Abstract
Background
Chagas disease (CD) presents an ominous prognosis. The predictive value of biomarkers and new echocardiogram parameters in adjusted models have not been well studied.
Methods and Results
There were 361 patients with chronic CD (57.6% men, 61±11 years of age, clinical forms: indeterminate 27.1%, cardiac 56.6%, digestive 3.6%, cardiodigestive 12.7%) included in this single‐center, observational, prospective longitudinal study. Echocardiographic evaluation included strain analyses of left atrial, left ventricular (LV), and right ventricular and 3‐dimensional analyses of left atrial and LV volumes. Biomarkers included cardiac troponin I, brain natriuretic peptide, transforming growth factor β1, tumor necrosis factor, matrix metalloproteinases, and Trypanosoma cruzi polymerase chain reaction. The studied end point was a composite of CD‐related mortality, heart transplant, hospital admission due to worsening heart failure, or new cardiac device insertion. Event‐free survival was analyzed by multivariable regression analyses adjusted for competing risks. P values <0.05 were considered significant. The composite event occurred in 79 patients after 4.9±2.0 years follow‐up. LV end‐diastolic volume (hazard ratio [HR], 1.01 [95% CI, 1.00–1.02]; P=0.02), peak negative global atrial strain (HR, 1.08 [95% CI, 1.00–1.17]; P=0.04), LV global circumferential strain (HR, 1.12 [95% CI, 1.04–1.21]; P=0.003), LV torsion (HR, 0.55 [95% CI, 0.35–0.81]; P=0.003), brain natriuretic peptide (HR, 2.03 [95% CI, 1.23–3.34]; P=0.005), and positive T cruzi polymerase chain reaction (HR, 1.80 [95% CI, 1.12–2.91]; P=0.01) were end point predictors independent from age, sex, 2‐dimensional echocardiographic indexes, hypertension, previous cardiac device, and CD cardiac form.
Conclusions
Two‐dimensional strain‐ and 3‐dimensional‐derived parameters, brain natriuretic peptide, and positive T cruzi polymerase chain reaction can be useful for prediction of CD cardiovascular events.
Keywords: 2‐dimensional strain analysis, 3‐dimensional echocardiography, Chagas disease, mortality, prognosis
Subject Categories: Clinical Studies, Heart Failure, Echocardiography, Prognosis, Biomarkers
Nonstandard Abbreviations and Acronyms
- CD
Chagas disease
- CHD
Chagas heart disease
- E′
peak early diastolic mitral annulus velocity
- E
peak early wave diastolic filling velocity
- GCS
global circumferential strain
- GEB
guanidine EDTA buffer
- GLS
global longitudinal strain
- LASct
peak negative globalleft atrial strain
- MMP
matrix metalloproteinase
- STE
speckle tracking echocardiography
- TGF‐β1
transforming growth factor β1
Clinical Perspective.
What Is New?
We evaluated for the first time in chronic Chagas disease the value of several biomarkers and new echocardiographic parameters to predict survival free of cardiovascular events independent from clinical and 2‐dimensional Doppler echocardiographic indexes.
Cardiac troponin I, brain natriuretic peptide, transforming growth factor β1, and tumor necrosis factor serum levels differed between patients with and without Chagas disease cardiac form.
Lower absolute peak negative global left atrial strain, left ventricular global circumferential strain and torsion, higher 3‐dimensional left ventricular end‐diastolic volume, brain natriuretic peptide, and positive Trypanosoma cruzi polymerase chain reaction were independent predictors of a poorer survival free of cardiovascular events.
What Are the Clinical Implications?
Left ventricular systolic and left atrial contractile strain parameters, 3‐dimensional left ventricular volume, brain natriuretic peptide, and a positive T cruzi quantitative polymerase chain reaction are predictors of survival free of cardiovascular events in chronic Chagas disease and represent potential tools to be incorporated in models of risk prediction in Chagas disease.
The independent value of a positive T cruzi quantitative polymerase chain reaction for cardiovascular outcome prediction suggests the importance of parasite persistence in Chagas disease pathophysiology and the need for further studies on trypanocidal treatment in chronic Chagas disease.
Chagas disease (CD) is recognized by the World Health Organization as a neglected tropical disease, mainly affecting millions of people in endemic areas of Latin America, with the majority living in Brazil and Argentina. 1 Furthermore, migration has resulted in a large number of infected individuals living in nonendemic countries, such as the United States, with an estimated 288 000 infected individuals, 2 and the European Union, with an estimated 120 000 infected individuals. 3 The combined prevalence of infection in Latin American migrants in Europe is 6.08%. 4 Moreover, chronic Chagas heart disease (CHD) is an important cause of hospital admission due to decompensated heart failure (HF), sudden cardiac arrest, arrhythmias needing cardiac device implantation, and stroke in endemic areas. 1 , 5 Thus, CD is still considered one of the main challenges of global health, causing >7000 deaths per year, and the parasitic disease with the highest economic and health burden in the Western hemisphere. 1
Chronic CD can present with 4 different clinical forms. The indeterminate form can last for years, represented by patients with a normal ECG, chest and digestive tract radiographs, and affects 60% to 70% of the patients. 6 The cardiac form has the worst prognosis due to arrhythmias, thromboembolic events, sudden death, and HF with reduced ejection fraction. 6 The digestive form is characterized by dilated esophagus and/or colon. The fourth form is when cardiac and digestive complications supervene in the same patient. 6
The pathophysiology that determines which patient will progress from the indeterminate to the cardiac form is still under investigation. The persistent low‐grade chronic fibrosing myocarditis driven by a persistent myocardial infection and an imbalance favoring an inflammatory milieu are considered key to CD progression. 1 , 7 , 8 In this regard, several studies compared serum biomarkers levels in patients with different CD clinical forms, 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 and explored the prognostic value of several biomarkers. 19 , 20 , 21 , 22 Moreover, cardiac exams also have a strong prognostic value among patients with the CD cardiac form. Echocardiography is the most studied, and left ventricular (LV) ejection fraction the most consistent mortality predictor across different follow‐up studies. 23 Other traditional 2‐dimensional (2D) Doppler echocardiographic parameters, such as LV diastolic function, 24 , 25 left atrial (LA) volume 26 and function, 25 and right ventricular (RV) function 27 were also described to be predictors of poor cardiovascular outcomes in CD. More recently, speckle tracking echocardiography (STE) enabled the study of myocardial contractility and independent prognostic value of LV global longitudinal strain (LV‐GLS), 28 LV global circumferential strain (LV‐GCS), and LV global radial strain and LV torsion 29 to predict cardiovascular events in CD were reported. In regard to biomarkers, brain natriuretic peptide (BNP), 21 and its pro form, 30 , 31 and high‐sensitivity troponin T 31 have been shown to have prognostic value, together with other biomarkers that can indicate an inflammatory profile, such as transforming growth factor β1 (TGF‐β1), 20 and matrix metalloproteinase (MMP). 21 Furthermore, the detection of the parasite in the bloodstream has been implicated in a worse clinical form and prognosis, both by hemoculture 32 and molecular methods. 33 However, few studies have explored in the same cohort the prognostic value of biomarkers and new echocardiographic techniques against traditional 2D Doppler echocardiographic parameters to try to understand if the addition of such tools to the patients' workup would add to prognosis prediction in CD.
Therefore, we aimed to investigate in this single‐center, longitudinal prospective study if biomarkers, including Trypanosoma cruzi polymerase chain reaction (PCR), and new echocardiographic tools can predict cardiovascular outcomes in patients with CD independent from clinical and traditional 2D Doppler echocardiographic parameters.
METHODS
Design and Study Subjects
The data that support the findings of this study are available from the corresponding author upon reasonable request.
This is a single‐center, observational, prospective longitudinal study that was approved by the local institutional review committee on January 27, 2014 under number 515851. This study conforms to standards currently applied by the Brazilian National Committee for Research Ethics and Resolution 466/2012 of the National Health Council, of the Ministry of Health, and to the Code of Ethics of the World Medical Association (Declaration of Helsinki). All subjects gave written informed consent before their participation.
Adult patients with chronic CD were randomly selected among those regularly followed at our institution between July 2014 and March 2017. CD diagnosis was based on positivity in 2 different serological tests (enzyme‐linked immunosorbent assay and indirect immunofluorescence). 25
From 402 included patients, 11 did not return for the index echocardiogram, and 30 were excluded due to concomitant heart disease not related to CD (n=10), cancer (n=6), coinfections (n=4), autoimmune disease (n=5), chronic use of corticosteroid or nonsteroidal anti‐inflammatory drugs (n=4), or splenomegaly (n=1). The final studied population was composed of 361 patients (Figure 1).
Figure 1. Flow diagram.
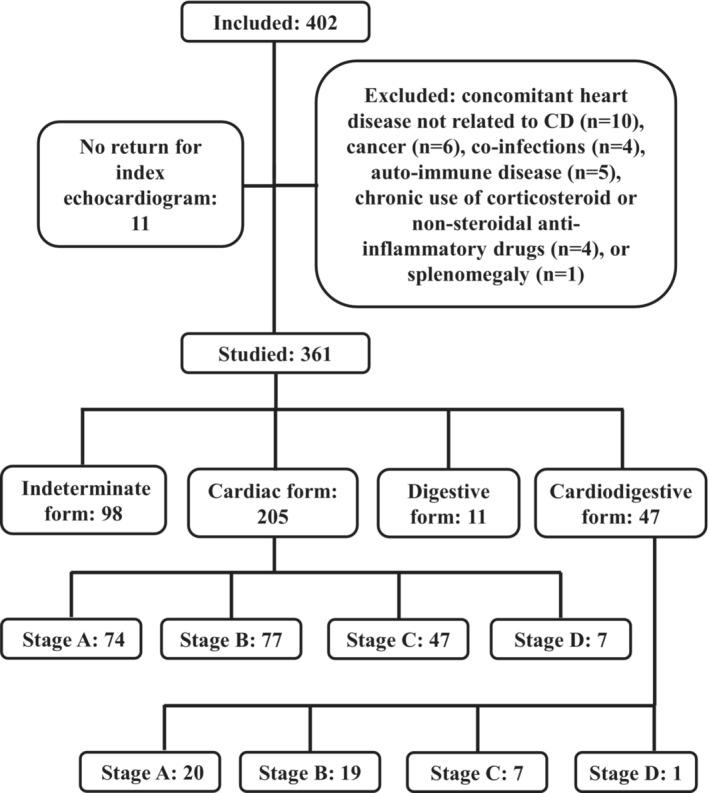
Flow diagram depicting recruitment, exclusion criteria, and studied groups at baseline. CD indicates Chagas disease.
