Abstract
Background
Although previous observational studies have shown an association between anemia and cardiovascular disease (CVD), the underlying causal relationship between anemia and CVD remains uncertain.
Methods and Results
We conducted a 2‐sample bidirectional Mendelian randomization (MR) study to assess the causal association between anemia and CVD. We extracted summary statistics data for anemia, heart failure (HF), coronary artery disease (CAD), atrial fibrillation, any stroke, and any ischemic stroke (AIS) from relevant published genome‐wide association studies. After rigorous quality control steps, independent single‐nucleotide polymorphisms for each disease were selected as instrumental variables. Inverse‐variance weighting was used as the primary method to estimate the causal association between anemia and CVD in the 2‐sample MR analysis. Simultaneously, we performed a series of multiple methods analyses (median weighting, maximum likelihood [MR robust adjusted profile score]), sensitivity analyses (Cochran's Q test and MR‐Egger intercept, leave‐one‐out test [MR pleiotropy residual sum and outlier]), instrumental variable strength evaluations (F statistic), and statistic power estimates to verify the robustness and reliability of our results. Furthermore, the associations between anemia and CVD from different studies, including the UK Biobank and FinnGen studies, were combined by meta‐analysis. The MR analysis showed that genetically predicted anemia was significantly associated with HF risk at the Bonferroni‐corrected significance level (odds ratio [OR], 1.11 [95% CI, 1.04–1.18]; P=0.002) and was suggestively associated with CAD risk (OR, 1.11 [95% CI, 1.02–1.22]; P=0.020). However, the associations between anemia and atrial fibrillation, any stroke, or AIS were not statistically significant. In the reverse MR analysis, we found that genetic susceptibility to HF, CAD, and AIS was significantly associated with anemia risk. The ORs of HF, CAD, and AIS were 1.64 (95% CI, 1.39–1.94; P=7.60E‐09), 1.16 (95% CI, 1.08–1.24; P=2.32E‐05), and 1.30 (95% CI, 1.11–1.52; P=0.001), respectively. Genetically predicted atrial fibrillation was suggestively associated with anemia (OR, 1.06 [95% CI, 1.01–1.12]; P=0.015). Sensitivity analyses found weak evidence of horizontal pleiotropy and heterogeneity, which ensured the robustness and reliability of the results. Meta‐analysis also showed the statistically significant association between anemia and HF risk.
Conclusions
Our study supports bidirectional causality between anemia and HF and significant associations between genetic predisposition to CAD and AIS with anemia, which contributes to the clinical management of both diseases.
Keywords: cardiovascular disease, anemia, Mendelian randomization, heart failure, coronary artery disease, atrial fibrillation, stroke, any ischemic stroke
Subject Categories: Heart Failure, Cardiovascular Disease, Diet and Nutrition
Nonstandard Abbreviations and Acronyms
- AIS
any ischemic stroke
- ARIC
Atherosclerosis Risk in Communities
- AS
any stroke
- IV
instrumental variable
- IVW
inverse‐variance weighting
- MR
Mendelian randomization
- PRESSO
pleiotropy residual sum and outlier
Clinical Perspective.
What Is New?
Although previous observational studies have shown an association between anemia and cardiovascular diseases, the underlying causal relationship between anemia and cardiovascular diseases remains uncertain.
We conducted a 2‐sample bidirectional Mendelian randomization study to assess the causal association between anemia and cardiovascular diseases.
What Are the Clinical Implications?
These findings support detection and treatment of anemia for preventing heart failure.
Cardiovascular diseases (CVDs) remains the leading cause of disease burden in the world, 1 with an 18.7% increase in deaths attributable to CVD from 2010 to 2020, making it the number 1 cause of death. 2 In addition to the well‐recognized common CVD risk factors (hypertension, hyperlipidaemia, obesity, diabetes, smoking, and alcohol consumption), accumulating observational studies have suggested that anemia is associated with an increased risk of CVD.
Anemia, defined by the World Health Organization as a hemoglobin concentration of <13 g/dL in men and <12 g/dL in women, affects nearly one‐third of the global population and is a clear public health burden and a public health issue. 3 Anemia is common among patients with CVD and is estimated to be prevalent in ≈10% to 43% of patients with acute coronary syndrome and 30% to 70% of patients with heart failure (HF). 4 The results of a prospective cohort study of 14 410 subjects without CVD at baseline showed that patients with anemia had a 41% increased risk of CVD compared with those without anemia during a mean follow‐up of 6.1 years. 5 Several observational studies have suggested that anemia is related to an increased risk of adverse prognostic events in patients with HF, coronary artery disease (CAD), atrial fibrillation (AF), and stroke. 6 , 7 , 8 , 9 However, one study showed that the relationship between anemia and CAD was not statistically significant after adjusting for traditional CAD factors and covariates. 10 Notably, these observational studies may be affected by potential confounding factors (eg, diabetes, obesity, and hyperlipidemia), which makes it difficult to ascertain the causality between anemia and CVD.
Under assumptions, Mendelian randomization (MR) can estimate the causal relationship between an exposure and outcome by using genetic variants as instrumental variables (IVs), which are strongly associated with the exposure and strengthen the causal inference by controlling for nonheritable environmental confounders and reverse causation. 11 Summary statistics data obtained from genome‐wide association studies (GWASs) provide reliable IVs for MR analyses. Previous MR analyses showed positive associations of hemoglobin concentration with the risk of CAD and venous thromboembolism, 12 which may be related to vasoconstriction attributable to scavenging of NO by hemoglobin and promotion of platelet adhesion or activation. 13 To date, there has been no genetic study on the relationship between anemia and CVD.
