Abstract
Molecular and clinical observations provide evidence for a potential role of parathyroid hormone (PTH) in colorectal cancer development. We therefore aimed to assess the association of PTH with regard to colorectal cancer precursor lesions. A cohort of 1432 participants, 777 men, 58.4 ± 9.6 years and 701 women, 59.1 ± 10.6 years, undergoing screening colonoscopy were allocated to PTH serum concentrations either above or below 55 ng/L. The number, localization, size, and histology of the polypoid lesions detected during screening colonoscopy were recorded according to PTH serum concentrations. Serum PTH concentrations were not different between men and women. Women with PTH serum concentrations above the cut-off had significantly more adenomas (13/40; 32.5 %) of the distal colon compared to women below the cut-off (91/659; 13.8 %; P = 0.001). Additionally, the rate of dysplasia in adenomas of the distal colon was higher in women with high compared to low PTH concentrations (P = 0.001). These findings remained robust after adjustments for serum vitamin D, age, plasma creatinine, BMI, diabetes, and liver steatosis. No associations were observed between serum PTH concentrations and colorectal lesions in men. These data suggest that elevated PTH serum concentrations might have a role in colorectal cancer development as indicated by higher rates of adenomas, specifically with dysplasia, in women. The role of PTH in colon carcinogenesis and its sex specificity deserve further study.
Keywords: Adenoma, Distal Colon, Colorectal Adenoma, Colon Carcinogenesis, Screen Colonoscopy
Introduction
Parathyroid hormone (PTH) is primarily known for its prominent role in bone metabolism and calcium and phosphate homeostasis together with vitamin D (VitD) [1]. Although the associations of low concentrations of VitD with malignancies and metabolic diseases have been extensively investigated in recent years, the potential involvement of PTH in this respect has been only marginally considered [2]. PTH serum concentrations rise as serum VitD decreases and may thereby contribute to the associations observed between low VitD and various diseases [1].
Higher serum PTH concentrations have been associated with mortality independent of VitD, age, and other co-variates [3, 4]. Additionally, PTH serum concentrations increase with age and this increase likewise occurs independent of VitD status, renal function, or phosphate in men and women [5]. Although gender differences with regard to PTH increases with age and its biological effects have been suggested [6], both short- and long-term changes are observed in men and women alike [5, 7].
Several molecular observations suggest that PTH may potentially be involved in colon cancer development. The PTH receptor (PTH1R) is highly expressed in colorectal adenomas and cancers but less in normal colonic mucosa from the same patients [8, 9]. Additionally, direct PTH actions have been demonstrated in carcinogenesis mainly via co-mitogenic and anti-apoptotic activities in the mammary gland [10]. However, in the human colon cancer cell line Caco-2, PTH induced G0/G1 phase arrest by affecting proteins involved in the PKC pathway [11]. PTH increases insulin-like-growth factor I which is considered a growth-promoting agent in colon carcinogenesis [12, 13]. PTH-related peptide, which acts via the same PTH1R, has been demonstrated to enhance colon cancer cell proliferation and adhesion [14]. Its expression is markedly enhanced in colon cancer tissue compared to adenomas and normal mucosa [15]. In summary, the pleiotropic effects of PTH on cell cycle regulation together with the diversity of its receptor expression encourage clinical research regarding a potential role of PTH in cancer development.
However, data investigating the relevance of these molecular and cell culture findings in the pathogenesis of human diseases are scarce. Case reports and case series have repeatedly suggested a link between primary hyperparathyroidism and colon cancer [16–19]. Farr et al. reported malignancies in one third and benign tumors primarily of the breast, stomach, and colon or premalignant conditions of various sites in another third of their patients from Sloan-Kettering Cancer Center [20]. In a recent nested case-control study from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, the incidence of colon cancer but not of rectal cancer was significantly related to pre-diagnostic serum PTH concentrations in men, but not in women, and the link was strongest in overweight subjects [21]. This analysis represented the first epidemiological report on an association between colonic neoplasia and serum PTH concentrations.
Since available molecular data and clinical observations support a role of PTH in colon cancerogenesis, we aimed to investigate the potential association of PTH with precursor lesions of colorectal cancers in a screening colonoscopy cohort. To our knowledge, this is the first study showing that elevated serum PTH concentrations may be relevant for the development of colorectal neoplasia and, in particular, for malignant transformation at early stages.
Material and Methods
Study Concept
The study has been designed as an ongoing evaluation of subjects undergoing screening colonoscopy, details of which have been reported previously [22]. From all study participants, a detailed drug and medical history and a routine physical examination were obtained. Following an overnight, fast venous blood was collected and an oral glucose tolerance test was performed. On the following day, subjects underwent colonoscopy.