Patients were classified at the time of their inclusion according to the Brazilian CD consensus 6 into indeterminate (no evidence of cardiac involvement), stage A (no HF symptoms with isolated changes in the ECG), stage B (no HF symptoms with segmental or global LV systolic dysfunction), stage C (symptomatic HF), or stage D (end‐stage HF). ECGs were analyzed by cardiologists with extensive experience in CD and classified using the Minnesota Code criteria, modified for CD. 34 The ECG changes considered compatible with CD cardiac form followed previously published criteria. 6
Study End Point and Follow‐Up
The studied end point was a composite event (mortality related to CD, heart transplant, hospital admission due to worsening HF, or insertion of a new cardiac device [pacemaker, implantable cardioverter‐defibrillator, or cardiac resynchronization therapy device], whichever occurred first). Death was classified as related to CD (sudden, due to stroke, or HF), unrelated to CD, or unknown cause. Death was considered sudden when it occurred within 1 hour after of symptoms onset, during sleep, or unwitnessed in a previously stable patient. 35 Death was considered due to HF when it occurred associated to a period of worsening HF clinical status. In cases of patients who did not return for medical appointments, mortality data were also retrieved from registries of death certificates available at the Department of Justice of the Rio de Janeiro state (http://www4.tjrj.jus.br/Portal‐Extrajudicial/CNO/).
The diagnosis of worsening HF depended on the fulfilling of all of following criteria: symptoms of HF, LV systolic dysfunction by echocardiography, and response to treatment directed toward HF. 36 Insertion of a new cardiac device and use of cardiovascular medications followed Brazilian guidelines. 6 Patients were followed as previously described 29 until December 2021.
Echocardiography
Studies were performed using a phased‐array ultrasound system (Vivid 7; GE Medical Systems, Milwaukee, WI) equipped with M4S phased‐array and 1.5‐ to 4‐MHz 4‐matrix‐array transducers by a single experienced echocardiographer. Cardiac dimensions, measured using M‐mode and 2D echocardiography, and Doppler measurements were obtained as recommended. 37 2D LV ejection fraction and 2D maximum LA volume were determined using the modified Simpson rule with images obtained from apical 4‐ and 2‐chamber views. Echocardiograms were reviewed offline, and strain (ε) and 3‐dimensional echocardiogram (3DE) analyses were performed with Echopac PC workstation software version 108.1.12 (GE Medical Systems) with the 4‐dimensional LV‐volume analysis plug‐in (Tomtec Imaging Systems, Unterschleissheim, Germany). All 2D clips analyzed were acquired at high frame rates (>60 frames/s).
3DE 4‐beat full volume images were acquired during breath hold in end expiration from apical views. Volume rate varied from 17 to 25 volumes/s. The Echopac software was used to determine the maximum and minimum LA volumes, and the precontraction LA volume was obtained from analysis of time‐volume curves. 25 3DE LV volumes and ejection fraction were measured using a similar approach. 25
LA ε was determined as previously described 29 using apical 4‐ and 2‐chamber views. The onset of the P wave was used as the reference point, and peak positive global LA ε, peak negative global LA ε (LASct), and the sum of those previous values were obtained.
LV‐GLS, LV‐GCS, LV global radial strain, and LV torsion and twist were analyzed as previously described. 25 , 29 RV longitudinal ε was calculated using a focused 4‐chamber apical view. RV free wall longitudinal ε was calculated as the average of the basal, mid, and apical RV free wall segments.
Biomarker Measurement
Biomarkers serum measurements were performed by researchers blinded to the clinical classification of the patients. TGF‐β1, tumor necrosis factor (TNF), MMP‐2, MMP‐9, and BNP measurements were performed using commercially available ELISA kits (TGF‐β1 and TNF: Quantikine ELISA Human ImmunoAssay; R&D Systems; MMP‐2 and MMP‐9: Picokine ELISA Human ImmunoAssay; Boster Biologics; BNP: ABCAM) according to the manufacturer's instructions using SpectraMax 190 absorbance microplate reader (Molecular Devices). Cardiac troponin I was determined by enzyme immunoassay (Siemens). For total TGF‐β1 dosage, all samples were assayed after acidic pH activation of latent TGF‐β1.
T cruzi PCR
Ten milliliters of whole blood samples were obtained from each patient and immediately mixed with an equal volume of 6 M guanidinium hydrochloride/0.2 M EDTA, pH 8.00 (guanidine EDTA buffer [GEB]). 38 The GEB samples were coded and delivered blinded to perform the molecular tests. Before DNA extraction, GEB samples were boiled for 15 minutes, and after 24 to 48 hours at room temperature, samples were stored at 4 °C for DNA extraction and PCR‐based analysis. Additionally, the blood of individuals without T cruzi infection (negative serology) from nonendemic CD areas were included as controls for DNA extraction and molecular assays. One negative control was used per DNA extraction of 11 GEB samples, and tested in parallel with the DNA patient samples in all quantitative PCR (qPCR) assays. Three hundred microliters of GEB samples were processed for DNA extraction using the High Pure PCR Template Preparation kit (Roche Diagnostics, Indianapolis, IN).
The qPCR assays were performed by absolute quantification to estimate the parasite load, based on standard curves constructed by 1:10 serial dilutions of DNA extracted from a seronegative‐GEB sample spiked with T cruzi epimastigotes (CL Brener), ranging from 104 to 0.5 parasite equivalents per milliliter of blood, as previously described. 39 To prepare the standard curve, the 1:10 serial dilution was done with DNA extracted from negative GEB samples. The standardized nuclear satellite DNA qPCR assay used the Cruzi 1/Cruzi 2 primers and Cruzi 3 probe reported by Piron et al 40 and the exogenous internal amplification control described by Duffy et al. 41 The parasite load was quantified in all GEB samples and expressed as the average between the duplicates for each patient. The DNA quality and absence of PCR inhibition were monitored by the threshold cycle value for the exogenous internal amplification control. The qPCR assays were performed with 5 μL of DNA, using the FastStart Universal Probe Master Mix (Roche Diagnostics, Mannheim, Germany) in a final volume of 20 μL. The cycling conditions for the multiplex TaqMan qPCR were a first step of 10 minutes at 95 °C, followed by 40 cycles at 95 °C for 15 seconds, and 58 °C for 1 minute. The amplifications were performed in an ABI 7500 Fast Real Time PCR device (Applied Biosystems).
Statistical Analysis
The corresponding author had full access to all of the data in the study and takes responsibility for their integrity and the data analysis. Calculations were done using MedCalc version 20.113 (MedCalc Software, Mariakerke, Belgium) and Stata version 13.0 (StataCorp, College Station, TX). Continuous variables are expressed as mean±standard deviation or median (interquartile range), and categorical variables as absolute and percentage values. Kolmogorov‐Smirnov tests provided support that continuous variables were normal, because P values were >0.10. Associations between studied variables and event‐free survival time were tested using univariable and multivariable Cox proportional hazards regression analysis. Separate multivariable Cox proportional hazards regression analyses were performed for each variable of interest to test if 3DE, 2D ε, and biomarker parameters were associated with the studied end point. Multivariable analyses were adjusted for parameters reported as independent mortality predictors in CD: age, 42 sex, 43 LV ejection fraction, 44 ratio of peak early wave diastolic filling velocity to early diastolic mitral annulus velocity ratio, 24 and RV systolic function 27 expressed by peak systolic tricuspid annulus velocity. Hypertension and presence of a cardiac device and cardiac form at baseline were also included among the variables of adjustment, because they presented a strong univariable association with the studied end point. Missing data were handled by listwise deletion. Schoenfeld residuals did not reject the proportional assumption of Cox proportional hazards regression analysis. Similar competing‐risk survival regression models using the Fine‐Gray method were also fitted to account for the possibility of competing risks for deaths from other causes.
The variance inflation factor was used to check for multicollinearity among the variables included in the multivariate models. Receiver operating characteristic (ROC) curves were generated to define cutoff values for each variable of interest with independent prognostic value with corresponding sensitivities and specificities for prediction of the composite event. The optimal cutoff obtained for an ROC curve corresponded to the maximum of the Youden index, defined as J=max[SEi+SPi−1], where SEi and SPi are the sensitivity and specificity over all possible threshold values. This value corresponded to the point on the ROC curve farthest from the diagonal line. Assessment of differences between areas under the ROC curves (AUC) was done by pairwise comparison, as previously described. 45 Cumulative survival curves dichotomized at optimal ROC were constructed using the Kaplan‐Meier method and compared using the log‐rank test. Patients whose follow‐up was lost were censored from statistical analyses at the time of the last medical appointment. The null hypothesis was rejected at P<0.05.
RESULTS
Patients
The patients studied presented the following CD clinical forms at the time of their enrollment: 98 (27.1%) presented the indeterminate form, 205 (56.6%) presented the cardiac form (stage A n=74 [20.5%], stage B n=77 [21.3%], stage C n=47 [13.0%], stage D n=7 [1.9%]), 11 (3.6%) presented digestive form, and 47 (12.7%) presented the cardiodigestive form (stage A n=20 [5.2%], stage B n=19 [5.2%], stage C n=7 [1.9%], stage D n=1 [0.3%]).
The clinical characteristics, laboratory results, and ECG findings of the included patients are depicted in Table 1. The most common ECG abnormalities were right bundle‐branch block, left anterior hemiblock, and primary T‐wave changes (Table 1). Fifty‐two patients (14.4%) had a cardiac device at baseline; 28 (7.8%) had a dual‐chamber pacemaker, 7 (1.9%) had a single‐chamber pacemaker, 12 (3.3%) had an ICD implant, 3 (0.8%) had a cardiac resynchronization therapy pacemaker device, and 2 (0.5%) had a cardiac resynchronization therapy defibrillator device.
Table 1.
