Abstract
Breast cancer is a hormone-dependent disease in which estrogen signaling targeting drugs fail in about 10 % due to resistance. Strong evidences highlighted the mitogen role of progesterone, its ligands, and the corresponding progesterone receptor (PR) isoforms in mammary carcinoma. Several PR antagonists have been synthesized; however, some of them are non-selective and led to side or toxic effects. Herein, we evaluated the anti-tumor activity of a commercially available PR modulator, ulipristal acetate (UPA), and a new selective and passive PR antagonist “APR19” in a novel preclinical approach based on patient-derived breast tumor (HBCx-34) xenografted in nude mice. As opposed to P4 that slightly reduces tumor volume, UPA and APR19 treatment for 42 days led to a significant 30 % reduction in tumor weight, accompanied by a significant 40 % retardation in tumor growth upon UPA exposure while a 1.5-fold increase in necrotic areas was observed in APR19-treated tumors. Interestingly, PR expression was upregulated by a 2.5-fold factor in UPA-treated tumors while APR19 significantly reduced expression of both PR and estrogen receptor α, indicating a potential distinct molecular mechanism among PR antagonists. Cell proliferation was clearly reduced in UPA group compared to vehicle conditions, as revealed by the significant reduction in Ki-67, Cyclin D1, and proliferating cell nuclear antigen (PCNA) expression. Likewise, an increase in activated, cleaved poly(ADP-ribose) polymerase (PARP) expression was also demonstrated upon UPA exposure. Collectively, our findings provide direct in vivo evidence for anti-progestin-mediated control of human breast cancer growth, given their anti-proliferative and pro-apoptotic activities, supporting a potential role in breast cancer therapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s12672-016-0255-4) contains supplementary material, which is available to authorized users.
Keywords: Progesterone Receptor, Proliferate Cell Nuclear Antigen, Progesterone Receptor Expression, Proliferate Cell Nuclear Antigen Expression, Ulipristal Acetate
Introduction
Over years, breast cancer care has evolved toward personalized treatment, including adapted chemotherapy, anti-hormone, and HER2-targeted drugs, since heterogeneity of the disease has been highlighted by large clinical trial results [22]. Earlier detection of breast cancer and currently available therapies led to a decrease in mortality rate. However, more than 10 % of patients will relapse within 60 months after treatment [4]. Among patients with hormone-dependent disease, recurrence is in part linked to early, or secondary acquired, anti-estrogen drug resistance [7], the mechanism of which still remains unclear.
The progesterone receptor (PR), progesterone, and progestins have been shown to be involved in breast carcinogenesis, in clinical studies as well as in vitro and animal models [5, 15, 17, 27]. Pharmacological strategies based on PR antagonists were thus developed to inhibit PR-mediated tumorigenesis. Data from preclinical and clinical studies using different PR antagonists or selective modulators (SPRM) suggested potential applications in breast cancer prevention and treatment [18, 26–28, 34, 59]. However, the relationship between altered PR signaling and anti-tumor properties of these molecules remains to be clearly established. Most of steroid PR antagonists contain bulky side chains. This favors recruitment of corepressors, leading to PR transcriptional inhibition by blocking induced conformational change in alpha-helix 12 of the ligand binding domain of PR [47]. Other chemical modifications of PR ligands such as 17-fluorination leading to EC304 compound were shown to block PR transcriptional activity and to inhibit breast cancer cell proliferation [43]. Nevertheless, these molecules could not be used on the long term either because they are non-selective drugs (anti-glucocorticoid activity) and/or because they are not approved for clinical use.
In order to circumvent these limitations, we decided to examine the effects of two new PR antagonists: ulipristal acetate (UPA) and a newly synthesized homosteroid 3, 17-fluorinated compound (APR19), in a preclinical breast cancer model. UPA is a recently released SPRM, used for emergency contraception and long-term treatment of leiomyoma, a benign hormone-dependent uterine tumor [11, 16], while APR19 belongs to a new class of highly selective and passive PR antagonists (APRn) [25].
Most currently available in vivo models, such as DMBA/MNU-induced mammary carcinogenesis in rats [2, 28, 44, 58, 59], MXT mouse models [28, 38], or breast cancer cell line xenografts (T47D, MCF-7) (reviewed in [28]), failed to mimic and reflect the phenotypic, genetic, and molecular profiling of the primary tumors and thus did not allow a relevant preclinical screening.
Recently, a new preclinical model of human breast tumor xenograft was established directly from patient biopsies without intermediary cell culture and aimed at preserving the histologic and molecular characteristics of the original patient’s tumors [9]. Well-characterized human breast tumor samples were grafted in immuno-deficient mice [35, 36, 42]. These xenograft models are used for pharmaceutical preclinical drug screening [1]. In order to evaluate the impact of anti-progestins’ treatment in breast cancer, we used the HBCx-34 patient-derived breast cancer xenograft model. This model is derived from a luminal invasive mammary ductal carcinoma expressing progesterone and estrogen receptors (PR and ERα), wild-type p53, and non-amplified HER2 expression. HBCx-34 has been already tested for its response to estrogen and endocrine therapies [8, 9].
Herein, we used this xenograft model to test for the first time the properties of progesterone (P4) and the potential anti-tumor effects of PR antagonists UPA and APR19. These molecules exhibited different mechanisms of action in terms of tumor weight and volume control and regulation of apoptotic and proliferation markers. Our results bring additional information on how PR ligands (agonists and antagonists) control human breast cancer growth during breast cancer therapy.
Materials and Methods
Animal Models
Six- to nine-week-old outbred athymic (nu/nu) female non-ovariectomized mice (20 g) (Hsd: Athymic Nude-Foxn1nu) (Harlan Laboratories, Gannat, France) were maintained in specific pathogen-free animal housing at the CERFE animal facility (Center for Exploration and Experimental Functional Research, Evry, France), authorized by the Direction des services vétérinaires, Ministère de l’Agriculture et de la Pêche (agreement no. B-91-228-107). Mice were housed in individually ventilated cages, with sterilized food, water, and dust-free bedding cobs, under controlled light–dark cycle, room temperature, and humidity in accordance with French regulatory legislation concerning the protection of laboratory animals (Xentech, Evry, France).
Tumor Xenograft Induction
Human breast cancer HBCx-34 tumor tissues of the same passage (n-1) were xenografted subcutaneously in donor mice (five to ten mice) [9]. Mice were sacrificed by cervical dislocation once tumors reached 1–2 × 103 mm3. Mammary tumors were aseptically excised, dissected, and cut into fragments measuring approximately 20 mm3 after removing necrotic areas. Recipient mice were anaesthetized with ketamine/xylazine, and skin was incised after application of a chlorhexidine solution, at the level of the interscapular region where tumor fragments were subcutaneously transplanted. Mice with homogenous tumor volume were allocated into different groups of treatment.
Study Groups and Treatment
Nude mice grafted subcutaneously with HBCx-34 tumor fragments were distributed into four different experimental groups as reported in Table 1 when their initial tumor volumes were 62.5–196 mm3. Human breast tumor-bearing mice kept drinking water containing β-estradiol (8.5 mg/L) during the whole study. Plasma samples were collected at D0 and D42 or at day of sacrifice (due to ethical criteria), in the UPA-treated group (group 3). Serum and tumor samples were collected in the four experimental groups at the end point (i.e., D42 or ethical sacrifice). Tumors were first weighed immediately after sacrifice. One half of each tumor was snap frozen in liquid nitrogen and then transferred to −80 °C for storage. The other half of each tumor was fixed in 10 % formalin for 24 h at 4 °C and then stored at room temperature (RT) in 70 % ethanol until paraffin embedding.
Table 1.
Drug doses, routes of administration, and schedules in this study
| Group | N | Compound | mg/kg | Route | Schedule |
|---|---|---|---|---|---|
| 1 | 10 | Controla | – | SC | 5 qwk × 6 |
| 2 | 10 | Progesterone | 50 | SC | |
| 3 | 10 | UPA | 130 | PO | |
| 4 | 10 | APRns19 | 20 | IP | q2d × 43 |
5 qwk × 6 dosed 5 days on, 2 days off for 6 weeks; q2d × 43 every other day during 43 days
aControl: vehicle corresponding to group 2 (10 % benzyl alcohol/90 % sesame oil)
Tumor Measurement and Criteria for Ethical Sacrifice
All animals were weighted twice a week during the treatment period. Tumor volume was evaluated by measuring tumor diameters, using a caliper, bi-weekly during the treatment period. The formula TV (mm3) = [length (mm) × width (mm)2] / 2 was used, where the length and the width are the longest and the shortest diameters of the tumor, respectively. Animals were sacrificed, following ethical criteria, according to their body loss (BWL ≥20 % as compared to the first day of treatment) and tumor volume (≥2 × 103 mm3). At D28, almost all the tumors were collected for the four groups.
