Abstract
The eglA gene, encoding a thermostable endoglucanase from the hyperthermophilic archaeon Pyrococcus furiosus, was cloned and expressed in Escherichia coli. The nucleotide sequence of the gene predicts a 319-amino-acid protein with a calculated molecular mass of 35.9 kDa. The endoglucanase has a 19-amino-acid signal peptide but not cellulose-binding domain. The P. furiosus endoglucanase has significant amino acid sequence similarities, including the conserved catalytic nucleophile and proton donor, with endoglucanases from glucosyl hydrolase family 12. The purified recombinant enzyme hydrolyzed β-1,4 but not β-1,3 glucosidic linkages and had the highest specific activity on cellopentaose (degree of polymerization [DP] = 5) and cellohexaose (DP = 6) oligosaccharides. To a lesser extent, EglA also hydrolyzed shorter cellodextrins (DP < 5) as well as the amorphous portions of polysaccharides which contain only β-1,4 bonds such as carboxymethyl cellulose, microcrystalline cellulose, Whatman paper, and cotton linter. The highest specific activity toward polysaccharides occurred with mixed-linkage β-glucans such as barley β-glucan and lichenan. Kinetics studies with cellooliogsaccharides and p-nitrophenyl-cellooligosaccharides indicated that the enzyme had three glucose binding subsites (−I, −II, and −III) for the nonreducing end and two glucose binding subsites (+I and +II) for the reducing end from the scissile glycosidic linkage. The enzyme had temperature and pH optima of 100°C and 6.0, respectively; a half-life of 40 h at 95°C; and a denaturing temperature of 112°C as determined by differential scanning calorimetry. The discovery of a thermostable enzyme with this substrate specificity has implications for both the evolution of enzymes involved in polysaccharide hydrolysis and the occurrence of growth substrates in hydrothermal vent environments.
The hyperthermophilic archaeon Pyrococcus furiosus is an obligately anaerobic heterotroph isolated from a shallow marine hydrothermal vent which grows optimally at 98 to 100°C (16). Until recently, P. furiosus was known to utilize a limited number of carbohydrates including starch, pullulan, maltose (8), and cellobiose (26). However, it was recently reported that P. furiosus could grow on the β-linked glucose polymers laminarin (β-1,3 linkages only) and lichenan (both β-1,4 and β-1,3 linkages) (22). A family of 16 laminarinase which hydrolyzed the β-1,3 bonds found in these substrates was identified in P. furiosus (22). The presence of a laminarinase in P. furiosus and the resulting growth of P. furiosus on lichenan suggested that P. furiosus might contain other glycosyl hydrolases capable of hydrolyzing the β-1,4 linkages of mixed-linkage β-glucans. In an effort to determine the full set of glycosyl hydrolases produced by this model hyperthermophilic archaeon, we have identified a novel family 12 endoglucanase which is capable of degrading the β-1,4 bonds of cellooligosaccharides, mixed-linkage β-glucans such as lichenan, and to a lesser extent cellulose.
Cellulose, the most abundant polysaccharide in the biosphere (29), is composed of d-glucose units linked together to form linear chains via β-1,4 glycosidic linkages (50). Although cellulose is found abundantly in plants, where it constitutes the major structural polysaccharide of cell walls, β-1,4 glucose polymers have also been identified in fungi (45); algae, such as Valonia macrophysa (6, 10, 31, 44); invertebrates (45); protists (45); bacteria, such as Acetobacter xylinum (58); and even occasionally animals (e.g., tunicin) (5). On the other hand, (1→3),(1→4)-β-d-glucan (β-glucan) is one of the major structural components of cereal endosperm cell walls (21). Although β-glucan accounts for only a small fraction of the total carbohydrate in barley kernels, it represents nearly 75% of the total carbohydrate in the cell walls of the endosperm (3). Mixed-linkage (1→3),(1→4)-β-d-glucans are also produced by some bacteria (2, 24), lichen (14, 28), and fungi (1). Although several bacteria that can produce exopolysaccharides (15, 41, 42, 43), which generally contain glucose, mannose, and galactose as well as uronic acids, hexosamines, and other monosaccharide derivatives, have been isolated from hydrothermal vent environments, neither β-glucans nor cellulose has been reported to occur in the hydrothermal vent environments from which P. furiosus was isolated.
Glycosyl hydrolase family 12 currently consists of endoglucanases from meosphilic (i.e., Erwinia carotovora) (48) and hyperthermophilic (i.e., Thermotoga species) bacteria (13, 32) as well as various fungi (17, 27, 39, 40, 49, 60). Family 12 enzymes catalyze the hydrolysis of β-1,4 glucosidic linkages in cereal β-glucans and, to a lesser extent, in various forms of cellulose, such as Avicel (37), acid-swollen Avicel (32, 60), and alkali-swollen Avicel (37), as well as arabinoxylans (32). The catalytic mechanism of family 12 enzymes results in retention of configuration at the anomeric carbon (19, 52). Also the catalytic amino acids, which function as nucleophile and proton donor in this mechanism, have been identified by comparison of family 12 endoglucanases and family 11 xylanases by hydrophobic cluster analysis (55). Recently, the first three-dimensional crystal structure of a family 12 endoglucanase was solved, revealing active site structure and key catalytic residues for family 12 endoglucanases (54). Here, we report the first endoglucanase identified in the Archaea which is capable of degrading the β-1,4 bonds of β-glucans and cellulose. The presence of an enzyme with this substrate specificity in a hyperthermophilic archaeon has implications for both the evolution of enzymes involved in polysaccharide hydrolysis and the occurrence of growth substrates in hydrothermal vent environments.
MATERIALS AND METHODS
Materials.
Laminarin from Laminaria digitata, lichenan from Cetraria islandica, curdlan from Agrobacterium faecalis, carboxymethyl cellulose (average substitution = 0.6), SIgmaCel type 20, SigmaCel type 101, oat spelt arabinoxylan, birchwood xylan, p-nitrophenyl-β-d-glucopyranoside, p-nitrophenyl-β-d-cellobiose, d-glucono-1,5-lactone, and glucose were purchased from Sigma (St. Louis, Mo.). Barley β-glucan, RBB-barley glucan, pachyman, and wheat arabinoxylan were purchased from Megazyme (Bray, County Wicklow, Ireland). Pure-grade cellobiose, cellotriose, cellotetraose, cellopentaose, cellohexaose, laminaribiose, laminaritriose, laminaritetraose, laminaripentaose, laminarihexaose, p-nitrophenyl-β-d-cellotriose, p-nitrophenyl-β-d-cellotetraose, and p-nitrophenyl-β-d-cellopentaose were obtained from Seikagaku (Tokyo, Japan). Aminex HPX-42A was obtained from Bio-Rad (Rockville Center, N.Y.). DEAD-Sepharose and Superdex 200 were obtained from Pharmacia (Uppsala, Sweden). Avicel PH101 (50 μm, microcrystalline cellulose) was obtained from FMC (Rockland, Maine). Whatman cellulose powder (CC31, degree of polymerization [DP] ∼ 210), cellunier F wood pulp (ITT Rayonier), and cotton linter (Buckeye type 1N) were kindly provided by Samuel Hudson, North Carolina State University College of Textiles.
