Abstract
Ovarian cancer is a highly metastatic disease. The metastatic potential is enhanced by epithelial to mesenchymal transition (EMT) in which αvβ3 integrin plays a role. Thyroid hormones (l-thyroxine, T4, and 3,5,3′-triiodo-l-thyronine, T3) bind this integrin, and we hypothesized that the thyroid hormone-αvβ3 axis may be involved in EMT activity in ovarian cancer. The transcription (mRNA), protein abundance (westerns), and protein localization (fluorescence microscopy) of several EMT markers were studied in ovarian cancer cells (OVCAR-3, A2780, and SKOV-3) treated with 1 nM T3 or 100 nM T4 for 1–24 h. The protein levels of β-catenin, and its downstream targets, zeb-1, slug, and vimentin, were significantly induced by both hormones, while the effect on transcription was limited. The pre-incubation of the cells overnight with two integrin inhibitors, RGD (0.1–10 μM) or αvβ3 blocking antibody (1–100 ng/mL), prevented the induction of β-catenin by T3 and zeb-1 by T4, indicating direct integrin involvement. The transcription of the mesenchymal markers, β-catenin, zeb-1, slug/snail, vimentin, and n-cadherin was hardly affected by T3 and T4, while that of the epithelial markers, e-cadherin and zo-1, was inhibited. Our results suggest a novel role for the thyroid hormone-αvβ3 axis in EMT, with possible implications for ovarian cancer metastasis.
Electronic supplementary material
The online version of this article (10.1007/s12672-017-0316-3) contains supplementary material, which is available to authorized users.
Keywords: αVβ3 Blocking Antibody, Vimentin, Mesenchymal Markers, Integrin Inhibitors, Ovarian Cancer Cell Model
Background
Ovarian cancer is a highly metastatic disease and the second leading cause of death from gynecologic malignancies [1]. Fewer than 20% of the cases are diagnosed when the tumor is localized. The 5-year survival of women diagnosed with late stage ovarian cancer is still less than 50% [2]. An improved understanding of the molecular changes associated with ovarian cancer metastasis could lead to the identification of new targets and therapeutic interventions.
Epithelial-mesenchymal transition (EMT) is a biological process that is essential to driving plasticity during embryonic development and to cancer invasiveness and metastasis [3], including these functions in ovarian cancer [4, 5]. In essence, it is a trans-differentiation process characterized by decreased epithelial markers (e.g., e-cadherin) and increased mesenchymal markers (e.g., vimentin). Cancer cells undergoing EMT lose their epithelial morphology, reorganize their cytoskeleton, and acquire a motile phenotype leading to enhanced migratory capacity, invasiveness, and increased resistance to apoptosis. Several proteins are up- or downregulated during EMT and have been recognized as EMT markers [6, 7]. These proteins have been shown to be involved in ovarian cancer progression and include β-catenin [8], vimentin [9, 10], zeb-1 (TCF8) [9, 10], slug (SNAI2) and snail (SNAI1) [5], n-cadherin [9] and e-cadherin, claudins, and zo-1 (D7D12) [10].
Integrins are cell surface heterodimeric transmembrane glycoproteins, which facilitate cell-cell and cell extracellular matrix (ECM) adhesion and migration [11]. An important member of this family is the αvβ3 integrin which participates in many essential cancer signaling pathways. αvβ3 is over-expressed by an array of cancer cells and was found to correlate with tumor progression [12]. Its expression is particularly prominent in metastatic tissues [11, 13]. This specific integrin is amply expressed by ovarian cancer cell lines and primary tumor tissues from ovarian cancer patients and has been found to correlate with tumor progression and metastasis [12, 14–22]. αvβ3 integrin interacts with ECM proteins primarily through an Arg-Gly-Asp (RGD) recognition site on the extracellular domain, but was recently shown to have a panel of small “non RGD” molecule ligands. Among these small molecule ligands are the thyroid hormones l-thyroxine (T4) and the principal intracellular hormone 3,5,3′-triiodo-l-thyronine (T3), which are important regulators of differentiation, growth, and metabolism of virtually all tissues [23] including the ovary [24–27]. In a population-based case-control study, a relationship between hyperthyroidism and risk of ovarian cancer has been suggested [28, 29], but other studies have not provided support for such an association [30, 31].
The receptor for these moieties on the integrin is close to, but independent of, the RGD recognition site [32, 33]. The discovery that both hormones bind and act at αvβ3, with a substantially higher affinity for T4, established that T4 is a hormone at this site, rather than serving as a prohormone for T3. The actions initiated by T4 or by T3 at the integrin are non-genomic, that is, they are initiated quickly (minutes-hours), do not require hormone interaction with the nuclear thyroid hormone receptors (TRs), and regulate intracellular events, including specific gene transcription by kinase transduction signaling, mainly, the mitogen-activated protein kinase (MAPK) pathway. Such non-genomically initiated effects by the thyroid hormone-αvβ3 axis were described by our group in multiple myeloma [34–36], melanoma [37], and ovarian cancer cell models [38]. These actions are induced by 100 nM T4 concentrations that yield physiologic free hormone concentrations4 and slightly supra-physiological levels of T3 (1 nM), highlighting the relevance of the thyroid-integrin axis in ovarian cancer patients with normal circulating hormone levels. Although involvement of the αvβ3 integrin in the acquisition of the EMT phenotype was recently reported in vitro and in vivo [39, 40], direct involvement of thyroid hormones in EMT has not been described. In the current work, we have studied in ovarian cancer cell lines the dynamic effects of thyroid hormones on central EMT markers and the involvement of αvβ3 integrin in this process.
Methods
Cell Lines
Human ovarian adenocarcinoma cells used in the study were OVCAR-3 (high-grade serous ovarian cancer cells, ATCC HTB-161), SKOV-3 (low-grade serous ovarian cancer cells, ATCC HTB-77), and A2780 (low-grade ovarian cancer, endometroid histology, Sigma-Aldrich, Steinheim, Germany) [41–43]. All cell lines were authenticated at the Genomics Core Facility of BioRap Technologies and the Rappaport Research Institute, Technion, Israel. The test was performed using the Promega PowerPlex 16 HS kit in order to determine short tandem repeat (STR) profile of 15 loci plus amelogenin for sex determination (X or XY). The results were analyzed using the 3130xl Genetic Analyzer (Life Technologies) and GeneMapper IDX software. Positive and negative controls were run and confirmed in each test. The cells were negative for mycoplasma contamination. Cells were cultured in RPMI1640 supplemented with 10% heat-inactivated fetal bovine serum and antibiotics. Before conducting an experiment, the cells were cultured for 24 h in RPMI1640 supplemented with 1.5% heat-inactivated charcoal-stripped fetal bovine serum and antibiotics. Bovine-source thyroid hormones were removed from the serum by stripping, as confirmed by measurements of total and free hormones levels using the IMMULITE system (Siemens, Erlangen, Germany).