Immunohistochemistry
Immunohistochemistry was performed on paraffin-embedded samples of mammary tumor tissues, using the Benchmark XT Roche Ventana automate Ki-67 immunohistochemistry, and manually for other markers’ immunohistochemistry. After microwave antigen retrieval in pH 6.0 citrate buffer for PR and for 30 min at 98 °C in an EDTA alkaline buffer for Ki-67, sections were then incubated either overnight with mouse monoclonal antibody anti-PR (NCL-L-PGR-312 Novocastra Laboratories, île Saint Martin, Nanterre, France), dilution 1:50, at 4 °C in a humid chamber or for 32 min at 37 °C with anti-Ki-67 antibody (clone MIB 1, DAKO, Les Ulis, France), dilution 1:150. After primary antibody incubation, endogenous peroxidases were quenched with 3 % H2O2 in phosphate-buffered saline (PBS) (pH 7.4) for 10 min and bound Igs were revealed with a commercial peroxidase immunolabeling kit (immPRESS Anti-Mouse Ig Reagent Kit; Vector Laboratories, Burlingame, CA). Finally, Dako AEC+ High Sensitivity Substrate was used as a chromogen for PR and Multimere Kit (UltraView Universal DAB detection Kit, Roche, Ventana, Boulogne Billancourt, France). Immunolabeled sections were examined by an observer in a blinded manner using six high-power fields (×40) with a conventional optical microscope (Provis; Olympus, Tokyo, Japan).
Quantitative Real-Time PCR
Total RNA was extracted from frozen mammary tumor fragments with TRI Reagent (Applied Biosystems) according to the manufacturer’s recommendations. One microgram of total RNA was processed for reverse-transcribed qPCR. Briefly, total RNA was treated with DNase I (Biolabs, Evry, France) and then reverse-transcribed using the High-Capacity complementary DNA (cDNA) Reverse Transcription Kit (Life Technologies). cDNA were analyzed by quantitative RT-PCR using the Power SYBR Green PCR Master Mix (Life Technologies) with the indicated primers (300 nM, final concentration) (Supplemental Table S1) and a StepOne Real-Time PCR System (Life Technologies). Relative gene expression was calculated as a ratio of attomoles normalized by ribosomal rRNA 18S expression in femtomoles.
Immunoblotting
Mammary tissues were lysed using TissueLyser LT (Qiagen) in lysis buffer (150 mM NaCl, 50 mM Tris-HCl (pH 7.5), 5 mM EDTA, 30 mM Na pyrophosphate, 50 mM Na fluoride, 1 % Triton X-100, and protease and phosphatase inhibitor cocktails (Sigma)) for 30 min on rotation at 4 °C, followed by a centrifugation at 12,000×g for 15 min at 4 °C to clear debris. Samples were resolved by 7.5 % sodium dodecyl sulfate gel electrophoresis and transferred onto nitrocellulose membranes. Primary antibodies used were anti-PR (PR Antibody (C-19) (sc-538, Santa Cruz Biotechnology, CA, USA), dilution 1:200; anti-PR (PR Antibody (C-20) (sc-539, Santa Cruz Biotechnology), dilution 1:200; anti-Bcl-XL antibody (E18, ab32370, Abcam, France), dilution 1:1,000; anti-proliferating cell nuclear antigen (PCNA) (FL-261, sc-7907, Santa Cruz Biotechnology), dilution 1:200; anti-poly(ADP-ribose) polymerase (PARP) antibody (#9542, Cell Signaling Technology), dilution 1:200; anti-Cyclin D1 antibody (H-295, sc-753, Santa Cruz Biotechnology), dilution 1: 200; and anti-α-tubulin, dilution 1:1000 (Sigma). Secondary antibodies were Goat Anti-Mouse IgG (H+L) Cross Adsorbed Secondary Antibody (DyLight 680 conjugated) and Goat Anti-Rabbit IgG (H+L) DyLight 800 conjugated, dilution 1:10,000 (Thermo Fisher Scientific, Rockford, IL, USA). Antibodies were diluted in PBS and 0.1 % Tween 20 buffer supplemented with 5 % non-fat milk and added to the membranes for 1 h at RT or overnight at 4 °C, followed by incubation with the indicated secondary antibody for 1 h at RT. Target proteins were detected using Odyssey® Fc, Dual-Mode Western Imaging (Li-Cor, Lincoln, NE, USA) by fluorescence and quantified.
Statistical Analysis
All data are mean ± SEM. Non-parametric Mann–Whitney statistical U tests were applied to determine all significant differences between experimental conditions, using the Prism 5 software (GraphPad Software, San Diego, CA). Statistical significances are indicated by asterisk or x (one to three symbols corresponding to P < 0.05 or <0.01 or <0.001, respectively).
Results
Characterization of Human Xenograft HBCx-34 Model
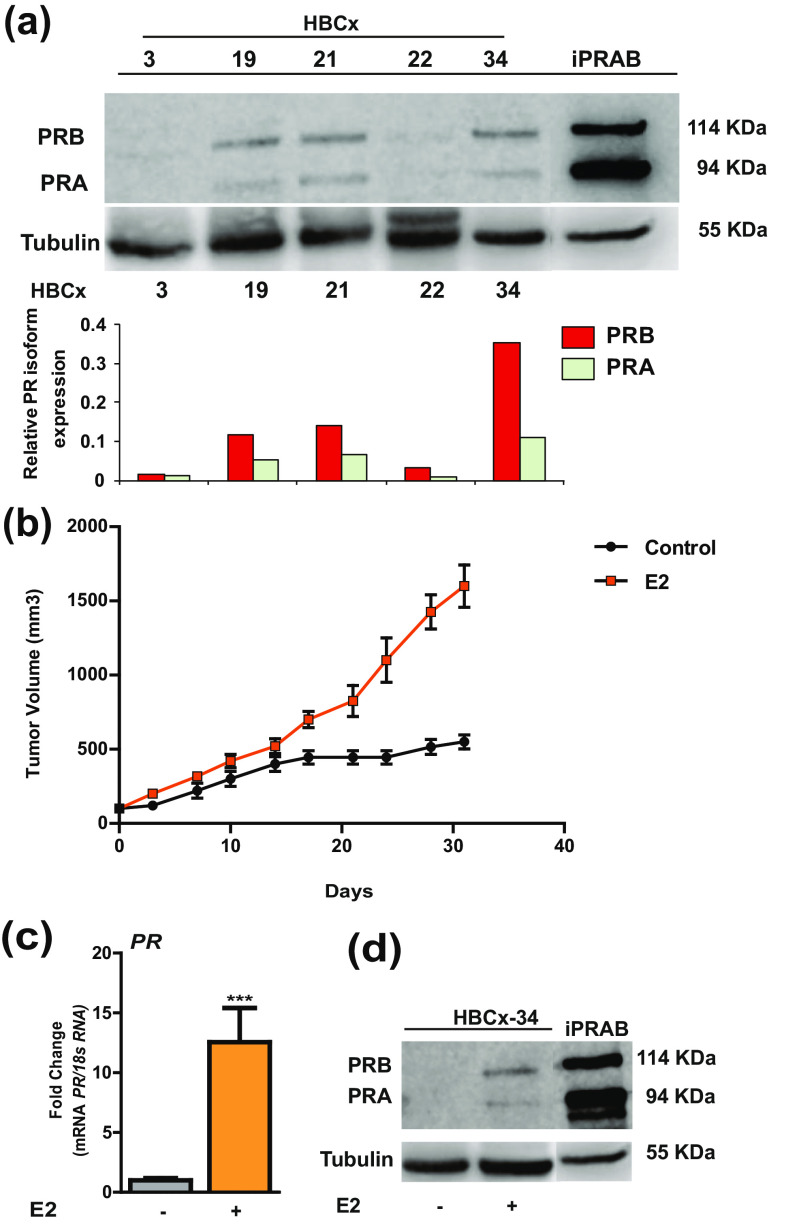
In order to evaluate anti-tumor effects of anti-progestins, it was necessary to choose human breast cancer tumors that do express substantial amounts of PR. Thus, different human-derived, breast tumor samples, derived from primary ER- and PR-expressing tumors, were obtained from Xentech (HBCx-3, 19, 21, 22, 34). After analyzing PR and ER expression by Western blot and RT-qPCR studies and proliferation rates (provided by Xentech company), we selected the HBCx-34 tumor for its sustained PR expression throughout grafting processes, as compared to HBCx-3, 19, 21, and 22 or to human bi-inducible MDA iPRAB cell model used as positive control [14, 24] (Fig. 1a). Of note, both PRA and PRB isoforms were expressed and identified as 94- and 114-kDa bands, respectively, PRB being more expressed than PRA (Fig. 1a). HBCx-34 is a primary mammary infiltrating ductal carcinoma expressing not only PR but also ERα (data not shown). HBCx-34 has a wild-type p53 and no HER2 overexpression. Proliferation rate of the HBCx-34 tumor was considered as relatively slow yet with growth rate higher than other HBCx tumor sample growth and an estimated doubling time around 17 days [9]. More importantly, tumor proliferation rate was estrogen-dependent since, in the absence of estrogen in drinking water, tumor volume failed to increase after 15 days, never reaching more than 500 mm3 (Fig. 1b). We also demonstrated that both PR isoforms are expressed in HBCx-34 tumor and presumably functional upon E2 exposure in terms of transcript (12.5× higher than in the group not exposed to E2) and protein (Fig. 1c, d). This finding is consistent with the notion that PR is an estrogen-regulated gene and its synthesis requires E2 and its receptor [6].