Clinical and Echocardiographic Characteristics of Studied Subjects (n=361)
| Variable | Value |
|---|---|
| Age, y | 61.4±10.6 |
| Sex, men | 208 (57.6) |
| Body mass index, kg/m2 | 27.2±5.1 |
| Hypertension | 245 (67.9%) |
| Diabetes | 81 (22.4%) |
| Electrocardiogram | |
| RBBB | 125 (34.6%) |
| LBBB | 17 (4.7%) |
| LAHB | 108 (29.9%) |
| Primary T‐wave changes | 113 (31.3%) |
| Electric inactive areas | 30 (8.3%) |
| Low voltage | 27 (7.5%) |
| Cardiac device | 52 (14.4%) |
| Blood tests | |
| Urea, mg/dL | 39±16 |
| Creatinine, mg/dL | 1.0±0.3 |
| Sodium, mmol/L | 140.2±2.2 |
| Potassium, mmol/L | 4.5±0.5 |
| Uric acid, mg/dL | 5.0±1.5 |
| Hemoglobin, g/dL | 13.5±1.4 |
| 2D echocardiogram | |
| LA, cm | 3.9±0.6 |
| LA volume, mL/m2 | 34.3±14.9 |
| LVd, cm | 5.5±0.8 |
| LVs, cm | 3.6±1.2 |
| LV ejection fraction, % | 57.9±13.9 |
| LV S′, cm/s | 7.2±1.9 |
| RV S′, cm/s | 12.6±2.8 |
| TAPSE, mm | 23.1±5.1 |
| PASP, mm Hg | 29.1±10.3 |
| E/A ratio | 1.1±0.7 |
| DT, ms | 192±55 |
| E′, cm/s | 7.7±2.7 |
| A′, cm/s | 8.9±2.7 |
| E/E′ ratio | 10.6±5.2 |
| LV aneurysm | 56 (15.5%) |
| 3DE | |
| Maximum LA volume, mL/m2 | 31.2±13.7 |
| Minimum LA volume, mL/m2 | 16.5±12.3 |
| Pre‐A LA volume, mL/m2 | 20.5±10.6 |
| LV end‐diastolic volume, mL/m2 | 67.0±27.1 |
| LV end‐systolic volume, mL/m2 | 34.7±24.1 |
| LV ejection fraction, % | 51.7±13.7 |
| Strain | |
| LASct, % | −11.4±3.6 |
| LAScd, % | 10.9±4.4 |
| LASr, % | 21.7±6.4 |
| Peak LV‐GLS, % | −15.9±4.5 |
| Peak LV‐GCS, % | −16.6±5.9 |
| Peak LV‐GRS, % | 37.1±17.9 |
| Peak twist, degrees | 10.9±6.8 |
| Peak torsion, degrees/cm | 1.3±0.9 |
| RV‐fwLS, % | −21.4±5.5 |
Values are mean±SD or n (%). 2D, indicates 2‐dimensional; 3DE, 3‐dimensional echocardiogram; A, peak late wave diastolic filling velocity; A′, peak late diastolic mitral annulus velocity; DT, deceleration time; E, peak early wave diastolic filling velocity; E′, peak early diastolic mitral annulus velocity; GCS, global circumferential strain; GLS, global longitudinal strain; GRS, global radial strain; LA, left atrial; LAHB, left anterior hemiblock; LAScd, peak positive global LA strain; LASct, peak negative global LA strain; LASr, total global LA strain; LBBB, left bundle‐branch block; LV, left ventricular; LVd, LV end‐diastolic diameter; LVs, LV end‐systolic diameter; PASP, pulmonary artery systolic pressure; RBBB, right bundle‐branch block; RV, right ventricular; RV‐fwLS, RV free wall longitudinal strain; S′, peak systolic mitral annulus velocity; and TAPSE, tricuspid annular plane excursion.
The frequency of use of cardiovascular medications was as follows in patients with no evidence of cardiac form: angiotensin‐converting enzyme inhibitor (ACEi) 29.4%, angiotensin receptor blocker (ARB) 25.7%, furosemide 1.8%, warfarin 0.9%, and amiodarone 2.7%. The frequency of use of cardiovascular medications was as follows in patients with cardiac form (with or without associated digestive form): stage A, carvedilol 2.1%, ACEi 38.3%, ARB 37.3%, digoxin 3.2%, warfarin 7.5%, and amiodarone 5.3%; stage B, carvedilol 43.7%, ACEi 56.2%, ARB 29.2%, spironolactone 6.2%, furosemide 6.2%, warfarin 20.8%, and amiodarone 17.7%; stage C/D, carvedilol 95.2%, ACEi 48.4%, ARB 43.5%, digoxin 37.1%, spironolactone 71.0%, furosemide 85.5%, warfarin 38.7%, and amiodarone 32.2%.
Echocardiography
The mean values for 2D Doppler echocardiographic parameters, LV and LA 3DE parameters, and LA, LV, and RV function analyses by STE analysis are depicted in Table 1.
Seven patients (1.9%) were excluded from LV‐GLS analysis, and 13 patients (3.6%) were excluded from LV‐GCS and LV global radial ε analyses due to poor imaging quality. Twenty‐five (6.9%) were excluded from LV twist and torsion analyses due to poor imaging quality for LV rotation analysis in either basal or apical short‐axis views. LA good imaging quality for RV ε analysis was not obtained in 23 patients (6.4%). LA ε parameters were obtained from all participants. Seven patients (1.9%) and 23 patients (6.4%) were excluded from LA and LV 3DE analyses, respectively, due to poor imaging quality.
The differences in 3DE and STE parameters among the different CD clinical forms were already described in previous articles from our group. 25 , 29 Although the current study represents a new sample of patients, we did not repeat this analysis.
Correlations between LV ejection fraction and 2D LV ε in a correlation matrix revealed that the correlation with LV ejection fraction was −0.81 for LV‐GCS, −0.84 for LV‐GLS, 0.70 for LV global radial strain, and 0.59 for LV torsion (P<0.0001 for all correlations).
Biomarkers
For biomarkers analyses, patients with indeterminate and digestive forms were grouped together in a group named no evidence of cardiac form, and patients with the cardiodigestive form were grouped together with patients with the cardiac form according to their stage.
Cardiac troponin I serum levels were higher among patients at stages B and C/D of the cardiac form than in patients with no evidence of the cardiac form or at stage A of the cardiac form. BNP serum levels were higher among patients at stages C/D of the cardiac form than in all other groups. TGF‐β1 serum levels were lower among patients at stages C/D of the cardiac form than in all other groups. TNF serum levels were higher among patients at stages C/D of the cardiac form than in patients with no evidence of the cardiac form (Table 2). MMP‐2, MMP‐9, and ratio serum levels were similar across the studied groups.
Table 2.
Biomarkers Serum Levels of Studied Subjects
| Variable | No evidence of cardiac form, n=109 | Cardiac form | ||
|---|---|---|---|---|
| Stage A, n=94 | Stage B, n=96 | Stages C/D, n=62 | ||
| Troponin I, ng/mL | 0.01 (0.0–0.04) | 0.00 (0.0–0.04) | 0.03 (0.0–0.07)* , † | 0.03 (0.0–0.08)* , † |
| BNP, pg/mL | 40.0 (31.7–53.4) | 37.7 (29.8–49.6) | 40.2 (33.6–63.3) | 76.8 (37.3–183.5)* , † , ‡ |
| TGF‐β1, ng/mL | 49.9±19.2 | 46.4±12.4 | 44.8±10.1 | 36.9±12.8* , † , ‡ |
| TNF, pg/mL | 4.2±2.4 | 4.7±2.9 | 5.2±4.1 | 6.2±6.0* |
| MMP‐2, ng/mL | 3.9 (2.5–7.6) | 3.7 (2.4–7.1) | 4.1 (2.5–8.4) | 4.1 (2.9–8.9) |
| MMP‐9, ng/mL | 6.5±3.8 | 5.9±3.8 | 6.2±3.9 | 6.7±3.8 |
| MMP‐2/MMP‐9 ratio | 0.74 (0.38–1.66) | 0.89 (0.35–1.79) | 0.92 (0.40–1.75) | 0.79 (0.42–1.65) |
| T cruzi PCR, % | 37 (35.9) | 43 (47.8) | 33 (37.1) | 24 (47.1) |
| Parasitic load, par.Eq./mL | 0.41 (0.10–2.09) | 0.44 (0.08–2.70) | 0.17 (0.04–1.25) | 0.20 (0.05–0.82) |
Values are mean±standard deviation, median (interquartile range), or n (%). BNP indicates brain natriuretic peptide; MMP, matrix metalloproteinase; par.Eq., parasite equivalents per milliliter of blood; PCR, polymerase chain reaction; T cruzi, Trypanosoma cruzi; TGF‐β1, transforming growth factor β1; and TNF, tumor necrosis factor.
P<0.05 vs no evidence of cardiac form.
P<0.05 vs stage A.
P<0.05 vs stage B.
PCR for T cruzi DNA was positive in 137 out of 333 (41.1%) patients whose samples were tested for T cruzi PCR. The T cruzi PCR positivity and the parasitic load were similar across the groups studied. Among those who were tested for T cruzi PCR, 40 had a previous history of trypanocidal treatment at baseline. The T cruzi PCR positivity was 10% (4/40) among those with previous history of trypanocidal treatment against 45.4% (133/293) among those without previous history of trypanocidal treatment (P<0.0001).
Event‐Free Survival Time
The mean follow‐up period was 4.9±2.0 years. Patients lost to follow‐up (n=18, 5.0%) were censored from analysis at the time of their last clinic visit. The composite event occurred in 79 patients (45.1 per 1000 patient‐years). A total of 49 patients died due to CD‐related problems, 2 underwent a heart transplant, 33 were admitted due to worsening HF, and 26 had a cardiac device implant (dual‐chamber pacemaker [n=16], single‐chamber pacemaker [n=1], cardiac resynchronization therapy pacemaker device [n=8], and cardiac resynchronization therapy defibrillator [n=1]) during the study follow‐up. The cause of death related to CD was HF in 29 patients (59.2%), sudden death in 18 patients (36.7%), and stroke in 2 patients (4.1%). Sixteen patients died due to events not related to CD, and 8 patients died due to ignored causes. These patients were censored from analysis. Additionally, 3 patients with the indeterminate form were treated with benznidazole during the study follow‐up and were censored by the date their treatment started, because benznidazole treatment can change the status of positive to negative T cruzi PCR.
The numbers of composite events according to the clinical forms at baseline were as follows: indeterminate form (n=2), cardiac form (stage A n=9, stage B n=18, stage C n=30, stage D n=7), and cardiodigestive form (stage A n=3, stage B n=3, stage C n=6, stage D n=1). No patient with the digestive form at baseline presented the studied end point during the study follow‐up. A survival curve analysis according to the clinical form at baseline was performed with patients with indeterminate and digestive forms grouped together in a group named no evidence of cardiac form, and patients with the cardiodigestive form were grouped together with patients with the cardiac form according to their stage. There was a worse outcome for patients at stage A (hazard ratio [HR], 7.2 [95% CI, 4.1–12.7]), B (HR, 13.3 [95% CI, 7.5–23.4]), C (HR, 58.0 [95% CI, 26.6–126.5]), and D (HR, 165.2 [95% CI, 14.0–1943.4]) compared with patients with no cardiac form at baseline, a worse outcome for patients at stage B (HR, 1.8 [95% CI, 1.01–3.2]), C (HR, 8.0 [95% CI, 3.6–17.8]), and D (HR, 22.8 [95% CI, 1.9–269.5]) compared with patients at stage A of the cardiac form at baseline, and a worse outcome for patients at stage C (HR, 4.4 [95% CI, 2.0–9.8]), and D (HR, 12.5 [95% CI, 1.05–35.7]) compared with patients at stage B of the cardiac form at baseline (P<0.0001; Figure 2).
Figure 2. Event‐free survival curves according to the Chagas disease clinical form at baseline.
