Abstract
Background
The impact of major changes in the treatment practice of pulmonary embolism (PE), such as limited indications for systemic thrombolysis and the introduction of direct oral anticoagulants, is not well documented. This study aimed to describe annual trends in the treatment patterns and outcomes in patients with PE.
Methods and Results
Using the Japanese Diagnosis Procedure Combination inpatient database from April 2010 to March 2021, we identified hospitalized patients with PE. Patients with high‐risk PE were defined as those admitted for out‐of‐hospital cardiac arrest or who received cardiopulmonary resuscitation, extracorporeal membrane oxygenation, vasopressors, or invasive mechanical ventilation on the day of admission. The remaining patients were defined as patients with non–high‐risk PE. The patient characteristics and outcomes were reported with fiscal year trend analyses. Of 88 966 eligible patients, 8116 (9.1%) had high‐risk PE, and the remaining 80 850 (90.9%) had non–high‐risk PE. Between 2010 and 2020, in patients with high‐risk PE, the annual proportion of extracorporeal membrane oxygenation use significantly increased from 11.0% to 21.3%, whereas that of thrombolysis use significantly decreased from 22.5% to 15.5% (P for trend <0.001 for both). In‐hospital mortality significantly decreased from 51.0% to 43.7% (P for trend=0.04). In patients with non–high‐risk PE, the annual proportion of direct oral anticoagulant use increased from 0.0% to 38.3%, whereas that of thrombolysis use significantly decreased from 13.7% to 3.4% (P for trend <0.001 for both). In‐hospital mortality significantly decreased from 7.9% to 5.4% (P for trend <0.001).
Conclusions
Substantial changes in the PE practice and outcomes occurred in patients with high‐risk and non–high‐risk PE.
Keywords: epidemiology, extracorporeal membrane oxygenation, factor Xa inhibitors, mortality, pulmonary embolism, thrombolytic therapy
Subject Categories: Epidemiology, Thrombosis, Embolism
Nonstandard Abbreviations and Acronyms
- DOAC
direct oral anticoagulant
- OHCA
out‐of‐hospital cardiac arrest
Clinical Perspective.
What Is New?
To the best of our knowledge, this is the first nationwide study on the annual trends in the treatment patterns and outcomes in patients with high‐risk and non–high‐risk pulmonary embolism (PE) in Japan between 2010 and 2020.
In the patients with high‐risk PE, the annual proportions showed an increasing trend in extracorporeal membrane oxygenation use and a decreasing trend in thrombolysis use and in‐hospital mortality.
In the patients with non–high‐risk PE, the annual proportions showed a remarkably increasing trend in direct oral anticoagulant use and decreasing trends in thrombolysis use and in‐hospital mortality.
What Are the Clinical Implications?
Given that more than half of the patients with high‐risk PE who received extracorporeal membrane oxygenation had extracorporeal membrane oxygenation alone, an aggressive additional surgical embolectomy or catheter‐directed therapy in combination with extracorporeal membrane oxygenation might be warranted to reduce high in‐hospital mortality in those patients.
In Japan, where there are no dedicated devices for catheter‐directed thrombolysis or embolectomy, surgical embolectomy should be further considered for patients with high‐risk PE who have bleeding concerns.
Venous thromboembolism contributes to a major global burden of disease, and the incidence of venous thromboembolism increases with increasing age. 1 Acute pulmonary embolism (PE) is the most serious clinical manifestation of venous thromboembolism. 2 In addition, acute PE has a wide range of clinical presentations and is classified into low, intermediate, or high risk of early death, depending on the hemodynamic instability, right ventricular dysfunction, and comorbidities. 3 High‐risk PE presents an immediate life‐threatening situation defined by hemodynamic instability, including cardiac arrest, obstructive shock, or persistent hypotension. 3
The guidelines consistently have placed systemic thrombolysis as the first‐line reperfusion treatment for patients with high‐risk PE but have limited the indications for systemic thrombolysis for a rescue reperfusion treatment in patients with intermediate‐risk PE in the United States, Europe, and Japan since 2012, 2014, and 2017, respectively. 4 , 5 Direct oral anticoagulants (DOACs) have become commonly used in patients with low‐ and intermediate‐risk PE in Japan since their approval for venous thromboembolism; edoxaban was approved in September 2014, rivaroxaban in September 2015, and apixaban in December in 2015. 6 At present, it is unclear whether these major changes in the PE practice have affected the treatment patterns and clinical outcomes.
Capturing the trends in the treatment practice resulting from changes in the guideline recommendations and the introduction of new anticoagulants is important for understanding the current state of PE practice and to establish evidence‐based medicine in the future. The present study therefore aimed to describe the annual proportions of treatment patterns and outcomes, and their trends in patients with PE, using a nationwide inpatient database in Japan.
Methods
Data Availability
The data sets analyzed during the current study are not publicly available due to contracts with the hospitals providing the data to the database.