Fig. 1.
Characterization of human xenograft tumor models. a PR isoform protein expression by western blot analysis of tumor tissue extracts performed using anti-PR rabbit polyclonal antibody recognizing both PR isoforms (PR, C-19) and relative PR expression normalized to tubulin in HBCx xenograft tumor models (HBCx-3, 19, 21, 22 and 34) compared to the breast cancer cell line MDA-iPRAB [24]. b Evolution of tumor volume of both control- and estrogen-treated group, measured bi-weekly with a caliper, during the treatment period. The formula TV (mm3) = [length (mm) × width (mm)2] / 2 was used, where the length and the width are the longest and the shortest diameters of the tumor, respectively. c mRNA expression levels of PR gene determined by RT-qPCR in HBCx-34 tumors collected from mice receiving E2-supplemented drinking water vs control mice. d Western blot analysis of estrogen-dependent PR isoform expression in HBC-34 tumor xenograft carried out with anti-PR (C-19). Data are expressed as fold induction compared to control condition, arbitrarily set at 1, and are means ± SEM. *** indicates p < 0.001 compared to the control group (non-parametric Mann-Whitney U tests)
Impact of P4, UPA, and APR19 on HBCx-34 Tumor Growth Parameters
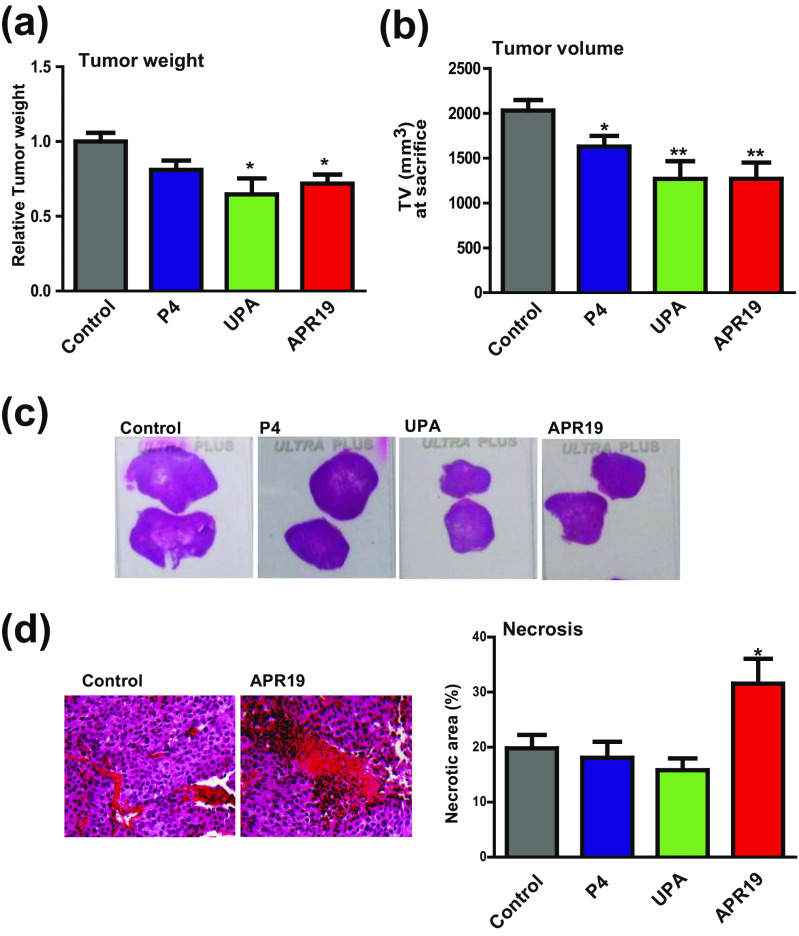
We next examined the effects of UPA and APR19 on HBCx-34 tumor growth. After transplanting HBCx-34 tumors (see “Materials and Methods” section), mice were treated for 42 days with progesterone (P4), UPA, and APR19 as detailed in Table 1. Tumor weight was measured at day 42 or at sacrifice (due to ethical criteria) for treated groups and control group. A significant 30 % reduction of tumor weight was found in both anti-progestin-treated groups as compared to control group (1.4 ± 0.40 vs 2.0 ± 0.37 g, respectively, p < 0.05), (Fig. 2a), while no significant difference was observed between the P4-treated group and control group. Figure 2b shows a slight reduction of 20 % in tumor volume in P4-treated group and a reduction of up to 40 % upon UPA and APR19 treatment. The T/C ratio (%), which represents the ratio between the mean tumor volume of the treated group (T) and the mean tumor volume of the control group (C), generally constitutes a reliable index of anti-tumor activity according to NCI criteria [3]. This ratio was calculated bi-weekly during the treatment period until D28 where almost all mice for each group were still alive (see “Materials and Methods” section) and is illustrated in Supplemental Fig. S1a. The T/C (%) at D28 demonstrates an approximately 30, 40, and 20 % decrease in tumor volume of P4-, UPA-, and APR19-treated groups, respectively. As anticipated, a positive, linear, and highly significant correlation was observed between tumor weight and volume (Supplemental Fig. S1b). We next analyzed the macroscopic and histopathologic parameters of the collected tumors. Figure 2c shows four representative tissue sections of mammary tumors after hematoxylin-eosin staining, indicating that the tumor surface was smaller in anti-progestin-treated groups in accordance with the reduction in tumor growth and tumor weight of the corresponding groups. Moreover, necrosis is a well-regulated process activated in tumor cells; large necrotic areas are considered as a good sign of therapeutic efficiency [28, 49]. Figure 2d (left panel) shows two mammary tumor sections, belonging to control and APR19 groups. Mean necrotic surface (%) was calculated and normalized to the total tumor surface. Interestingly, we found a 1.5-fold increase in necrotic index in APR19-treated groups as compared to the control group (30 vs 20 %, Fig. 2d, right panel, p < 0.05) while there was no significant difference in P4 and UPA groups. Collectively, both anti-progestins significantly reduce human breast cancer tumor growth whereas, in addition, APR19 drastically increases necrotic areas.
Fig. 2.