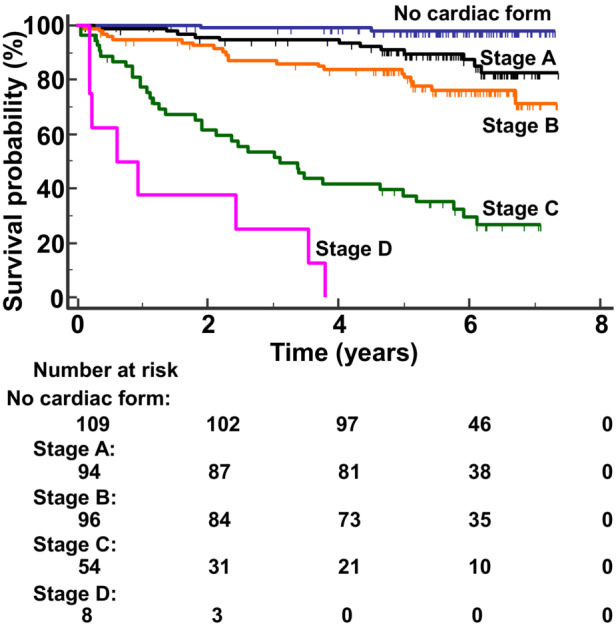
The Kaplan–Meier analysis showed a progressively worse outcome across the different Chagas disease clinical forms from patients with no cardiac form (best survival) to patients at stage D of the cardiac form at baseline (worst outcome; P<0.0001).
Nonadjusted Analyses
Clinical characteristics associated in the univariable analysis with the studied end point were CD cardiac form, body mass index, hypertension, right bundle‐branch block, primary T‐wave changes, electric inactive areas, and presence of a cardiac device. Blood tests associated in the univariable analysis with the studied end point were urea, creatinine, potassium, and uric acid serum levels. All 2D echocardiographic and Doppler studied parameters were also associated in the univariable analysis with the studied end point. LA and LV 3DE parameters and LA, LV, and RV ε parameters were associated with the occurrence of the composite event by univariable analysis (Table 3).
Table 3.
Nonadjusted Associations Between Clinical, Echocardiographic, and Biomarkers Characteristics of Studied Subjects and the Studied Composite Event
| Variable | HR | 95% CI | P value | Harrell C index (95% CI) |
|---|---|---|---|---|
| Age, y | 1.00 | 0.98–1.03 | 0.63 | 0.50 (0.44–0.56) |
| Sex, men | 1.08 | 0.69–1.68 | 0.74 | 0.50 (0.45–0.56) |
| Body mass index, kg/m2 | 0.95 | 0.91–0.99 | 0.01 | 0.59 (0.52–0.66) |
| Hypertension | 0.48 | 0.30–0.74 | 0.001 | 0.59 (0.53–0.64) |
| Diabetes | 0.87 | 0.50–1.50 | 0.61 | 0.51 (0.46–0.58) |
| CD cardiac form | 19.8 | 4.9–80.5 | <0.0001 | 0.65 (0.63–0.68) |
| Electrocardiogram | ||||
| RBBB | 1.96 | 1.26–3.04 | 0.0028 | 0.57 (0.51–0.62) |
| LBBB | 175 | 0.76–4.02 | 0.19 | 0.51 (0.49–0.54) |
| LAHB | 1.45 | 0.92–2.29 | 0.11 | 0.52 (0.47–0.58) |
| Primary T‐wave changes | 1.81 | 1.16–2.83 | 0.0085 | 0.56 (0.51–0.62) |
| Electric inactive areas | 2.81 | 1.58–5.02 | 0.0005 | 0.55 (0.51–0.59) |
| Low voltage | 1.82 | 0.94–3.54 | 0.076 | 0.52 (0.49–0.56) |
| Cardiac device | 2.96 | 1.82–4.80 | <0.0001 | 0.59 (0.54–0.64) |
| Blood tests | ||||
| Urea, mg/dL | 1.02 | 1.01–1.03 | <0.0001 | 0.67 (0.61–0.73) |
| Creatinine, mg/dL | 2.50 | 1.55–4.04 | 0.0002 | 0.62 (0.56–0.68) |
| Sodium, mmol/L | 0.97 | 0.88–1.07 | 0.53 | 0.54 (0.47–0.60) |
| Potassium, mmol/L | 2.00 | 1.25–3.20 | 0.0039 | 0.58 (0.51–0.65) |
| Uric acid, mg/dL | 1.21 | 1.05–1.38 | 0.006 | 0.58 (0.52–0.65) |
| Hemoglobin, g/dL | 0.96 | 0.81–1.14 | 0.64 | 0.52 (0.45–0.59) |
| 2D echocardiogram | ||||
| LA, cm | 2.79 | 2.093.72 | <0.0001 | 0.72 (0.67–0.77) |
| LA volume, mL/m2 | 1.04 | 1.03–1.04 | <0.0001 | 0.76 (0.71–0.81) |
| LVd, cm | 3.23 | 2.57–4.06 | <0.0001 | 0.79 (0.75–0.84) |
| LVs, cm | 2.46 | 2.10–2.90 | <0.0001 | 0.82 (0.77–0.87) |
| LV ejection fraction, % | 0.93 | 0.92–0.94 | <0.0001 | 0.81 (0.76–0.87) |
| LV S′, cm/s | 0.49 | 0.42–0.57 | <0.0001 | 0.80 (0.75–0.85) |
| RV S′, cm/s | 0.76 | 0.69–0.83 | <0.0001 | 0.67 (0.61–0.73) |
| TAPSE, mm | 0.88 | 0.84–0.92 | <0.0001 | 0.65 (0.58–0.72) |
| PASP, mm Hg | 1.06 | 1.04–1.07 | <0.0001 | 0.71 (0.65–0.78) |
| E/A ratio | 1.98 | 1.63–2.42 | <0.0001 | 0.61 (0.53–0.68) |
| DT, ms | 0.99 | 0.98–0.99 | 0.0005 | 0.62 (0.56–0.69) |
| E′, cm/s | 0.68 | 0.61–0.77 | <0.0001 | 0.74 (0.68–0.79) |
| A′, cm/s | 0.69 | 0.64–0.75 | <0.0001 | 0.78 (0.73–0.84) |
| E/E′ ratio | 1.15 | 1.12–1.18 | <0.0001 | 0.74 (0.68–0.81) |
| LV aneurysm | 1.76 | 1.05–2.916 | 0.03 | 0.54 (0.50–0.59) |
| 3DE | ||||
| Maximum LA volume, mL/m2 | 1.03 | 1.02–1.04 | <0.0001 | 0.72 (0.66–0.78) |
| Minimum LA volume, mL/m2 | 1.03 | 1.03–1.04 | <0.0001 | 0.77 (0.71–0.82) |
| Pre‐A LA volume, mL/m2 | 1.07 | 1.05–1.08 | <0.0001 | 0.77 (0.72–0.83) |
| LV end‐diastolic volume, mL/m2 | 1.03 | 1.02–1.03 | <0.0001 | 0.80 (0.75–0.86) |
| LV end‐systolic volume, mL/m2 | 1.03 | 1.03–1.04 | <0.0001 | 0.82 (0.77–0.87) |
| LV ejection fraction, % | 0.93 | 0.91–0.94 | <0.0001 | 0.79 (0.74–0.85) |
| Strain | ||||
| LASct, % | 1.31 | 1.23–1.39 | <0.0001 | 0.73 (0.67–0.79) |
| LAScd, % | 0.87 | 0.82–0.92 | <0.0001 | 0.68 (0.62–0.74) |
| LASr, % | 0.85 | 0.82–0.88 | <0.0001 | 0.76 (0.70–0.81) |
| Peak LV‐GLS, % | 1.25 | 1.20–1.31 | <0.0001 | 0.79 (0.74–0.84) |
| Peak LV‐GCS, % | 1.25 | 1.20–1.31 | <0.0001 | 0.82 (0.77–0.87) |
| Peak LV‐GRS, % | 0.94 | 0.92–0.95 | <0.0001 | 0.77 (0.72–0.83) |
| Peak twist, degrees | 0.86 | 0.83–0.89 | <0.0001 | 0.76 (0.70–0.81) |
| Peak torsion, degrees/cm | 0.26 | 0.19–0.36 | <0.0001 | 0.78 (0.73–0.83) |
| RV‐fwLS, % | 1.06 | 1.01–1.10 | 0.01 | 0.59 (0.53–0.66) |
| Biomarkers | ||||
| Troponin I, ng/mL | 1.22 | 1.07–1.41 | 0.004 | 0.57 (0.51–0.64) |
| BNP, ng/mL | 2.75 | 1.74–4.35 | <0.0001 | 0.67 (0.60–0.73) |
| TGF‐β1, ng/mL | 0.96 | 0.94–0.97 | <0.0001 | 0.64 (0.58–0.71) |
| TNF, pg/mL | 1.05 | 1.01–1.10 | 0.02 | 0.53 (0.46–0.59) |
| MMP‐2, ng/mL | 1.03 | 0.98–1.08 | 0.21 | 0.53 (0.47–0.60) |
| MMP‐9, ng/mL | 1.00 | 0.94–1.06 | 0.99 | 0.50 (0.43–0.57) |
| MMP‐2/MMP‐9 ratio | 1.00 | 0.98–1.02 | 0.96 | 0.48 (0.41–0.55) |
| T cruzi PCR, % | 1.47 | 0.93–2.34 | 0.099 | 0.53 (0.47–0.59) |
| Parasitic load, par.Eq./mL | 1.03 | 1.00–1.06 | 0.058 | 0.54 (0.47–0.60) |
2D indicates 2‐dimensional; 3DE, 3‐dimensional echocardiogram; A, peak late wave diastolic filling velocity; A′, peak late diastolic mitral annulus velocity; BNP, brain natriuretic peptide; CD, Chagas disease; DT, deceleration time; E, peak early wave diastolic filling velocity; E′, peak early diastolic mitral annulus velocity; GCS, global circumferential strain; GLS, global longitudinal strain; GRS, global radial strain; HR, hazard ratio; LA, left atrial; LAHB, left anterior hemiblock; LAScd, peak positive global LA strain; LASct, peak negative global LA strain; LASr, total global LA strain; LBBB, left bundle‐branch block; LV, left ventricular; LVd, LV end‐diastolic diameter; LVs, LV end‐systolic diameter; MMP, matrix metalloproteinase; par.Eq., parasite equivalents per milliliter of blood; PASP, pulmonary artery systolic pressure; PCR, polymerase chain reaction; RBBB, right bundle‐branch block; RV, right ventricular; RV‐fwLS, RV free wall longitudinal strain; S′, peak systolic mitral annulus velocity; TAPSE, tricuspid annular plane excursion; T cruzi, Trypanosoma cruzi; TGF‐β1, transforming growth factor β1; and TNF, tumor necrosis factor.
Among biomarkers, although cardiac troponin, BNP, TGF‐β1, and TNF serum levels were associated with the occurrence of the composite event by univariable analysis, MMP‐2 and MMP‐9 serum levels and their ratio, T cruzi PCR positivity, and parasitic load were not associated (Table 3).
Adjusted Analyses
Multivariable Cox proportional hazards regression analyses revealed that 3DE LV end‐diastolic volume, LASct, peak LV‐GCS, peak torsion and twist, and a positive T cruzi PCR were independent predictors of the composite event (Table 4). The full description of these models is depicted in Tables S1 through S6. The mean variance inflation factor of the multivariable models ranged from 1.37 to 1.99. BNP presented a close to significant independent association with the composite event (Table 4).
