Change history
4/20/2020
The Editors-in-Chief are currently investigating this article [Tabassi, Z., Bagheri, S., Samimi, M. et al. Clinical and Metabolic Response to Vitamin D Supplementation in Endometrial Hyperplasia: a Randomized, Double-Blind, Placebo-Controlled Trial. HORM CANC 8, 185–195 (2017). https://doi.org/10.1007/s12672-017-0290-9] as concerns have been raised about integrity of the clinical trial reported here. There is also an ongoing investigation by the Iranian National Committee for Ethics in Biomedical Researches. Further editorial action will be taken as appropriate once the investigation into the concerns is complete and all parties have been given an opportunity to respond in full.
Abstract
There was inconsistent evidence showing that vitamin D intake may be associated with reduced cancer risk due to optimized metabolic profile and reduced oxidative stress. However, we are not aware of any study evaluating the effects of vitamin D supplementation on clinical response and metabolic status of patients with endometrial hyperplasia (EH). This research was done to evaluate the effects of vitamin D supplementation on clinical response and metabolic status of patients with EH. This randomized, double-blind, placebo-controlled trial was conducted among 60 women diagnosed with EH. EH diagnosis was made based on specific diagnostic procedures of biopsy. Participants were randomly assigned into two groups to intake either 50,000 IU vitamin D3 supplements (n = 30) or placebo (n = 30) every 2 weeks for 12 weeks. After the 12-week intervention, compared with the placebo, vitamin D supplementation increased serum-25(OH) vitamin D levels (+12.0 ± 10.4 vs. +1.9 ± 7.1 ng/mL, P < 0.001). In addition, vitamin D administration was associated with significant decreases in fasting plasma glucose (FPG) (−1.6 ± 7.0 vs. +2.1 ± 6.1 mg/dL, P = 0.03), serum insulin levels (−0.8 ± 1.9 vs. +1.1 ± 3.5 μIU/mL, P = 0.01), homeostasis model of assessment-insulin resistance (HOMA-IR) (−0.2 ± 0.6 vs. +0.3 ± 0.8, P = 0.01), and a significant increase in the quantitative insulin sensitivity check index (QUICKI) (+0.003 ± 0.01 vs. −0.01 ± 0.02, P = 0.02) compared with the placebo. Additionally, a significant decrease in serum high-sensitivity C-reactive protein (hs-CRP) (−1.9 ± 2.8 vs. −0.003 ± 2.0 μg/mL, P = 0.003) and a significant rise in plasma total antioxidant capacity (TAC) values (+62.5 ± 53.5 vs. +7.5 ± 34.1 mmol/L, P < 0.001) were observed following supplementation with vitamin D compared with the placebo. In conclusion, vitamin D3 supplementation for 12 weeks among women with EH had beneficial effects on glucose metabolism, serum hs-CRP, and plasma TAC concentrations. In addition, vitamin D may have played an indirect role in reducing complications of EH due to its effect on improved glycemic control, hs-CRP, and TAC concentrations.
Keywords: Endometrial Cancer, Fast Plasma Glucose, Total Antioxidant Capacity, Endometrial Hyperplasia, Endometrial Biopsy
Introduction
Endometrial hyperplasia (EH) is one of the most common gynecologic diseases in the world, which is defined as an increased ratio of endometrial glands to stroma greater than one to one [1]. In simple EH, the ratio is only slightly increased and the glands may be cystically dilated [1]. EH is among the most common cause of morbidity of all gynecological problems among women of childbearing age, causing 10 to 18% of women hospitalized every year [2]. It is estimated that up to 50% of women with EH may develop endometrial cancer [3]. Previous studies have shown that EH has an intimate relationship with insulin resistance and metabolic disorders [4, 5]. Moreover, increased inflammatory cytokines and parameters of oxidative stress in EH may be considered as a factor in the progression of pathology, as well as an attributed risk factor for malignancy in EH [6, 7].
Conversion of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D, the active form of vitamin D, occurs in the endometrium [8] and kidney [9]. Furthermore, endometrial tissue expresses the vitamin D receptor [10], a 1,25-dihydroxyvitamin D-activated nuclear transcription factor that regulates the production of proteins involved in cell proliferation and differentiation [11]. Therefore, these data support the hypothesis that vitamin D plays a role in the etiology of endometrial hyperplasia and cancer. However, data on the relation between vitamin D and EH are scarce and inconsistent; few studies have reported the association between vitamin D and endometrial cancer. In a study by Zeleniuch-Jacquotte et al. [12], it was observed that circulating levels of 25-hydroxyvitamin D were not associated with risk of endometrial cancer in nested case-control study based on seven cohorts. About the effects of vitamin D supplementation on metabolic status, we have previously demonstrated that beneficial effects of vitamin D supplementation on metabolic profiles in patients with gestational diabetes (GDM) [13] and diabetic foot ulcer (DFU) [14].
Vitamin D intake may reduce hyperplasia and cancer risk through modulating calcium metabolism, inhibiting cellular proliferation [15, 16] and induction of apoptosis in cancer cells through downregulation of telomerase activity [17]. We hypothesized that metabolic profiles, biomarkers of inflammation, and oxidative stress of patients with EH might be improved by vitamin D supplementation. We are not aware of any study assessing the effects of vitamin D administration on treatment, glucose metabolism, lipid concentrations, biomarkers of inflammation, and oxidative stress in patients with EH. The aim of current study was to assess the effects of vitamin D supplementation on treatment and metabolic status of patients diagnosed with EH.
Subjects and Methods
Participants
This randomized double-blind placebo-controlled clinical trial, registered in the Iranian website for registration of clinical trials (http://www.irct.ir: IRCT201606275623N86), was done among 60 premenopausal women with EH who had an endometrial biopsy in the past year and aged 35–55 years old referred to the Naghavi Clinic in Kashan, Iran, between June 2016 and October 2016. All participates provided written informed consent according to the study protocol approved by the Committee for the Protection of Human Subjects at the Kashan University of Medical Sciences (KUMS), Kashan, Iran, which complies with the Declaration of Helsinki standards for human subject research. Exclusion criteria included menopausal women, symptoms or personal history of cardiovascular disease, diabetes mellitus, fasting plasma glucose (FPG) > 126 mg/dL, the consumption of antihyperglycemic agents including metformin, triglycerides levels > 500 mg/dL, systolic blood pressure ≥ 160 mmHg, and/or diastolic blood pressure ≥ 110 mmHg, untreated thyroid disease.
