Abstract
Consumption of methylxanthine alkaloids appears to induce activities by antagonizing adenosine receptors, implicated in breast cancer behavior in vitro. Our goal was to evaluate expression of genes for methylxanthine receptors and metabolizing enzymes to assess risk of breast carcinoma recurrence. Clinical outcomes, estrogen/progestin receptor results, and gene expression assays guided selection. RNA was isolated from laser capture microdissection-procured carcinoma cells for microarray using established protocols. Gene expression levels of eight methylxanthine receptors, eight metabolizing enzymes, and various phosphodiesterases were retrieved from microarray results. Univariable Cox regressions and Kaplan-Meier plots were determined for each gene with R software. Individually, lower expressions of PDE4A, CYP2A6, or CYP2E were related to decreased progression-free survival (PFS) and overall survival (OS). PDE1A over-expression predicted decreased PFS and OS. ADORA2B and RYR1 over-expressions predicted diminished OS. ER+ cancers exhibited lower ADORA1, ADORA2B, and RYR1 and elevated PDE4A, CYP2A6, and CYP2E expressions. Of PR+ carcinomas, diminished ADORA2B and RYR1 and elevated expressions of ADORA3, PDE4A, CYP2C8, and CYP2E were noted. Least absolute shrinkage and selection operator (LASSO) revealed that CYP2E, PDE1A, and PDE4A expressions collectively predicted PFS whereas ADORA1, CYP2E, PDE1A, PDE1B, and PDE4A expressions jointly predicted OS. Models were clinically significant when validated externally. LASSO also derived a six-gene model and five-gene model that predicted PFS of ER− or PR− carcinomas, respectively. Similarly, five-gene and four-gene models predicted OS in ER− or PR− carcinomas, respectively. Collectively, expression of genes involved in methylxanthine action and metabolism in single-cell types predicted clinical outcomes of breast carcinoma indicating promise for developing diagnostics and design of new therapeutics.
Keywords: Least Absolute Shrinkage And Selection Operator (LASSO), Adorption, PDE5A Expression, Breast Cancer, Antagonize Adenosine Receptors
Introduction
The microenvironment, particularly with regard to the stroma and associated cells in which breast cancer cells emerge and grow, appears to play a significant role in local invasion and metastasis [1–7]. Certain cells termed cancer-associated fibroblasts have been of particular interest to our studies because caffeine is reported to inactivate them and diminish expression of several cytokines (e.g., TGF-β and SDF-1) while enhancing expression of a number of critical tumor suppressor proteins, e,g, PTEN, p53, and p21 [2, 6, 8, 9]. Myofibroblasts, which appear to be sensitive to caffeine exposure (e.g., [3]), represent a large component of stroma in breast carcinomas that has been proposed to contribute to progression including metastasis [1, 4, 6, 8]. Although association between consumption of methylxanthines in the form of either coffee, tea, or chocolate and breast cancer continues to be investigated rigorously [10–17], conclusions are not uniform.
Our investigation circumvents apparent problems of cellular heterogeneity which may confound the goal to determine interrelationships between expression of genes for methylxanthine receptors and certain enzymes involved in their metabolism with clinical outcomes of human breast carcinomas by utilizing gene expression results from laser capture microdissection (LCM)-procured cancer cells. Since gene expression features of stromal components in breast carcinomas [1, 4, 6, 8] would compromise our investigative goals to predict disease progression including metastasis, only LCM-procured cancer cells were employed. Earlier, we reported [18] significant differences in gene expression profiles in stromal cells compared to those of human breast carcinoma cells, when each cell type was collected independently by LCM (e.g., [19]).
Furthermore, the absorbing conclusions of Gerlinger and co-workers [20] of intratumoral heterogeneity of renal carcinomas and implications of significant genomic variability within a single lesion support our approach of using only breast carcinoma cells isolated by LCM for deriving molecular signatures predicting risk of recurrence. To the best of our knowledge, no study has analyzed collectively expression patterns of genes for receptors of methylxanthines and their metabolizing enzymes as well as certain phosphodiesterases in relationship to a patient’s risk of breast cancer progression. Our investigations are complemented by associating the ER or PR status of primary breast cancer biopsies with LCM-procured carcinoma cells to discern small panels of genes (emerging molecular signatures) that are associated with prediction of clinical outcome of breast cancer.
Methods and Materials
Databases and Microarray Analyses
Results described in these investigations were collected from de-identified primary breast cancer carcinomas obtained from 1988 to 1996, in IRB-approved studies and stored in de-identified databases of our laboratory which holds CLIA and Commonwealth of Kentucky licenses. Selection and examination of the patient population were performed using the REMARK criteria [21] as described previously [18, 22–26]. Patients were treated with standard of care at time of diagnosis. Patient-related characteristics and tissue-based properties (Table 6, Appendix), stored as de-identified parameters in our unique comprehensive databases, were explored to determine relationships between relative gene expression and clinical parameters.
Table 6.
Clinico-pathological properties of the study population of primary breast carcinomas and patients
| Continuous variables | Mean (SD) |
| Age (years) | 58.4 (14.9) |
| Tumor size (mm) | 29.8 (16.3) |
| Median (IQR) | |
| Progression-free survival (months) | 57 (26.0–82.5) |
| Overall survival (months) | 65 (41.0–89.5) |
| Discrete variables | n (%) |
| Menopausal status | |
| Premenopausal | 58 (23.5) |
| Postmenopausal | 135 (54.6) |
| Unknown | 54 (21.9) |
| Race | |
| White | 211 (85.4) |
| Black | 34 (13.8) |
| Other | 2 (0.8) |
| PFS events | 96 (38.9) |
| PFS censored | 151 (61.1) |
| OS events | 75 (30.4) |
| OS censored | 172 (69.6) |
| Pathology of primary | |
| IDC | 201 (81.4) |
| ILC | 15 (6.1) |
| IDC + ILC | 2 (0.8) |
| Other histologic types | 29 (11.7) |
| Nodal status | |
| N 0 | 126 (51.0) |
| N 1–3 | 55 (22.3) |
| N > 3 | 46 (18.6) |
| Unknown | 20 (8.1) |
| Tumor grade | |
| 1 | 14 (5.7) |
| 2 | 69 (27.9) |
| 3 | 94 (38.1) |
| 4 | 1 (0.4) |
| Unknown | 69 (27.9) |
| Tumor stage | |
| 0 | 3 (1.2) |
| 1 | 60 (24.3) |
| 2 | 140 (56.7) |
| 3 | 35 (14.2) |
| 4 | 4 (1.6) |
| Unknown | 5 (2.0) |
| Steroid receptor status | |
| ER+ | 146 (59.1) |
| ER− | 101 (40.9) |
| PR+ | 151 (61.1) |
| PR− | 96 (38.9) |
| HER2 status* (n = 45) | |
| HER2+ | 28 (62.2) |
| HER2− | 17 (37.8) |
Cutoff values utilized for the NEN/DuPont ELISA was 1.7 hnu/μg protein (7+ of 14 biopsies), while those used for the TRITON Diagnostics EIA was 129.9 hnu/mg protein (21+ of 31 biopsies)
Briefly, the tissue sections of frozen de-identified tissue biopsies were processed previously for LCM with a PixCell IIe™ instrument (Arcturus/Thermo Fisher), which allowed collection of only breast carcinoma cells in a non-destructive manner for microarray analyses [18, 19, 27]. Total RNA was extracted from LCM-procured cells using PicoPure™ RNA isolation kits (Arcturus Engineering) and amplified (Arcturus RiboAmp™ kit) before microarray as described earlier [28, 29]. Normalized Cy5 and Cy3 fluorescent intensities from microarray were employed to represent relative gene expression of the LCM-procured cells [28, 29]. Relative expression levels of each of 22,000 genes obtained from microarray uniquely represented only those mRNA species of breast carcinoma cells [18, 19, 23, 26–29].
Preliminary Gene Selection
An extensive review of published papers related to methylxanthine receptors, associated phosphodiesterases, and metabolizing enzymes (e.g., [30–34]) revealed 23 genes as study candidates (Table 1). Pathways involving these molecules of interest are illustrated by PharmGKB (https://www.pharmgkb.org/pathway/PA165884757#tabview=tab0&subtab; https://www.pharmgkb.org/pathway/PA165958541#). Of these, eight genes were for methylxanthine receptors, seven were for phosphodiesterases, and eight were for enzymes involved in the metabolism of these pharmacologically active ligands. Examination of the extensive repository of microarray expression levels for ~ 22,000 genes in LCM-procured breast carcinoma cells indicated that results were available for each of the 23 gene candidates. The 247 tissue biopsies of primary invasive ductal carcinoma of the breast served as the principal study population of patients (Table 6, Appendix).
Table 1.