Expression screening and subcloning.
A library of the P. furiosus genome was constructed by shearing and size selecting chromosomal DNA followed by ligation to EcoRI linkers. The chromosomal fragments were cloned into EcoRI-digested lambda gt11 arms and packaged according to the manufacturer’s instructions (Stratagene Cloning Systems, La Jolla, Calif.). The gt11 library was used to transfect Y1090 cells (Stratagene) and plated in molten 0.7% NZ agar–0.2% RBB-barley glucan (Megazyme) onto 1.5% NZ agar plates and screened essentially according to the protocol of Chen et al. (11). When a gt11 plaque expresses an enzyme that breaks down barley glucan, the blue RBB indicator dye is released and a clearing zone will be observed around the plaque. A positive plaque was identified in this manner and subsequently cored from the plate by using a sterile pipette tip, resuspended in sterile medium, and replated as before for single-plaque isolation.
PCR amplification of the insert was performed from the purified lambda gt11 clone with primers which anneal to the lambda sequences flanking either side of the EcoRI site (5′-GGTGGCGACTCCTGGAGCAGCCCG-3′ and 5′-TTGACACCAGACCAACTGGTATG-3′). The PCR product was agarose gel isolated and cloned into the plasmid pCR2.1 with a TOPO TA cloning kit (Invitrogen, Carlsbad, Calif.) according to the manufacturer’s instructions. The nucleotide sequence was determined with an ABI Prism sequencing kit and an ABI377 sequencer (Perkin-Elmer/Applied Biosystems Division, Foster City, Calif.). The open reading frame was identified by BLAST analysis. Primers were designed for PCR amplification of the gene, and the amplified product was cloned into the QE30 plasmid system (Qiagen, Chatsworth, Calif.) for overexpression.
Production and purification of recombinant protein.
Recombinant P. furiosus eglA was expressed in Escherichia coli and purified as follows. Protein was expressed in E. coli M15 with the QE30 expression system (Qiagen). Cells were grown overnight in 200 ml of Luria broth with 100 μg of ampicillin per ml, 80 μg of methicillin per ml, and 50 μg of kanamycin per ml (LBamk) at 37°C. This culture was used to inoculate 2 liters of LBamk. Cultures were grown at 37°C until optical density at 600 nm was 0.8, IPTG (isopropyl-β-d-thiogalactopyranoside) was added to a final concentration of 1 mM. Cultures were harvested after 6 h and centrifuged (30 min, 10,000 × g). The cell pellet was resuspended in 20 ml of 50 mM sodium phosphate buffer (pH 8.0), passed twice through a French press (SLM Instruments), and centrifuged (20 min, 30,000 × g).The supernatant was heated at 80°C for 20 min and centrifuged (20 min, 30,000 × g). The heat-treated supernatant was loaded onto a column of DEAE-Sepharose CL-6B (5 by 40 cm) which had been previously equilibrated with 50 mM sodium phosphate, pH 8.0. The column was developed with a 4.0-liter linear gradient of 0 to 1 M sodium chloride in the starting buffer. Fractions were assayed for endoglucanase activity at 95°C with 2% RBB-barley glucan (35). Fractions containing endoglucanase activity were pooled, equilibrated to 50 mM sodium phosphate buffer (pH 7.0) containing 150 mM NaCl, and loaded onto a column (1.6 by 60 cm) of Superdex 200 (Pharmacia). Fractions containing endoglucanase activity were pooled and concentrated. The enzyme was judged to be homogeneous by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Protein concentrations were determined by a dye-binding method (7) with bovine serum albumin as standard.
Assay of enzyme activity on β-glucan polysaccharides.
Unless indicated otherwise, enzyme assays were done in triplicate at 95°C in 1.0-ml reaction mixtures containing 50 mM sodium phosphate buffer, pH 6.0, and 0.5% (wt/vol) solutions of soluble polysaccharide substrates (e.g., laminarin, lichenan, barley glucan, curdlan, carboxymethyl cellulose, pachyman, and arabionxylans). Slurries (1% [wt/vol] of nonsoluble substrates (e.g., Avicel, SigmaCels, and Whatman CC31) were mixed by mechanical agitation at 100 rpm. Whatman 42 was cut into 7-mm-diameter circles with a hole punch and used at 25 mg/ml, cotton linter was used at 50 mg/ml, and Cellunier F was cut into a 1- by 1- by 7-mm strip (25 mg). The enzymatic activity was measured by monitoring the release of reducing sugars (38, 53). One unit of enzyme activity was defined as the amount of enzyme required to release 1 μmol of glucose-equivalent reducing groups per min. Nonenzymatic hydrolysis of the substrates at elevated temperatures was corrected for with the appropriate blanks.
HPLC analysis of reaction products.
Enzyme-catalyzed hydrolysis reactions of 10 mM cellulose and laminarin oligosaccharides were performed in triplicate at 95°C in 0.05 ml of deionized water. Hydrolysis reactions were terminated at various time intervals by placing samples on ice. Samples were applied to an Aminex HPX-42A high-performance liquid chromatography (HPLC) column (7.8 by 300 mm), equilibrated in 60°C deionized water at 0.2 ml/min and prefitted with an Aminex guard cartridge (125-0507). Identification of hydrolysis products (DP < initial substrate) and transglycosylation products (DP > initial substrate) was done with a refractive index detector (Shimadzu, Kyoto, Japan), relative to cellulose and laminarin oligosaccharide concentration standards (33). Oligosaccharide standard curves for carmelization (30) effects were also used to correct for changes in sample color analyzed by the refractive index detector. One unit of enzyme activity was defined either as the amount of enzyme required to degrade 1 μmol of oligosaccharide substrate per min or as Up, the amount of enzyme required to release 1 μmol of oligosaccharide product per min.
Kinetic analyses and inhibitor studies.
