Abstract
Thymic carcinoids are rare neuroendocrine tumors that occur in 1–5 % of patients with multiple endocrine neoplasia type 1 (MEN1) and are a major cause of morbidity and mortality. The few published reports associate these tumors with male sex and smoking. Our objective was to describe cases of these tumors treated at our institution. We performed a retrospective chart review of all patients diagnosed with MEN1 at our tertiary referral center from 1980 to 2014. Patients with a histopathologic, fine-needle aspiration, or clinical diagnosis of a thymic carcinoid were included. Two hundred ninety-one patients fulfilled the criteria for a diagnosis of MEN1. Clinicopathologic characteristics, MEN1 genetic testing results, treatments, and survival rates were analyzed. Nine patients had a thymic carcinoid, six men (67 %) and three women (33 %). Six patients were non-smokers (67 %). Two patients had synchronous (22 %) and eight patients (89 %) had metachronous distant metastasis. The 10-year overall survival rate was 45 % (lower 95 % upper 95 % CI 20–100 %). The 10-year disease-free survival rate was 42 % (lower 95 % upper 95 % CI 15–100 %). Five patients had MEN1 genetic testing, and the genotypes of affected individuals were p.W341X, c.275_286delGCTTCACCGCCC, p.R98X, c.1350+(1_11)del11, and partial duplication of exons 9 and 10. A higher percentage of MEN1-related thymic carcinoids can occur in women and in non-smokers than previously reported. Both novel and known mutations were present in our cohort. Eighty nine percent of patients developed a metachronous metastasis from the thymic carcinoid. Patients with MEN1 and thymic carcinoids should be followed closely.
Electronic supplementary material
The online version of this article (doi:10.1007/s12672-016-0269-y) contains supplementary material, which is available to authorized users.
Keywords: Synchronous Metastasis, Metachronous Metastasis, Large Cell Neuroendocrine Carcinoma, Thymic Carcinoid, Ominous Prognosis
Introduction
Multiple endocrine neoplasia type 1 (MEN1) is an autosomal dominant disorder that presents as a constellation of tumors involving mainly the parathyroid glands, anterior pituitary, and pancreas; however, other tumors can present in MEN1 such as carcinoid tumors, adrenocortical tumors, and meningiomas [1]. The estimated prevalence of MEN1 is 2–3/100,000 [2]. MEN1 is caused by loss-of-function germline mutations in the tumor suppressor gene MEN1, which encodes the menin protein. Compared to non-MEN1 patients with the same tumors, MEN1 patients face poorer prognoses and decreased life expectancy due to the multiplicity and aggressiveness of their tumors [3, 4].
Carcinoid tumors diagnosed in the context of MEN-1 originate in the thymus, the bronchi, and/or the foregut of the gastrointestinal system. The incidence of thymic carcinoids in patients with MEN1 has been reported to be 3.6–8.4 % [5, 6], and 25 % of all thymic carcinoids occur in patients with MEN1 [7]. Thymic carcinoids in MEN1 patients have been reported to exhibit a predilection for men over women, with a male/female ratio of 20:1 [8]; however, one study from Japan showed this ratio to be markedly reduced (male/female ratio of 2:1) [7]. Furthermore, thymic carcinoids have been associated in the literature with a history of smoking [9, 10]. MEN1-related thymic carcinoids carry an ominous prognosis due to their aggressive nature and potential for metastasis, and they are associated with increased mortality in patients with MEN1 (hazard ratio = 4.29) [4, 11, 12].
Owing to the significant morbidity and mortality of thymic carcinoids in MEN1, a better understanding of the natural course of this disease, its presentation, and the associated risk factors is needed. With only 101 cases of thymic carcinoids in patients with MEN1 reported in the literature, primarily in small retrospective case series, all available information on these tumors is valuable [13]. To add to this body of knowledge, we identified a series of patients with MEN1 with thymic carcinoids treated at our institution and investigated their clinicopathologic characteristics, genotype, management, and survival.
Materials and Methods
This study was approved by the institutional review board. The surgical endocrinology departmental database of patients with possible MEN1 of prospectively collected data was searched for patients with thymic carcinoids who underwent at least part of their evaluation or treatment at our institution from January 1980 through December 2014. The patients included in this study fulfilled the clinical, genetic, and/or familial criteria for MEN1 from the clinical practice guidelines described by Thakker et al. in 2012 [1] and had a clinical, histopathologic, or fine-needle aspiration diagnosis of a thymic carcinoid. Patients with only a suspected diagnosis of MEN1 were excluded.