List of candidate genes whose protein products are associated with methylxanthine receptors, enzymes that metabolize these pharmacologically active agents and certain phosphodiesterase targets
| Gene symbol | Aliases | Gene ID | |
|---|---|---|---|
| Methylxanthine alkaloid receptors | |||
| Adenosine A1 receptor | ADORA1 | RDC7 | 134 |
| Adenosine A2a receptor | ADORA2A | A2aR, ADORA2, RDC8 | 135 |
| Adenosine A2b receptor | ADORA2B | ADORA2 | 136 |
| Adenosine A3 receptor | ADORA3 | A3AR | 140 |
| Dopamine receptor D2 | DRD2 | D2DR, D2R | 1813 |
| Calcium channel, voltage-dependent | CACNA1S | CACNL1A3, CCHL1A3, Cav1.1, HOKPP, HOKPP1, MHS5, TTPP1, hypoPP | 779 |
| Ryanodine receptor 1 (skeletal) | RYR1 | CCO, MHS, MHS1, PPP1R137, RYDR, RYR, RYR-1, SKRR | 6261 |
| Antigen with calponin homology | SPECC1L | CYTSA, GBBB2, OBLFC1 | 23384 |
| Phosphodiesterases | |||
| Phosphodiesterase 1A | PDE1A | CAM-PDE-1A, HCAM-1, HCAM1, HSPDE1A | 5136 |
| Phosphodiesterase 1B | PDE1B | HEL-S-79p1, PDES1B, PDE1B | 5153 |
| Phosphodiesterase 1C | PDE1C | Hcam3, cam-PDE 1C, hCam-3 | 5137 |
| Phosphodiesterase 3A | PDE3A | CGI-PDE, CGI-PDE A, CGI-PDE-A, HTNB | 5139 |
| Phosphodiesterase 4A | PDE4A | DPDE2, PDE4, PDE46 | 5141 |
| Phosphodiesterase 4B | PDE4B | DPDE4, PDEIVB | 5142 |
| Phosphodiesterase 5A | PDE5A | CGB-PDE, CN5A, PDE5 | 8654 |
| Metabolizing enzymes | |||
|
Cytochrome P450, family 1, subfamily A, polypeptide 2 |
CYP1A2 | CP12, P3–450, P450(PA) | 1544 |
|
Cytochrome P450, family 2, subfamily A, polypeptide 6 |
CYP2A6 | CPA6, CYP2A, CYP2A3, CYPIIA6, P450C2A, P450PB | 1548 |
|
Cytochrome P450, family 3, subfamily A, polypeptide 4 |
CYP3A4 | CP33, CP34, CYP3A, CYP3A3, CYPIIIA3, CYPIIIA4, HLP, NF-25, P450C3, P450PCN1 | 1576 |
|
Cytochrome P450, family 2, subfamily C, polypeptide 8 |
CYP2C8 | CPC8, CYPIIC8, MP-12/MP-20 | 1558 |
|
Cytochrome P450, family 2, subfamily C, polypeptide 9 |
CYP2C9 | CPC9, CYP2C, CYP2C10, CYPIIC9, P450IIC9 | 1559 |
|
Cytochrome P450, family 2, subfamily E, polypeptide 1 |
CYP2E1 | CPE1, CYP2E, P450-J, P450C2E | 1571 |
|
N-acetyltransferase 2 (arylamine N-acetyltransferase) |
NAT2 | AAC2, NAT-2, PNAT | 10 |
| Xanthine dehydrogenase | XDH | XAN1, XO, XOR | 7498 |
Tumor Marker Detection
Estrogen receptor and progestin receptor protein levels were determined using either the Abbott enzyme immunoassay (EIA kit) or the radio-labeled ligand-binding assay (NEN/DuPont kit) on freshly prepared cytosols as described previously [27, 35–37]. Using the latter FDA-approved kits which employ either [3H]estradiol-17β or [3H]R5020 depending upon the receptor type being determined, specific ligand-binding capacity, reflecting both receptor levels, expressed as femtomole per milligram cytosol protein, and activity expressed as the apparent dissociation constant (Kd value as M) were determined by the Scatchard analysis. Determination of ER and PR levels by EIA employed an FDA-approved kit formerly distributed by Abbott Laboratories [36, 37]. This protocol utilized beads coated with anti-receptor monoclonal antibodies (either to ER or PR) which were incubated with the tissue extracts [27, 35–37]. The receptor level (mass) was expressed as femtomole per milligram cytosol protein. Each kit was approved by the FDA for clinical assays; a cutoff value of 15 fmol/mg cytosol protein was used in the investigations described. The distribution according to ER and PR statuses of the primary breast carcinomas used in these investigations is given in Table 6 of the Appendix.
Although HER2/neu status was not considered in this investigation, the presence of the oncoprotein in 45 primary breast cancers is reported in Appendix Table 6 using results from two experimental antibody-based assays. Cutoff values utilized for NEN/DuPont ELISA (which became Oncogene Science Diagnostics) was 1.7 hnu/μg protein (7+ of 14 biopsies), while that used for the TRITON Diagnostics EIA was 129.9 hnu/mg protein (21+ of 31 biopsies).
Univariable Cox Regression and Kaplan-Meier Analyses
Statistical computations, violin plots, and Kaplan-Meier plots were performed using R version 3.2.5. Utilizing commands from the R package survival [38], univariable Cox regressions of expression levels of each gene candidate estimated the hazard ratio (HR) p values to determine significant genes. p values were adjusted for multiple comparisons using the Benjamini and Hochberg (BH) method with < 0.30 selected as the “discovery” cutoff as applied earlier [23, 25, 26].
Univariable Cox regression was performed on each gene candidate using relative expression levels to discern relationships with progression-free survival (PFS) and overall survival (OS). This allows the use of relative gene expression values as a single covariate to investigate the extent to which expression levels of a single gene in the cohort predicted PFS or OS of breast cancer patients. Hazard ratios were derived from univariable Cox regression models and were calculated for each of the 23 candidate genes.
Relative gene expression levels determined with LCM-procured carcinoma cells from 247 patients were stratified above and below the median for each univariable significant gene candidate. Adjusted p values (BH method) were derived from log-rank tests comparing survival times between groups. Since ER and PR statuses of a breast cancer are related to a patient’s prognosis [35, 36, 39], analyses of candidate gene expression in LCM-procured cells according to steroid hormone receptor status of the primary carcinoma biopsy were performed. Survival curves for various groups were visualized in Kaplan-Meier plots with the number of patients at risk at various time points displayed in a table below each plot.
Relationships of ER and PR Statuses with Gene Expression Levels
Univariable Cox regression of relative gene expression was performed for patient survival stratified by the receptor protein status of their primary breast cancer, e.g., either ER− vs ER+ and either PR− vs PR+. Relative gene expression values were examined as a single covariate and to investigate relationships of expression levels of each gene according to either ER or PR protein status. Univariable Cox regression models were used to calculate hazard ratios (HR) for each of the 23 candidate genes.
For each of the 23 candidate genes, violin plots juxtaposed distributions of relative gene expression values of ER+ vs ER− and PR+ vs PR− primary breast cancers to examine potential regulation by these sex steroid hormones. Distributions were compared using an unpaired independent two-sample Mann-Whitney-Wilcoxon test.
To investigate relationships among expression levels of each candidate gene and either ER or PR protein status of a carcinoma, Kaplan-Meier plots were also constructed to discern influence on PFS and OS of patients. Kaplan-Meier plots displayed survival curves for groups split by the median relative expression for each gene in either ER+ or ER− lesions, without regard to PR status and vice versa.
Least Absolute Shrinkage and Selection Operator
As noted by Daniels et al. 2017 [25], large numbers of variables precluded stepwise selection methods to determine significant genes in a model, which was reported to be infeasible [40]. Using an alternative method, least absolute shrinkage and selection operator (LASSO) evaluated the 23 candidate genes in primary breast carcinoma cells of 247 patients in models for PFS and OS. LASSO penalizes the size of the beta coefficient vector and removes genes whose coefficients are close to zero. We followed the suggestion of Daniels et al. [25], since maximum likelihood estimates of are derived by maximizing the penalized Cox log partial likelihood with the form, , where l (β) represents the standard log Cox log partial likelihood and λ is the shrinkage parameter. The optimum value for λ was determined by tenfold cross-validation [23, 26]. The LASSO-penalized Cox model was used to fit multivariable models for PFS and OS based on biomarker profiles. Models were fitted using the R package penalized [41, 42] or glmnet [43, 44].
External Validation of Candidate Genes Identified by LASSO Using Breast Cancer Metabase
SurvExpress, a web resource for biomarker validation for cancer gene expression that may be employed to examine survival analysis [45], was used for external validation of the significant collective of genes derived from the LASSO-penalized Cox model for PFS and OS. Expression levels of the LASSO model-identified sets of genes were extracted from the Breast Cancer Metabase (10 cohorts: 22,000 genes) and were used to validate clinical outcomes (PFS and OS) in Kaplan-Meier plots. SurvExpress determined risk groups by the median split of ordered linear predictors.