Design and Ethical Statements
This was a retrospective cohort study using an inpatient administrative database, and the study conformed to the REporting of studies Conducted using Observational Routinely‐collected health Data statement reporting guidelines. 7 This study was conducted in accordance with the amended Declaration of Helsinki and was approved by the institutional review board of the University of Tokyo (approval number, 3501‐[5]; May 19, 2021). Because the data were anonymous, the institutional review board waived the requirement for informed consent. No information about individual patients, hospitals, or treating physicians was available.
Data Source
We used the Japanese Diagnosis Procedure Combination inpatient database, which contained administrative claims data and discharge abstracts from >1500 acute care hospitals and covers ≈90% of all tertiary emergency hospitals in Japan. 8 The database includes the following patient‐level data for all hospitalizations: age, sex, diagnoses (main diagnosis, admission‐precipitating diagnosis, most resource‐consuming diagnosis, second‐most resource‐consuming diagnosis, comorbidities present on admission, and complications arising after admission) recorded with the International Classification of Diseases, Tenth Revision (ICD‐10) codes, daily procedures recorded using Japanese medical procedure codes, daily drug administration, and discharge status. 8 A previous validation study showed that the specificity of the recorded diagnoses in the database exceeded 96%, the sensitivity of the diagnoses ranged from 50% to 80%, and the specificity and sensitivity of procedures both exceeded 90%. 9
Study Population
Using the database from July 2010 to March 2021, which was the maximum period available at that time, we identified hospitalized patients with the primary diagnosis of PE (ICD‐10 code, I26). We did not include patients with a suspected PE diagnosis and patients who developed PE as a complication after hospitalization. Patients who were admitted for out‐of‐hospital cardiac arrest (OHCA) (ICD‐10 code, I46) or who received cardiopulmonary resuscitation, extracorporeal membrane oxygenation (ECMO), vasopressors (adrenaline, noradrenaline, dopamine, or vasopressin), or invasive mechanical ventilation on the day of admission were defined as patients with high‐risk PE in accordance with the guidelines, 3 and the remaining patients were defined as patients with non–high‐risk PE in this study.
Variables and Outcomes
The variables included the age, sex, body mass index at admission, Japan Coma Scale at admission, 10 OHCA, comorbidities (coronary artery disease, heart failure, chronic lung disease, hypertension, diabetes, chronic kidney disease, cancer, and sepsis), ambulance use, weekend admissions, intensive care unit admissions, high‐dependency care unit admissions, procedures on the day of admission (cardiopulmonary resuscitation, ECMO, surgical embolectomy, thrombolysis, inferior vena cava filter, and invasive mechanical ventilation), resuscitative drugs on the day of admission (vasopressors or dobutamine), and anticoagulant agents on the day of admission (DOACs [rivaroxaban, apixaban, or edoxaban], warfarin, or heparin).
The primary outcome was in‐hospital mortality. The secondary outcomes were the length of hospital stay, total hospitalization cost, major bleeding, and total blood transfusion volume during the hospitalization. Major bleeding was defined as the presence of intracranial bleeding (ICD‐10 code, I61), intraspinal bleeding (G951), pericardial hematomas (I312), intra‐abdominal or retroperitoneal hematomas (K661), intra‐articular bleeding (M250), intraocular bleeding (H448), and compartment syndrome (M622), which was in accordance with the International Society of Thrombosis and Hemostasis definitions. 11
Statistical Analysis
Continuous variables are presented as the mean and SD and categorical variables as the number and percentage. Continuous variables were compared using the Student t test, and categorical variables were compared using the χ2 test. To evaluate the trends by the fiscal year, we performed Cochran‐Armitage tests for the binary variables and Jonckheere‐Terpstra tests for the continuous variables. The trends in the incidence, patient characteristics, and outcomes were separately assessed among the patients with high‐risk and non–high‐risk PE. Patients with high‐risk PE were categorized into the following 8 treatment categories on the day of admission: (1) none, (2) thrombolysis alone, (3) ECMO alone, (4) surgical embolectomy alone, (5) thrombolysis+ECMO, (6) ECMO+surgical embolectomy, (7) thrombolysis+surgical embolectomy, and (8) thrombolysis+ECMO+surgical embolectomy. We then presented the age, sex, and outcomes stratified by the treatment categories among the patients with high‐risk PE. We presented the trends in the patient characteristics and outcomes stratified by the age category in the overall patients with PE. Finally, we separately assessed the trends in in‐hospital mortality stratified by the age category in the overall patients and the patients with high‐risk and non–high‐risk PE. The body mass index and total hospitalization cost had missing values as shown in Table S1–S6. We considered all reported P values as 2‐sided and a P<0.05 as statistically significant. All analyses were performed using Stata/SE 17.0 software (StataCorp).