Data extracted included demographic characteristics (sex and age); smoking status; MEN1-related diseases; presenting symptomatology at the time of the thymic carcinoid diagnosis; pre-operative diagnostic imaging and biochemical workup; types of operations performed; histopathology results for the resected thymic carcinoids and their metastases; MEN1 genetic testing results; adjuvant therapies (chemotherapy and radiotherapy); and follow-up details including timing of metastases (synchronous or metachronous) from thymic carcinoids and other MEN1-related primary tumors, recurrence, and survival. Patients were considered non-smokers in this study only if their clinical notes specifically mentioned that the patient did not smoke. When there was nothing mentioned regarding the smoking status, the patient was classified as having an unknown smoking status.
Statistical Analysis
Demographic and clinical characteristics were summarized. Time-to-event analysis was conducted using the Kaplan-Meier method to estimate disease-specific survival and overall survival rates. Patients with an unknown cause of death were not included in the analysis of disease-specific survival. All statistical analyses were performed using R version 3.2.2.
Results
Two hundred ninety-one patients fulfilled the clinical, genetic, and/or familial criteria for a diagnosis of MEN1. Nine (3.1 %) of these patients with MEN1 were diagnosed with a thymic carcinoid. Two more patients with thymic carcinoids had only a suspected diagnosis of MEN1 at the time of the analysis according to our assessment and were excluded from the study (did not meet the clinical or genetic or familial criteria for MEN1).
The demographic, genetic, and MEN1-related characteristics of the nine patients with thymic carcinoids and MEN1 are summarized in Table 1. The male/female ratio was 2:1. Among the MEN1-related diseases [primary hyperparathyroidism (PHPT), pancreatic neuroendocrine tumor (PNET), and pituitary tumor (PIT)], PHPT had the highest penetrance (89 % of thymic carcinoid cases) and, on average, was the earliest presenting characteristic among MEN1 patients. One patient met only the genetic criteria for MEN1. The median time to metachronous metastasis from the date of diagnosis of a thymic carcinoid was 4.4 years (standard deviation [SD] 5.5 years).
Table 1.
Demographic, genetic, and MEN1-related features of patients with MEN1 with thymic carcinoids (n = 9)
| Characteristic | Value | |
|---|---|---|
| Sex n | Male | 6 |
| Female | 3 | |
| Criteria used to diagnose MEN1 (clinical or genetic)a n | PHPT and PIT | 3 |
| PHPT and PNET | 2 | |
| PHPT, PNET, and PIT | 3 | |
| Genetic criteria | 1 | |
| MEN1-related disease penetrance n | PHPT | 8 |
| PNET | 5 | |
| PIT | 6 | |
| Familial MEN1 n | Yes | 5 |
| No | 4 | |
| Age at date of diagnosis (years)b mean (SD), range | Thymic carcinoid | 38.6 (8.8), 22–47 |
| PNET | 42.4 (6.8), 32–48 | |
| PHPT | 37.3 (10.1), 20–48 | |
| PIT tumor | 47.8 (3.9), 44–55 | |
| Time to metachronous metastasis from thymic carcinoid diagnosis (years) median, range | 4.4, 0.3–15.8 | |
| Follow-up (months) mean (SD), range | 55.3 (47.4), 1–178 | |
aAccording to Thakker et al.
bData only for cases with the respective disease
The presentation, biochemical findings, and diagnostic imaging for each patient at the time of their diagnosis with a thymic carcinoid are shown in Table 2. These data were not available for two of the nine patients. Five of the seven patients with these data available had an incidental diagnosis of a thymic carcinoid without any symptomatology.
Table 2.
Clinical characteristics, presentation, biochemical findings, and diagnostic imaging at the time of diagnosis of a thymic carcinoid
| Patient | Year of diagnosis | Diagnosis | Symptomatology | Biochemistry findings | Radiology findings |
|---|---|---|---|---|---|
| I | 1982 | Incidental | None |
CgA 139 ng/ml (<64) IGF-1 172 ng/ml (126–382) Serotonin 74 ng/ml (22–180) Cortisol 8.6 μg/dl (6.2–19.4) |
– |
| II | 1987 | – | – | – | – |
| III | 1990 | Incidental (on workup for persistent PHPT) | None | – | – |
| IV | 1994 | – | – | – | – |
| V | 1997 | Incidental (left shoulder pain workup) | None | – | – |
| VI | 2003 | Symptomatic | Nausea, vomiting, diarrhea, dehydration, hypokalemia, anemia, weight loss | 5-HIAA 4.7 mg/24 h (<6) |
CXR: + Chest CT: + Octreotide scan: unknown |
| VII | 2006 | Symptomatic | Chest pain | – | – |
| VIII | 2007 | Incidental (on routine CXR) | None |
CgA 15.1 ng/ml (<64) IGF-1 242 ng/ml (126–382) 5-HIAA 3.6 mg/24 h (<6) Serotonin <10 ng/ml (22–180) |
CXR: + Chest CT: + PET/CT: + (SUV 25.7) |
| IX | 2013 | Incidental (on workup for hypercalcemia and HTN) | None |
ACTH 11 pg/ml (7–69) Cortisol 10.7 μg/dl (6.2–19.4) |
SPECT/CT technetium sestamibi: + |
“–” signifies that there are no available records for review. Normal values of laboratory tests are in parentheses
USCXR chest X-rays, HTN hypertension, CT computed tomography, PET positron emission tomography, CgA chromogranin A, IGF-1 insulin-like growth factor 1, 5-HIAA 5-hydroxyindoleacetic acid, ACTH adrenocorticotropic hormone, SPECT single-photon emission computed tomography, SUV standardized uptake values
The operations performed and their timing can be found in Table 3. All patients except one had a thoracotomy and a thymectomy. One patient had debulking thymectomy owing to tumor invading the pericardium. Two patients underwent reoperation for local recurrence of thymic carcinoids. These patients also underwent operations for PHPT, PNET, and/or PIT. Four patients (cases I, III, V, and VII) had a parathyroidectomy for PHPT before their surgery for the thymic carcinoid, but we have no data to indicate whether or not they had a prophylactic thymectomy at the time of the PHPT surgery (Table 3).