Tumor growth in HBCx-34 xenograft model (tumor weight and volume) following PR ligand exposure. a HBCx-34 mammary tumors were weighted (g) at D42 or ethical sacrifice according to ethical criteria (body weight loss (BWL) ≥20 % compared to the first day of treatment for 48 consecutive hours (three measurements); general alteration of behavior or clinical signs and tumor volume ≥2000 mm3). b Histograms of tumor volume (TV) in cubic millimeter measured by a caliper at sacrifice (end of treatment or ethical sacrifice) (the formula TV (mm3) = [length (mm) × width (mm)2] / 2). c Images of representative xenograft tissue tumors by hematoxylin-eosin staining of the four groups of the study. d Images of necrotic zones of xenograft tumors of the four groups colored by hematoxylin-eosin staining. In the right panel, analysis of the % of necrotic zones calculated by Calopix software reported to the tumor total surface. Data are means ± SEM. *, **, and *** indicate p < 0.05, 0.01, and 0.001, respectively, compared to the control condition (non-parametric Mann-Whitney U tests)
UPA Plasma Levels
In order to confirm that UPA treatment (130 mg/kg) was adequately administrated, mean plasma levels of UPA were measured at the day of sacrifice by liquid chromatography–mass spectrometry (LC-MS/MS) and reached 6919 ± 2820 ng/mL. These pharmacological doses of UPA in mice led to extremely high levels of circulating anti-progestin that are not comparable to what is clinically used for women. Interestingly enough, these values did not lead to neither any undesirable side effects nor any overt toxicity in the UPA-treated animals.
PR Analysis: Opposite Action of UPA and APR19
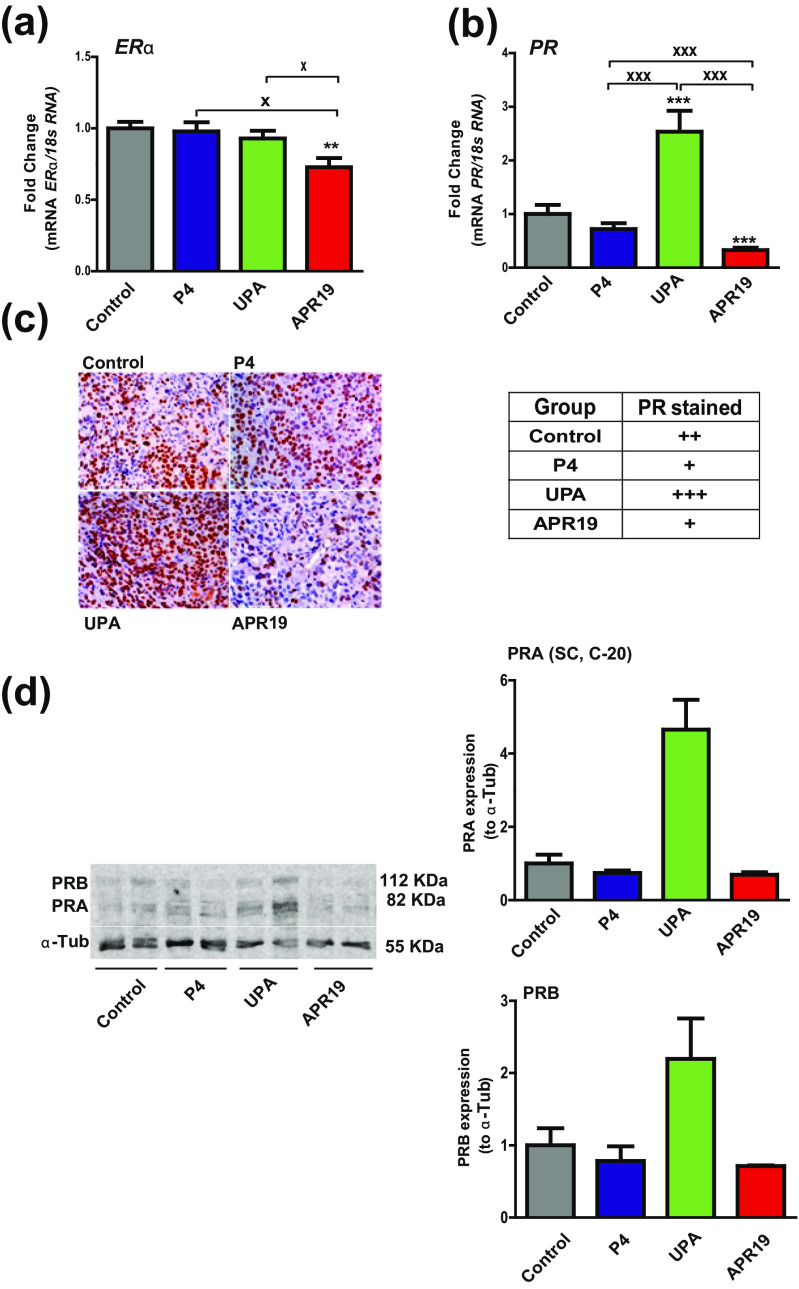
We next evaluated whether ERα and PR expression was modified during the treatment procedure in the four groups. By means of RT-qPCR analysis, we showed that ERα transcript levels were significantly reduced only in the APR19-treated group while PR expression was markedly decreased by 70 % in this group as compared to control group (Fig. 3a, b). In sharp contrast, PR mRNA expression was increased by 2.5-fold in the UPA-treated group compared with control group. No significant variation was found in the P4 group (Fig. 3b). PR protein expression was also investigated using immunohistochemical PR staining (Fig. 3c) and quantified by measuring positively stained cells normalized to the total cell number. Semiquantitative results for PR expression in collected tumors of the four groups are summarized in Fig. 3c, right panel, and classified from + for moderate staining (10–29 % PR-positive cells), ++ for strong staining (30–69 %) to +++ representing a very high PR staining (>70 %). Consistent with PR mRNA expression levels, PR protein expression was slightly reduced by P4 and APR19 treatments but drastically increased in UPA-treated tumors. PR isoform protein expression was also evaluated by Western blot analyses of two representative tumor samples per group using an anti-PR antibody recognizing both PR isoforms (PR C-20, Santa Cruz antibody) (Fig. 3d). We found, as anticipated, that both PRA and PRB expressions were downregulated by 30 % in P4- and APR19-treated groups as compared to vehicle-treated group. In contrast, UPA highly stimulates by 2–5-fold factor, PRA and PRB expressions.
Fig. 3.