Results
During the study period, we identified 88 966 patients with a primary diagnosis of PE. Of those, 8116 (9.1%) were identified as having high‐risk PE and the remaining 80 850 (90.9%) as having non–high‐risk PE. The annual incidence of overall PE per 100 000 hospitalizations significantly increased from 100.9 in 2010 to 124.2 in 2020 (P for trend <0.001) (Figure S1 and Table S2). The annual incidence of high‐risk and non–high‐risk PE per 100 000 hospitalizations also significantly increased from 9.6 and 91.3 in 2010 to 11.0 and 113.2 in 2020, respectively.
The patient characteristics differed widely between the patients with high‐risk and non–high‐risk PE (Table 1). The patients with high‐risk PE were more likely to be women, have poor consciousness, and receive thrombolysis on the day of admission and less likely to have comorbidities or receive DOACs on the day of admission. In patients with high‐risk PE, 33.2% had OHCA, and 36.7%, 16.3%, and 78.2% received cardiopulmonary resuscitation, ECMO, and vasopressors on the day of admission, respectively.
Table 1.
Patient Characteristics
| Characteristic | Overall | High‐risk PE | Non–high‐risk PE | P value |
|---|---|---|---|---|
| (n=88 966) | (n=8116) | (n=80 850) | ||
| Age, y, mean (SD) | 68.7 (15.7) | 69.3 (16.3) | 68.6 (15.7) | <0.001 |
| Men, n (%) | 36 546 (41.1) | 3088 (38.0) | 33 458 (41.4) | <0.001 |
| Body mass index on the day of admission, kg/m2, mean (SD) | 23.8 (4.6) | 23.7 (5.1) | 23.8 (4.6) | 0.006 |
| Japan Coma Scale on the day of admission, n (%) | ||||
| 0, alert | 74 078 (83.3) | 2927 (36.1) | 71 151 (88.0) | <0.001 |
| 1–3, dizzy | 8889 (10.0) | 1109 (13.7) | 7780 (9.6) | |
| 10–30, somnolent | 1877 (2.1) | 574 (7.1) | 1303 (1.6) | |
| 100–300, coma | 4119 (4.6) | 3505 (43.2) | 614 (0.8) | |
| Out‐of‐hospital cardiac arrest, n (%) | 2698 (3.0) | 2698 (33.2) | 0 (0.0) | <0.001 |
| Comorbidities, n (%) | ||||
| Coronary artery disease | 5482 (6.2) | 418 (5.2) | 5064 (6.3) | <0.001 |
| Heart failure | 17 155 (19.3) | 1527 (18.8) | 15 628 (19.3) | 0.26 |
| Chronic lung disease | 5249 (5.9) | 336 (4.1) | 4913 (6.1) | <0.001 |
| Hypertension | 25 497 (28.7) | 1440 (17.7) | 24 057 (29.8) | <0.001 |
| Diabetes | 11 430 (12.8) | 767 (9.5) | 10 663 (13.2) | <0.001 |
| Chronic kidney disease | 2234 (2.5) | 231 (2.8) | 2003 (2.5) | 0.04 |
| Cancer | 16 663 (18.7) | 740 (9.1) | 15 923 (19.7) | <0.001 |
| Sepsis | 43 340 (48.7) | 2678 (33.0) | 40 662 (50.3) | <0.001 |
| Ambulance use, n (%) | 34 393 (38.7) | 6733 (83.0) | 27 660 (34.2) | <0.001 |
| Weekend admission, n (%) | 13 687 (15.4) | 1949 (24.0) | 11 738 (14.5) | <0.001 |
| Intensive care unit admission, n (%) | 13 733 (15.4) | 4073 (50.2) | 9660 (11.9) | <0.001 |
| High‐dependency care unit admission, n (%) | 13 978 (15.7) | 1875 (23.1) | 12 103 (15.0) | <0.001 |
| Procedures on the day of admission, n (%) | ||||
| Cardiopulmonary resuscitation | 2977 (3.3) | 2977 (36.7) | 0 (0.0) | <0.001 |
| Extracorporeal membrane oxygenation | 1326 (1.5) | 1326 (16.3) | 0 (0.0) | <0.001 |
| Surgical embolectomy | 500 (0.6) | 452 (5.6) | 48 (0.1) | <0.001 |
| Thrombolysis | 7868 (8.8) | 1602 (19.7) | 6266 (7.8) | <0.001 |
| Inferior vena cava filter | 7703 (8.7) | 479 (5.9) | 7224 (8.9) | <0.001 |
| Invasive mechanical ventilation | 4974 (5.6) | 4974 (61.3) | 0 (0.0) | <0.001 |
| Resuscitative drugs on the day of admission, n (%) | ||||
| Vasopressors | 6346 (7.1) | 6346 (78.2) | 0 (0.0) | <0.001 |
| Dobutamine | 1882 (2.1) | 1376 (17.0) | 506 (0.6) | <0.001 |
| Anticoagulant agents on the day of admission, n (%) | ||||
| Rivaroxaban | 5308 (6.0) | 123 (1.5) | 5185 (6.4) | <0.001 |
| Apixaban | 5943 (6.7) | 121 (1.5) | 5822 (7.2) | <0.001 |
| Edoxaban | 3537 (4.0) | 68 (0.8) | 3469 (4.3) | <0.001 |
| Warfarin | 11 339 (12.7) | 323 (4.0) | 11 016 (13.6) | <0.001 |
| Heparin | 55 202 (62.0) | 5943 (73.2) | 49 259 (60.9) | <0.001 |
PE indicates pulmonary embolism.