In the present study, we performed a 2‐sample bidirectional MR study to explore the causal relationship between anemia and major CVD as well as the causal effect of CVD on anemia.
METHODS
Study Design
All data and materials used in this MR study are publicly available, and specific sources can be found in the article. An overview of the bidirectional MR study design is shown in Figure 1; we conducted the bidirectional MR study based on the 3 assumptions. 14 Informed consent and ethical approval were obtained for the original GWASs. This MR study was performed following the latest STROBE‐MR (Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomisation) guideline. 15
Figure 1. Assumptions and study design flowchart of the MR study.
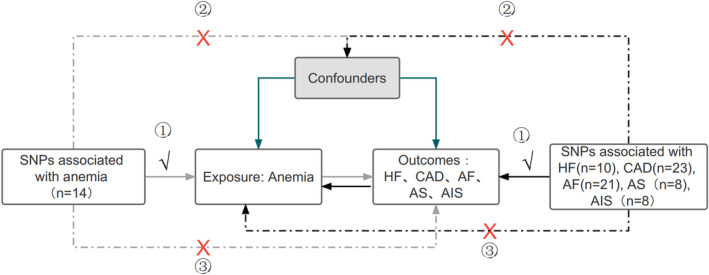
The MR method is based on 3 hypotheses: (1) instrumental variables directly affect the exposure; (2) instrumental variables are independent of any confounding factor; and (3) instrumental variables affect the results only via exposure but not through other pathways. Gray lines show the relationship across instrumental variables, exposure, and outcomes in the MR study examining the effects of anemia on cardiovascular diseases, and black lines show these relationships in the reverse MR study. AF indicates atrial fibrillation; AIS, any ischemic stroke; AS, any stroke; CAD, coronary artery disease; HF, heart failure; MR, Mendelian randomization; and SNP, single‐nucleotide polymorphism.
Data Sources and Single‐Nucleotide Polymorphism Selection for Anemia
Single‐nucleotide polymorphisms (SNPs) were used as our IVs to conduct the 2‐sample bidirectional MR study. 16 Summary‐level data for anemia were obtained from FinnGen (FREEZE 7; https://r7.finngen.fi/), including 19 885 cases and 76 464 controls, with samples mainly from European individuals. Anemia was defined according to the International Classification of Diseases, Tenth Revision (ICD‐10), codes (D50–D53, D55–D59, and D60–D64).
A series of screening procedures were conducted to select eligible IVs. First of all, because few SNPs identified by GWASs for anemia reached the genome‐wide significance levels of P<5×10−8, SNPs with suggestive genome‐wide significance (P<5×10−6) were selected as the IVs for anemia. Second, the clumping process (r 2<0.001 and clump window=10 000‐kb) was conducted to select independent SNPs without linkage disequilibrium as the IVs (Table S1). Third, we retrieved the secondary phenotype of each SNP in the PhenoScanner database with a threshold of P<1×10−5 and removed SNPs associated with outcome confounders (Table S2) to avoid potential pleiotropic effects. 17 Fourth, SNPs with palindromic alleles and SNPs that were not available in the outcome GWASs were eliminated. Finally, we evaluated variance (R 2) and the F statistic to detect whether the selected IVs were weakly correlated with the exposure. 18 , 19 R 2 indicates variability explained by genetic instruments. The R 2 was calculated using the formula 20 : R 2=β2(1−EAF)×2EAF. EAF is the frequency of mutated genes. F statistics were calculated using the formula 19 : F=R 2(N−K−1)/[K(1−R2)]. K is the number of SNP‐exposure association, and N is the sample size of the GWAS for the SNP‐exposure association. SNPs with F statistics >10 were defined as reliable and valid IVs, which could prevent the MR results from being influenced by weak instrument bias.
Data Sources and SNP Selection for CVDs
Summary statistical data for 5 CVD outcomes were extracted from large genetic consortia. 21 , 22 , 23 , 24 Notably, we selected independent data sets for 2‐sample MR analysis to avoid bias caused by overlapping samples. A description of the demographics and GWASs included in this study is shown in Table 1 and Table S1. Summary statistical data for HF (47 309 cases and 930 014 controls) were obtained from the Heart Failure Molecular Epidemiology for Therapeutic Targets Consortium. 21 Summary statistical data for CAD were extracted from a published GWAS meta‐analysis of 48 studies, including 60 801 cases and 123 504 controls. 22 A summary data set for AF was retrieved from the Atrial Fibrillation Genetics Consortium, including 60 620 cases and 970 216 controls. 23 Summary‐level data sets for any stroke (AS; 40 585 cases and 406 111 controls) and any ischemic stroke (AIS; 34 217 cases and 406 111 controls) were derived from the MEGASTROKE Consortium. 24 Ten SNPs associated with HF, 23 SNPs associated with CAD, 21 SNPs associated with AF, 8 SNPs associated with AS, and 8 SNPs associated with AIS reached the genome‐wide significance level (P<5×10−8) and were selected as IVs for the corresponding exposure factors in the reverse‐direction MR analysis.
Table 1.