Effects of PR ligands on ERα and PR expression. mRNA transcript levels of ERα (a) and PR (b) genes analyzed by RT-qPCR in HBCx-34 xenograft tumors treated with control, P4, UPA, and APR19. c Images of tissues sections obtained by immunohistochemical analysis of PR expression from xenografted tumors (×40) (left panel). A semiquantitative evaluation of PR expression is presented on the right panel and is classified from + for moderate staining (10–29 %), ++ for strong staining (30–69 % PR-positive cells), to +++ representing a very high PR positively stained cells (>70 %). d Western blot analysis of PR expression of xenografted tumor protein extracts (50 μg) was performed using anti-PR antibody recognizing both PR isoforms (C-20, Santa Cruz) and anti-α-tubulin antibody for sample loading control (left panel) (two samples/group of treatment). PRA and PRB protein expressions were quantified relatively to α-tubulin (right panel). Data are expressed as fold induction compared to control condition arbitrarily set at 1 and are means ± SEM. In a, b,*, **, and *** indicate p < 0.05, 0.01, and 0.001, respectively, compared to the control condition and x, xx, and xxx symbols indicate p < 0.05, 0.01, and 0.001, respectively, and represent the comparison between the three treated groups (P4, UPA, APR19) (non-parametric Mann-Whitney U tests)
UPA Exerts Anti-Proliferative Effects
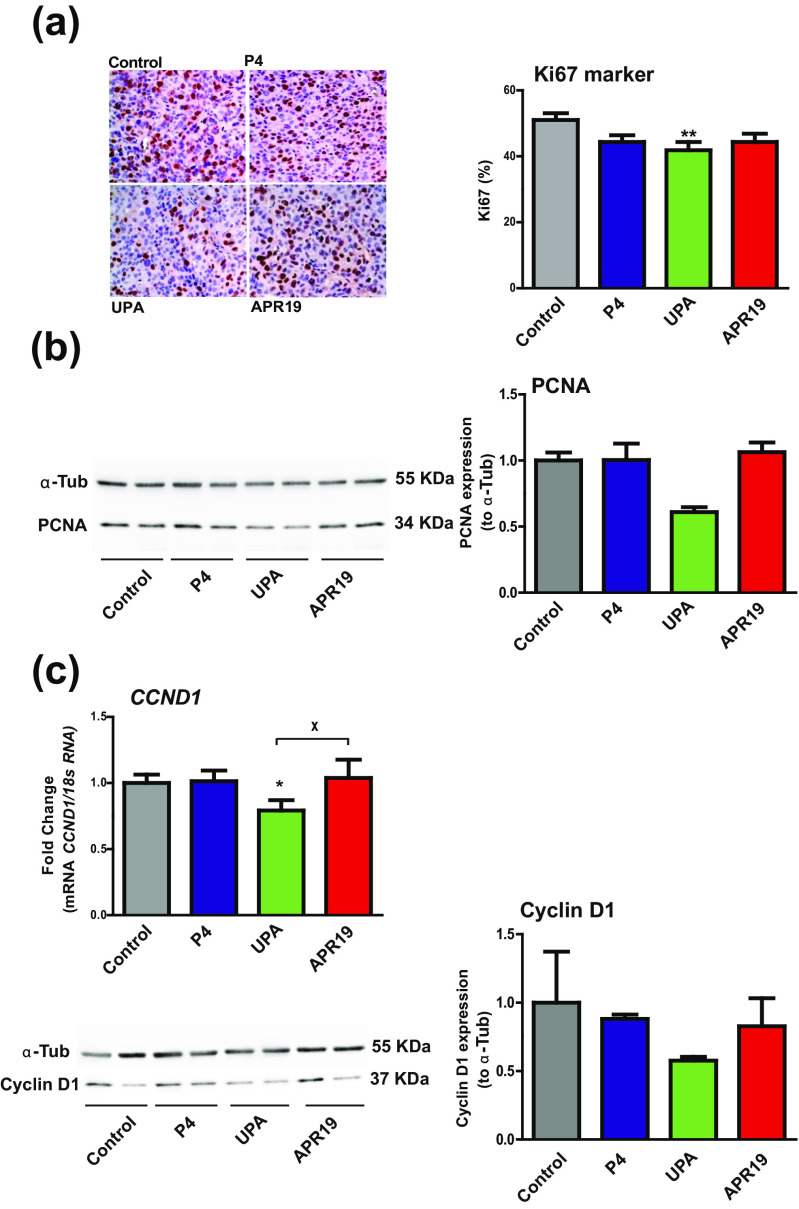
Cell proliferation is a hallmark when evaluating an anti-tumor activity of any given compound. To investigate PR ligand effects, several proliferation markers (Ki-67, PCNA, and Cyclin D1) were evaluated in collected tumors of each group by either immunohistochemical studies, Western blot experiments, or RT-qPCR analyses. Immunohistochemical staining of the nuclear marker Ki-67 is illustrated for one representative control-, P4-, UPA-, and APR19-treated tumor (Fig. 4a, left panel). The resulting Ki-67 index, as defined in the “Materials and Methods” section, was significantly decreased by 20 % in the UPA group vs control group while no significant difference was found for the two other groups (Fig. 4a, right panel). PCNA is a cell-related nuclear protein, highly expressed in proliferating cells during DNA synthesis phase of the cell cycle. PCNA protein expression of two representative tumor samples per group was quantified by Western blot (Fig. 4b, left panel) and normalized to the tubulin loading control (Fig. 4b, right panel). PCNA expression was reduced by 40 % in the UPA-treated group, while in the P4- and APR19-treated group, no variation in PCNA expression was observed (Fig. 4b, right panel). Finally, Cyclin D1, required for progression through G1 phase of the cell cycle, is another important proliferative marker. Cyclin D1 expression analyses revealed a 25 % decrease in the UPA-treated group (Fig. 4c upper panel), consistent with the already observed reduction of Ki-67 and PCNA expression upon UPA exposure. Similarly, Cyclin D1 protein expression of two representative tumor samples per group was quantified by Western blot (Fig. 4c, left panel) and normalized to the tubulin loading control (Fig. 4c, right panel). Cyclin D1 expression was reduced by 20 % in the P4- and APR19-treated groups, while it was decreased by almost 50 % in the UPA-treated group (Fig. 4b, right panel), in accordance to the reduction of mRNA abundance previously demonstrated.
Fig. 4.
Anti-proliferative activity of UPA. a Immunohistochemical analysis of nuclear Ki-67 expression as depicted in left panel by images of tissue sections from the four xenografted tumor groups. In the right panel, analysis of the % of Ki-67 calculated by counting the marked cells (nuclei) compared to the total number of cells (×40). Data are expressed as % according to the total cell number. b Western blot analysis of xenografted tumor protein extracts (50 μg) was performed using anti-PCNA and anti-α-tubulin for sample loading control (left panel) (two samples/group of treatment). PCNA protein expression (34 kDa) was quantified relatively to α-tubulin loading control (55 kDa) (right panel). c mRNA transcript levels of CCND1 (CYCLIN D1) gene analyzed by RT-qPCR in HBCx-34 xenograft tumors treated with control, P4, UPA, and APR19 (upper panel). Western blot analysis of xenografted tumor protein extracts (50 μg) was performed using anti-Cyclin D1 and anti-α-tubulin for sample loading control (left panel) (two samples/group of treatment). Cyclin D1 protein expression (37 kDa) was quantified relatively to α-tubulin loading control (55 kDa) (right panel). Data are expressed as fold induction compared to control condition arbitrarily set at 1 and are mean ± SEM. In a, c, *, **, and *** indicate p < 0.05, 0.01, and 0.001, respectively, compared to the control condition (non-parametric Mann-Whitney U tests)
Pro-Apoptotic Effects of UPA
Apoptosis may also constitute another important hallmark of an anti-tumor activity of an anti-progestin. Several apoptotic markers exerting either an anti-apoptotic effect (Clusterin (CLU), BCL2-L1) or a pro-apoptotic action (PARP) were also examined in the collected tumors of each group. Indeed, clusterin is an anti-apoptotic intracellular marker that binds to the pro-apoptotic marker Bax and inhibits apoptosis [50]. CLU transcript levels were quantified by RT-qPCR analysis in the four treated groups. A significant increase of 35 % in CLU expression was observed in the APR19 group. However, neither progesterone nor UPA seemed to affect the CLU gene expression as compared to the control group (Fig. 5a). Another well-known anti-apoptotic marker BCL2-L1 was also investigated in terms of transcript (Fig. 5b) and protein (Fig. 5c) expression in the four groups. BCL 2 -L 1 mRNA was increased by 50 % in the APR19-treated group vs control group (Fig. 5b). However, BCL2-L1 protein expression was increased in all treated groups as revealed by Western blot analysis (Fig. 5c). PARP is a protein involved in programmed cell death and DNA repair. During apoptosis, the full-length PARP (116 kDa) is cleaved into an 89-kDa fragment. Western blot analysis of cleaved PARP expression of two representative tumor samples per group (Fig. 5d, left panel) demonstrated an increase by 1.7-fold in P4-treated group, 4-fold in UPA-treated group, and 3.5-fold in APR19-treated group, respectively (Fig. 5d, right panel). Collectively, these findings indicate that UPA exerts pro-apoptotic effects associated with remarkable anti-tumor growth effects.
Fig. 5.
Apoptotic marker analysis of HBCx-34 xenograft tumor model. mRNA transcript levels of CLU (CLUSTERIN) (a) and BCL 2 -L 1 (b) genes analyzed by RT-qPCR in HBCx-34 xenograft tumors treated with control, P4, UPA, and APR19. c Western blot analysis of xenografted tumor protein extracts (50 μg) was performed using anti-Bcl-XL antibody and anti-α-tubulin antibody for sample loading control (left panel) (two samples/group of treatment). BCL2-L1 (26 kDa) protein expression was quantified relatively to α-tubulin loading control (right panel). d Western blot analysis of PARP (two samples/group of treatment). Cleaved-PARP (89 kDa) protein expression was quantified relatively to α-tubulin loading control. Data are expressed as fold induction compared to control condition arbitrarily set at 1 and are means ± SEM. In a, b, *, **, and *** indicate p < 0.05, 0.01, and 0.001, respectively, compared to the control (non-parametric Mann-Whitney U tests)
Discussion
In this study, we evaluated the effects of progesterone and two PR antagonists, ulipristal acetate (UPA) and APR19 [25], in patient-derived breast tumor tissue, HBCx-34, xenografted in nude mice. Patient-derived xenograft (PDX) is a reliable preclinical model to test the efficacy of a drug treatment, due to the preservation of genome, tumor behavior, heterogeneity, and histologic and metastatic features of the original tumor [35, 36, 57]. HBCx-34 is an estrogen-dependent tumor and responds to endocrine therapies; however, the role of P4 and PR antagonists has not been elucidated, yet.
