Abstract
The human prostate gland is an endocrine organ where dysregulation of various hormonal factors may play a pivotal role in the pathogenesis of prostate cancer. There is emerging epidemiological data to support the role of components of metabolic syndrome, namely, obesity, hypercholesterolaemia, diabetes and hyperinsulinaemia on the development and/or the progression of prostate cancer. Although the exact mechanisms behind the relationship between metabolic syndrome and prostate cancer remain largely unknown, various in vitro and animal experiments of metabolic syndrome models have been shown to promote survival, mitogenesis, metastasis and treatment resistance pathways, through various adaptive responses such as intracellular steroidogenesis and lipogenesis. Also, in a large proportion of men with metabolic syndrome, alteration in levels of hormones such as testosterone, leptin and adiponectin has been shown to contribute towards the aggression of prostate cancer. Whilst the exact bio-pathophysiological mechanisms between metabolic syndrome and prostate cancer are yet to be fully elucidated, medications that target specific components of metabolic syndrome have further provided evidence for the inter-relationship between metabolic syndrome, its components and prostate cancer. Emerging in vitro and molecular data is likely to bring us closer to utilizing this knowledge to target particular cancer survival pathways and improving outcomes for men with prostate cancer.
Keywords: Metabolic Syndrome, Metformin, Androgen Deprivation Therapy, Biochemical Recurrence, Dutasteride
Introduction
Prostate cancer (PCa) and obesity are both monumental public health problems with rising prevalence. In 2012 alone, more than 1.1 million cases of PCa were recorded, accounting for around 8 % of all new cancer cases and 15 % of cancer diagnosed in men in the USA. PCa remains the most common solid organ tumour and the second leading cause of cancer deaths in men [1].
On the other hand, more than one third of adults in the world are classified as overweight or obese, with Australia boasting greater than 60 % population prevalence [2]. Obesity is often accompanied by other related medical disorders such as hypertension, hypercholesterolaemia and hyperglycaemia. Collectively, the concomitant conditions are clustered under a medical umbrella labelled ‘metabolic syndrome’ (MS) [3]. There are numerous ways by which this can be defined; however, the definition of Alberti et al. is one of the most commonly adopted (Table 1). In Australia alone, MS affects approximately 30 % of the population with most diabetic men qualifying for the syndrome [4]. Recognition of obesity and MS as a chronic illness is important, as it is associated with high risk of cardiovascular disease, diabetes, and chronic kidney disease. In fact, men with MS have an absolute cardiovascular disease risk of 10–15 % in 5 years with all-cause mortality risk increased by 1.5-fold [5, 6]. In addition, MS has gained publicity recently as an independent risk factor for the development of cancer and clinical outcomes of both localized and metastatic PCa.
Table 1.
Definition of metabolic syndrome
| Measure | Categorical cut points |
|---|---|
| Elevated waist circumference | Population- and country-specific definitions |
| Elevated triglycerides (drug treatment for elevated triglycerides is an alternate indicator) | ≥150 mg/dL (1.7 mmol/L) |
| Reduced HDL-C (drug treatment for reduced HDL-C is an alternate indicator) | <40 mg/dL (1.0 mmol/L) in males; <50 mg/dL (1.3 mmol/L) in females |
| Elevated blood pressure (anti-hypertensive drug treatment in a patient with a history of hypertension is an alternate indicator) | Systolic ≥130 and/or diastolic ≥85 mmHg |
| Elevated fasting glucose (drug treatment of elevated glucose is an alternate indicator) | ≥100 mg/dL |
With advances in our understanding of the basic cancer biology, new insights into the association between MS and PCa have become evident [7]. In this review, we aim to provide contemporary evidence around the association between MS and PCa, and how the knowledge is applicable in the clinical setting.
Evidence Synthesis
Relevant articles were searched using databases including MEDLINE, Pubmed, EMBASE, CINAHL, and clinicaltrials.gov. Articles between January 1990 and September 2015 were searched using the following terms: metabolic syndrome, obesity, adiposity, body mass index, PCa, insulin, adipokines, leptin, adiponectin, IL-6, exercise, nutrition, calorie and diet. Only English-language-based articles were considered.
Epidemiological Association (Fig. 1)
Fig. 1.
Proposed mechanisms of action in prostate cancer progression and survival in men with metabolic syndrome
Body mass index (BMI) as a measure of tissue adiposity has been shown to carry adverse outcomes in a variety of malignancies including breast, ovarian, colorectal, bladder, kidney, and endometrial cancers [8, 9]. In Finland where approximately 50 % of adult population are considered to have abnormally high BMI, Laukkanen et al. reported that in men with MS, there is an increased risk of PCa diagnosis by 1.9-fold (CI 1.1–3.5, 95 % confidence) [10]. The ‘Cancer of the Prostate Strategic Urologic Research Endeavour (CaPSURE)’ database found that men with elevated BMI were more likely to be diagnosed with PCa at a younger age and with more aggressive cancer [11]. In addition, two large randomized controlled clinical trial subgroup analyses also demonstrated positive association between PCa and MS. In the ‘PCa Prevention Trial (PCPT)’, elevated C-peptide was associated with twofold increase in the risk of aggressive PCa in the placebo group [12]. C-peptide is a by-product of insulin production, which is often used as a surrogate measure of endogenous insulin secretion. Similarly the ‘Reduction by Dutasteride of PCa Events (REDUCE)’ study showed that men with MS were more likely to have lower prostate-specific antigen (PSA) and high-grade PCa. In a meta-analysis investigating these observations, it was estimated that there would be 15 % higher risk of PCa mortality with each 5 kg/m2 increase in body mass index [13].