Kinetic studies with aryl glycoside substrates were performed at 95°C as described elsewhere (12). The buffer employed for all kinetic experiments was 50 mM sodium phosphate buffer, pH 6.0. Rates were determined at 7 to 10 different substrate concentrations, ranging from approximately 0.15 times the value of the Km ultimately determined to 7 times its value, when possible. Values of Km and kcat were determined from these rates by means of nonlinear regression analysis (59). Ki values for inhibitors were determined by first estimating the approximate Ki value by varying the inhibitor concentration at a fixed concentration of substrate (1.0 mM PNPGlu3) and then determining the parameters from Dixon plots. A full Ki determination was then carried out at a series of 7 to 10 different substrate concentrations bracketing the Km,app value, with three to five inhibitor concentrations bracketing the approximate Ki value. All such data were fitted by nonlinear regression analysis (59).
Temperature and pH optima and thermostability.
Kinetic parameters were determined for EglA with PNPGlu3 at temperatures from 35 to 105°C. The temperature dependence of the enzyme was also determined by measuring the specific activity of the enzyme with a 0.5% barley β-glucan solution in 50 mM sodium phosphate buffer, pH 6.0, at various temperatures. The pH dependence of the enzyme was investigated by determining the kinetic parameters for the enzyme with PNPGlu3 as well as the specific activities of the enzyme with 0.5% barley β-glucan. This was done for a series of pH values between 3.6 and 5.6 with 50 mM sodium acetate buffer, between 5.4 and 8.4 with 50 mM sodium phosphate buffer, and between 8.6 and 10.0 with 50 mM glycine-NaOH. The pH values were determined by pH meter (Fisher Accumet 15) at the appropriate temperature. Thermostability was determined by incubating the purified enzyme for various lengths of time at 95 or 105°C in 50 mM sodium phosphate buffer, pH 6.0, covered with Ampliwax (Perkin-Elmer) and determining the residual activity.
Calorimetry.
Differential scanning calorimetry analysis was carried out on a Nanoscan microcalorimeter (Calorimetry Sciences, Salt Lake City, Utah) operating in the temperature range of 25 to 125°C. The cell was pressurized to 3.00 atm to allow operation above 100°C. Purified β-1,4 endoglucanase was dialyzed extensively against 10 mM sodium phosphate buffer, pH 6.0. The equilibrated enzyme was scanned at 1.0°C/min with a concentration of 15 μM. The enzyme was scanned three times under identical conditions. All enzyme scans were corrected with a buffer-buffer baseline. The partial specific volume was determined from the amino acid composition (34). The excess molar heat capacity was calculated after baseline subtraction (17), i.e., the baseline was obtained from the linear temperature dependence of the native-state heat capacity.
RESULTS
Sequence analysis.
Sequencing of the 3.0-kb insert from an isolated clone containing endoglucanase activity revealed a 319-amino-acid open reading frame (Fig. 1). The enzyme had significant homology with glycosyl hydrolases from family 12 (23) especially in the region of the catalytic acid-base and nucleophile (Fig. 2). Ten amino acids were absolutely conserved among the 14 family 12 enzymes. Of these 10 amino acids, two residues (E197 and E290 in Pfu EglA) had previously been identified as possibly being the active site nucleophile and proton donor, respectively, based on comparison between family 12 endoglucanases and family 11 xylanase by hydrophobic cluster analysis (56). These two amino acids are the only absolutely conserved residues in family 12 that have the proper functionalities (i.e., carboxlates) to act as the catalytic residues. This strengthens the previous assertion that these residues are involved in catalysis. Of the eight additional absolutely conserved amino acids (N82, W84, G131, M199, W201, P209, G211, and F264 in EglA), three were aromatic residues which may interact with sugar moieties as has been seen for other carbohydrate-binding proteins (47, 61), including some endoglucanases (56). As is the case with several other family 12 enzymes (13, 32, 40, 49, 60), EglA has a signal peptide. EglA also contained a 22-amino-acid region (amino acids 28 to 49) which was rich in proline and hydroxyamino acids. Similar stretches of sequence have been shown to connect different domains in glycosyl hydrolases having multiple domains (20). Unlike the family 12 endoglucanases from Streptomyces lividans (60) and Streptomyces rochei (40), EglA did not contain a cellulose binding domain.
FIG. 1.
Deduced amino acid sequences for eglA from P. furiosus. The putative signal peptide is underlined. The proline- and hydroxyamino-acid-rich region is double underlined. The putative catalytic nucleophile and acid-base are identified by asterisks.
FIG. 2.
Alignment of the amino acid sequences for family 12 endoglucanases in the region of the catalytic nucleophile (†) and acid-base (‡). Residues that occur in at least 10 of the 14 sequences are in boldface. The numbers refer to the amino acids in the following proteins (accession numbers, where available, are given in parentheses): pf eglA, P. furiosus endoglucanase; tm celA, T. maritima endoglucanase A (Z69341); tm celB, T. maritima endoglucanase B (Z69341); tn celA, T. neapolitana endoglucanase A (U93354); tn celB, T. neapolitana endoglucanase A (U93354); rt celA, R. marinus cellulase (U72637); ak celA, Aspergillus kawachii endoglucanase A (D12901); ao cel, Aspergillus oryzae endoglucanase (D83731); aa gun, Aspergillus aculeatus endoglucanase (P22669); tr gun, Trichoderma reesei endoglucanase (AB003694); sr, eglS, S. rochei endoglucanase S (X73953); sl celB, S. lividans cellulase B (U04629); sh celA2, Streptomyces halstedii cellulase A2 (U51222); ec celS, E. carotovora cellulase S (P16630); mt rv1090, Mycobacterium tuberculosis putative endoglucanase (AL021897). The percentages to the right indicate the amino acid sequence identities between the indicated enzyme and the P. furiosus endoglucanase as determined by using BESTFIT (gap creation penalty, 1; gap extension penalty, 0.3).
Substrate specificity.
Purified EglA had the highest specific activity toward cellulose oligosaccharides, specifically cellohexaose and cellopentaose (Table 1). The specific activities of the P. furiosus endoglucanase on these substrates are similar to those observed for other family 12 enzymes (25, 32, 37, 60). Like other family 12 endoglucanases, the Pfu enzyme had significant activity toward (1→3),(1→4)-β-d-glucans, such as barley β-glucan and lichenan. The enzyme was also capable of hydrolyzing polysaccharides with only β-1,4 linkages, such as carboxymethyl cellulose, microcrystalline cellulose, Whatman paper, and cotton linter, although with a specific activity over 2 to 3 orders of magnitude lower than that for cellopentaose and cellohexaose. Furthermore, similar to several other family 12 endoglucanases (32, 48). EglA could degrade arabinoxylans but to a much lesser extent. No activity was detected on solely β-1,3-linked oligosaccharides or polysaccharides.