In the patients with high‐risk PE, the annual proportion of OHCA significantly increased from 26.2% in 2010 to 38.2% in 2020 (P for trend <0.001) (Table S3). The annual proportion of ECMO use significantly increased from 11.0% in 2010 to 21.3% in 2020 (P for trend <0.001). However, the annual proportion of thrombolysis use significantly decreased from 22.5% in 2010 to 15.5% in 2020 (P for trend <0.001) (Figure 1). In‐hospital mortality significantly decreased from 51.0% in 2010 to 43.7% in 2020 (P for trend=0.04).
Figure 1. Annual proportions of in‐hospital mortality, thrombolysis use, and ECMO use in patients with high‐risk pulmonary embolism.
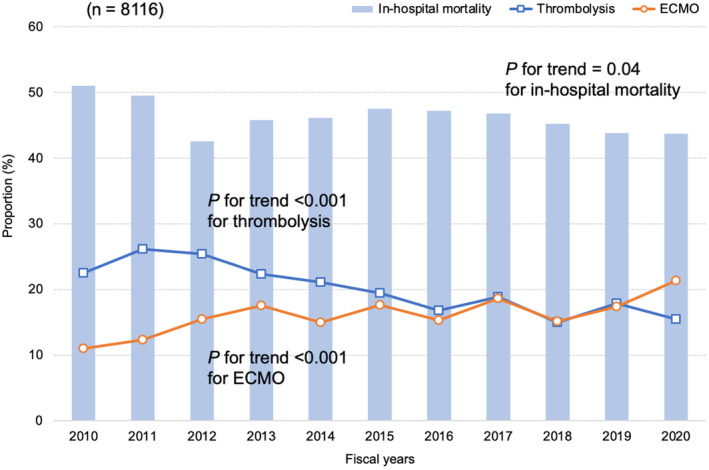
Each fiscal year started on April 1 and ended on March 31. ECMO indicates extracorporeal membrane oxygenation.
In the patients with non–high‐risk PE, the annual proportion of DOAC use increased from 0.0% in 2010 to 38.3% in 2020 (P for trend <0.001). However, the annual proportion of warfarin use decreased from 27.4% in 2010 to 2.1% in 2020 (P for trend <0.001) (Table S4). The annual proportion of thrombolysis use significantly decreased from 13.7% in 2010 to 3.4% in 2020 (P for trend <0.001) (Figure 2). In‐hospital mortality significantly decreased from 7.9% in 2010 to 5.4% in 2020 (P for trend <0.001). Both the length of hospital stay and total hospitalization cost significantly decreased (P for trend <0.001) (Table S4).
Figure 2. Annual proportions of in‐hospital mortality, thrombolysis use, and DOAC use in patients with non–high‐risk pulmonary embolism.
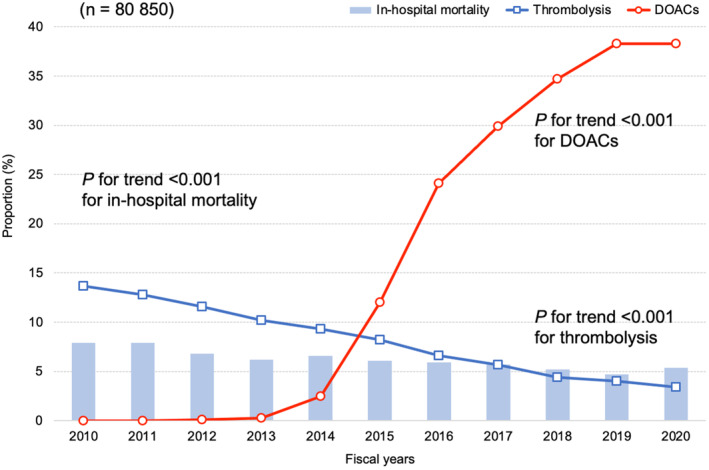
Each fiscal year started on April 1 and ended on March 31. DOAC indicates direct oral anticoagulant.
The patient characteristics and outcomes across treatment categories in patients with high‐risk PE are shown in Table 2. In‐hospital mortality was the lowest in patients who underwent surgical embolectomy and the highest in those who received ECMO alone (17.8% and 58.7%, respectively). Major bleeding occurred most frequently in patients who received thrombolysis and surgical embolectomy (5.0%) but not in those who received ECMO and surgical embolectomy.
Table 2.