Reagents and Chemicals
T3 (1 nM); T4 (100 nM), dissolved in 0.04 N KOH in 4% propylene glycol (PG); and RGD and RGE (Arg-Gly-Glu) peptides were from Sigma-Aldrich. The αvβ3 blocking antibody (clone LM609) was obtained from Merck Millipore, Darmstadt, Germany. Primary rabbit antibodies against human β-catenin, vimentin, and GAPDH were from the EMT Western blot cocktail (Abcam, Cambridge, UK). Primary antibodies against slug, Zo-1, N-cadherin, E-cadherin, Snail, Claudin-1, and Zeb-1 were from Cell Signaling (Leiden, The Netherlands).
Western Blotting
Whole-cell lysates um from each experiment were separated on 10–12.5% polyacrylamide gels, fast transferred to polyvinylidene difluoride membranes (PVDF), incubated with the indicated antibodies, and visualized using horseradish peroxidase conjugated secondary antibody (1:10,000, Jackson ImmunoResearch Laboratories, West Grove, PA, USA), followed by enhanced chemiluminescence detection (Biological Industries, Beit HaEmek, Israel). Integrated optical densities of the bands were measured by the Image reader Las3000, Multi-gauge v3.0 software.
Fluorescence Microscopy
Cells were treated, fixed, and permeabilized with 0.1% Triton X-100 for 5 min at room temperature and then incubated with the indicated primary rabbit anti human antibodies for 1 h. A secondary NL-493 donkey anti-rabbit immunoglobulin G was used (R&D Systems, Minneapolis, MN, USA). Hoechst 33342 was used for nuclear staining (Sigma-Aldrich). Cells were visualized by a fluorescence microscope equipped with a camera (model IX71; Olympus) with a ×40/0.50 objective lens and Cell^A (version 3.1) Olympus software imaging.
RNA Extraction and cDNA Generation
RNA was extracted using NucleoSpin RNA II kit (Macherey-Nagel, Düren, Germany) and eluted in 40 μL RNase-free water. RNA concentration and purity were measured using NanoDrop™ 1000 Spectrophotometer (Thermo Scientific, Wilmington, DE, USA). RNA (200 ng) was reverse transcribed using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA), according to the manufacturer’s instructions.
Real-Time PCR
mRNA levels were measured by Real-Time PCR (7500 Fast system, Applied Biosystems, Carlsbad, CA, USA), using Fast SYBR Green Master Mix (Applied Biosystems). Results were calculated as fold change using the comparative threshold cycle method (2−ΔΔCT) relative to control cells, i.e., controls are assigned a value of 1 by definition. GAPDH was used for normalization. Primers (Hylabs, Rehovot, Israel) were designed (Primer Express software, Applied Biosystems) in different exons to minimize DNA contamination:
β-catenin reverse primer: GATTCCTGAGAGTCCAAAGACAG
β-catenin forward primer: CACAAGCAGAGTGCTGAAGGTG
Zeb-1 reverse primer: TGGGCGGTGTAGAATCAGAGTC
Zeb-1 forward primer: GGCATACACCTACTCAACTACGG
Claudin-1 reverse primer: CACCTCATCGTCTTCCAAGCAC
Claudin-1 forward primer: GTCTTTGACTCCTTGCTGAATCTG
Zo-1 reverse primer: TGGGCGGTGTAGAATCAGAGTC
Zo-1 forward primer: GGCATACACCTACTCAACTACGG
Slug reverse primer: GAGCCCTCAGATTTGACCTGTC
Slug forward primer: ATCTGCGGCAAGGCGTTTTCCA
Snail reverse primer: GGGACAGGAGAAGGGCTTCTC
Snail forward primer: TGCCCTCAAGATGCACATCCGA
E-cadherin reverse primer: TGGCAGTGTCTCTCCAAATCCG
E-cadherin forward primer: GCCTCCTGAAAAGAGAGTGGAAG
N-cadherin reverse primer: GTGGCGATGGTTCCTTCATTTCC
N-cadherin forward primer: CTGTCACTGCTCAAGACCTGGA
Vimentin reverse primer: ATCTGGCGTTCCAGGGACTCAT
Vimentin forward primer: AGGCAAAGCAGGAGTCCACTGA
GAPDH reverse primer: GTCTCCTCTGACTTCAACAGCG
GAPDH forward primer: ACCACCCTGTTGCTGTAGCCAA
Statistical Analysis
Experiments were conducted in triplicate at least three times, and results were analyzed by a Student’s unpaired t test (p < 0.05).
Results
Basal Levels of EMT Markers in Different Ovarian Cancer Cell Models
The choice of the three ovarian cancer cell models was based on a recent genomic profile evaluation [43] in order to represent different histological, molecular subtypes and grades of ovarian cancer. OVCAR-3 represents type II high-grade serous ovarian tumors, while SKOV-3 and A2780 correlate with type I low-grade tumors of different histology. The basal level of nine EMT markers was examined in the cells. These included Zo-1, Zeb-1, N-cadherin and E-cadherin, β-catenin, vimentin, slug, snail, and claudin-1. Results (Supplementary Figure 1) indicate that the cells express different basal levels of these protein markers. Specifically, zeb-1, β-catenin, and slug were higher in OVCAR-3 cells in comparison to the other two cell models, while a low vimentin level, which is typically more abundant in mesenchymal-like cells, was observed in these cells. In 2780 cells, in contrast, very low levels of β-catenin, slug, snail, and claudin-1 and high vimentin levels were clearly observed. Based on initial results, four of these EMT markers were selected for further analysis. The first, β-catenin, is a key EMT player in cancer [6, 7], including ovarian cancer [8]. β-catenin regulates a panel of downstream targets, which themselves operate as transcription factors. We have selected to focus on two of these proteins, zeb-1 [44] and slug [9]. Lastly, vimentin, a key mesenchymal marker [6, 7], was chosen for further analysis.
Thyroid Hormones Affect Central EMT Proteins in Ovarian Cancer
Next, we evaluated the levels of four key EMT markers, zeb-1, β-catenin, vimentin, and slug in response to treatment with thyroid hormones in the three ovarian cancer cell models. To note, the three ovarian cancer cell models are negative for the nuclear thyroid receptor beta 1 (TRβ1), through which T3 exerts its classical genomic effects [38].