Results
Univariable Cox Regression Analyses of Relative Gene Expression Without Regard to ER or PR Status
Cox regression analyses of the 23 genes of interest revealed that expression levels of genes for phosphodiesterases PDE1A and PDE4A, in LCM-procured breast carcinoma cells, were significant at an adjusted p value of < 0.30 for predicting PFS (Table 2). Of these, PDE4A expression was also significant for predicting OS at an adjusted p value of < 0.30 (Table 3). In addition, expression of the gene for a methylxanthine metabolizing enzyme, CYP2E, predicted OS at an adjusted p value of < 0.30 (Table 3). None of the genes for eight methylxanthine alkaloid receptors examined exhibited expression levels that correlated with either PFS or OS.
Table 2.
Summary of univariable Cox regression analyses of expression levels of candidate genes in breast carcinomas associated with progression-free survival (PFS) without regard to ER or PR status
| Gene symbol | β | HR | 95% CI (HR) | p value | Adjusted p value |
|---|---|---|---|---|---|
| Methylxanthine alkaloid receptors | |||||
| ADORA1 | 0.08 | 1.08 | (0.93, 1.25) | 0.325 | 0.772 |
| ADORA2A | 0.00 | 1.00 | (0.84, 1.20) | 0.976 | 0.976 |
| ADORA2B | 0.06 | 1.06 | (0.93, 1.20) | 0.382 | 0.772 |
| ADORA3 | 0.01 | 1.01 | (0.83, 1.22) | 0.949 | 0.976 |
| DRD2 | 0.11 | 1.11 | (0.95, 1.30) | 0.195 | 0.748 |
| CACNA1S | 0.11 | 1.11 | (0.83, 1.49) | 0.478 | 0.786 |
| RYR1 | − 0.05 | 0.96 | (0.76, 1.20) | 0.696 | 0.942 |
| SPECC1L | − 0.11 | 0.90 | (0.64, 1.26) | 0.529 | 0.811 |
| Phosphodiesterases | |||||
| PDE1A | 0.17 | 1.19 | (1.02, 1.38) | 0.025 | 0.289 |
| PDE1B | 0.23 | 1.26 | (0.92, 1.71) | 0.149 | 0.748 |
| PDE1C | 0.30 | 1.35 | (0.75, 2.45) | 0.321 | 0.772 |
| PDE3A | 0.21 | 1.23 | (0.31, 4.88) | 0.765 | 0.976 |
| PDE4A | − 0.29 | 0.75 | (0.63, 0.89) | 0.001 | 0.016 |
| PDE4B | 0.00 | 1.00 | (0.89, 1.13) | 0.939 | 0.976 |
| PDE5A | − 0.13 | 0.87 | (0.62, 1.22) | 0.434 | 0.772 |
| Enzymes metabolizing methylxanthines | |||||
| CYP1A2 | − 0.03 | 0.97 | (0.38, 2.48) | 0.951 | 0.976 |
| CYP2A6 | − 0.07 | 0.93 | (0.81, 1.07) | 0.312 | 0.772 |
| CYP3A4 | − 0.13 | 0.88 | (0.63, 1.22) | 0.436 | 0.772 |
| CYP2C8 | − 0.10 | − 0.90 | (0.78, 1.05) | 0.169 | 0.748 |
| CYP2C9 | 0.04 | 1.04 | (0.76, 1.41) | 0.809 | 0.976 |
| CYP2E | − 0.18 | 0.83 | (0.69, 1.00) | 0.049 | 0.379 |
| NAT2 | − 0.03 | 0.97 | (0.85, 1.11) | 0.667 | 0.942 |
| XDH | − 0.07 | 0.93 | (0.80, 1.09) | 0.380 | 0.772 |
Genes with expression levels that were significant at a discovery cutoff and adjusted p value < 0.30 are shown in bold
Table 3.
Summary of univariable Cox regression analyses of expression levels of candidate genes in breast carcinomas associated with overall survival (OS) without regard to ER or PR status
| Gene symbol | β | HR | 95% CI (HR) | p value | Adjusted p value |
|---|---|---|---|---|---|
| Methylxanthine alkaloid receptors | |||||
| ADORA1 | 0.14 | 1.15 | (0.97, 1.36) | 0.097 | 0.334 |
| ADORA2A | − 0.08 | 0.93 | (0.76, 1.13) | 0.457 | 0.790 |
| ADORA2B | 0.12 | 1.13 | (0.98, 1.30) | 0.087 | 0.334 |
| ADORA3 | 0.05 | 1.06 | (0.85, 1.31) | 0.623 | 0.790 |
| DRD2 | 0.11 | 1.11 | (0.93, 1.33) | 0.252 | 0.604 |
| CACNA1S | 0.15 | 1.16 | (0.84, 1.60) | 0.366 | 0.790 |
| RYR1 | − 0.05 | 0.95 | (0.73, 1.23) | 0.699 | 0.790 |
| SPECC1L | − 0.10 | 0.90 | (0.61, 1.33) | 0.606 | 0.790 |
| Phosphodiesterases | |||||
| PDE1A | 0.16 | 1.17 | (0.99, 1.38) | 0.074 | 0.334 |
| PDE1B | 0.32 | 1.38 | (0.97, 1.96) | 0.072 | 0.334 |
| PDE1C | 0.16 | 1.17 | (0.58, 2.36) | 0.657 | 0.790 |
| PDE3A | 0.42 | 1.52 | (0.34, 6.84) | 0.584 | 0.790 |
| PDE4A | − 0.34 | 0.72 | (0.59, 0.86) | 0.001 | 0.010 |
| PDE4B | − 0.02 | 0.98 | (0.85, 1.12) | 0.724 | 0.790 |
| PDE5A | − 0.05 | 0.95 | (0.65, 1.39) | 0.804 | 0.839 |
| Enzymes metabolizing methylxanthines | |||||
| CYP1A2 | − 0.02 | 0.98 | (0.33, 2.89) | 0.965 | 0.965 |
| CYP2A6 | − 0.17 | 0.84 | (0.70, 1.01) | 0.069 | 0.334 |
| CYP3A4 | − 0.11 | 0.90 | (0.62, 1.29) | 0.554 | 0.790 |
| CYP2C8 | − 0.11 | 0.89 | (0.76, 1.06) | 0.187 | 0.499 |
| CYP2C9 | 0.10 | 1.10 | (0.80, 1.52) | 0.558 | 0.790 |
| CYP2E | − 0.29 | 0.75 | (0.61, 0.93) | 0.008 | 0.095 |
| NAT2 | − 0.03 | 0.97 | (0.84, 1.13) | 0.712 | 0.790 |
| XDH | − 0.05 | 0.95 | (0.80, 1.13) | 0.562 | 0.790 |
Genes with expression levels that were significant at a discovery cutoff and adjusted p-value < 0.30 are shown in bold
To relate the expression of genes of interest in a breast carcinoma to patient survival over time, Kaplan-Meier plots using the log-rank test were constructed for each of the 23 gene candidates (Table 1). Kaplan-Meier plots were constructed for genes with expression levels exhibiting an adjusted p value < 0.30 for the discovery cutoff regardless of ER or PR protein status of the lesion. Expression levels of 4 of the 23 gene candidates, PDE1A, PDE4A, CYP2A6, and CYP2E, predicted both PFS and OS of breast cancer patients (Fig. 1a–h). In addition, expression of two methylxanthine receptor genes ADORA2B and RYR1 predicted OS (Fig. 1i, j).
Fig. 1.
