for heterogeneity was performed using the I2 metric with value <25 % indicating minimal heterogeneity, 25–75 % indicating intermediate, and >75 % indicating significant heterogeneity. Fixed-effect model was used when I2 was low (<25 %) and P value was >0.05 and random-effect model was used when I2 was >25 % and P value <0.05.
TERT Promoter Mutations in Cancer
A significant number of human cancers have a high expression of TERT [8]. In 2013, two reports described high rates of TERT promotor mutations in familial and sporadic cases of melanomas [22, 23]. Horn and colleagues identified a germline TERT promotor mutation in a melanoma prone family and somatic TERT promoter mutations in a significant number of sporadic melanomas. These mutations occurred more frequently in the metastatic lesions than the primary melanomas [22]. Huang and colleagues reported TERT promotor mutations in 71 % of melanomas and in 16 % of 150 cancer cell lines derived from diverse tumor types with high frequencies in gliomas, bladder cancer, hepatocellular carcinomas, and thyroid cancer [23]. These mutations generate consensus binding domains for the E-Twenty six (ETS) transcription factors leading to 2 to 4-fold increase in the transcriptional activity [22, 23]. Since its original description in these two reports, a large number of studies reported variable rates of TERT promotor mutations in different types of cancers [1, 20]. The following sections describe in details their frequency and prognostic and diagnostic roles in TC.
TERT Promoter Mutations in Thyroid Cancer
Normal thyroid cells are low proliferating cells with almost absent TERT activity in the resting state [24, 25]. In addition, TC generally has less TERT activity than many other types of cancer [24]. Within TC itself, there is variable expression of TERT among the different subtypes of TC [24]. Anaplastic and poorly differentiated TCs have higher activities than the more differentiated types of TC [24, 26]. Not only that the level of TERT activity varies but the proportion of TCs with increased TERT activity is least common in the well-differentiated papillary type and most frequent in anaplastic thyroid cancer [26]. Benign adenomas and hyperplastic nodules have none or minimal TERT activity. With discovery of TERT promoter mutations in some types of cancers, it was also investigated in TC and found to be common, especially in the less differentiated subtypes [21, 27]. Subsequent studies on TERT promoter mutations in TC focused on two aspects, the frequency of these mutations in different subtypes of TC and their association with histopathological features and outcome of TC. We will discuss these two aspects in details in the following sections.
Frequency of TERT Promoter Mutations in Benign and Malignant Thyroid Tumors
We have identified 15 studies that reported the frequency of TERT promoter mutations in thyroid tumors [20, 21, 27–39]. In all except one of these studies [38], polymerase chain reaction and direct sequencing of the PCR products were used to identify TERT promoter mutations. De Biase et al. used next generation sequencing to identify TERT promoter mutations in thyroid microcarcinomas [38]. These studies included in total 4165 samples of benign (552 samples) and malignant (3613 samples) thyroid tumors of different subtypes (Table 1). C228T mutation was found in 424 (10.2 %) of them. C250T mutation was investigated in 12 studies (Table 1) which included 3549 patients and was found positive in 78 (2.2 %) of them [20, 21, 27–30, 32, 34–39]. In all the studies that included C250T mutation screening, C228T was also studied and the two mutations were always found mutually exclusive. Collectively, C228T or C250T mutations were reported in 502 cases of a total of 4165 patients in whom either the two mutations were studied (4165 cases) or C228T mutation alone was studied (3549 cases) (Table 1). The details of those studies are summarized in the following sections.
Table 1.
Studies reporting rates of TERT promoter mutations in benign and malignant thyroid tumors
| No. | Study | Thyroid cancer | Benigna | Totala | ||
|---|---|---|---|---|---|---|
| C228Ta | C250Ta | Botha | ||||
| 1 | Vinagre et al. [20] | 22/291 (7.6) | 5/291 (1.7) | 27/291 (9.3) | 0/81 | 27/372 (7.3) |
| 2 | Liu et al. [21] | 65/414 (15.7) | 4/414 (0.97) | 69/414 (16.7) | 0/85 | 69/499 (13.8) |
| 3 | Landa et al. [27] | 41/183 (22.4) | 21/183 (11.5) | 62/183 (33.8) | 62/183 (33.9) | |
| 4 | Liu and Xing [32] | 7/129 (5.4) | 2/129 (1.6) | 9/129 (7.0) | 0/179 | 9/308 (2.9) |
| 5 | Xing et al. [33] | 61/507 (12.0) | 61/507 (12.0) | 61/507 (12.0) | ||
| 6 | Wang et al. [34] | 8/52 (15.4) | 1/52 (1.92) | 9/52 (17.3) | 4/76 (5.3) | 13/128 (10.2) |
| 7 | Liu et al. [35] | 46/430 (10.7) | 8/430 (1.9) | 54/430 (12.6) | 0/44 | 54/474 (11.4) |
| 8 | Melo et al. [36] | 48/469 (10.2) | 10/469 (2.13) | 58/469 (12.4) | 0/81 | 58/550 (10.5) |
| 9 | Liu et al. [37] | 27/144 (18.8) | 4/144 (2.8) | 31/144 (21.5) | 31/144 (21.5) | |
| 10 | Shi et al. [28] | 37/106 (34.9) | 4/106 (3.8) | 41/106 (38.7) | 41/106 (38.7) | |
| 11 | Gandolfi et al. [29] | 12/121 (9.9) | 5/121 (4.1) | 17/121 (14.1) | 17/121 (14.1) | |
| 12 | Muzza et al. [30] | 24/254 (9.4) | 6/254 (2.4) | 30/254 (11.8) | 0/6 | 30/260 (11.5) |
| 13 | Chindris et al. [31] | 8/61 (13.1) | 8/61 (13.1) | 8/61 (13.1) | ||
| 14 | Crescenzi et al. [39] | 3/48 (6.3) | 3/48 (6.3) | 3/48 (6.3) | ||
| 15 | De Biase et al. [38] | 11/404 (2.7) | 8/404 (2.0) | 19/404 (4.7) | 19/404 (4.7) | |
| 16 | Total | 420/3613 (11.6) | 78/2997 (2.6) | 498/3613 (13.8) | 4/552 (0.72) | 502/4165 (12.1) |
aNumbers represent numbers of cases with mutations/total number of cases tested (%)
TERT Promoter Mutations in Benign Thyroid Tumors
In seven studies, a number of benign thyroid adenomas were included [20, 21, 30, 32, 34–36]. The total number of adenomas studied in these seven series was 552 cases (Table 1). Only one study reported positive C228T mutation in four out of 58 adenomas included in that study, three of them were atypical adenomas [34]. The single case with typical thyroid adenoma developed a scar carcinoma and died later on of follicular thyroid cancer (FTC) [34]. With the exception of these four cases of doubtful benign nature, none of the other studies (a total of 548 cases) reported TERT promoter mutations in benign thyroid tumors (Table 1). This unexpected finding of TERT promotor mutations in four benign nodules in a single study could be inaccurate either due to technical errors that are not uncommon in laboratory work such as PCR contamination or mixing of samples or in the pathological designation of the nodules. The latter is supported by the fact that three of those nodules were atypical and the fourth nodule developed scar carcinoma on follow-up. In our view, unless future studies show TERT promoter mutations in clearly benign thyroid nodules, these mutations should be considered cancer-specific for the time being.