Table 3.
Surgeries in MEN1 patients with thymic carcinoids (n = 9)
| Surgery-related feature | Value | |
|---|---|---|
| Surgery for thymic carcinoid (n = 9) | Yes | 8 |
| No | 1 | |
| Surgery for PHPT (n = 8) | Yes | 8 |
| No | 0 | |
| Surgery for PNET (n = 5) | Yes | 3 |
| No | 2 | |
| Surgery for PIT (n = 6) | Yes | 1 |
| No | 5 | |
| First thymic carcinoid surgery (n = 8) | Total thymectomy | 7 |
| Debulking thymectomy | 1 | |
| Second thymic carcinoid surgery (n = 2) | Local recurrence excision | 1 |
| Mediastinal lymph node excision | 1 | |
| Age at date of first surgery (years)a mean (SD), range | Thymic carcinoid | 40.3 (9.0), 22–48 |
| PNET | 39.3 (7.5), 32–47 | |
| PHPT | 38.5 (10.0), 20–48 | |
| PIT | 55.1 (N/A), N/A | |
| Timing of first surgeries for PHPT and thymic carcinoid for each patient | ||
| I: | 1981: PHPT, 1982: thymic carcinoid | |
| II: | 1987: thymic carcinoid, 1989: PHPT | |
| III: | 1990: PHPT, 1991: thymic carcinoid | |
| IV: | 1994 (Jan): thymic carcinoid, 1994 (Oct): PHPT | |
| V: | 1988: PHPT | |
| VI: | 2001: thymic carcinoid, 2005: PHPT | |
| VII: | 1981: PHPT, 2006: thymic carcinoid | |
| VIII: | 2007: thymic carcinoid | |
| IX: | 2013: thymic carcinoid and PHPT (simultaneous) | |
aData only for cases with the respective disease
The histopathologic characteristics of the thymic carcinoids and their metastases (surgical biopsy or fine needle aspiration), wherever available, are shown in Supplementary Table 1. Immunohistochemical staining was positive for chromogranin A and synaptophysin in the primary tumor in two cases and in metastases in three cases. Necrosis was present in four cases.
The timing of the development of distant metastasis and the locations of distant metastases according to the site of the primary tumor (thymic carcinoid vs. PNET) are shown in Table 4. Two patients (22.2 %) presented with a synchronous metastasis from a thymic carcinoid, and eight patients (88.9 %) eventually developed a metachronous metastasis from the thymic carcinoid. Patients also had a significant burden of disease from metastasis from PNET, mainly in liver and bone (Table 4).
Table 4.
Timing and locations of distant metastasis by primary tumor site
| Primary tumor site | Metastasis site | Synchronous metastasis n (%) | Metachronous metastasis n (%) |
|---|---|---|---|
| Thymic carcinoid n = 9 | Lung/mediastinum | 0 | 3 |
| Bone | 0 | 2 | |
| Lymph nodes | 0 | 1 | |
| Other (brain) | 0 | 1 | |
| Lung/mediastinum and bone | 1 | 1 | |
| Lymph nodes and bone | 1 | 0 | |
| Total number of patients with metastasis from thymic carcinoid at any site | 2 (22.2) | 8 (88.9) | |
| PNET n = 5 | Lung | 0 | 0 |
| Bone | 0 | 1 | |
| Liver | 0 | 1 | |
| Other (abdominal lymph nodes) | 0 | 1 | |
| Lungs and bone | 1 | 0 | |
| Liver and bone | 0 | 1 | |
| Total number of patients with metastasis from PNET at any site | 1 (20.0)a | 4 (80.0)b |
aOne patient had synchronous lung and bone metastasis
bOne patient had metachronous metastasis to liver and bones
An overview of the characteristics of the individual patients can be seen in Table 5. At the end of follow-up, only one patient (11.1 %) was alive. Six patients (66.6 %) were non-smokers, one patient (11.1 %) was a smoker, one patient (11.1 %) had a history of chewing tobacco, and one patient (11.1 %) had an unknown smoking status. MEN1 genetic testing results were available in five patients; the remaining four patients did not undergo MEN1 genetic testing or did so at an outside institution and lacked available results. Seven patients received adjuvant chemotherapy, and eight received postoperative adjuvant radiation therapy.