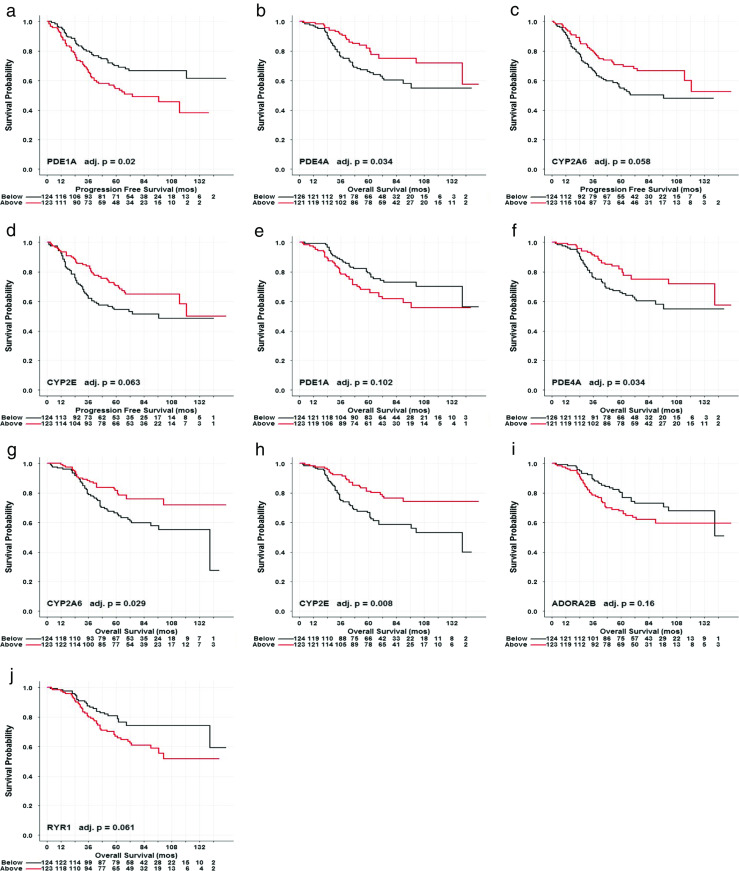
Representative Kaplan-Meier plots of median-split gene expression of methylxanthine receptors and metabolizing enzymes from breast carcinomas predicting PFS and OS without regard to steroid receptor status. High expression levels of PDE1A and low expression levels of PDE4A, CYP2A6, and CYP2E were associated with poorer outcomes of lower PFS. a–d High expression levels of PDE1A, ADORA2B, and RYR1 and low expression levels of PDE4A, CYP2A6, and CYP2E were associated with poorer outcomes of lower OS (e–j)
Relationship of ER Status of Breast Carcinoma and Gene Expression
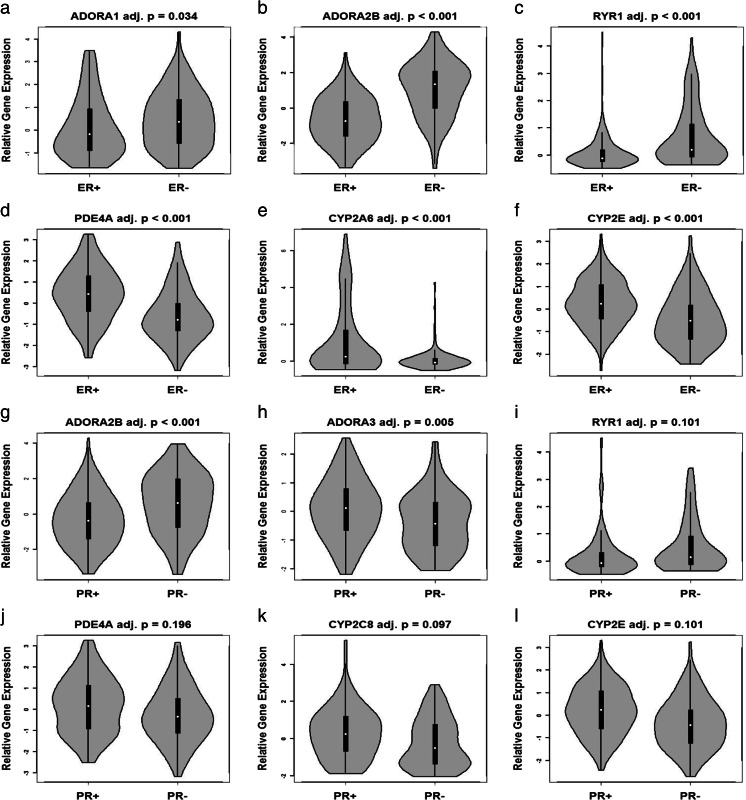
Since ER and PR are used routinely as biomarkers to predict primary breast cancer behavior, e.g., metastasis and overall survival, as well as treatment selection [35, 39], we examined the relative expression of each of the 23 genes of interest in LCM-procured carcinoma cells according to either ER or PR protein status of the primary lesion. Violin plots of relative gene expression were constructed for the 23 candidate genes according to either ER+ or ER− and PR+ or PR− status (Fig. 2).
Fig. 2.
Representative violin plots of gene expression of methylxanthine receptors and enzymes comparing distributions by estrogen or progesterone status. Plots display the kernel of the distribution of log-relative gene expression for either ER or PR subtypes from breast carcinomas. Median of log-relative gene expression is indicated by a white circle, and the interquartile range is denoted with a black bar
Thirteen of the 23 candidate genes, ADORA1, ADORA2A, ADORA2B, ADORA3, DRD2, RYR1, PDE4A, PDE4B, CYP2A6, CYP2C8, CYP2E, NAT2, and XDH exhibited significant differences according to either ER+ or ER− status of the primary lesion. Figure 2a–f depicts representative violin plots of expression differences for six candidate genes that exhibited an adjusted p value < 0.30. ER+ breast carcinoma cells overexpressed PDE4A, CYP2A6, and CYP2E compared to ER− cells that were procured by LCM (p value < 0.001). In contrast to ER+ cells, elevated expression of ADORA1, ADORA2B, and RYR1 were detected in ER− breast cancer cells.
Relationship of PR Status of Breast Carcinoma and Gene Expression
Violin plots of expression levels of 10 of the 23 candidate genes, ADORA2B, ADORA3, RYR1, PDE3A, PDE4A, PDE4B, CYP2A6, CYP2C8, CYP2E, and XDH, exhibited differences according to either PR+ or PR− status. Six representative plots of candidate genes exhibiting an adjusted p value < 0.30 are displayed (Fig. 2g–l). PR+ breast carcinoma cells overexpressed ADORA3, PDE4A, CYP2C8, and CYP2E compared to PR− cells that were procured by LCM (p value < 0.001). In contrast to PR+ cells, elevated expressions of ADORA2B and RYR1 were detected in PR− breast cancer cells. Independently, expression of ADORA2B, ADORA3, RYR1, PDE4A, PDE4B, CYP2A6, CYP2C8, CYP2E, and XDH was significantly associated with either ER or PR status of the primary.
Univariable Cox Regression Analyses of Microarray Results of Carcinomas Stratified According to ER and PR Protein Statuses
Since differences in gene expression levels were noted according to ER or PR status of the primary breast cancers, Cox regression analyses were performed to assess their relationships with clinical outcomes. Of the 23 genes of interest, expression levels of genes CACNA1S, PDE1A, and RYR1 of ER− lesions were significant at an adjusted p value < 0.30 for predicting PFS and OS (Table 4). Expression levels of candidate genes PDE4A and XDH of ER− lesions were significant for predicting PFS (Table 4). Expression of the gene for a methylxanthine metabolizing enzyme, CYP2E, of ER+ lesions was significant for predicting OS. Expression levels of genes CACNA1S, PDE4A, and SPECC1L were significant for predicting PFS and OS of PR− lesions. In contrast, univariable Cox regressions of patient survival and genes of interest indicated that expression levels did not correlate with PFS of ER+ lesions or with PFS or OS of PR+ lesions.
Table 4.
Univariable Cox regression analyses of microarray results of carcinomas stratified according to either ER or PR protein status
| Gene symbol | ER− | ER+ | PR− | PR+ | ||||
|---|---|---|---|---|---|---|---|---|
| PFS | OS | PFS | OS | PFS | OS | PFS | OS | |
| ADORA1 | 0.865 | 0.616 | 0.689 | 0.683 | 0.643 | 0.345 | 0.689 | 0.651 |
| ADORA2A | 0.865 | 0.756 | 0.561 | 0.713 | 0.643 | 0.414 | 0.561 | 0.728 |
| ADORA2B | 0.865 | 0.866 | 0.908 | 0.683 | 0.994 | 0.684 | 0.908 | 0.924 |
| ADORA3 | 0.911 | 0.812 | 0.561 | 0.656 | 0.994 | 0.834 | 0.561 | 0.651 |
| CACNA1S | 0.288 | 0.095 | 0.671 | 0.683 | 0.058 | 0.005 | 0.671 | 0.728 |
| CYP1A2 | 0.865 | 0.756 | 0.671 | 0.683 | 0.643 | 0.643 | 0.671 | 0.728 |
| CYP2A6 | 0.947 | 0.866 | 0.973 | 0.683 | 0.800 | 0.834 | 0.973 | 0.651 |
| CYP2C8 | 0.865 | 0.756 | 0.588 | 0.553 | 0.994 | 0.954 | 0.588 | 0.651 |
| CYP2C9 | 0.865 | 0.866 | 0.973 | 0.457 | 0.994 | 0.845 | 0.973 | 0.924 |
| CYP2E | 0.914 | 0.866 | 0.561 | 0.069 | 0.994 | 0.834 | 0.561 | 0.428 |
| CYP3A4 | 0.959 | 0.866 | 0.671 | 0.683 | 0.994 | 0.834 | 0.671 | 0.879 |
| DRD2 | 0.959 | 0.886 | 0.668 | 0.683 | 0.800 | 0.834 | 0.668 | 0.651 |
| NAT2 | 0.865 | 0.661 | 0.908 | 0.683 | 0.994 | 0.716 | 0.908 | 0.879 |
| PDE1A | 0.129 | 0.095 | 0.561 | 0.713 | 0.643 | 0.684 | 0.561 | 0.576 |
| PDE1B | 0.865 | 0.409 | 0.561 | 0.683 | 0.994 | 0.684 | 0.561 | 0.631 |
| PDE1C | 0.852 | 0.866 | 0.908 | 0.683 | 0.436 | 0.643 | 0.908 | 0.728 |
| PDE3A | 0.959 | 0.866 | 0.561 | 0.357 | 0.994 | 0.845 | 0.561 | 0.631 |
| PDE4A | 0.288 | 0.409 | 0.561 | 0.436 | 0.127 | 0.091 | 0.561 | 0.576 |
| PDE4B | 0.513 | 0.616 | 0.908 | 0.683 | 0.826 | 0.716 | 0.908 | 0.877 |
| PDE5A | 0.865 | 0.866 | 0.561 | 0.683 | 0.994 | 0.834 | 0.561 | 0.728 |
| RYR1 | 0.288 | 0.210 | 0.908 | 0.683 | 0.436 | 0.600 | 0.908 | 0.924 |
| SPECC1L | 0.690 | 0.616 | 0.561 | 0.713 | 0.127 | 0.257 | 0.561 | 0.631 |
| XDH | 0.288 | 0.409 | 0.671 | 0.818 | 0.800 | 0.684 | 0.671 | 0.877 |
Results of PFS and OS for carcinomas classified as ER− (n = 101)/ER+ (n = 146) or PR− (n = 96)/PR+ (n = 151) are displayed. Values shown represent adjusted p values. Those values in bold identify genes exhibiting expression levels within the limits of adjusted p values at the discovery cutoff
Kaplan-Meier plots were constructed for genes with expression levels exhibiting an adjusted p value < 0.30 for the discovery cutoff according to ER protein status of the primary lesion. Expression of phosphodiesterase PDE1A of either ER− or ER+ carcinomas predicted PFS (Fig. 3a, d). In contrast, expression of phosphodiesterases PDE1A and PDE1B of ER− carcinomas predicted OS (Fig. 3b, c), which was not observed in ER+ lesions. Expression of methylxanthine receptor CYP3A4 of ER+ carcinomas predicted PFS (Fig. 3g). Kaplan-Meier plots of OS indicated that phosphodiesterase PDE4A and methylxanthine receptor CYP2E expression in ER+ carcinomas were related to clinical outcomes (Fig. 3e, f).