Once men are diagnosed with PCa, treatment outcomes may also be influenced by MS. Longitudinal population studies such as the ‘Physician Health Study’ with 30 years of follow-up showed that obese men with elevated C-peptide protein were more likely to die from PCa after diagnosis (hazard ratio (HR) 2.66, CI 1.62–4.39) [14]. Analysis of both CaPSURE and Northwest Veterans Integrated Services Network databases showed that biochemical recurrence following PCa therapy was higher in patients with high BMI and hyperglycaemia [15, 16]. In another surgical cohort, hypertension and obesity were independent markers of biochemical recurrence following surgery [17]. Meta-analysis by Cao et al. confirmed that a 5 kg/m2 increase in BMI was associated with a 21 % increase risk of biochemical recurrence after surgery [13]. Interestingly, similar results were also observed among men with PCa who received radiotherapy. Subgroup analysis of the Radiation Therapy Oncology Group trial 85–31 found that greater baseline BMI was an independent marker for cancer-specific mortality over 5 years with hazard ratio (HR) of 1.64 (95 % CI 1.01–2.66) [18].
Whilst components of MS are associated with increased cancer diagnosis and worse treatment outcomes, MS also adversely impacts on the prognosis of men with metastatic PCa. In men treated with androgen deprivation therapy, the presence of elevated C-peptide prior to treatment with androgen deprivation therapy (ADT) was associated with significantly shorter time to development of castrate-resistant PCa (CRPC) (16 vs 36 months) [19]. Furthermore, men with the highest insulin-like growth factor 1 (IGF-1)/insulin-like growth factor binding protein 1 (IGFBP-1) ratio compared to those in the lowest tertile after 3 months of starting ADT had a significant reduction in time to progression to CRPC in a recent retrospective study (12.4 vs 21.9 months; [20].
Conflicting Studies
Unfortunately, current literature is controversial and at times contradictory. In a recent study by Haggstrom et al., men with MS were observed to have a lower risk of PCa diagnosis without significant adverse changes to cancer outcomes [21]. In another meta-analysis of case–control and cohort studies of childhood and young adulthood obesity, BMI was not associated with future risk of advanced PCa [22]. It is likely that there are several reasons for these discrepancies.
First, BMI as a surrogate for adiposity may not be entirely accurate. In MS, central adiposity is considered the more accurate assessment and predictor of cardiovascular events. However, in patients with cancer, total body fat mass has been proposed to be a better predictor of cancer outcomes [23]. Second, obese and diabetic men demonstrate hormonal milieu substantially different to that of the normal population. Testosterone levels are usually lower, with correspondingly lower PSA levels [24]. Lower PSA levels may result in detection bias where men are less likely to be diagnosed with early localized disease. For example, diminished PSA values correlate to the severity of obesity: 7 % in overweight, 14 % in obese and 18 % in morbidly obese men [25]. Haemodilution, increased conversion of testosterone to oestradiol by adipose aromatase and hypothalamic suppression are some of the observed causes of low PSA in obese men [26]. Third, larger prostates in obese men may lead to under-detection of PCa during prostate biopsy [27]. In a retrospective study by Freeland et al., the median prostate weight during radical prostatectomy was 34 versus 41 g in men with BMI less than 25 and greater than 30 respectively. Finally, diabetic men may be subjected to anti-diabetic medications such as metformin and the usual suspects such as aspirin, statins and anti-hypertensives. Metformin has recently received significant attention for its ability to reverse cancer survival mechanisms in vitro [28, 29]. Both metformin and statins have been shown to reduce cancer risk and death in several meta-analyses [30–32].
Pathophysiological Mechanisms
There are numerous theories on the role of adiposity and MS in cancer development and progression. Obesity was initially linked epidemiologically with the diagnosis of higher-grade PCa [33], positive surgical margin [34], cancer recurrence [35], shorter time to castrate-resistant PCa (CRPC) [14] and cancer-specific mortality [20]. Studies of biomarkers of obesity then revealed the importance of other metabolic aberrations such as hyperinsulinaemia which has incidence of 50–70 % in obese patients [36, 37].
More specifically, elevated insulin levels and other surrogate markers such as IGF-1, IGFBP-1 and C-peptide have now been identified as important predictors of diagnosis and cancer-specific morbidity and mortality. The highest quartile of C-peptide level was associated with a hazard ratio of 4.12 for cancer-specific death [14]. The insulin superfamily is a group of growth-promoting peptides that are essential for metabolic regulation. Their effects are ubiquitous and have the ability to trigger cascades of various signal transduction pathways including phosphatidylinositol 3 kinase (PI3K)/Akt, Ras/MAPK, mTOR, cyclooxygenase (COX)-2 and S6 kinase pathways. These pathways activate a host of genes/enzymes for lipogenesis, cholesterol synthesis and de novo steroidogenesis [38, 39]. Mutations in downstream pathways of the insulin receptor are well documented in CRPC and high-grade tumours [40–42], such as the PI3K signalling pathway, commonly associated with a loss of the negative regulator PTEN [7]. Recent research has also revealed various survival mechanisms that are thought to be initiated by insulin. Insulin appears to mediate lipogenesis and steroidogenesis in PCa cells, much like the characteristics exhibited by CRPC cells [42, 43]. Despite the body of research on insulin, adipocytes are collectively an endocrine organ that can greatly influence the body through a variety of hormones. Large volumes of adipocytes result not only in elevated insulin but also in elevated insulin-like growth factor (IGF)-1, oestrogen and leptin. Adiponectin and testosterone, however, are often reduced. Whilst such variations in hormone levels make it challenging to interpret clinical data, in vitro experiments have shown that individual hormones such as leptin appear to promote proliferation, migration and reduce apoptosis [44], whilst adiponectin promotes the opposite [45].