Study Subjects
From a total of 891 initially screened male subjects, 59 (6.6 %) were excluded from the study because of incomplete colonoscopies (N = 15), a history of previous colorectal polypectomy (N = 27), newly diagnosed and to date asymptomatic inflammatory bowel disease (N = 3), other extraintestinal malignancies (N = 11), or systemic autoimmune diseases (N = 3). Fifty-two (8.9 %) women of 851 initially screened were excluded because of incomplete colonoscopies (N = 20), a history of previous colorectal polypectomy (N = 21), to date asymptomatic inflammatory bowel disease (N = 2), extraintestinal malignancies (N = 3), or systemic autoimmune disease (N = 6). After exclusion of 93 subjects on VitD and/or calcium supplementation and 21 subjects with missing PTH levels, 1517 subjects (807 men, 710 women) were included. Among women, 3 were further excluded due to PTH >100 and 6 due to plasma creatinine above 1.2 mg/dL. Among men, 3 were excluded due to PTH >100 ng/L and 27 due to creatinine >1.2 mg/dL. This exclusion was performed as the aim of the evaluation was to assess potential associations of PTH with colorectal adenoma strictly within healthy ranges. No low PTH cut-off was used since 8 men and 20 women had concentrations below 15 ng/L with normal calcium and VitD concentrations, and exclusion of these subjects did not alter results. Finally, data from 701 women and 777 men were used for the analysis. All subjects underwent colonoscopy for colorectal cancer screening according to national screening recommendations for colorectal cancer at a single center from October 2010 to January 2013 and allocated to high or normal PTH defined by a cut-off of 55 ng/L which was identified as detailed below. The study was approved by the local ethics committee, and informed consent was obtained from all participants.
Laboratory Assessment
Full blood counts were obtained in all subjects by standard laboratory methods. Erythrocyte sedimentation rate (ESR) was measured in citrate plasma. Serum PTH concentrations (normal range 15–65 ng/L) were measured using the Cobas® Parathormon—PTH intact electrochemiluminescence immuno-assay obtained from Roche (Roche Diagnostics, Mannheim, Germany). Inter-assay variability of PTH measurements was below 7.0 % as determined with two control samples at target values of 55 and 60 ng/L. No significant between-day drift, time drifts, or other trends were observed. 25-Hydroxyvitamin D3 (25(OH)D3) (normal range 20–60 ng/mL) was measured by the electrochemiluminescence-based Cobas e411 analyzer (TM) employing the respective Elecsys (TM) reagents (Roche Diagnostics, Mannheim, Germany). The laboratory performed regular quality control measurements, including a pooled serum sample analysis with batches of study samples to monitor precision and identify possible laboratory shifts over time, as well as testing duplicates in different batches. The inter-assay coefficient of variation was <6 % for 25(OH)D3. All analyses were conducted in a blinded fashion. A standardized oral glucose tolerance test (OGTT) was performed with 75 g of glucose in 300 mL of water. HbA1c (normal range <6.5 %) was measured by HPLC using Adamts H-8160 (Menarini, Florence, Italy). Type 2 diabetes was classified as use of diabetes medication, HbA1C ≥6.5 %, fasting glucose >7.0 mmol/L, or 2-h oral glucose tolerance test values >11.1 mmol/L.
As reported previously [22], right upper quadrant US examination was performed (ATL HDI 5000; Phillips Medical Systems, Vienna, Austria). The liver was considered “normal” if the echogenicity was homogenous and similar to or slightly higher than the echogenicity of the renal parenchyma. The liver was considered “fatty liver” if increased echogenicity in relation to the renal parenchyma was found. The severity of sonographic steatosis was not graded [23]. The diagnosis of nonalcoholic fatty liver was based on specific findings on right upper quadrant US examination (as noted above) and after exclusion of viral, autoimmune, and hereditary (Wilson disease, hereditary hemochromatosis, alpha-1 antitrypsin deficiency) liver disease and excess alcohol consumption of >20 g/day [24].
Colonoscopy
The laxative Klean–Prep® (containing macrogol 59.0 g, sodium sulfate 5.68 g, sodium bicarbonate 1.68 g, NaCl 1.46 g, and potassium chloride 0.74 g; Norgine, Marburg, Germany) was used for bowel preparation before colonoscopy. Colonoscopic findings were classified as tubular adenoma, advanced adenoma, i.e., villous or tubulovillous features, size ≥1 cm or high-grade dysplasia, or carcinoma after a combined analysis of macroscopic and histological results. Hyperplastic adenomas were counted but not considered for further analyses [25]. Lesions were classified by location (i.e., proximal colon including caecum, ascending colon and transverse colon, distal colon ranging from the splenic flexure to the sigmoid and rectum alone).