Patient Characteristics and Outcomes Across the Treatment Categories in Patients With High‐Risk Pulmonary Embolism
| Characteristics and outcomes | Total | Treatment category | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| None | Thrombolysis alone | ECMO alone | Surgical embolectomy alone | Thrombolysis+ECMO | ECMO+surgical embolectomy | Thrombolysis+surgical embolectomy | Thrombolysis+ECMO+surgical embolectomy | |||
| (n=8116) | (n=5259) | (n=1191) | (n=857) | (n=320) | (n=357) | (n=78) | (n=20) | (n=34) | ||
| Age, y, mean (SD) | 69 (16) | 73 (16) | 69 (15) | 60 (15) | 62 (16) | 59 (16) | 59 (16) | 64 (13) | 52 (17) | <0.001 |
| Men, n (%) | 3088 (38.0) | 1960 (37.3) | 416 (34.9) | 344 (40.1) | 156 (48.8) | 142 (39.8) | 39 (50.0) | 12 (60.0) | 19 (55.9) | <0.001 |
| Outcomes | ||||||||||
| In‐hospital mortality, n (%) | 3737 (46.0) | 2595 (49.3) | 342 (28.7) | 503 (58.7) | 57 (17.8) | 186 (52.1) | 31 (39.7) | 5 (25.0) | 18 (52.9) | <0.001 |
| Length of hospital stay, d, mean (SD) | 22.5 (36.9) | 20.6 (34.5) | 25.1 (35.4) | 23.8 (33.2) | 29.5 (25.1) | 28.3 (72.4) | 28.6 (26.7) | 27.5 (26.2) | 36.9 (73.3) | <0.001 |
| Total hospitalization cost, ×103 dollars, mean (SD) | 16.1 (20.1) | 11.3 (16.3) | 14.2 (13.9) | 27.9 (22.2) | 45.3 (25.7) | 29.2 (24.6) | 47.8 (30.4) | 25.0 (11.5) | 43.7 (39.3) | <0.001 |
| Major bleeding in a critical area or organ, n (%) | 79 (1.0) | 24 (0.5) | 10 (0.8) | 26 (3.0) | 4 (1.3) | 13 (3.6) | 0 (0.0) | 1 (5.0) | 1 (2.9) | <0.001 |
| Intracranial bleeding, n (%) | 40 (0.5) | 14 (0.3) | 6 (0.5) | 11 (1.3) | 4 (1.3) | 3 (0.8) | 0 (0.0) | 1 (5.0) | 1 (2.9) | <0.001 |
| Blood transfusions, mL, mean (SD) | ||||||||||
| Red blood cells | 949 (5885) | 425 (7150) | 313 (1239) | 2675 (2785) | 2901 (3795) | 2758 (2653) | 4319 (4597) | 966 (1513) | 3426 (3151) | <0.001 |
| Fresh‐frozen plasma | 491 (1619) | 171 (1226) | 117 (826) | 1460 (2007) | 2078 (2811) | 1377 (2128) | 2845 (3150) | 384 (844) | 1813 (3035) | <0.001 |
| Platelet concentrate | 107 (1006) | 50 (1223) | 21 (170) | 300 (533) | 400 (650) | 247 (535) | 637 (1088) | 23 (70) | 319 (559) | <0.001 |
ECMO indicates extracorporeal membrane oxygenation.
In the overall patients with PE, the proportions of thrombolysis and ECMO use decreased with increasing age from 13.4% and 3.1% in patients aged 0 to 39 years to 3.3% and 0.2% in patients aged ≥90 years, respectively (Figure 3). On the other hand, in‐hospital mortality increased with increasing age from 5.5% in patients aged 0 to 39 years to 21.9% in patients aged ≥90 years. The detailed patient characteristics and outcomes across age categories are shown in Table S5. There were no specific trends by the age category in patients who received ECMO, thrombolysis, and DOACs (Table S6).
Figure 3. Proportions of in‐hospital mortality, thrombolysis use, and ECMO use according to the age category in the overall patients with pulmonary embolism.
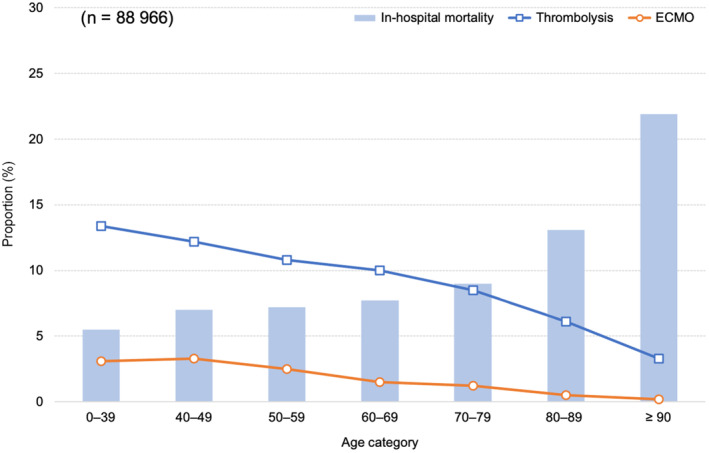
ECMO indicates extracorporeal membrane oxygenation.