Table 4.
Adjusted Associations Between 3DE, Strain, and Biomarkers Characteristics of Studied Subjects and the Studied Composite Event
| Variable | Adjusted analyses | |||
|---|---|---|---|---|
| HR | 95% CI | P value | Harrell C index (95% CI) | |
| 3DE | ||||
| Maximum LA volume, mL/m2 | 1.01 | 0.99–1.02 | 0.41 | 0.84 (0.80–0.88) |
| Minimum LA volume, mL/m2 | 1.01 | 0.99–1.02 | 0.32 | 0.84 (0.80–0.88) |
| Pre‐A LA volume, mL/m2 | 1.02 | 0.99–1.04 | 0.16 | 0.85 (0.81–0.89) |
| LV end‐diastolic volume, mL/m2 | 1.01 | 1.00–1.02 | 0.019 | 0.85 (0.81–0.89) |
| LV end‐systolic volume, mL/m2 | 1.01 | 0.99–1.02 | 0.21 | 0.85 (0.80–0.89) |
| LV ejection fraction, % | 1.01 | 0.97–1.04 | 0.75 | 0.84 (0.80–0.89) |
| Strain | ||||
| LASct, % | 1.09 | 1.01–1.18 | 0.026 | 0.85 (0.81–0.89) |
| LAScd, % | 1.00 | 0.93–1.08 | 0.90 | 0.84 (0.80–0.88) |
| LASr, % | 0.97 | 0.92–1.02 | 0.24 | 0.84 (0.80–0.88) |
| Peak LV‐GLS, % | 0.98 | 0.88–1.09 | 0.71 | 0.84 (0.79–0.88) |
| Peak LV‐GCS, % | 1.13 | 1.04–1.21 | 0.002 | 0.85 (0.81–0.89) |
| Peak LV‐GRS, % | 0.99 | 0.97–1.02 | 0.67 | 0.84 (0.79–0.88) |
| Peak twist, degrees | 0.94 | 0.90–0.99 | 0.022 | 0.84 (0.79–0.88) |
| Peak torsion, degrees/cm | 0.56 | 0.37–0.84 | 0.006 | 0.84 (0.80–0.88) |
| RV‐fwLS, % | 0.97 | 0.92–1.02 | 0.23 | 0.85 (0.81–0.89) |
| Biomarkers | ||||
| Troponin I, ng/mL | 0.99 | 0.84–1.16 | 0.88 | 0.83 (0.79–0.88) |
| BNP, ng/mL | 1.94 | 0.99–3.82 | 0.05 | 0.84 (0.80–0.88) |
| TGF‐β1, ng/mL | 0.99 | 0.97–1.01 | 0.51 | 0.85 (0.81–0.89) |
| TNF, pg/mL | 1.02 | 0.97–1.06 | 0.48 | 0.83 (0.79–0.88) |
| MMP‐2, ng/mL | 1.01 | 0.97–1.06 | 0.51 | 0.83 (0.79–0.88) |
| MMP‐9, ng/mL | 1.00 | 0.94–1.07 | 0.95 | 0.83 (0.79–0.88) |
| T cruzi PCR, % | 1.77 | 1.09–2.86 | 0.02 | 0.84 (0.79–0.88) |
| Parasitic load, par.Eq./mL | 1.01 | 0.98–1.03 | 0.55 | 0.83 (0.79–0.88) |
Multivariate models were adjusted for age, sex, LV ejection fraction, peak early wave diastolic filling velocity and peak early diastolic mitral annulus velocity ratio, RV peak systolic mitral annulus velocity, hypertension, and presence of a cardiac device and cardiac form at baseline. 3DE indicates 3‐dimensional echocardiogram; BNP, brain natriuretic peptide; GCS, global circumferential strain; GLS, global longitudinal strain; GRS, global radial strain; HR, hazard ratio; LA, left atrial; LAScd, peak positive global LA strain; LASct, peak negative global LA strain; LASr, total global LA strain; LV, left ventricular; MMP, matrix metalloproteinase; par.Eq., parasite equivalents per milliliter of blood; PCR, polymerase chain reaction; RV, right ventricular; RV‐fwLS, RV free wall longitudinal strain; T cruzi, Trypanosoma cruzi; TGF‐β1, transforming growth factor β1; and TNF, tumor necrosis factor.
Because 24 patients died due to causes unrelated to CD or due to ignored causes, we included those deaths as competing events and did a competing risk survival analysis for all variables of interest. The analyses revealed that 3DE LV end‐diastolic volume (HR, 1.01 [95% CI, 1.00–1.02]; P=0.02), LASct (HR, 1.08 [95% CI, 1.00–1.17]; P=0.04), LV‐GCS (HR, 1.12 [95% CI, 1.04–1.21]; P=0.003), LV twist (HR, 0.94 [95% CI, 0.89–0.99]; P=0.01), LV torsion (HR, 0.55 [95% CI, 0.35–0.81]; P=0.003), BNP (HR, 2.03 [95% CI, 1.23–3.34]; P=0.005), and positive T cruzi PCR (HR, 1.80 [95% CI, 1.12–2.91]; P=0.01) were independently associated with the composite event. The full multivariate analyses adjusted for competing events of the variables of interest with a significant result are presented in Tables S7 and S8.
Another multivariable regression model including all the variables of interest with independent association with the composite event adjusted for the adjusting variables and competing risks showed that 3DE LV end‐diastolic volume (HR, 1.01 [95% CI, 1.00–1.02]; P=0.03), LASct (HR, 1.10 [95% CI, 1.03–1.18]; P=0.0007), LV‐GCS (HR, 1.13 [95% CI, 1.02–1.25]; P=0.01), LV torsion (HR, 0.57 [95% CI, 0.34–0.97]; P=0.038), BNP (HR, 3.9 [95% CI, 1.8–8.3]; P<0.001), and positive T cruzi PCR (HR, 1.79 [95% CI, 1.07–2.99]; P=0.026) remained independently associated with the studied end point.
Optimal cutoff values to predict the composite event for 3DE LV end‐diastolic volume was 74.8 mL/m2 (AUC, 0.82; sensitivity, 69.4%; specificity, 86.1%; P<0.0001), for LASct was −10.5% (AUC, 0.74; sensitivity, 70.3%; specificity, 71.0%; P<0.0001), for peak LV‐GCS was −14.7% (AUC, 0.85; sensitivity, 79.2%; specificity, 78.2%; P<0.0001), for LV twist was 9.3° (AUC, 0.79; sensitivity, 78.9%; specificity, 66.0%; P < 0.0001), for LV torsion was 1.11 degrees/cm (AUC 0.81; sensitivity, 84.2%; specificity, 66.0%; P<0.0001), and for BNP was 51.6 pg/mL (AUC, 0.65; sensitivity, 57.7%; specificity, 73.0%; P=0.0001). Peak LV‐GCS presented a larger AUC than LASct (P=0.003) and BNP (P<0.0001). 3DE LV end‐diastolic volume also presented a larger AUC than LASct (P=0.02) and BNP (P<0.0001). LV torsion also presented a larger AUC than LASct (P=0.04) and BNP (P=0.0002) (Figure 3).
Figure 3. Comparison of the areas under the ROC curves generated for the independent predictors of the composite event.
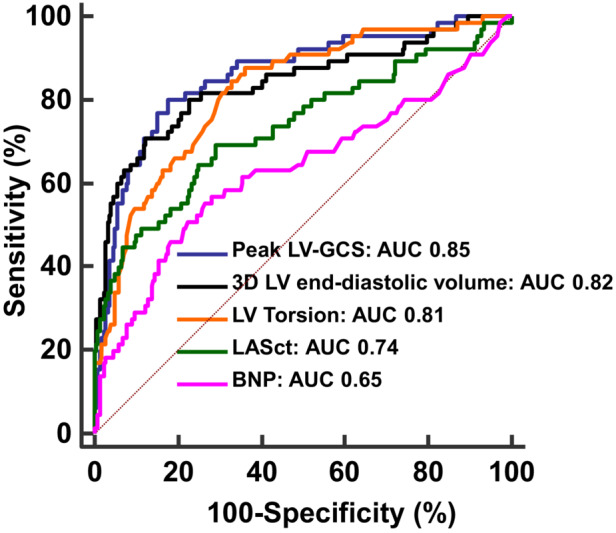
Comparison of ROC curves generated for LV‐GCS, 3DE LV end‐diastolic volume, LV torsion, LASct, and BNP. Peak LV‐GCS, 3DE LV end‐diastolic volume, and LV torsion presented a larger AUC than LASct (P=0.003, P=0.02, P=0.04, respectively), and BNP (P<0.0001, P<0.0001, P= 0.0002, respectively). Differences in areas under the ROC curves were assessed by pairwise comparison, as previously described. 45 3D indicates 3‐dimensional; 3DE, 3‐dimensional echocardiogram; AUC, area under the receiver operating characteristic curve; BNP, brain natriuretic peptide; GCS, global circumferential strain; LA, left atrial; LASct, peak negative global LA strain; LV, left ventricular; and ROC, receiver operating characteristic.
Survival curves were constructed for the parameters with the largest AUC according to ROC analyses. LV‐GCS absolute value ≤14.7% (HR, 11.6 [95% CI, 7.05–19.0]; P<0.0001; Figure 4A), 3 DE LV end‐diastolic volume ≥74.8 mL/m2 (HR, 24.9 [95% CI, 13.9–44.8]; P<0.0001; Figure 4B), and LV torsion ≤1.11 degrees/cm (HR, 6.3 [95% CI, 4.0–10.0]; P<0.0001; Figure 4C) were associated with lower survival free of the composite event.
Figure 4. Event‐free survival curves of patients with chronic Chagas disease.