Details of Data Sources Included in the Study
| Diseases | Data source | Population | Sample size (cases/controls) | Covariates adjusted in GWAS |
|---|---|---|---|---|
| Anemia | FinnGen | European | 19 885/76464 | Age, sex, and genotyping batch |
| Heart failure | HERMES | European | 47 309/930014 | Age, sex, and principal components |
| Atrial fibrillation | AFGen | European | 60 620/970216 | Birth year, sex, and genotype batch |
| Coronary artery disease | CARDIoGRAMplusC4D | Mixed | 60 801/123504 | Sex, age, and generation |
| Any stroke | MEGASTROKE | European | 40 585/406111 | Age, sex |
| Any ischemic stroke | MEGASTROKE | European | 34 217/406111 | Age, sex |
AFGen indicates Atrial Fibrillation Genetics; CARDIoGRAMplusC4D, Coronary Artery Disease Genome‐Wide Replication and Meta‐Analysis Plus the Coronary Artery Disease Genetics; FinnGen, FinnGen consortium; GWAS, genome‐wide association study; HERMES, Heart Failure Molecular Epidemiology for Therapeutic Targets; and MEGASTROKE, MEGASTROKE consortium.
Statistical Analysis
In the MR analysis, we used inverse‐variance weighting (IVW) as the primary analysis method, which calculated and combined the Wald ratio of each SNP through meta‐analysis to estimate the overall effect of the exposure on the outcome. 25 Simultaneously, we conducted the following multiple analysis methods to assess the robustness and reliability of the results: (1) the median weighting method, which could provide robust estimates and draw a reliable conclusion when more than half of the SNPs were valid IVs 26 ; (2) the MR‐Egger method, which could identify horizontal pleiotropy and correct the multiple effects, although it consumes statistical power 27 ; (3) the maximum‐likelihood method, which could combine data on multiple genetic variants into a single causal estimate, which has better finite‐sample type 1 error rates and is complementary to the MR‐Egger regression method 28 ; (4) the MR robust adjusted profile score, which could correct horizontal pleiotropy and reduce the bias caused by pleiotropy effects 29 ; and (5) the MR pleiotropy residual sum and outlier (MR‐PRESSO) method, which could detect horizontal pleiotropy and correct horizontal pleiotropy via outlier removal. 30 Collectively, we used comprehensive methods to investigate the causal relationship between anemia and CVD to ensure the robustness and reliability of the results.
Sensitivity Analyses
We conducted sensitivity analyses using a variety of methods. First, the Cochran Q test was performed to assess the heterogeneity between the SNP‐specific estimates. If the heterogeneity was not statistically significant in the results (P>0.05), the fixed‐effects model of IVW was used for MR analysis; otherwise, the random‐effects model was used. 31 Second, the MR‐Egger intercept method was conducted to assess the horizontal pleiotropy of SNPs. 27 Third, the leave‐one‐out sensitivity analysis was applied to detect whether the results were influenced by any single SNP. Fourth, we plotted funnel plots to visually judge the pleiotropy of SNPs and scatterplots to show associations between the exposure and outcome. Finally, we extracted genetic variants associated with anemia from the UK Biobank study and performed 2‐sample MR analysis. The associations between anemia and CVDs from different databases, including the UK Biobank and FinnGen studies, were combined by meta‐analysis.
The Bonferroni correction method was used to identify false‐positive results caused by multiple tests. Associations with P<0.005 (0.05 divided by 10) were considered statistically significant, whereas associations with P>0.005 and P<0.05 were defined as suggestive associations. All statistical analyses were conducted using the “TwoSampleMR,” “mr.raps,” and “MR‐PRESSO” packages in R software, version 4.2.0.
RESULTS
Characteristics of the Selected SNPs
We derived IVs that were significantly associated with anemia from the GWAS and removed some SNPs (rs2476601, rs707926, rs3129761, and rs7184768) after searching the PhenoScanner database, because they were associated with potential confounders, including “diabetes type 1,” “systolic blood pressure,” “self‐reported type 1 diabetes,” and “whole body fat‐free mass” (Table S2). In addition, we removed palindromic SNPs (ie, A/T or G/C) and SNPs that were not available in the outcome. Finally, the screened SNPs with F‐statistics all >10 (Table S1), which showed no weak tool bias, were selected as the IVs for the MR analyses (Tables S3–S8).
Causal Effects of Anemia on CVD
The results of MR analyses are presented in Figure 2 and Figures S1 through S3. The results showed that genetically predicted anemia was significantly associated with an increased risk of HF (odds ratio [OR], 1.11 [95% CI, 1.04–1.18]; P=0.002) in the fixed‐effects IVW model. The result was consistent in the maximum likelihood, weighted median, MR‐PRESSO, and robust adjusted profile score analyses, although it was nonsignificant in the MR‐Egger analysis. MR‐PRESSO analysis detected no outliers, suggesting that the results were robust and reliable (Table S9). No heterogeneity among estimates of individual SNPs was detected in the Cochran Q test (Q=12.8; P=0.236), or no pleiotropy was observed in the MR‐Egger regression analysis (intercept P=0.444; Table 2). Moreover, leave‐one‐out analysis suggested that the causal effect of anemia on HF was not driven by any single SNP (Figure S1).
Figure 2. Associations of genetically predicted anemia with risk of cardiovascular disease.

AF indicates atrial fibrillation; AIS, any ischemic stroke; AS, any stroke; CAD, coronary artery disease; HF, heart failure; IVW, inverse‐variance weighting; IVW(fixed), fixed‐effects IVW; MR, Mendelian randomization; OR, odds ratio; PRESSO, pleiotropy residual sum and outlier; RAPS, robust adjusted profile score; and SNP, single‐nucleotide polymorphism.
Table 2.