Statistical Analysis
For all analyses, SigmaStat 3.1 or STATA 8.0 software packages were used. Data are presented as mean ± standard deviation (SD), unless otherwise indicated. ANOVA was used for comparison of continuous variables. The Pearson chi-square test was used to calculate rates and proportions. In multivariate regression analysis, the number of adenomas in the distal colon was used as dependent variable and age, BMI, creatinine, VD, liver steatosis, T2DM, and PTH concentrations < or >55 ng/L as independent variables. To determine predictors of the presence/absence of colonic adenomas or dysplasias, logistic regression analysis was used using independent variables as above. Throughout, a two-tailed P value <0.05 was considered statistically significant.
Results
Characteristics of the Study Cohort
As detailed above, data from 777 males and 701 females were included in the analysis. The clinical and biochemical characteristics of these subjects are summarized in Table 1. Average values for BMI, VitD, and creatinine were higher in men than in women, while no difference was noted in average values for age, PTH, and HbA1c. The prevalence of type 2 diabetes was similar between men and women, but liver steatosis was more prevalent in men than in women. Data on the associations between VitD, corrected for seasonal variation, and colorectal adenomas in this cohort have been published in detail previously [26].
Table 1.
Clinical and biochemical characteristics of men and women
| Variable | Men (N = 777) | Women (N = 701) | P |
|---|---|---|---|
| Age (years) | 58.4 ± 9.6 | 59.1 ± 10.6 | 0.427 |
| Subjects with any neoplasia (y/n) | 384/383 | 232/469 | <0.001 |
| BMI (kg/m2) | 27.7 ± 4.1 | 26.1 ± 5.5 | <0.001 |
| Diabetes (y/n) | 128/649 | 96/605 | 0.359 |
| HbA1c (%) | 5.76 ± 0.64 | 5.76 ± 0.68 | 0.891 |
| Steatosis (y/n) | 469/308 | 286/415 | <0.001 |
| 25(OH)D3 (ng/mL)a | 24.6 ± 10.8 | 23.0 ± 11.6 | 0.007 |
| PTH (ng/L) | 33.3 ± 11.3 | 33.6 ± 12.1 | 0.687 |
| Creatinine, mg/dL | 0.89 ± 0.13 | 0.74 ± 0.13 | <0.001 |
BMI body mass index, HbA1c glycated hemoglobin A1c
aSeasonally adjusted serum concentrations
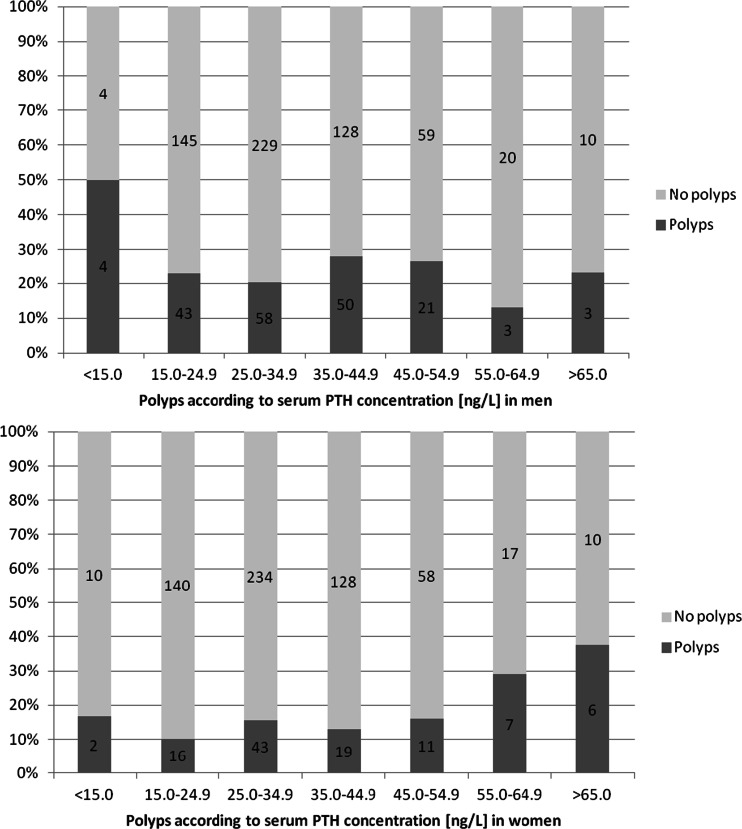
Among the variables shown in Table 1, PTH was associated with age (R = 0.090; P = 0.017), BMI (R = 0.212; P < 0.001), and VitD (R = −0.282; P < 0.001) in women and with age (R = 0.073; P = 0.040) and VitD (R = −0.197; P < 0.001, Spearman rank correlation analysis) in men. Women with T2DM or liver steatosis had higher PTH serum concentrations compared to those without T2DM or steatosis, respectively (36.7 ± 14.8 vs. 33.1 ± 11.6 ng/L; P = 0.007 or 35.9 ± 13.3 vs. 32.0 ± 11.0, respectively), but no differences were observed in men. Overall, the rate of colorectal adenomas was higher in men as compared to women. To determine possible associations of PTH concentrations with colorectal lesions, we plotted sex-specific rates of colorectal lesions against PTH increments and observed an abrupt significant increase of rates for the presence of adenomas (P = 0.034) and dysplastic lesions (P = 0.012) in the distal colon at PTH values above 55 ng/L in women. However, a cut-off value for any of the adenoma categories could not be identified in men (Fig. 1). Nevertheless, we used 55 ng/L as the cut-off value for PTH serum concentrations in both sexes for comparison. We found that 5.1 % of our study participants (40/701; 5.7 % of women and 36/777; 4.6 % of men) were above of 55 ng/L. The number of total adenoma and sessile adenoma showed borderline significance in the comparison of men with low compared to high PTH serum concentrations. However, no dose-effect relationship could be identified in men as opposed to women.