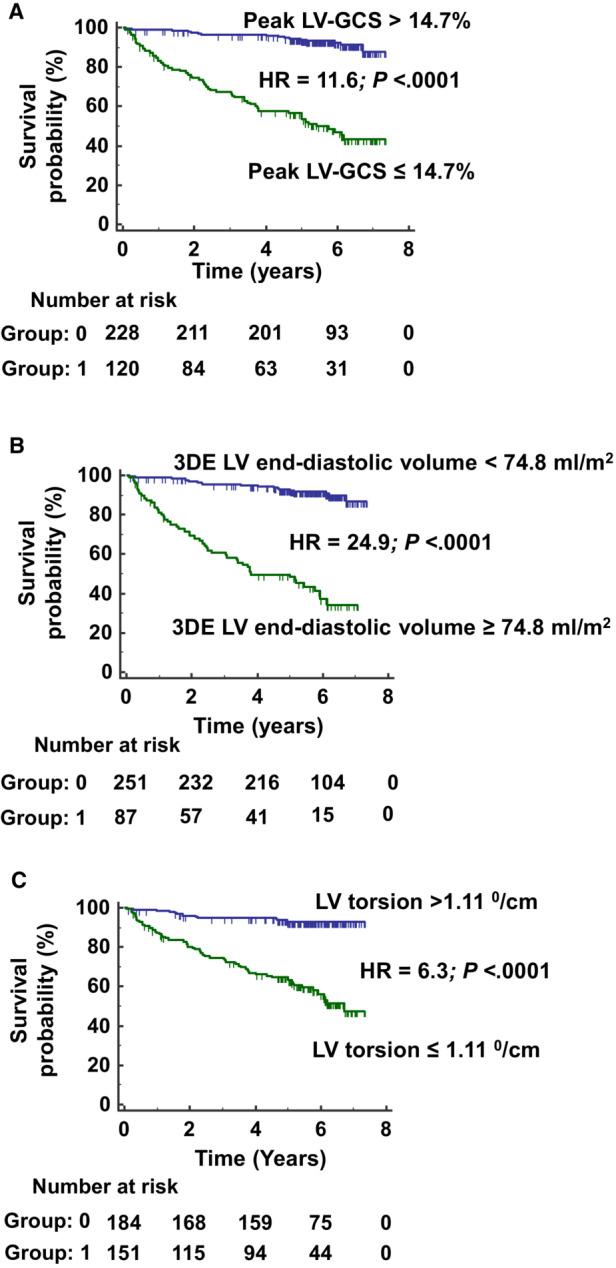
Kaplan–Meier curves of the event‐free survival time according to LV‐GCS (A), 3DE LV end‐diastolic volume (B), and LV torsion (C). 3DE indicates 3‐dimensional echocardiogram; GCS, LV global circumferential strain; HR, hazard ratio; and LV, left ventricular.
DISCUSSION
The dismal prognosis of patients with chronic CHD drives the research for new biomarkers of progression and prognosis in CD. The insight obtained from cross‐sectional studies and some longitudinal studies points out that a complex interplay of immunological mediators, parasite persistence, and LV fibrosis and remodeling interacts for the pathophysiology and slow progressive nature of CHD. In this study, we evaluated the prognostic value of biomarkers, parasite persistence, and new echocardiographic indexes against 2D Doppler echocardiographic indexes already tested as prognostic predictors in CD. We showed that parasite persistence, BNP serum levels, 3DE LV volume, and LA and LV contractility measured by STE were predictors of cardiovascular events independent from age, sex, presence of a cardiac device or cardiac form at baseline, and 2D Doppler echocardiographic indexes of LV systolic and diastolic function, and RV systolic function.
Immunological biomarkers are pursued as a marker or a panel of markers that could explain CD progression and identify the group of patients at higher risk to develop CHD. Several cross‐sectional studies including both cytokine serum levels and genetic polymorphisms 46 indicated that biomarkers, such as interleukin‐10, 14 , 16 , 17 interleukin‐17, 17 MMP‐9, 11 TGF‐β1, 13 , 17 , 18 TNF, 14 , 16 , 17 , 18 interferon‐δ, 14 , 16 , 17 interleukin‐6, 14 , 16 , 17 and interleukin‐1β, 14 could have a role in CD progression. However, cross‐sectional studies have limited power to prove causality, and longitudinal studies are still scarce in CD. 19 , 20 , 21 In our article, we included a large sample of patients followed up to 7 years. Although univariable analysis showed an association of troponin I, BNP, TNF, and TGF‐β1 with the occurrence of cardiovascular events, only BNP was associated with cardiovascular events after multivariable analysis and adjustment for competing risks. Abnormal NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) was shown to have association with mortality independent from LV ejection fraction, RV end‐diastolic area, ratio of early diastolic filling to early diastolic mitral annulus peak velocities, and LA volume index. 47 Other articles also showed that NT‐proBNP was a predictor of a composite outcome (mortality, heart transplant, or use of an LV assist device) independent of age and LV ejection fraction 31 or mortality independent from age and sex 21 in CD. In our article, we further adjusted our results to several factors that can be associated with BNP serum levels, such as age, 48 sex, 48 hypertension, 49 and 2D echocardiographic indexes of LV systolic and diastolic function, 18 and BNP serum levels remained as an independent cardiovascular outcome predictor in CD, which demonstrates the importance of its use in clinical practice. In regard to troponin, our article did not show an independent association of troponin I with cardiovascular events; however, another article found an association of high‐sensitivity troponin T with a composite outcome of mortality, heart transplant, or use of an LV assist device, but the results were adjusted only to age and LV ejection fraction. 31 In regard to MMP‐2, our article did not show an association with cardiovascular events, whereas others showed a significant association with mortality after adjusting for age and sex. 21 In regard to TGF‐β1, our longitudinal study including patients with indeterminate form and all stages of cardiac form did not confirm an association with mortality described by us in a small retrospective sample including patients without moderate to severe LV systolic dysfunction. 20 However, it is important to stress that a negative finding on multivariable analyses does not mean that cytokine serum levels would not have an important role in CHD pathogenesis. This finding can be interpreted as the clinical and echocardiogram exams being able to detect the cardiovascular changes that affect prognosis, or that the measurement of biomarkers in peripheral blood do not accurately represent the local effects of those cytokines in the heart.
The parasite persistence has been increasingly recognized as a key element for the ongoing relentless chronic fibrosing myocarditis characteristic of CD. 1 , 5 , 6 The identification of the parasite in the peripheral blood is more frequent in patients with the cardiac form 16 , 32 and has been implicated in a worse prognosis. 32 In this article, we added the important information that a positive T cruzi qPCR identified patients with a worse cardiovascular outcome independent from clinical and 2D Doppler echocardiographic parameters. However, the parasite load was not associated with a worse outcome. Another study did not find an association between a positive qPCR and the occurrence of a composite outcome of mortality, heart transplant, and LV assist device after a 2‐year follow‐up in patients with CHD,33 but on the other hand showed that a group with lower parasitemia had a higher risk of presenting the studied combined outcome. However, this finding was only adjusted for age, whereas LV ejection fraction was lower in this group than in those with higher parasitemia. 33 Furthermore, the BENEFIT (BENznidazole Evaluation for Interrupting Trypanosomiasis) trial did not show a significant difference in the occurrence of a composite outcome of cardiovascular events between patients with CHD treated or not treated with benznidazole, although there was a significant decrease in the number of hospitalizations for cardiovascular causes. The main finding of the BENEFIT trial was also observed in the subgroup with a positive T cruzi PCR at baseline. 50 Thus, the role of the parasite in CD progression or in the occurrence of cardiovascular events must have a complex mechanism including the triggering of chronic myocardial inflammation.
In our article, we confirmed our previous finding that peak LV‐GCS and LV torsion are independent predictors of an adverse cardiovascular outcome in patients with CD.29 This is an important finding, because this is an internal validation of our previous finding using a different group of patients with echocardiograms obtained in a different period of time. 29 We also found that LA contractility measured by STE is an independent predictor of an adverse cardiovascular outcome, as we also described previously.25 Although peak LV‐GLS presented a high C value in nonadjusted analysis, LV‐GLS did not remain as an independent predictor after adjusted analysis, because LV‐GLS and LV ejection fraction presented a high correlation. Other recent studies with smaller group of patients also studied the prognostic value of strain and 3DE. A study that enrolled 177 patients and studied a combined outcome of all‐cause mortality, heart transplant, or LV assist device implantation described that LV‐GLS, LV ejection fraction, and peak early wave diastolic filling velocity to early diastolic mitral annulus velocity ratio were the best outcome predictors. 28 However, the comparison with our results is difficult, because the variables that were included in the multivariate models were not clear, and some continuous echocardiographic variables were analyzed as dichotomic variables. Another smaller study that included 72 patients who were followed for 60 months for the occurrence of hospitalization for HF, complex ventricular arrhythmias, heart transplant, and all‐cause mortality described that 2D LV‐GLS, 2D LV‐GCS, 3‐dimensional LV area strain, and RV‐GLS were univariable predictors of the studied composite end point. 51
Clinical Implications
Biomarkers of CD progression, prognosis, and/or therapeutic response are pursued by several groups. 52 The current article shows that strain parameters that evaluate LV systolic function and torsion mechanisms are independent from clinical and 2D Doppler echocardiographic parameters and confirms previous findings of our group. 29 This internal validation strengthens the value of LV‐GCS and torsion as new parameters for cardiovascular prediction in CD. Additionally, LV end‐diastolic volume evaluated by 3DE was also an independent parameter associated with the studied outcome. Moreover, the adjusted models including these parameters presented a high Harrell C index. Additionally, LV‐GCS was also pointed out by a different group as a cardiovascular outcome predictor in CD51 and presented the higher AUC in ROC curve comparison analysis. Among biomarkers, our article shows that markers of myocardial damage, such as cardiac troponin, or increased LV end‐diastolic pressure, such as BNP, or immunological activity, such as TGF‐β1 and TNF, differ among the different CD groups and are associated on univariable analysis with cardiovascular outcome. Moreover, BNP remained associated with cardiovascular events after multivariable analysis with adjustment for competing risks. This shows the importance of this biomarker in clinical scenarios in the absence of echocardiogram tools.
Another clinically relevant result is that a positive T cruzi qPCR was an independent cardiovascular outcome predictor. This suggests the importance of parasite persistence in CD pathophysiology and the need for further studies on trypanocidal treatment for patients with chronic CD.
The use of cardiovascular medications by the study participants was in accordance with the published recommendations, 6 as demonstrated by their high frequency of use among patients at stages C or D of the cardiac form, and frequent use of carvedilol and ACEi/ARB in patients at stage B. The frequency of use of those medications in patients with no cardiac form or at stage A was lower, as expected, and more related to the comorbidities these patients presented.
Limitations
Our study limitations on the echocardiographic technique used in this article were discussed in a previous publication by our group. 29 Another limitation of the present study is that external validation of our findings, mainly on LV torsion and LA strain, is still required. Also, LV‐GLS is load‐dependent, and a global work index has been recommended as an alternative to overcome this limitation. 53 However, this novel index was not measured at the time the echocardiograms were acquired.
Another limitation is that findings on 24‐hour Holter monitoring were not included among the variables of adjustment.
Although the qPCR positivity was an independent cardiovascular outcome predictor, the parasitic load was not. However, the mean parasitic load found in this article was under the limit of detection described for this technology 41 but within the range for the parasitic load described by others in Brazilian patients. 54 Therefore, the parasitic load measurement precision was low, which limits the use of this variable in comparisons among different study groups and in prediction models.
The number of adjusting variables included in the Cox survival models followed the classical rule published by Peduzzi et al, 55 which considers the need of 10 events per variable. Despite some authors questioning this approach, 56 this rule is still widely accepted, with some authors suggesting that even a lower number of events could adequately estimate the risk. 57
Conclusions
2D ε‐ and 3DE‐derived parameters, BNP serum levels, and a positive T cruzi PCR predicted a composite event of mortality related to CD, heart transplant, or hospital admission due to worsening HF in patients with CD independent of clinical and 2D‐Doppler echocardiographic indexes. Our findings suggest the potential usefulness of new echocardiographic tools, BNP, and parasitological exams in CD prediction and the importance of further studies on treatment for the causes of CD.