Associations of Genetically Predicted Anemia With Risk of CVD in Sensitivity Analyses in FinnGen Study
| CVD | Heterogeneity test MR‐Egger | Heterogeneity test IVW | Pleiotropy test | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Q | Q df | Q P value | Q | Q df | Q P value | Egger intercept | SE | P value | |
| Heart failure | 12.8 | 10 | 0.236 | 13.6 | 11 | 0.256 | 0.006 | 0.008 | 0.444 |
| Coronary artery disease | 3.4 | 10 | 0.969 | 6.0 | 11 | 0.875 | 0.014 | 0.009 | 0.142 |
| Atrial fibrillation | 12.7 | 11 | 0.312 | 12.8 | 12 | 0.383 | 0.003 | 0.012 | 0.788 |
| Any stroke | 11.7 | 12 | 0.474 | 19.5 | 13 | 0.109 | 0.022 | 0.008 | 0.016 |
| Any ischemic stroke | 11.3 | 12 | 0.506 | 18.0 | 13 | 0.158 | 0.022 | 0.009 | 0.024 |
CVD indicates cardiovascular disease; IVW, inverse‐variance weighting; MR, Mendelian randomization; Q, heterogeneity statistic Q; and Q df, Q degree of freedom.
Anemia was suggestively associated with CAD (OR, 1.11 [95% CI, 1.02–1.22]; P=0.020) in the fixed‐effects IVW model. The heterogeneity (P value of Cochran Q>0.05), pleiotropy (P value for intercept >0.05), or outliers in the CAD analysis were not statistically significant (Table 2 and Table S9). Leave‐one‐out analysis showed that 1 outlier SNP was detected in the analysis of CAD (Figure S1).
There was no evidence of an association between genetic susceptibility to anemia and AF, AS, or AIS in the fixed‐effects IVW analysis. However, robust adjusted profile score analysis suggested that anemia was suggestively associated with AF (OR, 1.14 [95% CI, 1.01–1.30]; P=0.040). The pleiotropy or heterogeneity was not statistically significant in the AF analysis (Table 2 and Table S9). Further studies are required to confirm the association between anemia and AF.
Causal Effects of CVD on Anemia
After removing palindromic and unavailable SNPs, we selected 10 SNPs for HF, 23 SNPs for CAD, 21 SNPs for AF, 8 SNPs for AS, and 8 SNPs for AIS as the IVs in the analysis of the causal effects of CVDs on anemia (Tables S10–S15). The F‐statistics of the screened SNPs were all >10 to avoid bias caused by weak IVs. The statistical results of reverse‐direction MR are presented in Figure 3 and Figures S4 through S6. In the reverse‐direction MR analyses, we found that genetic susceptibility to HF, CAD, and AIS was significantly associated with anemia. The ORs of HF, CAD, and AIS were 1.64 (95% CI, 1.39–1.94; P=7.60E‐09), 1.16 (95% CI, 1.08–1.24; P=2.32E‐05), and 1.31 (95% CI, 1.14–1.50; P=1.36E‐04), respectively, in the fixed‐effects IVW model. We observed that genetic susceptibility to AF was suggestively associated with anemia (OR, 1.06 [95% CI, 1.01–1.12]; P=0.015). The MR‐PRESSO analysis detected no outliers in HF, CAD, or AF, and detected 1 and 2 outliers in AS and AIS, respectively (Table S16). The OR of AIS was 1.30 (95% CI, 1.11–1.52; P=0.001) in the fixed‐effects IVW model after correction for 2 outliers in the MR‐PRESSO analysis. Although genetic susceptibility to AS was suggestively associated with anemia in the fixed‐effects IVW analysis (OR, 1.22 [95% CI, 1.05–1.42]; P=0.009), no association was observed after the removal of an outlier (OR, 1.13 [95% CI, 0.91–1.42]; P=0.275) in the random‐effect analysis (Table S16). The heterogeneity among the estimates of individual SNPs was not statistically significant in the Cochran Q test, and the pleiotropy was not statistically significant in the MR‐Egger regression analysis for 5 CVDs after correction for outliers in the MR‐PRESSO analysis (Table 3 and Table S16). Moreover, leave‐one‐out analysis suggested that the causal relationships between HF, CAD, or AS with anemia were not driven by any single SNP, but 3 outlier SNPs were detected in the AIS analysis (Figure S4). In conclusion, the inverse MR analysis showed that higher genetic predictors of HF, CAD, and AIS were significantly and positively associated with increased risk of anemia after sensitivity and pleiotropy analyses at the Bonferroni‐corrected significance level (P<0.005).
Figure 3. Associations of genetically predicted cardiovascular disease with risk of anemia.