Table 5.
Overview of characteristics of individual patients with MEN1 with thymic carcinoids (n = 9)
| Patient | Sex | Smoking status | Vital status | Total follow-up (months) | MEN1 genetic testing | MEN1-related diseases | Thymic carcinoid diagnosis | Diagnosis age of thymic carcinoid (years) | Surgery for thymic carcinoid | Adjuvant chemotherapy | Adjuvant radiotherapy | Metastasis from thymic carcinoid | Overall survivalb (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | Male | No | Dead | 27 | Not done |
PHPT: + PNET: + |
Incidental | 32 | Thymectomy |
5-FU STZ Doxorubicin Leucovorin |
XRT | Lymph nodes: metachronous | 22 |
| II | Female | No | Dead | 99 | R98X (non-sense-deleterious, exon 2) |
PHPT: + PNET: + Pituitary: + |
Unknown | 44 | Thymectomy |
5-FU STZ Doxorubicin |
XRT | Lung: metachronous | 19 |
| III | Male | No | Dead | 1 | 275_286delGCTTCACCGCCC (deletion-deleterious, exon 2) |
PHPT: + PNET: + Pituitary: + |
Incidental | 40 | Thymectomy |
5-FU Leucovorin |
XRT | Bones: metachronous | 17 |
| IV | Male | No | Dead | 38 | Not done |
PHPT: + PNET: + Pituitary: + |
Unknown | 46 | Thymectomy | None | XRT | Lung: metachronous | 5 |
| V | Female | Unknown | Dead | 16 | Not done |
PHPT: + PNET: + |
Incidental | 41 | Not operated |
Cisplatin Etoposide Carboplatin |
XRT | Bones and lymph nodes: synchronous | 6 |
| VI | Female | Noa | Dead | 85 | Not done |
PHPT: + Pituitary: + |
Symptomatic | 48 | Thymectomy | None | None | Bones: metachronous | 8 |
| VII | Male | Chewing tobacco use | Dead | 1 | W341X (non-sense-deleterious, exon 7) |
PHPT: + Pituitary: + |
Symptomatic | 45 | Thymectomy |
Etoposide Carboplatin Denosumab |
XRT | Lung and bones: metachronous | 9 |
| VIII | Male | No | Dead | 55 | Duplication of exons 9, 10 + (insertion-deleterious, exons 9 and 10) | None | Incidental | 23 | Thymectomy |
Etoposide Cisplatin Bevacizumab AKT inhibitor (MK-2206) |
IMRT |
Lung, bone: synchronous Brain: metachronous |
7 |
| IX | Male | Yes | Alive | 20 | c.1350+(1_11)del11 (splice-deleterious, exon 9) |
PHPT: + Pituitary: + |
Incidental | 45 | Debulking thymectomy |
Carboplatin Etoposide |
XRT | Lung: metachronous | – |
XRT X-ray radiation therapy, IMRT intensity-modulated radiotherapy, 5-FU 5-fluorouracil, STZ streptozocin
aPatient smoked very rarely in puberty and never smoked as an adult
bFrom time of thymic carcinoid diagnosis
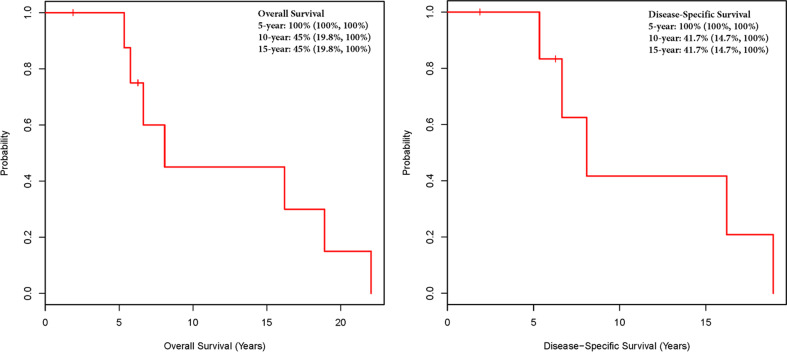
Estimates of 5-, 10-, and 15-year overall and disease-specific survival rates can be seen in Fig. 1. The 10-year overall survival and disease-specific survival were 45.0 and 41.7 %, respectively.
Fig. 1.