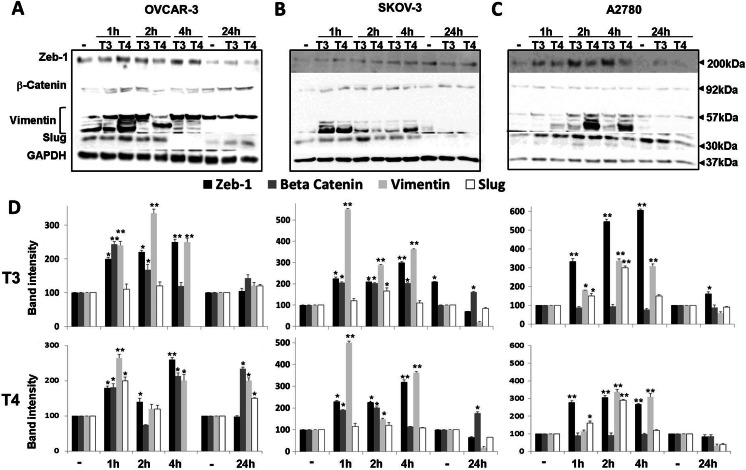
The cells (2 × 106 cells/well in six well plates) were grown in media containing hormone-negative 1.5% charcoal-stripped serum overnight, to minimize any residual effect of medium thyroid hormones from prior culturing and were incubated the next day with T3 (1 nM, slightly supra-physiological concentration) or T4 (100 nM, which yields a physiological concentration of free hormone in the medium) for 1, 2, 4, and 24 h. At the indicated times, total proteins were extracted and Western blot analysis was conducted using primary antibodies against the human zeb-1, β-catenin, vimentin, and slug. Representative blots for OVCAR-3 (Fig. 1a), SKOV-3 (Fig. 1b), and A2780 (Fig. 1c) are shown. The calculated normalized results for T3 and T4 are presented in Fig. 1d. Analysis of zeb-1, a zinc finger protein that transcribes an array of genes involved in EMT, reveals clear induction of the protein by both hormones in the three cell models. This effect was generally observed between 1 and 4 h of incubation with the hormones, decreasing in all cells after 24 h of treatment. Analysis of β-catenin protein levels indicated early induction in OVCAR-3 and SKOV-3 cells by both hormones, an effect which was sustained for 24 h. In A2780 cells, which express low basal β-catenin levels, no effect on β-catenin level was documented. The third mesenchymal marker examined, vimentin, was induced by both thyroid hormones in all three cell lines, although with different time patterns. Slug protein was induced in parallel with vimentin, but there was a difference between the actions of T3 and T4. For this specific protein in OVCAR-3 cells, T3 minimally affected the abundance of slug, whereas T4 caused an increase at 1–2 h that was completely lost by 4 h; a second peak was apparent at 24 h only with T3 treatment, whereas in A2780 cells, both hormones produced early increases in the level of this protein.
Fig. 1.
Induction of EMT proteins by T3 or T4 treatment of ovarian cancer cell lines. a OVCAR-3, b SKOV-3, and c A2780 cell lines were treated with T3 (1 nM) or T4 (100 nM) for short (1–4 h) or long (24 h) incubation periods, after which Western blots analyses for the protein levels of zeb-1, β-catenin, vimentin, and slug were performed on whole-cell lysates. Shown is one representative experiment of three performed. d Representative quantification of band intensity of the corresponding Western blots is presented as ratio of target protein over GAPDH intensity. Values are means ± SD. Densitometry is expressed as percentage compared with the vehicle treated group (considered as 100%). *, p < 0.05; **, p < 0.005
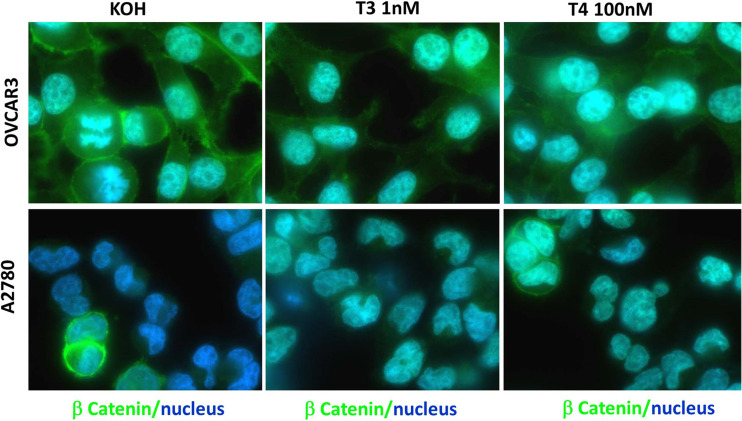
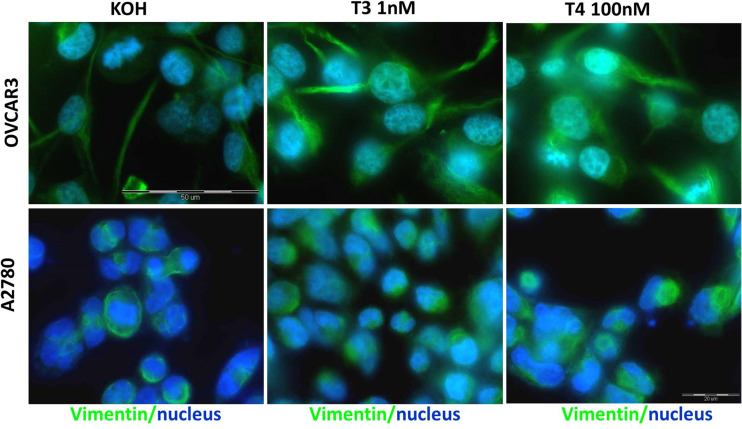
To define hormonal effects on the EMT markers in the setting of intact cells, we carried out immunofluorescence (IF) microscopy experiments. OVCAR-3 and A2780 cells were treated with 1 nM T3 or 100 nM T4 for 2 h. Following fixation and permeabilization, the cells were incubated with primary antibodies specific for β-catenin, vimentin, or zeb-1, followed by incubation with appropriate secondary antibodies. The cells were examined under a light microscopy as well as fluorescent microscopy. The background stain of an isotype control is presented in Supplementary Figure 2. Representative merged images of the proteins examined (green) and nuclear staining (blue, Hoechst nuclear dye) are shown in both ovarian cancer cell lines for β-catenin (Fig. 2), vimentin (Fig. 3), or zeb-1 (Fig. 4). In details, β-catenin (Fig. 2) was observed in OVCAR-3 cells mainly in the cytosol and nucleus, with a moderate membrane expression. Following the addition of T3, and more so T4, a clear accumulation of nuclear β-catenin localization was documented. In A2780, a low basal membrane expression of β-catenin was documented and was slightly induced in the nucleus by both thyroid hormones. Images of the various channels (light, green, and blue) in both cell models are shown in Supplementary Figure 3. Regarding vimentin (Fig. 3), in OVCAR-3 cells, cytosolic vimentin was shown to be induced by T3, while T4 triggered both cytosolic and nuclear vimentin localizations. In A2780, a strong cytoplasmic stain was observed, which was induced by T3 and to a lesser extent by T4. Images of the various channels (light, green, and blue) in both cell models are shown in Supplementary Figure 4. Lastly, for zeb-1 (Fig. 4), basal nuclear staining was evident in both cell models. Following T3 and T4 treatments, enhancement in fluorescence was observed in the cell nucleus of OVCAR-3, while in A2780, this was particularly evident following T3 treatment and corresponds with the Western blot results. Images of the various channels (light, green, and blue) in both cell models are shown in Supplementary Figure 5.