Study Design
Firstly, participants were stratified according their BMI (<30 and ≥30 kg/m2) and age (<35 and ≥35 year), and then patients were randomly allocated into two groups to receive either 50,000 IU vitamin D3 supplements (n = 30) or placebo (n = 30) every 2 weeks for 12 weeks. Vitamin D supplements and its placebos were made by Zahravi Pharmaceutical Company (Tabriz, Iran) and Barij Essence Pharmaceutical Company (Kashan, Iran), respectively. Both vitamin D supplements and placebo tablets had similar packaging and patients and researchers were unaware of the content of the package until the end of study. In addition, all participants were received equal treatment protocol based on guideline. Participants were requested not to change their routine physical activity and not to take any nutritional supplements during the 12-week trial. Compliance with the consumption of supplements and placebos was determined by examining the tablet containers as well as by the measurement of serum 25-hydroxyvitamin D values with the enzyme-linked immunosorbent assay (ELISA) method. All patients completed 3-day food records and three physical activity records as metabolic equivalents (METs) in hours per day [18] at baseline, weeks 3, 6, and 9 of the intervention and end-of-trial. Daily macro- and micro-nutrient intakes were analyzed by nutritionist IV software (First Databank, San Bruno, CA).
Assessment of Anthropometric Measures
Weight and height of participants were measured in an overnight fasting status using a standard scale (Seca, Hamburg, Germany) at baseline and after the 12-week intervention. BMI was calculated as weight in kilograms divided by height in meters squared. All anthropometric measures were done by a trained midwife.
Clinical Assessment
In the current study, diagnosis of EH was determined through biopsy and pathological diagnosis at baseline and after the 12-week intervention. Endometrial biopsies were done using suction pipelles. Endometrial biopsies were fixed in 10% neutral buffered formalin, routinely processed, and paraffin embedded. Serial 5-μm sections were stained with hematoxylin and eosin and evaluated under the light microscope by a pathologist. Assessment of the pathological diagnosis was performed as blindness by a single experienced pathologist at baseline and 12 weeks after the intervention. Informed consent was taken from all participants for biopsy both baseline and after the 12-week intervention.
Biochemical Assessment
Ten milliliters fasting blood samples were collected at baseline and after the 12-week intervention at Kashan reference laboratory. Then, the samples were stored at −80 °C before analysis. Circulating levels of insulin were quantified with the use of available ELISA kit (DiaMetra, Milano, Italy) with inter- and intra-assay coefficient variances (CVs) of 3.5 to 5.1%, respectively. The homeostasis model of assessment-insulin resistance (HOMA-IR), β-cell function (HOMA-B), and the quantitative insulin sensitivity check index (QUICKI) were determined according to the suggested formulas [19]. As few studies have reported abnormal glucose metabolism, insulin resistance in patients with EH, and endometrial cancer [20, 21], we measured these variables in the current study. Enzymatic kits (Pars Azmun, Tehran, Iran) were used to quantify FPG, serum triglycerides, VLDL-, total-, LDL-, and HDL-cholesterol values. All inter- and intra-assay CVs for FPG and lipid values were less than 5%. Circulating levels of serum high-sensitivity C-reactive protein (hs-CRP) were assessed by commercial ELISA kit (LDN, Nordhorn, Germany) with inter- and intra-assay CVs of 4.6 to 6.5%, respectively. The plasma nitric oxide (NO) concentrations using Griess method [22], total antioxidant capacity (TAC) by the method of ferric reducing antioxidant power developed by Benzie and Strain [23], total glutathione (GSH) using the method of Beutler et al. [24], and malondialdehyde (MDA) values by the thiobarbituric acid reactive substances spectrophotometric test [25] were determined. TAC considers the occurrence of a synergic function of all antioxidants present in organic fluids, providing an integrative system between such compounds [26]. Thus, TAC has a higher predictive capacity and biological relevance when compared to the activity of a single antioxidant. In addition, MDA is the breakdown product of the most important chain reactions leading to the oxidation of polyunsaturated fatty acids and therefore serves as a reliable oxidant marker of oxidative stress-mediated lipid peroxidation [27]. As the existence of atypical cells in a hyperplasia induces significant modifications of lipid peroxidation especially MDA [7], we quantified MDA in this study. All inter- and intra-assay CVs for NO, TAC, GSH, and MDA concentrations were less than 5%.
Randomization
Randomization assignment was performed using computer-generated random numbers. Randomization and allocation were concealed from the researchers and patients until the final analyses were completed. The randomized allocation sequence, enrolling patients and allocating them to interventions were done by a trained midwife at the gynecology clinic.
Statistical Methods
To evaluate whether the study variables were normally distributed or not, we used the Kolmogrov-Smirnov test. The analyses were done in all randomized subjects according to the intention-to-treat (ITT) principle. Missing values were treated based on last-observation-carried-forward (LOCF) method [28]. LOCF ignores whether the participant’s condition was improving or deteriorating at the time of dropout but instead freezes outcomes at the value observed before dropout (i.e., last observation). To detect differences in anthropometric measures as well as in macro- and micro-nutrient intakes between the two groups, we applied Student’s t test to independent samples. The Pearson Chi-square test was used to compare EH regression. Paired-samples t test was used to detect within-group differences. To determine the effects of vitamin D supplementation on glucose metabolism, lipid fractions, biomarkers of inflammation, and oxidative stress, we used one-way repeated measures analysis of variance. In this analysis, the treatment (vitamin D vs. placebo group) was regarded as a between-subject factor and time with two time-points (baseline and after the 12-intervention) was considered as within-subject factor. Adjustment for changes in baseline values of biochemical values, age, and baseline BMI was performed by analysis of covariance (ANCOVA) using general linear models. The P value of <0.05 were considered statistically significant. All statistical analyses used the Statistical Package for Social Science version 18 (SPSS Inc., Chicago, IL, USA).
Results
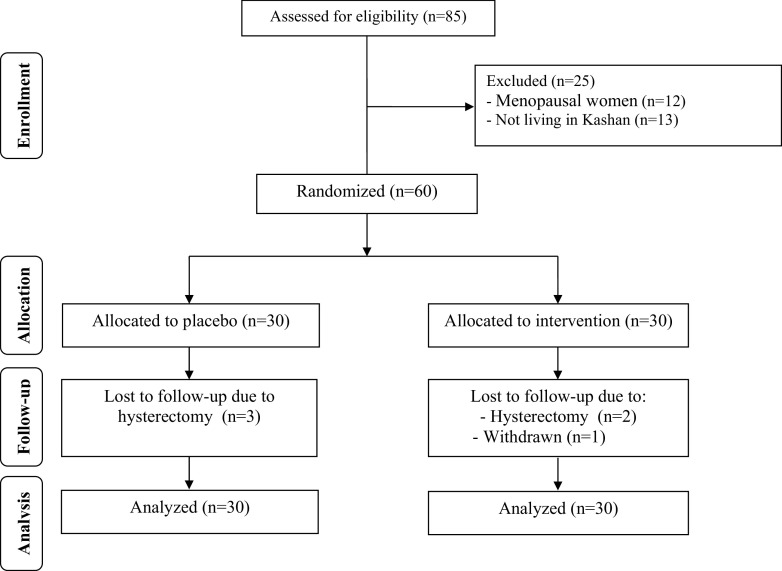
As demonstrated in the study flow diagram (Fig. 1), during the intervention phase of the study, 3 patients were excluded from the vitamin D group [hysterectomy due to persistent hyperplasia (n = 1) and secretory endometrium (n = 1), and withdrawn due to personal reasons (n = 1)] and 3 [hysterectomy due to persistent hyperplasia (n = 2) and proliferative endometrium (n = 1)] from the placebo group. Hysterectomy was done due to abnormal uterine bleeding (AUB) resistant to medical treatment. However, as the analysis was done based on ITT principle, all 60 patients were included in the final analysis.