Fig. 1.
a, b Proportions of subjects with adenomas in the colon according to increasing serum PTH concentrations. An increase of the proportion of subjects with adenomas was detected in women above 55 ng/L but not in men. The figures in the bars represent the absolute numbers of subjects in the respective groups
Number, histology, morphology, and localization of colorectal lesions observed in men and women were determined above and below 55 ng/L (Table 2). In univariate analysis, the rates for the presence of any adenomas and sessile adenomas were higher below as compared to the respective rates above 55 ng/L PTH. In women, univariate analyses showed higher rates for the total number of adenomas, advanced and tubulovillous adenomas, adenomas with dysplasia, and the number of adenomas in distal colon above as compared to the respective rates below the cut-off value.
Table 2.
Summary of colorectal lesions found in men and women analyzed with regard to number, histology, morphology, and localization according to serum PTH concentrations
| PTH (ng/ml) | Men (N = 777) | Women (N = 701) | ||||
|---|---|---|---|---|---|---|
| <55 (N = 741) | >55 (N = 36) | P value | <55 (N = 661) | >55 (N = 40) | P value | |
| Number (total adenoma) | ||||||
| >0 | 372 (50.2) | 12 (33.3) | 0.048 | 216 (32.7) | 16 (40.0) | 0.339 |
| 1 | 186 (25.1) | 8 (22.2) | 134 (20.3) | 4 (10.0) | ||
| ≥2 | 186 (25.1) | 4 (11.1) | 0.091* | 82 (12.4) | 12 (30.0) | 0.004* |
| Advanced | 60 (8.1) | 2 (5.6) | 0.582 | 25 (3.8) | 5 (12.5) | 0.008 |
| Advanced proximal colon | 25 (3.4) | 0 (0) | 0.263 | 14 (2.0) | 4 (10.0) | 0.002 |
| Advanced distal colon | 24 (3.2) | 2 (5.6) | 0.450 | 9 (1.4) | 2 (5.0) | 0.072 |
| Advanced rectum | 14 (1.8) | 0 (0) | 0.405 | 3 (0.5) | 1 (2.5) | 0.095 |
| N, histology | ||||||
| >0, tubular | 249 (33.6) | 8 (22.2) | 0.156 | 124 (18.8) | 12 (30.0) | 0.081 |
| 1, tubular | 148 (20.0) | 6 (16.7) | 93 (14.1) | 7 (17.5) | ||
| ≥2, tubular | 101 (13.6) | 2 (5.6) | 0.281* | 31 (4.7) | 5 (12.5) | 0.068* |
| Tubulovillous | 22 (3.0) | 0 (0.0) | 0.165 | 13 (2.0) | 3 (7.5) | 0.023 |
| With any dysplasia | 83 (11.2) | 1 (2.8) | 0.112 | 46 (7.0) | 7 (17.5) | 0.014 |
| Dysplasia proximal colon | 46 (6.2) | 0 (0) | 0.123 | 27 (4.1) | 6 (15.0) | 0.002 |
| Dysplasia distal colon | 34 (4.6) | 1 (2.8) | 0.609 | 18 (2.7) | 5 (12.5) | 0.001 |
| Dysplasia rectum | 22 (3.0) | 0 (0) | 0.294 | 7 (1.1) | 1 (2.5) | 0.405 |
| Morphology of adenoma | ||||||
| Flat | 63 (8.5) | 2 (5.6) | 0.533 | 45 (6.8) | 4 (10.0) | 0.442 |
| Sessile | 334 (45.1) | 10 (27.8) | 0.041 | 175 (26.5) | 15 (37.5) | 0.128 |
| Pedunculated | 48 (6.3) | 1 (2.3) | 0.277 | 17 (2.6) | 4 (8.9) | 0.015 |
| Localization | ||||||
| >0, proximal colon | 191 (25.8) | 5 (13.9) | 0.109 | 119 (18.0) | 8 (20.0) | 0.750 |