Hypercholesterolaemia is a core component of MS that may promote cancer growth by providing substrates for cellular signalling, proliferation and migration [7]. Again, there is significant contradiction within the literature regarding the relationship. In a recent meta-analysis of population-based studies, relative risk of PCa diagnosis was 1.05 (CI 0.71–1.14, p = 0.21) for elevated total cholesterol, 0.93 (CI 0.8–1.1, p = 0.4) for elevated HDL and 1.17 (CI 0.88–1.55, p = 0.51) for elevated LDL, none of which were statistically significant [46]. The cardio-protective nature of HDL also appeared to confer some protection for PCa diagnosis and recurrence [47]. With elevated triglycerides, a recent population-based study reported that high levels were inversely associated with PCa risk (HR 0.78, CI 0.66–0.93) [48] whilst others reported increased risk of biochemical recurrence after surgery [49]. Despite the inconsistencies, in vitro studies have uniformly reported changes in PCa cell behaviour by hypercholesterolaemia via lipid mediators of inflammation such as arachidonic acid, eicosanoids, prostanoids and leukotrienes [50]. Adipocyte has also been found to produce local hypercholesterolaemia by providing free fatty acids and triglycerides in a paracrine fashion. In bone marrow, adipocyte promotes growth of disseminated cancer cells by supplying lipids for metabolism and through pro-inflammatory mechanisms [51]. Similar effects may be exerted by periprostatic fat [52, 53] such that the volume of periprostatic fat could be correlated with high-risk PCa [54]. As a local endocrine organ, periprostatic fat is likely to also contribute towards local inflammatory pathways to be explained below [53].
The inflammatory theory has been intrinsically linked to adiposity and cancer behaviour. The pro-inflammatory influences of adipose tissue have been found to be exerted by a variety of mediators such as interleukin 1, 6, 8 and 10, and chemokines such as CXCL8, CCL2, MCP-1, CXCL10 and IFN-inducible protein-10, and growth factors such as nerve growth factor, VEGF and TNF alpha [55]. Such factors create a hypoxic environment, which induces altered energy environment, which affects cancer cell behaviour [56]. Hypoxia-inducible factor (HIF) has been known to be overexpressed in a pro-inflammatory environment. Men treated with non-specific HIF-1-alpha inhibitors (digoxin, metformin and angiotensin 2 receptor blockers) have exhibited reduced incidence of metastatic disease and increased time to cancer progression [57]. Furthermore, inflammatory mediators such as interleukin-6 have been shown to contribute towards insulin-mediated cancer growth and Wnt5a-mediated metabolic dysfunction in both in vitro and in vivo experiments [58, 59].
Emerging Therapeutic Strategies for MS and PCa
Insulin-Sensitizing Agents
Metformin is one of the most commonly prescribed hypoglycemic agent and has been used extensively to treat components of MS such as obesity, insulin resistance and dyslipidaemia [60]. Metformin has bimodal mechanisms of action in patients through both systemic and intracellular systems. While, systemically, insulin and cholesterol levels may normalize following administration of metformin, within cancer cells, this drug exerts multiple cellular effects such as (1) reduced hormonal signalling and energy regulating pathways via AMP-activated protein kinase (AMPK), (2) reduced energy production via complex 1 inhibition and promotion of fatty acid oxidation in mitochondria, (3) reduced cellular proliferation via inhibition of mTOR, and (4) increased tumour suppression via activation of p53-p21 tumour suppressor axis [28, 29]. The downstream effects of AMPK includes the inhibition of PI3K-AKT pathway, and ultimately RAPTOR, mTORC1 and p70S6Kinase 1 pathways. Inhibitors of PI3K/AKT signalling and promoters of its negative regulators such as PTEN are currently being explored for the development small-molecule inhibitors [60, 61]. The AMPK-independent pathways of tumour growth inhibition also involve mTORC1 inhibition via inactivation of the RAG family of GTPases [60]. Metformin can also interfere with AR protein synthesis via MID1-alpha4/PP2A complex [62].
In a systematic analysis, metformin was shown to have numerous clinical benefits ranging from the treatment of type 2 diabetes to a reduced incidence of cancer and decreased overall mortality [63]. Libby et al. reported that the risk of cancer diagnosis in patients treated with metformin was significantly lower (hazard ratio of 0.63), and metformin use was associated with lower cancer-related mortality [64]. In a recent retrospective analysis, the use of metformin in diabetic patients with PCa was associated with a decrease in biochemical recurrence, PCa-specific mortality and development of CRPC, and an increase in overall survival over 8.7 years [65]. Apart from a cancer-specific advantage, a prospective clinical trial of metformin in conjunction with ADT, caloric restriction and exercise also reported a reduction in adverse effects of ADT such as alteration in abdominal girth, weight, body mass index and systolic blood pressure [66]. In a recent phase II trial of chemotherapy-naïve CRPC patients, metformin use was associated with prolongation of PSA doubling time, decline of PSA and decrease in the homeostatic model assessment index, IGF-1 and IGFBP-3 levels [67]. The prospect of repurposing a commonly used medication to combat cancer is certainly exciting, and this has resulted in the recent registration of many clinical trials involving metformin in the treatment of cancer (62–65).
Another class of insulin-sensitizing agents, the thiazolinidinediones, also provides another potential avenue for metabolic agents in cancer therapy. Thiazolinidinediones have been shown to improve insulin sensitivity via peroxisome-proliferator-activated receptor gamma (PPAR gamma) resulting in increased glucose uptake, lower gluconeogenesis and greater free fatty acid uptake by fat cells [68]. A recent meta-analysis reported the potential benefit of pioglitazone in reducing overall cancer risk (relative risk (RR) of 0.95; CI 0.1–0.99) [69].