Fig. 2.
Immunofluorescence analysis of β-catenin following T3 or T4 treatment of ovarian cancer cell lines. a A2780 and b OVCAR-3 cells were treated with 1 nM T3 or 100 nM T4 for 2 h, followed by fixation and permeabilization. β-catenin is depicted in green and the nucleus in blue (Hoechst nuclear dye). The images were obtained by a fluorescence microscope equipped with a camera (model IX71; Olympus) using emersion oil (×63) objective lens and Cell^A (version 3.1) Olympus software imaging (Color figure online)
Fig. 3.
Immunofluorescence analysis of vimentin following T3 or T4 treatment of ovarian cancer cell lines. a A2780 and b OVCAR-3 cells were treated with 1 nM T3 or 100 nM T4 for 2 h, followed by fixation and permeabilization. Vimentin is depicted in green and the nucleus in blue (Hoechst nuclear dye). The images were obtained by a fluorescence microscope equipped with a camera (model IX71; Olympus) using emersion oil (×63) objective lens and Cell^A (version 3.1) Olympus software imaging (Color figure online)
Fig. 4.
Immunofluorescence analysis of zeb-1 following T3 or T4 treatment of ovarian cancer cell lines. a A2780 and b OVCAR-3 cells were treated with 1 nM T3 or 100 nM T4 for 2 h, followed by fixation and permeabilization. Zeb-1 is depicted in green and the nucleus in blue (Hoechst nuclear dye). The images were obtained by a fluorescence microscope equipped with a camera (model IX71; Olympus) using emersion oil (×63) objective lens and Cell^A (version 3.1) Olympus software imaging (Color figure online)
In parallel to the protein analyses, OVCAR-3 and A2780 cells (0.5 × 106 cells/well in six-well plates) were incubated with T3 (1 nM) or T4 (100 nM) for the indicated times (1–4 and 24 h) and RNA was then extracted in order to perform RQ-PCR analysis. To that end, specific primers for the identification of the four selected genes, zeb-1, β-catenin, vimentin, and slug were designed. GAPDH was used as a loading control. In general, we did not observe a consistent effect on the transcription of these genes by the two hormones in the two cell lines (Supplementary Figure 6). Moreover, no clear correlation with their effect on protein expression was documented. Additional genes that are tightly regulated by the three transcription factors detailed previously, β-catenin, slug, and zeb-1, were also examined for hormonal responsiveness. The potential of T3 and T4 to affect n-cadherin, e-cadherin, zo-1, claudin-1, and snail is presented in Supplementary Figure 7. Similar to the previously detailed genes, this panel of genes was hardly affected by the hormones, excluding two important markers for the epithelial phenotype, e-cadherin (Supplementary Figure 7B), and zo-1 (Supplementary Figure 7C), which were reduced by an average 50–70%. It is important to note that e-cadherin was completely absent from A2780, cells that are not considered epithelial in nature. Taken together, the effect of the thyroid hormones on the transcription of some EMT genes was minimal in these ovarian cancer models and did not correlate with the hormonal effect on protein levels which was more substantial. These collective results suggest that the effect by the hormones is via regulation of protein turnover, rather than transcription/translation initiation.
Involvement of the Thyroid Hormones-αvβ3 Integrin Axis in the Elevation of EMT Markers in Ovarian Cancer
The basal level of the αvβ3 integrin expression in different human ovarian cancer cell models has been previously analyzed by our group [38]; the results indicate that all cell systems studied are integrin positive, with the highest expression demonstrated by OVCAR-3. In order to validate the involvement of the αvβ3 integrin in the accumulation of EMT markers by the thyroid hormones, the cells (2.5 × 106 cells/six well plates) were pre-incubated overnight with two commonly used αvβ3 integrin antagonists. The first, RGD peptide, is a blocker of the RGD-recognition site upon the integrin (RGE peptide served as negative control). This site is located at close proximity to the thyroid hormone binding site and therefore may hinder hormone binding to the integrin allosterically. In parallel, a functional blocking antibody against the αvβ3 integrin dimer was used. In this work, we used the clone LM609, which is well characterized and a widely used blocking antibody. Following incubation with the two antagonists at increasing concentrations, the cells were treated with T3 (1 nM) or T4 (100 nM) for 2 h and total protein was extracted for Western blot analysis. Results indicate that β-catenin induction by T3 (Fig. 5a), but not by T4 (Fig. 5b), was effectively blocked by RGD, as well as by the functional integrin blocker at various concentrations. In contrast, the induction in zeb-1 levels by T4, but not by T3, was similarly inhibited in the presence of the two integrin antagonists. The induction of vimentin or slug by both thyroid hormones was not affected by the two integrin inhibitors. The calculated normalized results for T3 and T4 are presented in Fig. 5c. In parallel, the effect of the antagonists alone, in the absence of hormones, was examined (Supplementary Figure 8). Reduction of zeb-1 and vimentin was observed by RGD, but not by RGE, at selected concentrations. The functional αvβ3 blocking antibody inhibited at specific concentrations zeb-1, vimentin, and slug protein expressions, with no effect on β-catenin.
Fig. 5.
Involvement of the thyroid hormone-αvβ3 integrin axis in the elevation of EMT markers in ovarian cancer. OVCAR-3 cells were pre-incubated overnight with increasing concentrations of RGD or functional blocking αvβ3 antibody. Following incubation, the cells were treated with a T3 (1 nM) or b T4 (100 nM) for 2 h, total proteins were extracted, and Western blot analysis for the levels of zeb-1, β-catenin, vimentin, and slug was performed. Shown here is a representative result of three experiments performed. c Representative quantification of band intensity of the corresponding Western blots is presented as the ratio of target protein over GAPDH intensity. Values are means ± SD. Densitometry is expressed as percentage compared with the vehicle treated group (considered as 100%). *p < 0.05 by t test. #, significant inhibition compared to hormone-treated cells
Discussion
Ovarian cancer is highly aggressive with the majority of cases diagnosed after distant metastasis has occurred. Improved understanding of the molecular basis for disease progression is important in this illness.
The αvβ3 integrin plays a role in cancer progression [21], including in ovarian cancer [12, 14–22]. Epithelial-to-mesenchymal transition is a phenomenon that, when occurs in malignant cells, enhances metastatic potential. [7]. The αvβ3 integrin is pivotal in the acquisition of the EMT phenotype, but limited published data exist regarding its role in ovarian cancer [16]. The thyroid hormones T4 and T3 bind to αvβ3 at a specific receptor and participate in the oncogenic process in a number of ways [33]. We therefore hypothesized that there may be a previously unrecognized role for the thyroid hormone-αvβ3 axis in the induction of EMT, and we have examined this hypothesis in ovarian cancer cell models. We used three human ovarian cancer cells lines representing type II high-grade serous ovarian tumors (OVCAR-3 cells) and type I low-grade tumors (SKOV-3, serous and A2780, undefined histology) [43]. In all three cell lines, the αvβ3 integrin was expressed, with OVCAR-3 exhibiting the highest integrin level, while in the lower grade cells, a lower expression of αvβ3 was evident. This pattern of integrin expression among the different cells correlates with published results, suggesting higher integrin expression in late disease stages [20, 22].