TERT Promoter Mutations in Thyroid Cancer
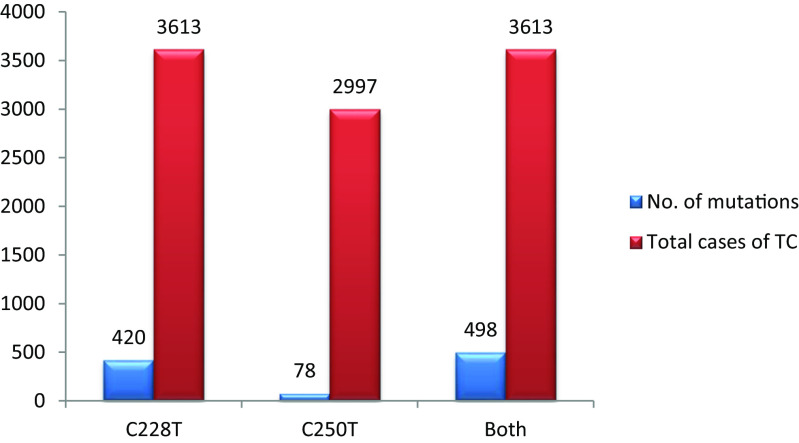
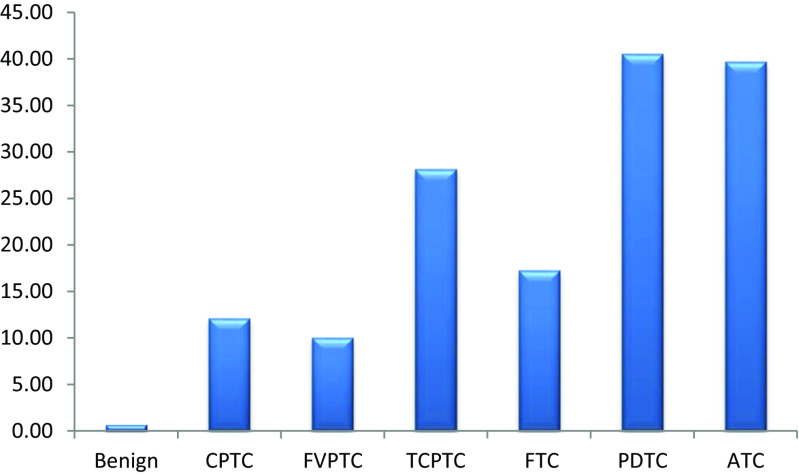
The rate of TERT promoter mutations was reported in 15 studies that included 3613 cases of thyroid cancer of different subtypes (Table 1). C228T was positive in 420 (11.6 %) of these cases. C250T was reported in 12 of these studies which included 2997 cases of thyroid cancer. Of these cases, 78 (2.6 %) harbored C250T mutations. The two mutations were mutually exclusive. Overall, C228T or C250T mutations were positive in 498 cases out of a total of 3613 (13.8 %) thyroid cancers of different subtypes (Fig. 1). The frequencies of TERT promoter mutations increase as the tumors become less differentiated with much higher rates in the undifferentiated and anaplastic subtypes compared to the well-differentiated subtypes (Fig. 2).
Fig. 1.
Total numbers of TERT promoter mutations reported in 15 studies that included 3613 thyroid cancer patients
Fig. 2.
Percentages of patients with TERT promoter mutations in different subtypes of TC. CPTC classical papillary thyroid cancer, FVPTC follicular variant papillary thyroid cancer, TCPTC tall cell variant papillary thyroid cancer, FTC follicular thyroid cancer, PDTC poorly differentiated thyroid cancer, ATC anaplastic thyroid cancer
TERT Promoter Mutations in Papillary Thyroid Cancer
TERT promoter mutations were reported in 11 studies that included a total of 2622 PTCs of different subtypes [20, 21, 27, 29, 30, 32, 33, 35–38]. Of these cases, 273 (10.4 %) cases harbored TERT promoter mutations (Table 2). Although some studies did not report on the different PTC subtypes, the majority of cases included in these studies were of the classical subtype (CPTC) where a total of 725 CPTCs were tested for TERT promoter mutations and 88 (12.14 %) of them were found to harbor TERT promoter mutations [29, 30, 32, 33]. The next most common subtype of PTC is the follicular variant PTC (FVPTC). TERT promoter mutations in FVPTC were reported in four studies that included 219 FVPTCs of which 22 cases (10.0 %) were positive for these mutations [29, 30, 32, 33] (Table 2). Tall cell variant PTC (TC-PTC) is relatively rare. Only two studies that included 32 cases of TC-PTC reported a relatively high frequency of TERT promoter mutation with nine (28 %) of these 32 cases harboring TERT promoter mutations [32, 33].
Table 2.