Statins
The statins are another commonly prescribed medications for MS that may have an impact on cancer outcome [70]. Statins appear to have two separate pathways to influence cancer cells. First, HMGCoA reductase reduces serum total cholesterol and LDL and increase HDL, which may ultimately reduce substrates for cellular signalling, proliferation and migration. The findings are consistent with a recent meta-analysis of 27 observational studies, where the statin use was found to correlate with reduced risk of PCa diagnosis by 7 % (RR = 0.93; CI 0.87–0.99) and clinically advanced PCa by 20 % (RR = 0.8; CI 0.70–0.90) [32]. Loeb et al. also reviewed pathology specimens of 504 statin users who underwent radical prostatectomy and concluded that statin users had less adverse pathology features with lower PSA, despite the higher age and BMI [71]. Conversely, in a population-based cohort study of 185,000 men in Sweden, statin use was associated with reduced PSA (−4.6 %; CI −6.2 to −2.9) although no change in cancer diagnosis was observed [72]. The second mechanism of statins, however, appears to be directed on individual cancer cells. In in vitro studies, treatment of cells with atorvastatin has shown to reduce cancer proliferation, promote apoptosis and inhibit local invasion by inhibition of PI3K/Akt pathways and counteracting the effects of ATP [73, 74].
Immunophillin Ligands
Advances in understanding the relationship between MS and PCa have allowed for the development of novel therapeutic agents such as figitumumab, which is currently in phase II clinical trials. It is an immunoglobulin G2 monoclonal antibody that acts against IGF-1 receptor. Whilst its adverse effects such as hyperglycaemia preclude its extensive use, studies have reported that its use in men with localized PCa was associated with PSA decline and that 30 % of patients had a PSA decline greater than 50 % [75].
Anti-inflammatory Agents (e.g., Aspirin)
Consistent with the inflammatory theory of adiposity, several epidemiological studies have shown benefit in the use of prophylactic aspirin. In a recent retrospective review by Jacobs et al., aspirin use of more than 2 years was associated with a lower risk of PCa-specific mortality in men with high risk disease (71). Meta-analysis of 24 epidemiological studies showed that regular aspirin use was associated with reduced risk of diagnosis of new and/or advanced PCa (RR = 0.86, CI 0.81–0.92; and RR = 0.83, CI 0.75–0.91) (72). In men with metastatic PCa who received ADT, some studies showed positive benefit for aspirin consumption in cardiovascular disease vulnerable group (73).
Physical Exercise and Weight Loss
There is limited evidence regarding the role of exercise as an intervention in the prevention or treatment of PCa. In a recent Australian study, increasing body size or weight change from the age of 18 resulted in increase in PCa risk (HR 1.06, 95 % CI 1.00–1.13, per 5 kg) and PCa mortality (HR 1.12, 95 % CI 1.01–1.23 per 5 kg) [76]. Given the high incidence of cardiovascular morbidity and mortality in men with PCa, it seems logical to recommend engaging in activities that will potentially reduce both cancer and cardiovascular risks. Furthermore, there is substantive evidence to demonstrate the benefits of exercise during and after cancer treatments to improve quality of life, cancer-related fatigue and physical functioning ([77, 78].
Most men on ADT are usually older (60–80), overweight (87 %), hypertensive (61 %) and diabetic (25 %) with hypercholesterolaemia (56 %) and impaired fasting glucose (16 %) [79]. These patients are particularly susceptible to cardiovascular disease and adverse treatment effects such as diabetes, osteoporosis and sexual/physical/cognitive dysfunction [80]. It is in this group of patient that clinical studies have demonstrated significant benefits of early exercise intervention with preservation of total body weight, lean body mass and fat mass, as well as bone mineral density and improvement in cholesterol and blood sugar levels [81, 82].
Nutrition
Currently, there is no consensus on the type of diet and caloric intake required for prevention and aiding treatment of PCa [83]. However, some organizational bodies such as the ‘National Vascular Disease Prevention Alliance’ have published guidelines on nutritional intake and lifestyle changes that would be helpful in maintaining total body weight and reducing cardiovascular disease in both men with and without PCa [84]. Certain food groups such as isothiozyanate containing vegetables (broccoli, Brussels sprouts, cabbage and cauliflower), allium vegetables (garlic, leaks, chives and shallots) and phytooestrogen- and phytochemical-containing food (tomato, turmeric, pomegranates and coffee) have received recent interest as chemo-preventative agents. Although the prospect of cancer prevention through consuming particular food groups is attractive, clear evidence has not yet been established. At this present time, it is likely that judicious caloric intake control and maintenance of lower BMI is advisable.
Anti-hypertensives
Hypertension as a solitary risk or a part of constellation that makes up MS has been associated with increased risk of PCa [85]. In a large case–control study by Perron et al., beta blockers were associated with reduction in PCa risk (OR = 0.86, CI 0.77–0.96) [86], whilst a meta-analysis of 27 retrospective studies reported that anti-hypertensives were not generally associated with cancer diagnosis [87].