Fig. 1.
Summary of patient flow diagram
Mean age and height, baseline weight and BMI, as well as their means before and after the 12-week intervention of participants were not statistically different between vitamin D and placebo groups (Table 1). In addition, supplementation with vitamin D for 12 weeks did not affect EH regression.
Table 1.
General characteristics of study participants
| Placebo group (n = 30) | Vitamin D group (n = 30) | P a | |
|---|---|---|---|
| Age (years) | 43.2 ± 4.4 | 41.5 ± 6.3 | 0.24 |
| Height (cm) | 161.5 ± 8.1 | 158.9 ± 5.1 | 0.13 |
| Weight at study baseline (kg) | 77.8 ± 11.6 | 74.7 ± 12.5 | 0.31 |
| Weight at end-of-trial (kg) | 77.9 ± 11.5 | 75.0 ± 13.0 | 0.37 |
| Weight change (kg) | 0.1 ± 0.9 | 0.3 ± 1.5 | 0.32 |
| BMI at study baseline (kg/m2) | 29.8 ± 4.1 | 29.7 ± 5.1 | 0.88 |
| BMI at end-of-trial (kg/m2) | 29.9 ± 4.1 | 29.8 ± 5.4 | 0.96 |
| BMI change (kg/m2) | 0.1 ± 0.3 | 0.1 ± 0.5 | 0.31 |
| EH regression (%) | 21 (77.8) | 24 (88.9) | 0.27† |
Data are means ± SDs.
EH endometrial hyperplasia
†Obtained from Pearson Chi-square test
aObtained from independent t test
Based on the 3-day dietary records obtained throughout the intervention, no significant differences were observed between the two groups in macro- and micronutrients (Data not shown).
After the 12-week intervention, compared with the placebo, vitamin D supplementation increased serum-25(OH) vitamin D levels (+12.0 ± 10.4 vs. +1.9 ± 7.1 ng/mL, P < 0.001) (Fig. 2). In addition, vitamin D administration was associated with significant decreases in FPG (−1.6 ± 7.0 vs. +2.1 ± 6.1 mg/dL, P = 0.03), serum insulin levels (−0.8 ± 1.9 vs. +1.1 ± 3.5 μIU/mL, P = 0.01), HOMA-IR (−0.2 ± 0.6 vs. +0.3 ± 0.8, P = 0.01), and a significant increase in QUICKI (+0.003 ± 0.01 vs. −0.01 ± 0.02, P = 0.02) compared with the placebo. Additionally, a significant decrease in serum hs-CRP (−1.9 ± 2.8 vs. −0.003 ± 2.0 μg/mL, P = 0.003) and a significant rise in plasma TAC values (+62.5 ± 53.5 vs. +7.5 ± 34.1 mmol/L, P < 0.001) were observed following supplementation with vitamin D compared with the placebo (Figs. 3, 4, 5, 6, 7, and 8).
Fig. 2.
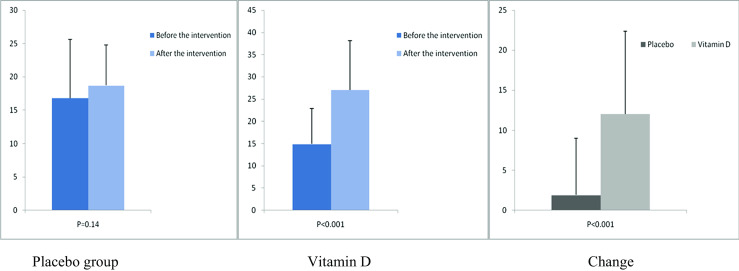
Before, after, and changes in (means ± standard deviation) of serum vitamin D levels in women with endometrial hyperplasia that received either vitamin D supplements or placebo
Fig. 3.
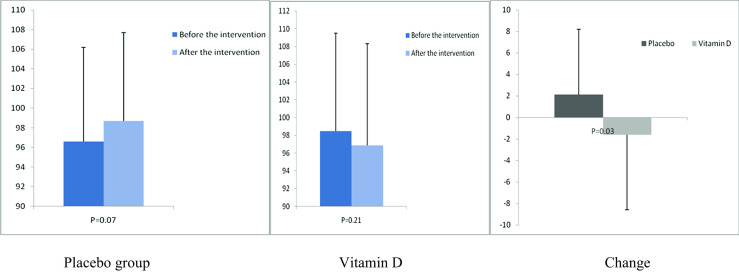
Before, after, and changes in (means ± standard deviation) of fasting plasma glucose in women with endometrial hyperplasia that received either vitamin D supplements or placebo
Fig. 4.
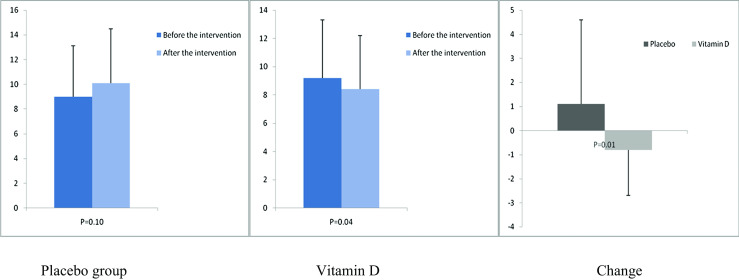
Before, after, and changes in (means ± standard deviation) of serum insulin levels in women with endometrial hyperplasia that received either vitamin D supplements or placebo
Fig. 5.
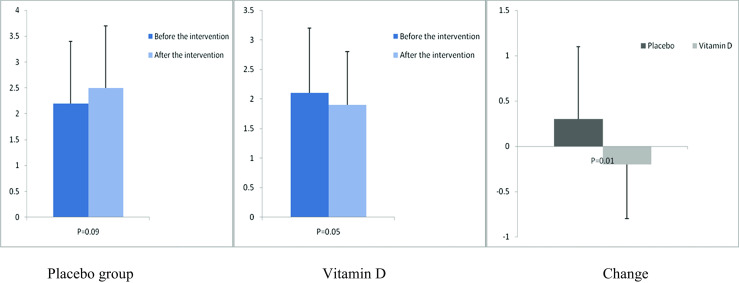
Before, after, and changes in (means ± standard deviation) of homeostasis model of assessment-estimated insulin resistance in women with endometrial hyperplasia that received either vitamin D supplements or placebo
Fig. 6.