The interplay between thyroid hormones and EMT in ovarian cancer was assessed on a selected set of protein markers. Several proteins are known to be up- and downregulated during EMT [3, 7]. A key driver of EMT in cancer, including ovarian cancer [8], is β-catenin [6, 7]. Our current work provides data indicating that β-catenin is potently and rapidly induced by T4 and T3 at the protein level. Induction by T3 of both transcription and translation of β-catenin has been documented in normal cells [45] and in animal models [46]. However, two reports in cancer cells [47, 48] have indicated an opposite effect, but these studies depended upon higher hormone concentrations and longer incubation times than those that we used. Furthermore, these studies did not examine the effect of T4 on EMT markers. We have further provided data, by utilizing two common integrin blockers, that the induction of β-catenin protein synthesis by both hormones is initiated at the αvβ3 integrin and can therefore be considered the first EMT marker to be subject to non-genomic control. These rapid onset actions have been confirmed to exist in ovarian cancer cells lacking the classical nuclear thyroid hormone receptor [38], suggesting for non-genomic effects by the hormones. Support for the role of the thyroid hormone-αvβ3 axis on β-catenin regulation came from two studies in which a specific T4 analog, tetraiodoacetic acid (tetrac), which selectively blocks the thyroid hormone binding site on the integrin, inhibited β-catenin expression in two cancer models [49, 50].
β-catenin acts as a master transcription factor, regulating a panel of downstream targets, which themselves operate as transcription factors. These include zeb-1 [44] and slug/snail [9], mesenchymal markers that amplify the transcription of β-catenin via a positive feedback loop. These transcription factors further regulate the transcription of several EMT markers [6, 7], including upregulation of the mesenchymal markers, vimentin, caludin-1, and n-cadherin and the inhibition of expression of epithelial markers such as e-cadherin and zo-1. Our results provide evidence for an induction in zeb-1, slug, and vimentin protein synthesis by both T3 and T4. Among these three EMT markers, zeb-1 regulation by T4 was shown to be integrin mediated. Lastly, we have shown the both T3 and T4 inhibit the transcription of the epithelial markers, e-cadherin, and zo-1. These actions were initiated quickly (within 1–4 h), consistent with non-genomic actions by the hormones. Of note, there are no data currently suggesting an effect of thyroid hormones on the abovementioned transcription factors and/or EMT genes; however, an association between the αvβ3 integrin and β-catenin [51–53], Zo-1 [54], vimentin [55, 56], Slug [57, 58], and e-cadherin [59] has been reported.
To note, a hormonal effect on the protein levels of the various EMT markers was validated by two complementary methods. Nevertheless, no clear correlation was documented with the transcription of these genes. Evidence exists that thyroid hormone can increase efficiency of mRNA translation without necessarily an increase in specific mRNA abundance, as is the case in the current study. Some specific examples of this are thyroid hormone action on histone generation [60], on GH production [61] and on NOS (nitric oxide synthase) isoform translation [62]. We have also shown that the half-lives of specific mRNAs may be decreased by thyroid hormone, but with sufficient increase in translation efficiency to cause increased protein abundance in response to the hormone [63]. In another study, we recently reported that a small (0.3-fold) increase in specific (PD-L1) mRNA abundance in response to T4 caused a 2.7-fold increase in the PD-L1 protein abundance [64]. Similar lack of correlation between the protein and the mRNA levels was reported before for beta catenin [65, 66], vimentin [65, 67], and zeb-1 [68]. The failure to find a perfect correlation between the EMT proteins examined in this current work and their RNA levels can be further clarified by post-transcriptional mechanisms. Specifically, epithelial-mesenchymal transition is known to be regulated through epigenetic and post-translational modifications [69]. Such post-translation modifications were reported for beta catenin [70], vimentin [71], and zeb-1 [68] and suggest that regulation at the protein level, rather than de novo RNA and protein synthesis, is the common path. To note, genome-wide correlation between expression levels of mRNA and protein is notoriously poor, hovering around 40% explanatory power across many studies [65, 72–74]. Given the numerous ways proteins are controlled post-translationally, together with mRNA translation regulation by microRNAs, the low correlation with mRNA levels does not seem unreasonable [74, 75]. Interestingly, poor correlation between mRNA and protein was expected for the most important and interesting regulators of cellular division and differentiation, such as the EMT markers examined in this work, while a relatively good correlation is expected for housekeeping proteins [74].
We demonstrated an effect of thyroid hormones on EMT markers in both low-grade and high-grade ovarian cancer cell lines. However, the hormones caused different expression patterns of the markers that may reflect inherent biologic differences between the cells. A pivotal transcription factor in EMT, β-catenin, was hormonally induced more rapidly and to a greater extent in the high-grade OVCAR-3 cells than in the low-grade cells, while vimentin was more profoundly affected in the low-grade SKOV-3 and A2780 cells.
We also examined the localization pattern within cells of zeb-1, β-catenin, and vimentin following hormonal treatment. Zeb-1 and β-catenin were induced in the nuclei of OVCAR-3 and A2780 cell models, whereas vimentin accumulated in the cytosol. In the case of β-catenin, nuclear accumulation indicates the activation of its oncogenic form [8]. Such nuclear localization was observed in T4-treated OVCAR-3 cells, supporting the hormone’s previously reported mitogenic effects in this high-grade cell model. Low-grade serous carcinoma represents less than 10% of all cases of serous ovarian carcinomas and tends to be confined to the ovary and to behave in an indolent manner [76]. Nevertheless, women with low-grade disease may exhibit chemotherapy resistance and remain at significant risk for recurrence and cancer-related death [77]. The observation that thyroid hormones may take part in the induction of central EMT proteins—not only in high-grade but also in low-grade ovarian cancer models—may explain in part an apparent role for the hormones, as part of the disease microenvironment in recurrence of low-grade cases.
To conclude, we hypothesized that there is a previously unrecognized role for the thyroid hormone-αvβ3 axis in the induction of EMT in ovarian cancer. Our data support this hypothesis and define a novel mechanism that may play a central role in ovarian cancer metastasis. Nano-particle antagonists of thyroid-integrin binding that are currently being developed by our group may represent novel agents that may limit the metastatic potential of ovarian carcinoma.