Studies reporting rates of TERT promoter mutations in different subtypes of thyroid cancer
| No. | Study | All PTCsa | All FTCsa | CPTCa | FVPTCa | TCPTCa | PDTCa | ATCa |
|---|---|---|---|---|---|---|---|---|
| 1 | Vinagre et al. [20] | 13/169 (7.7) | 9/64 (14.1) | 3/14 (21.4) | 2/16 (12.6) | |||
| 2 | Liu et al. [21] | 30/257 (11.7) | 11/79 (13.9) | 23/178 (12.9) | 2/56 (3.6) | 4/13 (30.8) | 3/8 (37.5) | 25/54 (46.3) |
| 3 | Landa et al. [27] | 18/80 (22.5) | 30/58 (51.7) | 10/20 (50.0) | ||||
| 4 | Liu & Xing [32] | 5/111 (4.5) | 4/18 (22.2) | |||||
| 5 | Xing et al. [33] | 61/507 (12.1) | 47/383 (12.3) | 8/103 (7.8) | 5/19 (26.3) | |||
| 6 | Wang et al. [34] | 9/52 (17.3) | ||||||
| 7 | Liu et al. [35] | 46/408 (11.3) | 8/22 (36.4) | |||||
| 8 | Melo et al. [36] | 25/332 (7.5) | 12/70 (17.1) | 9/31 (29.0) | 12/36 (33.3) | |||
| 9 | Liu et al. [37] | 13/51 (25.5) | 8/36 (22.2) | 10/20 (50.0) | ||||
| 10 | Shi et al. [28] | 41/106 (38.7) | ||||||
| 11 | Gandolfi et al. [29] | 21/121 (17.4) | 6/21 (28.6) | 2/21 (9.5) | ||||
| 12 | Muzza et al. [30] | 22/182 (12.1) | 8/58 (13.8) | 12/143 (8.4) | 10/39 (25.6) | |||
| 13 | De Biase et al. [38] | 19/404 (4.7) | ||||||
| Total | 273/2622 (10.4) | 69/399 (17.3) | 88/725 (12.1) | 22/219 (10.0) | 9/32 (28.1) | 45/111 (40.5) | 100/252 (39.7) |
PTC papillary thyroid cancer, FTC follicular thyroid cancer, CPTC conventional PTC, FVPTC follicular variant PTC, TCPTC tall cell PTC, PDTC poorly differentiated thyroid cancer, ATC anaplastic thyroid cancer
aNumbers represent numbers of cases with mutations/total number of cases tested (%)
TERT Promoter Mutations in Poorly Differentiated and Anaplastic Thyroid Cancers
Four studies included 111 cases of PDTC for TERT promoter mutation testing [20, 27, 32, 36]. Of these cases, 45 (40.5 %) tumors harbored these mutations (Table 2 and Fig. 2). TERT promoter mutations were screened in six studies including 252 cases of ATC (Table 2 and Fig. 2). One hundred (39.7 %) of these tumors harbored TERT promoter mutations [20, 27, 28, 32, 36, 37] (Table 2 and Fig. 2).
TERT Promoter Mutations in Follicular Thyroid Cancer
Eight studies included 399 cases of FTC and reported TERT promoter mutations in 69 (17.3 %) of them [20, 21, 30, 32, 34–37] (Table 2). Hurthle cell thyroid cancer (HCC), which is considered a more aggressive variant of FTC, was screened for TERT promoter mutations in two studies that included 82 cases with 12 (14.6 %) of them harboring TERT promoter mutations [27, 31]. In one study, eight of 61 (13.1 %) HCC were found to carry TERT promoter mutation [31]. In the other study, four of 21 (19 %) HCC were positive for TERT promoter mutations [27].
TERT Promoter Mutations in Other Types of Thyroid Cancer
Medullary thyroid cancer (MTC) was included in three studies [32, 36, 37]. A total of 79 cases were studied and none of them harbored TERT promoter mutations. Columnar cell variant PTC is very rare and only three tumors were included in two studies with a single tumor harboring C228T mutation [32, 33].
Association of TERT Promoter Mutations with Histopathological Features and Outcome of Thyroid Cancer
Several studies reported an association between TERT promoter mutations and a number of demographic and histopathological features and outcome of thyroid cancer. Table 3 summarizes the overall associations between these factors and TERT promoter mutations as reported in previous studies. In the following sections, we will review the overall results for each factor in PTC and FTC and will describe the findings in details for those studies that reported an association between these factors and TERT promoter mutations.
Table 3.
Summary of studies that reported associations between TERT promoter mutations and clinical and histopathological features, BRAF V600E mutation, and outcome of different types of thyroid cancer
| Study | Total no. of tumors | Age | Size | ETE | Vascular invasion | LNM | Distant mets | TNM stage | Recurrence | BRAF | Death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shi et al. [28] | 106 ATC | Yes | No | No | No | Yes | Yes | ||||
| Gandolfi et al. [29] | 121 | Yes | No | No | Yes | Yes | Yes | No | |||
| Muzza et al. [30] | 240 | Yes | No | No | No | No | Yes | No | |||
| Liu & Xing [32] | 129 | Yes | Yes | Yes | Yes | ||||||
| Xing et al. [33] | 507 | Yes | No | No | Yes | Yes | No | No | No | Yes | |
| Wang et al. [34] | 52 FTC | Yes | No | No | Yes | ||||||
| Liu et al. [35] | 430 Chinese | Yes | Yes | Yes | No | Yes | Yes | ||||
| Melo et al. [36] | 469 | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes |
| Liu et al. [37] | 144 | Yes | No | Yes | Yes | ||||||
| Vinagre et al. [20] | 263 | Yes | Yes | Yes | |||||||
| Liu et al. [21] | 414 | Yes |
Age
Consistent data from different studies showed an association between TERT promoter mutations and old age at the time of diagnosis of TC [20, 28–30, 33–37] (Table 3). As can be seen in Table 4, five large studies showed a significant association between TERT promoter mutations and age in PTC [30, 33, 35–37]. In all of these studies, the mean or median age was significantly higher than 50 years in the TERT promoter mutation positive subgroups compared with ≤45 years in those with wild-type TERT (Table 4). However, in a study that included 404 thyroid microcarcinomas, De biase et al. did not find a significant difference in age between TERT promoter mutations and wild-type TERT [38] (Table 4). However, this study included only microcarcinomas which might be more prevalent in young age group and the rate of TERT mutations in this study was quite low (4.7 %). In FTC, similar associations between TERT promoter mutations and age at diagnosis were found in four studies [30, 34, 36, 37] (Table 5). It is of note that the age of patients in FTC was more than 50 years in both TERT promoter mutation positive and wild-type TERT. This is consistent with the generally older age group of patients in FTC compared with PTC. A meta-analysis that included four studies which reported details of the age of patients at the diagnosis and TERT promoter mutations in PTC showed a significant association between age and TERT promoter mutations (Fig. 3).