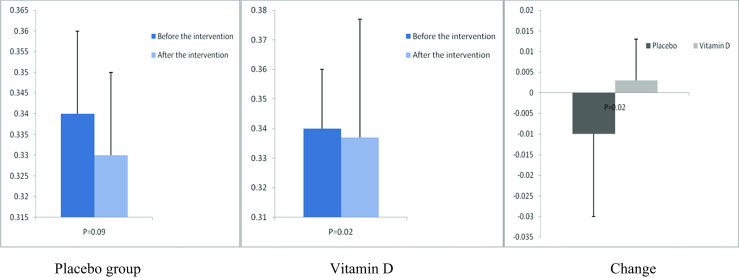
Before, after, and changes in (means ± standard deviation) of quantitative insulin sensitivity check index in women with endometrial hyperplasia that received either vitamin D supplements or placebo
Fig. 7.
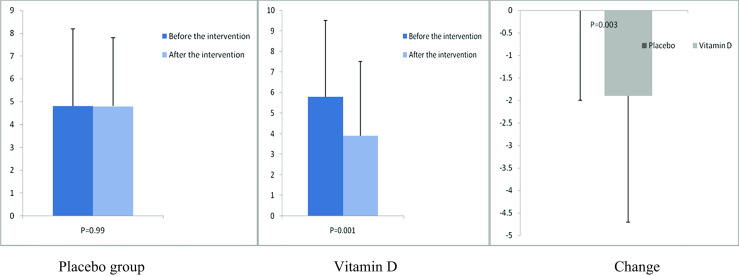
Before, after, and changes in (means ±standard deviation) of serum high-sensitivity C-reactive protein in women with endometrial hyperplasia that received either vitamin D supplements or placebo
Fig. 8.
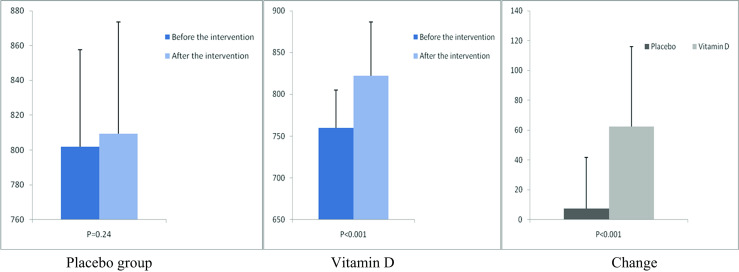
Before, after, and changes in (means ± standard deviation) of plasma total antioxidant capacity in women with endometrial hyperplasia that received either vitamin D supplements or placebo
We did not observe any significant effect of supplementation with vitamin D on lipid profiles, other biomarkers of inflammation and oxidative (Table 2).
Table 2.
Metabolic profiles, biomarkers of inflammation, and oxidative stress at baseline and after the 12-week intervention in women with endometrial hyperplasia that received either vitamin D supplements or placebo
| Placebo group (n = 30) | Vitamin D group (n = 30) | P b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ideal levels | Wk0 | Wk12 | Change | P a | Wk0 | Wk12 | Change | P a | |||
| HOMA-B | ND | 30.2 ± 12.9 | 33.6 ± 14.5 | 3.3 ± 13.4 | 0.18 | 29.7 ± 17.6 | 27.9 ± 17.5 | −1.8 ± 6.9 | 0.16 | 0.06 | |
| Triglycerides (mg/dL) | <150 | 128.6 ± 79.9 | 135.0 ± 74.5 | 6.4 ± 22.3 | 0.12 | 129.8 ± 60.2 | 137.2 ± 67.0 | 7.4 ± 66.0 | 0.54 | 0.94 | |
| VLDL-cholesterol (mg/dL) | <30 | 25.7 ± 16.0 | 27.0 ± 14.9 | 1.3 ± 4.5 | 0.12 | 26.0 ± 12.0 | 27.4 ± 13.4 | 1.4 ± 13.2 | 0.54 | 0.94 | |
| Total cholesterol (mg/dL) | <200 | 186.4 ± 35.2 | 192.2 ± 36.5 | 5.8 ± 26.3 | 0.23 | 188.0 ± 36.8 | 192.2 ± 34.9 | 4.2 ± 21.3 | 0.28 | 0.80 | |
| LDL-cholesterol (mg/dL) | <130 | 115.3 ± 34.4 | 118.2 ± 34.2 | 2.9 ± 25.5 | 0.53 | 113.9 ± 37.3 | 115.2 ± 34.2 | 1.2 ± 20.9 | 0.76 | 0.77 | |
| HDL-cholesterol (mg/dL) | >50 | 45.4 ± 8.9 | 46.9 ± 11.3 | 1.5 ± 5.9 | 0.16 | 48.1 ± 10.0 | 49.6 ± 11.1 | 1.5 ± 5.0 | 0.11 | 0.96 | |
| NO (μmol/L) | ND | 41.0 ± 4.7 | 41.5 ± 4.5 | 0.5 ± 3.7 | 0.50 | 39.4 ± 3.2 | 40.9 ± 3.4 | 1.5 ± 3.9 | 0.04 | 0.31 | |
| GSH (μmol/L) | ND | 543.4 ± 61.4 | 547.3 ± 65.2 | 3.9 ± 74.3 | 0.77 | 561.5 ± 48.1 | 578.5 ± 73.0 | 26.0 ± 87.7 | 0.11 | 0.29 | |
| MDA (μmol/L) | ND | 2.4 ± 0.3 | 2.3 ± 0.3 | −0.1 ± 0.3 | 0.71 | 2.3 ± 0.3 | 2.2 ± 0.2 | −0.1 ± 0.3 | 0.56 | 0.89 | |
Data are means ± SDs
aObtained from paired-samples t tests
bObtained from repeated measures ANOVA test
GSH total glutathione, HOMA-B homeostasis model of assessment-estimated b cell function, MDA malondialdehyde, NO nitric oxide, ND not determined
There was a significant difference in the baseline levels of TAC between the two groups. Therefore, we adjusted the analysis for baseline values of biochemical parameters, age and baseline BMI. After controlling for these potential confounders, the difference in changes in plasma GSH (P = 0.04) became statistically significant (Table 3). Other findings did not alter significantly after this adjustment.
Table 3.