Discussion
To the best of our knowledge, this is the first nationwide study on the annual trends in the treatment patterns and outcomes in patients with high‐risk and non–high‐risk PE in Japan. In the patients with high‐risk PE, the annual proportions showed an increasing trend in ECMO use and decreasing trend in thrombolysis use and in‐hospital mortality between 2010 and 2020. In the patients with non–high‐risk PE, the annual proportions showed a remarkably increasing trend in DOAC use and decreasing trends in thrombolysis use and in‐hospital mortality between 2010 and 2020.
In the patients with high‐risk PE, the annual proportion of ECMO use exhibited an increasing trend, which was consistent with that in the previous reports from the United States and Germany. 12 , 13 The proportion of ECMO use in the patients with high‐risk PE in Japan (between 2010 and 2020) reached 19.2%, which was remarkably high as compared with 2.8% in Germany (between 2005 and 2018) and 0.3% in the United States (between 2005 and 2013). 12 , 13 In addition, the patients with high‐risk PE with ECMO in Japan (mean age of 60 years) were older than those with a mean age of 42 years in the United States and those with a median age of 55 years in Germany. 12 , 13 In the present study, among all the ECMO users, the percentage of patients with high‐risk PE aged ≥70 years was >30%. These results may indicate a high resource availability and low threshold for ECMO implementation in Japan. In‐hospital mortality in patients with high‐risk PE with ECMO was similar across Japan, the United States, and Germany (58.7%, 61.6%, and 61.8%, respectively). Given that previous reports showed the potential benefit of ECMO for patients with high‐risk PE aged ≤60 years, 14 careful selection in older patients for ECMO may be needed to reduce the high in‐hospital mortality. Furthermore, in the present study, more than half (65%) of patients who received ECMO had ECMO alone. Based on a concept that ECMO is a bridge to reperfusion therapy, 13 , 15 , 16 aggressive additional surgical embolectomy or catheter‐based therapy in combination with ECMO might be warranted. 17
Although the guidelines recommend systemic thrombolysis as the first‐line reperfusion treatment for patients with high‐risk PE, 3 , 18 the present study showed a decreasing trend in the annual proportion of thrombolysis use. Systemic thrombolysis promotes resolution of PE and a rapid hemodynamic improvement 19 , 20 but increases major bleeding events. 21 Concern about the bleeding risk and poor prognosis after bleeding events 22 may have led to a decreasing trend in thrombolysis use in Japan, a super‐aging society. The proportion of thrombolysis use was lower in the older patients, and the finding was consistent with that in a previous report from Germany. 23 In regard to surgical embolectomy, the annual proportion remained low. In particular, the proportion of surgical embolectomy in combination with ECMO was 1.4%. This figure was considerably low as compared with 20.4% in the United States and 17.1% in Germany, 12 , 13 even considering that the proportion in the present study was based only on the day of admission. Given the effectiveness of surgical embolectomy for patients with high‐risk PE with ECMO, 24 there is a need to encourage a setup with multidisciplinary PE response teams 25 and perform surgical embolectomy for appropriately selected patients with high‐risk PE in Japan.
The present study had several strengths. First, the present study was based on one of the largest databases, which covered ≈90% of all tertiary emergency hospitals in Japan. Second, our database included data before and after the change in the indications for systemic thrombolysis and the introduction of DOACs.
The present study had several limitations. First, our definition of high‐risk PE depended on the diagnosis of PE and OHCA using the ICD‐10 codes or procedures such as cardiopulmonary resuscitation, ECMO, or vasopressors on the day of admission, because the database did not contain detailed clinical information such as the vital signs. Therefore, misclassifications may have led to a bias in our study. Second, we could not classify non–high‐risk PE into intermediate‐ and low‐risk PE due to a lack of data such as the vital signs, laboratory tests, and imaging examinations. Third, the outcomes may be overestimated by rehospitalization for the primary diagnosis of PE. Fourth, the proportion of major bleeding events in the present study was considerably lower than that in the previous studies. 13 , 15 , 16 Given that the sensitivity of the diagnosis might have been low in our database, 9 there was a possibility of having underreported the major bleeding events. Finally, dedicated devices for catheter‐based thrombolysis or embolectomy are not available in Japan, suggesting caution should be taken when applying our results to those in other countries where dedicated devices for catheter‐based thrombolysis or embolectomy are available.
Conclusions
The present study using a nationwide inpatient administrative database showed substantial changes in the clinical practice and outcomes in patients with high‐risk and non–high‐risk PE. In the patients with high‐risk PE, the annual proportion of ECMO use increased, that of thrombolysis use decreased, and that of in‐hospital mortality remained high over time. In patients with non–high‐risk PE, the annual proportion of DOAC use remarkably increased, and that of thrombolysis use and in‐hospital mortality decreased over time.