TABLE 1.
Substrate specificity of EglA
| Substrate | Backbone linkage(s) | Sp act (U/mg) |
|---|---|---|
| Glucan oligosaccharidesa | ||
| Cellobiose | β-1,4 only | 7.5 |
| Cellotriose | β-1,4 only | 8.4 |
| Cellotetraose | β-1,4 only | 208.1 |
| Cellopentaose | β-1,4 only | 864.8 |
| Cellohexaose | β-1,4 only | 960.9 |
| Laminarin oligosaccharides (1 < DP < 7) | β-1,3 only | 0 |
| Glucan polysaccharidesb | ||
| Barley glucan | β-1,3/4 | 58.0 |
| Lichenan | B-1,3/4 | 53.0 |
| Carboxymetnyl cellulose | β-1,4 only | 7.1 |
| Whatman 42 filter paper | β-1,4 only | 0.52 |
| Cotton linter | β-1,4 only | 0.052 |
| Avicel PH101 | β-1,4 only | 0.049 |
| SigmaCel type 20 | β-1,4 only | 0.042 |
| SigmaCel type 101 | β-1,4 only | 0.039 |
| Cellunier F | β-1,4 only | 0.029 |
| Whatman microcrystalline | β-1,4 only | 0.016 |
| Pachyman, curdlan, laminarin | β-1,3 only | 0 |
| Xylansb | ||
| Wheat arabinoxylan | β-1,4 only | 0.062 |
| Oat spelt arabinoxylan | β-1,4 only | 0.048 |
| Birchwood xylan | β-1,4 only | 0.045 |
One unit of activity is defined as 1 μmol of initial oligosaccharide substrate degraded per min. All assays were performed at 95°C, in deionized water.
One unit of activity is defined as 1 μmol of glucose equivalents (for glucan substrates) or xylose equivalents (for xylan substrates) released per min. All assays were performed at 95°C, pH 6.0. The two units of activity measurement are equivalent.
Kinetic parameters and subsite mapping characterization.
HPLC analysis of the degradation of cellooligosaccharides from cellobiose to cellohexaose (only cellopentaose degradation shown in Fig. 3) and steady-state kinetic parameters for the release of para-nitrophenol (PNP) from PNP-cellooligosaccharides (up to DP 5 shown in Table 2) were determined. HPLC analysis of cellooligosaccharide reaction products revealed that hydrolysis and transglycosylation reactions occurred simultaneously (Fig. 3). Transglycosylation has been observed previously for the family 12 endoglucanase from Rhodothermus marinus (25). The rates of the transglycosylation reactions were quantified from cellooligosaccharide standard curves. Transglycosylation reactions were noticeable with all cellooligosaccharide substrate concentrations (0.5 to 10 mM) studied (data not shown). Hydrolytic activity toward each cellooligosaccharide substrate was determined by accounting for contributions from synthesis reactions involving the initial substrate and products. Likewise, quantification of hydrolysis products (DP < initial substrate) was determined after considering the amount of each product that had undergone a synthesis reaction with the initial substrate to produce larger (DP > initial substrate) cellooligosaccharides. For example, 1 μmol of cellobiose was assumed to combine with 1 μmol of initial substrate to synthesize an oligosaccharide with DP 2 greater than the initial substrate. The 1 μmol of cellobiose, which undergoes the synthesis reaction, was added back to the amount of cellobiose detected in the hydrolysis reaction. Similarly, the 1-μmol initial substrate, which undergoes the synthesis reaction, was added back to the initial substrate still present upon termination of the reaction.
FIG. 3.
HPLC analysis of the initial degradation of 10 mM cellopentaose with EglA at 98°C. Cellooligosaccharide DPs are identified by number. For hydrolysis products, DP < 5; for synthesis products DP > 5. The dotted line represents control incubated for the same period of time in the absence of enzyme. RI, refractive index.
TABLE 2.
Kinetic parameters for the release of PNP from the reducing end of PNP-cellooligosaccharides by EglA
| Substrate | Km (mM) | kcat (s−1) | kcat/Km (s−1 mM−1) |
|---|---|---|---|
| PNP-glucoside | 0.06 | ||
| PNP-cellobiose | 0.15 | 0.79 | 8.7 |
| PNP-cellotriose | 0.061 | 8.3 | 220 |
| PNP-cellotetraose | 0.12 | 3.6 | 49 |
| PNP-cellopentaose | 0.36 | 1.4 | 6.5 |
The enzyme showed no preference for degrading cellotetraose to 45% (molar basis) glucose-cellotriose and 55% cellobiose (two equivalents). However, cellopentaose hydrolysis resulted in 74% cellobiose-cellotriose and 26% glucose-cellotetraose. Likewise, cellohexaose hydrolysis produced an unequal distribution of products: 60% cellobiose-cellotetraose, 26% glucose-cellopentaose, and only 14% cellotriose (two equivalents). Extended periods of incubation produced predominantly cellotriose and cellobiose (data not shown). Glucose levels remained lower than those of cellobiose due to synthesis reactions involving glucose (data not shown).
Steady-state kinetic parameters for the release of PNP from the reducing end of PNP-cellooligosaccharide are given in Table 2. The maximum catalytic efficiency (kcat/Km) occurred for PNP-cellotriose. The contribution of single subsites to transition-state stabilization can be calculated from the second-order rate constants (kcat/Km). This stabilization can be expressed by the difference in transition-state activation energy between two substrates differing in one glucopyranose unit according to the following equation (35):
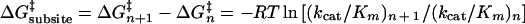 |
The values calculated from the kinetic data for the hydrolysis of the PNP-cellooligosaccharides are presented below. ΔG‡ values (in kilocalories per mole) for subsites are as follows: −I, not determined; −II, −3.6 ± 0.2; −III, −2.4 ± 0.2; −IV, +1.1 ± 0.2; −V, +1.5 ± 0.2. Binding of glucopyranose moieties to subsites −II and −III has a stabilizing effect on the enzyme-carbohydrate transition-state complex, with a larger contribution from subsite −II (−3.6 kcal/mol). This degree of transition-state stabilization for subsites −II and −III (−2.1 and −3.5 kcal/mol, respectively) was observed for the 1,3-1,4-β-glucanase from Bacillus licheniformis (35). By a similar analysis, binding at subsites −IV and −V appears to have a destabilizing effect on the enzyme-carbohydrate transition state (+1.1 and +1.5 kcal/mol, respectively). However, this analysis does not account for the substrate binding which results in the hydrolysis of glycosidic linkages other than the one linking the glycone moiety and the chromophoric aglycone. Both the HPLC-analyzed cellooligosaccharide degradation and spectrophotometrically determined PNP released from PNP-cellooligosaccharide support the notion that the Pfu family 12 endoglucanase contains a carbohydrate-binding cleft consisting of three glucopyranose-binding subsites on the nonreducing end and two glucopyranose-binding subsites on the reducing end from the scissile glycosidic linkage.