Adjusted changes in metabolic variables in women with endometrial hyperplasia who were or were not supplemented with vitamin D for 12 weeks
| Placebo group (n = 30) | Vitamin D group (n = 30) | P a | |
|---|---|---|---|
| Vitamin D (ng/mL) | 2.3 ± 1.5 | 11.6 ± 1.5 | <0.001 |
| FPG (mg/dL) | 2.0 ± 1.2 | -1.5 ± 1.2 | 0.04 |
| Insulin (μIU/mL) | 1.1 ± 0.5 | -0.8 ± 0.5 | 0.006 |
| HOMA-IR | 0.3 ± 0.1 | -0.2 ± 0.1 | 0.006 |
| HOMA-B | 3.4 ± 1.9 | -1.8 ± 1.9 | 0.06 |
| QUICKI | -0.007 ± 0.003 | 0.003 ± 0.003 | 0.009 |
| Triglycerides (mg/dL) | 4.8 ± 8.5 | 9.0 ± 8.5 | 0.72 |
| VLDL-cholesterol (mg/dL) | 1.0 ± 1.7 | 1.8 ± 1.7 | 0.72 |
| Total cholesterol (mg/dL) | 5.5 ± 4.2 | 4.4 ± 4.2 | 0.85 |
| LDL-cholesterol (mg/dL) | 3.3 ± 4.0 | 0.9 ± 4.0 | 0.67 |
| HDL-cholesterol (mg/dL) | 1.6 ± 1.0 | 1.4 ± 1.0 | 0.89 |
| hs-CRP (μg/mL) | -0.2 ± 0.4 | -1.7 ± 0.4 | 0.007 |
| NO (μmol/L) | 0.8 ± 0.6 | 1.1 ± 0.6 | 0.73 |
| TAC (mmol/L) | 10.0 ± 8.7 | 59.9 ± 8.7 | <0.001 |
| GSH (μmol/L) | -3.7 ± 12.8 | 33.6 ± 12.8 | 0.04 |
| MDA (μmol/L) | 0.02 ± 0.04 | -0.07 ± 0.04 | 0.12 |
All values are means ± SEs. Values are adjusted for baseline values, age, and BMI at baseline
FPG fasting plasma glucose, GSH total glutathione, HOMA-IR homeostasis model of assessment-estimated insulin resistance, HOMA-B homeostasis model of assessment-estimated b cell function, hs-CRP high-sensitivity C-reactive protein, MDA malondialdehyde, NO nitric oxide, QUICKI quantitative insulin sensitivity check index, TAC total antioxidant capacity
aObtained from ANCOVA
Discussion
To our knowledge, the effects of vitamin D supplementation on regression and metabolic status of EH have not been evaluated previously. For the first time, we have demonstrated that in patients with EH taking one dose of 50,000 IU vitamin D supplement every 2 weeks for 12 weeks compared with the placebo resulted in (1) decreased FPG and other markers of insulin metabolism and (2) improved few biomarkers of inflammation and oxidative stress.
Patients with EH are susceptible to endometrial cancer in a linear fashion [3]. Our study had shown that vitamin D supplementation among patients with EH did not affect EH regression. Although data on the association between vitamin D and EH are limited, a previous pooled study using circulating 25(OH) D levels measured at a single time point found no association with endometrial cancer incidence [12]. In addition, in 3 case-control studies that have evaluated the relationship between dietary intake of vitamin D and risk of endometrial cancer [29–31] concluded that the evidence available did not support an association. In a systematic review of the literature conducted by McCullough et al. [32], no relation between endometrial cancer in the ranges of dietary vitamin D was also shown.
The current study demonstrated that taking vitamin D in women with EH for 12 weeks resulted in significant reductions in FPG, serum insulin values, and HOMA-IR, and a significant rise in QUICKI, but had no effects on HOMA-B and lipid concentrations. We have previously shown that 50,000 IU vitamin D supplementation every 2 weeks for 12 weeks among patients with DFU had beneficial effects on glucose homeostasis parameters, total-, LDL-, and total-/HDL-cholesterol, but did not influence other lipid concentration [14]. Likewise, vitamin D supplementation at dosage of 420 IU for 1 year effectively improved fasting glucose values and insulin resistance in healthy Japanese adults [33]. Similarly, the administration of 50,000 IU vitamin D supplements every 2 weeks for 12 weeks among pregnant women at risk of pre-eclampsia led to a significant difference in markers of insulin metabolism [34]. In a meta-analysis study conducted by Wang et al. [35], no significant effects following supplementation with vitamin D on total-, HDL-cholesterol, and triglycerides were observed. However, some researchers did not report favorable effects of vitamin D supplementation on glucose metabolism. For instance, supplementation with 50,000 IU vitamin D weekly for 4 months among subjects with metabolic syndrome improved vitamin D status, but did not affect glucose homeostasis parameters [36]. Moreover, the same findings were observed following supplementation with vitamin D among patients with type 2 diabetes mellitus (T2DM) after 24 weeks [37] and after 6 months [38]. Previous studies have demonstrated that high glucose levels and insulin resistance have potential modifying effects on endometrial cancer development [39, 40]. Hyperinsulinemia may act directly on endometrial tissue as a mitogenic and anti-apoptotic growth factor [41]. In addition, higher prevalence of hypertension in subjects with endometrial carcinoma may be related to hyperinsulinemia and insulin resistance [42]. Increased metabolic profiles stimulate cell growth and cell division [43]. Goldman et al. [39] demonstrated that high glucose concentrations could increase cancer risk by acting as an energy source for the proliferation of tumor cells generating free radicals, or causing oxidative damage to DNA and DNA repair enzymes. Therefore, vitamin D due to their beneficial effects on markers of insulin metabolism may be useful to decrease metabolic disorders in patients with EH. Nonetheless, we believe that future studies are needed to confirm our findings. The anti-inflammatory effects of vitamin D [44], decreased parathyroid hormone (PTH) concentrations [33], and increased expression of the insulin receptor and/or proteins of the insulin-signaling cascade by vitamin D [45] may result in improved insulin sensitivity and decreased insulin resistance of vitamin D. The absence of significant effect of vitamin D administration on lipid fractions in this study may be due to various dosages of vitamin D supplements and characteristics of the participants.