Sources of Funding
This research was funded by grants from the Ministry of Health, Labour and Welfare, Japan, grant numbers 19AA2007 and H30‐Policy‐Designated‐004, and the Ministry of Education, Culture, Sports, Science and Technology, Japan, grant number 17H04141.
Disclosures
Dr Nishimoto received lecture fees from Bayer Healthcare, Bristol‐Myers Squibb, Pfizer, and Daiichi‐Sankyo. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S6
Figure S1
Acknowledgments
The authors would like to express their gratitude to J. Martin for his grammatical assistance. Yuji Nishimoto: Conceptualization, software, formal analysis, investigation, writing–original draft; Hiroyuki Ohbe: Conceptualization, methodology, software, formal analysis, investigation, writing–review and editing; Hiroki Matsui: Software, formal analysis, investigation, data curation; Mikio Nakajima: Conceptualization, investigation, writing–review and editing, supervision; Yusuke Sasabuchi: Investigation, writing–review and editing, supervision; Yukihito Sato: Software, investigation, resources, supervision; Tetsuya Watanabe: Software, investigation, resources, supervision; Takahisa Yamada: Software, investigation, resources, supervision; Masatake Fukunami: Software, investigation, resources, supervision; Hideo Yasunaga: Writing–review and editing, supervision. All authors read and approved the final article.
This article was sent to Yen‐Hung Lin, MD, PhD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028981
For Sources of Funding and Disclosures, see page 8.
REFERENCES
- 1. Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, Gallus A, Hunt BJ, Hylek EM, Kakkar A, Konstantinides SV, McCumber M, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34:2363–2371. doi: 10.1161/ATVBAHA.114.304488 [DOI] [PubMed] [Google Scholar]
- 2. Yamashita Y, Murata K, Morimoto T, Amano H, Takase T, Hiramori S, Kim K, Oi M, Akao M, Kobayashi Y, et al. Clinical outcomes of patients with pulmonary embolism versus deep vein thrombosis: from the COMMAND VTE Registry. Thromb Res. 2019;184:50–57. doi: 10.1016/j.thromres.2019.10.029 [DOI] [PubMed] [Google Scholar]
- 3. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing G‐J, Harjola V‐P, Huisman MV, Humbert M, Jennings CS, Jiménez D, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405 [DOI] [PubMed] [Google Scholar]
- 4. Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e419S–e496S. doi: 10.1378/chest.11-2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, Gibbs JS, Huisman MV, Humbert M, Kucher N, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–3069, 3069a–3069k. doi: 10.1093/eurheartj/ehu283 [DOI] [PubMed] [Google Scholar]
- 6. Yamashita Y, Morimoto T, Yoshikawa Y, Yaku H, Sumita Y, Nakai M, Ono K, Kimura T. Temporal trends in the practice pattern for venous thromboembolism in Japan: insight from JROAD‐DPC. J Am Heart Assoc. 2020;9:e014582. doi: 10.1161/JAHA.119.014582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, Sørensen HT, von Elm E, Langan SM. The REporting of studies conducted using observational routinely‐collected health data (RECORD) statement. PLoS Med. 2015;12:e1001885. doi: 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yasunaga H. Real world data in Japan: chapter II the diagnosis procedure combination database. Ann Clin Epidemiol. 2019;1:76–79. doi: 10.37737/ace.1.3_76 [DOI] [Google Scholar]
- 9. Yamana H, Moriwaki M, Horiguchi H, Kodan M, Fushimi K, Yasunaga H. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol. 2017;27:476–482. doi: 10.1016/j.je.2016.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shigematsu K, Nakano H, Watanabe Y. The eye response test alone is sufficient to predict stroke outcome—reintroduction of Japan coma scale: a cohort study. BMJ Open. 2013;3:e002736. doi: 10.1136/bmjopen-2013-002736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schulman S, Angerås U, Bergqvist D, Eriksson B, Lassen MR, Fisher W; Subcommittee on control of anticoagulation of the scientific and standardization Committee of the International Society on thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8:202–204. doi: 10.1111/j.1538-7836.2009.03678.x [DOI] [PubMed] [Google Scholar]