Sources of Funding
This work was supported by the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro, Brazil (FAPERJ) (grant numbers E‐26/201.561/2014 and E‐26/110.176/2014 to Dr Saraiva), and Brazilian National Council for Scientific and Technological Development (CNPq) (grant number 305088/2013‐0 to Dr Saraiva). O.C.M. and C.B. are researcher fellows of CNPq (311 539/2020‐3), CNPq (304 308/2019‐3), and FAPERJ (CNE, E‐26/201.096/2022 and E‐26/201.213/2022). A.S.S. is a research fellow of FAPERJ (CNE 260003/004257/2021). This study was partially supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–CAPES–Finance Code 001. Funding sources had no role in the conduct of the research, preparation of the article, and in the decision to submit the article for publication.
Disclosures
None.
Supporting information
Tables S1–S8
Acknowledgments
R.M.S. conceived and designed the study. R.M.S., L.R., V.G.M., A.C.B.d.L., R.R.F., A.R.C., L.H.C.S., F.S.N.S.M., A.S.S., H.H.V., M.T.H., A.M.H.‐M. acquired the data. R.M.S., G.M.S.d.S., M.F.F.M. contributed to data analysis. R.M.S., M.F.F.M., M.C.W., L.R.G., O.C.M., C.B., A.M.H.‐M. contributed to interpretation and contextualization of the results and article drafting. All authors have contributed to the critical revision of the article, and read and approved the final article.
This article was sent to Erik B. Schelbert, MD, MS, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028810
For Sources of Funding and Disclosures, see page 14.
References
- 1. Nunes MCP, Beaton A, Acquatella H, Bern C, Bolger AF, Echeverria LE, Dutra WO, Gascon J, Morillo CA, Oliveira‐Filho J, et al. Chagas cardiomyopathy: an update of current clinical knowledge and management: a scientific statement from the American Heart Association. Circulation. 2018;138:e169–e209. doi: 10.1161/CIR.0000000000000599 [DOI] [PubMed] [Google Scholar]
- 2. Irish A, Whitman JD, Clark EH, Marcus R, Bern C. Updated estimates and mapping for prevalence of Chagas disease among adults, United States. Emerg Infect Dis. 2022;28:1313–1320. doi: 10.3201/eid2807.212221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Basile L, Jansa JM, Carlier Y, Salamanca DD, Angheben A, Bartoloni A, Seixas J, Van GT, Canavate C, Flores‐Chavez M, et al. Chagas disease in European countries: the challenge of a surveillance system. Euro Surveill. 2011;16:19968. doi: 10.2807/ese.16.37.19968-en [DOI] [PubMed] [Google Scholar]
- 4. Velasco M, Gimeno‐Feliu LA, Molina I, Salas‐Coronas J, Sola I, Monge‐Maillo B, Torrus‐Tendero D, Cayla J, de Guzman EN, Arellano JP, et al. Screening for Trypanosoma cruzi infection in immigrants and refugees: systematic review and recommendations from the Spanish Society of Infectious Diseases and Clinical Microbiology. Euro Surveill. 2020;25:1900393. doi: 10.2807/1560-7917.ES.2020.25.8.1900393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saraiva RM, Mediano MFF, Mendes FS, Sperandio da Silva GM, Veloso HH, Sangenis LHC, da Silva PS, Mazzoli‐Rocha F, Sousa AS, Holanda MT, et al. Chagas heart disease: an overview of diagnosis, manifestations, treatment, and care. World J Cardiol. 2021;13:654–675. doi: 10.4330/wjc.v13.i12.654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dias JC, Ramos AN Jr, Gontijo ED, Luquetti A, Shikanai‐Yasuda MA, Coura JR, Torres RM, Melo JR, Almeida EA, Oliveira W Jr, et al. 2 nd Brazilian consensus on Chagas disease, 2015. Rev Soc Bras Med Trop. 2016;49(suppl 1):3–60. doi: 10.1590/0037-8682-0505-2016 [DOI] [PubMed] [Google Scholar]
- 7. Gonzalez FB, Villar SR, Pacini MF, Bottasso OA, Perez AR. Immune‐neuroendocrine and metabolic disorders in human and experimental T. cruzi infection: new clues for understanding Chagas disease pathology. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165642. doi: 10.1016/j.bbadis.2019.165642 [DOI] [PubMed] [Google Scholar]
- 8. Dutra WO, Menezes CA, Magalhaes LM, Gollob KJ. Immunoregulatory networks in human Chagas disease. Parasite Immunol. 2014;36:377–387. doi: 10.1111/pim.12107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rocha IH, Ferreira Marques AL, Moraes GV, Alves da Silva DA, Silva MVD, Rodrigues V, Cunha DFD, Correia D. Metabolic and immunological evaluation of patients with indeterminate and cardiac forms of Chagas disease. Medicine (Baltimore). 2020;99:e23773. doi: 10.1097/MD.0000000000023773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saravia SG, Haberland A, Bartel S, Araujo R, Valda G, Reynaga DD, Ramirez ID, Borges AC, Wallukat G, Schimke I. Cardiac troponin T measured with a highly sensitive assay for diagnosis and monitoring of heart injury in chronic Chagas disease. Arch Pathol Lab Med. 2011;135:243–248. doi: 10.5858/135.2.243 [DOI] [PubMed] [Google Scholar]
- 11. Fares RC, Gomes JA, Garzoni LR, Waghabi MC, Saraiva RM, Medeiros NI, Oliveira‐Prado R, Sangenis LH, Chambela MC, de Araujo FF, et al. Matrix metalloproteinases 2 and 9 are differentially expressed in patients with indeterminate and cardiac clinical forms of Chagas disease. Infect Immun. 2013;81:3600–3608. doi: 10.1128/IAI.00153-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vilas‐Boas F, Feitosa GS, Soares MB, Pinho‐Filho JA, Nascimento T, Barojas MM, Andrade MV, Ribeiro‐Dos‐Santos R, Bocchi E. Invasive and noninvasive correlations of B‐type natriuretic peptide in patients with heart failure due to Chagas cardiomyopathy. Congest Heart Fail. 2008;14:121–126. doi: 10.1111/j.1751-7133.2008.08166.x [DOI] [PubMed] [Google Scholar]
- 13. Araujo‐Jorge TC, Waghabi MC, Hasslocher‐Moreno AM, Xavier SS, Higuchi ML, Keramidas M, Bailly S, Feige JJ. Implication of transforming growth factor‐beta1 in Chagas disease myocardiopathy. J Infect Dis. 2002;186:1823–1828. doi: 10.1086/345882 [DOI] [PubMed] [Google Scholar]
- 14. Sousa GR, Gomes JA, Fares RC, Damasio MP, Chaves AT, Ferreira KS, Nunes MC, Medeiros NI, Valente VA, Correa‐Oliveira R, et al. Plasma cytokine expression is associated with cardiac morbidity in Chagas disease. PLoS ONE. 2014;9:e87082. doi: 10.1371/journal.pone.0087082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pissetti CW, Correia D, Braga T, Faria GE, Oliveira RF, Ribeiro BM, Rodrigues DB, Rodrigues V. Association between the plasma levels of TNF‐alpha, IFN‐gamma, IL‐10, nitric oxide and specific IgG isotypes in the clinical forms of chronic Chagas disease. Rev Soc Bras Med Trop. 2009;42:425–430. doi: 10.1590/S0037-86822009000400013 [DOI] [PubMed] [Google Scholar]
- 16. Keating SM, Deng X, Fernandes F, Cunha‐Neto E, Ribeiro AL, Adesina B, Beyer AI, Contestable P, Custer B, Busch MP, et al. Inflammatory and cardiac biomarkers are differentially expressed in clinical stages of Chagas disease. Int J Cardiol. 2015;199:451–459. doi: 10.1016/j.ijcard.2015.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perez AR, Silva‐Barbosa SD, Berbert LR, Revelli S, Beloscar J, Savino W, Bottasso O. Immunoneuroendocrine alterations in patients with progressive forms of chronic Chagas disease. J Neuroimmunol. 2011;235:84–90. doi: 10.1016/j.jneuroim.2011.03.010 [DOI] [PubMed] [Google Scholar]
- 18. Curvo EO, Ferreira RR, Madeira FS, Alves GF, Chambela MC, Mendes VG, Sangenis LHC, Waghabi MC, Saraiva RM. Correlation of transforming growth factor‐beta1 and tumour necrosis factor levels with left ventricular function in Chagas disease. Mem Inst Oswaldo Cruz. 2018;113:e170440. doi: 10.1590/0074-02760170440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heringer‐Walther S, Moreira MC, Wessel N, Saliba JL, Silvia‐Barra J, Pena JL, Becker S, Siems WE, Schultheiss HP, Walther T. Brain natriuretic peptide predicts survival in Chagas' disease more effectively than atrial natriuretic peptide. Heart. 2005;91:385–387. doi: 10.1136/hrt.2003.026856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saraiva RM, Waghabi MC, Vilela MF, Madeira FS, da Silva GM, Xavier SS, Feige JJ, Hasslocher‐Moreno AM, Araujo‐Jorge TC. Predictive value of transforming growth factor‐beta1in Chagas disease: towards a biomarker surrogate of clinical outcome. Trans R Soc Trop Med Hyg. 2013;107:518–525. doi: 10.1093/trstmh/trt050 [DOI] [PubMed] [Google Scholar]
- 21. Sherbuk JE, Okamoto EE, Marks MA, Fortuny E, Clark EH, Galdos‐Cardenas G, Vasquez‐Villar A, Fernandez AB, Crawford TC, Do RQ, et al. Biomarkers and mortality in severe Chagas cardiomyopathy. Glob Heart. 2015;10:173–180. doi: 10.1016/j.gheart.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bautista‐Lopez NL, Morillo CA, Lopez‐Jaramillo P, Quiroz R, Luengas C, Silva SY, Galipeau J, Lalu MM, Schulz R. Matrix metalloproteinases 2 and 9 as diagnostic markers in the progression to Chagas cardiomyopathy. Am Heart J. 2013;165:558–566. doi: 10.1016/j.ahj.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 23. Rassi A Jr, Rassi A, Rassi SG. Predictors of mortality in chronic Chagas disease: a systematic review of observational studies. Circulation. 2007;115:1101–1108. doi: 10.1161/CIRCULATIONAHA.106.627265 [DOI] [PubMed] [Google Scholar]
- 24. Nunes MP, Colosimo EA, Reis RC, Barbosa MM, da Silva JL, Barbosa F, Botoni FA, Ribeiro AL, Rocha MO. Different prognostic impact of the tissue Doppler‐derived E/e' ratio on mortality in Chagas cardiomyopathy patients with heart failure. J Heart Lung Transplant. 2012;31:634–641. doi: 10.1016/j.healun.2012.01.865 [DOI] [PubMed] [Google Scholar]