Recommendations
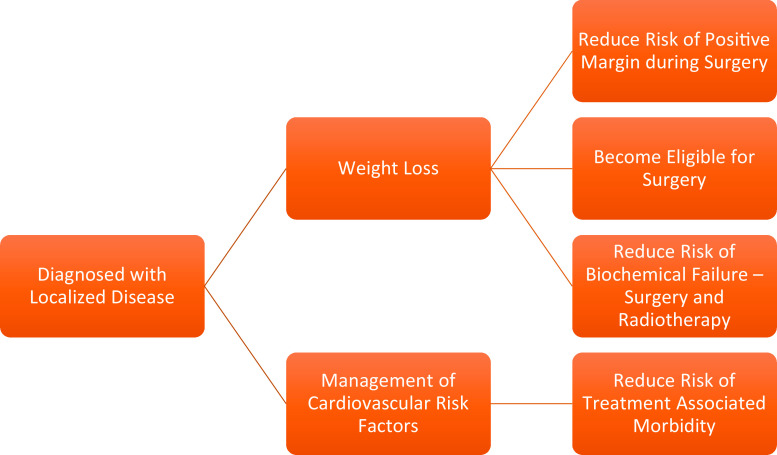
Although the evidence between MS and PCa is still accruing, it is likely that there is a causal relationship between MS and PCa. When cancer is viewed as a metabolic disorder, it creates many opportunities to influence the future of cancer prevention and treatment. However, given the obvious morbidity and mortality associated with MS in both cancer and non-cancer patients, it is reasonable to adopt strategies to combat MS such as caloric restriction, a diet composed of fresh fruit and vegetables, and regular moderate-intensity exercise, to improve the overall health and potentially minimize cancer risk and progression (Figs. 2 and 3).
Fig. 2.
Recommendations for a patient diagnosed with localized PCa
Fig. 3.
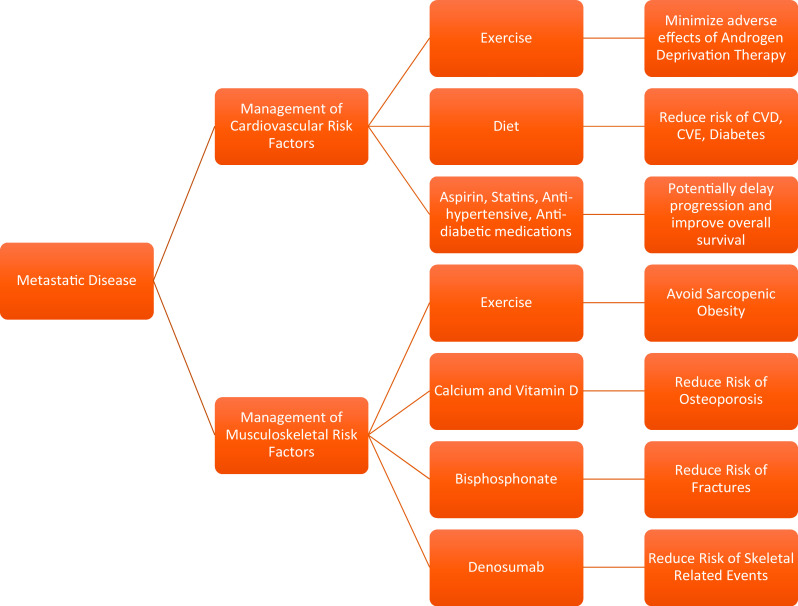
Recommendations for a patient diagnosed with metastatic PCa (NB: PCa = prostate cancer)
Conclusions
The apparent relationship between MS and PCa is complex, and the bio-pathophysiological mechanisms are yet to be fully elucidated. The published evidence to date in the areas of epidemiology, in vitro and animal experimental studies, and interventional studies has demonstrated a close relationship between MS and PCa in terms of cancer diagnosis, progression and recurrence. Emerging in vitro and molecular data is likely to bring us closer to utilizing this knowledge to target particular cancer survival pathways and improving outcomes for men with PCa.
Compliance with ethical standards
Funding
None.
Conflict of interest
None.
Disclosure
None.
References
- 1.Siegel R , Miller KD, Jemal A. et al (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):5–29 [DOI] [PubMed]
- 2.WHO. Overweight/obesity 2008. 2014 31/10/2014]; Available from: http://gamapserver.who.int/gho/interactive_charts/ncd/risk_factors/overweight_obesity/atlas.html
- 3.Alberti KG, Eckel RH, Grundy SM et al (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16):1640–5 [DOI] [PubMed]
- 4.Janus ED, Laatikainen T, Dunbar JA et al (2007) Overweight, obesity and metabolic syndrome in rural southeastern Australia. Med J Aust 187(3):147–52 [DOI] [PubMed]
- 5.Tonkin A, Barter P, Best J et al (2005) National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: position statement on lipid management—2005. Heart Lung Circ 14(4):275–91 [DOI] [PubMed]
- 6.Mottillo S, Filion KB, Genest J et al (2010) The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol 56(14):1113–32 [DOI] [PubMed]
- 7.Zadra G, Photopoulos C, Loda M (2013) The fat side of prostate cancer. Biochim Biophys Acta 1831(10):1518–32 [DOI] [PMC free article] [PubMed]
- 8.Renehan AG1, Tyson M, Egger M, Heller RF, Zwahlen M (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371(9612):569–78 [DOI] [PubMed]
- 9.Bhaskaran K, Douglas I, Forbes H et al (2014) Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5 · 24 million UK adults. Lancet 384(9945):755–765 [DOI] [PMC free article] [PubMed]
- 10.Laukkanen JA, Laaksonen DE, Niskanen L et al (2004) Metabolic syndrome and the risk of prostate cancer in Finnish men: a population-based study. Cancer Epidemiol Biomarkers Prev 13(10):1646–50 [PubMed]
- 11.Kane CJ, Bassett WW, Sadetsky N et al (2005) Obesity and prostate cancer clinical risk factors at presentation: data from CaPSURE. J Urol 173(3):732–6 [DOI] [PubMed]
- 12.Neuhouser ML, Till C, Kristal A et al (2010) Finasteride modifies the relation between serum C-peptide and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Cancer Prev Res (Phila) 3(3):279–89 [DOI] [PMC free article] [PubMed]
- 13.Cao Y, Ma J (2011) Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila) 4(4):486–501 [DOI] [PMC free article] [PubMed]
- 14.Ma J, Li H, Giovannucci E et al (2008) Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol 9(11):1039–47 [DOI] [PMC free article] [PubMed]
- 15.Bassett WW, Cooperberg MR, Sadestsky N et al (2005) Impact of obesity on prostate cancer recurrence after radical prostatectomy: data from CaPSURE. Urology 66(5):1060–5 [DOI] [PubMed]
- 16.Wright JL, Plymate SR, Porter MP et al (2013) Hyperglycemia and prostate cancer recurrence in men treated for localized prostate cancer. Prostate Cancer Prostatic Dis 16(2):204–208 [DOI] [PMC free article] [PubMed]
- 17.Asmar R, Beebe-Dimmer JL, Korgavkar K, Keele GR, Cooney KA (2013) Hypertension, obesity and prostate cancer biochemical recurrence after radical prostatectomy. Prostate Cancer Prostatic Dis 16(1):62–6 [DOI] [PMC free article] [PubMed]