Table 4.
Summary of five large studies that reported associations between TERT promoter mutations and clinicopathological features of PTC
| Muzza et al. 2015 [30] | Xing M et al. 2014 [33] | Liu X et al. 2014 [35] | Melo M et al. 2014 [36] | Liu T et al. 2014 [37] | De Biase et al. 2015 [38] | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M (22) | W (160) | P value | M (61) | W (446) | P value | M (42) | W (325) | P value | M (25) | W (307) | P value | M (18) | W (38) | P value | M (19) | W (385) | P value | |
| Age | 57.6 (29–82) | 44.2 (14–80) | 0.004 | 51.7 ± 15.7 | 45.1 ± 13.6 | <0.001 | 53.40 ± 16.14 | 43.66 ± 12.91 | 0.00 | 58.4 ± 13.2 | 43.6 ± 15.3 | <0.001 | 68 (49–84) | 36 (22–97) | <0.0001 | 50.2 ± 11.5 | 47.8 ± 12.8 | 0.39 |
| Size | 2.2 | 1.93 | 0.34 | 2.3 (1.2–3.5) | 1.8 (1.1–3) | 0.048 | 3.14 ± 1.62 | 2.48 ± 1.58 | 0.03 | 3.2 ± 2.2 | 2.3 ± 1.4 | 0.005 | 5.9 ± 2.9 | 6.2 ± 2.5 | 0.68 | |||
| ETE | 11/22 (50) | 85/160 (53.1) | 0.9 | 27/58 (46.5) | 66/439 (15) | <0.001 | 9/32 (28.1) | 17/207 (8.2) | 0.00 | 14/18 (77.8) | 162/250 (64.8)) | 0.26 | ||||||
| Vascular invasion | 14/54 (25.9) | 63/437 (14.1) | 0.028 | 5/16 (31.3) | 98/241 (40.7) | 0.46 | ||||||||||||
| LNM | 11/22 (50) | 83/160 (51.8) | 0.9 | 31/59 (50.8) | 122/438 (27.8) | <0.001 | 7/26 (26.9) | 68/213 (31.9) | 0.60 | 18/23 (6) | 184/275 (66.9) | 0.26 | 4/12 (33.3) | 53/254 (20.8) | 0.29 | |||
| Distant Mets | 12/61 (19.7) | 10/446 (2.2) | <0.001 | 5/18 (27.8) | 31/275 (14.7) | 0.07 | 7/13 (53.8) | 3/38 (7.9) | 0.001 | |||||||||
| Higher TNM III/IV | 10/22 (45.5) | 49/160 (30.6) | 0.09 | 29/61 (47.5) | 77/446 (17.5) | <0.001 | 11/26 (42.31) | 52/213 (24.41) | 0.05 | 10/14 (71.4) | 66/211 (31.3) | 0.02 | 4/15 (26.7) | 61/332 (18.4) | 0.62 | |||
| Recurrence | 10/22 (45) | 29/160 (18) | 0.002 | 29/61 (47.5) | 51/466 (11.4) | <0.001 | 10/16 (62.5) | 48/195 (24.6) | 0.001 | 1/12 (8.3) | 6/276 (2.2) | 0.20 | ||||||
| BRAF V600E | 10/64 (15.6) | 54/160 (33.8) | 0.34 | 35/61 (57.8) | 159/446 (35.750 | 0.007 | 40/46 (86.9) | 210/362 (58) | <0.001 | 18/24 (75) | 130/277 (46.9) | 0.008 | ||||||
| Death | 2/19 (10.5) | 3/256 (1.1) | 0.001 | 11/13 (84.6) | 8/38 (21.1) | <0.0001 | ||||||||||||
ETE extrathyroidal extension/invasion, LNM lymph node metastases, Mets metastases, TNM tumor node metastasis staging system
Table 5.
Summary of a number of large studies that reported associations between TERT promoter mutations and clinicopathological features of FTC or ATC
| FTC | ATC | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muzza et al. 2015 [30] | Melo M et al. 2014 [36] | Wang N et al. 2014 [34] | Liu T et al. 2014 [37] | Shi X et al. 2015 [28] | |||||||||||
| Mutant (8) | Wild (50) | P value | Mutant (12) | Wild (58) | P value | Mutant (9) | Wild (42) | P value | Mutant (8) | Wild (28) | P value | Mutant (18) | Wild (28) | P value | |
| Age | 66 (48–81) | 56 (18–85) | 0.04 | 63.8 ± 11.0 | 49.3 ± 16.3 | 0.004 | 66 ± 16 | 54 ± 19 | 0.052 | 69 (31–83) | 54 (17–77) | 0.05 | 68.7 ± 12.5 | 60.4 ± 11.2 | 0.023 |
| Size | 5.4 | 4.09 | 0.1 | 4.4 ± 2.5 | 4.4 ± 2.6 | 0.96 | 5.0 (3.5–6.7) | 6.0 (3.5–7.5) | 0.46 | ||||||
| ETE | 5/8 (62.5 %) | 24/50 (48 %) | 0.7 | 3/8 (37.5 %) | 5/41 (12.2 %) | 0.08 | 18/18 (100 %) | 22/24 (91.6 %) | 0.49 | ||||||
| Vascular invasion | 8/10 (80 %) | 31/43 (72.1 %) | 0.61 | ||||||||||||
| LNM | 7/8 (87.5 %) | 46/50 (92 %) | 0.7 | 3/12 (25 %) | 5/35 (14.3 %) | 0.39 | 11/17 (64.7 %) | 13/21 (61.9 %) | 0.86 | ||||||
| Distant mets | 5/5 (100 %) | 3/26 (11.5 %) | 0.001 | 15/18 (83.3 %) | 8/26 (30.8 %) | 0.001 | |||||||||
| TNM III and IV | 6/8 (75 %) | 23/50 (46 %) | 0.02 | 5/6 (83.3 %) | 8/23 (34.8 %) | 0.007 | |||||||||
| Recurrence | 5/8 (62.5 %) | 11/50 (22 %) | 0.03 | ||||||||||||
| Death | 2/12 (16.7 %) | 0/58 (0) | <0.001 | ||||||||||||
ETE extrathyroidal extension/invasion, LNM lymph node metastases, Mets metastases, TNM tumor node metastasis staging system
Fig. 3.