Other biochemical and biophysical properties of the endoglucanase.
Several compounds were tested for their ability to inhibit the activity of EglA. Of these, glucose was weakly inhibitory (Ki = 90 mM). However, cellobiose and laminaribiose were found to be moderate inhibitors (Ki = 3.0 and 1.6 mM, respectively) of EglA, while gluconolactone was the most potent inhibitor of this enzyme (Ki = 100 μM).
The temperature optimum for both the degradation of barley glucan and PNPGlu3 was 100°C (Fig. 4). This is the highest temperature optimum yet reported for an endoglucanase. The activation energy (EA), calculated from the Arrhenius plot (Fig. 4 inset) for the enzyme-catalyzed reaction of PNPGlu3, was 15.9 kcal/mol (based on kcat). The pH optimum for the hydrolysis of both barley glucan and PNPGlu3 was approximately 6.0. This is nearly identical to that of the family 12 endoglucanase (celA) from Thermotoga maritima (31). The enzyme was extremely thermostable with half-lives of 40 h at 95°C and 1.6 h at 105°C (data not shown) and a denaturation temperature of 112°C as determined by differential scanning calorimetry (data not shown).
FIG. 4.
Temperature dependence for the hydrolysis of barley glucan (open circles) and PNPGlu3 (closed circles) by EglA. Inset: Arrhenius plot for the hydrolysis of PNPGlu3.
DISCUSSION
Evolutionary implications for the presence of glucans and glycosyl hydrolases in hydrothermal vent environments.
Although this is the second endoglucanase discovered in P. furiosus, the two enzymes (LamA and EglA) have distinctly different amino acid sequences and substrate specificities. For instance, the two enzymes have no significant sequence homology. The laminarinase has been classified as a member of glucosyl hydrolase family 16 based on amino acid sequence similarities (22), whereas the β-1,4 endoglucanase clearly belongs in glycosyl hydrolase family 12. Furthermore, the laminarinase, like many enzymes from family 16, cleaves the β-1,3 bonds of the β-1,3 polymer laminarin. It is also capable of degrading the β-1,3 bonds of the mixed linkage β-glucan, lichenan, but with a specific activity that is 1 order of magnitude lower (22). On the other hand, EglA is incapable of degrading the β-1,3 bonds in these substrates and instead degrades the β-1,4 bonds of both β-glucans and cellulose. These two extracellular enzymes are capable of working in a concerted-synergistic fashion to efficiently hydrolyze mixed-linkeage β-glucans to a variety of short oligosaccharides which can further be hydrolyzed by the P. furiosus intracellular family 1 β-glucosidase (4, 57) to produce glucose (14a).
Although a putative endoglucanase was identified in the genome of Methanococcus jannashii (9), this gene product has not been characterized. However, the P. furiosus eglA and lamA genes have no significant homology with the putative M. jannashii endoglucanase, a family 60 glycosyl hydrolase. In fact, the extracellular family 12 endoglucanases from THermotoga neapolitana (14) and T. maritima (32) are most closely related (40 and 38% identical, respectively) to EglA. The presence of family 12 and 16 enzymes in P. furiosus and the absence of similar enzymes in the genome of M. jannashii indicate that either P. furiosus has acquired the genes for these enzymes’ specificity or M. jannaschii has lost the genes for these activities with the divergence of the Thermococcales and the methanogenic archaea. A similar argument cannot yet be used for the presence or absence of family 60 enzymes in these organisms, since the complete genome of P. furiosus has not been reported.
EglA clearly has the highest specific activity on cellulose oligosaccharides, specifically cellopentaose and cellohexaose. This activity is over 2 orders of magnitude higher than that on the soluble β-1,4 polysaccharide carboxymethyl cellulose and from 3 to 5 orders of magnitude higher than that on insoluble forms of cellulose, such as Whatman paper, cotton linter, and Avicel. While the enzyme cannot efficiently degrade cellulose alone, from the cellulose oligosaccharide degradation data presented here, it is likely that it could work synergistically with a blend of other cellulases to enhance the overall rate of cellulose degradation. EglA has a higher specific activity on the more soluble (1→3),(1→4)-β-d-glucans, such as barley glucan and lichenan, than on the insoluble (1→4)-β-d-cellulose polysaccharides. Unlike some endoglucanases (51), EglA clearly does not require β-1,3 bonds to be present to effectively cleave mixed-linkage β-glucans. The inability of the enzyme to efficiently hydrolyze insoluble cellulose, and yet its ability to degrade soluble cellulose oligosaccharides and mixed-linkage β-glucans, indicates that it likely attacks the β-1,4 amorphous regions within the insoluble cellulose. This was confirmed by cellulose binding studies which indicated that the endoglucanase did not significantly bind to cotton linter or Avicel (data not shown). This is not surprising since the enzyme lacks a cellulose-binding domain.
The crystal structure from the family 12 endoglucanase in S. lividans indicates that the substrate binding cleft is 35 Å long with the catalytic nucleophile and Brønsted acid-base 15 Å from one end of the cleft. This implies that the enzyme likely binds to five, possibly six, glucopyranose units with two being in the reducing-end subsites (+I and +II) and three or four units being in the nonreducing-end subsites (−I, −II, −III, and −IV) (54). Unfortunately, the three-dimensional structure reported for the family 12 endoglucanase in S. lividans does not clearly reveal enzyme conformational changes upon interaction with substrates. If we knew more about the conformational changes, the actual number of subsites that participate in substrate binding could be elucidated. In this regard, the number of subsites occupied within the substrate binding cleft remains unclear. However, hydrolysis product analysis and substrate specificity analysis reported here indicate three subsites for the nonreducing end of oligosaccharides and two subsites for the reducing end of oligosaccharides within the substrate binding cleft of the P. furiosus family 12 endoglucanase.