Overall and disease-specific survival in years and estimates of disease-specific survival at 5 years for MEN1 patients with thymic carcinoids. Vertical lines represent censored patients
Discussion
We reported our experience of treating patients with MEN1 with thymic carcinoids and described the clinical courses of these patients. The male/female ratio was 2:1, and 67 % of the patients were non-smokers. The thymic carcinoids carried an ominous prognosis: 22 % of the patients developed synchronous distant metastases, and 89 % of the patients developed metachronous distant metastases. The median time to a metachronous metastasis from the diagnosis of the thymic carcinoid was 4.4 years, with metastases appearing as much as 15 years after the initial diagnosis. These patients’ unfavorable outcomes suggest the need for close follow-up in other patients with MEN1 with thymic carcinoids.
The incidence of thymic carcinoids in our series of MEN1 patients was 3.1 % (9/291), which is similar to rates that other groups have previously published (3–6 %) [8–10, 12–21]. The age of presentation with a thymic carcinoid in this series matched the reported literature (between about 30 and 50 years old), with a mean age at diagnosis of 38.6 years (SD 8.8 years) [13, 15, 18]. Thymic carcinoids have been reported almost exclusively in males in the literature (male/female ratio of as high as 20:1) [3, 9, 10, 13, 22], whereas in our series, the male/female ratio was 2:1 (three of the nine patients were women [33.3 %]). A similar sex ratio to that in our series was reported by Sakurai et al. in Japan in 2012 (37 % of patients with MEN1 and thymic carcinoids were women, totaling 10 out of a total of 27 patients) [7]. In the same study from Sakurai et al., it was postulated that there could be an ethnic difference behind the higher ratio of thymic carcinoids in women compared to the western population. Our study has shown that ethnic differences could play a much smaller role, if any, than what was previously thought. Thymic carcinoids are rare tumors, and published literature is limited; hence, it would be prudent to remember that they can also occur in women, albeit with a lower incidence than in men. Alternative explanation for the predilection of thymic carcinoids for men could be attributed to the effect of the sex hormones on thymocyte proliferation and maturation [23], including the finding that estrogens have a protective role in which they prevent the growth of thymic tumors [24].
The relationship between thymic carcinoids and smoking also remains to be elucidated. Thymic carcinoids have been associated with smoking in some published case series [9, 10, 22]; however, most of the patients in our study were non-smokers (at least 67 %). Other studies supporting our findings include a case series from Sakurai et al., who reported that 60 % of their female patients were non-smokers [7], and from Ospina et al., who reported that 57 % of their patients were non-smokers [13]. Tobacco consumption has been shown to cause mutations in the tumor suppressor P53, and these mutations are frequently present in small cell lung carcinomas and large cell neuroendocrine carcinomas [25]. Menin is also a tumor suppressor (acting in the JunD pathway), and mutations in the MEN1 gene result in the development of the constellation of endocrine tumors that comprise MEN1 [26]. It remains to be seen whether tobacco acts synergistically with MEN1 mutations to create an environment of suppressed anti-neoplastic agents.
The natural history of MEN1 almost always starts with PHPT at a young age. In our cohort of patients, a PHPT diagnosis preceded the thymic carcinoid diagnosis by approximately 1 year (mean ages of 37 years for PHPT diagnoses and 39 years for thymic carcinoid diagnoses), whereas PNET and PIT developed later.
In the majority of the patients in our cohort, the diagnosis of the thymic carcinoid was incidental (71 %—5 out of 7 patients with available data). A similar clinically silent picture has been reported in several studies in the literature [9, 10, 12], while Ospina et al. reported that 57 % (4 out of 7 patients) were symptomatic [13]. Interestingly, none of the two symptomatic patients in our cohort presented with synchronous metastasis, while 40 % (2 out of 5) of patients with an incidental thymic carcinoid diagnosis presented with a synchronous metastasis. The lack of symptomatology could possibly contribute to the late diagnosis and unfavorable prognosis, when the tumor has already metastasized.
During the time period that this study covers, the clinical practices in diagnostic imaging in treating patients with thymic carcinoids changed to accommodate new available technology for testing and screening (PET/CT). Biochemical testing has been proven to yield a low diagnostic accuracy both in our cohort and in the literature [10].
One of the patients in our cohort was diagnosed with metastatic thymic carcinoid at the age of 22 years and died 7 years later without demonstrating any other MEN1-related diseases (genetic testing revealed partial duplication of exons 9 and 10). Partial or whole-gene deletions/duplications account for up to 4–6 % of MEN1 pathogenic mutations in the literature [5, 27, 28]. Similar cases of very young patients with MEN1 and only thymic carcinoids, without any other MEN1-related diseases, have previously been reported [10]. The mutations p.W341X [29], p.R98X [30], and h275_286delGCTTCACCGCCC [31] have been previously described in patients with MEN1 and appeared in our cohort as well.