Our study shown that taking vitamin D in women with EH for 12 weeks resulted in a significant decrease in serum hs-CRP and a significant increase in plasma TAC concentrations compared with the placebo, but unchanged other biomarkers of inflammation and oxidative stress. In line with our study, a meta-analysis study conducted by Chen et al. [46] has shown that vitamin D supplementation was related to decreased levels of hs-CRP. However, the results from clinical trials on vitamin D supplementation and its effects on CRP concentrations have been inconsistent. We have previously shown that intake of 400 IU vitamin D supplements per day in healthy pregnant women for 9 weeks was linked to significant decreases in serum hs-CRP, and significant increases in plasma TAC and GSH levels [47]. However, a 6-week supplementation with 1000 mg calcium per day and 50,000 IU vitamin D3 every 3 weeks did not change hs-CRP and NO concentrations among patients with GDM for 6 weeks [48]. Even in another study, hs-CRP levels were significantly increased among overweight and obese subjects who received 40,000 IU vitamin D per week or 20,000 IU vitamin D per week for 1 year [49]. Supplementation with 50,000 IU vitamin D3 every 2 weeks for 16 weeks among adult patients with non-alcoholic fatty liver disease led to amelioration in MDA values, but unchanged TAC concentrations [50]. Increased inflammatory factors can result in disorders in the regulation of cell division, leading to excessive mitosis, decreased apoptosis, mutations, and therefore the initiation and promotion of neoplastic transformation [51]. In addition, EH may be able to activate compensatory mechanisms of oxidative stress [7]. In this sense, one of the possible mechanisms that hyperplasia can employ to reduce oxidative stress could be by means of an increase in antioxidant activity. Increased biomarkers of oxidative stress are capable of changing the expression of proteins directly related to apoptosis [52]. Furthermore, reactive oxygen species (ROS) are capable of inhibiting mismatches or DNA repair mechanisms [53]. The relevance of our findings, in particular with regard to the possible decrease in serum hs-CRP and increase in TAC levels, may lie in the fact that malignant tissues have a need to reduce oxidative stress to permit tumor promotion and progression and thus acquire the ability to metastasize [54]. Moreover, the increase in TAC levels, whether due to the induction of antioxidant enzymes (catalase, glutathione, or superoxide dismutase) or for any other reason, seems to transfer cellular resistance to the ROS [7], which might be a reflection of tumor adaptation mechanisms. Less production of PTH [55] induced superoxide dismutase activity [56] and upregulation of antioxidant systems including glutathione peroxidase via its nuclear receptors [57] by vitamin D might explain anti-inflammatory and antioxidant effects of vitamin D.
Our study had some limitations. Sample size and duration of intervention were low in our study. Furthermore, due to limited funding, we did not examine the effect of supplementation on signaling pathway related to inflammation and oxidative stress. We did not evaluate hemoglobin A1C and serum calcium levels in all patients, and estradiol levels in post-menopausal patients. In addition, we did not stratify participants according their vitamin D, metabolic profiles, biomarkers of inflammation, and oxidative stress at baseline. Therefore, this should be taken into considered in the interpretation of our findings.
In conclusion, vitamin D3 supplementation for 12 weeks among women with EH had beneficial effects on glucose metabolism, serum hs-CRP, and plasma TAC concentrations, but did not affect EH regression, lipid profiles, other biomarkers of inflammation, and oxidative. In addition, vitamin D may have played an indirect role in reducing complications of EH due to its effect on improved glycemic control, hs-CRP, and TAC concentrations.
Acknowledgement
The authors would like to thank the staff of Naghavi Clinic (Kashan, Iran) for their assistance in this project and all women who were participated in this study.
Authors’ Contributions
ZA contributed in conception, design, statistical analysis, and drafting of the manuscript. ZT, SB, MS, HGh, MCh, and FB contributed in conception, data collection, and manuscript drafting. All authors approved the final version for submission.
Compliance with Ethical Standards
Funding
The current research was supported by a grant from the Vice-chancellor for Research, KUMS, and Iran.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Clinical Trial Registration Number
www.irct.ir: IRCT201606275623N86.
Guarantor
Z.A. is the guarantor of this work.
References
- 1.Montgomery BE, Daum GS, Dunton CJ. Endometrial hyperplasia: a review. Obstet Gynecol Surv. 2004;59:368–378. doi: 10.1097/00006254-200405000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Benyuk V, Kurochka V, Vynyarskyi Y, Goncharenko V. Diagnostic algorithm endometrial pathology using hysteroscopy in reproductive age women. Women Health (ZdorovyaZhinky) 2009;6:54–56. [Google Scholar]
- 3.Mittal K, Sebenik M, Irwin C, Yan Z, Popiolek D, Curtin J, Palazzo J. Presence of endometrial adenocarcinoma in situ in complex atypical endometrial hyperplasia is associated with increased incidence of endometrial carcinoma in subsequent hysterectomy. Mod Pathol. 2009;22:37–42. doi: 10.1038/modpathol.2008.138. [DOI] [PubMed] [Google Scholar]
- 4.Shan W, Wang C, Zhang Z, Gu C, Ning C, Luo X, Zhou Q, Chen X. Conservative therapy with metformin plus megestrol acetate for endometrial atypical hyperplasia. J Gynecol Oncol. 2014;25:214–220. doi: 10.3802/jgo.2014.25.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Guo YR, Lin JF, Feng Y, Billig H, Shao R. Combination of Diane-35 and metformin to treat early endometrial carcinoma in PCOS women with insulin resistance. J Cancer. 2014;5:173–181. doi: 10.7150/jca.8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubyshkin AV, Aliev LL, Fomochkina II, Kovalenko YP, Litvinova SV, Filonenko TG, Lomakin NV, Kubyshkin VA, Karapetian OV. Endometrial hyperplasia-related inflammation: its role in the development and progression of endometrial hyperplasia. Inflamm Res. 2016;65:785–794. doi: 10.1007/s00011-016-0960-z. [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Zubeldia MA, Bazo AP, Gabarre JJ, Nogales AG, Palomino JC. Oxidative stress in endometrial hyperplasia. Menopause. 2008;15:363–368. doi: 10.1097/gme.0b013e318093e646. [DOI] [PubMed] [Google Scholar]