| 1, proximal colon | 123 (16.6) | 4 (11.1) | 90 (13.6) | 6 (13.3) | ||
| ≥2, proximal colon | 68 (9.2) | 1 (2.8) | 0.239* | 29 (4.4) | 2 (4.4) | 0.950* |
| >0, distal colon | 176 (23.8) | 6 (16.7) | 0.327 | 91 (13.8) | 13 (32.5) | 0.001 |
| 1, distal colon | 121 (816.3) | 2 (5.6) | 67 (10.1) | 11 (27.5) | ||
| ≥2, distal colon | 55 (7.4) | 4 (11.1) | 0.187* | 24 (3.6) | 2 (5.0) | 0.003* |
| >0, rectum | 150 (20.2) | 3 (8.3) | 0.079 | 65 (9.8) | 6 (15.0) | 0.293 |
| 1, rectum | 113 (15.3) | 3 (8.7) | 53 (8.0) | 4 (10.0) | ||
| ≥2, rectum | 37 (5.0) | 0 (0) | 0.173* | 12 (1.8) | 2 (5.0) | 0.331* |
The figures in the columns refer to the respective number of adenomas detected. Percentage values are in parentheses. In the distal colon, a significantly increased rate of adenomas, particularly those with dysplasia, was found in women with higher PTH concentration. The size of adenomas in the various groups is not presented as there were no differences in either men or women. Advanced adenomas include adenomas with villous features (>25 %), size of 1.0 cm or more, high-grade dysplasia, or early invasive cancer
PTH parathyroid hormone
*P value referring to the comparison for the number of adenomas (coded = 0, 1, or ≥2) (two and more) between high and low PTH; other P values referring to the comparison of presence or absence of the respective adenomas between the defined groups
High Serum PTH Concentrations are Independently Associated with Dysplasia
To determine whether the associations of high PTH serum concentrations observed in women remain robust after correction for potential confounders, we performed logistic regression analyses. Associations with PTH strata were found with age, BMI, plasma creatinine, VitD, T2DM, and hepatic steatosis as detailed in Table 3. These variables were therefore adjusted for in the multivariate model.
Table 3.
Associations of binary PTH groups with clinical characteristics of female study participants
| Trait | PTH level (ng/L) | P | |
|---|---|---|---|
| <55 | >55 | ||
| Age [years] | 58.8 (10.5) | 64.0 (10.8) | 0.003 |
| BMI [kg/m2] | 26.5 (5.4) | 28.7 (5.9) | 0.012 |
| Creatinin [mg/dL] | 0.73 (0.12) | 0.78 (0.14) | 0.019 |
| VitD [ng/ml] | 23.6 (11.6) | 14.7 (9.0) | <0.001 |
| Diabetes [y/n] | 86/575 | 10/30 | 0.032 |
| Steatosis [y/n] | 260/401 | 26/14 | 0.001 |
Univariate logistic regression analysis showed that PTH was associated with dysplasia in adenomas of the distal colon with an OR of 5.1 (95 % CI 1.8–14.5; P = 0.002). This association remained significant after adjustment for potential confounding variables (Table 4). Additionally, a significant association was noted for advanced adenoma (P = 0.032) and the location of adenomas in the distal colon (P = 0.018). Borderline significance (P = 0.054) for the association of tubulovillous adenoma of all locations with PTH strata was observed after adjustment for creatinine, age, BMI, VitD, steatosis, and T2DM. In multivariate regression analysis, the number of adenomas in the distal colon displayed a borderline association (P = 0.062). Furthermore, the significance of the association between adenomas with dysplasia in the proximal colon and serum PTH concentrations (P = 0.002) was not maintained after adjustment for VitD and the confounders described.
Table 4.