In agreement with the literature [9, 12, 13, 22], our study demonstrated a significant number of synchronous (22 %) and metachronous (89 %) metastases in patients with MEN1 and a thymic carcinoid. Thymic carcinoids in patients with MEN1 carry significant morbidity and mortality and pose a diagnostic and treatment challenge, as 21 % of patients with this disease have been observed to develop PNET-related metastasis [31] (both synchronous and metachronous PNET-related metastases developed in our case series). Hypercalcemia in a patient with MEN1 might be mistaken for a sign of PHPT; if PHPT has not been already diagnosed, therefore, hypercalcemia in this setting should also prompt early investigations for the diagnosis of bone metastasis. Computed tomography, magnetic resonance imaging, or 99mTc bone scintigraphy can be used to investigate the possibility of bone metastasis.
Although most patients with MEN1 with thymic carcinoids already have metastases at presentation, these patients still undergo surgery to remove the thymic carcinoid, even if only for debulking reasons, to alleviate local compression on adjacent mediastinal structures and to facilitate adjuvant therapies [18]. Adjuvant radiotherapy and chemotherapy are part of standardized treatment for thymic carcinoids. A variety of chemotherapeutic agents were administered in our cohort, most commonly etoposide and 5-fluorouracil. Only one patient in our cohort did not undergo surgery for the primary tumor and was treated with only chemotherapy and radiotherapy.
As the surgical treatment of PHPT is the first surgery usually performed in these patients, some surgeons, including the authors of this study, advocate performing a prophylactic thymectomy concurrently with the initial parathyroidectomy. This combination has a dual benefit, removing any supranumerary parathyroid glands hidden in the thymus and reducing the risk of a thymic carcinoid. Since most of these procedures are performed through a cervical collar incision, only the superior aspect of the thymus can be removed, leaving the mediastinal part of the thymus in place. This remaining thymus may explain why some studies showed no significant advantage of prophylactic thymectomy in patients with MEN1 with and without thymic carcinoids [9, 10, 32, 33]. A Dutch series reported no thymic carcinoids in a series of 97 patients with MEN1 who underwent prophylactic transcervical thymectomy during parathyroidectomy after a follow-up of 8 years (range 0–40 years) [22], and similar findings were reported by Libutti et al. [34] with 66 patients with MEN1 undergoing routine transcervical thymectomy and in a review by Teh [35]. In our cohort, three patients had a parathyroidectomy before the diagnosis of a thymic carcinoid, but none of them, to our knowledge, had prophylactic thymectomy, although our information is limited because the initial operation was not performed at our institution.
Unfortunately, the prognosis for patients with MEN1 with thymic carcinoids remains poor, with a 10-year overall survival rate of 54 % in our series and 10-year overall survival rate of 25–36 % in the literature [10, 12, 18, 22]. In our cohort, all patients but one were dead by the end of follow-up. The aggressiveness of these tumors and their late detection, when the disease has already disseminated, could account for the poor outcomes observed. It seems self-evident that earlier detection and better screening are needed for these tumors. Although a very small subset of patients with MEN1 will eventually develop a thymic carcinoid, their ominous prognosis warrants active monitoring for thymic carcinoids as soon as patients are diagnosed with MEN1. The consensus statement on the management of thymic carcinoids in patients with MEN1 from Brandi et al. in 2001 advocated the use of computed tomography or magnetic resonance imaging for the early diagnosis of thymic or bronchial carcinoid every 3–5 years [3]. However, computed tomography and/or magnetic resonance imaging as part of a screening process carries a non-negligible radiation exposure and significant cost, respectively, generating a difficult conundrum regarding the cost/benefit ratio of a widespread screening for thymic carcinoids in the MEN1 population. Ospina et al. tried to address this dilemma, proposing multicenter randomized trials and a screening strategy that involve baseline evaluation (with computed tomography or magnetic resonance imaging) followed by follow-up with alternating computed tomography or magnetic resonance imaging of the chest every 1–2 years, which aligns with the guidelines from the Endocrine Society [1, 36].
The authors acknowledge the limitations of this study, which include those inherent to a retrospective review and the limited sample size. Given the rarity of MEN1 with thymic carcinoids, case reports and small cohorts of patients can provide valuable guidance to the clinician and contribute to the pool of data for a future systematic review. The high mortality of this disease—all but one of the patients in our series are now deceased—was the reason that we were unable to obtain approval to access data about or tissue from the thymic carcinoids collected outside our institution.
In summary, our study presents the clinicopathologic features of thymic carcinoids in a series of patients with MEN1. We have shown that a higher percentage of thymic carcinoids can occur in women and in non-smokers than previously reported. Almost half of the patients with thymic carcinoids already had a distant metastasis at the time of their diagnosis with a thymic carcinoid, which may explain the poor prognoses observed. Close follow-up of patients with MEN1 and thymic carcinoids could help detect these disease metastases early. The role of prophylactic transcervical thymectomy during PHPT surgery in patients with MEN1 to prevent the development of a thymic carcinoid is still uncertain, and its potential benefit requires more outcome-based research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 33 kb)
Acknowledgments
This work was supported by the National Institutes of Health/National Cancer Institute under award number P30CA016672 (and using the Clinical Trials Support Resource) and by the Cancer Center Support Grant (National Cancer Institute Grant P30CA016672).