- 12. Elbadawi A, Mentias A, Elgendy IY, Mohamed AH, Syed MH, Ogunbayo GO, Olorunfemi O, Gosev I, Prasad S, Cameron SJ. National trends and outcomes for extra‐corporeal membrane oxygenation use in high‐risk pulmonary embolism. Vasc Med. 2019;24:230–233. doi: 10.1177/1358863X18824650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hobohm L, Sagoschen I, Habertheuer A, Barco S, Valerio L, Wild J, Schmidt FP, Gori T, Münzel T, Konstantinides S, et al. Clinical use and outcome of extracorporeal membrane oxygenation in patients with pulmonary embolism. Resuscitation. 2022;170:285–292. doi: 10.1016/j.resuscitation.2021.10.007 [DOI] [PubMed] [Google Scholar]
- 14. Karami M, Mandigers L, Miranda DDR, Rietdijk WJR, Binnekade JM, Knijn DCM, Lagrand WK, den Uil CA, Henriques JPS, Vlaar APJ, et al. Survival of patients with acute pulmonary embolism treated with venoarterial extracorporeal membrane oxygenation: a systematic review and meta‐analysis. J Crit Care. 2021;64:245–254. doi: 10.1016/j.jcrc.2021.03.006 [DOI] [PubMed] [Google Scholar]
- 15. Meneveau N, Guillon B, Planquette B, Piton G, Kimmoun A, Gaide‐Chevronnay L, Aissaoui N, Neuschwander A, Zogheib E, Dupont H, et al. Outcomes after extracorporeal membrane oxygenation for the treatment of high‐risk pulmonary embolism: a multicentre series of 52 cases. Eur Heart J. 2018;39:4196–4204. doi: 10.1093/eurheartj/ehy464 [DOI] [PubMed] [Google Scholar]
- 16. Pozzi M, Metge A, Martelin A, Giroudon C, Lanier Demma J, Koffel C, Fornier W, Chiari P, Fellahi JL, Obadia JF, et al. Efficacy and safety of extracorporeal membrane oxygenation for high‐risk pulmonary embolism: a systematic review and meta‐analysis. Vasc Med. 2020;25:460–467. doi: 10.1177/1358863X20944469 [DOI] [PubMed] [Google Scholar]
- 17. Nishimoto Y, Ohbe H, Matsui H, Nakajima M, Sasabuchi Y, Sato Y, Watanabe T, Yamada T, Fukunami M, Yasunaga H. Effectiveness of systemic thrombolysis on clinical outcomes in high‐risk pulmonary embolism patients with venoarterial extracorporeal membrane oxygenation: a nationwide inpatient database study. J Intensive Care. 2023;11:4. doi: 10.1186/s40560-023-00651-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stevens SM, Woller SC, Kreuziger LB, Bounameaux H, Doerschug K, Geersing G‐J, Huisman MV, Kearon C, King CS, Knighton AJ, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545–e608. doi: 10.1016/j.chest.2021.07.055 [DOI] [PubMed] [Google Scholar]
- 19. Goldhaber SZ, Kessler CM, Heit J, Markis J, Sharma GV, Dawley D, Nagel JS, Meyerovitz M, Kim D, Vaughan DE, et al. Randomised controlled trial of recombinant tissue plasminogen activator versus urokinase in the treatment of acute pulmonary embolism. Lancet. 1988;2:293–298. doi: 10.1016/S0140-6736(88)92354-9 [DOI] [PubMed] [Google Scholar]
- 20. Goldhaber SZ, Haire WD, Feldstein ML, Miller M, Toltzis R, Smith JL, Taveira da Silva AM, Come PC, Lee RT, Parker JA, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right‐ventricular function and pulmonary perfusion. Lancet. 1993;341:507–511. doi: 10.1016/0140-6736(93)90274-K [DOI] [PubMed] [Google Scholar]
- 21. Marti C, John G, Konstantinides S, Combescure C, Sanchez O, Lankeit M, Meyer G, Perrier A. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta‐analysis. Eur Heart J. 2015;36:605–614. doi: 10.1093/eurheartj/ehu218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamashita Y, Morimoto T, Klok FA, Barco S, Nishimoto Y, Kato T, Ono K, Kimura T; COMMAND VTE Registry Investigators . Anticoagulation strategies and clinical outcomes after bleeding events during anticoagulation therapy for venous thromboembolism in the practice‐based Japanese registry. J Thromb Thrombolysis. 2022;54:524–534. doi: 10.1007/s11239-022-02665-x [DOI] [PubMed] [Google Scholar]
- 23. Keller K, Hobohm L, Ebner M, Kresoja K‐P, Münzel T, Konstantinides SV, Lankeit M. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur Heart J. 2020;41:522–529. doi: 10.1093/eurheartj/ehz236 [DOI] [PubMed] [Google Scholar]
- 24. Chopard R, Nielsen P, Ius F, Cebotari S, Ecarnot F, Pilichowski H, Schmidt M, Kjaergaard B, Sousa‐Casasnovas I, Ghoreishi M, et al. Optimal reperfusion strategy in acute high‐risk pulmonary embolism requiring extracorporeal membrane oxygenation support: a systematic review and meta‐analysis. Eur Respir J. 2022;60:2102977. doi: 10.1183/13993003.02977-2021 [DOI] [PubMed] [Google Scholar]
- 25. Dudzinski DM, Piazza G. Multidisciplinary pulmonary embolism response teams. Circulation. 2016;133:98–103. doi: 10.1161/CIRCULATIONAHA.115.015086 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6
Figure S1
Data Availability Statement
The data sets analyzed during the current study are not publicly available due to contracts with the hospitals providing the data to the database.