Electronic Supplementary Material
(PDF 1.14 MB)
Author Contributions
OAF designed, analyzed, and interpreted the experimental data. CW, YJ, DM, and AA preformed the experimental data. RYT preformed the real-time PCR experiments. AH, PJD, ME, and OAF wrote the manuscript. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with Ethical Standards
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Su Z, Graybill WS, Zhu Y. Detection and monitoring of ovarian cancer. Clin Chim Acta. 2013;415:341–345. doi: 10.1016/j.cca.2012.10.058. [DOI] [PubMed] [Google Scholar]
- 3.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 4.Davidson B, Trope CG, Reich R. Epithelial-mesenchymal transition in ovarian carcinoma. Front Oncol. 2012;2:33. doi: 10.3389/fonc.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim MK, Kim MA, Kim H, Kim YB, Song YS. Expression profiles of epithelial-mesenchymal transition-associated proteins in epithelial ovarian carcinoma. Biomed Res Int. 2014;2014:495754. doi: 10.1155/2014/495754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arend RC, Londono-Joshi AI, Straughn JM, Jr, Buchsbaum DJ. The Wnt/beta-catenin pathway in ovarian cancer: a review. Gynecol Oncol. 2013;131(3):772–779. doi: 10.1016/j.ygyno.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 9.Miow QH, Tan TZ, Ye J, Lau JA, Yokomizo T, Thiery JP, Mori S. Epithelial-mesenchymal status renders differential responses to cisplatin in ovarian cancer. Oncogene. 2015;34(15):1899–1907. doi: 10.1038/onc.2014.136. [DOI] [PubMed] [Google Scholar]
- 10.Wu DI, Liu L, Ren C, Kong D, Zhang P, Jin X, Wang T, Zhang G. Epithelial-mesenchymal interconversions and the regulatory function of the ZEB family during the development and progression of ovarian cancer. Oncol Lett. 2016;11(2):1463–1468. doi: 10.3892/ol.2016.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo W, Giancotti F. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5(10):816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 13.Desgrosellier JS, Barnes LA, Shields DJ, Huang M, Lau SK, Prevost N, Tarin D, Shattil SJ, Cheresh DA. An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat Med. 2009;15(10):1163–1169. doi: 10.1038/nm.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boger C, Kalthoff H, Goodman SL, Rocken C. Validation and comparison of anti-alphavbeta3 and anti-alphavbeta5 rabbit monoclonal versus murine monoclonal antibodies in four different tumor entities. Appl Immunohistochem Mol Morphol. 2013;21(6):553–560. doi: 10.1097/PAI.0b013e318284a03a. [DOI] [PubMed] [Google Scholar]
- 15.Cannistra SA, Ottensmeier C, Niloff J, Orta B, DiCarlo J. Expression and function of beta 1 and alpha v beta 3 integrins in ovarian cancer. Gynecol Oncol. 1995;58(2):216–225. doi: 10.1006/gyno.1995.1214. [DOI] [PubMed] [Google Scholar]
- 16.Carduner L, Leroy-Dudal J, Picot CR, Gallet O, Carreiras F, Kellouche S. Ascites-induced shift along epithelial-mesenchymal spectrum in ovarian cancer cells: enhancement of their invasive behavior partly dependant on alphav integrins. Clin Exp Metastasis. 2014;31(6):675–688. doi: 10.1007/s10585-014-9658-1. [DOI] [PubMed] [Google Scholar]
- 17.Cruet-Hennequart S, Maubant S, Luis J, Gauduchon P, Staedel C, Dedhar S. alpha(v) integrins regulate cell proliferation through integrin-linked kinase (ILK) in ovarian cancer cells. Oncogene. 2003;22(11):1688–1702. doi: 10.1038/sj.onc.1206347. [DOI] [PubMed] [Google Scholar]
- 18.Hapke S, Kessler H, Luber B, Benge A, Hutzler P, Hofler H, Schmitt M, Reuning U. Ovarian cancer cell proliferation and motility is induced by engagement of integrin alpha(v)beta3/Vitronectin interaction. Biol Chem. 2003;384(7):1073–1083. doi: 10.1515/BC.2003.120. [DOI] [PubMed] [Google Scholar]
- 19.Landen CN, Kim TJ, Lin YG, Merritt WM, Kamat AA, Han LY, Spannuth WA, Nick AM, Jennnings NB, Kinch MS, Tice D, Sood AK. Tumor-selective response to antibody-mediated targeting of alphavbeta3 integrin in ovarian cancer. Neoplasia. 2008;10(11):1259–1267. doi: 10.1593/neo.08740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liapis H, Adler LM, Wick MR, Rader JS. Expression of alpha(v)beta3 integrin is less frequent in ovarian epithelial tumors of low malignant potential in contrast to ovarian carcinomas. Hum Pathol. 1997;28(4):443–449. doi: 10.1016/S0046-8177(97)90033-2. [DOI] [PubMed] [Google Scholar]
- 21.Rathinam R, Alahari SK. Important role of integrins in the cancer biology. Cancer Metastasis Rev. 2010;29(1):223–237. doi: 10.1007/s10555-010-9211-x. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Liu J, Lin B, Wang C, Li Q, Liu S, Yan L, Zhang S, Iwamori M. Study on the expression and clinical significances of Lewis y antigen and integrin alphav, beta3 in epithelial ovarian tumors. Int J Mol Sci. 2011;12(12):3409–3421. doi: 10.3390/ijms12063409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pascual A, Aranda A. Thyroid hormone receptors, cell growth and differentiation. Biochim Biophys Acta. 2013;1830(7):3908–3916. doi: 10.1016/j.bbagen.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Dittrich R, Beckmann MW, Oppelt PG, Hoffmann I, Lotz L, Kuwert T, Mueller A. Thyroid hormone receptors and reproduction. J Reprod Immunol. 2011;90(1):58–66. doi: 10.1016/j.jri.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31(5):702–755. doi: 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- 26.Rae MT, Gubbay O, Kostogiannou A, Price D, Critchley HO, Hillier SG. Thyroid hormone signaling in human ovarian surface epithelial cells. J Clin Endocrinol Metab. 2007;92(1):322–327. doi: 10.1210/jc.2006-1522. [DOI] [PubMed] [Google Scholar]
- 27.Verga Falzacappa C, Panacchia L, Bucci B, Stigliano A, Cavallo MG, Brunetti E, Toscano V, Misiti S. 3,5,3′-triiodothyronine (T3) is a survival factor for pancreatic beta-cells undergoing apoptosis. J Cell Physiol. 2006;206(2):309–321. doi: 10.1002/jcp.20460. [DOI] [PubMed] [Google Scholar]