- 18.Efstathiou JA, Bae K, Shipley WU et al (2007) Obesity and mortality in men with locally advanced prostate cancer. Cancer 110(12):2691–2699 [DOI] [PMC free article] [PubMed]
- 19.Flanagan J, Gray PK, Hahn N et al (2011) Presence of the metabolic syndrome is associated with shorter time to castration-resistant prostate cancer. Ann Oncol 22(4):801–7 [DOI] [PubMed]
- 20.Sharma J, Gray KP, Evan C et al (2014) Elevated insulin-like growth factor binding protein-1 (IGFBP-1) in men with metastatic prostate cancer starting androgen deprivation therapy (ADT) is associated with shorter time to castration resistance and overall survival. Prostate 74(3):225–34 [DOI] [PubMed]
- 21.Haggstrom C, Stocks T, Nagel G et al (2014) Prostate cancer, prostate cancer death, and death from other causes, among men with metabolic aberrations. Epidemiology 25(6):823–8 [DOI] [PMC free article] [PubMed]
- 22.Robinson WR, Poole C, Godley PA (2008) Systematic review of prostate cancer's association with body size in childhood and young adulthood. Cancer Causes Control 19(8):793–803 [DOI] [PubMed]
- 23.Rickles AS, Iannuzzi JC, Mironov O et al (2013) Visceral obesity and colorectal cancer: are we missing the boat with BMI? J Gastrointest Surg 17(1):133–43, discussion p 143 [DOI] [PubMed]
- 24.Fukui M, Tanaka M, Kadono M et al (2008) Serum prostate-specific antigen levels in men with type 2 diabetes. Diabetes Care 31(5):930–1 [DOI] [PubMed]
- 25.Banez LL, Hamilton RJ, Partin AW et al (2007) Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA 298(19):2275–80 [DOI] [PubMed]
- 26.Gunter JH, Lubik AA, McKenzie I, Pollak M, Nelson CC (2012) The Interactions between Insulin and Androgens in Progression to Castrate-Resistant Prostate Cancer. Adv Urol 2012:248607 [DOI] [PMC free article] [PubMed]
- 27.Freedland SJ, Platz EA, Presi JC Jr et al (2006) Obesity, serum prostate specific antigen and prostate size: implications for prostate cancer detection. J Urol 175(2):500–4, discussion 504 [DOI] [PubMed]
- 28.Sanli T, Steinberg GR, Singh G, Tsakiridis T (2014) AMP-activated protein kinase (AMPK) beyond metabolism: a novel genomic stress sensor participating in the DNA damage response pathway. Cancer Biol Ther 15(2):156–69 [DOI] [PMC free article] [PubMed]
- 29.Larsson O, Morita M, Topisirovic I et al (2012) Distinct perturbation of the translatome by the antidiabetic drug metformin. Proc Natl Acad Sci U S A 109(23):8977–82 [DOI] [PMC free article] [PubMed]
- 30.Zhang P, Li H, Tan X, Chen L, Wang S (2013) Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol 37(3):207–18 [DOI] [PubMed]
- 31.Decensi A, Puntoni M, Goodwin P et al (2010) Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res 3(11):1451–61 [DOI] [PubMed]
- 32.Bansal D, Undela K, D'Cruz S, Schifano F (2012) Statin use and risk of prostate cancer: a meta-analysis of observational studies. PLoS ONE 7(10), e46691 [DOI] [PMC free article] [PubMed]
- 33.Whittemore AS, Kolonel LN, Wu AH et al (1995) Prostate cancer in relation to diet, physical activity, and body size in blacks, whites, and Asians in the United States and Canada. J Natl Cancer Inst 87(9):652–61 [DOI] [PubMed]
- 34.SEARCH Database Study Group, Jayachandran J, Aronson WJ, Terris MK et al (2008) Obesity and positive surgical margins by anatomic location after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital database. BJU Int 102(8):964–8 [DOI] [PMC free article] [PubMed]
- 35.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR (2007) Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer 109(6):1192–202 [DOI] [PubMed]
- 36.Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG (2006) Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care 29(11):2427–32 [DOI] [PubMed]
- 37.Kocelak P, Chudek J, Olszanecka-Glinianowicz M (2012) Prevalence of metabolic syndrome and insulin resistance in overweight and obese women according to the different diagnostic criteria. Minerva Endocrinol 37(3):247–54 [PubMed]
- 38.Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R (2009) Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev 30(6):586–623 [DOI] [PubMed]
- 39.Mounier C, Posner BI (2006) Transcriptional regulation by insulin: from the receptor to the gene. Can J Physiol Pharmacol 84(7):713–24 [DOI] [PubMed]
- 40.Heni M, Hennenlotter J, Scharpf M et al (2012) Insulin receptor isoforms A and B as well as insulin receptor substrates-1 and −2 are differentially expressed in prostate cancer. PLoS ONE 7(12), e50953 [Electronic Resource] [DOI] [PMC free article] [PubMed]
- 41.Cox ME, Gleave ME, Zakikhani M et al (2009) Insulin receptor expression by human prostate cancers. Prostate 69:33–40 [DOI] [PubMed]