- 8.Becker S, Cordes T, Diesing D, Diedrich K, Friedrich M. Expression of 25 hydroxyvitamin D3-1alpha-hydroxylase in human endometrial tissue. J Steroid Biochem Mol Biol. 2007;103:771–775. doi: 10.1016/j.jsbmb.2006.12.075. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 10.Vienonen A, Miettinen S, Blauer M, Martikainen PM, Tomas E, Heinonen PK, Ylikomi T. Expression of nuclear receptors and cofactors in human endometrium and myometrium. J Soc Gynecol Investig. 2004;11:104–112. doi: 10.1016/j.jsgi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Uitterlinden A, Fang Y, Van Meurs J, Pols H. Genetic vitamin D receptor polymorphisms and risk of disease. Vitamin D. 2005;2:1121–1157. [Google Scholar]
- 12.Zeleniuch-Jacquotte A, Gallicchio L, Hartmuller V, Helzlsouer KJ, McCullough ML, Setiawan VW, Shu XO, Weinstein SJ, Weiss JM, Arslan AA, De Vivo I, Gao YT, Hayes RB, Henderson BE, Horst RL, Koenig KL, Patel AV, Purdue MP, Snyder K, Steplowski E, Yu K, Zheng W, Hankinson SE. Circulating 25-hydroxyvitamin D and risk of endometrial cancer: cohort consortium vitamin D pooling project of rarer cancers. Am J Epidemiol. 2010;172:36–46. doi: 10.1093/aje/kwq114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asemi Z, Hashemi T, Karamali M, Samimi M, Esmaillzadeh A. Effects of vitamin D supplementation on glucose metabolism, lipid concentrations, inflammation, and oxidative stress in gestational diabetes: a double-blind randomized controlled clinical trial. Am J Clin Nutr. 2013;98:1425–1432. doi: 10.3945/ajcn.113.072785. [DOI] [PubMed] [Google Scholar]
- 14.Razzaghi R, Pourbagheri H, Momen-Heravi M, Bahmani F, Shadi J, Soleimani Z, Asemi Z. The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. J Diabetes Complications doi. 2016 doi: 10.1016/j.jdiacomp.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Miettinen S, Ahonen MH, Lou YR, Manninen T, Tuohimaa P, Syvala H, Ylikomi T. Role of 24-hydroxylase in vitamin D3 growth response of OVCAR-3 ovarian cancer cells. Int J Cancer. 2004;108:367–373. doi: 10.1002/ijc.11520. [DOI] [PubMed] [Google Scholar]
- 16.Ziegler RG, Jones CJ, Brinton LA, Norman SA, Mallin K, Levine RS, Lehman HF, Hamman RF, Trumble AC, Rosenthal JF. Diet and the risk of in situ cervical cancer among white women in the United States. Cancer Causes Control. 1991;2:17–29. doi: 10.1007/BF00052357. [DOI] [PubMed] [Google Scholar]
- 17.Jiang F, Bao J, Li P, Nicosia SV, Bai W. Induction of ovarian cancer cell apoptosis by 1,25-dihydroxyvitamin D3 through the down-regulation of telomerase. J Biol Chem. 2004;279:53213–53221. doi: 10.1074/jbc.M410395200. [DOI] [PubMed] [Google Scholar]
- 18.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 19.Pisprasert V, Ingram KH, Lopez-Davila MF, Munoz AJ, Garvey WT. Limitations in the use of indices using glucose and insulin levels to predict insulin sensitivity: impact of race and gender and superiority of the indices derived from oral glucose tolerance test in African Americans. Diabetes Care. 2013;36:845–853. doi: 10.2337/dc12-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kacalska-Janssen O, Rajtar-Ciosek A, Zmaczynski A, Wyroba J, Milewicz T, Krzyczkowska-Sendrakowska M, Krzysiek J. Markers of insulin resistance in perimenopausal women with endometrial pathology. Ginekol Pol. 2013;84:922–929. doi: 10.17772/gp/1661. [DOI] [PubMed] [Google Scholar]
- 21.Mitsuhashi A, Uehara T, Hanawa S, Shozu M. Prospective evaluation of abnormal glucose metabolism and insulin resistance in patients with atypical endometrial hyperplasia and endometrial cancer. Support Care Cancer doi. 2016 doi: 10.1007/s00520-016-3554-y. [DOI] [PubMed] [Google Scholar]
- 22.Tatsch E, Bochi GV, Pereira Rda S, Kober H, Agertt VA, de Campos MM, Gomes P, Duarte MM, Moresco RN. A simple and inexpensive automated technique for measurement of serum nitrite/nitrate. Clin Biochem. 2011;44:348–350. doi: 10.1016/j.clinbiochem.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 24.Beutler E, Gelbart T. Plasma glutathione in health and in patients with malignant disease. J Lab Clin Med. 1985;105:581–584. [PubMed] [Google Scholar]
- 25.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 26.Serafini M, Del Rio D. Understanding the association between dietary antioxidants, redox status and disease: is the total antioxidant capacity the right tool? Redox Rep. 2004;9:145–152. doi: 10.1179/135100004225004814. [DOI] [PubMed] [Google Scholar]
- 27.Uzar E, Koyuncuoglu HR, Uz E, Yilmaz HR, Kutluhan S, Kilbas S, Gultekin F. The activities of antioxidant enzymes and the level of malondialdehyde in cerebellum of rats subjected to methotrexate: protective effect of caffeic acid phenethyl ester. Mol Cell Biochem. 2006;291:63–68. doi: 10.1007/s11010-006-9196-5. [DOI] [PubMed] [Google Scholar]
- 28.Lachin JM. Fallacies of last observation carried forward analyses. Clin Trials. 2016;13:161–168. doi: 10.1177/1740774515602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbone F, Austin H, Partridge EE. Diet and endometrial cancer: a case-control study. Am J Epidemiol. 1993;137:393–403. doi: 10.1093/oxfordjournals.aje.a116687. [DOI] [PubMed] [Google Scholar]
- 30.Negri E, La Vecchia C, Franceschi S, Levi F, Parazzini F. Intake of selected micronutrients and the risk of endometrial carcinoma. Cancer. 1996;77:917–923. doi: 10.1002/(sici)1097-0142(19960301)77:5<917::aid-cncr17>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Salazar-Martinez E, Lazcano-Ponce E, Sanchez-Zamorano LM, Gonzalez-Lira G, Escudero DELRP, Hernandez-Avila M. Dietary factors and endometrial cancer risk. Results of a case-control study in Mexico. Int J Gynecol Cancer. 2005;15:938–945. doi: 10.1111/j.1525-1438.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- 32.McCullough ML, Bandera EV, Moore DF, Kushi LH. Vitamin D and calcium intake in relation to risk of endometrial cancer: a systematic review of the literature. Prev Med. 2008;46:298–302. doi: 10.1016/j.ypmed.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun X, Cao ZB, Tanisawa K, Ito T, Oshima S, Higuchi M. Vitamin D supplementation reduces insulin resistance in Japanese adults: a secondary analysis of a double-blind, randomized, placebo-controlled trial. Nutr Res. 2016;36:1121–1129. doi: 10.1016/j.nutres.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Karamali M, Beihaghi E, Mohammadi AA, Asemi Z. Effects of high-dose vitamin D supplementation on metabolic status and pregnancy outcomes in pregnant women at risk for pre-eclampsia. Horm Metab Res. 2015;47:867–872. doi: 10.1055/s-0035-1548835. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Xia N, Yang Y, Peng DQ. Influence of vitamin D supplementation on plasma lipid profiles: a meta-analysis of randomized controlled trials. Lipids Health Dis. 2012;11:42. doi: 10.1186/1476-511X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salekzamani S, Mehralizadeh H, Ghezel A, Salekzamani Y, Jafarabadi MA, Bavil AS, Gargari BP. Effect of high-dose vitamin D supplementation on cardiometabolic risk factors in subjects with metabolic syndrome: a randomized controlled double-blind clinical trial. J Endocrinol Investig. 2016;39:1303–1313. doi: 10.1007/s40618-016-0507-8. [DOI] [PubMed] [Google Scholar]