Analyses of cell proliferation markers, necrotic areas, and apoptosis markers are the hallmarks of a potential anti-tumor activity following treatment. In the present study, we found that UPA and APR19 reduced tumor weight and volume, while P4 slightly reduced tumor volume without affecting tumor weight. The effects of PR antagonists are in agreement with other in vivo studies, in which anti-progestin treatment inhibited estrogen-induced mammary tumor growth. Mifepristone (RU486), a PR modulator (PRM), alone or combined with the anti-estrogen tamoxifen, induced tumor regression and reduction in tumor volume in several experimental animal models, including DMBA- and MNU-induced mammary rat carcinogenesis models (reviewed in [26, 28]). Another anti-progestin CDB-4124 exerted a similar inhibitory action on DMBA- and MNU-induced mammary carcinogenesis [59]. Anti-progestin-induced tumor regression is accompanied by an increase in cytostasis, apoptosis, and differentiation (reviewed in [28]). In this study, UPA treatment increased apoptosis by upregulating and activating PARP into cleaved PARP protein expression. PARP is known as a key DNA repair enzyme, PARP inhibitors being evaluated as therapeutic agents either in patient-derived triple-negative breast cancer xenograft model or combined with radiotherapy in treating triple-negative breast cancer [54, 56]. On the other hand, P4 and APR19 slightly upregulated cleaved PAPR expression. Previously, it has been demonstrated that BCL-X is overexpressed in invasive breast cancer [45] and is associated with ER expression [37]. In in vitro models, progesterone upregulated the anti-apoptotic factor BCL-XL, thus inhibiting apoptosis in T47D human breast cancer cell line [51], and in cultured leiomyoma cells while promoting their growth by upregulating epidermal growth factor [60]. In this latter study, a pro-apoptotic activity of UPA through a decrease in Bcl-2 protein expression, an increase in cleaved caspase-3, and PARP protein expression has been observed in cultured leiomyoma cells [60]. In vivo studies reported similar results [20, 61]. Apoptosis has been also shown to be induced by RU486 in cancer cells, including breast and endometrial cells [12, 32, 53].
In our study, increased apoptosis index following UPA treatment is associated with an anti-proliferative effect of UPA combined with a decrease in Ki-67, PCNA, and Cyclin D1 expression. Cyclin D1 is a P4-regulated target gene correlating with ER expression. In our model, progesterone does not seem to affect Cyclin D1 expression. The amplification of Cyclin D1 is observed in 15 % of breast cancers and is associated with tamoxifen resistance [52]. Proliferating cell nuclear antigen (PCNA) is a cell cycle-related nuclear protein whose expression is elevated in late G1 and S phases of proliferating cells [46]. Herein, PCNA expression was downregulated in the UPA-treated group, while neither P4 nor APR19 modified its expression, supporting distinct mechanism of action of PR ligands (antagonists or P4). A previous study demonstrated an increase in PCNA expression following treatment with the CDB-4124 SPRM in cultured leiomyoma cells [33], while in another study, PCNA expression was downregulated after UPA treatment of cultured leiomyoma cells, reversing the stimulatory effect of P4. P4-induced PCNA expression was not observed in our study in the P4-treated group. Ki-67-positive cells (normal breast epithelial proliferating cells) are significantly reduced in RU486-treated patients [13]; the same inhibitory effect was observed when treating leiomyoma cells with CDB-4124 (a steroid SPRM) [59]. Treatment of endometriosis, a benign hormone-dependent invasive disease, in rat models by UPA led to an increase in Bax/Bcl2 ratio and in cytochrome c expression, in addition to a decrease in Ki-67 expression [21].
In the present study, APR19 inhibited ER expression, as well as PR expression. This is consistent with ER-dependent regulation of PR expression. However, ER expression was not affected by UPA while PR expression was strongly upregulated. In previous studies, after CDB-4124 treatment, ER alpha expression was decreased in T47D cells but PR expression was not affected [59]. This finding contrasts with the effect of CDB-4124 that decreases PR-positive cells in MNU-induced mammary carcinogenesis [10], with the effect of RU486 that inhibits PR expression in BT474 luminal human breast cancer cell line xenografted in nude mice [30, 31], and with the inhibition of PR activation by its antagonists [29]. In the present study, PR expression was differentially affected by PR ligands. We found that PRA and PRB followed the same expression pattern as revealed by qPCR, immunohistochemistry, and western blot analyses. Both PR isoforms are highly upregulated by UPA, while P4 and APR19 downregulate PR isoform protein expression. PRA and PRB isoforms are co-expressed in equal amounts in normal human breast cells. However, an alteration in PRA/PRB ratio with a predominant expression of PRA isoform has been reported to constitute an early event of breast carcinogenesis, accompanied by a poorer disease-free survival rates, tamoxifen resistance [19, 39], and progestin and anti-progestin responsiveness [40, 55]. An increase in PRA expression is observed in normal breast tissue of BRCA1 or BRCA2 mutation carriers [41]. Along the same line, RU486 was shown to prevent mammary carcinogenesis in double transgenic mice carrying BRCA1/P53 mutations [23, 48], which suggests that anti-progestin treatment in breast cancer prevention may lead to a beneficial effect for BRCA1 mutation carriers. Taking apart the role of progesterone, we found that it only diminishes slightly tumor volume without affecting tumor weight, the necrotic index, the Ki-67 and Cyclin D1 expression, and the apoptotic markers.
In conclusion, we have evaluated the effects of two different PR antagonists in a preclinical breast cancer model. Our results revealed anti-proliferative and pro-apoptotic activities for UPA in breast cancer. However, defining the main role and properties of the selective and passive APR19 needs further investigation. Our data suggest that UPA could be of interest as an adjuvant endocrine therapeutic drug or in the prevention of hormone-dependent breast cancer. However, confirmation in the clinical setting is required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 257 kb)
(DOCX 50 kb)
Acknowledgments
This study was supported by fundings from Institut National de la Santé et de la Recherche Médicale (Inserm), Université Paris Sud, and a grant from La Ligue Nationale Contre le Cancer (to FJ and ML). The authors would like to thank Pr Jean-Daniel Brion, Dr Marie-Edith Rafestin-Oblin, and Dr Junaid Ali Khan for their help and comments during the initial phase of this work.
The funder HRA Pharma provided grant together with the national agency for Research (ANRT/HRA pharma CIFRE, i.e., Conventions Industrielles de Formation par la Recherche, in English Research Training Industrial Grant Contract) for [NE], support in the form of salary for one author [MRR], but did not have any additional role in the study design, data collection and analysis, or preparation of the manuscript.
Abbreviations
- P4
Progesterone
- PR
Progesterone receptor
- UPA
Ulipristal acetate
- PR antagonists
APRn
Compliance with Ethical Standards
Conflict of Interest
MRR is an employee of HRA Pharma.
NE is a recipient of a joint ANRT/HRA Pharma grant.
NCB is a member of the European board of Gedeon Richter, without personal income.