A meta-analysis for an association between TERT promoter mutations and different clinical, histopathological, and outcome of papillary thyroid cancer showing a significant association with age, tumor size, extrathyroidal tumor extension/invasion, distant metastasis, high tumor stage (III/IV), BRAF V600E mutation, persistent/recurrent disease, and cancer-specific mortality. The analysis showed no significant associations between TERT promoter mutations and vascular invasion and lymph node metastasis. The test of heterogeneity is at the left lower corner of each graph. Fixed-effect model was used when I2 was <25 % and P value >0.05. In other situations, a random-effect model was used for the meta-analysis
Tumor Size
The association between tumor size and TERT promoter mutations is not as consistent as age at diagnosis. It was reported in seven studies [20, 28, 30, 33, 35–37] (Table 3). In four of these studies, no significant association was detected [28, 30, 33, 37]. In the other three studies, a significant association was reported [20, 35, 36] (Tables 4 and 5).
In PTC, three studies reported a significant association [33, 35, 36] and two studies showed no significant association [30, 38] between TERT promoter mutations and tumor size (Table 4). A meta-analysis of two studies that included a large number of PTC [36, 37] showed a significant association between tumor size and TERT promoter mutations (Fig. 3). In FTC, no association between TERT promoter mutation and tumor size was found in two studies in which it was reported [30, 36] (Table 5). Similarly, a study that included 106 ATC patients did not find an association between tumor size and TERT promoter mutations [28] (Table 5).
Extrathyroidal Tumor Invasion
The relationship between TERT promoter mutations and extra thyroidal tumor extension/invasion was reported in seven studies [21, 28, 30, 33–36] (Table 3). Only two of them showed a significant association between these two features of TC [32, 35]. In PTC, the overall effect of TERT promoter mutations was in favor of a strong association of these mutations with extrathyroidal extension with odd ratios of 3.02 (95 % CI, 2.02–4.5, P < 0.001) (Fig. 3). In FTC, two studies showed no association between TERT promoter mutation and extrathyroidal extension [30, 36] (Table 5).
Vascular Invasion
The relationship of vascular invasion and TERT promoter mutations was reported in three studies [29, 33, 36]. Only one of these studies showed an association between TERT promoter mutations and vascular invasion in PTC [33] (Table 4). Xing et al. reported 25.9 % occurrence of vascular invasion in 54 cases of PTC with TERT promoter mutation vs. only 14.4 % of 437 cases of PTC with wild-type TERT (P 0.028). This relationship was borderline in CPTC (P 0.052) but was stronger in cases with combined TERT and BRAF mutations (P = 0.022) [33]. Two other studies and a meta-analysis (Fig. 3) showed no association between TERT promoter mutations and vascular invasion, one included patients with PTC and the other included FTC patients [29, 36] (Table 5).
Lymph Node Metastasis
The association between TERT promoter mutation and lymph node metastasis was reported in eight studies [20, 21, 28–30, 33, 35, 36] (Table 3). Only three of these studies showed significant associations [20, 21, 33]. Five studies included patients with PTC [30, 33, 35, 36, 38], and only one of them showed an association between TERT promoter mutations and lymph node metastasis [33] (Table 4). There was no significant association between TERT promoter mutations and lymph node metastasis in two studies [30, 36] that included patients with FTC and one study that included ATC [28] (Table 5).
Liu et al. studied 129 cases of PTC and found a strong association between TERT promoter mutations and a number of histopathological features including lymph node metastasis [21]. Xing et al. reported lymph node metastasis in 52.5 % of TERT mutation positive compared with 27.8 % of cases of DTC with wild-type TERT (P < 0.001) [33] (Table 4). In CPTC, the rates were even higher with 60 % in TERT promoter mutation positive vs. 32.5 % in TERT promoter mutation negative groups (<0.001) [33]. Vinagre et al. found a strong association between TERT promoter mutations and lymph node metastasis in the CPTC subtype (P 0.03) [20]. A meta-analysis of five studies that included a large number of PTC showed non-significant association between TERT promoter mutations and lymph node metastases (Fig. 3).
Distant Metastasis
TERT promoter mutations were found to be associated with distant metastasis of TC in five [21, 28, 29, 36, 37] and not associated in two studies [33, 34] (Table 3).
In PTC, two studies showed an association [33, 37] and one study did not show such an association between TERT promoter mutations and distant metastasis [36] (Table 4). In a meta-analysis that included PTC patients in three studies, there was a significant association between TERT promoter mutations and distant metastases (odds ratio 7.2, 95 % CI, 2.9–18.0, Fig. 3).
In TERT positive FTC [36] and ATC [28], each showed an association with distant metastasis in one study (Table 5). Shi et al. reported that TERT promoter mutation positive ATCs were more likely to have distant metastasis (83.3 %) compared with wild-type TERT (83.3 vs. 30.8 %, P 0.001) [28] (Table 5).