PNP-cellotetraose and -cellpentaose studies indicate that the fourth and fifth glucopyranose moieties toward the nonreducing end of the cleavage site have a destabilizing effect on the enzyme-carbohydrate transition stae. This would suggest that only three nonreducing-end subsites (−I, −II, and −II) exist. Cellulose oligosaccharide (7 >DP > 1) initial degradation rates increase as DP increases from cellobiose and cellopentaose. Activity remains constant as DP increases by one more glucose moiety from cellopentaose to cellohexaose, from which it may be concluded that there are a total of five subsites which can contribute to transition-state stabilization. In addition, a major product of the initial degradation of cellooligosaccharides is cellobiose, indicating that these two units are bound at the nonreducing side of the cleavage site and released during hydrolysis. Cellobiose, along with glucose, also appears to contribute as a major reactant product in the reverse synthesis reaction (Fig. 3). The fate of cellobiose helps to clarify the number of reducing-end subsites within the carbohydrate binding cleft, suggesting that there are only two (+I and +II).
The presence of a β-1,4 cleaving endoglucanase, as well as a β-1,3 hydrolyzing laminarinase, in the genome of the hyperthermophilic archaeon P. furiosus implies that this organism likely encounters and utilizes β-1,3 and/or β-1,4 glucans in its native environment. The presence of polysaccharides or even oligosaccharides with this structure has not yet been reported for the hydrothermal vent environments from which P. furiosus was isolated. Although no archaea have yet been shown to synthesize extracellular β-glucans, the hyperthermophilic archaeon Thermococcus litoralis generates an extracellular polysaccharide composed of (modified) mannose (46). Therefore, it appears that P. furiosus is capable of utilizing soluble β-glucans that occur within its native hydrothermal vent environment. Whether these oligosaccharides or polysaccharides are produced by other thermophilic archaea or bacteria or whether they are produced in mesophilic environments and subsequently diffuse into the hydrothermal vent areas remains to be seen.
ACKNOWLEDGMENTS
R.M.K. acknowledges the support of the National Science Foundation and the U.S. Department of Agriculture for the work reported here. M.W.B. acknowledges the support of a Department of Education GAANN Fellowship.
We also acknowledge Jun Gao from the Department of Chemical Engineering at NCSU for help with manuscript preparation.
REFERENCES
- 1.Allard B, Tazi A. Influence of growth status on composition of extracellular polysaccharides from two Chlamydomonas species. Phytochemistry. 1993;32:41–47. doi: 10.1016/0031-9422(92)80103-l. [DOI] [PubMed] [Google Scholar]
- 2.Anderson M A, Stone B A. New substrate for investigating the specificity of β-glucan hydrolases. FEBS Lett. 1975;52:202–207. doi: 10.1016/0014-5793(75)80806-4. [DOI] [PubMed] [Google Scholar]
- 3.Bamforth C W. Barley β-glucans—their role in malting and brewing. Brew Dig. 1982;35:22–27. [Google Scholar]
- 4.Bauer M W, Bylina E, Swanson R, Kelly R M. Comparison of a beat-glucosidase and a beta-mannosidase from the hyperthermophilic archaeon Pyrococcus furiosus: purification, characterization, gene cloning, and sequence analysis. J Biol Chem. 1996;271:23749–23755. doi: 10.1074/jbc.271.39.23749. [DOI] [PubMed] [Google Scholar]
- 5.Belton P S, Tanner S F, Cartier N, Chanzy H. High resolution solid-state C-13 nuclear magnetic-resonance spectroscopy of tunicin, an animal cellulose. Macromolecules. 1989;22:1615–1617. [Google Scholar]
- 6.Bourret A, Chanzy H, Lazaro R. Crystallite features of Valonia cellulose by electron diffraction and DAK-field electron microscopy. Biopolymers. 1972;11:113–118. [Google Scholar]
- 7.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 8.Brown S H, Costantino H R, Kelly R M. Characterization of amylolytic enzyme activities associated with the hyperthermophilic archaebacterium Pyrococcus furiosus. Appl Environ Microbiol. 1990;56:1985–1991. doi: 10.1128/aem.56.7.1985-1991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bult C J, White O, Olsen G J, Zhou L, Fleischmann R D, Sutton G G, Blake J A, Fitzgerald L M, Clayton R A, Gocayne J D, Kerlavage A R, Dougherty B A, Tomb J F, Adams M D, Reich C I, Overbeek R, Kirkness E F, Weinstock K G, Merrick J M, Glodek A, Scott J L, Geoghagen N S M, Venter J C. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 10.Chanzy H, Henrissat B, Vuong R, Schülein M. The action of 1,4-β-d-glucan cellobiohydrolase of Valonia cellulose microcrystals. An electron microscopic study. FEBS Lett. 1983;153:113–118. [Google Scholar]
- 11.Chen C-C, Adolphson R, Dean J F D, Ericksson K-E, Adams M W W, Westpheling J. Release of lignin from kraft pulp by a hyperthermophilic xylanase from Thermotoga maritima. Enzyme Microb Technol. 1997;20:39–45. [Google Scholar]
- 12.Costantino H R, Brown S H, Kelly R M. Purification and characterization of an α-glucosidase from a hyperthermophilic archaebacterium, Pyrococcus furiosus, exhibiting a temperature optimum of 105 to 115°C. J Bacteriol. 1990;172:3654–3660. doi: 10.1128/jb.172.7.3654-3660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahkova O N, Kurepina N E, Zverlov V V, Svetlichnyi V A, Velikodvorskaya G A. Cloning and expression in Escherichia coli of Thermotoga neapolitana genes coding for enzymes of carbohydrate substrate degradation. Biochem Biophys Res Commun. 1993;194:1359–1364. doi: 10.1006/bbrc.1993.1974. [DOI] [PubMed] [Google Scholar]
- 14.da Silva M, D, Gorin L A, Iacomini M. Unusual carbohydrates from the lichen, Parmotrema certratum. Phyochemistry. 1993;34:715–717. doi: 10.1016/0031-9422(93)85345-r. [DOI] [PubMed] [Google Scholar]
- 14a.Driskill, L. E. et al. Unpublished results.