Logistic regression analysis of dysplasia in colorectal adenomas with PTH above or below 55 ng/L in women
| OR | CI (95 %) | P | |
|---|---|---|---|
| PTH, ng/L, >55/<55 | 4.486 | 1.443–13.937 | 0.009 |
| Age, years | 1.043 | 0.996–1.093 | 0.069 |
| BMI, kg/m2 | 0.893 | 0.804–0.992 | 0.034 |
| VitD, ng/mL | 1.00 | 0.968–1.042 | 0.818 |
| Creatinine, mg/dL | 9.553 | 0.502–181.85 | 0.133 |
| Liver steatosis, y/n | 2.354 | 0.873–6.347 | 0.090 |
| Diabetes, y/n | 0.911 | 0.271–3.056 | 0.879 |
Discussion
Lately, the role of the calcium/VitD homeostatic network has been studied in various diseases. Overall, data suggest that VitD deficiency is involved in the pathogenesis of multiple diseases including malignancies besides its mere role in bone integrity and calcium homeostasis. Since previously published literature provides at least circumstantial evidence that primary hyperparathyroidism was associated with an increased cancer rate particularly of the colon, we aimed to investigate whether PTH was linked to the presence, number, and histological characteristics of colorectal adenomas in subjects undergoing screening colonoscopies.
The main findings of our study were that high serum PTH concentrations were associated with colon adenomas in women but not in men. In detail, this relation was due to a higher rate of adenomas in the distal colon. Regression analysis confirmed that the association of PTH with distal adenomas remained intact after adjustment for potential confounders. With regard to the gender difference of the association of PTH with colon adenomas, we do not have plausible explanation why this was observed in women and not in men. So far, the only epidemiological study by Fedirko et al., a sub-analysis of the EPIC cohort, suggested that higher PTH serum concentrations were particularly related to colon cancer in men but only non-significantly in women [21]. It is worth mentioning that the cut-off used by Fedirko et al. was 65 ng/L, however, we found very few subjects with concentrations above 65 ng/L. One may speculate that effects in men are observed at higher levels of PTH and that other factors were more important in the PTH range observed in our male study population. Taking these data together, we suggest that the link of PTH with colonic neoplasia may in fact occur in men and women even though but the two available studies failed to detect these differences in women or men, respectively. Furthermore, PTH effects may have some gender-specific differences in experimental animal settings [27], but to our knowledge, no data are available that would justify the assumption of a true biological gender difference in the association of PTH and colonic neoplasia.
Higher PTH concentrations were also associated with dysplasia in the distal colon. This adds further weight to the finding that elevated serum PTH concentrations may contribute to cancer development. Although PTH was borderline associated with dysplasias of adenomas also in the proximal colon, this association was abolished after adjustment for VitD. We published findings on the association of VitD and findings at screening colonoscopy previously and found that in this cohort, lower VitD serum concentrations were linked to a higher rate of colonic adenomas in the proximal colon. This difference in relation to the colonic sub-site suggests that low VitD does not lead to a higher rate of colonic adenomas via consecutively raised PTH concentrations. From previous investigations, it can be concluded that PTHrP, and thus likewise PTH, may promote cell proliferation and adhesion to the extracellular matrix [14]. Additionally, higher PTH concentrations may aid in inhibition of apoptosis. Thus, rather than being mutagenic by itself, higher PTH may offer a survival benefit for dysplastic cells, increasing the rate of dysplastic adenomas and thereby also cancers in the long term. However, these data were not confirmed in another study questioning the role of PTH fragments in colon carcinogenesis [28].
A vast body of literature has been published on the involvement of VitD in the development of multiple common diseases such as malignancies or cardiovascular disease. However, two aspects need to raise caution with regard to the extensive research in extra-osseous VitD roles. First, although broad epidemiological research has been performed, evidence for causality has not been demonstrated and is therefore still questionable. Second, the VitD homeostatic system is complex with interaction of multiple factors like PTH, phosphate, fibroblast growth factor 23, and klotho directly or indirectly involved, but research has almost exclusively focused on VitD serum concentrations leaving the potential involvement of these tightly related factors uninvestigated.
In addition, the relatively small sample size may be considered a limitation of our study. Admittedly, sample size may underlie the observed gender differences between our study and others [16]. However, the study participants have been characterized in detail regarding clinical, biochemical, and endoscopic findings. This meticulous patient characterization allowed for reliable adjustment for co-morbidities and VitD. Moreover, for this analysis, we rigorously excluded subjects with elevated creatinine above normal limits to avoid renal insufficiency as a cause of elevated PTH concentration. Hence, future studies will need to investigate colonoscopy findings in patients with a broader spectrum of renal disease in order to analyze the effect of elevated PTH serum concentrations over often many years.