References
- 1.Aparicio S, Hidalgo M, Kung AL. Examining the utility of patient-derived xenograft mouse models. Nature Reviews Cancer. 2015;15(5):311–316. doi: 10.1038/nrc3944. [DOI] [PubMed] [Google Scholar]
- 2.Bakker GH, Setyono-Han B, Henkelman MS, de Jong FH, Lamberts SW, van der Schoot P, Klijn JG. Comparison of the actions of the antiprogestin mifepristone (RU486), the progestin megestrol acetate, the LHRH analog buserelin, and ovariectomy in treatment of rat mammary tumors. Cancer Treatment Reports. 1987;71(11):1021–1027. [PubMed] [Google Scholar]
- 3.Bissery MC, Chabot GG. History and new development of screening and evaluation methods of anticancer drugs used in vivo and in vitro. Bull Cancer. 1991;78(7):587–602. [PubMed] [Google Scholar]
- 4.Brewster AM, Hortobagyi GN, Broglio KR, Kau SW, Santa-Maria CA, Arun B, Buzdar AU, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst. 2008;100(16):1179–1183. doi: 10.1093/jnci/djn233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brisken C. Progesterone signalling in breast cancer: a neglected hormone coming into the limelight. Nature Reviews Cancer. 2013;13(6):385–396. doi: 10.1038/nrc3518. [DOI] [PubMed] [Google Scholar]
- 6.Chabbert-Buffet N, Meduri G, Bouchard P, Spitz IM. Selective progesterone receptor modulators and progesterone antagonists: mechanisms of action and clinical applications. Hum Reprod Update. 2005;11(3):293–307. doi: 10.1093/humupd/dmi002. [DOI] [PubMed] [Google Scholar]
- 7.Clarke R, Liu MC, Bouker KB, Gu Z, Lee RY, Zhu Y, Skaar TC, et al. Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene. 2003;22(47):7316–7339. doi: 10.1038/sj.onc.1206937. [DOI] [PubMed] [Google Scholar]
- 8.Cottu P, Bieche I, Assayag F, El Botty R, Chateau-Joubert S, Thuleau A, Bagarre T, et al. Acquired resistance to endocrine treatments is associated with tumor-specific molecular changes in patient-derived luminal breast cancer xenografts. Clin Cancer Res. 2014;20(16):4314–4325. doi: 10.1158/1078-0432.CCR-13-3230. [DOI] [PubMed] [Google Scholar]
- 9.Cottu P, Marangoni E, Assayag F, de Cremoux P, Vincent-Salomon A, Guyader C, de Plater L, et al. Modeling of response to endocrine therapy in a panel of human luminal breast cancer xenografts. Breast Cancer Res Treat. 2012;133(2):595–606. doi: 10.1007/s10549-011-1815-5. [DOI] [PubMed] [Google Scholar]
- 10.Cui X, Schiff R, Arpino G, Osborne CK, Lee AV. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol. 2005;23(30):7721–7735. doi: 10.1200/JCO.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Donnez J, Tomaszewski J, Vazquez F, Bouchard P, Lemieszczuk B, Baro F, Nouri K, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366(5):421–432. doi: 10.1056/NEJMoa1103180. [DOI] [PubMed] [Google Scholar]
- 12.El Etreby MF, Liang Y, Wrenn RW, Schoenlein PV. Additive effect of mifepristone and tamoxifen on apoptotic pathways in MCF-7 human breast cancer cells. Breast Cancer Res Treat. 1998;51(2):149–168. doi: 10.1023/A:1006078032287. [DOI] [PubMed] [Google Scholar]
- 13.Engman M, Skoog L, Soderqvist G, Gemzell-Danielsson K. The effect of mifepristone on breast cell proliferation in premenopausal women evaluated through fine needle aspiration cytology. Hum Reprod. 2008;23(9):2072–2079. doi: 10.1093/humrep/den228. [DOI] [PubMed] [Google Scholar]
- 14.Esber N, Le Billan F, Resche-Rigon M, Loosfelt H, Lombes M, Chabbert-Buffet N. Ulipristal acetate inhibits progesterone receptor isoform A-mediated human breast cancer proliferation and BCl2-L1 expression. PLoS One. 2015;10(10):e0140795. doi: 10.1371/journal.pone.0140795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giulianelli S, Molinolo A, Lanari C. Targeting progesterone receptors in breast cancer. Vitam Horm. 2013;93:161–184. doi: 10.1016/B978-0-12-416673-8.00009-5. [DOI] [PubMed] [Google Scholar]
- 16.Glasier AF, Cameron ST, Fine PM, Logan SJ, Casale W, Van Horn J, Sogor L, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375(9714):555–562. doi: 10.1016/S0140-6736(10)60101-8. [DOI] [PubMed] [Google Scholar]
- 17.Hagan CR, Lange CA. Molecular determinants of context-dependent progesterone receptor action in breast cancer. BMC Medicine. 2014;12:32. doi: 10.1186/1741-7015-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han SJ, Tsai SY, Tsai MJ, O’Malley BW. Distinct temporal and spatial activities of RU486 on progesterone receptor function in reproductive organs of ovariectomized mice. Endocrinology. 2007;148(5):2471–2486. doi: 10.1210/en.2006-1561. [DOI] [PubMed] [Google Scholar]
- 19.Hopp TA, Weiss HL, Hilsenbeck SG, Cui Y, Allred DC, Horwitz KB, Fuqua SA. Breast cancer patients with progesterone receptor PR-A-rich tumors have poorer disease-free survival rates. Clin Cancer Res. 2004;10(8):2751–2760. doi: 10.1158/1078-0432.CCR-03-0141. [DOI] [PubMed] [Google Scholar]
- 20.Horak P, Mara M, Dundr P, Kubinova K, Kuzel D, Hudecek R, Chmel R. Effect of a selective progesterone receptor modulator on induction of apoptosis in uterine fibroids in vivo. Int J Endocrinol. 2012;2012:436174. doi: 10.1155/2012/436174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huniadi CA, Pop OL, Antal TA, Stamatian F. The effects of ulipristal on Bax/Bcl-2, cytochrome c, Ki-67 and cyclooxygenase-2 expression in a rat model with surgically induced endometriosis. Eur J Obstet Gynecol Reprod Biol. 2013;169(2):360–365. doi: 10.1016/j.ejogrb.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 23.Kariagina A, Aupperlee MD, Haslam SZ. Progesterone receptor isoform functions in normal breast development and breast cancer. Crit Rev Eukaryot Gene Expr. 2008;18(1):11–33. doi: 10.1615/CritRevEukarGeneExpr.v18.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan JA, Bellance C, Guiochon-Mantel A, Lombes M, Loosfelt H. Differential regulation of breast cancer-associated genes by progesterone receptor isoforms PRA and PRB in a new bi-inducible breast cancer cell line. PLoS One. 2012;7(9):e45993. doi: 10.1371/journal.pone.0045993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan JA, Tikad A, Fay M, Hamze A, Fagart J, Chabbert-Buffet N, Meduri G, et al. A new strategy for selective targeting of progesterone receptor with passive antagonists. Mol Endocrinol. 2013;27(6):909–924. doi: 10.1210/me.2012-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klijn JG, Setyono Han B, Foekens JA. Progesterone antagonists and progesterone receptor modulators in the treatment of breast cancer. Steroids. 2000;65(10–11):825–830. doi: 10.1016/S0039-128X(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 27.Knutson TP, Lange CA. Tracking progesterone receptor-mediated actions in breast cancer. Pharmacol Ther. 2014;142(1):114–125. doi: 10.1016/j.pharmthera.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanari C, Wargon V, Rojas P, Molinolo AA. Antiprogestins in breast cancer treatment: are we ready? Endocrine-Related Cancer. 2012;19(3):R35–50. doi: 10.1530/ERC-11-0378. [DOI] [PubMed] [Google Scholar]
- 29.Leonhardt SA, Edwards DP. Mechanism of action of progesterone antagonists. Exp Biol Med (Maywood) 2002;227(11):969–980. doi: 10.1177/153537020222701104. [DOI] [PubMed] [Google Scholar]
- 30.Liang Y, Benakanakere I, Besch-Williford C, Hyder RS, Ellersieck MR, Hyder SM. Synthetic progestins induce growth and metastasis of BT-474 human breast cancer xenografts in nude mice. Menopause. 2010;17(5):1040–1047. doi: 10.1097/gme.0b013e3181d3dd0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang Y, Besch-Williford C, Brekken RA, Hyder SM. Progestin-dependent progression of human breast tumor xenografts: a novel model for evaluating antitumor therapeutics. Cancer Res. 2007;67(20):9929–9936. doi: 10.1158/0008-5472.CAN-07-1103. [DOI] [PubMed] [Google Scholar]