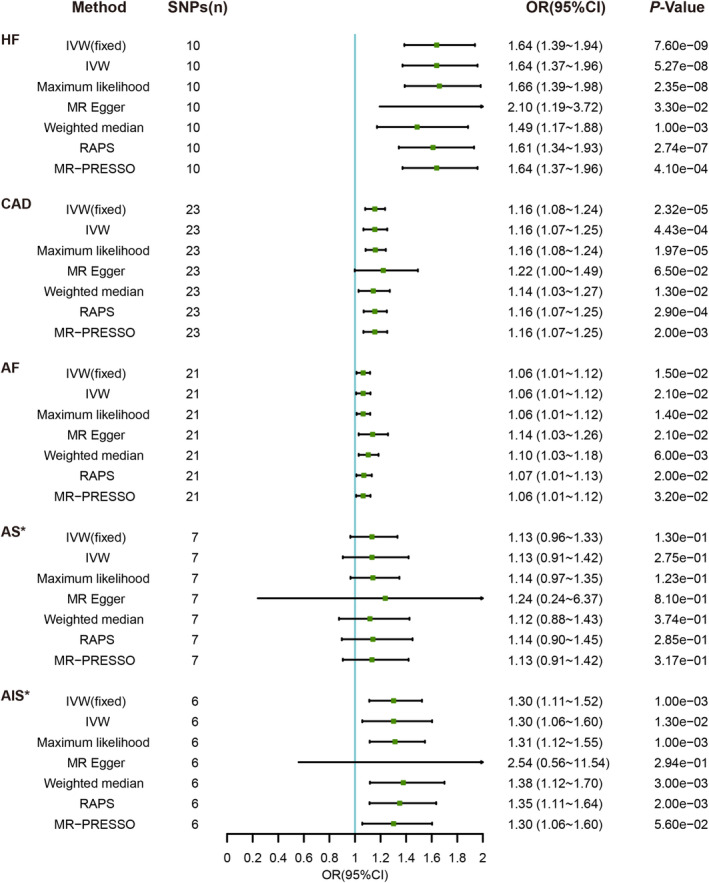
AF indicates atrial fibrillation; AIS*, any ischemic stroke, “rs2634074” and “rs4942561” were removed as outliers in the MR‐PRESSO analysis; AS*, any stroke, “rs10774624” was removed as an outlier in the MR‐PRESSO analysis; CAD, coronary artery disease; HF, heart failure; IVW, inverse‐variance weighting; IVW(fixed), fixed‐effects IVW; MR, Mendelian randomization; OR, odds ratio; PRESSO, pleiotropy residual sum and outlier; RAPS, robust adjusted profile score; and SNP, single‐nucleotide polymorphism.
Table 3.
Associations of Genetically Predicted CVD With Risk of Anemia in Sensitivity Analyses in FinnGen Study
| CVD | Heterogeneity test MR‐Egger | Heterogeneity test IVW | Pleiotropy test | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Q | Q df | Q P value | Q | Q df | Q P value | Egger intercept | SE | P value | |
| Heart failure | 9.2 | 8 | 0.327 | 10.1 | 9 | 0.339 | −0.017 | 0.019 | 0.389 |
| Coronary artery disease | 31.4 | 21 | 0.067 | 32.0 | 22 | 0.079 | −0.006 | 0.010 | 0.561 |
| Atrial fibrillation | 19.9 | 19 | 0.403 | 22.3 | 20 | 0.324 | −0.010 | 0.007 | 0.143 |
| Any stroke | 18.0 | 6 | 0.006 | 18.0 | 7 | 0.012 | 0.011 | 0.065 | 0.875 |
| Any stroke* | 11.5 | 5 | 0.042 | 11.6 | 6 | 0.072 | −0.006 | 0.058 | 0.920 |
| Any ischemic stroke | 23.5 | 6 | 0.001 | 24.0 | 7 | 0.001 | −0.028 | 0.086 | 0.757 |
| Any ischemic stroke† | 7.3 | 4 | 0.119 | 8.7 | 5 | 0.120 | −0.052 | 0.059 | 0.431 |
CVD indicates cardiovascular disease; IVW, inverse‐variance weighting; MR, Mendelian randomization; Q, heterogeneity statistic Q; and Q df, Q degree of freedom.
Any stroke, “rs10774624” was removed as an outlier in the MR pleiotropy residual sum and outlier analysis.
Any ischemic stroke, “rs2634074” and “rs4942561” were removed as outliers in the MR pleiotropy residual sum and outlier analysis.
In sensitivity analyses, we conducted the MR‐Steiger test in 2‐sample bidirectional MR analysis in FinnGen study, and the results showed that the directions of causal association between anemia and HF and between HF and anemia were statistically significant (Table S17). Furthermore, we also extracted IVs associated with anemia from the UK Biobank study (Table S18). MR analysis showed that genetically predicted anemia was not significantly associated with CVD, whereas genetically predicted HF, AS, and AIS were significantly associated with anemia (Tables S19–S22). We conducted a meta‐analysis to synthesize results on the association between anemia and CVD from different data sources, including the FinnGen and UK Biobank studies, and the analysis showed a significant association between genetically predicted anemia and HF and between genetically predicted AS and anemia (Tables S23 and S24).
DISCUSSION
We used a bidirectional 2‐sample MR study to investigate the causal relationships between anemia and a broad range of CVDs for the first time. The results showed that a genetic predisposition to anemia was significantly associated with HF risk and was suggestively associated with CAD risk. However, there was no evidence of an association between anemia and AF, AS, or AIS. The reverse‐direction MR analysis revealed significant positive associations of higher genetically predicted HF, CAD, and AIS with increased risk of anemia. Furthermore, genetic susceptibility to AF was suggestively associated with anemia risk. There is no MR evidence to support a potential causal effect of AS on anemia risk. Meta‐analyses from different databases, including the FinnGen and UK Biobank studies, showed some differences but generally similar results, which may be related to differences in ethnic background. In general, there are genetic associations between anemia and CVD, and further exploration is needed to explore the underlying mechanisms.