In summary, we report a link of higher serum PTH concentrations with distal colonic adenomas and a higher rate of dysplasia therein in females undergoing screening colonoscopy. To our knowledge, this is the first report of a significant association of PTH with colorectal pathologies in a relatively healthy cohort undergoing screening colonoscopy. We suggest that the role of PTH in colon carcinogenesis should be investigated in more diverse patient cohorts and also with regard to molecular mechanisms.
Acknowledgments
The authors would like to extend their many thanks to ABBOTT Diagnostics (Vienna, Austria) for the partial provision of the reagents for some laboratory tests.
The authors gratefully acknowledge technical support by Carmen Winkler and Elke Albrecht, Department of Internal Medicine, Oberndorf Hospital, Austria. Support from Spar Austria to Christian Datz is gratefully acknowledged.
Conflict of Interest
The authors declare that they have no competing interests.
Author Contributions
EA contributed to this manuscript through the study of the concept, analysis of data, and drafting and writing of the manuscript. AS, UHS, JZ, EHM, DN, SE, FS, CP, and GS contributed to this manuscript through the acquisition of data and critical revision of the manuscript for important intellectual content. WP contributed to this manuscript through data analysis and interpretation, statistical analysis, and outlining and revising the manuscript. CD contributed to this manuscript through the study the concept and outlining and revising the manuscript.
References
- 1.D’Amour P. Acute and chronic regulation of circulating PTH: significance in health and in disease. Clin Biochem. 2012;45:964–969. doi: 10.1016/j.clinbiochem.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 2.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 3.Bjorkman MP, Sorva AJ, Tilvis RS. Elevated serum parathyroid hormone predicts impaired survival prognosis in a general aged population. Eur J Endocrinol. 2008;158:749–753. doi: 10.1530/EJE-07-0849. [DOI] [PubMed] [Google Scholar]
- 4.Sambrook PN, Chen JS, March LM, Cameron ID, Cumming RG, Lord SR, et al. Serum parathyroid hormone is associated with increased mortality independent of 25-hydroxy vitamin d status, bone mass, and renal function in the frail and very old: a cohort study. J Clin Endocrinol Metab. 2004;89:5477–5481. doi: 10.1210/jc.2004-0307. [DOI] [PubMed] [Google Scholar]
- 5.Carrivick SJ, Walsh JP, Brown SJ, Wardrop R, Hadlow NC. Does PTH increase with age, independent of 25-hydroxyvitamin D, phosphate, renal function and ionized calcium? J Clin Endocrinol Metab 2015:jc20144370 [DOI] [PubMed]
- 6.Di Monaco M, Castiglioni C, Vallero F, Di Monaco R, Tappero R. Parathyroid hormone response to severe vitamin D deficiency is sex associated: an observational study of 571 hip fracture inpatients. J Nutr Health Aging. 2013;17:180–184. doi: 10.1007/s12603-012-0088-y. [DOI] [PubMed] [Google Scholar]
- 7.Haden ST, Brown EM, Hurwitz S, Scott J, El-Hajj FG. The effects of age and gender on parathyroid hormone dynamics. Clin Endocrinol (Oxf) 2000;52:329–338. doi: 10.1046/j.1365-2265.2000.00912.x. [DOI] [PubMed] [Google Scholar]
- 8.Carron JA, Fraser WD, Gallagher JA. PTHrP and the PTH/PTHrP receptor are co-expressed in human breast and colon tumours. Br J Cancer. 1997;76:1095–1098. doi: 10.1038/bjc.1997.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Seitz PK, Thomas ML, Selvanayagam P, Rajaraman S, Cooper CW. Widespread expression of the parathyroid hormone-related peptide and PTH/PTHrP receptor genes in intestinal epithelial cells. Lab Invest. 1995;73:864–870. [PubMed] [Google Scholar]
- 10.Liang H, Zhong Y, Huang Y, Chen G. Type 1 receptor parathyroid hormone (PTH1R) influences breast cancer cell proliferation and apoptosis induced by high levels of glucose. Med Oncol. 2012;29:439–445. doi: 10.1007/s12032-011-9851-x. [DOI] [PubMed] [Google Scholar]