- 42.Lubik AA, Gunter JH, Hendy SC et al (2011) Insulin increases de novo steroidogenesis in prostate cancer cells. Cancer Res 71(17):5754–64 [DOI] [PubMed]
- 43.Lubik AA, Gunter JH, Hollier BG et al (2013) IGF2 increases de novo steroidogenesis in prostate cancer cells. Endocr Relat Cancer 20(2):173–86 [DOI] [PubMed]
- 44.Huang CY, Yu HS, Lai TY et al (2011) Leptin increases motility and integrin up-regulation in human prostate cancer cells. J Cell Physiol 226(5):1274–82 [DOI] [PubMed]
- 45.Collins CC, Volik SV, Lapuk AV et al (2012) Next generation sequencing of prostate cancer from a patient identifies a deficiency of methylthioadenosine phosphorylase, an exploitable tumor target. Mol Cancer Ther 11(3):775–83 [DOI] [PMC free article] [PubMed]
- 46.YuPeng L, LuXue Z, PengFei L et al (2015) Cholesterol levels in blood and the risk of prostate cancer: a meta-analysis of 14 prospective studies. Cancer Epidemiol Biomarkers Prev 24(7):1086–93 [DOI] [PubMed]
- 47.Bhindi B, Locke J, Alibhai SM et al. (2014) Dissecting the association between metabolic syndrome and prostate cancer risk: analysis of a large clinical cohort. Eur Urol 67(10):64–70 [DOI] [PubMed]
- 48.Harding J, Sooriyakumaran M, Anstey KJ et al. (2015) The metabolic syndrome and cancer: is the metabolic syndrome useful for predicting cancer risk above and beyond its individual components? Diabetes Metab. doi:10.1016/j.diabet.2015.04.006 [DOI] [PubMed]
- 49.Kang M, Jeong CW, Ku JH, Kwak C, Kim HH (2015) Hypertriglyceridemia is a potential preoperative predictor for biochemical recurrence after radical prostatectomy. PLoS ONE 10(3), e0122438 [DOI] [PMC free article] [PubMed]
- 50.Iyer A, Fairlie DP, Prins JB, Hammock BD, Brown L (2010) Inflammatory lipid mediators in adipocyte function and obesity. Nat Rev Endocrinol 6(2):71–82 [DOI] [PubMed]
- 51.Herroon MK, Rajagurubandara E, Hardaway AL et al (2013) Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget 4(11):2108–23 [DOI] [PMC free article] [PubMed]
- 52.Venkatasubramanian PN, Brendler CB, Plunkett BA et al (2014) Periprostatic adipose tissue from obese prostate cancer patients promotes tumor and endothelial cell proliferation: a functional and MR imaging pilot study. Prostate 74(3):326–35 [DOI] [PubMed]
- 53.Finley DS, Calvert VS, Inokuchi J et al (2009) Periprostatic adipose tissue as a modulator of prostate cancer aggressiveness. J Urol 182(4):1621–7 [DOI] [PubMed]
- 54.van Roermund JG, Hinnen KA, Tolman CJ et al (2011) Periprostatic fat correlates with tumour aggressiveness in prostate cancer patients. BJU Int 107(11):1775–9 [DOI] [PubMed]
- 55.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348(17):1625–38 [DOI] [PubMed]
- 56.Marignol L, rivera-Figueroa K, Lynch T, Hollywood D (2013) Hypoxia, notch signalling, and prostate cancer. Nat Rev Urol 10(7):405–13 [DOI] [PMC free article] [PubMed]
- 57.Ranasinghe WK, Sengupta S, Williams S et al (2014) The effects of nonspecific HIF1alpha inhibitors on development of castrate resistance and metastases in prostate cancer. Cancer Med 3(2):245–51 [DOI] [PMC free article] [PubMed]
- 58.Fuster JJ, Zuriaga MA, Ngo DT et al. (2014) Non-canonical Wnt signaling promotes obesity-induced adipose tissue inflammation and metabolic dysfunction independent of adipose tissue expansion. Diabetes, [DOI] [PMC free article] [PubMed]
- 59.Rojas A, Liu G, Coleman I et al (2011) IL-6 promotes prostate tumorigenesis and progression through autocrine cross-activation of IGF-IR. Oncogene 30(20):2345–55 [DOI] [PMC free article] [PubMed]
- 60.Pernicova I, Korbonits M (2014) Metformin--mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol 10(3):143–56 [DOI] [PubMed]
- 61.Shi M, Zhou X, Zhang Z et al (2014) A novel PI3K inhibitor displays potent preclinical activity against an androgen-independent and PTEN-deficient prostate cancer model established from the cell line PC3. Toxicology Letters 228(3):133–9 [DOI] [PubMed]
- 62.Demir U, Koehler A, Scnieder R, Schweiger S, Klocker H (2014) Metformin anti-tumor effect via disruption of the MID1 translational regulator complex and AR downregulation in prostate cancer cells. BMC Cancer 14:52 [DOI] [PMC free article] [PubMed]
- 63.Saenz A, Fernandez-Esteban I, Mataix A et al. (2005) Metformin monotherapy for type 2 diabetes mellitus. Cochrane Database Syst Rev, (3): p. CD002966 [DOI] [PubMed]
- 64.Libby G, Donnelly LA, Donnan PT et al (2009) New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 32(9):1620–5 [DOI] [PMC free article] [PubMed]