The adverse effect of anemia on the development of HF has been consistently confirmed in observational studies. 32 , 33 , 34 A large cohort study of community‐dwelling patients with congestive heart failure proposed that anemia was a common and independent prognostic factor for mortality. 6 Patients with severe anemia tend to be characterized by the exacerbation of HF, including more extensive left ventricular remodeling, higher levels of inflammatory markers, and worse renal function. 35 This may be related to the mechanism by which long‐term chronic anemia can increase preload and decrease afterload, resulting in increased cardiac output, which can lead to undesirable left ventricular hypertrophy and left heart enlargement. 36 The MR analysis results verify a significant positive association of genetically predicted anemia with HF, which indicates that further studies are required to explore the biological mechanism of anemia on HF.
In patients with CAD, the relationships between anemia and adverse outcomes are less consistent, with some but not all studies showing an adverse effect of anemia on CAD outcomes. 37 , 38 , 39 The ARIC (Atherosclerosis Risk in Communities) study revealed that participants with anemia had a risk of CVD that was 41% higher than that of people without anemia. 5 A comprehensive analysis of 41 637 patients with acute coronary syndrome from 16 clinical trials found that cardiovascular mortality increased by 21% for every 1‐g/dL decrease in hemoglobin below 14 g/dL over 30 days. 7 However, there was no significant association between anemia and CAD mortality after adjusting for traditional CVD risk factors and other covariates in the NHANES (National Health and Nutrition Examination Survey) II Mortality Study. 10 In our MR study, the results showed a suggestive association between anemia and CAD. Mechanistically, anemia may also exacerbate cardiac ischemia and contribute to the progression of CAD because of a decreased oxygen supply or increased demand. However, the effect and mechanism of anemia on CAD need to be further verified.
At present, a few observational studies have suggested that anemia is an independent risk factor for new‐onset AF. 8 Anemia has been detected in 13% to 34% of patients with nonvalvular AF. 40 , 41 The treatment of patients with anemia with oral anticoagulants may increase the risk of bleeding because some patients may have hidden bleeding sites. 41 , 42 A retrospective study assessing the association of iron deficiency anemia with clinical outcomes in hospitalized patients with AF found that admission attributable to iron deficiency anemia was associated with a higher incidence of acute myocardial infarction but not with all‐cause mortality. 43 Our MR analysis showed no association between anemia and AF. The conclusion that anemia is associated with the risk of AF in observational studies may be influenced by confounders, such as age and concomitant diseases (eg, CAD and HF) because, similar to AF, the incidence of anemia increases with increasing age. 3
Several observations and systematic analyses suggested an equivocal relationship between anemia and stroke. A systematic retrospective study showed that increased anemia severity was associated with increased in‐hospital mortality in patients with AIS. 44 Another retrospective study found that high hemoglobin, not low hemoglobin, was related to an increased risk of recurrent stroke and composite vascular events. 45 This MR analysis provided no evidence that genetically predicted anemia was related to AS or AIS.
Notably, people may pay more attention to the cardiovascular harm caused by anemia but ignore that CVDs can also cause or aggravate anemia in clinical practice, which further promotes the occurrence of adverse cardiovascular outcomes. Therefore, we performed reverse MR analysis, and the results showed that higher genetically predicted HF, CAD, and AIS risks were significantly positively associated with anemia risk, and higher genetically predicted AF risk was suggestively associated with anemia risk. HF may increase the risk of anemia through several underlying pathophysiological mechanisms, including hemodilution, renal insufficiency, inflammatory activation, and depressed bone marrow function. 36 Patients with CAD and AIS may take antiplatelet drugs for a long time, which may lead to a decrease in the absorption capacity of iron in the stomach and increase the risk of anemia. Appropriate anticoagulant therapy reduces the risk of stroke attributable to AF by ≈70%, 46 and gastrointestinal tract bleeding from anticoagulants may be a possible mechanism of iron deficiency and anemia in patients with AF. Further studies are required to explore the mechanism by which CVDs promote anemia. Collectively, attention should be given to the more severe adverse cardiovascular outcomes associated with anemia attributable to CVDs.
In brief, this bidirectional MR analysis not only investigated the causal relationship between anemia and CVD but also analyzed the causal effect of CVD on anemia in reverse. To better understand and manage both diseases in clinical practice, further studies are needed to explore and elucidate the biological mechanism between anemia and CVD. Considering the poor prognosis associated with the causal relationship, it is recommended to further clarify the cause of patients with anemia in time and give intervention to prevent the occurrence of CVDs. At the same time, it is also necessary to strengthen the detection and prevention of anemia in patients with CVDs.
Our study has several strengths and limitations. First, this is the first study to investigate the bidirectional causal relationship between anemia and CVD through a 2‐sample MR analysis that is not affected by confounders and reverse causation compared with observational studies. In addition, multiple sensitivity analyses, and IV strength evaluations, were conducted to ensure the robustness and effectiveness of the results. There are also some limitations. The MR analysis is based on specific assumptions that cannot be assessed. The present study was mainly conducted on individuals of European ancestry, which limits the generality of our results to other populations. Although we explored the association between anemia and CVD from a genetic perspective, the underlying mechanisms are not clear and require further investigation.
CONCLUSIONS
In conclusion, we applied a bidirectional 2‐sample MR study for the first time to investigate the causality between anemia and CVD. The present MR study supports that there is bidirectional causality between anemia and HF and demonstrates that CAD and AIS are potential risk factors for anemia. However, further original studies are still required to profoundly elucidate the exact association between anemia and CVD and the mechanism of such an association. Considering the poor prognosis associated with causality, it is recommended to strengthen the prevention and treatment of anemia in CVD.
Sources of Funding
This work was supported by grants from the National Natural Science Foundation of China (82070400).
Disclosures
None.