Fig. 3.
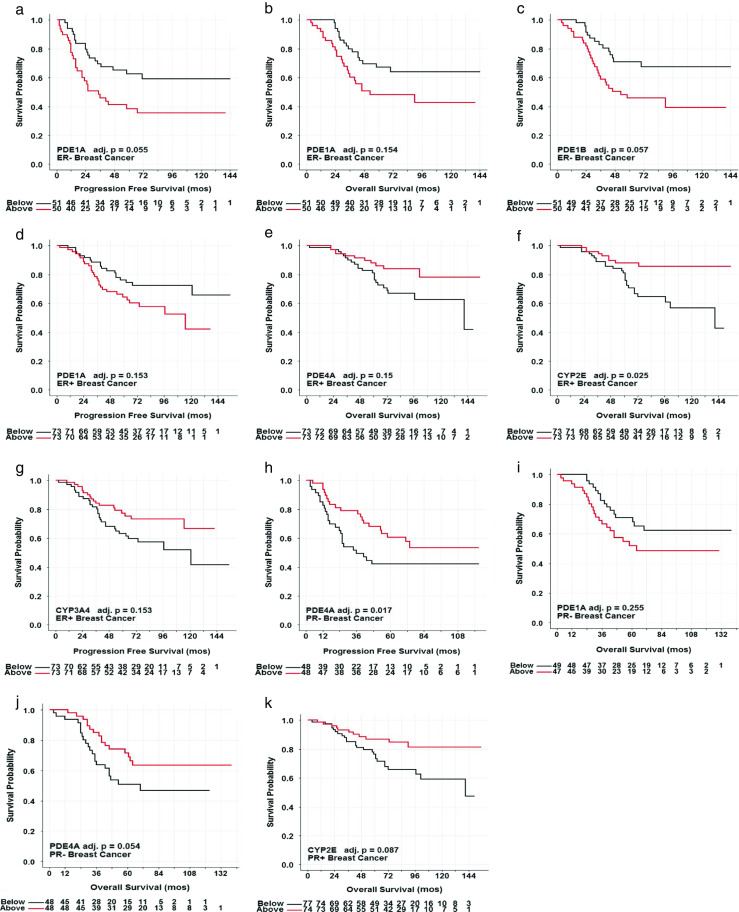
Representative Kaplan-Meier plots of median-split gene expression of methylxanthine receptors and metabolizing enzymes from breast carcinomas predicting PFS and OS with regard to steroid receptor status. High expression levels of PDE1A were associated with poorer outcomes of lower PFS (a) and high expression levels of PDE1A and PDE1B were associated with poorer outcomes of lower OS (b, c) of ER− breast carcinomas. High expression levels of PDE1A and low expression of CYP3A4 were associated with poorer outcomes of lower PFS (d, g), and low expression levels of PDE4A and CYP2E were associated with poorer outcomes of lower OS (e, f) of ER− breast carcinomas. High expression levels of PDE1A were associated with poorer outcomes of lower OS (h), and low expression levels of PDE4A were associated with poorer outcomes of lower PFS and OS (i, j) of PR− breast carcinomas. Low expression levels of CYP2E were associated with poorer outcomes of lower OS (k) of PR+ breast cancer
Similarly, Kaplan-Meier plots were constructed for genes with expression levels exhibiting an adjusted p value < 0.30 for the discovery cutoff according to PR protein status of the lesion. Expression of phosphodiesterase PDE4A of PR− carcinomas predicted PFS (Fig. 3h). In addition, expression of phosphodiesterases PDE1A and PDE4A of PR− carcinomas predicted OS (Fig. 3i, j). Expression of methylxanthine receptor CYP2E predicted OS of PR+ carcinomas (Fig. 3k).
Identification of Molecular Signatures Without Regard to ER or PR Status
To discern a molecular signature, LASSO evaluated the gene expression levels of the 23 gene candidates collectively to determine the minimal gene subset for optimal prediction of PFS and OS. Using R version 3.2.5, LASSO was performed using the package penalized without regard to either ER or PR status of the breast carcinoma biopsy. LASSO derived a significant model composed of PDE1A, CYP2E, and PDE4A expressions predicting PFS for breast carcinomas regardless of their ER or PR status (Table 5). Genes are shown according to increased risk (positive beta coefficient) and decreased risk (negative beta coefficient) of developing recurrent breast cancer.
Table 5.
Gene symbols and beta coefficients of gene subsets determined by LASSO analysis without or with regard to sex steroid receptors status
| PFS | OS | ||
| Gene symbol | β | Gene symbol | β |
| CYP2E | − 0.16 | CYP2E | − 0.08 |
| PDE1A | 0.67 | PDE1A | 0.03 |
| PDE4A | − 1.85 | PDE4A | − 0.18 |
| PDE1B | 0.12 | ||
| ADORA1 | 0.01 | ||
| PFS ER− | PFS PR− | ||
| Gene symbol | β | Gene symbol | β |
| PDE4A | 1.24 | PDE4A | − 0.81 |
| CACNA1S | 0.07 | CACNA1S | 0.47 |
| RYR1 | − 0.56 | RYR1 | − 0.22 |
| PDE1A | − 0.33 | PDE1C | 0.03 |
| PDE4B | − 0.59 | SPECC1L | − 0.76 |
| XDH | − 0.13 | ||
| OS ER− | OS PR− | ||
| Gene symbol | β | Gene symbol | β |
| PDE4A | − 0.21 | PDE4A | − 1.03 |
| CACNA1S | 0.20 | CACNA1S | 0.76 |
| PDE1A | 1.06 | ADORA1 | 0.08 |
| RYR1 | − 0.83 | SPECC1L | − 0.52 |
| XDH | − 0.05 | ||
PFS and OS models (n = 247) without regard to sex steroid receptor status. PFS models with regard to either ER− (n = 101) or PR− (n = 96) carcinomas. OS models with regard to either ER− (n = 101) or PR− (n = 96) carcinomas
When LASSO was performed using the R package glmnet [43, 44], another significant model was derived that predicted OS for patients regardless of ER or PR status of their breast carcinomas. LASSO models employing data of candidate genes and clinical outcomes identified genes PDE1B, PDE1A, ADORA1, CYP2E, and PDE4A expressions collectively predicted OS (Table 5).
Identification of Molecular Signatures with Regard to ER or PR Status
LASSO was also employed to discern molecular signatures using expression results of the 23 gene candidates collectively for optimal prediction of PFS and OS. LASSO was performed with regard to either ER or PR status of the breast carcinoma biopsy using R package version 3.2.5 and package penalized. Considering ER− cancers, LASSO derived a six-gene model composed of PDE4A, CACNA1S, XDH, PDE1A, RYR1, and PDE4B expression predicting PFS while a five-gene model for PR− breast carcinomas consisted of CACNA1S, PDE1C, RYR1, SPECC1L, and PDE4A (Table 5). Genes are shown according to increased risk (positive beta coefficient) and decreased risk (negative beta coefficient) of developing recurrent breast cancer.