- 37.Ryu OH, Chung W, Lee S, Hong KS, Choi MG, Yoo HJ. The effect of high-dose vitamin D supplementation on insulin resistance and arterial stiffness in patients with type 2 diabetes. Korean J Intern Med. 2014;29:620–629. doi: 10.3904/kjim.2014.29.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strobel F, Reusch J, Penna-Martinez M, Ramos-Lopez E, Klahold E, Klepzig C, Wehrle J, Kahles H, Badenhoop K. Effect of a randomised controlled vitamin D trial on insulin resistance and glucose metabolism in patients with type 2 diabetes mellitus. Horm Metab Res. 2014;46:54–58. doi: 10.1055/s-0033-1358453. [DOI] [PubMed] [Google Scholar]
- 39.Goldman NA, Katz EB, Glenn AS, Weldon RH, Jones JG, Lynch U, Fezzari MJ, Runowicz CD, Goldberg GL, Charron MJ. GLUT1 and GLUT8 in endometrium and endometrial adenocarcinoma. Mod Pathol. 2006;19:1429–1436. doi: 10.1038/modpathol.3800656. [DOI] [PubMed] [Google Scholar]
- 40.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 41.Cust AE, Kaaks R, Friedenreich C, Bonnet F, Laville M, Tjønneland A, Olsen A, Overvad K, Jakobsen MU, Chajès V, Clavel-Chapelon F, Boutron-Ruault MC, Linseisen J, Lukanova A, Boeing H, Pischon T, Trichopoulou A, Christina B, Trichopoulos D, Palli D, Berrino F, Panico S, Tumino R, Sacerdote C, Gram IT, Lund E, Quirós JR, Travier N, Martínez-García C, Larrañaga N, Chirlaque MD, Ardanaz E, Berglund G, Lundin E, Bueno-de-Mesquita HB, van Duijnhoven FJ, Peeters PH, Bingham S, Khaw KT, Allen N, Key T, Ferrari P, Rinaldi S, Slimani N, Riboli E. Metabolic syndrome, plasma lipid, lipoprotein and glucose levels, and endometrial cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Endocr Relat Cancer. 2007;14:755–767. doi: 10.1677/ERC-07-0132. [DOI] [PubMed] [Google Scholar]
- 42.Rosato V, Zucchetto A, Bosetti C, Dal Maso L, Montella M, Pelucchi C, Negri E, Franceschi S, La Vecchia C. Metabolic syndrome and endometrial cancer risk. Ann Oncol. 2011;22:884–889. doi: 10.1093/annonc/mdq464. [DOI] [PubMed] [Google Scholar]
- 43.Faulds MH, Dahlman-Wright K. Metabolic diseases and cancer risk. Curr Opin Oncol. 2012;24:58–61. doi: 10.1097/CCO.0b013e32834e0582. [DOI] [PubMed] [Google Scholar]
- 44.Al-Sofiani ME, Jammah A, Racz M, Khawaja RA, Hasanato R, El-Fawal HA, Mousa SA, Mason DL. Effect of vitamin D supplementation on glucose control and inflammatory response in type II diabetes: a double blind, randomized clinical trial. Int J Endocrinol Metab. 2015;13:e22604. doi: 10.5812/ijem.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient—a randomised, placebo-controlled trial. Br J Nutr. 2010;103:549–555. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 46.Chen N, Wan Z, Han SF, Li BY, Zhang ZL, Qin LQ. Effect of vitamin D supplementation on the level of circulating high-sensitivity C-reactive protein: a meta-analysis of randomized controlled trials. Nutrients. 2014;6:2206–2216. doi: 10.3390/nu6062206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asemi Z, Samimi M, Tabassi Z, Shakeri H, Esmaillzadeh A. Vitamin D supplementation affects serum high-sensitivity C-reactive protein, insulin resistance, and biomarkers of oxidative stress in pregnant women. J Nutr. 2013;143:1432–1438. doi: 10.3945/jn.113.177550. [DOI] [PubMed] [Google Scholar]
- 48.Asemi Z, Karamali M, Esmaillzadeh A. Effects of calcium-vitamin D co-supplementation on glycaemic control, inflammation and oxidative stress in gestational diabetes: a randomised placebo-controlled trial. Diabetologia. 2014;57:1798–1806. doi: 10.1007/s00125-014-3293-x. [DOI] [PubMed] [Google Scholar]
- 49.Beilfuss J, Berg V, Sneve M, Jorde R, Kamycheva E. Effects of a 1-year supplementation with cholecalciferol on interleukin-6, tumor necrosis factor-alpha and insulin resistance in overweight and obese subjects. Cytokine. 2012;60:870–874. doi: 10.1016/j.cyto.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 50.Sharifi N, Amani R, Hajiani E, Cheraghian B. Does vitamin D improve liver enzymes, oxidative stress, and inflammatory biomarkers in adults with non-alcoholic fatty liver disease? A randomized clinical trial. Endocrine. 2014;47:70–80. doi: 10.1007/s12020-014-0336-5. [DOI] [PubMed] [Google Scholar]
- 51.Gao Y, Li S, Li Q. Uterine epithelial cell proliferation and endometrial hyperplasia: evidence from a mouse model. Mol Hum Reprod. 2014;20:776–786. doi: 10.1093/molehr/gau033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Devadas S, Hinshaw JA, Zaritskaya L, Williams MS. Fas-stimulated generation of reactive oxygen species or exogenous oxidative stress sensitize cells to Fas-mediated apoptosis. Free Radic Biol Med. 2003;35:648–661. doi: 10.1016/s0891-5849(03)00391-5. [DOI] [PubMed] [Google Scholar]
- 53.Starcevic SL, Diotte NM, Zukowski KL, Cameron MJ, Novak RF. Oxidative DNA damage and repair in a cell lineage model of human proliferative breast disease (PBD) Toxicol Sci. 2003;75:74–81. doi: 10.1093/toxsci/kfg154. [DOI] [PubMed] [Google Scholar]
- 54.Anasagasti MJ, Martin JJ, Mendoza L, Obrador E, Estrela JM, McCuskey RS, Vidal-Vanaclocha F. Glutathione protects metastatic melanoma cells against oxidative stress in the murine hepatic microvasculature. Hepatology. 1998;27:1249–1256. doi: 10.1002/hep.510270510. [DOI] [PubMed] [Google Scholar]
- 55.Brandi L. 1alpha (OH) D3 One-alpha-hydroxy-cholecalciferol—an active vitamin D analog. Clinical studies on prophylaxis and treatment of secondary hyperparathyroidism in uremic patients on chronic dialysis. Dan Med Bull. 2008;55:186–210. [PubMed] [Google Scholar]
- 56.Somjen D, Katzburg S, Grafi-Cohen M, Knoll E, Sharon O, Posner GH. Vitamin D metabolites and analogs induce lipoxygenase mRNA expression and activity as well as reactive oxygen species (ROS) production in human bone cell line. J Steroid Biochem Mol Biol. 2011;123:85–89. doi: 10.1016/j.jsbmb.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 57.Hamden K, Carreau S, Jamoussi K, et al. 1Alpha,25 dihydroxyvitamin D3: therapeutic and preventive effects against oxidative stress, hepatic, pancreatic and renal injury in alloxan-induced diabetes in rats. J Nutr Sci Vitaminol (Tokyo) 2009;55:215–222. doi: 10.3177/jnsv.55.215. [DOI] [PubMed] [Google Scholar]