TNM Staging
Association between advanced TNM stage and TERT promoter mutations was reported in five studies [29, 30, 33, 35, 36] (Table 3). In PTC, three of these studies showed significant associations [29, 35, 36] and two did not show such an association [30, 33] (Table 4). A meta-analysis also showed a significant association between TERT promoter mutations and high stage (stages III and IV) in PTC with odds ratio of 2.96 (95 % CI, 1.9–4.6, Fig. 3). In FTC, two of these studies showed an association [30, 36] (Table 5). In a total of 121 cases of PTC, Gandolfi et al. reported a higher percentage of advanced TNM stage (III and IV) in patients with TERT promoter mutations than in patients with wild-type TERT [29]. This relationship was not evident when the analysis was limited to patients with or without distant metastasis [29]. Melo et al. showed a significantly high rate of advanced TNM stage in 402 cases of TC (Table 4). This association remains significant when the analysis was performed in PTC only, FTC only but not in UTC subgroups [36] (Tables 4 and 5). Liu et al. also showed that the rate of advanced stages of PTC was 42.3 % in the TERT promoter mutation group and 24.4 % in the wild-type TERT promoter mutation group (P 0.05) [35].
BRAFV600E Mutation
BRAF V600E mutation is the most common mutation in PTC [40]. It has been associated with aggressive histopathological features of TC, recurrence, and mortality [41–43]. In six studies, association and interaction of BRAF V600E mutation with TERT promoter mutations were reported [21, 28–30, 36, 40] (Table 3). In four of these studies, a higher rate of TERT mutations was seen in cases with BRAF V600E mutation than in tumors with wild-type BRAF [21, 28, 36, 40] (Table 4). In a large study, Xing et al. showed that TERT promoter and BRAF V600E mutations not only frequently coexist but also cooperate to confer a higher risk of recurrence of TC and when occurring together are frequently associated with aggressive histopathological features [33]. In that study, 507 PTC of different subtypes were tested for BRAF V600E and TERT promoter mutations. BRAF V600E mutation was significantly more prevalent in TERT promoter mutation cases than in the cases with wild-type TERT [40] (Table 4). Conversely, TERT promoter mutation C228T was more significantly present in cases with BRAF V600E mutation than in the wild-type BRAF. Both mutations occurred in 35 of 507 PTC (6.9 %) and in 28 of 383 CPTC (7.3 %). Patients harboring both mutations had a much higher recurrence rate than patients without either mutation. Double mutation was also associated with high-risk histopathological features [33]. Melo et al. studied 402 DTC cases of which 332 cases were PTC, 70 FTC, and 67 UTC [36]. In the whole group, no significant association between BRAF V600E and TERT promoter mutations was found (Table 4). Shi et al. studied 106 ATC and reported that TERT promoter mutations occurred in 9/16 (56.3 %) BRAF V600E positive ATC compared with 28/90 (31.1 %) in ATC with wild-type BRAF [28] (Table 5). Similarly, BRAF V600E mutation occurred in 9/37 (24.3 %) TERT promoter mutation positive group compared with 7/69 cases (10 %) in the TERT mutation negative subgroup (P 0.05) [28]. Interestingly, Gandlofi et al. compared PTC cases with distant metastasis and a control group of PTC without metastasis. They found no association between BRAF V600E mutation and TERT mutations except in the subgroup of patients with distant metastasis [29]. Muzza et al. also found that the prevalence of TERT mutations was not different between BRAF V600E and wild-type BRAF but TERT promoter mutations were more strongly associated with the outcome than BRAF V600E mutation and both mutations did not seem to be stronger than TERT promoter mutations alone [30]. A meta-analysis also showed a significant association between TERT promoter mutations and BRAF V600E mutation with odd ratio of 1.8 (95 % CI, 1.27–2.64, Fig. 3).
Diagnostic Value of TERT Promoter Mutations
Two studies evaluated the diagnostic value of TERT promoter mutations using DNA extracted from FNAB samples of thyroid nodules [32, 44]. Liu and Xing examined 308 FNAB samples obtained preoperatively for patients who subsequently underwent thyroidectomy. No TERT promoter mutation was detected in any of the 179 benign thyroid nodules. On the other hand, nine cases of the remaining 129 cases with thyroid cancer were positive for TERT promoter mutations. C228 mutation was positive in four out of 111 cases of PTC and in three out of 18 cases of FTC. C250T mutation was positive in 1 PTC and 1 FTC. BRAF V600E mutation was positive in 42 out of 111 PTC and in none of 18 FTC or 179 benign adenomas. This gave a sensitivity of 7 % and specificity of 100 % for TERT promoter mutations alone. When results of TERT promoter mutations were added to BRAF V600E mutations, the sensitivity increased to 38 % and the specificity remained at 100 % for detection of malignancy on FNAB samples. Three of the samples that were positive for TERT promoter mutations showed indeterminate cytology [32]. Since TERT promoter mutations and BRAF V600E mutation detection is technically easy, this represents a useful diagnostic test for FNAB samples, especially those with indeterminate cytology. Nikiforov et al. used next generation sequencing technology with a gene panel (ThyroSeq v2) which simultaneously tested for point mutations in 13 genes and 42 fusion genes [44]. They examined 143 FNAB samples with cytological diagnosis of follicular neoplasm/suspicious for follicular neoplasms. The final histological diagnosis of the 143 nodules revealed 104 benign and 39 malignant neoplasms. The ThyroSeq v2 had a sensitivity, specificity, positive, and negative predictive values and an overall accuracy of 90, 93, 83, 96, and 92 %, respectively, for the diagnosis of thyroid cancer in nodules with follicular neoplasm/suspicious for follicular neoplasm category of the Bethesda FNAB cytological classification scheme [45]. TERT promoter mutations were positive in four cases (all were C228T mutation) which turned out to be malignant on final histological examination showing 100 % specificity but only 10.3 % sensitivity for TERT promoter mutations. In two of the four TERT positive nodules, an NRAS mutation was also detected [33].