R package penalized was also employed for LASSO analysis to determine models of gene subsets that predicted OS for patients according to ER− or PR− status of their breast carcinomas. LASSO analyses employing expression data of candidate genes and clinical outcomes identified a five-gene model (PDE1A, CACNA1S, XDH, PDE4A, and RYR1) which collectively predicted OS in ER− breast cancers (Table 5). Two of these genes, PDE4A and CACNA1S, also appeared in the four-gene model with ADORA1 and SPECC1L that predicted OS in patients with PR− breast carcinomas. Noteworthy, collective analyses of the 23 gene candidates with LASSO did not reveal any models for predicting PFS or OS in either ER+ or PR+ breast cancers.
External Validation of LASSO Models
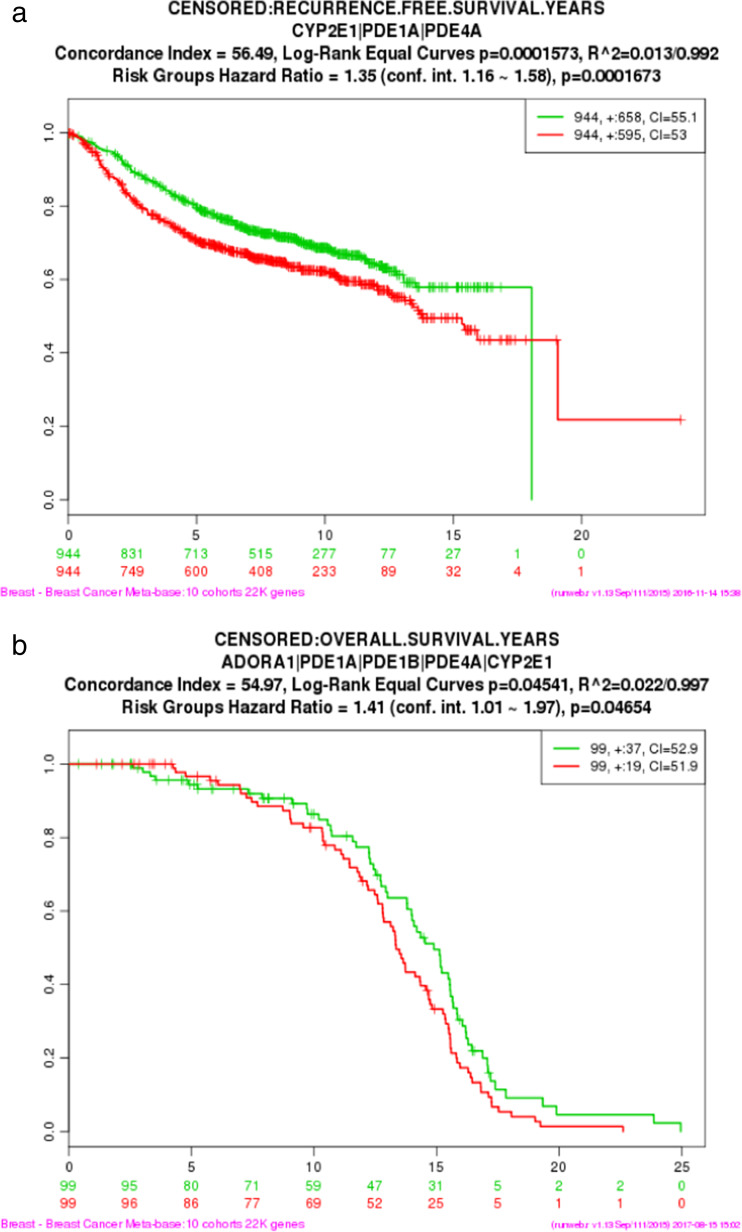
External validation of the gene subsets obtained from LASSO models was performed with the Breast Cancer Metabase, available through an online tool SurvExpress (http://bioinformatica.mty.itesm.mx:8080/Biomatec/SurvivaX.jsp). External validation of the relationship between the three-gene subset (PDE1A, PDE4A, and CYP2E) derived in our study was performed using gene expression from cancers of the 1888 patients with PFS results in the metabase (Fig. 4a). In the same manner, external validation of the relationship between the five-gene subset (ADORA1, PDE1A, PDE1B, PDE4A, and CYP2E) deciphered in our investigation was performed using the metabase gene expression results available for OS of 198 patients (Fig. 4b). These critical analyses revealed that expression of PDE1A, PDE4A, and CYP2E in primary breast carcinomas collectively predicted PFS (Fig. 4a) in concert with our discovery using LCM-procured breast carcinoma cells. Whereas the five-gene subset composed of ADORA1, PDE1A, PDE1B, PDE4A, and CYP2E collectively predicted OS in this publicly available database (Fig. 4b), further validating our original findings.
Fig. 4.
SurvExpress, a publicly available tool for computation and analysis, was utilized to perform external validation of the three-gene model for predicting PFS (a) and the five-gene model for predicting OS (b) derived from LASSO without regard to ER or PR status. Expression levels of the LASSO model-identified sets of genes were extracted from the Breast Cancer Metabase (10 cohorts: 22,000 genes) and used to validate clinical outcomes represented by the Kaplan-Meier plots (n = 1888 patients for PFS and n = 198 patients for OS). SurvExpress determined risk groups, indicated by red or green in the plots, by median split of ordered linear predictors. The abscissa indicates time to event in months, and the ordinate is survival probability (Color figure online)
Summary and Conclusions
It is widely accepted that naturally active methylxanthine alkaloids such as caffeine, theophylline, and theobromine are associated with many physiological and pharmacological activities as described briefly in the “Introduction” section. Among these, a weak association has been reported (e.g., [10–17]) between consumption of coffee or tea and breast cancer risk, which evolved since the early report of Stocks [46]. Because of reports suggesting that caffeine inactivates breast cancer-associated myofibroblasts while decreasing expression of certain cytokines (e.g., TGF-β and SDF-1) and elevating levels of tumor suppressor proteins, e,g, PTEN, p53, and p21 (e.g., [2, 9, 47]), our study was designed to evaluate gene expression only in breast carcinoma cells. This was accomplished as explained earlier by first isolating pure populations of human breast carcinoma cells using LCM [18, 19, 25, 28, 29]. Caffeine appears to induce its biological activities by antagonizing adenosine receptors and other targets, which have been implicated in breast cancer cell behavior in vitro [16, 34, 48]. The innovative study of Catalona et al. [34], evaluating both protein and messenger RNA levels, reported that PDE5 expression improved the invasive potential of breast cancer cell activity. To our knowledge, collective expression of genes for certain receptor proteins and enzymes involved in the actions or pathways of pharmacologically active methylxanthines and breast cancer behavior have not been explored.
We first evaluated expression levels of genes for eight methylxanthine receptors, eight metabolizing enzymes, and seven phosphodiesterases in relationship to prediction of risk of breast cancer recurrence. As described, unique aspects of this investigation relate to the use of gene expression results obtained from only LCM-procured breast carcinoma cells without the contributions of non-neoplastic (normal) cells in tissue biopsies (e.g., stroma, macrophages, lymphocytes) [18, 19, 28, 29]. Furthermore, concentrations of both ER and PR proteins of primary breast cancers were quantified by FDA-approved assays unlike the semi-quantification of IHC measurements [39]. Our approach has revealed clinically relevant relationships that appear to be applicable to breast carcinoma management.
As documented, univariable Cox regressions of PDE4A and PDE1A expression levels in LCM-procured carcinoma cells were related to PFS while PDE4A and CYP2E expressions were related to OS at adjusted p values < 0.3 (discovery limit). These clinically relevant genes were derived without considering the ER or PR protein status of the primary lesions. Phosphodiesterases, with synthesis directed by more than 20 genes, are widely reported to be involved in contraction and relaxation of vascular smooth muscle and cardiac myocytes [47], as a major physiological function. These enzymes catalyze the degradation of the second messengers, cAMP and cGMP, thereby playing critical roles modulating signal transduction in a variety of pathways. In an interesting study of a large number of phosphodiesterase by Dong et al. [30], expression of genes for PDE1A and PDE4A as well as many other phosphodiesterases were observed in breast cancer cell lines and in human tissue biopsies. Associated with our findings, they reported that breast cancer cell migration in vitro was inhibited by activation of cAMP-regulated signaling. In a comprehensive study of another phosphodiesterase, PDE5 [34], the enzyme was purported to play a significant role in regulating breast cancer cell behavior in vitro. The authors suggest that PDE5 expression may serve as a prognostic biomarker and possibly as a candidate for therapy development. In a related study, elevated cAMP at sustained levels was achieved by blocking its efflux and degradation and suppressed triple-negative breast cancer development in vivo [49].