Supporting information
Table S1–S24
Figures S1–S6
Acknowledgments
The authors thank the Heart Failure Molecular Epidemiology for Therapeutic Targets Consortium, Coronary Artery Disease Genome‐Wide Replication and Meta‐Analysis Plus The Coronary Artery Disease Genetics, Atrial Fibrillation Genetics Consortium, MEGASTROKE Consortium, FinnGen Consortium, and the UK Biobank study.
This article was sent to Julie K. Freed, MD, PhD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 9.
Contributor Information
Yan Wang, Email: tomato82@126.com.
Zhaohui Wang, Email: wuxiaohongtian@163.com.
References
- 1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore‐Mensah Y, et al. Heart disease and stroke statistics‐2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/cir.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 3. Safiri S, Kolahi AA, Noori M, Nejadghaderi SA, Karamzad N, Bragazzi NL, Sullman MJM, Abdollahi M, Collins GS, Kaufman JS, et al. Burden of anemia and its underlying causes in 204 countries and territories, 1990–2019: results from the global burden of disease study 2019. J Hematol Oncol. 2021;14:185. doi: 10.1186/s13045-021-01202-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tang YD, Katz SD. Anemia in chronic heart failure: prevalence, etiology, clinical correlates, and treatment options. Circulation. 2006;113:2454–2461. doi: 10.1161/circulationaha.105.583666 [DOI] [PubMed] [Google Scholar]
- 5. Sarnak MJ, Tighiouart H, Manjunath G, MacLeod B, Griffith J, Salem D, Levey AS. Anemia as a risk factor for cardiovascular disease in the atherosclerosis risk in communities (ARIC) study. J Am Coll Cardiol. 2002;40:27–33. doi: 10.1016/s0735-1097(02)01938-1 [DOI] [PubMed] [Google Scholar]
- 6. Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new‐onset heart failure. Circulation. 2003;107:223–225. doi: 10.1161/01.cir.0000052622.51963.fc [DOI] [PubMed] [Google Scholar]
- 7. Sabatine MS, Morrow DA, Giugliano RP, Burton PB, Murphy SA, McCabe CH, Gibson CM, Braunwald E. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042–2049. doi: 10.1161/01.Cir.0000162477.70955.5f [DOI] [PubMed] [Google Scholar]
- 8. Xu D, Murakoshi N, Sairenchi T, Irie F, Igarashi M, Nogami A, Tomizawa T, Yamaguchi I, Yamagishi K, Iso H, et al. Anemia and reduced kidney function as risk factors for new onset of atrial fibrillation (from the Ibaraki prefectural health study). Am J Cardiol. 2015;115:328–333. doi: 10.1016/j.amjcard.2014.10.041 [DOI] [PubMed] [Google Scholar]
- 9. Chang JY, Lee JS, Kim BJ, Kim JT, Lee J, Cha JK, Kim DH, Cho YJ, Hong KS, Lee SJ, et al. Influence of hemoglobin concentration on stroke recurrence and composite vascular events. Stroke. 2020;51:1309–1312. doi: 10.1161/strokeaha.119.028058 [DOI] [PubMed] [Google Scholar]
- 10. Brown DW, Giles WH, Croft JB. Hematocrit and the risk of coronary heart disease mortality. Am Heart J. 2001;142:657–663. doi: 10.1067/mhj.2001.118467 [DOI] [PubMed] [Google Scholar]
- 11. Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070 [DOI] [PubMed] [Google Scholar]
- 12. Wang K, Shi X, Zhu Z, Hao X, Chen L, Cheng S, Foo RSY, Wang C. Mendelian randomization analysis of 37 clinical factors and coronary artery disease in east Asian and European populations. Genome Med. 2022;14:63. doi: 10.1186/s13073-022-01067-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet (London, England). 1987;2:1057–1058. doi: 10.1016/s0140-6736(87)91481-4 [DOI] [PubMed] [Google Scholar]
- 14. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. 2017;318:1925–1926. doi: 10.1001/jama.2017.17219 [DOI] [PubMed] [Google Scholar]
- 15. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, VanderWeele TJ, Higgins JPT, Timpson NJ, Dimou N, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE‐MR statement. JAMA. 2021;326:1614–1621. doi: 10.1001/jama.2021.18236 [DOI] [PubMed] [Google Scholar]
- 16. Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. 2016;27:3253–3265. doi: 10.1681/asn.2016010098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, Butterworth AS, Staley JR. PhenoScanner V2: an expanded tool for searching human genotype‐phenotype associations. Bioinformatics. 2019;35:4851–4853. doi: 10.1093/bioinformatics/btz469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two‐sample Mendelian randomization analyses using MR‐egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45:1961–1974. doi: 10.1093/ije/dyw220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40:755–764. doi: 10.1093/ije/dyr036 [DOI] [PubMed] [Google Scholar]
- 20. Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shah S, Henry A, Roselli C, Lin H, Sveinbjörnsson G, Fatemifar G, Hedman ÅK, Wilk JB, Morley MP, Chaffin MD, et al. Genome‐wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun. 2020;11:163. doi: 10.1038/s41467-019-13690-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, Saleheen D, Kyriakou T, Nelson CP, Hopewell JC, et al. A comprehensive 1,000 genomes‐based genome‐wide association meta‐analysis of coronary artery disease. Nat Genet. 2015;47:1121–1130. doi: 10.1038/ng.3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, Herron TJ, McCarthy S, Schmidt EM, Sveinbjornsson G, et al. Biobank‐driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. 2018;50:1234–1239. doi: 10.1038/s41588-018-0171-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, Rutten‐Jacobs L, Giese AK, van der Laan SW, Gretarsdottir S, et al. Multiancestry genome‐wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. 2018;50:524–537. doi: 10.1038/s41588-018-0058-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133–1163. doi: 10.1002/sim.3034 [DOI] [PubMed] [Google Scholar]