- 28.Ness R, Grisso J, Cottreau C, Klapper J, Vergona R, Wheeler J, Morgan M, Schlesselman J. Factors related to inflammation of the ovarian epithelium and risk of ovarian cancer. Epidemiology. 2000;11(2):111–117. doi: 10.1097/00001648-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Minlikeeva AN, Freudenheim JL, Cannioto RA, Eng KH, Szender JB, Mayor P, Etter JL, Cramer DW, Diergaarde B, Doherty JA. History of thyroid disease and survival of ovarian cancer patients: results from the Ovarian Cancer Association Consortium, a brief report. Br J Cancer. 2017;117(7):1063–1069. doi: 10.1038/bjc.2017.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brinton LA, Sakoda LC, Frederiksen K, Sherman ME, Kjaer SK, Graubard BI, Olsen JH, Mellemkjaer L. Relationships of uterine and ovarian tumors to pre-existing chronic conditions. Gynecol Oncol. 2007;107(3):487–494. doi: 10.1016/j.ygyno.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang JH, Kueck AS, Stevens R, Curhan G, De Vivo I, Rosner B, Alexander E, Tworoger SS. A large cohort study of hypothyroidism and hyperthyroidism in relation to gynecologic cancers. Obstet Gynecol Int. 2013;2013:743721. doi: 10.1155/2013/743721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergh JJ, Lin HY, Lansing L, Mohamed SN, Davis FB, Mousa S, Davis PJ. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology. 2005;146(7):2864–2871. doi: 10.1210/en.2005-0102. [DOI] [PubMed] [Google Scholar]
- 33.Davis PJ, Goglia F, Leonard JL. Nongenomic actions of thyroid hormone. Nat Rev Endocrinol. 2016;12(2):111–121. doi: 10.1038/nrendo.2015.205. [DOI] [PubMed] [Google Scholar]
- 34.Cohen K, Ellis M, Khoury S, Davis PJ, Hercbergs A, Ashur-Fabian O. Thyroid hormone is a MAPK-dependent growth factor for human myeloma cells acting via alphavbeta3 integrin. Mol Cancer Res. 2011;9(10):1385–1394. doi: 10.1158/1541-7786.MCR-11-0187. [DOI] [PubMed] [Google Scholar]
- 35.Cohen K, Ellis M, Shinderman E, Khoury S, Davis PJ, Hercbergs A, Ashur-Fabian O. Relevance of the thyroid hormones-alphavbeta3 pathway in primary myeloma bone marrow cells and to bortezomib action. Leuk Lymphoma. 2015;56(4):1107–1114. doi: 10.3109/10428194.2014.947612. [DOI] [PubMed] [Google Scholar]
- 36.Cohen K, Flint N, Shalev S, Erez D, Baharal T, Davis PJ, Hercbergs A, Ellis M, Ashur-Fabian O. Thyroid hormone regulates adhesion, migration and matrix metalloproteinase 9 activity via alphavbeta3 integrin in myeloma cells. Oncotarget. 2014;5(15):6312–6322. doi: 10.18632/oncotarget.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fabian ID, Rosner M, Fabian I, Vishnevskia-Dai V, Zloto O, Shinderman Maman E, Cohen K, Ellis M, Lin HY, Hercbergs A, et al. Low thyroid hormone levels improve survival in murine model for ocular melanoma. Oncotarget. 2015;6(13):11038–11046. doi: 10.18632/oncotarget.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shinderman-Maman E, Cohen K, Weingarten C, Nabriski D, Twito O, Baraf L, Hercbergs A, Davis PJ, Werner H, Ellis M, Ashur-Fabian O. The thyroid hormone-alphavbeta3 integrin axis in ovarian cancer: regulation of gene transcription and MAPK-dependent proliferation. Oncogene. 2016;35(15):1977–1987. doi: 10.1038/onc.2015.262. [DOI] [PubMed] [Google Scholar]
- 39.Mori S, Kodaira M, Ito A, Okazaki M, Kawaguchi N, Hamada Y, Takada Y, Matsuura N. Enhanced expression of integrin alphavbeta3 induced by TGF-beta is required for the enhancing effect of fibroblast growth factor 1 (FGF1) in TGF-beta-induced epithelial-mesenchymal transition (EMT) in mammary epithelial cells. PLoS One. 2015;10(9):e0137486. doi: 10.1371/journal.pone.0137486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah PP, Fong MY, Kakar SS. PTTG induces EMT through integrin alphaVbeta3-focal adhesion kinase signaling in lung cancer cells. Oncogene. 2012;31(26):3124–3135. doi: 10.1038/onc.2011.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beaufort CM, Helmijr JC, Piskorz AM, Hoogstraat M, Ruigrok-Ritstier K, Besselink N, Murtaza M, van IJcken WF, Heine AA, Smid M, et al. Ovarian cancer cell line panel (OCCP): clinical importance of in vitro morphological subtypes. PLoS One. 2014;9(9):e103988. doi: 10.1371/journal.pone.0103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coscia F, Watters KM, Curtis M, Eckert MA, Chiang CY, Tyanova S, Montag A, Lastra RR, Lengyel E, Mann M. Integrative proteomic profiling of ovarian cancer cell lines reveals precursor cell associated proteins and functional status. Nat Commun. 2016;7:12645. doi: 10.1038/ncomms12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez-Tillo E, de Barrios O, Siles L, Cuatrecasas M, Castells A, Postigo A. Beta-catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci U S A. 2011;108(48):19204–19209. doi: 10.1073/pnas.1108977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Shao YY, Ballock RT. Thyroid hormone interacts with the Wnt/beta-catenin signaling pathway in the terminal differentiation of growth plate chondrocytes. J Bone Miner Res. 2007;22(12):1988–1995. doi: 10.1359/jbmr.070806. [DOI] [PubMed] [Google Scholar]
- 46.Plateroti M, Kress E, Mori JI, Samarut J. Thyroid hormone receptor alpha1 directly controls transcription of the beta-catenin gene in intestinal epithelial cells. Mol Cell Biol. 2006;26(8):3204–3214. doi: 10.1128/MCB.26.8.3204-3214.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dentice M, Luongo C, Ambrosio R, Sibilio A, Casillo A, Iaccarino A, Troncone G, Fenzi G, Larsen PR, Salvatore D. Beta-catenin regulates deiodinase levels and thyroid hormone signaling in colon cancer cells. Gastroenterology. 2012;143(4):1037–1047. doi: 10.1053/j.gastro.2012.06.042. [DOI] [PubMed] [Google Scholar]
- 48.Moriggi G, Verga Falzacappa C, Mangialardo C, Michienzi S, Stigliano A, Brunetti E, Toscano V, Misiti S. Thyroid hormones (T3 and T4): dual effect on human cancer cell proliferation. Anticancer Res. 2011;31(1):89–96. [PubMed] [Google Scholar]
- 49.Davis PJ, Lin HY, Sudha T, Yalcin M, Tang HY, Hercbergs A, Leith JT, Luidens MK, Ashur-Fabian O, Incerpi S, Mousa SA. Nanotetrac targets integrin alphavbeta3 on tumor cells to disorder cell defense pathways and block angiogenesis. OncoTargets Ther. 2014;7:1619–1624. doi: 10.2147/OTT.S67393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glinskii AB, Glinsky GV, Lin HY, Tang HY, Sun M, Davis FB, Luidens MK, Mousa SA, Hercbergs AH, Davis PJ. Modification of survival pathway gene expression in human breast cancer cells by tetraiodothyroacetic acid (tetrac) Cell Cycle. 2009;8:3554–3562. doi: 10.4161/cc.8.21.9963. [DOI] [PubMed] [Google Scholar]