- 25. Nascimento CA, Gomes VA, Silva SK, Santos CR, Chambela MC, Madeira FS, Holanda MT, Brasil PE, Sousa AS, Xavier SS, et al. Left atrial and left ventricular diastolic function in chronic Chagas disease. J Am Soc Echocardiogr. 2013;26:1424–1433. doi: 10.1016/j.echo.2013.08.018 [DOI] [PubMed] [Google Scholar]
- 26. Nunes MC, Barbosa MM, Ribeiro AL, Colosimo EA, Rocha MO. Left atrial volume provides independent prognostic value in patients with Chagas cardiomyopathy. J Am Soc Echocardiogr. 2009;22:82–88. doi: 10.1016/j.echo.2008.11.015 [DOI] [PubMed] [Google Scholar]
- 27. Nunes MC, Rocha MO, Ribeiro AL, Colosimo EA, Rezende RA, Carmo GA, Barbosa MM. Right ventricular dysfunction is an independent predictor of survival in patients with dilated chronic Chagas' cardiomyopathy. Int J Cardiol. 2008;127:372–379. doi: 10.1016/j.ijcard.2007.06.012 [DOI] [PubMed] [Google Scholar]
- 28. Echeverría LE, Rojas LZ, Rueda‐Ochoa OL, Gómez‐Ochoa SA, Mayer MA, Becerra‐Motta LP, Luengas C, Chaves AM, Rodríguez JA, Morillo CA. Longitudinal strain by speckle tracking and echocardiographic parameters as predictors of adverse cardiovascular outcomes in chronic Chagas cardiomyopathy. Int J Cardiovasc Imaging. 2022;38:1245–1255. doi: 10.1007/s10554-021-02508-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saraiva RM, Mediano MFF, Quintana MSB, Sperandio da Silva GM, Costa AR, Sousa AS, Sangenis LHC, Mendes FSNS, Veloso HH, Xavier SS, et al. Two‐dimensional strain derived parameters provide independent predictors of progression to Chagas cardiomyopathy and mortality in patients with Chagas disease. Int J Cardiol Heart Vasc. 2022;38:100955. doi: 10.1016/j.ijcha.2022.100955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Di Lorenzo OC, Nunes MCP, Colosimo EA, de Lima EM, Cardoso CS, Ferreira AM, de Oliveira LC, Moreira CHV, Bierrenbach AL, Haikal DAS, et al. Risk score for predicting 2‐year mortality in patients with Chagas cardiomyopathy from endemic areas: SaMi‐Trop cohort study. J Am Heart Assoc. 2020;9:e014176. doi: 10.1161/JAHA.119.014176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Echeverría LE, Rojas LZ, Gómez‐Ochoa SA, Rueda‐Ochoa OL, Sosa‐Vesga CD, Muka T, Januzzi JL, Marcus R, Morillo CA. Cardiovascular biomarkers as predictors of adverse outcomes in chronic Chagas cardiomyopathy. PLoS ONE. 2021;16:e0258622. doi: 10.1371/journal.pone.0258622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nielebock MAP, de Freitas Campos Miranda L, Americano do Brasil PEA, de Jesus S. Pereira TO, da Silva AF, Hasslocher‐Moreno AM, Sangenis LHC, Saraiva RM. Blood culture positivity rate for Trypanosoma cruzi in patients with chronic Chagas disease differs among different clinical forms. Trans R Soc Trop Med Hyg. 2021;115:720–725. doi: 10.1093/trstmh/traa121 [DOI] [PubMed] [Google Scholar]
- 33. Echeverría LE, Rojas LZ, Rueda‐Ochoa OL, Gómez‐Ochoa SA, González Rugeles CI, Díaz ML, Marcus R, Morillo CA. Circulating Trypanosoma cruzi load and major cardiovascular outcomes in patients with chronic Chagas cardiomyopathy: a prospective cohort study. Trop Med Int Health. 2020;25:1534–1541. doi: 10.1111/tmi.13487 [DOI] [PubMed] [Google Scholar]
- 34. Prineas RJ, Crow RS, Zhang ZM. The Minnesota Code Manual of Electrographic Findings. 2nd ed. Springer; 2010. doi: 10.1007/978-1-84882-778-3 [DOI] [Google Scholar]
- 35. Deyell MW, Krahn AD, Goldberger JJ. Sudden cardiac death risk stratification. Circ Res. 2015;116:1907–1918. doi: 10.1161/CIRCRESAHA.116.304493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, Tavazzi L, Smiseth OA, Gavazzi A, Haverich A, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): the Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–1140. doi: 10.1093/eurheartj/ehi204 [DOI] [PubMed] [Google Scholar]
- 37. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 38. Schijman AG, Bisio M, Orellana L, Sued M, Duffy T, Jaramillo AMM, Cura C, Auter F, Veron V, Qvarnstrom Y, et al. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl Trop Dis. 2011;5:e931. doi: 10.1371/journal.pntd.0000931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ramírez JC, Parrado R, Sulleiro E, de la Barra A, Rodríguez M, Villarroel S, Irazu L, Alonso‐Vega C, Alves F, Curto MA, et al. First external quality assurance program for bloodstream real‐time PCR monitoring of treatment response in clinical trials of Chagas disease. PLoS One. 2017;12:e0188550. doi: 10.1371/journal.pone.0188550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Piron M, Fisa R, Casamitjana N, López‐Chejade P, Puig L, Vergés M, Gascón J, Gómez i Prat J, Portús M, Sauleda S. Development of a real‐time PCR assay for Trypanosoma cruzi detection in blood samples. Acta Tropica. 2007;103:195e200. doi: 10.1016/j.actatropica.2007.05.019 [DOI] [PubMed] [Google Scholar]
- 41. Duffy T, Cura CI, Ramirez JC, Abate T, Cayo NM, Parrado R, Bello ZD, Velazquez E, Muñoz‐Calderon A, Juiz NA, et al. Analytical performance of a multiplex real‐time PCR assay using TaqMan probes for quantification of Trypanosoma cruzi satellite DNA in blood samples. PLoS Negl Trop Dis. 2013;7:e2000. doi: 10.1371/journal.pntd.0002000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Salles G, Xavier S, Sousa A, Hasslocher‐Moreno A, Cardoso C. Prognostic value of QT interval parameters for mortality risk stratification in Chagas' disease: results of a long‐term follow‐up study. Circulation. 2003;108:305–312. doi: 10.1161/01.CIR.0000079174.13444.9C [DOI] [PubMed] [Google Scholar]
- 43. Rassi A Jr, Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG, Rassi GG, Hasslocher‐Moreno A, Sousa AS, Scanavacca MI. Development and validation of a risk score for predicting death in Chagas' heart disease. N Engl J Med. 2006;355:799–808. doi: 10.1056/NEJMoa053241 [DOI] [PubMed] [Google Scholar]
- 44. Bestetti RB, Dalbo CM, Freitas OC, Teno LA, Castilho OT, Oliveira JS. Noninvasive predictors of mortality for patients with Chagas' heart disease: a multivariate stepwise logistic regression study. Cardiology. 1994;84:261–267. doi: 10.1159/000176409 [DOI] [PubMed] [Google Scholar]
- 45. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 46. Acosta‐Herrera M, Strauss M, Casares‐Marfil D, Martín J; Chagas Genetics CYTED Network . Genomic medicine in Chagas disease. Acta Trop. 2019;197:105062. doi: 10.1016/j.actatropica.2019.105062 [DOI] [PubMed] [Google Scholar]
- 47. Maia MA, Sabino EC, Oliveira LC, Oliveira CDL, Cardoso CS, Maia AIN, Versiani FCPG, Silva JLPD, Ferreira AM, Ribeiro ALP, et al. Incremental prognostic value of echocardiography to brain natriuretic peptide in patients with Chagas cardiomyopathy from endemic areas. J Am Soc Echocardiogr. 2022;35:1002–1003. doi: 10.1016/j.echo.2022.05.008 [DOI] [PubMed] [Google Scholar]
- 48. Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC Jr. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40:976–982. doi: 10.1016/S0735-1097(02)02059-4 [DOI] [PubMed] [Google Scholar]
- 49. Rivera M, Taléns‐Visconti R, Salvador A, Bertomeu V, Miró V, García de Burgos F, Climent V, Cortés R, Payá R, Pérez‐Boscá JL, et al. NT‐proBNP levels and hypertension. Their importance in the diagnosis of heart failure. Rev Esp Cardiol. 2004;57:396–402. doi: 10.1016/S0300-8932(04)77124-9 [DOI] [PubMed] [Google Scholar]
- 50. Morillo CA, Marin‐Neto JA, Avezum A, Sosa‐Estani S, Rassi A Jr, Rosas F, Villena E, Quiroz R, Bonilla R, Britto C, et al. Randomized trial of Benznidazole for chronic Chagas' cardiomyopathy. N Engl J Med. 2015;373:1295–1306. doi: 10.1056/NEJMoa1507574 [DOI] [PubMed] [Google Scholar]
- 51. Hotta VT, Abduch MCD, Vieira MLC, de Andrade VA, Bocchi EA. Three and two‐dimensional cardiac mechanics by speckle tracking are predictors of outcomes in Chagas heart disease. Sci Rep. 2022;12:12237. doi: 10.1038/s41598-022-16379-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pinazo MJ, Thomas MC, Bua J, Perrone A, Schijman AG, Viotti RJ, Ramsey JM, Ribeiro I, Sosa‐Estani S, López MC, et al. Biological markers for evaluating therapeutic efficacy in Chagas disease, a systematic review. Expert Rev Anti Infect Ther. 2014;12:479–496. doi: 10.1586/14787210.2014.899150 [DOI] [PubMed] [Google Scholar]
- 53. Roemer S, Jaglan A, Santos D, Umland M, Jain R, Tajik AJ, Khandheria BK. The utility of myocardial work in clinical practice. J Am Soc Echocardiogr. 2021;34:807–818. doi: 10.1016/j.echo.2021.04.013 [DOI] [PubMed] [Google Scholar]
- 54. Melo MF, Moreira OC, Tenório P, Lorena V, Lorena‐Rezende I, Júnior WO, Gomes Y, Britto C. Usefulness of real time PCR to quantify parasite load in serum samples from chronic Chagas disease patients. Parasit Vectors. 2015;8:154. doi: 10.1186/s13071-015-0770-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–1510. doi: 10.1016/0895-4356(95)00048-8 [DOI] [PubMed] [Google Scholar]
- 56. Ogundimu EO, Altman DG, Collins GS. Adequate sample size for developing prediction models is not simply related to events per variable. J Clin Epidemiol. 2016;76:175–182. doi: 10.1016/j.jclinepi.2016.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and cox regression. Am J Epidemiol. 2007;165:710–718. doi: 10.1093/aje/kwk052 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S8