- 26. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–314. doi: 10.1002/gepi.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. 2015;44:512–525. doi: 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ‐TREE: a fast and effective stochastic algorithm for estimating maximum‐likelihood phylogenies. Mol Biol Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qingyuan Z, Jingshu W, Gibran H, Jack B, Dylan SS. Statistical inference in two‐sample summary‐data Mendelian randomization using robust adjusted profile score. Ann Stat. 2020;48:1742–1769. doi: 10.1214/19-AOS1866 [DOI] [Google Scholar]
- 30. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–698. doi: 10.1038/s41588-018-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two‐sample summary data Mendelian randomization. Stat Med. 2017;36:1783–1802. doi: 10.1002/sim.7221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Borenstein J. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol. 2002;39:1780–1786. doi: 10.1016/s0735-1097(02)01854-5 [DOI] [PubMed] [Google Scholar]
- 33. Mozaffarian D, Nye R, Levy WC. Anemia predicts mortality in severe heart failure: the prospective randomized amlodipine survival evaluation (PRAISE). J Am Coll Cardiol. 2003;41:1933–1939. doi: 10.1016/s0735-1097(03)00425-x [DOI] [PubMed] [Google Scholar]
- 34. Al‐Ahmad A, Rand WM, Manjunath G, Konstam MA, Salem DN, Levey AS, Sarnak MJ. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001;38:955–962. doi: 10.1016/s0735-1097(01)01470-x [DOI] [PubMed] [Google Scholar]
- 35. Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation. 2018;138:80–98. doi: 10.1161/circulationaha.118.030099 [DOI] [PubMed] [Google Scholar]
- 36. Anand IS. Anemia and chronic heart failure implications and treatment options. J Am Coll Cardiol. 2008;52:501–511. doi: 10.1016/j.jacc.2008.04.044 [DOI] [PubMed] [Google Scholar]
- 37. Lee PC, Kini AS, Ahsan C, Fisher E, Sharma SK. Anemia is an independent predictor of mortality after percutaneous coronary intervention. J Am Coll Cardiol. 2004;44:541–546. doi: 10.1016/j.jacc.2004.04.047 [DOI] [PubMed] [Google Scholar]
- 38. Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230–1236. doi: 10.1056/NEJMoa010615 [DOI] [PubMed] [Google Scholar]
- 39. Jurkovitz CT, Abramson JL, Vaccarino LV, Weintraub WS, McClellan WM. Association of high serum creatinine and anemia increases the risk of coronary events: results from the prospective community‐based atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2003;14:2919–2925. doi: 10.1097/01.asn.0000092138.65211.71 [DOI] [PubMed] [Google Scholar]
- 40. Bonde AN, Blanche P, Staerk L, Gerds TA, Gundlund A, Gislason G, Torp‐Pedersen C, Lip GYH, Hlatky MA, Olesen JB. Oral anticoagulation among atrial fibrillation patients with anaemia: an observational cohort study. Eur Heart J. 2019;40:3782–3790. doi: 10.1093/eurheartj/ehz155 [DOI] [PubMed] [Google Scholar]
- 41. Westenbrink BD, Alings M, Granger CB, Alexander JH, Lopes RD, Hylek EM, Thomas L, Wojdyla DM, Hanna M, Keltai M, et al. Anemia is associated with bleeding and mortality, but not stroke, in patients with atrial fibrillation: insights from the Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial. Am Heart J. 2017;185:140–149. doi: 10.1016/j.ahj.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 42. Bode C, Olivier CB, Duerschmied D. Anticoagulation and anaemia: old opponents from the era of VKA? Eur Heart J. 2019;40:3791–3792. doi: 10.1093/eurheartj/ehz628 [DOI] [PubMed] [Google Scholar]
- 43. Minhas AMK, Sagheer S, Shekhar R, Sheikh AB, Nazir S, Ullah W, Khan MZ, Shahid I, Dani SS, Michos ED, et al. Trends and inpatient outcomes of primary atrial fibrillation hospitalizations with underlying iron deficiency anemia: an analysis of the National Inpatient Sample Database from 2004–2018. Curr Probl Cardiol. 2022;47:101001. doi: 10.1016/j.cpcardiol.2021.101001 [DOI] [PubMed] [Google Scholar]
- 44. Li M, Liu X, Wang L, Shu L, Luan L, Yin J, Zhang J, Wang Q, Zhang Y, Xie T, et al. Admission hemoglobin is prognostic for in‐hospital mortality in oldest‐old patients with acute ischemic stroke. Gerontology. 2021;67:687–694. doi: 10.1159/000514678 [DOI] [PubMed] [Google Scholar]
- 45. Zhang R, Xu Q, Wang A, Jiang Y, Meng X, Zhou M, Wang Y, Liu G. Hemoglobin concentration and clinical outcomes after acute ischemic stroke or transient ischemic attack. J Am Heart Assoc. 2021;10:e022547. doi: 10.1161/jaha.121.022547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hohnloser SH. Stroke prevention versus bleeding risk in atrial fibrillation: a clinical dilemma. J Am Coll Cardiol. 2011;57:181–183. doi: 10.1016/j.jacc.2010.09.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S24
Figures S1–S6