- 15.Dubreucq G, Domon B, Fournet B. Structure determination of a novel uronic acid residue isolated from the exopolysaccharide produced by a bacterium originating from deep sea hydrothermal vents. Carbohydr Res. 1996;290:175–181. doi: 10.1016/0008-6215(96)00155-3. [DOI] [PubMed] [Google Scholar]
- 16.Fiala G, Stetter K O. Pyrococcus furiosus, new species represents a novel genus of marine heterotrophic archaebateria growing optimally at 100 Celsius. Arch Microbiol. 1986;145:56–60. [Google Scholar]
- 17.Freire E, Biltonen R L. Statistical mechanical deconvolution of thermal transitions in macromolecules. I. Theory and application to homogeneous systems. Biopolymers. 1978;17:463–479. [Google Scholar]
- 18.Gardas-Salas A L, Fernandez-Abalos J M, Sanchez P, Ruiz-Arribas A, Santamaria-Sanchez R I. Two genes encoding an endoglucanase and a cellulose-binding protein are clustered and co-regulated by a TTA codon in Streptomyces halstedii. Biochem J. 1997;324:403–411. doi: 10.1042/bj3240403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gebler J, Gilkes N R, Claeyssens M, Wilson D B, Beguin P, Wakarchuk W W, Kilburn D G, Miller R C, Warren A J, Withers S G. Stereoselective hydrolysis catalyzed by related beta-1,4-glucanases and beta-1,4-xylanases. J Biol Chem. 1992;267:12559–12561. [PubMed] [Google Scholar]
- 20.Gilkes N R, Henrissat B, Kilburn D G, Miller R C, Warren R A J. Domain in microbial β-1,4-glucanases: sequence conservation function and enzyme families. Microbiol Rev. 1991;55:303–315. doi: 10.1128/mr.55.2.303-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez C, Navarro A, Manzanares P, Horta A, Carbonell J V. Physical and structure properties of barley (1→3), (1→4)-beta-d-glucan. Part I. Determination of molecular weight and macromolecular radius by light scattering. Carbohydr Polym. 1997;32:7–15. [Google Scholar]
- 22.Gueguen Y, Voorhorst W G B, van der Oost J, deVos M W. Molecular and biochemical characterization of an endo-β-1,3-glucanase of the hyperthermophilic archaeon Pyrococcus furiosus. J Biol Chem. 1997;272:31258–31264. doi: 10.1074/jbc.272.50.31258. [DOI] [PubMed] [Google Scholar]
- 23.Henrissat B. A classification of glycosyl hydrolases based on amino-acid-sequence similarities. Biochem J. 1991;280:309–316. doi: 10.1042/bj2800309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou C T, Ahlgren J A, Brown W, Nicholson J J. Production of an extracellular polysaccharide by Agrobacterium sp. DS3 NRRL: B-14297 isolated from soil. J Ind Microbiol. 1996;16:129–133. doi: 10.1007/BF01570073. [DOI] [PubMed] [Google Scholar]
- 25.Hreggvidsson G O, Kaiste E, Holst O, Eggertsson G, Palsdottir A, Kristjansson J K. An extremely thermostable cellulase from the thermophilic eubacterium Rhodothermus marinus. Appl Environ Microbiol. 1996;62:3047–3049. [Google Scholar]
- 26.Kengen S W M, Luesink E J, Stams A J M, Zehnder A J B. Purification and characterization of an extremely thermostable beta-glucosidase from the hyperthermophilic archaeon Pyrococcus furiosus. Eur J Biochem. 1993;213:305–312. doi: 10.1111/j.1432-1033.1993.tb17763.x. [DOI] [PubMed] [Google Scholar]
- 27.Kitamoto N, Go M, Shibayama T, Kimura T, Kito Y, Ohmiya K, Tsukagoshi N. Molecular cloning, purification and characterization of two endo-1,4-beta-glucanases from Aspergillus oryzae KBN616. Appl Microbiol Biotechnol. 1996;46:538–544. doi: 10.1007/s002530050857. [DOI] [PubMed] [Google Scholar]
- 28.Kramer P, Wincierz U, Grubler J, Tschakert J, Voelter W, Mayer H. Rational approach to fractionation, isolation, and characterization of polysaccharides from the lichen Cetraria islandica. Arzneimittelforschung. 1995;45:726–731. [PubMed] [Google Scholar]
- 29.Krässig H A. Cellulose: structure, accessibility, and reactivity. Yverdon, Switzerland: Gordon and Breach Science Publishers; 1993. [Google Scholar]
- 30.Kroh L W. Carmelisation of food and beverages. Food Chem. 1994;51:373–379. [Google Scholar]
- 31.Kulshreshtha A K, Dweltz N E. Paracrystalline lattice disorder in cellulose. I. Reappraisal of the application of the two-phase hypothesis to the analysis of powder x-ray diffractograms of native and hydrolyzed cellulosic materials. J Polym Sci Polym Phys. 1973;11:487–497. [Google Scholar]
- 32.Liebl W, Ruile P, Bronnenmeier K, Reidel K, Lottspeich F, Greif I. Analysis of a Thermotoga maritima DNA fragment encoding two similar thermostable cellulases, CelA and CelB, and characterization of the recombinant enzymes. Microbiology. 1996;142:2533–2542. doi: 10.1099/00221287-142-9-2533. [DOI] [PubMed] [Google Scholar]
- 33.Lin J K, Jacobson b J, Pereira A N, Ladisch M R. Liquid-chromatography of carbohydrate monomers and oligomers. Methods Enzymol. 1988;160:145–149. [Google Scholar]
- 34.Makhatadze G I, Medvedkin V N, Privalov P L. Partial molar volumes of polypeptides and their constituent groups in aqueous solution over a broad temperature range. Biopolymers. 1990;30:1001–1010. doi: 10.1002/bip.360301102. [DOI] [PubMed] [Google Scholar]
- 35.Malet C, Planas A. Mechanism of Bacillus 1,3-1,4-β-d-glucan 4-glucanhydrolases: kinetics and pH studies with 4-methylumbelliferyl. Biochemistry. 1997;36:13838–13848. doi: 10.1021/bi9711341. [DOI] [PubMed] [Google Scholar]
- 36.McCleary B V, Shameer I. Assay of malt beta-glucanase using azo-barley glucans: an improved precipitant. J Inst Brew. 1987;93:87–90. [Google Scholar]
- 37.Murao S, Sakamoto R, Arai M. Cellulases of Aspergillus aculeatus. Methods Enzymol. 1988;160:274–299. [Google Scholar]
- 38.Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem. 1944;153:375–380. [Google Scholar]