- 11.Calvo N, de Boland AR, Gentili C. PTH inactivates the AKT survival pathway in the colonic cell line Caco-2. Biochim Biophys Acta. 1803;2010:343–351. doi: 10.1016/j.bbamcr.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Coxam V, Davicco MJ, Durand D, Bauchart D, Lefaivre J, Barlet JP. The influence of parathyroid hormone-related protein on hepatic IGF-1 production. Acta Endocrinol (Copenh) 1992;126:430–433. doi: 10.1530/acta.0.1260430. [DOI] [PubMed] [Google Scholar]
- 13.Johansson AG, Baylink DJ, af Ekenstam E, Lindh E, Mohan S, Ljunghall S. Circulating levels of insulin-like growth factor-I and -II, and IGF-binding protein-3 in inflammation and after parathyroid hormone infusion. Bone Miner. 1994;24:25–31. doi: 10.1016/S0169-6009(08)80128-6. [DOI] [PubMed] [Google Scholar]
- 14.Shen X, Falzon M. PTH-related protein enhances LoVo colon cancer cell proliferation, adhesion, and integrin expression. Regul Pept. 2005;125:17–27. doi: 10.1016/j.regpep.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 15.Malakouti S, Asadi FK, Kukreja SC, Abcarian HA, Cintron JR. Parathyroid hormone-related protein expression in the human colon: immunohistochemical evaluation. Am Surg. 1996;62:540–544. [PubMed] [Google Scholar]
- 16.Kawamura YJ, Kazama S, Miyahara T, Masaki T, Muto T. Sigmoid colon cancer associated with primary hyperparathyroidism: report of a case. Surg Today. 1999;29:789–790. doi: 10.1007/BF02482329. [DOI] [PubMed] [Google Scholar]
- 17.Feig DS, Gottesman IS. Familial hyperparathyroidism in association with colonic carcinoma. Cancer. 1987;60:429–432. doi: 10.1002/1097-0142(19870801)60:3<429::AID-CNCR2820600325>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson IL, Zedenius J, Yin L, Ekbom A. The association between primary hyperparathyroidism and malignancy: nationwide cohort analysis on cancer incidence after parathyroidectomy. Endocr Relat Cancer. 2007;14:135–140. doi: 10.1677/erc.1.01261. [DOI] [PubMed] [Google Scholar]
- 19.Artru P, Tournigand C, Mabro M, Lucchi E, Louvet C, De Gramont A, et al. Primary hyperparathyroidism associated with colon cancer. Gastroenterol Clin Biol. 2001;25:208–209. [PubMed] [Google Scholar]
- 20.Farr HW, Fahey TJ, Jr, Nash AG, Farr CM. Primary hyperparathyroidism and cancer. Am J Surg. 1973;126:539–543. doi: 10.1016/S0002-9610(73)80046-7. [DOI] [PubMed] [Google Scholar]
- 21.Fedirko V, Riboli E, Bueno-de-Mesquita HB, Rinaldi S, Pischon T, Norat T, et al. Prediagnostic circulating parathyroid hormone concentration and colorectal cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Cancer Epidemiol Biomarkers Prev. 2011;20:767–778. doi: 10.1158/1055-9965.EPI-10-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stadlmayr A, Aigner E, Steger B, Scharinger L, Lederer D, Mayr A, et al. Nonalcoholic fatty liver disease: an independent risk factor for colorectal neoplasia. J Intern Med. 2011;270:41–49. doi: 10.1111/j.1365-2796.2011.02377.x. [DOI] [PubMed] [Google Scholar]
- 23.Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed) 1986;292:13–15. doi: 10.1136/bmj.292.6512.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 25.Adams SV, Newcomb PA, Burnett-Hartman AN, White E, Mandelson MT, Potter JD. Circulating 25-hydroxyvitamin-D and risk of colorectal adenomas and hyperplastic polyps. Nutr Cancer. 2011;63:319–326. doi: 10.1080/01635581.2011.535960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aigner E, Stadlmayr A, Huber-Schonauer U, Zwerina J, Husar-Memmer E, Niederseer D, et al. Gender- and site-specific differences of colorectal neoplasia relate to vitamin D. Aliment Pharmacol Ther. 2014;40:1341–1348. doi: 10.1111/apt.12981. [DOI] [PubMed] [Google Scholar]
- 27.Babey M, Wang Y, Kubota T, Fong C, Menendez A, ElAlieh HZ, et al. Gender specific differences in the skeletal response to continuous PTH in mice lacking the IGF1 receptor in mature osteoblasts. J Bone Miner Res 2014. [DOI] [PMC free article] [PubMed]
- 28.Whitfield J, Bird RP, Morley P, Willick GE, Barbier JR, MacLean S, et al. The effects of parathyroid hormone fragments on bone formation and their lack of effects on the initiation of colon carcinogenesis in rats as indicated by preneoplastic aberrant crypt formation. Cancer Lett. 2003;200:107–113. doi: 10.1016/S0304-3835(03)00162-9. [DOI] [PubMed] [Google Scholar]