- 51.Hurt EM, Chan K, Serrat MA, Thomas SB, Veenstra TD, Farrar WL. Identification of vitronectin as an extrinsic inducer of cancer stem cell differentiation and tumor formation. Stem Cells. 2010;28(3):390–398. doi: 10.1002/stem.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabbah M, Emami S, Redeuilh G, Julien S, Prevost G, Zimber A, Ouelaa R, Bracke M, De Wever O, Gespach C. Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resist Updat. 2008;11(4-5):123–151. doi: 10.1016/j.drup.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Wu WB, Peng HC, Huang TF. Disintegrin causes proteolysis of beta-catenin and apoptosis of endothelial cells. Involvement of cell-cell and cell-ECM interactions in regulating cell viability. Exp Cell Res. 2003;286(1):115–127. doi: 10.1016/S0014-4827(03)00105-8. [DOI] [PubMed] [Google Scholar]
- 54.Yang SH, Lin HY, Changou CA, Chen CH, Liu YR, Wang J, Jiang X, Luh F, Yen Y. Integrin beta3 and LKB1 are independently involved in the inhibition of proliferation by lovastatin in human intrahepatic cholangiocarcinoma. Oncotarget. 2016;7(1):362–373. doi: 10.18632/oncotarget.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzales M, Weksler B, Tsuruta D, Goldman RD, Yoon KJ, Hopkinson SB, Flitney FW, Jones JC. Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol Biol Cell. 2001;12(1):85–100. doi: 10.1091/mbc.12.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsuruta D, Jones JC. The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. J Cell Sci. 2003;116(24):4977–4984. doi: 10.1242/jcs.00823. [DOI] [PubMed] [Google Scholar]
- 57.Desgrosellier JS, Lesperance J, Seguin L, Gozo M, Kato S, Franovic A, Yebra M, Shattil SJ, Cheresh DA. Integrin alphavbeta3 drives slug activation and stemness in the pregnant and neoplastic mammary gland. Dev Cell. 2014;30(3):295–308. doi: 10.1016/j.devcel.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knowles LM, Gurski LA, Engel C, Gnarra JR, Maranchie JK, Pilch J. Integrin alphavbeta3 and fibronectin upregulate Slug in cancer cells to promote clot invasion and metastasis. Cancer Res. 2013;73(20):6175–6184. doi: 10.1158/0008-5472.CAN-13-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohta H, Hamada J, Tada M, Aoyama T, Furuuchi K, Takahashi Y, Totsuka Y, Moriuchi T. HOXD3-overexpression increases integrin alpha v beta 3 expression and deprives E-cadherin while it enhances cell motility in A549 cells. Clin Exp Metastasis. 2006;23(7-8):381–390. doi: 10.1007/s10585-006-9047-5. [DOI] [PubMed] [Google Scholar]
- 60.Zambrano A, García-Carpizo V, Villamuera R, Aranda A. Thyroid hormone increases bulk histones expression by enhancing translational efficiency. Mol Endocrinol. 2015;29(1):68–75. doi: 10.1210/me.2014-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.da Silva FG, Giannocco G, Luchessi A, Curi R, Nunes M. T3 acutely increases GH mRNA translation rate and GH secretion in hypothyroid rats. Mol Cell Endocrinol. 2010;317(1-2):1–7. doi: 10.1016/j.mce.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 62.Carrillo-Sepúlveda MA, Ceravolo GS, Fortes ZB, Carvalho MH, Tostes RC, Laurindo FR, Webb RC, Barreto-Chaves MLM. Thyroid hormone stimulates NO production via activation of the PI3K/Akt pathway in vascular myocytes. Cardiovasc Res. 2009;85:560–570. doi: 10.1093/cvr/cvp304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin HY, Martino LJ, Wilcox BD, Davis FB, Gordinier JK, Davis PJ. Potentiation by thyroid hormone of human IFN-gamma-induced HLA-DR expression. J Immunol. 1998;161(2):843–849. [PubMed] [Google Scholar]
- 64.Lin H-Y, Chin Y-T, Nana AW, Shih Y-J, Lai H-Y, Tang H-Y, Leinung M, Mousa SA, Davis PJ. Actions of l-thyroxine and Nano-diamino-tetrac (Nanotetrac) on PD-L1 in cancer cells. Steroids. 2016;114:59–67. doi: 10.1016/j.steroids.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 65.Koussounadis A, Langdon SP, Um IH, Harrison DJ, Smith VA (2015) Relationship between differentially expressed mRNA and mRNA-protein correlations in a xenograft model system. Sci Rep 5. 10.1038/srep10775 [DOI] [PMC free article] [PubMed]
- 66.Demunter A, Libbrecht L, Degreef H, De Wolf-Peeters C, van den Oord JJ. Loss of membranous expression of β-catenin is associated with tumor progression in cutaneous melanoma and rarely caused by exon 3 mutations. Mod Pathol. 2002;15(4):454–461. doi: 10.1038/modpathol.3880546. [DOI] [PubMed] [Google Scholar]
- 67.Chen G, Gharib TG, Huang C-C, Taylor JM, Misek DE, Kardia SL, Giordano TJ, Iannettoni MD, Orringer MB, Hanash SM. Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics. 2002;1(4):304–313. doi: 10.1074/mcp.M200008-MCP200. [DOI] [PubMed] [Google Scholar]
- 68.Ellis AL, Wang Z, Yu X, Mertz JE. Either ZEB1 or ZEB2/SIP1 can play a central role in regulating the Epstein-Barr virus latent-lytic switch in a cell-type-specific manner. J Virol. 2010;84(12):6139–6152. doi: 10.1128/JVI.02706-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Serrano-Gomez SJ, Maziveyi M, Alahari SK. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol Cancer. 2016;15(1):18. doi: 10.1186/s12943-016-0502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shang S, Hua F, Hu Z-W. The regulation of β-catenin activity and function in cancer: therapeutic opportunities. Oncotarget. 2017;8:33972. doi: 10.18632/oncotarget.15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Snider NT, Omary MB. Post-translational modifications of intermediate filament proteins: mechanisms and functions. Nat Rev Mol Cell Biol. 2014;15(3):163–177. doi: 10.1038/nrm3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Mol BioSyst. 2009;5(12):1512–1526. doi: 10.1039/b908315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13(4):227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4(9):117. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maier T, Güell M, Serrano L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009;583(24):3966–3973. doi: 10.1016/j.febslet.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 76.Chen M, Jin Y, Bi Y, Yin J, Wang Y, Pan L. A survival analysis comparing women with ovarian low-grade serous carcinoma to those with high-grade histology. OncoTargets Ther. 2014;7:1891–1899. doi: 10.2147/OTT.S67812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmeler KM, Sun CC, Bodurka DC, Deavers MT, Malpica A, Coleman RL, Ramirez PT, Gershenson DM. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol. 2008;108(3):510–514. doi: 10.1016/j.ygyno.2007.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1.14 MB)