- 39.Ooi T, Shinmyo A, Okada H, Murao S, Kawagushi T, Arai M. Complete nucleotide sequence of a gene coding for Aspergillus aculeatus cellulase. Nucleic Acids Res. 1990;18:5884–5886. doi: 10.1093/nar/18.19.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perito B, Hanhart E, Irdani T, Iqbal M, McCarthy A, Mastromei G. Characterization and sequence analysis of a Streptomyces rochei A2 endoglucanase-encoding gene. Gene. 1994;148:119–124. doi: 10.1016/0378-1119(94)90244-5. [DOI] [PubMed] [Google Scholar]
- 41.Raguenes G, Pignet P, Gauthier G, Peres A, Christen r, Rougeaux H, Barbier G, Guezennec J. Description of a new polymer-secreting bacterium from a deep-sea hydrothermal vent, Alteromonas macleodii subsp. fijiensis, and preliminary characterization of the polymer. Appl Environ Microbiol. 1996;62:67–73. doi: 10.1128/aem.62.1.67-73.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raguenes G, Peres A, Ruimy R, Pignet P, Christen R, Loaec M, Rougeaux H, Barbier G, Guezennec J. Alteromonas infernus sp. nov., a new polysacchairde-producing bacterium from a deep-sea hydrothermal vent. J Appl Microbiol. 1997;82:422–430. doi: 10.1046/j.1365-2672.1997.00125.x. [DOI] [PubMed] [Google Scholar]
- 43.Raguenes G, Christen R, Guezennec J, Pignet P, Barbier G. Vibrio diabolicus sp. nov., a new polysaccharide-secreting organism isolated from a deep-sea hydrothermal vent polychaete annelid, ALvinella pompejana. Int J Syst Bacteriol. 1997;47:989–995. doi: 10.1099/00207713-47-4-989. [DOI] [PubMed] [Google Scholar]
- 44.Revol J F. On the cross-sectional shape of cellulose crystallites in Valonia ventricosa. Carbohydr Polym. 1982;2:123–134. [Google Scholar]
- 45.Richmond P A. Biosynthesis and biodegradation of cellulose. New York, N.Y: Marcel Dekker; 1991. pp. 5–23. [Google Scholar]
- 46.Rinker K D, Kelly R M. Growth physiology of the hyperthermophilic archaeon Thermococcus litoralis: development of a sulfur-free defined medium, characterization of an exopolysaccharide, and evidence of biofilm formation. Appl Environ Microbiol. 1996;62:4478–4485. doi: 10.1128/aem.62.12.4478-4485.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rouvinen J, Bergfors T, Teeri T, Knowles J K C, Jones T A. Three-dimensional structure of cellobiohydrolase II from Trichoderma reesei. Science. 1990;249:380–385. doi: 10.1126/science.2377893. [DOI] [PubMed] [Google Scholar]
- 48.Saarilahti H T, Henrissat B, Palva E T. CelS: a novel endoglucanase identified from Erwinia carotovora subsp. carotovora. Gene. 1990;90:9–14. doi: 10.1016/0378-1119(90)90433-r. [DOI] [PubMed] [Google Scholar]
- 49.Sakamoto, S. G., G. Tamura, K. Ito, T. Ishikawa, K. Iwano, and N. Nishiya. Cloning and sequencing of cellulase cDNA from Aspergillus kawachii and its expression in Saccharomyces cerevisiae. Curr. Genet. 27:435–439. [DOI] [PubMed]
- 50.Salmon S, Hudson S M. Crystal morphology, biosynthesis, and physical assembly of cellulose, chitin, and chitosan. Rev Macromol Chem Phys. 1997;C37:199–276. [Google Scholar]
- 51.Schimming S, Schwarz H, Staudenbauer W L. Structure of the Clostridium thermocellum gene licB and the encoded β-1,3-1,4-glucanase. Eur J Biochem. 1992;204:13–19. doi: 10.1111/j.1432-1033.1992.tb16600.x. [DOI] [PubMed] [Google Scholar]
- 52.Schou C, Rasmussen G, Kaltoft M-B, Henrissat B, Schülein M. Stereochemistry, specificity and kinetics of the hydrolysis of reduced cellodextrins by nine cellulases. Eur J Biochem. 1993;217:947–953. doi: 10.1111/j.1432-1033.1993.tb18325.x. [DOI] [PubMed] [Google Scholar]
- 53.Somogyi M. Notes of sugar determination. J Biol Chem. 1952;195:19–23. [PubMed] [Google Scholar]
- 54.Sulzenbacher G, Shareck F, Morosoli R, Dupont C, Davies G J. The Streptomyces lividans family 12 endoglucanase: construction of the catalytic core, expression, and X-ray structure at 1.75Å resolution. Biochemistry. 1997;36:16032–16039. doi: 10.1021/bi972407v. [DOI] [PubMed] [Google Scholar]
- 55.Törrönen A, Kubicek C P, Henrissat B. Amino acid sequence similarities between low molecular weight endo-1,4-beta-xylanases and family H cellulases revealed by clustering analysis. FEBS Lett. 1993;321:135–139. doi: 10.1016/0014-5793(93)80094-b. [DOI] [PubMed] [Google Scholar]
- 56.Varghese J N, Garrett T P J, Colman P M, Chen L, Høj P B, Fincher G B. Three-dimensional structures of two plant beta-glucan endohydrolases with distinct substrate specificities. Proc Natl Acad Sci USA. 1994;91:2785–2789. doi: 10.1073/pnas.91.7.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voorhorst W G B, Eggen R I L, Luesink E J, de Vos W M. Characterization of the CelB gene coding for beta-glucosidase from the hyperthermophilic archaeon Pyrococcus furiosus and its expression and site-directed mutation in Escherichia coli. J Bacteriol. 1995;177:7105–7111. doi: 10.1128/jb.177.24.7105-7111.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White A R, Brown M R. Enzymatic hydrolysis of cellulose: visual characterization of the process. Proc Natl Acad Sci USA. 1981;78:1047–1051. doi: 10.1073/pnas.78.2.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilkinson G N. Statistical estimations in enzyme kinetics. Biochem J. 1961;80:324. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wittmann S, Shareck F, Kluepfel D, Morosoli R. Purification and characterization of CelB endoglucanase from Streptomyces lividans 66 and DNA sequence of the encoding gene. Appl Environ Microbiol. 1994;60:1701–1703. doi: 10.1128/aem.60.5.1701-1703.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu G-Y, Ong E, Gilkes N R, Kilburn D G, Muhandiram D R, Harris-Brandts M, Carver J P, Kaym L E, Harvey T S. Solution structure of a cellulose-binding domain from Cellomonas fimi by nuclear-magnetic-resonance spectroscopy. Biochemistry. 1995;34:6993–7009. [PubMed] [Google Scholar]