Prognostic Value of TERT Promoter Mutations
Definitions of disease status were slightly different among different studies. However, disease free (remission) status was defined as absence of histologically or cytologically identifiable disease with negative radioiodine whole body scan and low or undetectable stimulated (<1 ng/dl) serum thyroglobulin in the absence of significant serum thyroglobulin autoantibodies. When the patient has never been free of disease since the initial surgery, the disease is persistent. When the disease reappears after a period of remission, the disease is recurrent. Four studies showed a significant association between TERT promoter mutations and recurrence rate of TC [21, 29, 30, 36] (Table 3). One study failed to show such an association between recurrence of thyroid microcarcinoma and TERT promoter mutations [38] (Table 4). In PTC, three studies showed an association between persistent/recurrent disease and TERT promoter mutations [30, 33, 36] (Table 4). In FTC, one study showed a significant association between TERT promoter mutations and persistent/recurrent disease [30] (Table 5). Gondolfi et al. studied 121 cases comprised of 43 cases with distant metastasis and 78 cases without distant metastasis [29]. In the whole group, there was a higher rate of patients living with disease in the TERT positive subgroup compared with TERT wild-type subgroup (4/21 vs. 14/100) [29]. Not only that there was a higher risk of recurrence but Kaplan Meier analysis showed a significant probability of mortality in TERT positive PTC compared with wild-type TERT group (P 0.001). However, this was not the case in the subgroup of distant metastases suggesting that the distant metastases are more powerful predictors of survival than the mutation status [29]. Similarly, Xing et al. performed Kaplan Meier analysis and showed a significant decline in recurrence-free survival in BRAF V600E or TERT promoter mutation positive compared with wild-type BRAF and TERT DTC, respectively [33]. This increased risk of recurrence was most pronounced when TERT and BRAF mutations co-occurred together compared with neither or either of these mutations. This was the case when all types of DTC were analyzed together and when the analysis was limited to CPTC only [33]. Muzza et al. showed in univariate and multivariate analyses of 182 PTCs that recurrence rate is significantly associated with TERT promoter mutations [30]. Ten of 22 (45 %) of the patients with TERT promoter mutations had persistent/recurrent PTC compared with 29/160 (18 %). In a multivariate analysis, the odds ratio for persistent/recurrent disease in patients with TERT promoter mutations was 3.40 (95 % CI, 1.29–8.96) [30]. Liu et al. reported a higher recurrence rate of PTC in 7/9 cases with TERT promoter mutations detected on FNAB specimens [32]. Melo et al. showed a significant association between TERT promoter mutations and persistent disease in 211 out of 469 cases of TC [36]. Persistent disease occurred in 62.4 % of cases with TERT promoter mutations compared with 24.6 % of cases with wild-type TERT (P 0.001). This association remained significant in a multivariate analysis (odds ratio 4.68, 95 % CI 1.54–14.27) [36]. A meta-analysis that included five studies of PTC showed a significant association between TERT promoter mutations and risk of persistent/recurrent disease (odds ratio, 5.7, 95 % CI 3.7–8.8, Fig. 3).
TERT promoter mutations were also found associated with mortality in four studies in which it was reported [34–37] (Table 3). In PTC, two studies showed a significant association between TERT promoter mutations and mortality [36, 37] (Table 4). A meta-analysis that included these two studies showed a significant association between TERT promoter mutations and thyroid cancer-specific mortality (Fig. 3). In FTC, Melo et al. reported a significant association between TERT promoter mutations and mortality [36] (Table 5). Wang et al. reported an association between TERT promoter mutations and mortality in 52 cases of FTC [34]. Melo et al. reported a significant association between TERT promoter mutations and mortality in 323 DTC, 284 PTC, and 70 FTC [36]. This remained significant after adjusting for age and gender with Hazard ratio of 10.35 (95 % CI 2.01–53.24, P = 0.005) in DTC and 23.81 (95 % CI, 1.36–415.76, P = 0.03) in PTC [36]. Kaplan Meier analysis showed a robust association between TERT promoter mutations and mortality in all types of follicular cell-derived thyroid cancer, PTC alone, FTC, and DTC (P values <0.001, 0.001, <0.001, and <0.001, respectively). In a study of 144 TC patients, of whom 51 had information on TERT promoter mutations and mortality, there was a significant association between mortality and TERT promoter mutations [37] (Table 4). Eleven of 13 patients with TERT promoter mutations died compared with 8/38 wild TERT subgroup [37].
Conclusions and Future Perspectives
Although overexpression of TERT in several types of cancer has been recognized for several years, the discovery of TERT promoter mutations and its association with aggressiveness in different types of cancer have opened a new avenue in our understanding of carcinogenesis. The potential use of these mutations in the diagnosis of patients with thyroid cancer has been briefly highlighted in this review. It is possible that the use of TERT promoter mutations in combination with other known mutations such as BRAF V600E would improve the accuracy of the molecular diagnosis of thyroid nodules. Detection of these mutations in the plasma of patients with thyroid cancer may lead to development of a molecular biomarker for the follow-up of these patients. Prognostically, there is substantial evidence that TERT promoter mutations are associated with more aggressive types of TC and they might be used alone or in combination with other mutations for evaluating the risk of recurrence and mortality and thus in decision making of therapeutic choices and frequency and tools for follow-up of TC. The use of drugs that inhibit the activity of TERT and control the highly TERT expressing cancer cells while sparing the low TERT activity normal cells is certainly an exciting aspect of the discovery of these mutations. Despite these major advances in this area of thyroid cancer pathogenesis and its potential translational applications, one has to recognize that our knowledge of the mechanisms by which these mutations contribute to carcinogenesis is still limited. It would take some time before a full understanding of the mechanisms, function, interactions, and impact of these mutations and the whole TERT pathways are fully understood with utilization of that understanding in developing tools and therapeutic measures for TC and other cancers in which TERT promoter mutations play roles.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
References
- 1.Vinagre J, et al. Telomerase promoter mutations in cancer: an emerging molecular biomarker? Virchows Arch. 2014;465(2):119–133. doi: 10.1007/s00428-014-1608-4. [DOI] [PubMed] [Google Scholar]