A member of the cytochrome P450 family of hemoproteins responsible for metabolism of many drugs, CYP2E, was identified in our early analyses to be associated with breast cancer progression. This enzyme has been implicated in breast cancer response to oxidative stress and also to cell migration [50]. These investigators raised the intriguing question whether cellular concentrations of CYP2E protein may be used as a biomarker of breast cancer progression. In the LASSO models that we derived of breast cancer cells procured by LCM from biopsies without regard to their ER or PR status, CYP2E was a key component of molecular signatures for predicting both PFS and OS.
Kaplan-Meier plots of candidate genes, using median-split gene expression, were constructed without regard to steroid receptor status as an additional means of assessing clinical relevance. These analyses indicated that elevated expression levels of PDE1A and low expression levels of PDE4A, CYP2A6, and CYP2E were associated with decreased PFS. Furthermore, increased expression levels of PDE1A, ADORA2B, and RYR1 and low expression levels of PDE4A, CYP2A6, and CYP2E were associated with diminished OS. Again, expression of many of these genes appear in the LASSO models predicting either PFS or OS further supporting their clinical utility in breast cancer management.
Since both ER and PR protein levels in the primary carcinoma are related to risk of disease progression [35–37, 39], it was enlightening to find that 13 of the 23 candidate genes, ADORA1, ADORA2A, ADORA2B, ADORA3, DRD2, RYR1, PDE4A, PDE4B, CYP2A6, CYP2C8, CYP2E, NAT2, and XDH exhibited significant differences in expression according to ER status. Expression levels of 10 of the 23 candidate genes, ADORA2B, ADORA3, RYR1, PDE3A, PDE4A, PDE4B, CYP2A6, CYP2C8, CYP2E, and XDH, exhibited differences according to PR status. Collectively, these results suggest that some of the genes involved in methylxanthine metabolism and action are associated with either estrogen and/or progestin action in breast cancer cells. Earlier, Rosendahl et al. [16] reported that caffeine and certain derivatives appear to sensitize either ER+ or ER− breast cancer cells to tamoxifen bringing about diminished proliferation. These results and those reported here suggest that further studies of estrogen or progestin regulation of genes identified are warranted.
Since ER and/or PR status of the primary lesion was related to expression levels of a number of genes, univariable Cox regressions showed that the expression of CACNA1S, PDE1A, and RYR1 independently predicted PFS and OS in ER− breast cancers. It was also observed that only PDE4A and XDH expressions predicted PFS in ER− breast cancers. However, the expression of CYP2E was the only gene identified in ER+ lesions that was significant for predicting OS. Individually, expression of CACNA1S, PDE4A, and SPECC1L predicted PFS and OS of PR− lesions. Of these genes, Kaplan-Meier plots confirmed relationships with clinical outcomes for PDE1A, PDE4A, and CYP2E. In addition, CYP3A4 and PDE1B expression levels were associated with poorer outcomes of lower PFS that was not observed by univariable Cox regression.
It was noted that PDE4A and CYP2E expression levels in breast carcinoma cells procured by LCM were elevated significantly in both ER+ and PR+ primary lesions. However, neither PDE1A nor PDE1B expression was associated with either ER or PR protein content of the primary carcinoma, although their expression examined individually was highly associated with clinical outcomes.
Next, we turned our investigations to analyzing the collective utility of gene subsets to predict clinical outcomes of breast carcinomas. Utilizing microarray results of candidate genes in LCM-procured carcinoma cells and clinical outcomes, LASSO models identified that collective expression of PDE1A, PDE4A, and CYP2E predicted PFS. Additionally, LASSO models employing the same expression levels of genes of interest also identified a five-gene signature composed of ADORA1, PDE1A, PDE1B, PDE4A, and CYP2E whose expression collectively predicted OS. External validation of the three-gene LASSO model derived in our analyses that predicted PFS was accomplished utilizing 1888 samples in the Breast Cancer Metabase (SurvExpress). Use of results in this public database confirmed that expression of PDE1A, PDE4A, and CYP2E collectively predicted PFS. Using overall survival data from a limited number of samples (n = 198) in SurvExpress, external validation of the five-gene LASSO model (ADORA1, PDE1A, PDE4A, PDE1B, and CYP2E) derived by our study indicated expression collectively was significant for predicting OS.
Perhaps the most significant findings of our studies are the deciphering of clinically relevant subsets of gene expression that predict PFS and OS of either ER− or PR− breast carcinomas. Since the historic studies of Fisher et al. [51, 52] showing the clinical relevance of determining ER and PR in primary breast carcinomas for assessing response to tamoxifen-associated therapeutic regimens, many studies [35, 36, 39] have shown that patients with ER−/PR− breast cancer exhibit poor prognosis and are not usually candidates for anti-hormonal therapies, such as tamoxifen. Breast cancers that do not contain ER and PR proteins, in general, show aggressive growth properties including early recurrences.
Noteworthy, we derived four molecular signatures using LASSO modeling that predict either PFS or OS in ER− and PR− breast carcinoma patients. This is particularly exciting in that there are few therapeutic regimens outside of combination chemotherapy without or with Herceptin, if the breast cancer biopsy is positive for the HER2/neu oncoprotein [53].Our findings are of particular clinical interest since the commercially available gene expression-based tests (e.g., OncoTypeDx™ and MammaPrint™) are largely focused on patients with ER+ breast cancer. Patients with ER−/PR− breast carcinomas in our investigations exhibited expression profiles of genes for certain phosphodiesterases and methylxanthine-metabolizing enzymes that jointly are associated with a patient’s risk of breast cancer recurrence. By employing unique gene expression results derived from LCM-procured breast carcinoma cells with de-identified clinical outcomes and quantified ER and PR measurements collected in our CLIA-approved laboratory, small panels of genes (emerging molecular signatures) have been deciphered that are associated with prediction of breast cancer progression. Collectively, expression of selective genes involved in methylxanthine action and metabolism in single-cell types predicted clinical outcomes of breast carcinoma patients suggesting promise for developing novel diagnostics for management and design of new therapeutics.
Acknowledgements
Preliminary results were presented at the 2017 Meeting of the American Association for Cancer Research in Washington, DC. SBS was the recipient of a Meritorious Honors Award in the AACR 2017 Undergraduate Poster Competition. MWD current address: Department of Biostatistics, University of Colorado, Aurora, CO 80045.