Author Contributions
Ioannis A. Christakis: collection, management, analysis, and interpretation of the data and preparation, review, or approval of the manuscript
Wei Qiu: collection, management, interpretation of the data and preparation, review, or approval of the manuscript
Angelica M. Silva Figueroa: collection, management, interpretation of the data and preparation, review, or approval of the manuscript
Samuel Hyde: analysis and interpretation of the data and preparation, review, or approval of the manuscript
Gilbert J. Cote: analysis and interpretation of the data and preparation, review, or approval of the manuscript
Naifa L. Busaidy: analysis and interpretation of the data and preparation, review, or approval of the manuscript
Michelle Williams: analysis and interpretation of the data and preparation, review, or approval of the manuscript
Jeffrey E. Lee: analysis and interpretation of the data and preparation, review, or approval of the manuscript
Nancy D. Perrier: analysis and interpretation of the data and preparation, review, or approval of the manuscript
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Thakker RV, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1) J Clin Endocrinol Metab. 2012;97(9):2990–3011. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 2.Marx SJ. Multiple endocrine neoplasia type 1. In: Scriver ALBCR, Sly WS, Valle D, editors. Multiple endocrine neoplasia type 1. New York: McGraw-Hill; 2001. pp. 943–966. [Google Scholar]
- 3.Brandi ML, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86(12):5658–5671. doi: 10.1210/jcem.86.12.8070. [DOI] [PubMed] [Google Scholar]
- 4.Goudet P, et al. Risk factors and causes of death in MEN1 disease. A GTE (Groupe d’Etude des Tumeurs Endocrines) cohort study among 758 patients. World J Surg. 2010;34(2):249–255. doi: 10.1007/s00268-009-0290-1. [DOI] [PubMed] [Google Scholar]
- 5.Stenson PD, et al. Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat. 2003;21(6):577–581. doi: 10.1002/humu.10212. [DOI] [PubMed] [Google Scholar]
- 6.Trump D, et al. Clinical studies of multiple endocrine neoplasia type 1 (MEN1) QJM. 1996;89(9):653–669. doi: 10.1093/qjmed/89.9.653. [DOI] [PubMed] [Google Scholar]
- 7.Sakurai A, et al. Multiple endocrine neoplasia type 1 in Japan: establishment and analysis of a multicentre database. Clin Endocrinol (Oxf) 2012;76(4):533–539. doi: 10.1111/j.1365-2265.2011.04227.x. [DOI] [PubMed] [Google Scholar]
- 8.Teh BT, et al. Clinicopathologic studies of thymic carcinoids in multiple endocrine neoplasia type 1. Medicine (Baltimore) 1997;76(1):21–29. doi: 10.1097/00005792-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Ferolla P, et al. Thymic neuroendocrine carcinoma (carcinoid) in multiple endocrine neoplasia type 1 syndrome: the Italian series. J Clin Endocrinol Metab. 2005;90(5):2603–2609. doi: 10.1210/jc.2004-1155. [DOI] [PubMed] [Google Scholar]
- 10.Goudet P, et al. Thymic neuroendocrine tumors in multiple endocrine neoplasia type 1: a comparative study on 21 cases among a series of 761 MEN1 from the GTE (Groupe des Tumeurs Endocrines) World J Surg. 2009;33(6):1197–1207. doi: 10.1007/s00268-009-9980-y. [DOI] [PubMed] [Google Scholar]
- 11.Giusti F, Marini F, Brandi ML (1993) Multiple endocrine neoplasia type 1. In: Pagon RA et al (ed) GeneReviews(R). Seattle (WA)
- 12.Gibril F, et al. Prospective study of thymic carcinoids in patients with multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 2003;88(3):1066–1081. doi: 10.1210/jc.2002-021314. [DOI] [PubMed] [Google Scholar]
- 13.Singh Ospina N, et al. Thymic and bronchial carcinoid tumors in multiple endocrine neoplasia type 1: the Mayo clinic experience from 1977 to 2013. Horm Cancer. 2015;6(5–6):247–253. doi: 10.1007/s12672-015-0228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duh QY, et al. Carcinoids associated with multiple endocrine neoplasia syndromes. Am J Surg. 1987;154(1):142–148. doi: 10.1016/0002-9610(87)90305-9. [DOI] [PubMed] [Google Scholar]
- 15.Blayney DW. Thymic carcinoid and multiple endocrine neoplasia. West J Med. 1990;152(4):426. [PMC free article] [PubMed] [Google Scholar]
- 16.Zahner J, et al. Thymus carcinoid in multiple endocrine neoplasms type I. Dtsch Med Wochenschr. 1994;119(5):135–140. doi: 10.1055/s-2008-1058672. [DOI] [PubMed] [Google Scholar]