- 65.Spratt DE, Zhang C, Zumsteg ZS et al (2013) Metformin and prostate cancer: reduced development of castration-resistant disease and prostate cancer mortality. Eur Urol 63(4):709–16 [DOI] [PMC free article] [PubMed]
- 66.Nobes JP, Langley SE, Klopper R, Russell-Jones D, Laing RW (2012) A prospective, randomized pilot study evaluating the effects of metformin and lifestyle intervention on patients with prostate cancer receiving androgen deprivation therapy. BJU Int 109(10):1495–502 [DOI] [PubMed]
- 67.Rothermundt C, Hayoz S, Templeton AJ et al. (2014) Metformin in Chemotherapy-naive Castration-resistant Prostate Cancer: A Multicenter Phase 2 Trial (SAKK 08/09). Eur Urol 66(3):468–74 [DOI] [PubMed]
- 68.Segawa Y, Yoshimura R, Hase T et al (2002) Expression of peroxisome proliferator-activated receptor (PPAR) in human prostate cancer. Prostate 51(2):108–16 [DOI] [PubMed]
- 69.Colmers IN, Bowker SL, Johnson JA (2012) Thiazolidinedione use and cancer incidence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab 38(6):475–84 [DOI] [PubMed]
- 70.Park YH, Seo SY, Lee E et al (2013) Simvastatin induces apoptosis in castrate resistant prostate cancer cells by deregulating nuclear factor-B pathway. J Urol 189(4):1547–52 [DOI] [PubMed]
- 71.Loeb S, Kan D, Helfand BT, Nadler RB, Catalona WJ (2010) Is statin use associated with prostate cancer aggressiveness? BJU Int 105(9):1222–5 [DOI] [PMC free article] [PubMed]
- 72.Nordstrom T, Clements M, Karlsson R, Adolfsson J, Gronberg H (2015) The risk of prostate cancer for men on aspirin, statin or antidiabetic medications. Eur J Cancer 51(6):725–33 [DOI] [PubMed]
- 73.Ghalali A, Wiklund F, Zheng H, Stenius U, Hogberg J (2014) Atorvastatin prevents ATP-driven invasiveness via P2X7 and EHBP1 signaling in PTEN-expressing prostate cancer cells. Carcinogenesis 35(7):1547–55 [DOI] [PubMed]
- 74.Babcook MA , Sramkoski RM, Fujioka H et al. (2014) Combination simvastatin and metformin induces G1-phase cell cycle arrest and Ripk1- and Ripk3-dependent necrosis in C4-2B osseous metastatic castration-resistant prostate cancer cells. Cell Death Dis e1536 [DOI] [PMC free article] [PubMed]
- 75.Chi KN, Gleave ME, Fazli L et al (2012) A phase II pharmacodynamic study of preoperative figitumumab in patients with localized prostate cancer. Clin Cancer Res 18(12):3407–13 [DOI] [PubMed]
- 76.Bassett JK, Severi G, Baglietto L et al (2012) Weight change and prostate cancer incidence and mortality. Int J Cancer 131(7):1711–9 [DOI] [PubMed]
- 77.Schmitz KH, Courneya KS, Matthews C et al (2010) American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 42(7):1409–26 [DOI] [PubMed]
- 78.Hayes SC, Spence RR, Galvao DA, Newton RU (2009) Australian Association for Exercise and Sport Science position stand: optimising cancer outcomes through exercise. J Sci Med Sport 12(4):428–34 [DOI] [PubMed]
- 79.Cheung AS, Pattison D, Bretherton I et al (2013) Cardiovascular risk and bone loss in men undergoing androgen deprivation therapy for non-metastatic prostate cancer: implementation of standardized management guidelines. Androl 1(4):583–9 [DOI] [PubMed]
- 80.Rhee H, Gunter JH, Heathcote P et al. (2014) Adverse effects of androgen deprivation therapy in prostate cancer and their management. BJU Int 15(Suppl5):3–13 [DOI] [PubMed]
- 81.Cormie P, Galvao GA, Spry N et al. (2014) Can Supervised exercise prevent treatment toxicity in prostate cancer patients initiating androgen deprivation therapy: a randomised controlled trial. BJU Int [DOI] [PubMed]
- 82.Gardner JR, Livingston PM, Fraser SF (2014) Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol 32(4):335–46 [DOI] [PubMed]
- 83.Masko EM, Allott EH, Freedland SJ (2013) The relationship between nutrition and prostate cancer: is more always better? Eur Urol 63(5):810–820 [DOI] [PMC free article] [PubMed]
- 84.Alliance, N.-N.V.D.P. Guidelines for the Management of Absolute Cardiovascular Disease Risk. 2012 [cited 2014 06/05]; Available from: http://strokefoundation.com.au/site/media/AbsoluteCVD_GL_webready.pdf
- 85.Esposito K, Chiodini P, Capuano A et al (2013) Effect of metabolic syndrome and its components on prostate cancer risk: meta-analysis. J Endocrinol Investig 36(2):132–9 [DOI] [PubMed]
- 86.Perron L, Bairati I, Harel F, Meyer F (2004) Antihypertensive drug use and the risk of prostate cancer (Canada). Cancer Causes Control 15(6):535–541 [DOI] [PubMed]
- 87.Coleman CI, Baker WL, Kluger J, White CM (2008) Antihypertensive medication and their impact on cancer incidence: a mixed treatment comparison meta-analysis of randomized controlled trials. J Hypertens 26(4):622–629 [DOI] [PubMed]