Appendix
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Natrajan R, et al. Microenvironmental heterogeneity parallels breast cancer progression: a histology-genomic integration analysis. PLoS Med. 2016;13(2):e1001961: 1–e1001961:19. doi: 10.1371/journal.pmed.1001961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Ansari MM, Aboussekhra A. Caffeine mediates sustained inactivation of breast cancer-associated myofibroblasts via up-regulation of tumor suppressor genes. PLoS One. 2014;9(3):e90907. doi: 10.1371/journal.pone.0090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aboussekhra A. Role of cancer-associated fibroblasts in breast cancer development and prognosis. Int J Dev Biol. 2011;55(7–9):841–849. doi: 10.1387/ijdb.113362aa. [DOI] [PubMed] [Google Scholar]
- 4.Downey CL, et al. The prognostic significance of tumour-stroma ratio in oestrogen receptor-positive breast cancer. Br J Cancer. 2014;110(7):1744–1747. doi: 10.1038/bjc.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franco OE, et al. Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol. 2010;21(1):33–39. doi: 10.1016/j.semcdb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 7.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7(2):139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 8.Dekker TJ, et al. Prognostic significance of the tumor-stroma ratio: validation study in node-negative premenopausal breast cancer patients from the EORTC perioperative chemotherapy (POP) trial (10854) Breast Cancer Res Treat. 2013;139(2):371–379. doi: 10.1007/s10549-013-2571-5. [DOI] [PubMed] [Google Scholar]
- 9.Miwa S, et al. Caffeine activates tumor suppressor PTEN in sarcoma cells. Int J Oncol. 2011;39(2):465–472. doi: 10.3892/ijo.2011.1051. [DOI] [PubMed] [Google Scholar]
- 10.Hashibe M, et al. Coffee, tea, caffeine intake, and the risk of cancer in the PLCO cohort. Br J Cancer. 2015;113(5):809–816. doi: 10.1038/bjc.2015.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh JK, et al. Prospective study of breast cancer in relation to coffee, tea and caffeine in Sweden. Int J Cancer. 2015;137(8):1979–1989. doi: 10.1002/ijc.29569. [DOI] [PubMed] [Google Scholar]
- 12.Jiang W, Wu Y, Jiang X. Coffee and caffeine intake and breast cancer risk: an updated dose-response meta-analysis of 37 published studies. Gynecol Oncol. 2013;129(3):620–629. doi: 10.1016/j.ygyno.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Fagherazzi G, et al. No association between coffee, tea or caffeine consumption and breast cancer risk in a prospective cohort study. Public Health Nutr. 2011;14(7):1315–1320. doi: 10.1017/S1368980011000371. [DOI] [PubMed] [Google Scholar]
- 14.Kotsopoulos J, et al. The CYP1A2 genotype modifies the association between coffee consumption and breast cancer risk among BRCA1 mutation carriers. Cancer Epidemiol Biomark Prev. 2007;16(5):912–916. doi: 10.1158/1055-9965.EPI-06-1074. [DOI] [PubMed] [Google Scholar]
- 15.Bageman E, et al. Coffee consumption and CYP1A2*1F genotype modify age at breast cancer diagnosis and estrogen receptor status. Cancer Epidemiol Biomark Prev. 2008;17(4):895–901. doi: 10.1158/1055-9965.EPI-07-0555. [DOI] [PubMed] [Google Scholar]
- 16.Rosendahl AH, et al. Caffeine and caffeic acid inhibit growth and modify estrogen receptor and insulin-like growth factor I receptor levels in human breast cancer. Clin Cancer Res. 2015;21(8):1877–1887. doi: 10.1158/1078-0432.CCR-14-1748. [DOI] [PubMed] [Google Scholar]
- 17.Ganmaa D, et al. Coffee, tea, caffeine and risk of breast cancer: a 22-year follow-up. Int J Cancer. 2008;122(9):2071–2076. doi: 10.1002/ijc.23336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andres SA, Wittliff JL. Relationships of ESR1 and XBP1 expression in human breast carcinoma and stromal cells isolated by laser capture microdissection compared to intact breast cancer tissue. Endocrine. 2011;40(2):212–221. doi: 10.1007/s12020-011-9522-x. [DOI] [PubMed] [Google Scholar]
- 19.Wittliff JL. Laser capture microdissection and its use in genomics & proteomics, in reliable lab solutions. In: Conn PM, editor. Techniques in confocal microscopy. Amsterdam: Elsevier Press; 2010. pp. 463–477. [Google Scholar]
- 20.Gerlinger M, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McShane LM, et al. Reporting recommendations for tumor marker prognostic studies (REMARK) Breast Cancer Res Treat. 2006;100(2):229–235. doi: 10.1007/s10549-006-9242-8. [DOI] [PubMed] [Google Scholar]
- 22.Kerr DA, 2nd, Wittliff JL. A five-gene model predicts clinical outcome in ER+/PR+, early-stage breast cancers treated with adjuvant tamoxifen. Horm Cancer. 2011;2(5):261–271. doi: 10.1007/s12672-011-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andres SA, et al. Interaction between smoking history and gene expression levels impacts survival of breast cancer patients. Breast Cancer Res Treat. 2015;152(3):545–556. doi: 10.1007/s10549-015-3507-z. [DOI] [PubMed] [Google Scholar]
- 24.Kruer TL, et al. Characterization of estrogen response element binding proteins as biomarkers of breast cancer behavior. Clin Biochem. 2013;46(16–17):1739–1746. doi: 10.1016/j.clinbiochem.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Daniels MW, Brock GN, Wittliff JL. Clinical outcomes linked to expression of gene subsets for protein hormones and their cognate receptors from LCM-procured breast carcinoma cells. Breast Cancer Res Treat. 2017;161(2):245–258. doi: 10.1007/s10549-016-4049-8. [DOI] [PubMed] [Google Scholar]
- 26.Andres SA, Brock GN, Wittliff JL. Interrogating differences in expression of targeted gene sets to predict breast cancer outcome. BMC Cancer. 2013;13:326–344. doi: 10.1186/1471-2407-13-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andres SA, Wittliff JL. Co-expression of genes with estrogen receptor-alpha and progesterone receptor in human breast carcinoma tissue. Horm Mol Biol Clin Investig. 2012;12(1):377–390. doi: 10.1515/hmbci-2012-0025. [DOI] [PubMed] [Google Scholar]
- 28.Ma X, et al. Breast cancer research and treatment: 2003. Dordrecht: Kluwer Academic Publ; 2003. Gene expression signatures associated with clinical outcome in breast cancer via laser capture microdissection; pp. S15–S15. [Google Scholar]
- 29.Ma XJ, et al. Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci U S A. 2003;100(10):5974–5979. doi: 10.1073/pnas.0931261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong H, et al. Inhibition of breast cancer cell migration by activation of cAMP signaling. Breast Cancer Res Treat. 2015;152(1):17–28. doi: 10.1007/s10549-015-3445-9. [DOI] [PubMed] [Google Scholar]
- 31.Ribeiro JA, Sebastiao AM. Caffeine and adenosine. J Alzheimers Dis. 2010;20(Suppl 1):S3–15. doi: 10.3233/JAD-2010-1379. [DOI] [PubMed] [Google Scholar]
- 32.Thorn CF, et al. PharmGKB summary: caffeine pathway. Pharmacogenet Genomics. 2012;22(5):389–395. doi: 10.1097/FPC.0b013e3283505d5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whirl-Carrillo M, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414–417. doi: 10.1038/clpt.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catalano S, et al. Expression and function of phosphodiesterase type 5 in human breast cancer cell lines and tissues: implications for targeted therapy. Clin Cancer Res. 2016;22(9):2271–2282. doi: 10.1158/1078-0432.CCR-15-1900. [DOI] [PubMed] [Google Scholar]
- 35.Fleisher M et al (2002) Practice guidelines and recommendations for use of tumor markers in the clinic. In: Tumor markers: physiology, pathobiology, technology and clinical applications, D. P. Diamandis, H. A. Fritsche, H. Lilja, D. W. Chan, M. K. Schwartz (eds.), AACC Press, Washington, D.C, p 33–63
- 36.Wittliff J, Pasic R, Bland K (1998) Steroid and peptide hormone receptors: methods, quality control and clinical use. In: The breast: comprehensive management of benign and malignant diseases, K. I. Bland and E. M. Copeland III (eds.), WB Saunders Co, Philadelphia, Chapter 25, p 458–498
- 37.Pasic R, Djulbegovic B, Wittliff JL. Comparison of sex steroid receptor determinations in human breast cancer by enzyme immunoassay and radioligand binding. J Clin Lab Anal. 1990;4(6):430–436. doi: 10.1002/jcla.1860040608. [DOI] [PubMed] [Google Scholar]
- 38.Therneau T (2013) A package for survival analysis in S. R package version 2.37–4. Box 980032:23298–0032, URL http://cran.R-project.org/package=survival
- 39.Hammond ME, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134(6):907–922. doi: 10.5858/134.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Austin PC, Tu JV. Automated variable selection methods for logistic regression produced unstable models for predicting acute myocardial infarction mortality. J Clin Epidemiol. 2004;57(11):1138–1146. doi: 10.1016/j.jclinepi.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Goeman JJ. L1 Penalized estimation in the cox proportional hazards model. Biom J. 2010;52(1):70–84. doi: 10.1002/bimj.200900028. [DOI] [PubMed] [Google Scholar]
- 42.Goeman JJ, et al.(2016) L1 and L2 penalized regression models. R Studio Version 0.9-47
- 43.Simon N, et al. Regularization paths for Cox’s proportional hazards model via coordinate descent. J Stat Softw. 2011;39(5):1–13. doi: 10.18637/jss.v039.i05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1–22. doi: 10.18637/jss.v033.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aguirre-Gamboa R, et al. SurvExpress: an online biomarker validation tool and database for cancer gene expression data using survival analysis. PLoS One. 2013;8(9):e74250: 1–e74250: 9. doi: 10.1371/journal.pone.0074250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stocks P. Cancer mortality in relation to national consumption of cigarettes, solid fuel, tea and coffee. Br J Cancer. 1970;24(2):215–225. doi: 10.1038/bjc.1970.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;100(3):309–327. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- 48.Burke TM, et al. Effects of caffeine on the human circadian clock in vivo and in vitro. Sci Transl Med. 2015;7(305):146–165. doi: 10.1126/scitranslmed.aac5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang W, et al. Triple negative breast cancer development can be selectively suppressed by sustaining an elevated level of cellular cyclic AMP through simultaneously blocking its efflux and decomposition. Oncotarget. 2016;7(52):87232–87245. doi: 10.18632/oncotarget.13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leung T, et al. Cytochrome P450 2E1 (CYP2E1) regulates the response to oxidative stress and migration of breast cancer cells. Breast Cancer Res. 2013;15(6):R107–R118. doi: 10.1186/bcr3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fisher B, et al. Treatment of primary breast cancer with chemotherapy and tamoxifen. N Engl J Med. 1981;305(1):1–6. doi: 10.1056/NEJM198107023050101. [DOI] [PubMed] [Google Scholar]
- 52.Fisher B, et al. Influence of tumor estrogen and progesterone receptor levels on the response to tamoxifen and chemotherapy in primary breast cancer. J Clin Oncol. 1983;1(4):227–241. doi: 10.1200/JCO.1983.1.4.227. [DOI] [PubMed] [Google Scholar]
- 53.Wolff AC, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]