- 17.Murat A, et al. Thymic and bronchial neuroendocrine tumors in multiple endocrine neoplasia type 1. GENEM1. Presse Med. 1997;26(34):1616–1621. [PubMed] [Google Scholar]
- 18.Teh BT, et al. Thymic carcinoids in multiple endocrine neoplasia type 1. Ann Surg. 1998;228(1):99–105. doi: 10.1097/00000658-199807000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosai J, Higa E. Mediastinal endocrine neoplasm, of probable thymic origin, related to carcinoid tumor. Clinicopathologic study of 8 cases. Cancer. 1972;29(4):1061–1074. doi: 10.1002/1097-0142(197204)29:4<1061::AID-CNCR2820290456>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Cooper RB, VanWay CW, 3rd, Robinson WA. Carcinoid tumor of the thymus. Rocky Mt Med J. 1979;76(5):238–240. [PubMed] [Google Scholar]
- 21.Gibril F, et al. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: a prospective study of 107 cases and comparison with 1009 cases from the literature. Medicine (Baltimore) 2004;83(1):43–83. doi: 10.1097/01.md.0000112297.72510.32. [DOI] [PubMed] [Google Scholar]
- 22.de Laat JM, et al. Natural course and survival of neuroendocrine tumors of thymus and lung in MEN1 patients. J Clin Endocrinol Metab. 2014;99(9):3325–3333. doi: 10.1210/jc.2014-1560. [DOI] [PubMed] [Google Scholar]
- 23.Seiki K, Sakabe K. Sex hormones and the thymus in relation to thymocyte proliferation and maturation. Arch Histol Cytol. 1997;60(1):29–38. doi: 10.1679/aohc.60.29. [DOI] [PubMed] [Google Scholar]
- 24.Ishibashi H, et al. Sex steroid hormone receptors in human thymoma. J Clin Endocrinol Metab. 2003;88(5):2309–2317. doi: 10.1210/jc.2002-021353. [DOI] [PubMed] [Google Scholar]
- 25.Walch AK, et al. Typical and atypical carcinoid tumors of the lung are characterized by 11q deletions as detected by comparative genomic hybridization. Am J Pathol. 1998;153(4):1089–1098. doi: 10.1016/S0002-9440(10)65653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisseler-Eckhoff A, Demes M. Neuroendocrine tumors of the lung. Cancers (Basel) 2012;4(3):777–798. doi: 10.3390/cancers4030777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cebrian A, et al. Mutational and gross deletion study of the MEN1 gene and correlation with clinical features in Spanish patients. J Med Genet. 2003;40(5):e72. doi: 10.1136/jmg.40.5.e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tham E, et al. Clinical testing for mutations in the MEN1 gene in Sweden: a report on 200 unrelated cases. J Clin Endocrinol Metab. 2007;92(9):3389–3395. doi: 10.1210/jc.2007-0476. [DOI] [PubMed] [Google Scholar]
- 29.Cote GJ, et al. Five novel mutations in the familial multiple endocrine neoplasia type 1 (MEN1) gene. Mutations in brief no. 188. Online Hum Mutat. 1998;12(3):219. [PubMed] [Google Scholar]
- 30.Mayr B, et al. Menin mutations in patients with multiple endocrine neoplasia type 1. Eur J Endocrinol. 1997;137(6):684–687. doi: 10.1530/eje.0.1370684. [DOI] [PubMed] [Google Scholar]
- 31.Kouvaraki MA, et al. Genotype-phenotype analysis in multiple endocrine neoplasia type 1. Arch Surg. 2002;137(6):641–647. doi: 10.1001/archsurg.137.6.641. [DOI] [PubMed] [Google Scholar]
- 32.Habbe N, et al. Multimodal treatment of sporadic and inherited neuroendocrine tumors of the thymus. Surgery. 2008;144(5):780–785. doi: 10.1016/j.surg.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Lim LC, et al. Thymic carcinoid in multiple endocrine neoplasia 1: genotype-phenotype correlation and prevention. J Intern Med. 2006;259(4):428–432. doi: 10.1111/j.1365-2796.2006.01619.x. [DOI] [PubMed] [Google Scholar]
- 34.Powell AC, et al. The utility of routine transcervical thymectomy for multiple endocrine neoplasia 1-related hyperparathyroidism. Surgery. 2008;144(6):878–883. doi: 10.1016/j.surg.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teh BT. Thymic carcinoids in multiple endocrine neoplasia type 1. J Intern Med. 1998;243(6):501–504. doi: 10.1046/j.1365-2796.1998.00329.x. [DOI] [PubMed] [Google Scholar]
- 36.Singh Ospina N et al (2015) Clinical question: when and how should patients with multiple endocrine neoplasia type 1 be screened for thymic and bronchial carcinoid tumors? Clin Endocrinol (Oxf) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 33 kb)