- 2.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn EH. Structure and function of telomeres. Nature. 1991;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 4.Greider CW. Telomeres. Curr Opin Cell Biol. 1991;3(3):444–451. doi: 10.1016/0955-0674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- 5.Gunes C, Rudolph KL. The role of telomeres in stem cells and cancer. Cell. 2013;152(3):390–393. doi: 10.1016/j.cell.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Muraki K, et al. Mechanisms of telomere loss and their consequences for chromosome instability. Front Oncol. 2012;2:135. doi: 10.3389/fonc.2012.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murnane JP. Telomere dysfunction and chromosome instability. Mutat Res. 2012;730(1–2):28–36. doi: 10.1016/j.mrfmmm.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim NW, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 9.Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet. 2010;11(5):319–330. doi: 10.1038/nrg2763. [DOI] [PubMed] [Google Scholar]
- 10.Kyo S, et al. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 2008;99(8):1528–1538. doi: 10.1111/j.1349-7006.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddel RR. Alternative lengthening of telomeres, telomerase, and cancer. Cancer Lett. 2003;194(2):155–162. doi: 10.1016/S0304-3835(02)00702-4. [DOI] [PubMed] [Google Scholar]
- 12.Heaphy CM, et al. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am J Pathol. 2011;179(4):1608–1615. doi: 10.1016/j.ajpath.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smekalova EM, et al. Telomerase RNA biosynthesis and processing. Biochemistry (Mosc) 2012;77(10):1120–1128. doi: 10.1134/S0006297912100045. [DOI] [PubMed] [Google Scholar]
- 14.Mocellin S, Pooley KA, Nitti D. Telomerase and the search for the end of cancer. Trends Mol Med. 2013;19(2):125–133. doi: 10.1016/j.molmed.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Cifuentes-Rojas C, Shippen DE. Telomerase regulation. Mutat Res. 2012;730(1–2):20–27. doi: 10.1016/j.mrfmmm.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montanaro L, et al. Relationship between dyskerin expression and telomerase activity in human breast cancer. Cell Oncol. 2008;30(6):483–490. doi: 10.3233/CLO-2008-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nandakumar J, Cech TR. Finding the end: recruitment of telomerase to telomeres. Nat Rev Mol Cell Biol. 2013;14(2):69–82. doi: 10.1038/nrm3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cong YS, Wen J, Bacchetti S. The human telomerase catalytic subunit hTERT: organization of the gene and characterization of the promoter. Hum Mol Genet. 1999;8(1):137–142. doi: 10.1093/hmg/8.1.137. [DOI] [PubMed] [Google Scholar]
- 19.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88(2):557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 20.Vinagre J, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. doi: 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013;20(4):603–610. doi: 10.1530/ERC-13-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horn S, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339(6122):959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 23.Huang FW, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339(6122):957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capezzone M, et al. Telomeres and thyroid cancer. Curr Genomics. 2009;10(8):526–533. doi: 10.2174/138920209789503897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soares P, et al. Genetic alterations in poorly differentiated and undifferentiated thyroid carcinomas. Curr Genomics. 2011;12(8):609–617. doi: 10.2174/138920211798120853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantara S, et al. Telomere abnormalities and chromosome fragility in patients affected by familial papillary thyroid cancer. J Clin Endocrinol Metab. 2012;97(7):E1327–E1331. doi: 10.1210/jc.2011-2096. [DOI] [PubMed] [Google Scholar]
- 27.Landa I, et al. Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab. 2013;98(9):E1562–E1566. doi: 10.1210/jc.2013-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi X. et al. (2015) Association of TERT promoter mutation 1,295,228 C > T with BRAF V600E mutation, older patient age, and distant metastasis in anaplastic thyroid cancer. J Clin Endocrinol Metab:jc20143606 [DOI] [PMC free article] [PubMed]
- 29.Gandolfi G, et al. TERT promoter mutations are associated with distant metastases in papillary thyroid carcinoma. Eur J Endocrinol. 2015;172(4):403–413. doi: 10.1530/EJE-14-0837. [DOI] [PubMed] [Google Scholar]
- 30.Muzza M, et al. Telomerase in differentiated thyroid cancer: promoter mutations, expression and localization. Mol Cell Endocrinol. 2015;399:288–295. doi: 10.1016/j.mce.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Chindris AM, et al. Clinical and molecular features of Hurthle cell carcinoma of the thyroid. J Clin Endocrinol Metab. 2015;100(1):55–62. doi: 10.1210/jc.2014-1634. [DOI] [PubMed] [Google Scholar]
- 32.Liu R, Xing M. Diagnostic and prognostic TERT promoter mutations in thyroid fine-needle aspiration biopsy. Endocr Relat Cancer. 2014;21(5):825–830. doi: 10.1530/ERC-14-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xing M, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014;32(25):2718–2726. doi: 10.1200/JCO.2014.55.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang N, et al. TERT promoter mutation as an early genetic event activating telomerase in follicular thyroid adenoma (FTA) and atypical FTA. Cancer. 2014;120(19):2965–2979. doi: 10.1002/cncr.28800. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J Clin Endocrinol Metab. 2014;99(6):E1130–E1136. doi: 10.1210/jc.2013-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melo M, et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2014;99(5):E754–E765. doi: 10.1210/jc.2013-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu T, et al. The age- and shorter telomere-dependent TERT promoter mutation in follicular thyroid cell-derived carcinomas. Oncogene. 2014;33(42):4978–4984. doi: 10.1038/onc.2013.446. [DOI] [PubMed] [Google Scholar]
- 38.de Biase D, et al. TERT promoter mutations in papillary thyroid microcarcinomas. Thyroid. 2015;25(9):1013–1019. doi: 10.1089/thy.2015.0101. [DOI] [PubMed] [Google Scholar]
- 39.Crescenzi A et al. (2015) Preoperative assessment of TERT promoter mutation on thyroid core needle biopsies supports diagnosis of malignancy and addresses surgical strategy. Horm Metab Res [DOI] [PubMed]
- 40.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12(2):245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 41.Xing M, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90(12):6373–6379. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 42.Xing M, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309(14):1493–1501. doi: 10.1001/jama.2013.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xing M, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015;33(1):42–50. doi: 10.1200/JCO.2014.56.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikiforov YE, et al. Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer. 2014;120(23):3627–3634. doi: 10.1002/cncr.29038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Thyroid. 2009;19(11):1159–1165. doi: 10.1089/thy.2009.0274. [DOI] [PubMed] [Google Scholar]