- 32.Liang Y, Hou M, Kallab AM, Barrett JT, El Etreby F, Schoenlein PV. Induction of antiproliferation and apoptosis in estrogen receptor negative MDA-231 human breast cancer cells by mifepristone and 4-hydroxytamoxifen combination therapy: a role for TGFbeta1. Int J Oncol. 2003;23(2):369–380. [PubMed] [Google Scholar]
- 33.Luo X, Yin P, Js Coon V, Cheng YH, Wiehle RD, Bulun SE. The selective progesterone receptor modulator CDB4124 inhibits proliferation and induces apoptosis in uterine leiomyoma cells. Fertil Steril. 2010;93(8):2668–2673. doi: 10.1016/j.fertnstert.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madauss KP, Grygielko ET, Deng SJ, Sulpizio AC, Stanley TB, Wu C, Short SA, et al. A structural and in vitro characterization of asoprisnil: a selective progesterone receptor modulator. Mol Endocrinol. 2007;21(5):1066–1081. doi: 10.1210/me.2006-0524. [DOI] [PubMed] [Google Scholar]
- 35.Marangoni E, Poupon MF. Patient-derived tumour xenografts as models for breast cancer drug development. Curr Opin Oncol. 2014;26(6):556–561. doi: 10.1097/CCO.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 36.Marangoni E, Vincent-Salomon A, Auger N, Degeorges A, Assayag F, de Cremoux P, de Plater L, et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res. 2007;13(13):3989–3998. doi: 10.1158/1078-0432.CCR-07-0078. [DOI] [PubMed] [Google Scholar]
- 37.Martin LA, Dowsett M. BCL-2: a new therapeutic target in estrogen receptor-positive breast cancer? Cancer Cell. 2013;24(1):7–9. doi: 10.1016/j.ccr.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Michna H, Schneider MR, Nishino Y, el Etreby MF. Antitumor activity of the antiprogestins ZK 98.299 and RU 38.486 in hormone dependent rat and mouse mammary tumors: mechanistic studies. Breast Cancer Res Treat. 1989;14(3):275–288. doi: 10.1007/BF01806299. [DOI] [PubMed] [Google Scholar]
- 39.Mote PA, Bartow S, Tran N, Clarke CL. Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat. 2002;72(2):163–172. doi: 10.1023/A:1014820500738. [DOI] [PubMed] [Google Scholar]
- 40.Mote PA, Gompel A, Howe C, Hilton HN, Sestak I, Cuzick J, Dowsett M, et al. Progesterone receptor A predominance is a discriminator of benefit from endocrine therapy in the ATAC trial. Breast Cancer Res Treat. 2015;151(2):309–318. doi: 10.1007/s10549-015-3397-0. [DOI] [PubMed] [Google Scholar]
- 41.Mote PA, Leary JA, Avery KA, Sandelin K, Chenevix-Trench G, Kirk JA, Clarke CL. Germ-line mutations in BRCA1 or BRCA2 in the normal breast are associated with altered expression of estrogen-responsive proteins and the predominance of progesterone receptor A. Genes Chromosom Cancer. 2004;39(3):236–248. doi: 10.1002/gcc.10321. [DOI] [PubMed] [Google Scholar]
- 42.Nemati F, Livartowski A, De Cremoux P, Bourgeois Y, Arvelo F, Pouillart P, Poupon MF. Distinctive potentiating effects of cisplatin and/or ifosfamide combined with etoposide in human small cell lung carcinoma xenografts. Clin Cancer Res. 2000;6(5):2075–2086. [PubMed] [Google Scholar]
- 43.Nickisch K, Nair HB, Kesavaram N, Das B, Garfield R, Shi SQ, Bhaskaran SS, Grimm SL, Edwards DP. Synthesis and antiprogestational properties of novel 17-fluorinated steroids. Steroids. 2013;78(9):909–919. doi: 10.1016/j.steroids.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Nishino T, Ishibashi K, Hirtreiter C, Nishino Y. Potentiation of the antitumor effect of tamoxifen by combination with the antiprogestin onapristone. J Steroid Biochem Mol Biol. 2009;116(3–5):187–190. doi: 10.1016/j.jsbmb.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Olopade OI, Adeyanju MO, Safa AR, Hagos F, Mick R, Thompson CB, Recant WM. Overexpression of BCL-x protein in primary breast cancer is associated with high tumor grade and nodal metastases. Cancer Journal from Scientific American. 1997;3(4):230–237. [PubMed] [Google Scholar]
- 46.Oue T, Fukuzawa M, Kamata S, Okada A. Immunohistochemical analysis of proliferating cell nuclear antigen expression in human neuroblastoma. J Pediatr Surg. 1995;30(4):528–532. doi: 10.1016/0022-3468(95)90123-X. [DOI] [PubMed] [Google Scholar]
- 47.Petit-Topin I, Fay M, Resche-Rigon M, Ulmann A, Gainer E, Rafestin-Oblin ME, Fagart J. Molecular determinants of the recognition of ulipristal acetate by oxo-steroid receptors. J Steroid Biochem Mol Biol. 2014;144(Pt B):427–435. doi: 10.1016/j.jsbmb.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Poole AJ, Li Y, Kim Y, Lin SC, Lee WH, Lee EY. Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science. 2006;314(5804):1467–1470. doi: 10.1126/science.1130471. [DOI] [PubMed] [Google Scholar]
- 49.Proskuryakov SY, Gabai VL. Mechanisms of tumor cell necrosis. Curr Pharm Des. 2010;16(1):56–68. doi: 10.2174/138161210789941793. [DOI] [PubMed] [Google Scholar]
- 50.Ranney MK, Ahmed IS, Potts KR, Craven RJ. Multiple pathways regulating the anti-apoptotic protein clusterin in breast cancer. Biochim Biophys Acta. 2007;1772(9):1103–1111. doi: 10.1016/j.bbadis.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277(7):5209–5218. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- 52.Rudas M, Lehnert M, Huynh A, Jakesz R, Singer C, Lax S, Schippinger W, et al. Cyclin D1 expression in breast cancer patients receiving adjuvant tamoxifen-based therapy. Clin Cancer Res. 2008;14(6):1767–1774. doi: 10.1158/1078-0432.CCR-07-4122. [DOI] [PubMed] [Google Scholar]
- 53.Schneider CC, Gibb RK, Taylor DD, Wan T, Gercel-Taylor C. Inhibition of endometrial cancer cell lines by mifepristone (RU 486) J Soc Gynecol Investig. 1998;5(6):334–338. doi: 10.1016/S1071-5576(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 54.Schreiber, V, Illuzzi G, Heberle E, Dantzer F. 2015. [From poly(ADP-ribose) discovery to PARP inhibitors in cancer therapy]. Bull Cancer. doi:10.1016/j.bulcan.2015.07.012 [DOI] [PubMed]
- 55.Wargon V, Riggio M, Giulianelli S, Sequeira GR, Rojas P, May M, Polo ML, et al. Progestin and antiprogestin responsiveness in breast cancer is driven by the PRA/PRB ratio via AIB1 or SMRT recruitment to the CCND1 and MYC promoters. Int J Cancer. 2015;136(11):2680–2692. doi: 10.1002/ijc.29304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weil MK, Chen AP. PARP inhibitor treatment in ovarian and breast cancer. Curr Probl Cancer. 2011;35(1):7–50. doi: 10.1016/j.currproblcancer.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whittle JR, Lewis MT, Lindeman GJ, Visvader JE. Patient-derived xenograft models of breast cancer and their predictive power. Breast Cancer Res. 2015;17:17. doi: 10.1186/s13058-015-0523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiehle RD, Christov K, Mehta R. Anti-progestins suppress the growth of established tumors induced by 7,12-dimethylbenz(a)anthracene: comparison between RU486 and a new 21-substituted-19-nor-progestin. Oncol Rep. 2007;18(1):167–174. [PubMed] [Google Scholar]
- 59.Wiehle R, Lantvit D, Yamada T, Christov K. CDB-4124, a progesterone receptor modulator, inhibits mammary carcinogenesis by suppressing cell proliferation and inducing apoptosis. Cancer Prev Res. 2011;4(3):414–424. doi: 10.1158/1940-6207.CAPR-10-0244. [DOI] [PubMed] [Google Scholar]
- 60.Xu Q, Takekida S, Ohara N, Chen W, Sitruk-Ware R, Johansson ED, Maruo T. Progesterone receptor modulator CDB-2914 down-regulates proliferative cell nuclear antigen and Bcl-2 protein expression and up-regulates caspase-3 and poly(adenosine 5′-diphosphate-ribose) polymerase expression in cultured human uterine leiomyoma cells. J Clin Endocrinol Metab. 2005;90(2):953–961. doi: 10.1210/jc.2004-1569. [DOI] [PubMed] [Google Scholar]
- 61.Yun BS, Seong SJ, Cha DH, Kim JY, Kim ML, Shim JY, Park JE. Changes in proliferating and apoptotic markers of leiomyoma following treatment with a selective progesterone receptor modulator or gonadotropin-releasing hormone agonist. Eur J Obstet Gynecol Reprod Biol. 2015;191:62–67. doi: 10.1016/j.ejogrb.2015.05.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 257 kb)
(DOCX 50 kb)